MicroRNA-125b-5p Drives MMP-2 Expression via Activation of RAGE-38MAPK-p65/p50NF-κB Axis: A Novel Mechanism in Human Lung Cancer Cells
Abstract
1. Introduction
2. Results
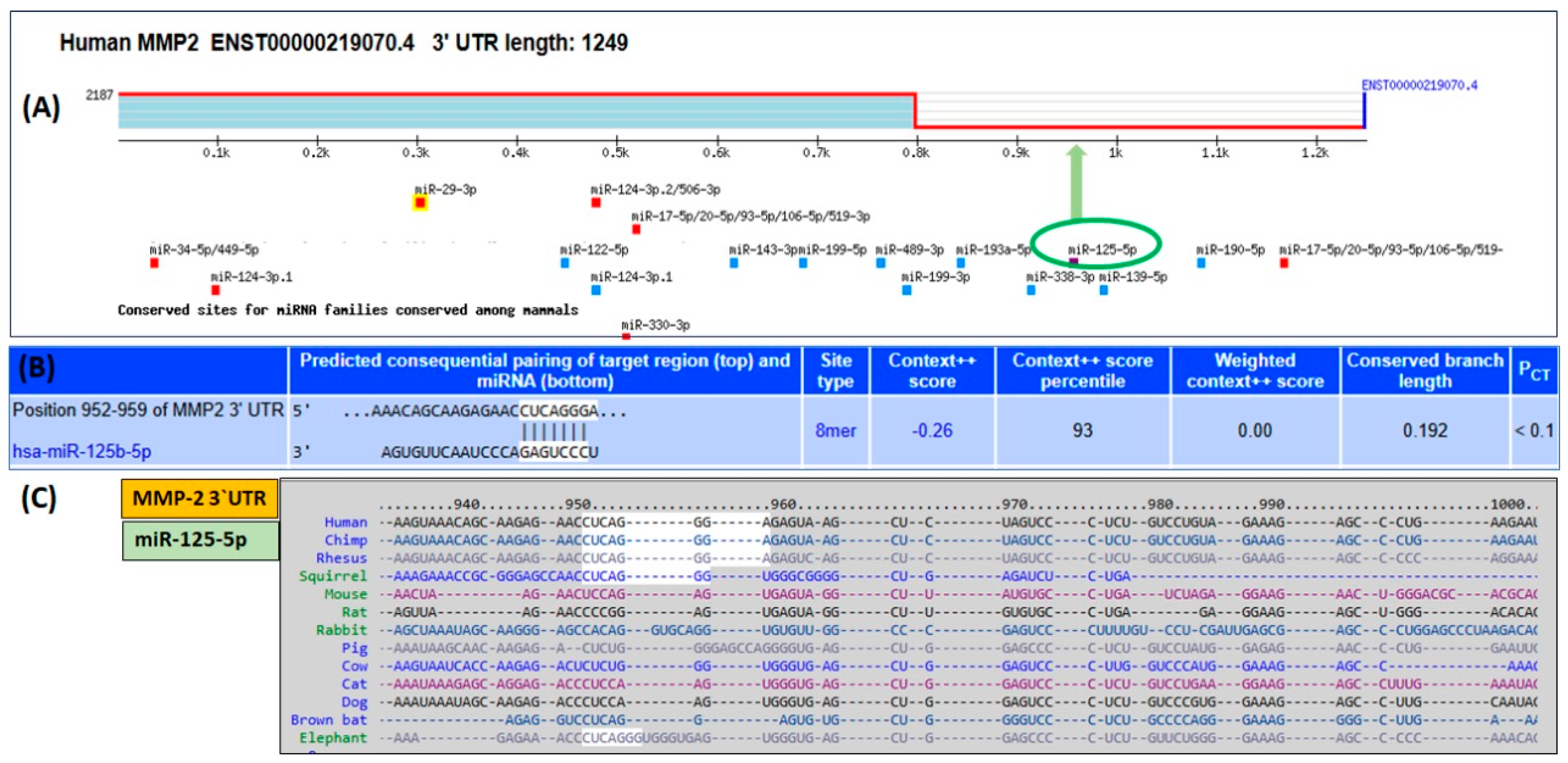
2.1. Bioinformatics Prediction of hsa-miR-125b-5p Binding to the 3′UTR of MMP-2 mRNA (ENST00000219070.4)
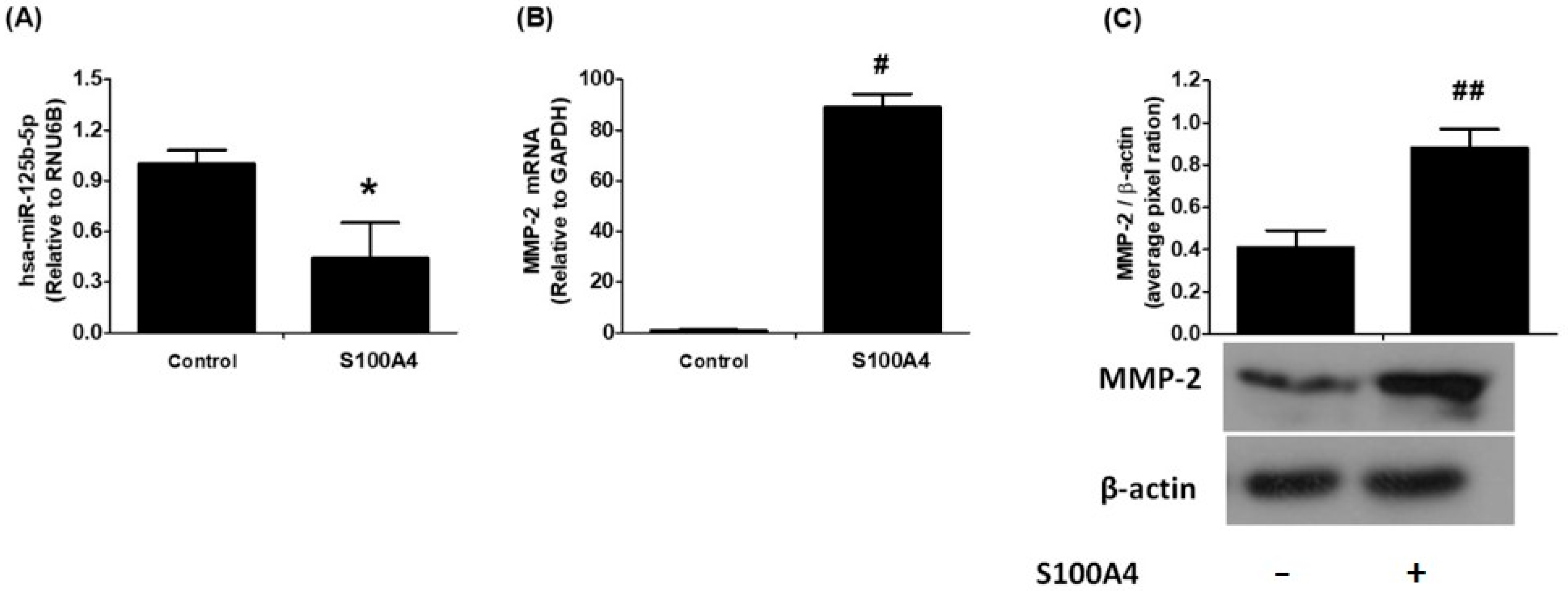
2.2. Relationship Between hsa-miR-125b-5p and MMP-2 in RAGE-Mediated S100A4 Stimulation of Human Lung Cancer Cells
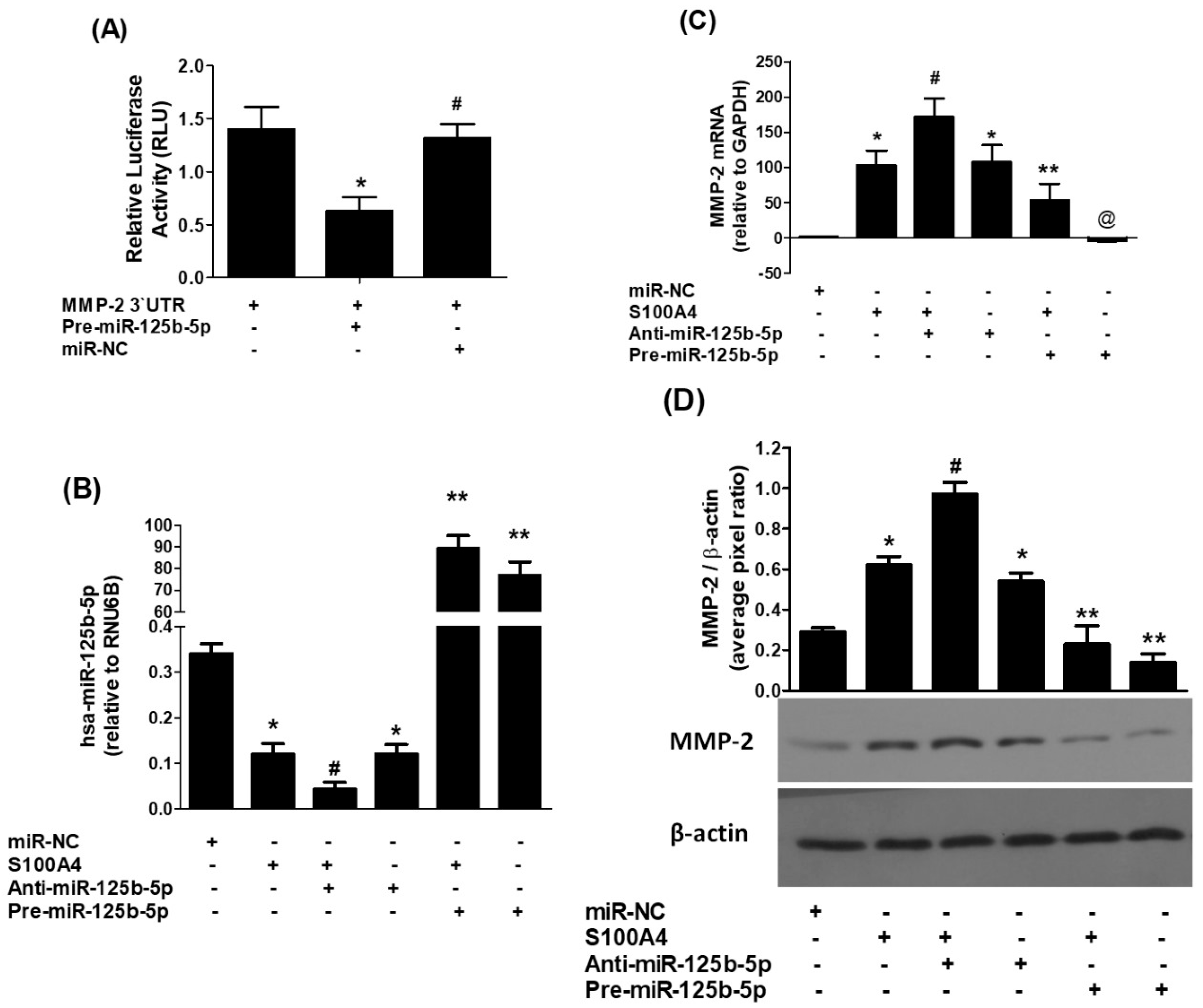
2.3. Validation of hsa-miR-125b-5p Interatcion to the Predicted 3′UTR Target Site of MMP-2 mRNA
2.4. Direct Regulation of MMP-2 Expression by hsa-miR-125b-5p in RAGE-Mediated Lung Cancer Cells
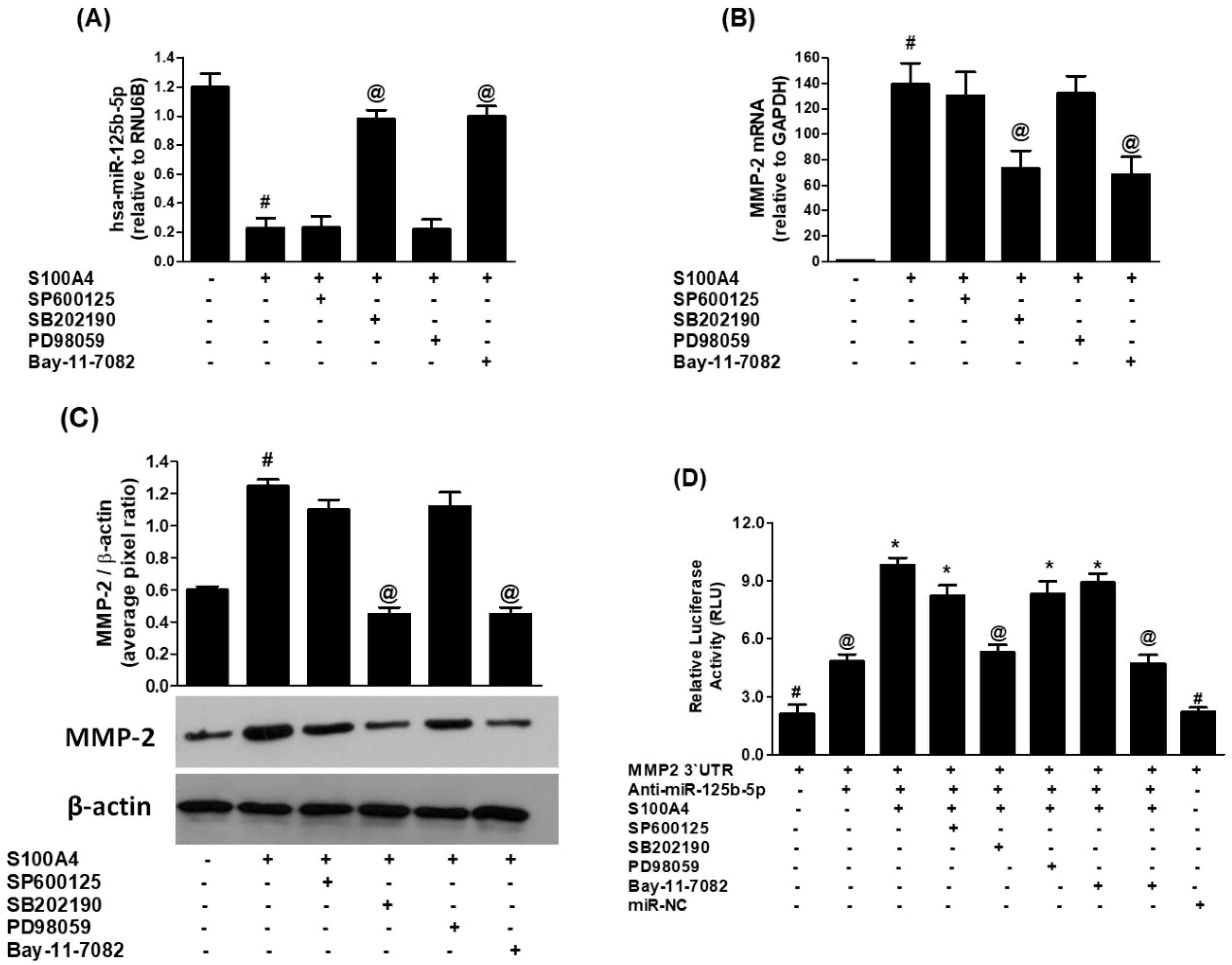
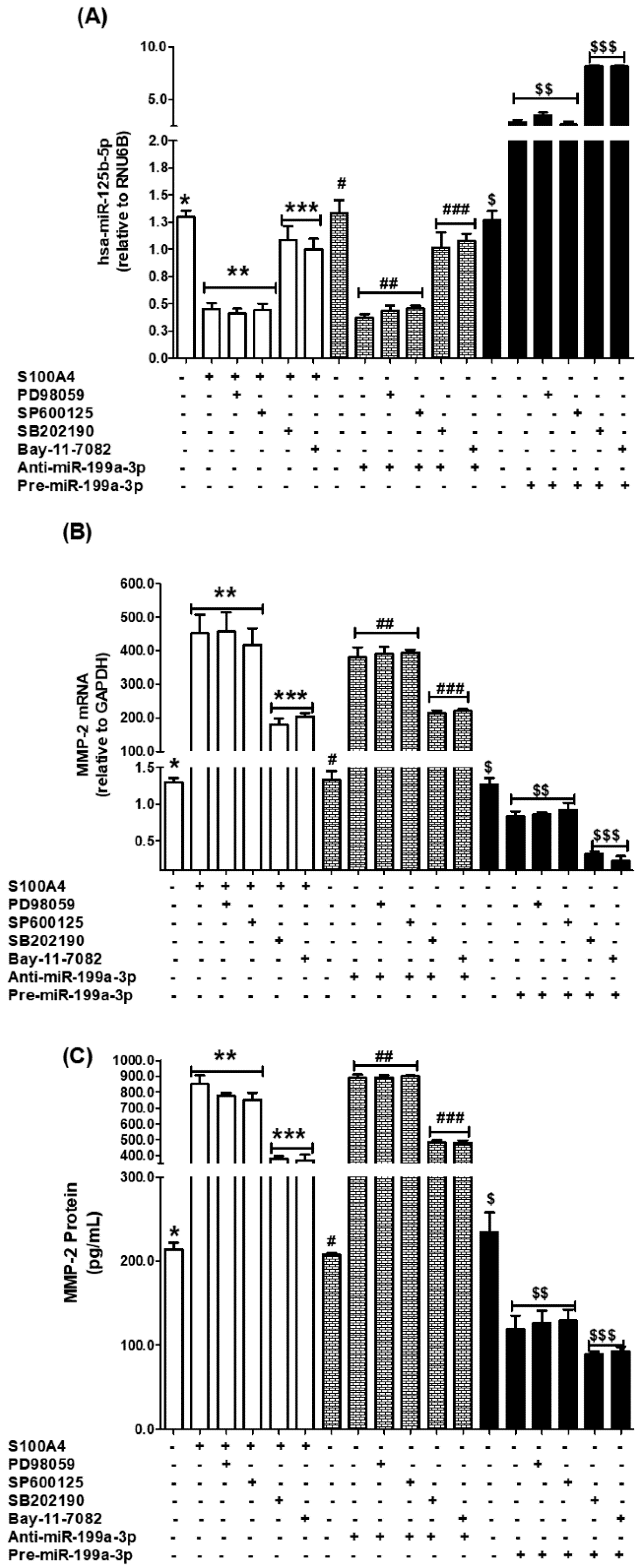
2.5. Role of p38-MAPKs in S100A4-RAGE-Mediated Modulation of hsa-miR-125b-5p and MMP-2
2.6. Role of JNK-MAPKs and ERK-MAPKs in S100A4-RAGE-Induced Modulation of hsa-miR-125b-5p and MMP-2
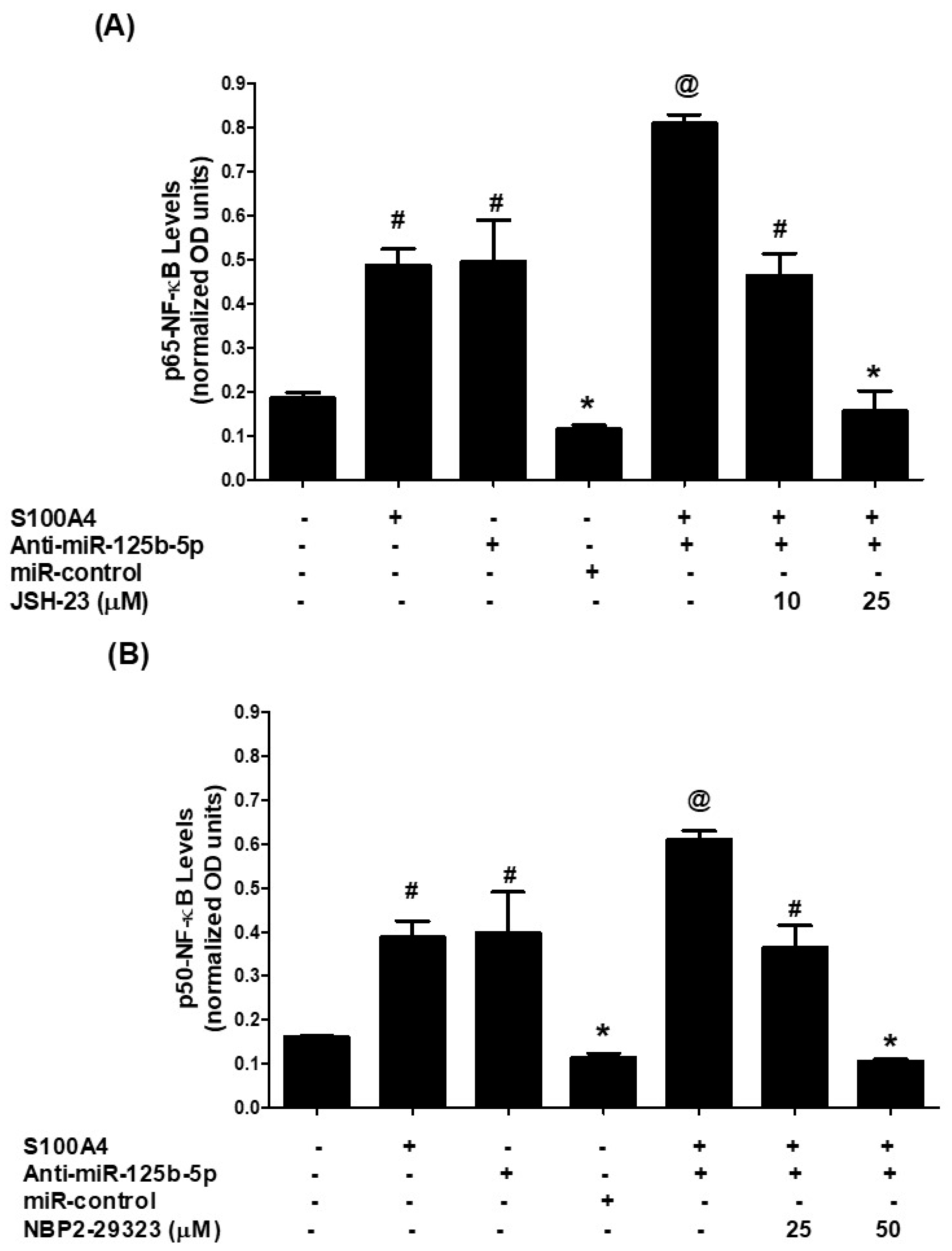
2.7. Role of NF-κB Transcription Factor in S100A4-RAGE-Induced Modulation of hsa-miR-125b-5p and MMP-2
2.8. Validation of Data Using SHP-77 Lung Cancer Cell Line
3. Discussion
4. Methods
4.1. Bioinformatics Analysis
4.2. Lung Cancer Cell Culture and S100A4 Treatment
4.3. RNA Extraction, cDNA Synthesis, and qPCR
4.4. Western Blot Analysis and Densitometry
4.5. Luciferase Reporter Assay
4.6. Cell Transfection and Inhibitor Treatments
4.7. Nuclear Extract Preparation and NF-κB Activity Assay
4.8. MMP-2 Quantification
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vicidomini, G. Current Challenges and Future Advances in Lung Cancer: Genetics, Instrumental Diagnosis and Treatment. Cancers 2023, 15, 3710. [Google Scholar] [CrossRef] [PubMed]
