Optimizing Positive End-Expiratory Pressure in Asymmetric Acute Lung Injury in a Porcine Model: The Role of Transpulmonary Pressure
Abstract
1. Introduction
2. Results
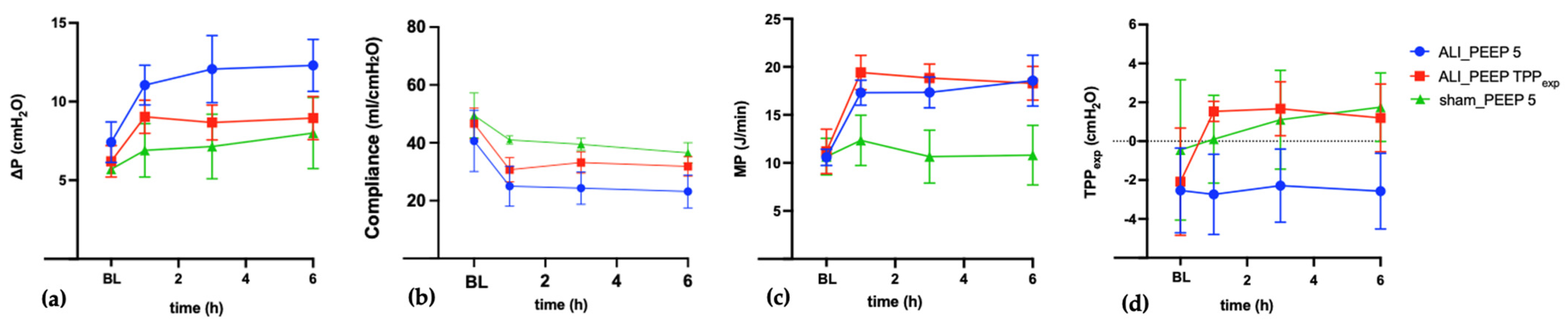
2.1. Respiratory and Hemodynamic Parameters
2.1.1. Baseline
2.1.2. t6 Results
2.1.3. Intergroup Differences in Respiratory Parameters
2.1.4. ALI_PEEP 5 vs. ALI_PEEP TPPexp
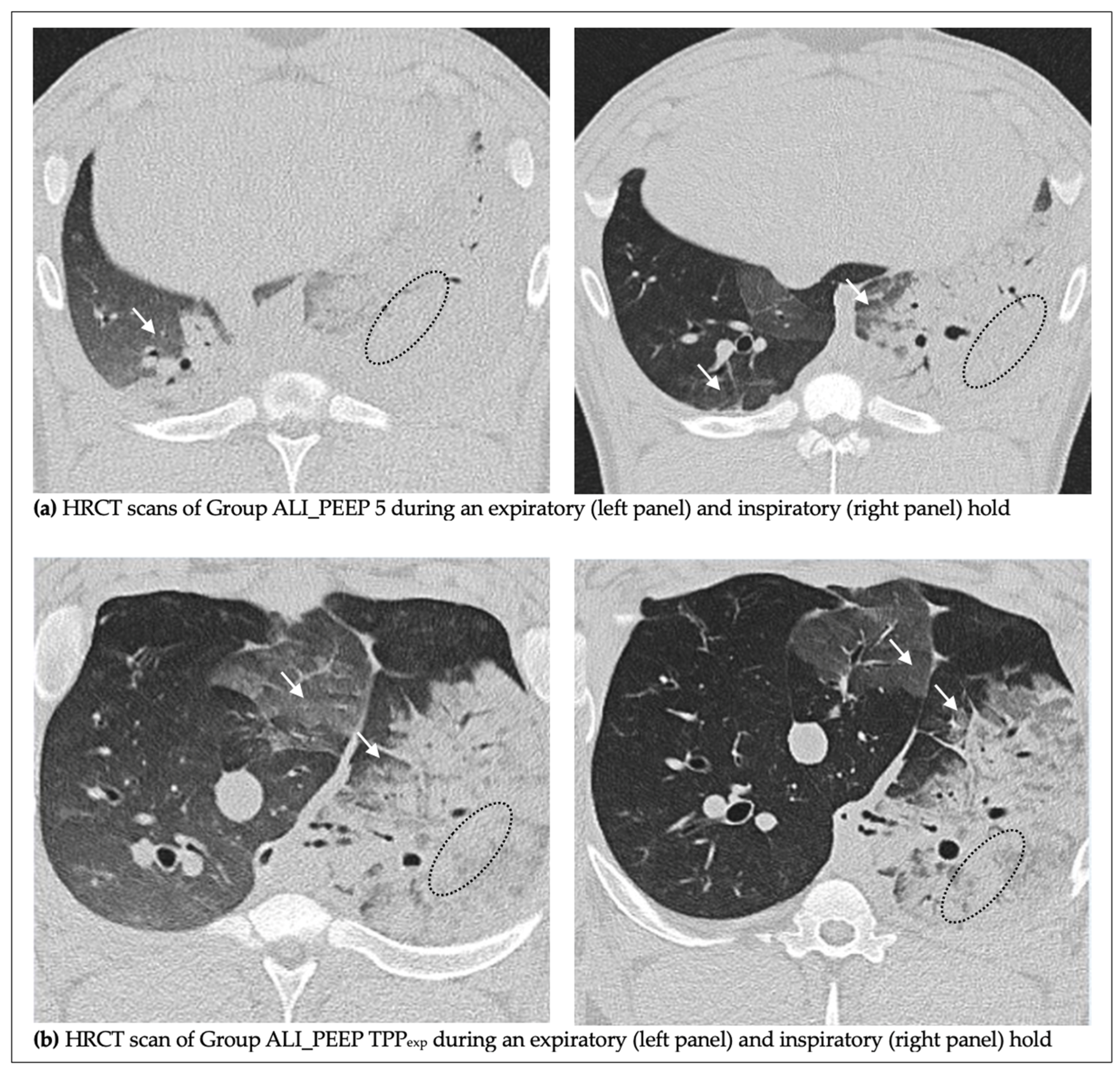
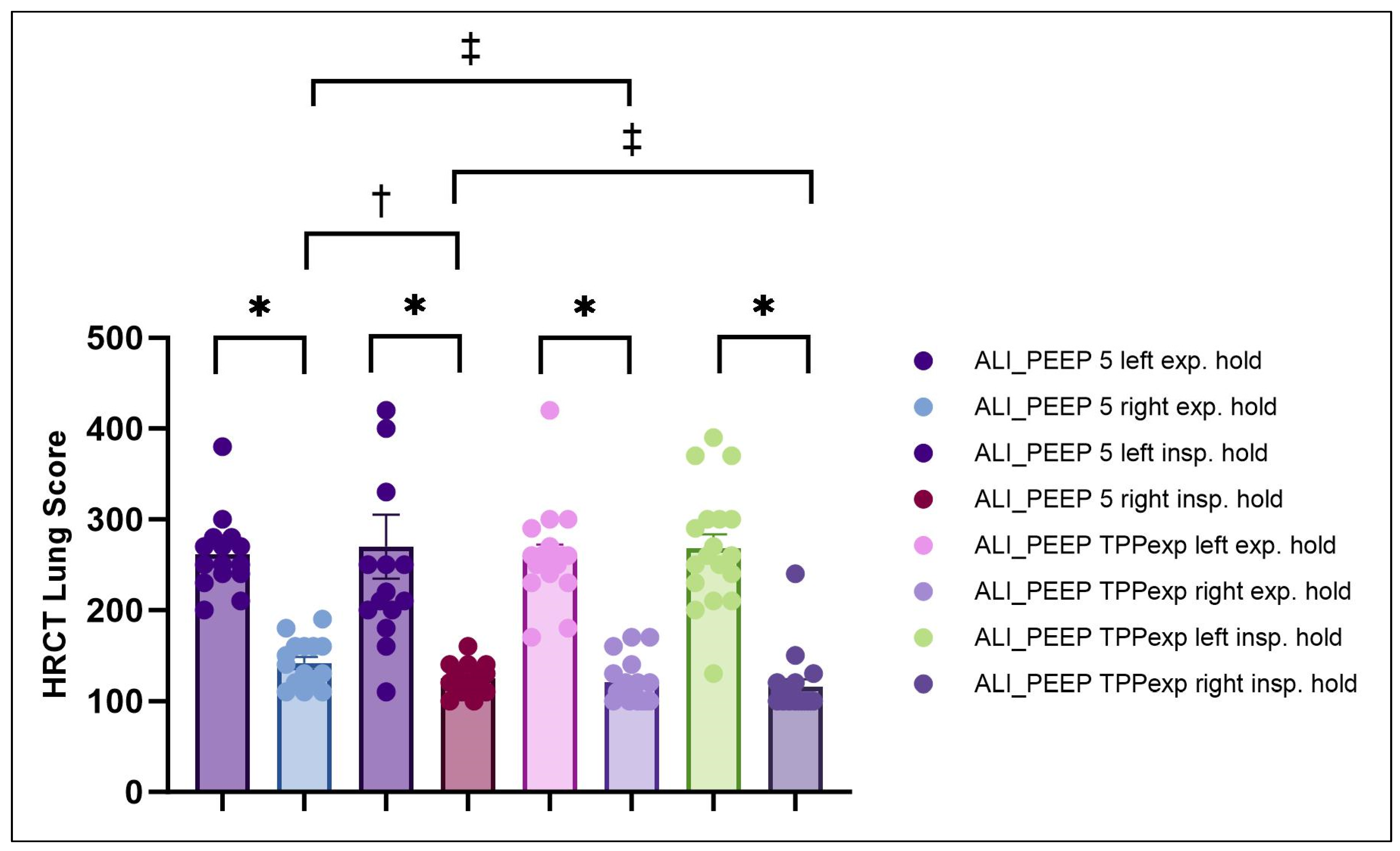
2.2. High-Resolution Computed Tomography Lung Injury Score
2.2.1. Left vs. Right Lung
2.2.2. Expiratory vs. Inspiratory Hold
2.2.3. ALI_PEEP 5 vs. ALI_PEEP TPPexp
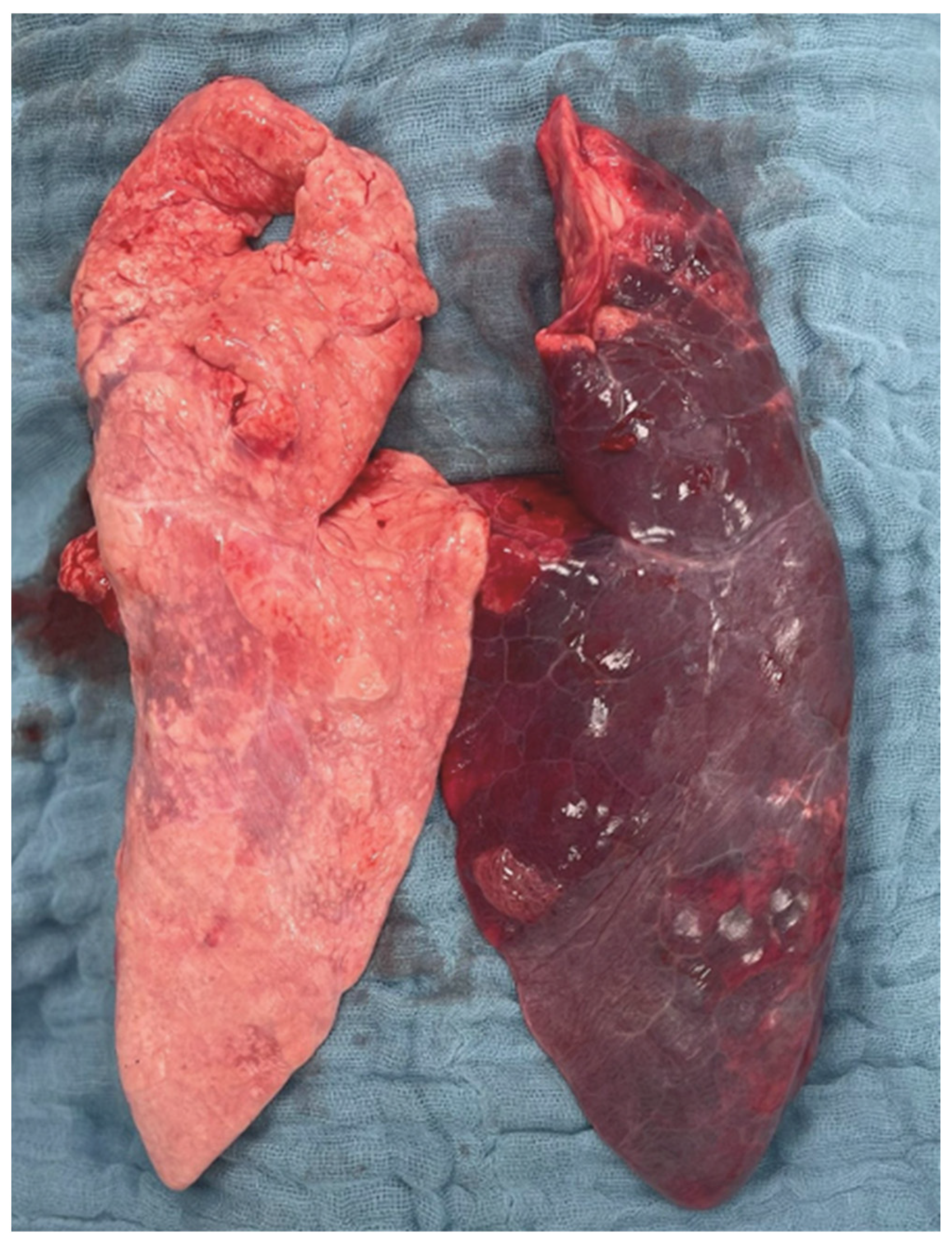
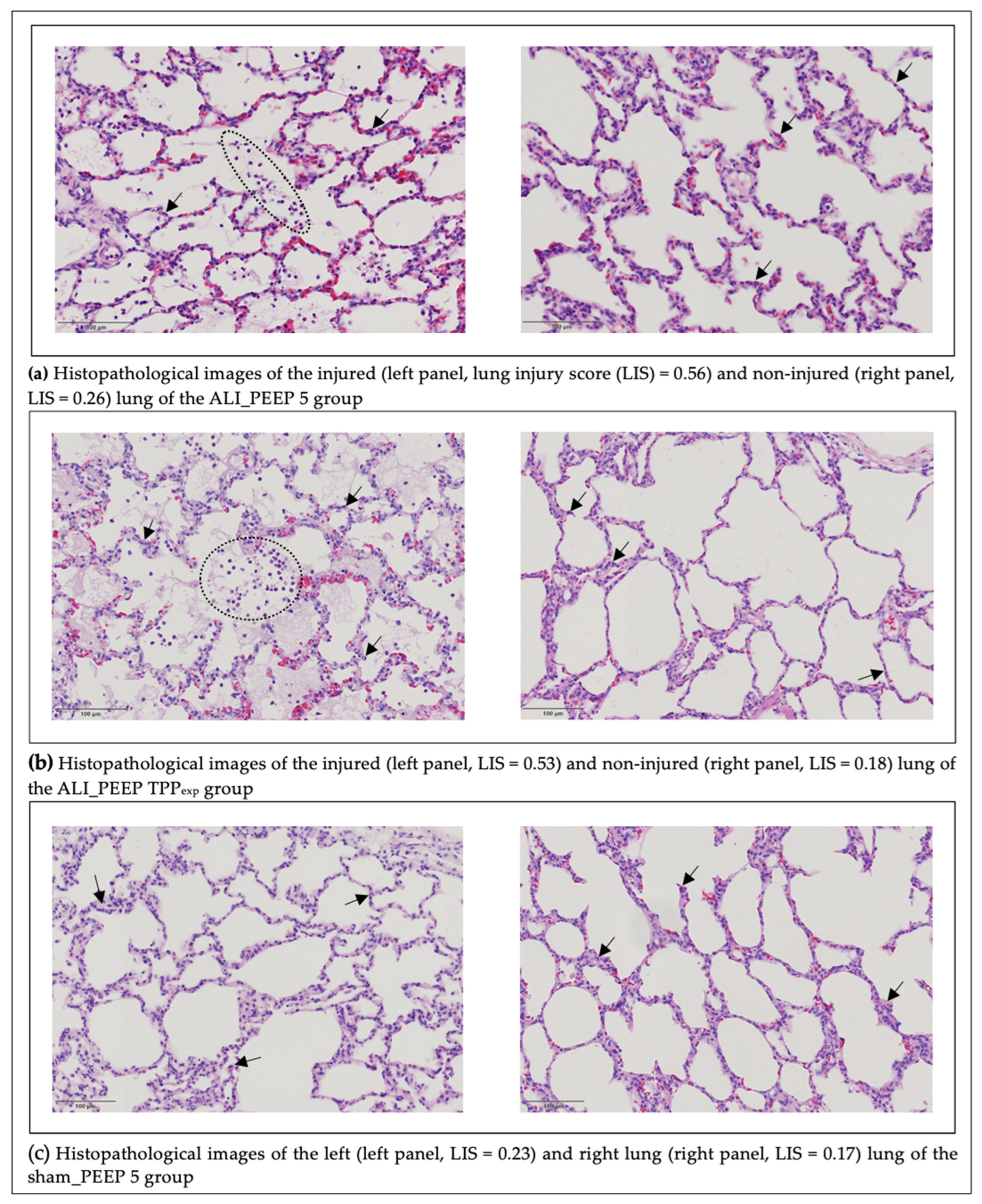
2.3. Histopathological Lung Injury Score
2.3.1. Left vs. Right Lung
2.3.2. Intergroup Differences
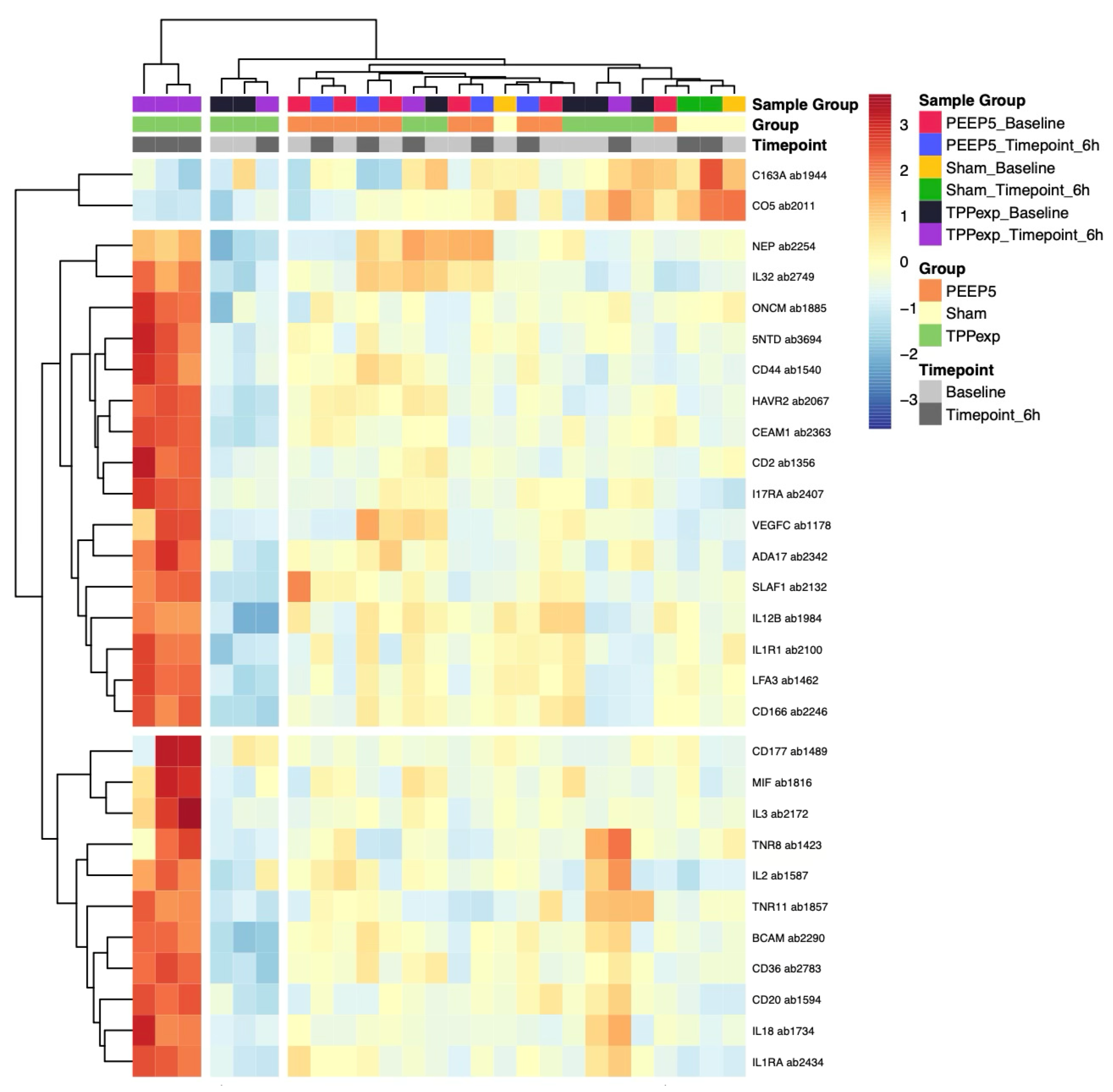
2.4. Exploratory Cytokine Profiling
2.4.1. Protein Concentration
2.4.2. Protein Regulation
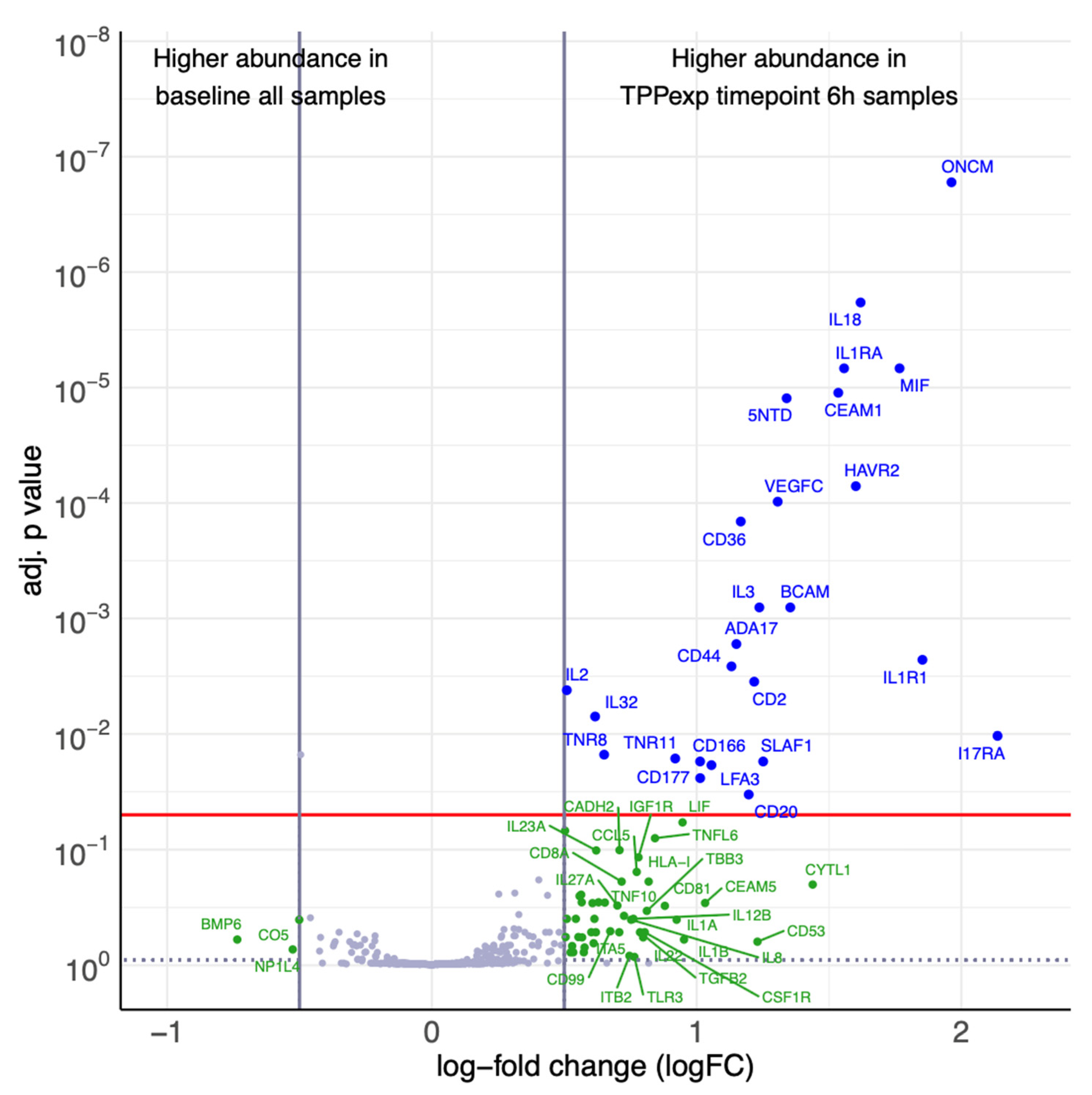
2.4.3. Timepoint-Dependent Biomarker Regulation
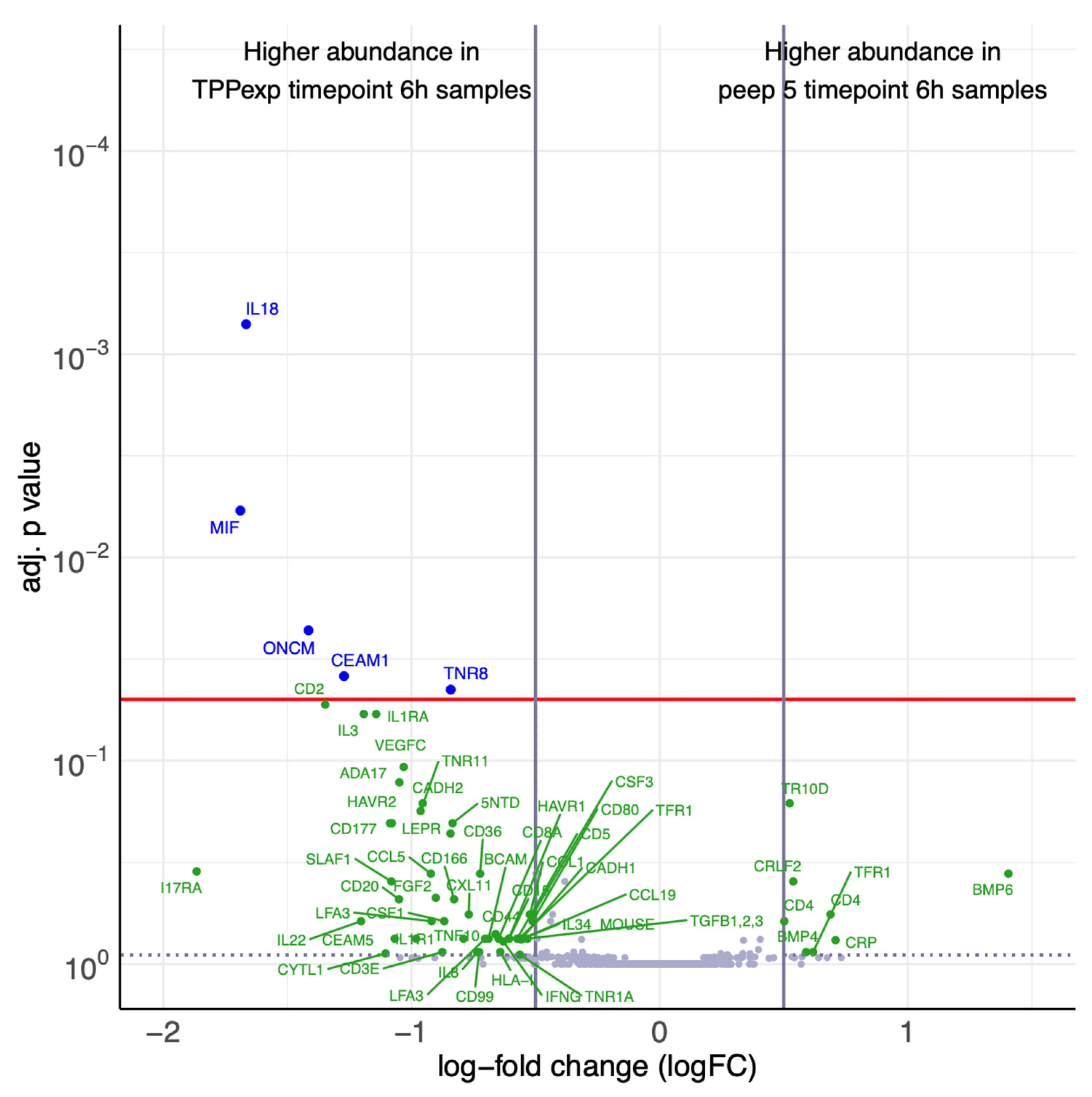
2.4.4. Ventilation Strategy-Dependent Biomarker Regulation
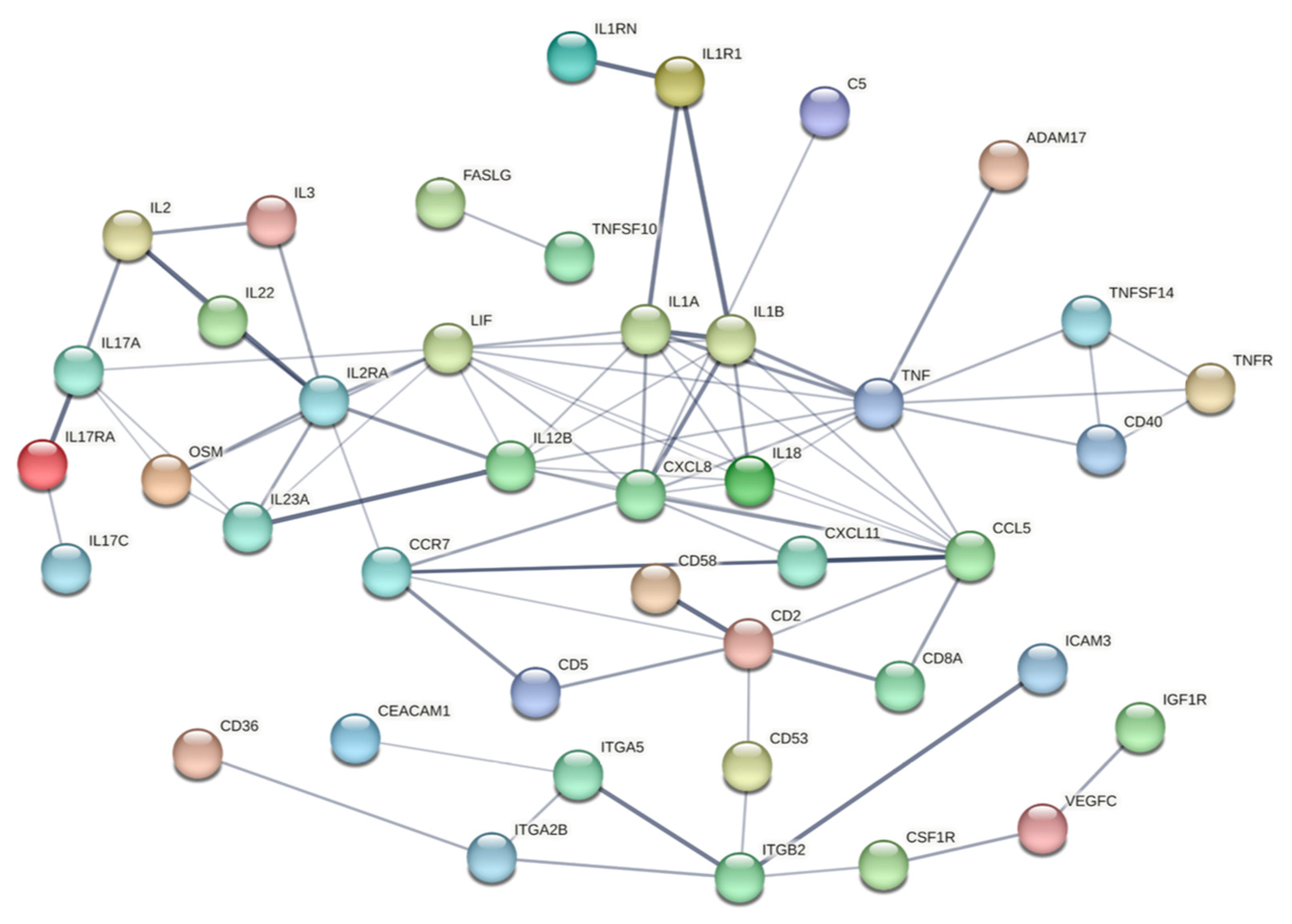
2.4.5. Protein Interaction Network Analysis
3. Discussion
3.1. Experimental Model
3.2. Respiratory Mechanics
3.3. HRCT Findings
3.4. Histopathological Findings
3.5. Exploratory Cytokine Profiling
3.6. Limitations
4. Materials and Methods
4.1. Study Settings
4.2. Ethics and Registry
4.3. Animal Preparation
4.4. Induction of Unilateral Acute Lung Injury
4.5. Experimental Protocol
4.6. Measurements of Respiratory Parameters
4.7. High-Resolution Computed Tomography
4.8. Histological Analysis
4.9. Plasma Biomarker Profiling
4.10. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALI | Acute Lung Injury |
| ANOVA | analysis of the variance |
| ARDS | Acute Respiratory Distress Syndrome |
| ATS | American thoracic society |
| bw | body weight |
| CRS | respiratory system compliance |
| CT | computed tomography |
| DAD | diffuse alveolar damage |
| DLtube | double-lumen tube |
| ΔP | driving pressure |
| ΔPL | transpulmonary pressure |
| ELWI | extravascular lung water index |
| FiO2 | inspiratory oxygen concentration |
| GEDI | global end-diastolic volume index |
| HI | heart index |
| HRCT | high-resolution computed tomography |
| I:E ratio | inspiratory-to-expiratory ratio |
| LIMMA | linear models for microarray data |
| LIS | lung injury score |
| logFC | log-fold change |
| MP | mechanical power |
| MV | mechanical ventilation |
| n | number |
| NVLI | non-ventilated lung injury |
| paCO2 | partial pressure of carbon dioxide |
| paO2 | partial pressure of oxygen |
| Ppeak | peak inspiratory pressure |
| PEEP | positive end-expiratory pressure |
| PES | esophageal pressure |
| PESexp | end-expiratory transpulmonary pressure |
| PESinsp | end-inspiratory transpulmonary pressure |
| Pplat | plateau pressure |
| RCT | randomized controlled trial |
| RR | respiratory rate |
| t6 | timepoint 6 h of mechanical ventilation |
| TPP | transpulmonary pressure |
| TPPexp | end-expiratory transpulmonary pressure |
| TPPinsp | end-inspiratory transpulmonary pressure |
| VILI | ventilator induced lung injury |
| vs. | versus |
| VT | tidal volume |
References
- Bellani, G.; Laffey, J.G.; Pham, T.; Fan, E.; Brochard, L.; Esteban, A.; Gattinoni, L.; van Haren, F.; Larsson, A.; McAuley, D.F.; et al. Epidemiology, Patterns of Care, and Mortality for Patients with Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016, 315, 788–800, Erratum in JAMA 2016, 316, 350. [Google Scholar] [CrossRef]
