Antihyperuricemic Effects of Cornus officinalis Extract via URAT1 Regulation and Renoprotective Mechanisms
Abstract
1. Introduction
2. Results
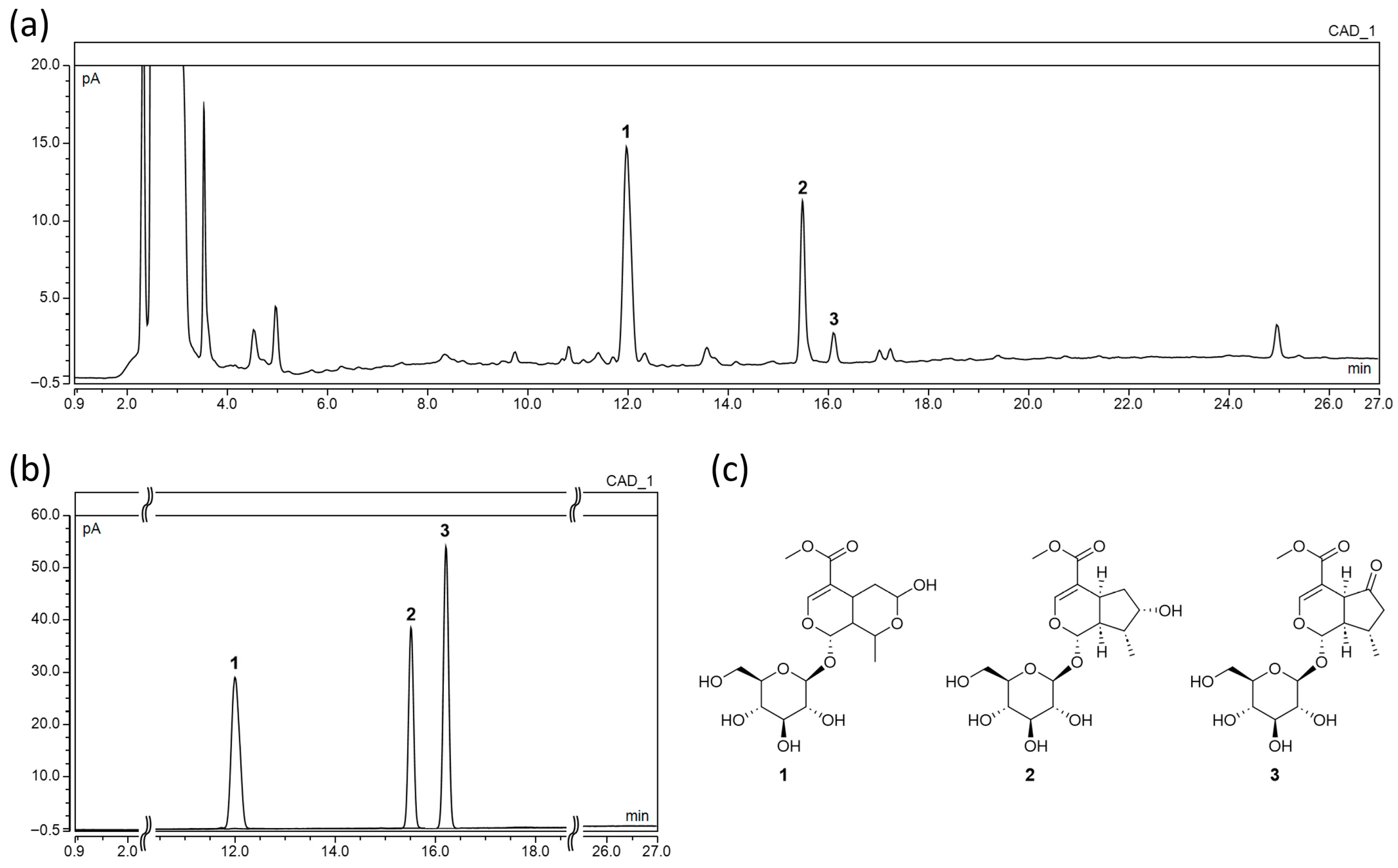
2.1. Chemical Profiling and Quantitative Analysis by UHPLC-CAD
2.2. Effects of COE on In Vitro XOD Activity
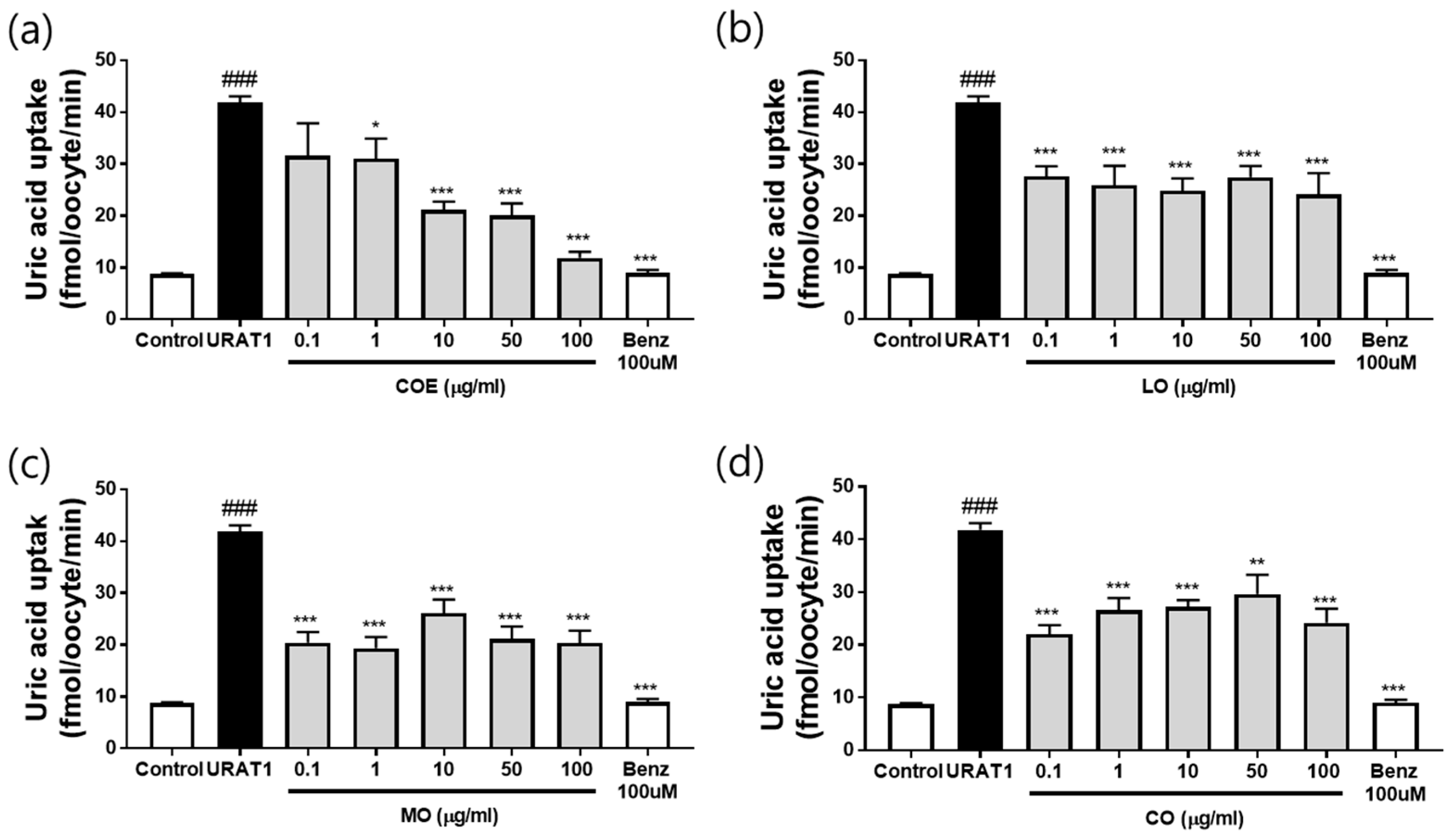
2.3. Effects of COE, LO, MO, and CO on Urate Excretion in In Vitro URAT1-Expressing Oocytes
2.4. Effects of COE, LO, and MO on Serum and Urine Uric Acid Levels
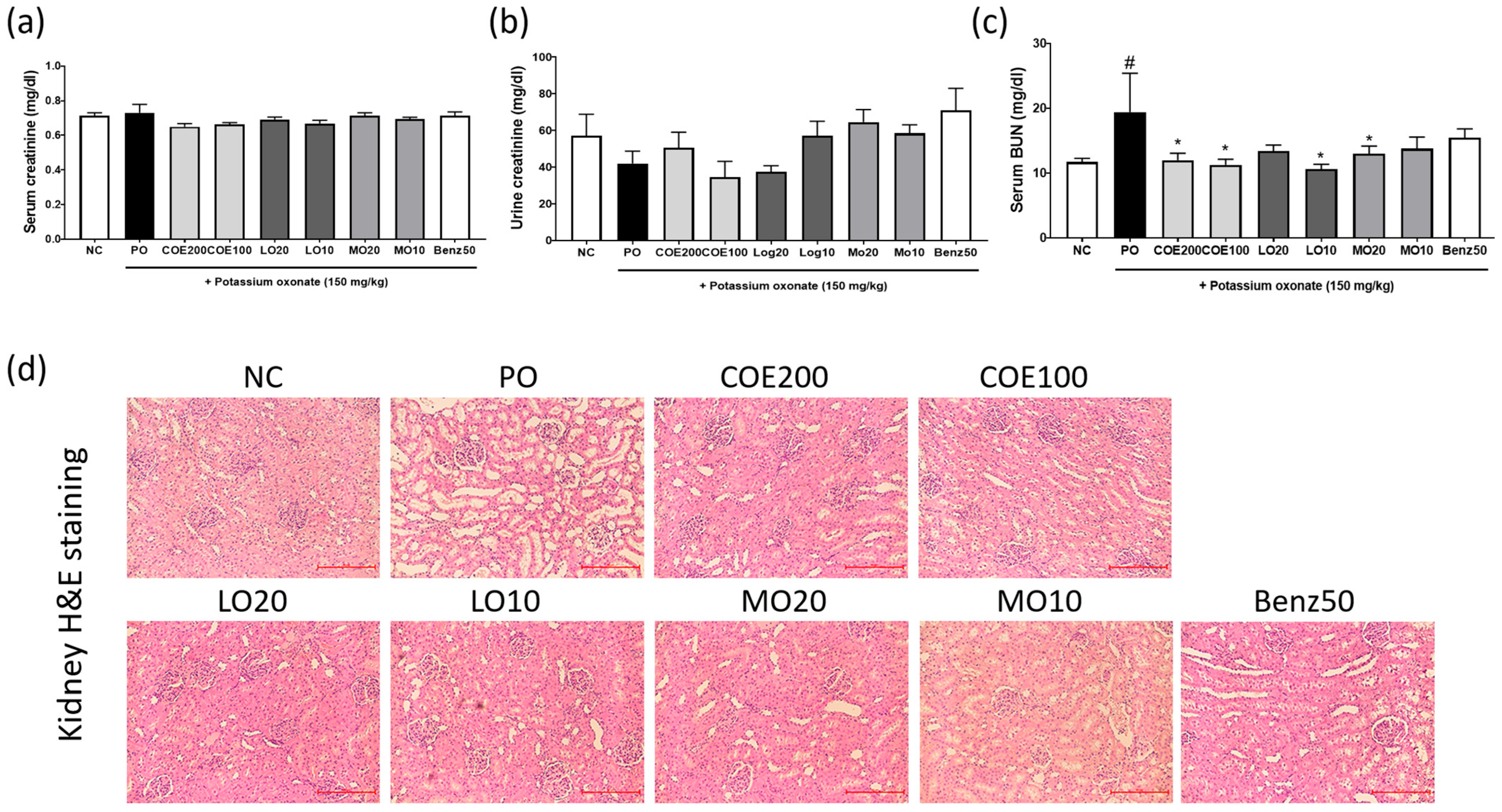
2.5. Effects of COE, LO, and MO on Kidney Function
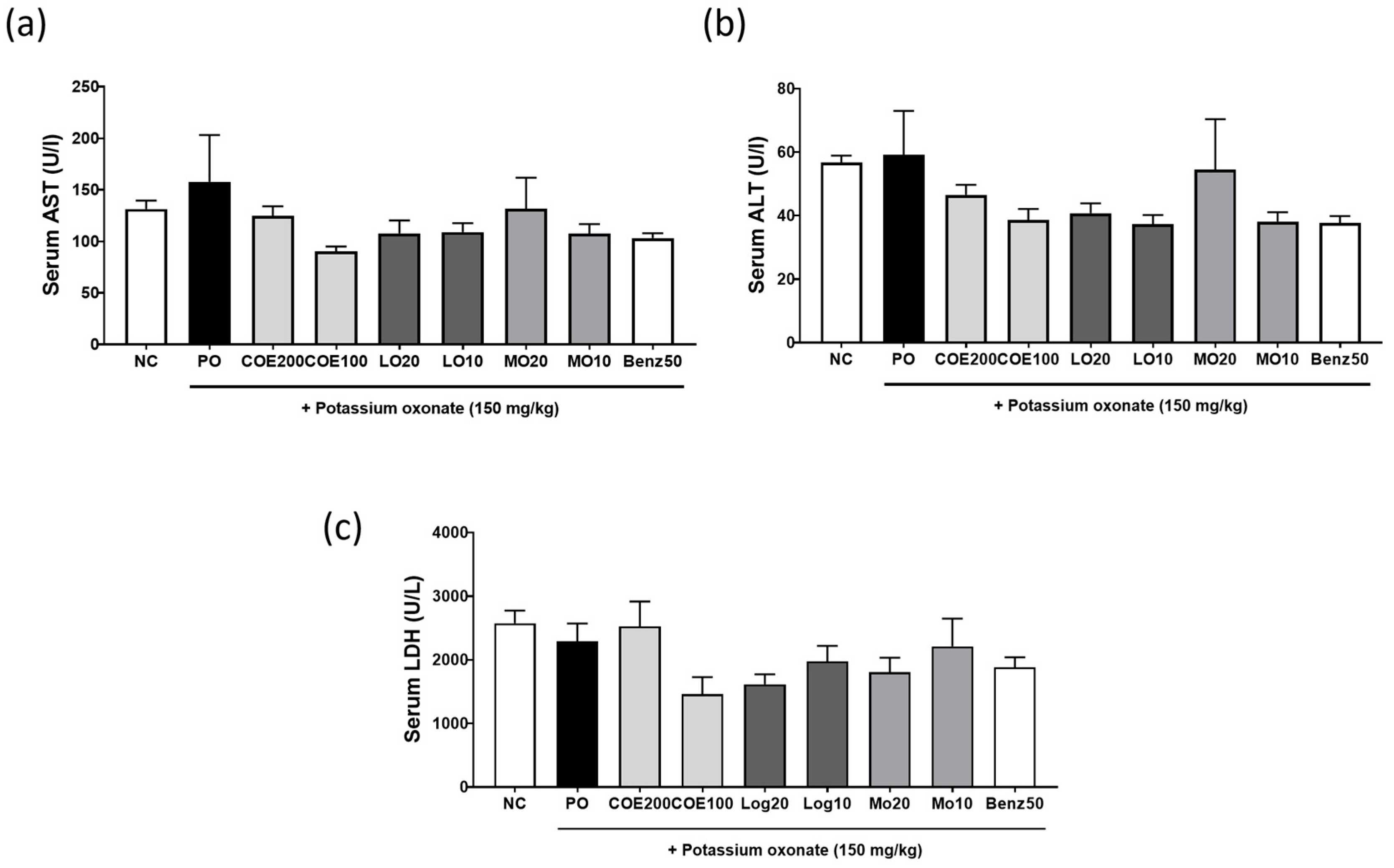