- Wei, C. The multifaceted roles of matrix metalloproteinases in lung cancer. Front. Oncol. 2023, 13, 1195426. [Google Scholar] [CrossRef] [PubMed]
- Merchant, N.; Nagaraju, G.P.; Rajitha, B.; Lammata, S.; Jella, K.K.; Buchwald, Z.S.; Lakka, S.S.; Ali, A.N. Matrix metalloproteinases: Their functional role in lung cancer. Carcinogenesis 2017, 38, 766–780. [Google Scholar] [CrossRef] [PubMed]
- Rojiani, M.V.; Alidina, J.; Esposito, N.; Rojiani, A.M. Expression of MMP-2 correlates with increased angiogenesis in CNS metastasis of lung carcinoma. Int. J. Clin. Exp. Pathol. 2010, 3, 775–781. [Google Scholar] [PubMed]
- Chetty, C.; Bhoopathi, P.; Rao, J.S.; Lakka, S.S. Inhibition of matrix metalloproteinase-2 enhances radiosensitivity by abrogating radiation-induced FoxM1-mediated G2/M arrest in A549 lung cancer cells. Int. J. Cancer 2009, 124, 2468–2477. [Google Scholar] [CrossRef]
- Abba, M.; Patil, N.; Allgayer, H. MicroRNAs in the Regulation of MMPs and Metastasis. Cancers 2014, 6, 625–645. [Google Scholar] [CrossRef]
- Rasheed, Z.; Rasheed, N.; Abdulmonem, W.A.; Khan, M.I. MicroRNA-125b-5p regulates IL-1β induced inflammatory genes via targeting TRAF6-mediated MAPKs and NF-κB signaling in human osteoarthritic chondrocytes. Sci. Rep. 2019, 9, 6882, Erratum in Sci. Rep. 2019, 9, 14729. [Google Scholar] [CrossRef]
- Tang, L.; Yuan, Y.; Zhai, H.; Wang, J.; Zhang, D.; Liang, H.; Shi, Y.; Duan, L.; Jiang, X. MicroRNA-125b-5p Correlates With Prognosis and Lung Adenocarcinoma Progression. Front. Mol. Biosci. 2022, 8, 788690. [Google Scholar] [CrossRef]
- Yuxia, M.; Zhennan, T.; Wei, Z. Circulating miR-125b is a novel biomarker for screening non-small-cell lung cancer and predicts poor prognosis. J. Cancer Res. Clin. Oncol. 2012, 138, 2045–2050. [Google Scholar] [CrossRef]
