Benzo[d]imidazole–Naphthalen-Arylmethanone Regioisomers as CB1 Ligands: Evaluation of Agonism via an Indirect Cytotoxicity-Based Approach
Abstract
1. Introduction
2. Results and Discussion
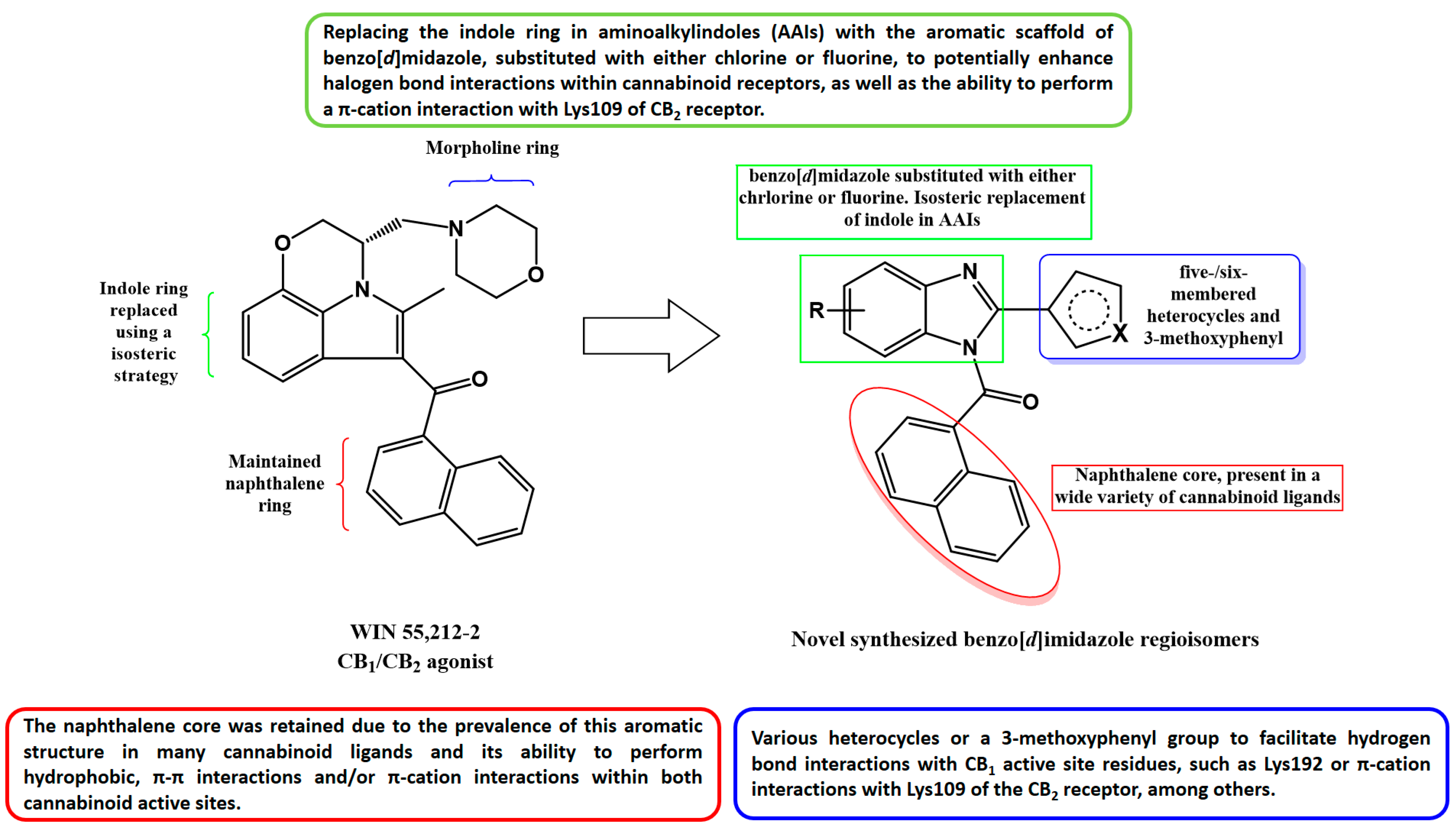
2.1. Subsection Design Criteria to Develop CB Ligands
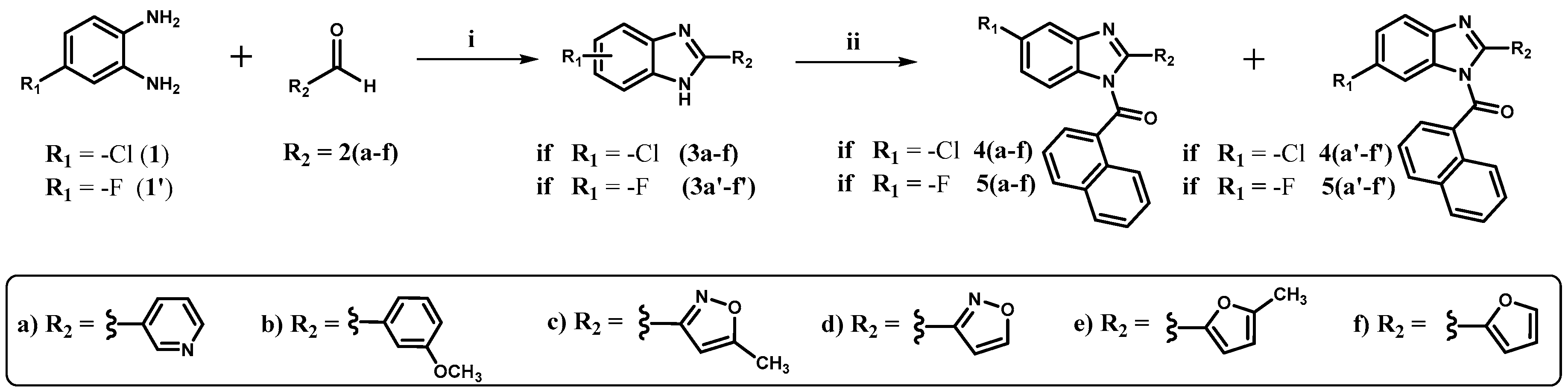
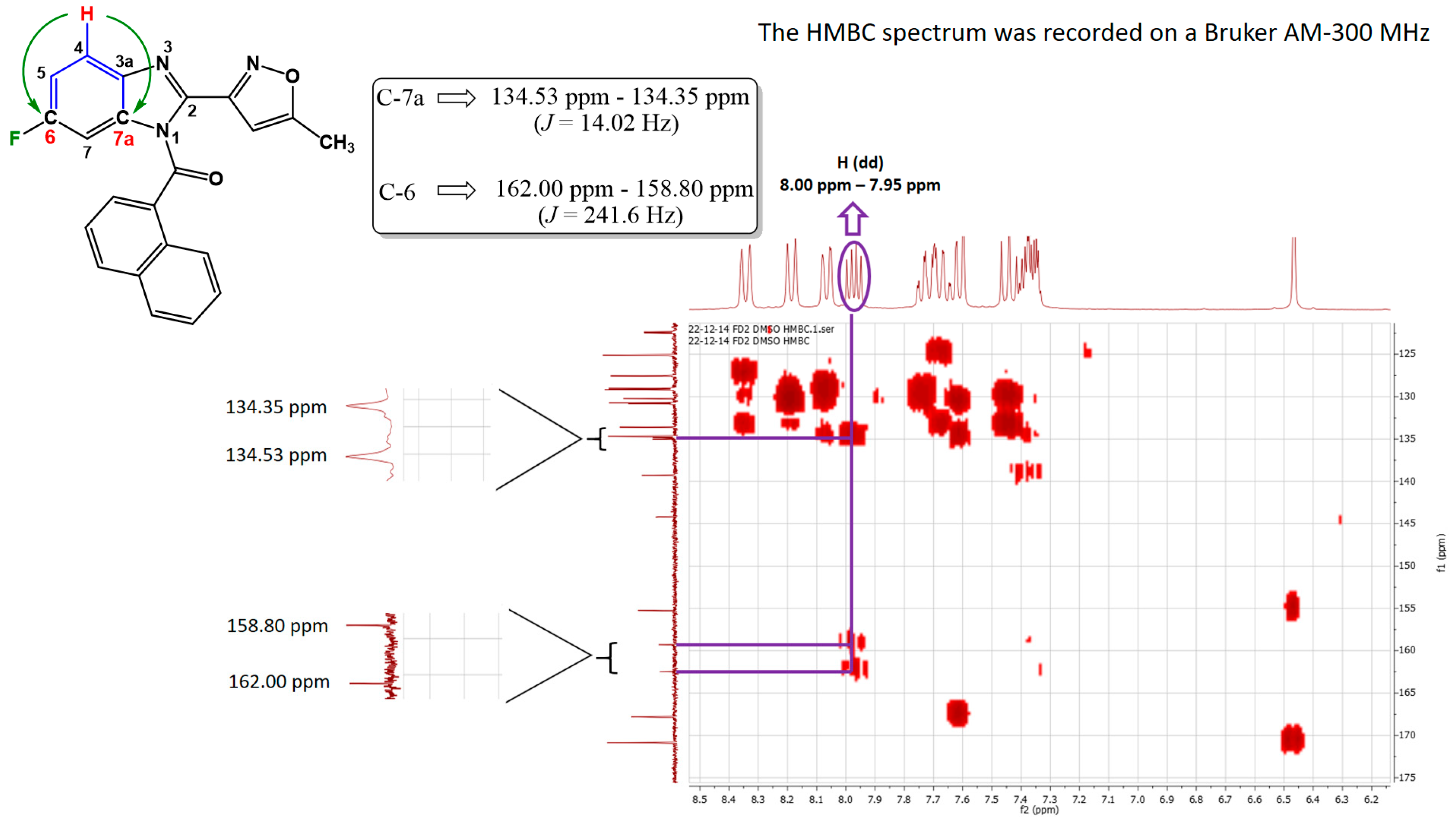
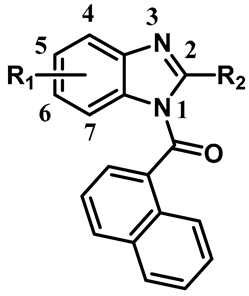
2.2. Chemistry
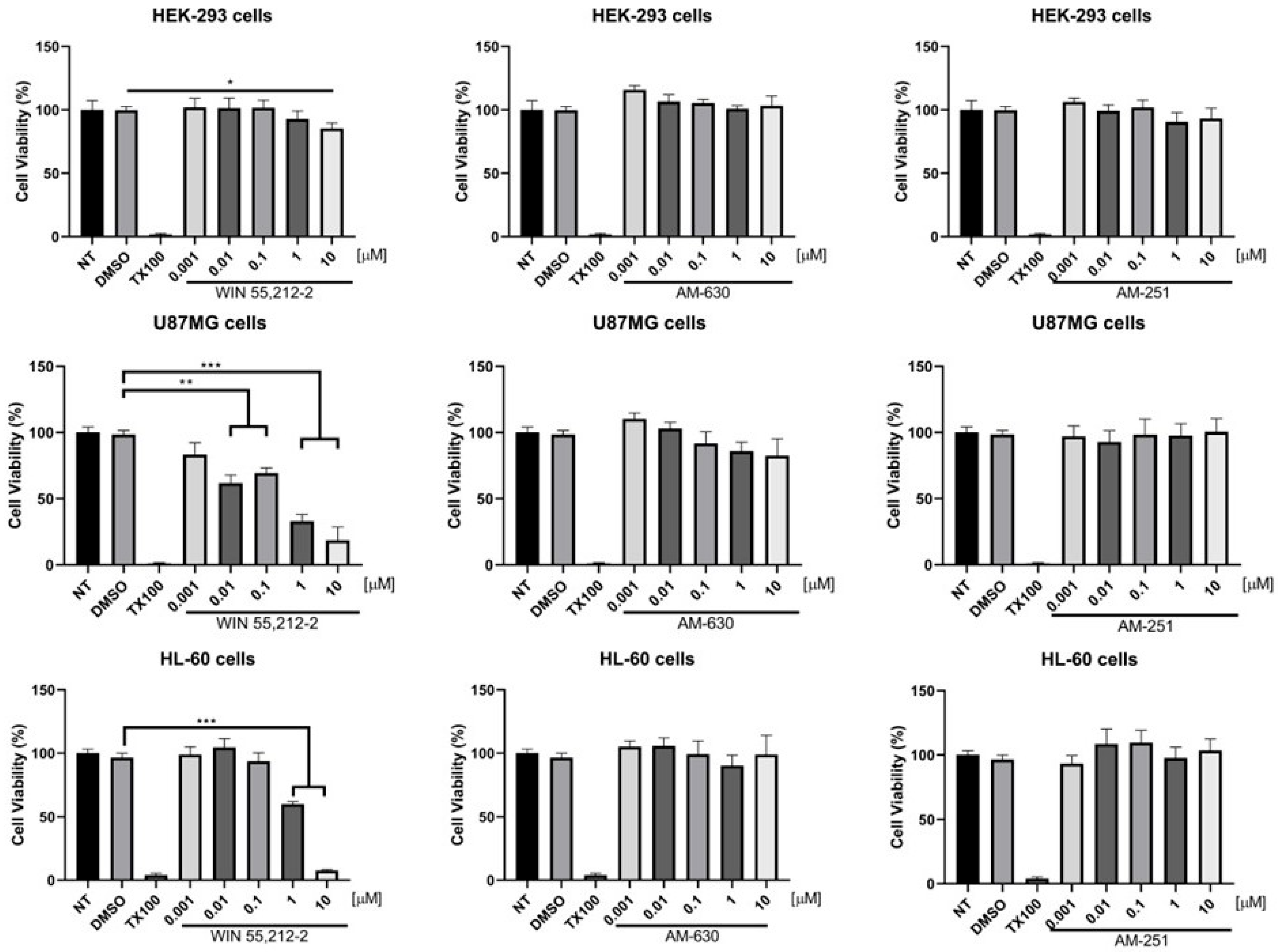
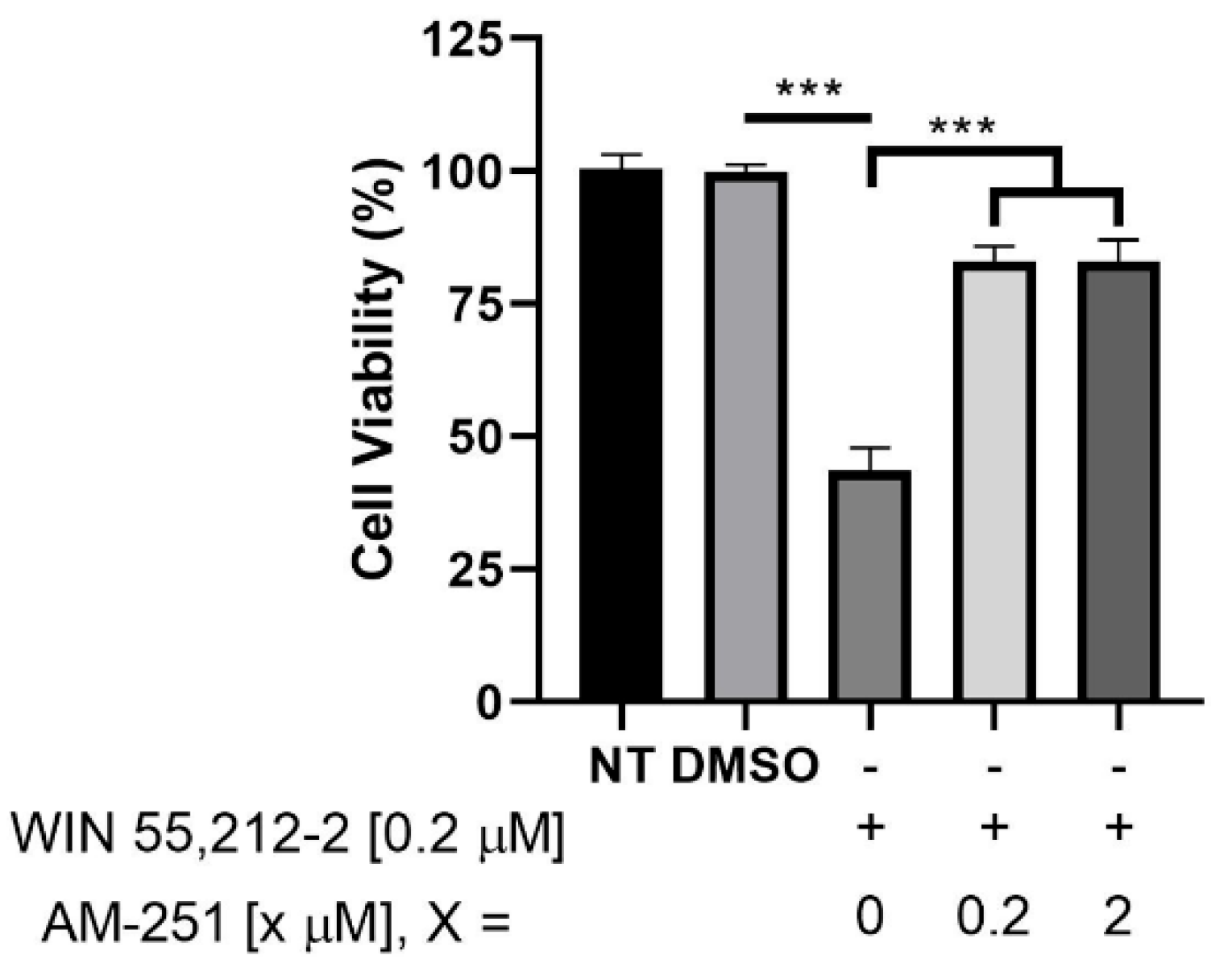