- Pham, T.; Pesenti, A.; Bellani, G.; Rubenfeld, G.; Fan, E.; Bugedo, G.; Lorente, J.A.; Fernandes, A.D.V.; Van Haren, F.; Bruhn, A.; et al. Outcome of acute hypoxaemic respiratory failure: Insights from the LUNG SAFE Study. Eur. Respir. J. 2021, 57, 2003317. [Google Scholar] [CrossRef] [PubMed]
- Force, A.D.T.; Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar]
- Fan, E.; Brodie, D.; Slutsky, A.S. Acute Respiratory Distress Syndrome: Advances in Diagnosis and Treatment. JAMA 2018, 319, 698–710. [Google Scholar] [CrossRef] [PubMed]
- Slutsky, A.S.; Ranieri, V.M. Ventilator-induced lung injury. N. Engl. J. Med. 2013, 369, 2126–2136. [Google Scholar] [CrossRef]
- Guerin, C.; Reignier, J.; Richard, J.C.; Beuret, P.; Gacouin, A.; Boulain, T.; Mercier, E.; Badet, M.; Mercat, A.; Baudin, O.; et al. Prone positioning in severe acute respiratory distress syndrome. N. Engl. J. Med. 2013, 368, 2159–2168. [Google Scholar] [CrossRef]
- Amato, M.B.; Barbas, C.S.; Medeiros, D.M.; Magaldi, R.B.; Schettino, G.P.; Lorenzi-Filho, G.; Kairalla, R.A.; Deheinzelin, D.; Munoz, C.; Oliveira, R.; et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N. Engl. J. Med. 1998, 338, 347–354. [Google Scholar] [CrossRef]
- Acute Respiratory Distress Syndrome Network; Brower, R.G.; Matthay, M.A.; Morris, A.; Schoenfeld, D.; Thompson, B.T.; Wheeler, A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N. Engl. J. Med. 2000, 342, 1301–1308. [Google Scholar]
- Amato, M.B.; Meade, M.O.; Slutsky, A.S.; Brochard, L.; Costa, E.L.; Schoenfeld, D.A.; Stewart, T.E.; Briel, M.; Talmor, D.; Mercat, A.; et al. Driving pressure and survival in the acute respiratory distress syndrome. N. Engl. J. Med. 2015, 372, 747–755. [Google Scholar] [CrossRef]
- Amigoni, M.; Bellani, G.; Zambelli, V.; Scanziani, M.; Farina, F.; Fagnani, L.; Latini, R.; Fumagalli, R.; Pesenti, A. Unilateral acid aspiration augments the effects of ventilator lung injury in the contralateral lung. Anesthesiology 2013, 119, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Santa Cruz, R.; Villarejo, F.; Irrazabal, C.; Ciapponi, A. High versus low positive end-expiratory pressure (PEEP) levels for mechanically ventilated adult patients with acute lung injury and acute respiratory distress syndrome. Cochrane Database Syst. Rev. 2021, 3, CD009098. [Google Scholar] [CrossRef] [PubMed]
- Dianti, J.; Tisminetzky, M.; Ferreyro, B.L.; Englesakis, M.; Del Sorbo, L.; Sud, S.; Talmor, D.; Ball, L.; Meade, M.; Hodgson, C.; et al. Association of Positive End-Expiratory Pressure and Lung Recruitment Selection Strategies with Mortality in Acute Respiratory Distress Syndrome: A Systematic Review and Network Meta-analysis. Am. J. Respir. Crit. Care Med. 2022, 205, 1300–1310. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Marini, J.J. In search of the Holy Grail: Identifying the best PEEP in ventilated patients. Intensive Care Med. 2022, 48, 728–731. [Google Scholar] [CrossRef]
- Blanch, L.; Murias, G.; Nahum, A. Lung recruitment in unilateral lung disease. Minerva Anestesiol. 2002, 68, 351–355. [Google Scholar] [PubMed]
- Meyer, N.J.; Gattinoni, L.; Calfee, C.S. Acute respiratory distress syndrome. Lancet 2021, 398, 622–637. [Google Scholar] [CrossRef]
- Gattinoni, L.; Tonetti, T.; Quintel, M. Regional physiology of ARDS. Crit. Care 2017, 21, 312. [Google Scholar] [CrossRef]