- Zheng, Z.; Qu, J.-Q.; Yi, H.-M.; Ye, X.; Huang, W.; Xiao, T.; Li, J.-Y.; Wang, Y.-Y.; Feng, J.; Zhu, J.-F.; et al. MiR-125b regulates proliferation and apoptosis of nasopharyngeal carcinoma by targeting A20/NF-κB signaling pathway. Cell Death Dis. 2017, 8, e2855. [Google Scholar] [CrossRef]
- Peng, B.; Theng, P.Y.; Le, M.T.N. Essential functions of miR-125b in cancer. Cell Prolif. 2021, 54, e12913. [Google Scholar] [CrossRef]
- Liu, L.; Qi, L.; Knifley, T.; Piecoro, D.W.; Rychahou, P.; Liu, J.; Mitov, M.I.; Martin, J.; Wang, C.; Wu, J.; et al. S100A4 alters metabolism and promotes invasion of lung cancer cells by up-regulating mitochondrial complex I protein NDUFS2. J. Biol. Chem. 2019, 294, 7516–7527. [Google Scholar] [CrossRef]
- Fei, F.; Qu, J.; Zhang, M.; Li, Y.; Zhang, S. S100A4 in cancer progression and metastasis: A systematic review. Oncotarget 2017, 8, 73219–73239. [Google Scholar] [CrossRef] [PubMed]
- Helfman, D.M.; Kim, E.J.; Lukanidin, E.; Grigorian, M. The metastasis associated protein S100A4: Role in tumour progression and metastasis. Br. J. Cancer 2005, 92, 1955–1958. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Yu, B.; Yuan, L.; Tu, J.; Shao, C.; Tang, Y. RAGE is a potential biomarker implicated in immune infiltrates and cellular senescence in lung adenocarcinoma. J. Clin. Lab. Anal. 2022, 36, e24382. [Google Scholar] [CrossRef]
- Bartling, B.; Hofmann, H.-S.; Weigle, B.; Silber, R.-E.; Simm, A. Down-regulation of the receptor for advanced glycation end-products (RAGE) supports non-small cell lung carcinoma. Carcinogenesis 2005, 26, 293–301. [Google Scholar] [CrossRef]