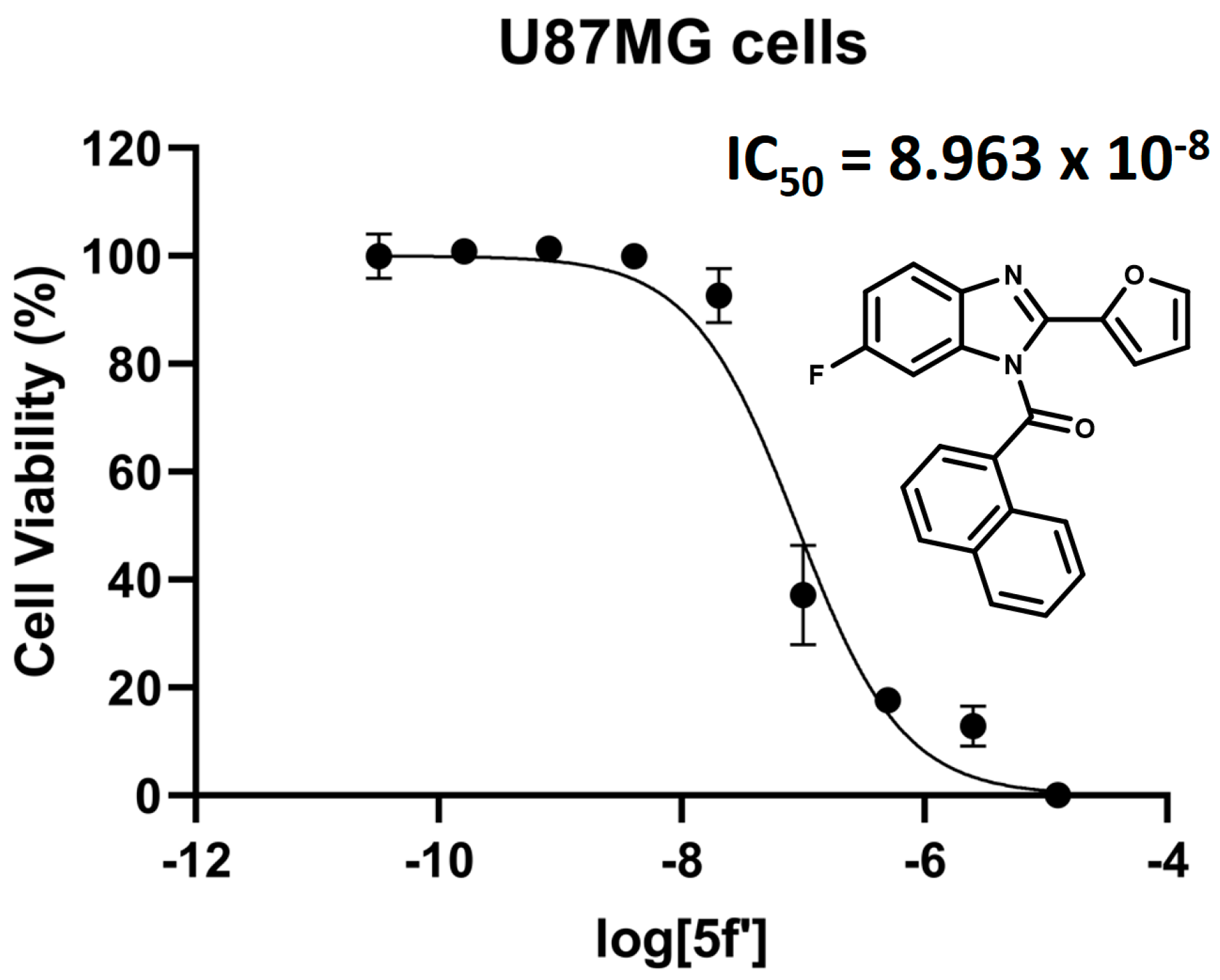
2.3. Biological Evaluations: Binding Assays and Cell Viability Experiments
2.4. Docking Simulations
2.5. Integrated SAR Analysis
2.5.1. Halogen Identity and Position on the Benzo[d]imidazole Core
2.5.2. Substitution at Position 2- of Benzo[d]imidazole: Furan Is Favored for CB1 Engagement
2.5.3. Binding vs. Functional Outcome (Indirect Agonism Readout)
- Affinity without CB1-mediated functional selectivity.
- 2.