- Chimenti, L.; Morales-Quinteros, L.; Puig, F.; Camprubi-Rimblas, M.; Guillamat-Prats, R.; Gomez, M.N.; Tijero, J.; Blanch, L.; Matute-Bello, G.; Artigas, A. Comparison of direct and indirect models of early induced acute lung injury. Intensive Care Med. Exp 2020, 8, 62. [Google Scholar] [CrossRef]
- Kulkarni, H.S.; Lee, J.S.; Bastarache, J.A.; Kuebler, W.M.; Downey, G.P.; Albaiceta, G.M.; Altemeier, W.A.; Artigas, A.; Bates, J.H.T.; Calfee, C.S.; et al. Update on the Features and Measurements of Experimental Acute Lung Injury in Animals: An Official American Thoracic Society Workshop Report. Am. J. Respir. Cell Mol. Biol. 2022, 66, e1–e14. [Google Scholar] [CrossRef]
- Bastia, L.; Engelberts, D.; Osada, K.; Katira, B.H.; Damiani, L.F.; Yoshida, T.; Chen, L.; Ferguson, N.D.; Amato, M.B.P.; Post, M.; et al. Role of Positive End-Expiratory Pressure and Regional Transpulmonary Pressure in Asymmetrical Lung Injury. Am. J. Respir. Crit. Care Med. 2021, 203, 969–976. [Google Scholar] [CrossRef]
- Matute-Bello, G.; Downey, G.; Moore, B.B.; Groshong, S.D.; Matthay, M.A.; Slutsky, A.S.; Kuebler, W.M. An official American Thoracic Society workshop report: Features and measurements of experimental acute lung injury in animals. Am. J. Respir. Cell Mol. Biol. 2011, 44, 725–738. [Google Scholar] [CrossRef]
- The UniProt Consortium. UniProt: The universal protein knowledgebase in 2021. Nucleic Acids Res. 2021, 49, D480–D489. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Nastou, K.C.; Lyon, D.; Kirsch, R.; Pyysalo, S.; Doncheva, N.T.; Legeay, M.; Fang, T.; Bork, P.; et al. The STRING database in 2021: Customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021, 49, D605–D612, Erratum in Nucleic Acids Res. 2021, 49, 10800. [Google Scholar] [CrossRef]
- Silva, P.L.; Scharffenberg, M.; Rocco, P.R.M. Understanding the mechanisms of ventilator-induced lung injury using animal models. Intensive Care Med. Exp. 2023, 11, 82. [Google Scholar] [CrossRef]
- Otahal, M.; Mlcek, M.; Vitkova, I.; Kittnar, O. A novel experimental model of acute respiratory distress syndrome in pig. Physiol. Res. 2016, 65, S643–S651. [Google Scholar] [CrossRef] [PubMed]
- Geilen, J.; Kainz, M.; Zapletal, B.; Geleff, S.; Wisser, W.; Bohle, B.; Schweiger, T.; Schultz, M.J.; Tschernko, E. Unilateral acute lung injury in pig: A promising animal model. J. Transl. Med. 2022, 20, 548. [Google Scholar] [CrossRef] [PubMed]
- Stenlo, M.; Hyllen, S.; Silva, I.A.N.; Bolukbas, D.A.; Pierre, L.; Hallgren, O.; Wagner, D.E.; Lindstedt, S. Increased particle flow rate from airways precedes clinical signs of ARDS in a porcine model of LPS-induced acute lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2020, 318, L510–L517. [Google Scholar] [CrossRef]
- Tomashefski, J.F., Jr. Pulmonary pathology of acute respiratory distress syndrome. Clin. Chest Med. 2000, 21, 435–466. [Google Scholar] [CrossRef] [PubMed]
- Grommes, J.; Soehnlein, O. Contribution of neutrophils to acute lung injury. Mol. Med. 2011, 17, 293–307. [Google Scholar] [CrossRef]
- Lorente, J.A.; Cardinal-Fernandez, P.; Munoz, D.; Frutos-Vivar, F.; Thille, A.W.; Jaramillo, C.; Ballen-Barragan, A.; Rodriguez, J.M.; Penuelas, O.; Ortiz, G.; et al. Acute respiratory distress syndrome in patients with and without diffuse alveolar damage: An autopsy study. Intensive Care Med. 2015, 41, 1921–1930. [Google Scholar] [CrossRef]