- Mathisen, B.; Lindstad, R.I.; Hansen, J.; El-Gewely, S.A.; Maelandsmo, G.M.; Hovig, E.; Fodstad, O.; Loennechen, T.; Winberg, J.-O. S100A4 regulates membrane induced activation of matrix metalloproteinase-2 in osteosarcoma cells. Clin. Exp. Metastasis 2003, 20, 701–711. [Google Scholar] [CrossRef]
- Ismail, T.M.; Bennett, D.; Platt-Higgins, A.M.; Al-Medhity, M.; Barraclough, R.; Rudland, P.S. S100A4 Elevation Empowers Expression of Metastasis Effector Molecules in Human Breast Cancer. Cancer Res. 2017, 77, 780–789. [Google Scholar] [CrossRef]
- El-Far, A.H.; Sroga, G.; Al Jaouni, S.K.; Mousa, S.A. Role and Mechanisms of RAGE-Ligand Complexes and RAGE-Inhibitors in Cancer Progression. Int. J. Mol. Sci. 2020, 21, 3613. [Google Scholar] [CrossRef]
- Swami, P.; Thiyagarajan, S.; Vidger, A.; Indurthi, V.S.K.; Vetter, S.W.; Leclerc, E. RAGE Up-Regulation Differently Affects Cell Proliferation and Migration in Pancreatic Cancer Cells. Int. J. Mol. Sci. 2020, 21, 7723. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, H.T. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002, 12, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Masliah-Planchon, J.; Garinet, S.; Pasmant, E. RAS-MAPK pathway epigenetic activation in cancer: miRNAs in action. Oncotarget 2016, 7, 38892–38907. [Google Scholar] [CrossRef]
- Zhang, T.; Ma, C.; Zhang, Z.; Zhang, H.; Hu, H. NF-κB signaling in inflammation and cancer. Medcomm 2021, 2, 618–653. [Google Scholar] [CrossRef]
- Farhana, A.; Alsrhani, A.; Nazam, N.; Ullah, M.I.; Khan, Y.S.; Rasheed, Z. Gold nanoparticles inhibit PMA-induced MMP-9 expression via microRNA-204-5p upregulation and deactivation of NF-κBp65 in breast cancer cells. Biology 2023, 12, 777. [Google Scholar] [CrossRef]