- Putative CB1 antagonist vs. CB1 agonist within a matched pair (regioisomers 5f vs. 5f′).
- 3.
- Chloro exception with CB1 agonist-like behavior (compound 4d).
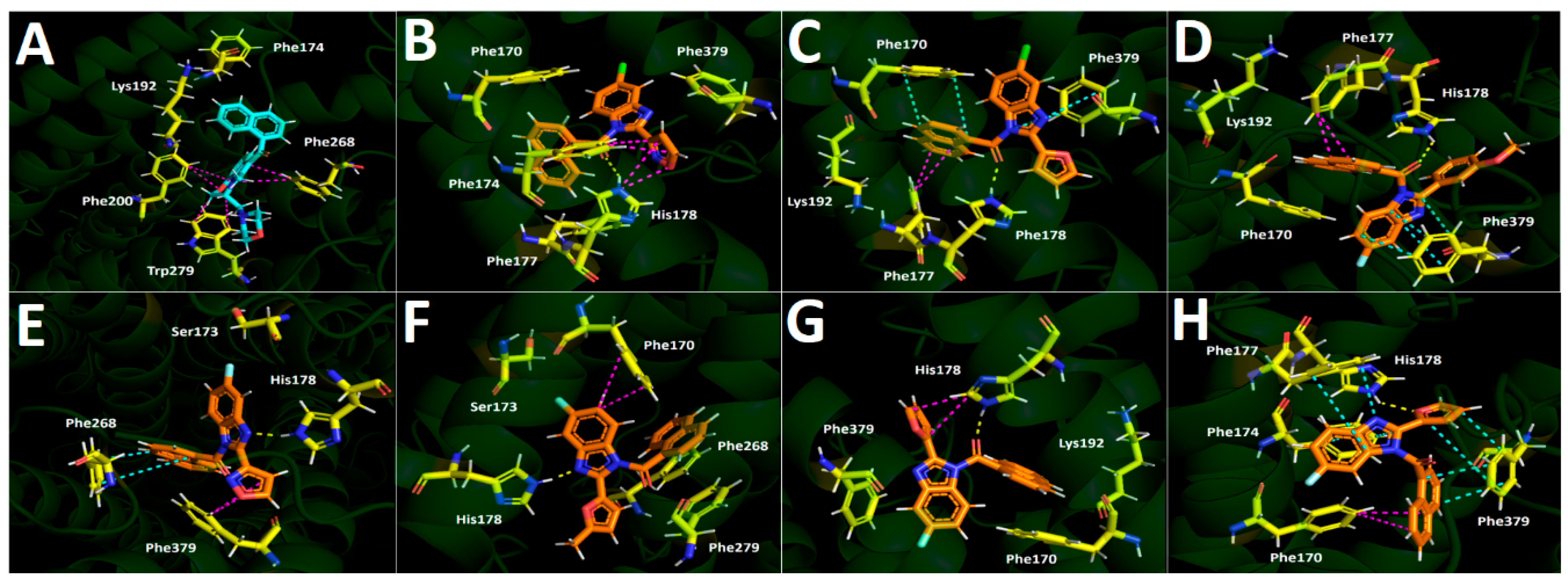
2.5.4. Binding-Mode Rationale
- Regioisomer 5f (antagonist-like): engages His178 via the carbonyl oxygen and displays a modest T-shaped contact pattern around Phe170/Phe177. This appears sufficient for affinity but insufficient for productive receptor activation within our model.
- Regioisomer 5f′ (agonist-like): shifts the His178 hydrogen bond donor–acceptor pair to the furan oxygen, which is topologically farther from the fluorine than in regioisomer 5f, mitigating inductive withdrawal from the hydrogen bond acceptor. Concomitantly, 5f′ builds a richer π-stacking network (dual π–π interaction to Phe379, plus π–π interactions to Phe177 and Phe174, and a T-shaped to Phe170). We hypothesize that this expanded aromatic engagement stabilizes an active-compatible pose, aligning with selective U87MG cytotoxicity.