- Ichikado, K.; Suga, M.; Gushima, Y.; Johkoh, T.; Iyonaga, K.; Yokoyama, T.; Honda, O.; Shigeto, Y.; Tomiguchi, S.; Takahashi, M.; et al. Hyperoxia-induced diffuse alveolar damage in pigs: Correlation between thin-section CT and histopathologic findings. Radiology 2000, 216, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Serpa Neto, A.; Filho, R.R.; Cherpanath, T.; Determann, R.; Dongelmans, D.A.; Paulus, F.; Tuinman, P.R.; Pelosi, P.; de Abreu, M.G.; Schultz, M.J. Associations between positive end-expiratory pressure and outcome of patients without ARDS at onset of ventilation: A systematic review and meta-analysis of randomized controlled trials. Ann. Intensive Care 2016, 6, 109. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, E.; Mauri, T. Alveolar Collapse as a Threat to Mechanically Ventilated Lungs. Am. J. Respir. Crit. Care Med. 2024, 209, 1418–1420. [Google Scholar] [CrossRef]
- Gattinoni, L.; Caironi, P.; Cressoni, M.; Chiumello, D.; Ranieri, V.M.; Quintel, M.; Russo, S.; Patroniti, N.; Cornejo, R.; Bugedo, G. Lung recruitment in patients with the acute respiratory distress syndrome. N. Engl. J. Med. 2006, 354, 1775–1786. [Google Scholar] [CrossRef]
- Spinelli, E.; Damia, A.; Damarco, F.; Gregori, B.; Occhipinti, F.; Busani, Z.; Leali, M.; Battistin, M.; Lonati, C.; Zhao, Z.; et al. Pathophysiological profile of non-ventilated lung injury in healthy female pigs undergoing mechanical ventilation. Commun. Med. 2024, 4, 18. [Google Scholar] [CrossRef]
- Battaglini, D.; Sottano, M.; Ball, L.; Robba, C.; Rocco, P.R.M.; Pelosi, P. Ten golden rules for individualized mechanical ventilation in acute respiratory distress syndrome. J. Intensive Med. 2021, 1, 42–51. [Google Scholar]
- Gattinoni, L.; Collino, F.; Camporota, L. Assessing lung recruitability: Does it help with PEEP settings? Intensive Care Med. 2024, 50, 749–751. [Google Scholar] [CrossRef]
- Chen, L.; Del Sorbo, L.; Grieco, D.L.; Shklar, O.; Junhasavasdikul, D.; Telias, I.; Fan, E.; Brochard, L. Airway Closure in Acute Respiratory Distress Syndrome: An Underestimated and Misinterpreted Phenomenon. Am. J. Respir. Crit. Care Med. 2018, 197, 132–136. [Google Scholar] [CrossRef]
- Grasselli, G.; Calfee, C.S.; Camporota, L.; Poole, D.; Amato, M.B.P.; Antonelli, M.; Arabi, Y.M.; Baroncelli, F.; Beitler, J.R.; Bellani, G.; et al. ESICM guidelines on acute respiratory distress syndrome: Definition, phenotyping and respiratory support strategies. Intensive Care Med. 2023, 49, 727–759. [Google Scholar] [PubMed]
- Beitler, J.R.; Sarge, T.; Banner-Goodspeed, V.M.; Gong, M.N.; Cook, D.; Novack, V.; Loring, S.H.; Talmor, D.; Group, E.P.-S. Effect of Titrating Positive End-Expiratory Pressure (PEEP) with an Esophageal Pressure-Guided Strategy vs an Empirical High PEEP-Fio2 Strategy on Death and Days Free From Mechanical Ventilation Among Patients with Acute Respiratory Distress Syndrome: A Randomized Clinical Trial. JAMA 2019, 321, 846–857. [Google Scholar] [PubMed]
- Sarge, T.; Baedorf-Kassis, E.; Banner-Goodspeed, V.; Novack, V.; Loring, S.H.; Gong, M.N.; Cook, D.; Talmor, D.; Beitler, J.R. Effect of Esophageal Pressure-guided Positive End-Expiratory Pressure on Survival from Acute Respiratory Distress Syndrome: A Risk-based and Mechanistic Reanalysis of the EPVent-2 Trial. Am. J. Respir. Crit. Care Med. 2021, 204, 1153–1163. [Google Scholar] [CrossRef]
- Writing Group for the Alveolar Recruitment for Acute Respiratory Distress Syndrome Trial Investigators. Effect of Lung Recruitment and Titrated Positive End-Expiratory Pressure (PEEP) vs Low PEEP on Mortality in Patients with Acute Respiratory Distress Syndrome: A Randomized Clinical Trial. JAMA 2017, 318, 1335–1345. [Google Scholar] [CrossRef]