- Roy, R.; Yang, J.; Moses, M.A. Matrix metalloproteinases as novel biomarkers and potential therapeutic targets in human cancer. J. Clin. Oncol. 2009, 27, 5287–5297. [Google Scholar] [CrossRef]
- Pandit, A.; Begum, Y.; Saha, P.; Srivastava, A.K.; Swarnakar, S. Approaches Toward Targeting Matrix Metalloproteases for Prognosis and Therapies in Gynecological Cancer: MicroRNAs as a Molecular Driver. Front. Oncol. 2022, 11, 720622. [Google Scholar] [CrossRef]
- Farhana, A.; Alsrhani, A.; Rasheed, N.; Rasheed, Z. Gold nanoparticles attenuate the interferon-γ induced SOCS1 expression and activation of NF-κB p65/50 activity via modulation of microRNA-155-5p in triple-negative breast cancer cells. Front. Immunol. 2023, 14, 1228458. [Google Scholar] [CrossRef]
- Passlick, B.; Sienel, W.; Seen-Hibler, R.; Wöckel, W.; Thetter, O.; Mutschler, W.; Pantel, K. Overexpression of matrix metalloproteinase 2 predicts unfavorable outcome in early-stage non-small cell lung cancer. Clin. Cancer Res. 2000, 6, 3944–3948. [Google Scholar]
- Khan, Y.S.; Farhana, A.; Mohammed, G.E.; Osman, A.A.; Alsrhani, A.; Shahid, S.M.; Kuddus, M.; Rasheed, Z. Thymoquinone Upregulates microRNA-199a-3p and Downregulates COX-2 Expression and PGE2 Production via Deactivation of p38/ERK/JNK-MAPKs and p65/p50-NF-κB Signaling in Human Lung Cancer Cells. Biology 2025, 14, 1348. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Khanbabapour Sasi, A.; Abak, A.; Shoorei, H.; Khoshkar, A.; Taheri, M. Contribution of miRNAs in the Pathogenesis of Breast Cancer. Front. Oncol. 2021, 11, 768949. [Google Scholar] [CrossRef] [PubMed]
- Mehrgou, A.; Ebadollahi, S.; Seidi, K.; Ayoubi-Joshaghani, M.H.; Ahmadieh Yazdi, A.; Zare, P.; Jaymand, M.; Jahanban-Esfahlan, R. Roles of miRNAs in Colorectal Cancer: Therapeutic Implications and Clinical Opportunities. Adv. Pharm. Bull. 2021, 11, 233–247. [Google Scholar] [CrossRef]