- Compound 4d (agonist-like despite chlorine): combines isoxazole-mediated edge-to-face contacts (Phe174/Phe177) with a carbonyl–His178 hydrogen bond, providing an alternative route to an activation-competent geometry even in a chloro series.
3. Materials and Methods
3.1. Binding Assays CB1/CB2 (Radioligand Displacement)
3.2. Cell Cultures
3.3. Cell Viability: ([MTT-(3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide)-formazan])
3.4. Molecular Docking Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garai, S.; Kulkarni, P.M.; Schaffer, P.C.; Leo, L.M.; Brandt, A.L.; Zagzoog, A.; Black, T.; Lin, X.; Hurst, D.P.; Janero, D.R.; et al. Application of Fluorine- and Nitrogen-Walk Approaches: Defining the Structural and Functional Diversity of 2-Phenylindole Class of Cannabinoid 1 Receptor Positive Allosteric Modulators. J. Med. Chem. 2020, 63, 542–568. [Google Scholar] [CrossRef] [PubMed]
- Gado, F.; Meini, S.; Bertini, S.; Digiacomo, M.; Macchia, M.; Manera, C. Allosteric Modulators Targeting Cannabinoid Cb1 and Cb2 Receptors: Implications for Drug Discovery. Future Med. Chem. 2019, 11, 2019–2037. [Google Scholar] [CrossRef] [PubMed]
- Devane, W.A.; Dysarz, F.A.; Johnson, M.R.; Melvin, L.S.; Howlett, A.C. Determination and Characterization of a Cannabinoid Receptor in Rat Brain. Mol. Pharmacol. 1988, 34, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Petrocellis, L.D.; Cascio, M.G.; Marzo, V.D. The Endocannabinoid System: A General View and Latest Additions. Br. J. Pharmacol. 2004, 141, 765–774. [Google Scholar] [CrossRef]
- Matsuda, L.A.; Lolait, S.J.; Brownstein, M.J.; Young, A.C.; Bonner, T.I. Structure of a Cannabinoid Receptor and Functional Expression of the Cloned cDNA. Nature 1990, 346, 561–564. [Google Scholar] [CrossRef]
- Munro, S.; Thomas, K.L.; Abu-Shaar, M. Molecular Characterization of a Peripheral Receptor for Cannabinoids. Nature 1993, 365, 61–65. [Google Scholar] [CrossRef]
- Romero, J.; Lastres-Becker, I.; de Miguel, R.; Berrendero, F.; Ramos, J.A.; Fernández-Ruiz, J. The Endogenous Cannabinoid System and the Basal Ganglia: Biochemical, Pharmacological, and Therapeutic Aspects. Pharmacol. Ther. 2002, 95, 137–152. [Google Scholar] [CrossRef]
- Guzmán, M. Cannabinoids: Potential Anticancer Agents. Nat. Rev. Cancer. 2003, 3, 745–755. [Google Scholar] [CrossRef]
- Cudaback, E.; Marrs, W.; Moeller, T.; Stella, N. The Expression Level of CB1 and CB2 Receptors Determines Their Efficacy at Inducing Apoptosis in Astrocytomas. PLoS ONE 2010, 5, e8702. [Google Scholar] [CrossRef]
- Downer, E.; Boland, B.; Fogarty, M.; Campbell, V. Δ9-Tetrahydrocannabinol Induces the Apoptotic Pathway in Cultured Cortical Neurones via Activation of the CB1 Receptor. NeuroReport 2001, 12, 3973–3978. [Google Scholar] [CrossRef]
- Maccarrone, M.; Finazzi-Agró, A. The Endocannabinoid System, Anandamide and the Regulation of Mammalian Cell Apoptosis. Cell Death. Differ. 2003, 10, 946–955. [Google Scholar] [CrossRef] [PubMed]
- Campbell, V.A. Tetrahydrocannabinol-Induced Apoptosis of Cultured Cortical Neurones Is Associated with Cytochrome c Release and Caspase-3 Activation. Neuropharmacology 2001, 40, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Rieder, S.A.; Chauhan, A.; Singh, U.; Nagarkatti, M.; Nagarkatti, P. Cannabinoid-Induced Apoptosis in Immune Cells as a Pathway to Immunosuppression. Immunobiology 2010, 215, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Mackie, K. Cannabinoid receptors as therapeutic targets. Annu. Rev. Pharmacol. Toxicol. 2006, 46, 101–122. [Google Scholar] [CrossRef]
- Lowe, H.; Toyang, N.; Steele, B.; Bryant, J.; Ngwa, W. The Endocannabinoid System: A Potential Target for the Treatment of Various Diseases. Int. J. Mol. Sci. 2021, 22, 9472. [Google Scholar] [CrossRef]
- Khan, M.I.; Sobocińska, A.A.; Czarnecka, A.M.; Król, M.; Botta, B.; Szczylik, C. The Therapeutic Aspects of the Endocannabinoid System (ECS) for Cancer and Their Development: From Nature to Laboratory. Curr. Pharm. Des. 2016, 22, 1756–1766. [Google Scholar] [CrossRef]
- Russo, E.B.; Guy, G.W.; Robson, P.J. Cannabis, Pain, and Sleep: Lessons from Therapeutic Clinical Trials of Sativex®, a Cannabis-Based Medicine. Chem. Biodivers. 2007, 4, 1729–1743. [Google Scholar] [CrossRef]
- Carley, D.W.; Prasad, B.; Reid, K.J.; Malkani, R.; Attarian, H.; Abbott, S.M.; Vern, B.; Xie, H.; Yuan, C.; Zee, P.C. Pharmacotherapy of Apnea by Cannabimimetic Enhancement, the PACE Clinical Trial: Effects of Dronabinol in Obstructive Sleep Apnea. Sleep 2018, 41, zsx184. [Google Scholar] [CrossRef]
- Palmer, S.L.; Thakur, G.A.; Makriyannis, A. Cannabinergic Ligands. Chem. Phys. Lipids 2002, 121, 3–19. [Google Scholar] [CrossRef]