- Wu, X.; Zheng, R.; Zhuang, Z. Effect of transpulmonary pressure-guided positive end-expiratory pressure titration on lung injury in pigs with acute respiratory distress syndrome. J. Clin. Monit. Comput. 2020, 34, 151–159. [Google Scholar] [CrossRef]
- Cressoni, M.; Chiumello, D.; Carlesso, E.; Chiurazzi, C.; Amini, M.; Brioni, M.; Cadringher, P.; Quintel, M.; Gattinoni, L. Compressive forces and computed tomography-derived positive end-expiratory pressure in acute respiratory distress syndrome. Anesthesiology 2014, 121, 572–581. [Google Scholar] [CrossRef]
- Huang, Y.; Tang, R.; Chen, Q.; Pan, C.; Liu, S.; Hui, X.; Li, Y.; Yang, Y.; Ranieri, V.M.; Qiu, H. How much esophageal pressure-guided end-expiratory transpulmonary pressure is sufficient to maintain lung recruitment in lavage-induced lung injury? J. Trauma Acute Care Surg. 2016, 80, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Sousa, M.L.A.; Katira, B.H.; Bouch, S.; Hsing, V.; Engelberts, D.; Amato, M.B.P.; Post, M.; Brochard, L.J. Limiting Overdistention or Collapse When Mechanically Ventilating Injured Lungs: A Randomized Study in a Porcine Model. Am. J. Respir. Crit. Care Med. 2024, 209, 1441–1452. [Google Scholar] [CrossRef]
- Ball, L.; Talmor, D.; Pelosi, P. Transpulmonary pressure monitoring in critically ill patients: Pros and cons. Crit. Care 2024, 28, 177. [Google Scholar] [CrossRef]
- Mauri, T.; Yoshida, T.; Bellani, G.; Goligher, E.C.; Carteaux, G.; Rittayamai, N.; Mojoli, F.; Chiumello, D.; Piquilloud, L.; Grasso, S.; et al. Esophageal and transpulmonary pressure in the clinical setting: Meaning, usefulness and perspectives. Intensive Care Med. 2016, 42, 1360–1373. [Google Scholar] [CrossRef] [PubMed]
- Chiumello, D.; Carlesso, E.; Brioni, M.; Cressoni, M. Airway driving pressure and lung stress in ARDS patients. Crit. Care 2016, 20, 276. [Google Scholar] [CrossRef]
- Gattinoni, L.; Tonetti, T.; Cressoni, M.; Cadringher, P.; Herrmann, P.; Moerer, O.; Protti, A.; Gotti, M.; Chiurazzi, C.; Carlesso, E.; et al. Ventilator-related causes of lung injury: The mechanical power. Intensive Care Med. 2016, 42, 1567–1575. [Google Scholar] [CrossRef] [PubMed]
- Ichikado, K.; Suga, M.; Muranaka, H.; Gushima, Y.; Miyakawa, H.; Tsubamoto, M.; Johkoh, T.; Hirata, N.; Yoshinaga, T.; Kinoshita, Y.; et al. Prediction of prognosis for acute respiratory distress syndrome with thin-section CT: Validation in 44 cases. Radiology 2006, 238, 321–329. [Google Scholar] [CrossRef]
- Ichikado, K.; Muranaka, H.; Gushima, Y.; Kotani, T.; Nader, H.M.; Fujimoto, K.; Johkoh, T.; Iwamoto, N.; Kawamura, K.; Nagano, J.; et al. Fibroproliferative changes on high-resolution CT in the acute respiratory distress syndrome predict mortality and ventilator dependency: A prospective observational cohort study. BMJ Open 2012, 2, e000545. [Google Scholar] [CrossRef]
- Constantin, J.M.; Jabaudon, M.; Lefrant, J.Y.; Jaber, S.; Quenot, J.P.; Langeron, O.; Ferrandiere, M.; Grelon, F.; Seguin, P.; Ichai, C.; et al. Personalised mechanical ventilation tailored to lung morphology versus low positive end-expiratory pressure for patients with acute respiratory distress syndrome in France (the LIVE study): A multicentre, single-blind, randomised controlled trial. Lancet Respir. Med. 2019, 7, 870–880. [Google Scholar] [CrossRef]
- Gattinoni, L.; Caironi, P.; Pelosi, P.; Goodman, L.R. What has computed tomography taught us about the acute respiratory distress syndrome? Am. J. Respir. Crit. Care Med. 2001, 164, 1701–1711. [Google Scholar] [CrossRef] [PubMed]