- Rojas, A.; González, I.; Araya, P. RAGE in Cancer Lung: The End of a Long and Winding Road is in Sight. Chin. J. Lung Cancer 2018, 21, 655–657. [Google Scholar] [CrossRef]
- Kozono, S.; Ohuchida, K.; Ohtsuka, T.; Cui, L.; Eguchi, D.; Fujiwara, K.; Zhao, M.; Mizumoto, K.; Tanaka, M. S100A4 mRNA expression level is a predictor of radioresistance of pancreatic cancer cells. Oncol. Rep. 2013, 30, 1601–1608. [Google Scholar] [CrossRef]
- Mudduluru, G.; Ilm, K.; Fuchs, S.; Stein, U. Epigenetic silencing of miR-520c leads to induced S100A4 expression and its mediated colorectal cancer progression. Oncotarget 2017, 8, 21081–21094. [Google Scholar] [CrossRef] [PubMed]
- Herwig, N.; Belter, B.; Wolf, S.; Haase-Kohn, C.; Pietzsch, J. Interaction of extracellular S100A4 with RAGE prompts prometastatic activation of A375 melanoma cells. J. Cell. Mol. Med. 2016, 20, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.-M.; Xu, W.-M.; Lin, J.-C.; Mo, L.-Q.; Hua, X.-X.; Chen, P.-X.; Wu, K.; Zheng, D.-D.; Feng, J.-Q. Activation of the p38 MAPK/NF-κB pathway contributes to doxorubicin-induced inflammation and cytotoxicity in H9c2 cardiac cells. Mol. Med. Rep. 2013, 8, 603–608. [Google Scholar] [CrossRef]
- Markopoulos, G.S.; Roupakia, E.; Tokamani, M.; Alabasi, G.; Sandaltzopoulos, R.; Marcu, K.B.; Kolettas, E. Roles of NF-κB Signaling in the Regulation of miRNAs Impacting on Inflammation in Cancer. Biomedicines 2018, 6, 40. [Google Scholar] [CrossRef]