- Alam, R.M.; Keating, J.J. Adding More “Spice” to the Pot: A Review of the Chemistry and Pharmacology of Newly Emerging Heterocyclic Synthetic Cannabinoid Receptor Agonists. Drug Test. Anal. 2020, 12, 297–315. [Google Scholar] [CrossRef]
- Worob, A.; Wenthur, C. DARK Classics in Chemical Neuroscience: Synthetic Cannabinoids (Spice/K2). ACS Chem. Neurosci. 2020, 11, 3881–3892. [Google Scholar] [CrossRef] [PubMed]
- Schoeder, C.T.; Hess, C.; Madea, B.; Meiler, J.; Müller, C.E. Pharmacological Evaluation of New Constituents of “Spice”: Synthetic Cannabinoids Based on Indole, Indazole, Benzimidazole and Carbazole Scaffolds. Forensic Toxicol. 2018, 36, 385–403. [Google Scholar] [CrossRef] [PubMed]
- Mella-Raipán, J.A.; Lagos, C.F.; Recabarren-Gajardo, G.; Espinosa-Bustos, C.; Romero-Parra, J.; Pessoa-Mahana, H.; Iturriaga-Vásquez, P.; Pessoa-Mahana, C.D. Design, Synthesis, Binding and Docking-Based 3D-QSAR Studies of 2-Pyridylbenzimidazoles—A New Family of High Affinity CB1 Cannabinoid Ligands. Molecules 2013, 18, 3972–4001. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Bustos, C.; Lagos, C.F.; Romero-Parra, J.; Zárate, A.M.; Mella-Raipán, J.; Pessoa-Mahana, H.; Recabarren-Gajardo, G.; Iturriaga-Vásquez, P.; Tapia, R.A.; Pessoa-Mahana, C.D. Design, Synthesis, Biological Evaluation and Binding Mode Modeling of Benzimidazole Derivatives Targeting the Cannabinoid Receptor Type 1. Arch. Pharm. 2015, 348, 81–88. [Google Scholar] [CrossRef]
- Romero-Parra, J.; Mella-Raipán, J.; Palmieri, V.; Allarà, M.; Torres, M.J.; Pessoa-Mahana, H.; Iturriaga-Vásquez, P.; Escobar, R.; Faúndez, M.; Di Marzo, V.; et al. Synthesis, Binding Assays, Cytotoxic Activity and Docking Studies of Benzimidazole and Benzothiophene Derivatives with Selective Affinity for the CB2 Cannabinoid Receptor. Eur. J. Med. Chem. 2016, 124, 17–35. [Google Scholar] [CrossRef]
- Romero-Parra, J.; Chung, H.; Tapia, R.A.; Faúndez, M.; Morales-Verdejo, C.; Lorca, M.; Lagos, C.F.; Di Marzo, V.; David Pessoa-Mahana, C.; Mella, J. Combined CoMFA and CoMSIA 3D-QSAR Study of Benzimidazole and Benzothiophene Derivatives with Selective Affinity for the CB2 Cannabinoid Receptor. Eur. J. Pharm. Sci. 2017, 101, 1–10. [Google Scholar] [CrossRef]
- Lorca, M.; Valdes, Y.; Chung, H.; Romero-Parra, J.; Pessoa-Mahana, C.D.; Mella, J. Three-Dimensional Quantitative Structure-Activity Relationships (3D-QSAR) on a Series of Piperazine-Carboxamides Fatty Acid Amide Hydrolase (FAAH) Inhibitors as a Useful Tool for the Design of New Cannabinoid Ligands. Int. J. Mol. Sci. 2019, 20, 2510. [Google Scholar] [CrossRef]
- Cho, A.Y.H.; Chung, H.; Romero-Parra, J.; Kumar, P.; Allarà, M.; Ligresti, A.; Gallardo-Garrido, C.; Pessoa-Mahana, H.; Faúndez, M.; Pessoa-Mahana, C.D. Motifs in Natural Products as Useful Scaffolds to Obtain Novel Benzo[d]Imidazole-Based Cannabinoid Type 2 (CB2) Receptor Agonists. Int. J. Mol. Sci. 2023, 24, 10918. [Google Scholar] [CrossRef]
- Tahlan, S.; Kumar, S.; Narasimhan, B. Pharmacological Significance of Heterocyclic 1H-Benzimidazole Scaffolds: A Review. BMC Chem. 2019, 13, 101. [Google Scholar] [CrossRef]
- Bansal, Y.; Silakari, O. The Therapeutic Journey of Benzimidazoles: A Review. Bioorg. Med. Chem. 2012, 20, 6208–6236. [Google Scholar] [CrossRef]
- Ajani, O.O.; Aderohunmu, D.V.; Ikpo, C.O.; Adedapo, A.E.; Olanrewaju, I.O. Functionalized Benzimidazole Scaffolds: Privileged Heterocycle for Drug Design in Therapeutic Medicine. Arch. Pharm. 2016, 349, 475–506. [Google Scholar] [CrossRef] [PubMed]
- Balenga, N.A.; Martínez-Pinilla, E.; Kargl, J.; Schröder, R.; Peinhaupt, M.; Platzer, W.; Bálint, Z.; Zamarbide, M.; Dopeso-Reyes, I.G.; Ricobaraza, A.; et al. Heteromerization of GPR55 and Cannabinoid CB2 Receptors Modulates Signalling. Br. J. Pharmacol. 2014, 171, 5387–5406. [Google Scholar] [CrossRef] [PubMed]
- Ciaglia, E.; Torelli, G.; Pisanti, S.; Picardi, P.; D’Alessandro, A.; Laezza, C.; Malfitano, A.M.; Fiore, D.; Pagano Zottola, A.C.; Proto, M.C.; et al. Cannabinoid Receptor CB1 Regulates STAT3 Activity and Its Expression Dictates the Responsiveness to SR141716 Treatment in Human Glioma Patients’ Cells. Oncotarget 2015, 6, 15464–15481. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.H.; Bonner, T.I. A Lysine Residue of the Cannabinoid Receptor Is Critical for Receptor Recognition by Several Agonists but Not WIN55212-2. Mol. Pharmacol. 1996, 49, 891–896. [Google Scholar] [CrossRef]
- Pan, X.; Ikeda, S.R.; Lewis, D.L. SR 141716A Acts as an Inverse Agonist to Increase Neuronal Voltage-Dependent Ca2+ Currents by Reversal of Tonic CB1 Cannabinoid Receptor Activity. Mol. Pharmacol. 1998, 54, 1064–1072. [Google Scholar] [CrossRef]