- Thille, A.W.; Esteban, A.; Fernandez-Segoviano, P.; Rodriguez, J.M.; Aramburu, J.A.; Vargas-Errazuriz, P.; Martin-Pellicer, A.; Lorente, J.A.; Frutos-Vivar, F. Chronology of histological lesions in acute respiratory distress syndrome with diffuse alveolar damage: A prospective cohort study of clinical autopsies. Lancet Respir. Med 2013, 1, 395–401. [Google Scholar] [CrossRef]
- Marongiu, I.; Spinelli, E.; Scotti, E.; Mazzucco, A.; Wang, Y.M.; Manesso, L.; Colussi, G.; Biancolilli, O.; Battistin, M.; Langer, T.; et al. Addition of 5% CO2 to Inspiratory Gas Prevents Lung Injury in an Experimental Model of Pulmonary Artery Ligation. Am. J. Respir. Crit. Care Med. 2021, 204, 933–942. [Google Scholar] [CrossRef] [PubMed]
- Haase, J.; Buchloh, D.C.; Hammermuller, S.; Salz, P.; Mrongowius, J.; Carvalho, N.C.; Beda, A.; Rau, A.; Starke, H.; Spieth, P.M.; et al. Mechanical Ventilation Strategies Targeting Different Magnitudes of Collapse and Tidal Recruitment in Porcine Acid Aspiration-Induced Lung Injury. J. Clin. Med. 2019, 8, 1250. [Google Scholar] [CrossRef]
- Schreiber, T.; Hueter, L.; Gaser, E.; Schmidt, B.; Schwarzkopf, K.; Rek, H.; Karzai, W. PEEP has beneficial effects on inflammation in the injured and no deleterious effects on the noninjured lung after unilateral lung acid instillation. Intensive Care Med. 2006, 32, 740–749. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, M.O.; Deutsch, B.L.; Simeliunas, E.; Diktanaite, D.; Harms, A.; Brune, M.; Uhle, F.; Weigand, M.; Brenner, T.; Kalenka, A. Effect of moderate elevated intra-abdominal pressure on lung mechanics and histological lung injury at different positive end-expiratory pressures. PLoS ONE 2020, 15, e0230830. [Google Scholar] [CrossRef]
- Araos, J.; Alegria, L.; Garcia, P.; Cruces, P.; Soto, D.; Erranz, B.; Amthauer, M.; Salomon, T.; Medina, T.; Rodriguez, F.; et al. Near-Apneic Ventilation Decreases Lung Injury and Fibroproliferation in an Acute Respiratory Distress Syndrome Model with Extracorporeal Membrane Oxygenation. Am. J. Respir. Crit. Care Med. 2019, 199, 603–612. [Google Scholar] [CrossRef]
- Halbertsma, F.J.; Vaneker, M.; Scheffer, G.J.; van der Hoeven, J.G. Cytokines and biotrauma in ventilator-induced lung injury: A critical review of the literature. Neth. J. Med. 2005, 63, 382–392. [Google Scholar]
- Zhou, K.; Lu, J. Progress in cytokine research for ARDS: A comprehensive review. Open Med. 2024, 19, 20241076. [Google Scholar] [CrossRef]
- Otto, C.M.; Markstaller, K.; Kajikawa, O.; Karmrodt, J.; Syring, R.S.; Pfeiffer, B.; Good, V.P.; Frevert, C.W.; Baumgardner, J.E. Spatial and temporal heterogeneity of ventilator-associated lung injury after surfactant depletion. J. Appl. Physiol. 2008, 104, 1485–1494. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xia, H.F.; Shang, Y.; Yao, S.L. Molecular Mechanisms of Ventilator-Induced Lung Injury. Chin. Med. J. 2018, 131, 1225–1231. [Google Scholar] [PubMed]
- Guldner, A.; Braune, A.; Ball, L.; Silva, P.L.; Samary, C.; Insorsi, A.; Huhle, R.; Rentzsch, I.; Becker, C.; Oehme, L.; et al. Comparative Effects of Volutrauma and Atelectrauma on Lung Inflammation in Experimental Acute Respiratory Distress Syndrome. Crit. Care Med. 2016, 44, e854–e865. [Google Scholar] [CrossRef]
- Cereda, M.; Kavanagh, B.P. Compartmentalization of lung injury--atelectasis versus overstretch. Crit. Care Med. 2014, 42, 223–224. [Google Scholar] [CrossRef]
- Wakabayashi, K.; Wilson, M.R.; Tatham, K.C.; O’Dea, K.P.; Takata, M. Volutrauma, but not atelectrauma, induces systemic cytokine production by lung-marginated monocytes. Crit. Care Med. 2014, 42, e49–e57. [Google Scholar] [CrossRef]