- Farhana, A.; Alsrhani, A.; Alghsham, R.S.; Derafa, W.; Khan, Y.S.; Rasheed, Z. Gold nanoparticles downregulate IL-6 expression/production by upregulating microRNA-26a-5p and deactivating the RelA and NF-κBp50 transcription pathways in activated breast cancer cells. Int. J. Mol. Sci. 2024, 25, 1404. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ai, X.; Shen, S.; Lu, S. NF-κB-mediated miR-124 suppresses metastasis of non-small-cell lung cancer by targeting MYO10. Oncotarget 2015, 6, 8244–8254. [Google Scholar] [CrossRef]
- Farhana, A.; Alsrhani, A.; Khan, Y.S.; Rasheed, Z. Cancer bioenergetics and tumor microenvironments—Enhancing chemotherapeutics and targeting resistant niches through nanosystems. Cancers 2023, 15, 3836. [Google Scholar] [CrossRef]
- Farhana, A. Enhancing skin cancer immunotheranostics and precision medicine through functionalized nanomodulators and nanosensors: Recent development and prospects. Int. J. Mol. Sci. 2023, 24, 3493. [Google Scholar] [CrossRef] [PubMed]
- Al Robaee, A.A.; Alzolibani, A.A.; Rasheed, Z. MicroRNA-183-5p regulates MITF expression in vitiligo skin depigmentation. Nucleosides Nucleotides Nucleic Acids 2022, 41, 703–723. [Google Scholar] [CrossRef] [PubMed]
- Al Abdulmonem, W.; Rasheed, Z.; Aljohani, A.S.M.; Omran, O.M.; Rasheed, N.; Alkhamiss, A.; Al Salloom, A.A.M.; Alhumaydhi, F.; Alblihed, M.A.; Al Ssadh, H.; et al. Absence of CD74 Isoform at 41kDa Prevents the Heterotypic Associations between CD74 and CD44 in Human Lung Adenocarcinoma-derived Cells. Immunol. Investig. 2021, 50, 891–905. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Rasheed, Z. MicroRNA-183-5p negatively regulates interleukin-8 expression in cervical cancer cells. Int. J. Health Sci. 2024, 18, 25–30. [Google Scholar]