- Chin, C.N.; Lucas-Lenard, J.; Abadji, V.; Kendall, D.A. Ligand Binding and Modulation of Cyclic AMP Levels Depend on the Chemical Nature of Residue 192 of the Human Cannabinoid Receptor 1. J. Neurochem. 1998, 70, 366–373. [Google Scholar] [CrossRef]
- Hurst, D.P.; Lynch, D.L.; Barnett-Norris, J.; Hyatt, S.M.; Seltzman, H.H.; Zhong, M.; Song, Z.-H.; Nie, J.; Lewis, D.; Reggio, P.H. N-(Piperidin-1-Yl)-5-(4-Chlorophenyl)-1-(2,4-Dichlorophenyl)-4-Methyl-1H-Pyrazole-3-Carboxamide (SR141716A) Interaction with LYS 3.28(192) Is Crucial for Its Inverse Agonism at the Cannabinoid CB1 Receptor. Mol. Pharmacol. 2002, 62, 1274–1287. [Google Scholar] [CrossRef]
- Xing, C.; Zhuang, Y.; Xu, T.-H.; Feng, Z.; Zhou, X.E.; Chen, M.; Wang, L.; Meng, X.; Xue, Y.; Wang, J.; et al. Cryo-EM Structure of the Human Cannabinoid Receptor CB2-Gi Signaling Complex. Cell 2020, 180, 645–654.e13. [Google Scholar] [CrossRef]
- Kumar, A.; Maurya, R.A.; Saxena, D. Diversity-oriented synthesis of benzimidazole, benzoxazole, benzothiazole and quinazolin-4 (3H)-one libraries via potassium persulfate–CuSO4-mediated oxidative coupling reactions of aldehydes in aqueous micelles. Mol. Divers. 2010, 14, 331–341. [Google Scholar] [CrossRef]
- Jung, M.H.; Park, J.M.; Lee, I.-Y.C.; Ahn, M. Synthesis of 2-(1-methyl-1, 2, 5, 6-tetrahydropyridin-3-yl) benzimidazoles. J. Heterocycl. Chem. 2003, 40, 37–44. [Google Scholar] [CrossRef]
- Siddiqui, H.; Farooq, R.; Marasini, B.P.; Malik, R.; Syed, N.; Moin, S.T.; Rahman, A.-U.; Choudhary, M.I. Synthesis and in vitro α-chymotrypsin inhibitory activity of 6-chlorobenzimidazole derivatives. Bioorg. Med. Chem. 2016, 24, 3387–3395. [Google Scholar] [CrossRef]
- Akande, A.A.; Salar, U.; Khan, K.M.; Syed, S.; Aboada, S.A.; Chigurupati, S.; Wadood, A.; Riaz, M.; Taha, M.; Bhatia, S.; et al. Substituted benzimidazole analogues as potential α-amylase inhibitors and radical scavengers. ACS Omega 2021, 6, 22726–22739. [Google Scholar] [CrossRef]
- Pham, E.C.; Le, T.V.T.; Truong, T.N. Design, synthesis, bio-evaluation, and in silico studies of some N-substituted 6-(chloro/nitro)-1 H-benzimidazole derivatives as antimicrobial and anticancer agents. RSC Adv. 2022, 12, 21621–21646. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.B. The Chemistry of the Benzimidazoles. Chem. Rev. 1951, 48, 397–541. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Prusoff, W.H. Relationship between the Inhibition Constant (K1) and the Concentration of Inhibitor Which Causes 50 per Cent Inhibition (I50) of an Enzymatic Reaction. Biochem. Pharmacol. 1973, 22, 3099–3108. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G. Pharmacological Actions of Cannabinoids. In Cannabinoids; Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2005; Volume 168, pp. 1–51. [Google Scholar] [CrossRef]
- An, D.; Peigneur, S.; Hendrickx, L.A.; Tytgat, J. Targeting Cannabinoid Receptors: Current Status and Prospects of Natural Products. Int. J. Mol. Sci. 2020, 21, 5064. [Google Scholar] [CrossRef]
- Yang, X.; Wang, X.; Xu, Z.; Wu, C.; Zhou, Y.; Wang, Y.; Lin, G.; Li, K.; Wu, M.; Xia, A.; et al. Molecular Mechanism of Allosteric Modulation for the Cannabinoid Receptor CB1. Nat. Chem. Biol. 2022, 18, 831–840. [Google Scholar] [CrossRef]
- Mosorov, V. The Lambert-Beer law in time domain form and its application. Appl. Radiat. Isot. 2017, 128, 1–5. [Google Scholar] [CrossRef]
- Schrödinger Release 2018-2: Maestro, Version 11.8; Schrödinger, LLC: New York, NY, USA, 2018.
- Rose, P.W.; Prlić, A.; Bi, C.; Bluhm, W.F.; Christie, C.H.; Dutta, S.; Green, R.K.; Goodsell, D.S.; Westbrook, J.D.; Woo, J.; et al. The RCSB Protein Data Bank: Views of structural biology for basic and applied research and education. Nucleic Acids Res. 2015, 43, D345–D356. [Google Scholar] [CrossRef]
- Sherman, W.; Day, T.; Jacobson, M.P.; Friesner, R.A.; Farid, R. Novel procedure for modeling ligand/receptor induced fit effects. J. Med. Chem. 2006, 49, 534–553. [Google Scholar] [CrossRef]
- Friesner, R.A.; Murphy, R.B.; Repasky, M.P.; Frye, L.L.; Greenwood, J.R.; Halgren, T.A.; Sanschagrin, P.C.; Mainz, D.T. Extra precision glide: Docking and scoring incorporating a model of hydrophobic enclosure for protein−ligand complexes. J. Med. Chem. 2006, 49, 6177–6196. [Google Scholar] [CrossRef]
- DeLano, W. PyMol: An Open-Source Molecular Graphics Tool. CCP4 Newsletter on Protein Crystallography. 2002. Available online: https://mozart.gmb.bio.br/uploads/6/4/3/6/64362047/pymol.pdf#page=44 (accessed on 6 October 2025).