- Blondonnet, R.; Constantin, J.M.; Sapin, V.; Jabaudon, M. A Pathophysiologic Approach to Biomarkers in Acute Respiratory Distress Syndrome. Dis. Markers 2016, 2016, 3501373. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Qin, Q.; Lu, J. Pathophysiological mechanisms of ARDS: A narrative review from molecular to organ-level perspectives. Respir. Res. 2025, 26, 54. [Google Scholar] [CrossRef]
- Swindle, M.M.; Makin, A.; Herron, A.J.; Clubb, F.J., Jr.; Frazier, K.S. Swine as models in biomedical research and toxicology testing. Vet. Pathol. 2012, 49, 344–356. [Google Scholar] [CrossRef]
- Baydur, A.; Behrakis, P.K.; Zin, W.A.; Jaeger, M.; Milic-Emili, J. A simple method for assessing the validity of the esophageal balloon technique. Am. Rev. Respir. Dis. 1982, 126, 788–791. [Google Scholar]
- Ichikado, K.; Suga, M.; Muller, N.L.; Taniguchi, H.; Kondoh, Y.; Akira, M.; Johkoh, T.; Mihara, N.; Nakamura, H.; Takahashi, M.; et al. Acute interstitial pneumonia: Comparison of high-resolution computed tomography findings between survivors and nonsurvivors. Am. J. Respir. Crit. Care Med. 2002, 165, 1551–1556. [Google Scholar] [CrossRef]
- Ichikado, K.; Johkoh, T.; Ikezoe, J.; Takeuchi, N.; Kohno, N.; Arisawa, J.; Nakamura, H.; Nagareda, T.; Itoh, H.; Ando, M. Acute interstitial pneumonia: High-resolution CT findings correlated with pathology. Am. J. Roentgenol. 1997, 168, 333–338. [Google Scholar] [CrossRef]
- Schroder, C.; Jacob, A.; Tonack, S.; Radon, T.P.; Sill, M.; Zucknick, M.; Rüffer, S.; Costello, E.; Neoptolemos, J.P.; Crnogorac-Jurcevic, T.; et al. Dual-color proteomic profiling of complex samples with a microarray of 810 cancer-related antibodies. Mol. Cell. Proteom. 2010, 9, 1271–1280. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Gillespie, M.; Jassal, B.; Stephan, R.; Milacic, M.; Rothfels, K.; Senff-Ribeiro, A.; Griss, J.; Sevilla, C.; Matthews, L.; Gong, C.; et al. The reactome pathway knowledgebase 2022. Nucleic Acids Res. 2022, 50, D687–D692. [Google Scholar] [PubMed]
- Martens, M.; Ammar, A.; Riutta, A.; Waagmeester, A.; Slenter, D.N.; Hanspers, K.; Miller, R.A.; Digles, D.; Lopes, E.N.; Ehrhart, F.; et al. WikiPathways: Connecting communities. Nucleic Acids Res. 2021, 49, D613–D621. [Google Scholar] [PubMed]
- Seybold, B.; Deutsch, A.M.; Deutsch, B.L.; Simeliunas, E.; Weigand, M.A.; Fiedler-Kalenka, M.O.; Kalenka, A. Differential Effects of Intra-Abdominal Hypertension and ARDS on Respiratory Mechanics in a Porcine Model. Medicina 2024, 60, 843. [Google Scholar] [CrossRef] [PubMed]
- Hufnagel, K.; Fathi, A.; Stroh, N.; Klein, M.; Skwirblies, F.; Girgis, R.; Dahlke, C.; Hoheisel, J.D.; Lowy, C.; Schmidt, R.; et al. Discovery and systematic assessment of early biomarkers that predict progression to severe COVID-19 disease. Commun. Med. 2023, 3, 51. [Google Scholar] [CrossRef]
- Sill, M.; Schroder, C.; Hoheisel, J.D.; Benner, A.; Zucknick, M. Assessment and optimisation of normalisation methods for dual-colour antibody microarrays. BMC Bioinform. 2010, 11, 556. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]


| Parameter | ALI_PEEP 5 Mean ± SEM | ALI_PEEP TPPexp Mean ± SEM | sham_PEEP 5 Mean ± SEM | p-Value ANOVA |
|---|---|---|---|---|
| SpO2 (%) | 99.2 ± 0.4 | 98.3 ± 0.7 | 99.0 ± 0.0 | 0.535 |
| paO2 (mmHg) | 152.4 ± 10.3 | 183.1 ± 7.6 | 186.9 ± 3.4 | 0.048 |
| paCO2 (mmHg) | 42.6 ± 1.0 | 50.1 ± 4.3 | 47.7 ± 5.2 | 0.267 |