- Rasheed, Z.; Anbazhagan, A.N.; Akhtar, N.; Ramamurthy, S.; Voss, F.R.; Haqqi, T.M. Green tea polyphenol epigallocatechin-3-gallate inhibits advanced glycation end product-induced expression of tumor necrosis factor-α and matrix metalloproteinase-13 in human chondrocytes. Arthritis Res. Ther. 2009, 11, R71. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, Y.S.; Farhana, A.; Kuddus, M.; Shahid, S.M.A.; Alsrhani, A.; Osman, A.A.; Mohammed, G.E.Y.; Ullah, M.I.; Rasheed, Z. MicroRNA-125b-5p Drives MMP-2 Expression via Activation of RAGE-38MAPK-p65/p50NF-κB Axis: A Novel Mechanism in Human Lung Cancer Cells. Int. J. Mol. Sci. 2025, 26, 9983. https://doi.org/10.3390/ijms26209983
Khan YS, Farhana A, Kuddus M, Shahid SMA, Alsrhani A, Osman AA, Mohammed GEY, Ullah MI, Rasheed Z. MicroRNA-125b-5p Drives MMP-2 Expression via Activation of RAGE-38MAPK-p65/p50NF-κB Axis: A Novel Mechanism in Human Lung Cancer Cells. International Journal of Molecular Sciences. 2025; 26(20):9983. https://doi.org/10.3390/ijms26209983
Chicago/Turabian StyleKhan, Yusuf Saleem, Aisha Farhana, Mohammed Kuddus, Syed Monowar Alam Shahid, Abdullah Alsrhani, Abuzar Abdulwahab Osman, Ghorashy E. Y. Mohammed, Muhammad Ikram Ullah, and Zafar Rasheed. 2025. "MicroRNA-125b-5p Drives MMP-2 Expression via Activation of RAGE-38MAPK-p65/p50NF-κB Axis: A Novel Mechanism in Human Lung Cancer Cells" International Journal of Molecular Sciences 26, no. 20: 9983. https://doi.org/10.3390/ijms26209983
APA StyleKhan, Y. S., Farhana, A., Kuddus, M., Shahid, S. M. A., Alsrhani, A., Osman, A. A., Mohammed, G. E. Y., Ullah, M. I., & Rasheed, Z. (2025). MicroRNA-125b-5p Drives MMP-2 Expression via Activation of RAGE-38MAPK-p65/p50NF-κB Axis: A Novel Mechanism in Human Lung Cancer Cells. International Journal of Molecular Sciences, 26(20), 9983. https://doi.org/10.3390/ijms26209983