 | ||||||||
| CB1 | CB2 | |||||||
| Compound | R1 | R2 | IC50 (µM) | Ki (µM) | Max Tested | IC50 (µM) | Ki (µM) | Max Tested |
| µM (% Displacement) | µM (% Displacement) | |||||||
| 4a | 5-Cl | pyridin-3-yl | >10 | >10 | 10 μM (36%) | 10 | 6.6 | 10 μM (35%) |
| 4a′ | 6-Cl | >10 | >10 | 10 μM (20%) | 10 | 6.6 | 10 μM (52%) | |
| 4b | 5-Cl | 3-methoxyphenyl | >10 | >10 | 10 μM (39%) | >10 | >10 | 10 μM (48%) |
| 4b′ | 6-Cl | >10 | >10 | 10 μM (43%) | 6.76 ± 3.09 | 4.46 ± 2.68 | 10 μM (58%) | |
| 4c | 5-Cl | 5-methylisoxazole | >10 | >10 | 10 μM (38%) | >10 | >10 | 10 μM (30%) |
| 4c′ | 6-Cl | >10 | >10 | 10 μM (44%) | >10 | >10 | 10 μM (29%) | |
| 4d | 5-Cl | isoxazole | 7.58 ± 4.21 | 1.86 ± 2.27 | 10 μM (55%) | >10 | >10 | 10 μM (15%) |
| 4d′ | 6-Cl | >10 | >10 | 10 μM (47%) | >10 | >10 | 10 μM (42%) | |
| 4e | 5-Cl | 5-methylfuran-2-yl | >10 | >10 | 10 μM (30%) | >10 | >10 | 10 μM (29%) |
| 4e′ | 6-Cl | >10 | >10 | 10 μM (38%) | >10 | >10 | 10 μM (52.8%) | |
| 4f | 5-Cl | furan-2-yl | >10 | >10 | 10 μM (56.5%) | >10 | >10 | 10 μM (46.5%) |
| 4f′ | 6-Cl | >10 | >10 | 10 μM (47.5%) | >10 | >10 | 10 μM (53.25%) | |
| 5a | 5-F | pyridin-3-yl | >10 | >10 | 10 μM (41%) | >10 | >10 | 10 μM (43%) |
| 5a′ | 6-F | >10 | >10 | 10 μM (9.5%) | >10 | >10 | 10 μM (16%) | |
| 5b | 5-F | 3-methoxyphenyl | 6.23 ± 3.80 | 1.53 ± 1.91 | 10 μM (58.25%) | >10 | >10 | 10 μM (45%) |
| 5b′ | 6-F | >10 | >10 | 10 μM (47%) | >10 | >10 | 10 μM (53.5%) | |
| 5c | 5-F | 5-methylisoxazole | >10 | >10 | 10 μM (54.5%) | >10 | >10 | 10 μM (24%) |
| 5c′ | 6-F | >10 | >10 | 10 μM (37%) | >10 | >10 | 10 μM (29%) | |
| 5d | 5-F | isoxazole | >10 | >10 | 10 μM (54%) | >10 | >10 | 10 μM (51%) |
| 5d′ | 6-F | >10 | >10 | 10 μM (34%) | >10 | >10 | 10 μM (26%) | |
| 5e | 5-F | 5-methylfuran-2-yl | 7.40 ± 3.28 | 1.82 ± 1.64 | 10 μM (58.25%) | >10 | >10 | 10 μM (38.5%) |
| 5e′ | 6-F | >10 | >10 | 10 μM (43%) | >10 | >10 | 10 μM (52%) | |
| 5f | 5-F | furan-2-yl | 6.03 ± 4.09 | 1.48 ± 2.03 | 10 μM (65.5) | >10 | >10 | 10 μM (54.5%) |
| 5f′ | 6-F | 8.65 ± 3.11 | 2.12 ± 1.55 | 10 μM (55%) | >10 | >10 | 10 μM (47%) | |
| % Toxicity | |||
|---|---|---|---|
| Compound | HEK293T | U87MG | HL-60 |
| 4a′ | NT | NT | NT |
| 4b′ | 50 ± 11 | 39± 20 | 52 ± 13 |
| 4d | 10 ± 9 | 50 ± 14 | NT |
| 4e′ | NT | NT | NT |
| 4f | NT | NT | NT |
| 4f′ | 50 ± 23 | NT | 10 ± 19 |
| 5b | 75 ± 17 | 25 ± 14 | 45 ± 17 |
| 5b′ | NT | NT | NT |
| 5d | NT | NT | NT |
| 5e | 55 ± 23 | 39 ± 18 | 50 ± 19 |
| 5f | NT | NT | NT |
| 5f′ | NT | 66 ± 17 | NT |
| IC50 (μM) | |||
| WIN-55212-2 | >10 | 0.22 | 1.39 |
| AM630 | >10 | >10 | >10 |
| AM251 | >10 | >10 | >10 |
| Compound | Binding Energy (kcal/mol) |
|---|---|
| 4d | −10.227 |
| 4f | −10.608 |
| 5b | −10.061 |
| 5d | −9.767 |
| 5e | −11.096 |
| 5f | −10.152 |
| 5f′ | −10.335 |
| WIN-55212-2 | −12.808 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwa Cho, A.Y.; Burgos Ravanal, R.; Zuñiga Salazar, V.; Mellado, M.; Lorca, M.; Pessoa-Mahana, D.; Mella, J.; Günther Sapunar, G.; Romero-Parra, J. Benzo[d]imidazole–Naphthalen-Arylmethanone Regioisomers as CB1 Ligands: Evaluation of Agonism via an Indirect Cytotoxicity-Based Approach. Int. J. Mol. Sci. 2025, 26, 9986. https://doi.org/10.3390/ijms26209986
Hwa Cho AY, Burgos Ravanal R, Zuñiga Salazar V, Mellado M, Lorca M, Pessoa-Mahana D, Mella J, Günther Sapunar G, Romero-Parra J. Benzo[d]imidazole–Naphthalen-Arylmethanone Regioisomers as CB1 Ligands: Evaluation of Agonism via an Indirect Cytotoxicity-Based Approach. International Journal of Molecular Sciences. 2025; 26(20):9986. https://doi.org/10.3390/ijms26209986
Chicago/Turabian StyleHwa Cho, Analia Young, Renato Burgos Ravanal, Valeria Zuñiga Salazar, Marco Mellado, Marcos Lorca, David Pessoa-Mahana, Jaime Mella, Germán Günther Sapunar, and Javier Romero-Parra. 2025. "Benzo[d]imidazole–Naphthalen-Arylmethanone Regioisomers as CB1 Ligands: Evaluation of Agonism via an Indirect Cytotoxicity-Based Approach" International Journal of Molecular Sciences 26, no. 20: 9986. https://doi.org/10.3390/ijms26209986
APA StyleHwa Cho, A. Y., Burgos Ravanal, R., Zuñiga Salazar, V., Mellado, M., Lorca, M., Pessoa-Mahana, D., Mella, J., Günther Sapunar, G., & Romero-Parra, J. (2025). Benzo[d]imidazole–Naphthalen-Arylmethanone Regioisomers as CB1 Ligands: Evaluation of Agonism via an Indirect Cytotoxicity-Based Approach. International Journal of Molecular Sciences, 26(20), 9986. https://doi.org/10.3390/ijms26209986