| Resp. rate (bpm) | 25.3 ± 0.8 | 24.7 ± 0.7 | 22.0 ± 2.0 | 0.162 |
| VT (mL) | 310 ± 10 | 300 ± 20 | 300 ± 40 | 0.915 |
| Ppeak (cmH2O) | 30.3 ± 1.3 | 29.8 ± 1.0 | 20.5 ± 0.5 | 0.003 |
| Pplat (cmH2O) | 17.5 ± 0.6 | 19.8 ± 0.8 | 13.2 ± 1.7 | 0.003 |
| PEEP (cmH2O) | 5.0 ± 0.0 | 10.8 ± 0.9 | 5.0 ± 0.0 | <0.001 * |
| ΔP (cmH2O) | 12.3 ± 0.7 | 9.0 ± 0.6 | 8.0 ± 1.6 | 0.005 ⨀ |
| Compliance (ml/cmH2O) | 23.2 ± 2.3 | 31.8 ± 1.4 | 36.5 ± 2.5 | 0.006 # |
| Resistance (cmH2O/L/s) | 16.2 ± 0.9 | 19.2 ± 3.4 | 14.0 ± 0.0 | 0.540 |
| Mech. Power (j/min) | 18.6 ± 1.1 | 18.3 ± 0.7 | 10.8 ± 2.2 | 0.004 |
| PESinsp (cmH2O) | 13.3 ± 0.7 | 13.6 ± 1.4 | 7.7 ± 0.9 | 0.046 |
| PESexp (cmH2O) | 7.6 ± 0.8 | 9.6 ± 1.3 | 3.3 ± 1.3 | 0.037 |
| TPPexp (cmH2O) | −2.6 ± 0.8 | 1.2 ± 0.7 | 1.8 ± 1.3 | 0.008 ✣ |
| ΔPL (cmH2O) | 6.7 ± 1.0 | 5.0 ± 0.8 | 3.8 ± 2.1 | 0.245 |
| Heart rate (bpm) | 70.5 ± 3.2 | 81.0 ± 5.7 | 65.0 ± 1 | 0.152 |
| MAP (mmHg) | 81.7 ± 3.2 | 75.0 ± 3.2 | 93.0 ± 0.0 | 0.037 |
| Lactate (mg/dL) | 10.7 ± 1.9 | 11.2 ± 2.3 | 7.6 ± 0.1 | 0.655 |
| Cardiac index (L/min/m2) | 3.6 ± 0.3 | 3.2 ± 0.3 | 3.1 ± 0.5 | 0.457 |
| GEDI (mL/m2) | 677.7 ± 36.5 | 537.5 ±34.8 | 677 ± 0.5 | 0.031 ↡ |
| ELWI (mL/kg) | 13.0 ± 1.4 | 12.3 ± 1.4 | 15.0 ± 1.0 | 0.615 |
| CVP (mmHg) | 12.3 ± 1.3 | 14.7 ± 1.5 | 12.5 ± 0.5 | 0.460 |
| Group | Lung Side | HRCT Score Expiratory Hold (5 cmH2O) | HRCT Score Inspiratory Hold (30 cmH2O) |
|---|---|---|---|
| ALI_PEEP 5 | Left | 262 ± 11 | 270 ± 35 |
| ALI_PEEP 5 | Right | 142 ± 7 * | 125 ± 4 *† |
| ALI_PEEP TPPexp | Left | 260 ± 12 | 268 ± 15 |
| ALI_PEEP TPPexp | Right | 121 ± 6 *‡ | 116 ± 8 *‡ |
| ALI_PEEP 5 Mean ± SEM | ALI_PEEP TPPexp Mean ± SEM | sham_PEEP 5 Mean ± SEM | p-Value ANOVA | |
|---|---|---|---|---|
| left lung | 0.56 ± 0.04 | 0.54 ± 0.04 | 0.33 ± 0.03 | 0.009 * |
| right lung | 0.30 ± 0.02 | 0.27 ± 0.02 | 0.32 ± 0.02 | 0.450 |
| p-value (Wilcoxon) | <0.001 † | < 0.001 † | 0.433 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mutschler, C.H.; Seybold, B.; Aschauer, S.; Englert, N.; Weis, C.-A.; Poth, T.; Cetiner, D.; Wielpütz, M.O.; Kehr, D.; Weigand, M.A.; et al. Optimizing Positive End-Expiratory Pressure in Asymmetric Acute Lung Injury in a Porcine Model: The Role of Transpulmonary Pressure. Int. J. Mol. Sci. 2025, 26, 9985. https://doi.org/10.3390/ijms26209985
Mutschler CH, Seybold B, Aschauer S, Englert N, Weis C-A, Poth T, Cetiner D, Wielpütz MO, Kehr D, Weigand MA, et al. Optimizing Positive End-Expiratory Pressure in Asymmetric Acute Lung Injury in a Porcine Model: The Role of Transpulmonary Pressure. International Journal of Molecular Sciences. 2025; 26(20):9985. https://doi.org/10.3390/ijms26209985
Chicago/Turabian StyleMutschler, Claudine H., Benjamin Seybold, Stefan Aschauer, Nils Englert, Cleo-Aron Weis, Tanja Poth, Defne Cetiner, Mark O. Wielpütz, Dorothea Kehr, Markus A. Weigand, and et al. 2025. "Optimizing Positive End-Expiratory Pressure in Asymmetric Acute Lung Injury in a Porcine Model: The Role of Transpulmonary Pressure" International Journal of Molecular Sciences 26, no. 20: 9985. https://doi.org/10.3390/ijms26209985
APA StyleMutschler, C. H., Seybold, B., Aschauer, S., Englert, N., Weis, C.-A., Poth, T., Cetiner, D., Wielpütz, M. O., Kehr, D., Weigand, M. A., Kalenka, A., & Fiedler-Kalenka, M. O. (2025). Optimizing Positive End-Expiratory Pressure in Asymmetric Acute Lung Injury in a Porcine Model: The Role of Transpulmonary Pressure. International Journal of Molecular Sciences, 26(20), 9985. https://doi.org/10.3390/ijms26209985