2.6. Effects of COE, LO, and MO on Liver Function
3. Discussion
4. Materials and Methods
4.1. COE Preparation and Compound Profiling
4.2. UHPLC-CAD Analysis of COE
4.3. In Vitro XOD Activity Assay
4.4. Urate Uptake in URAT1-Expressing Oocytes
4.5. Animal Model
4.6. Induction of Hyperuricemia and COE, LO, and MO Administration
4.7. Collection of Blood, Urine, and Tissue Samples
4.8. Biochemical Analysis of Serum and Urine
4.9. Histopathological Analysis of Kidney Tissues
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Du, L.; Zong, Y.; Li, H.; Wang, Q.; Xie, L.; Yang, B.; Pang, Y.; Zhang, C.; Gao, J. Hyperuricemia and its related diseases: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2024, 9, 212. [Google Scholar] [CrossRef]
- Srivastava, A.; Kaze, A.D.; McMullan, C.J.; Isakova, T.; Waikar, S.S. Uric acid and the risks of kidney failure and death in individuals with CKD. Am. J. Kidney Dis. 2018, 71, 362–370. [Google Scholar] [CrossRef]
- Li, X.; Yan, Z.; Tian, J.; Zhang, X.; Han, H.; Ye, F. Urate transporter URAT1 in hyperuricemia: New insights from hyperuricemic models. Ann. Clin. Lab. Sci. 2019, 49, 756–764. [Google Scholar] [PubMed]
- Wu, C.; Zhang, C.; Jin, S.; Wang, J.J.; Dai, A.; Xu, J.; Zhang, H.; Yang, X.; He, X.; Yuan, Q.; et al. Molecular mechanisms of urate transport by the native human URAT1 and its inhibition by anti-gout drugs. Cell Discov. 2025, 11, 15. [Google Scholar] [CrossRef]
- Ishii, T.; Hoshino, K.; Honda, M.; Yamana, Y.; Sasaki-Tanaka, R.; Kumagawa, M.; Kanezawa, S.; Mizutani, T.; Matsumoto, N.; Masuzaki, R.; et al. A case of recent liver injury induced by benzbromarone. Reports 2022, 5, 8. [Google Scholar] [CrossRef]
- Dong, Y.; Feng, Z.-L.; Chen, H.-B.; Wang, F.-S.; Lu, J.-H. Corni fructus: A review of chemical constituents and pharmacological activities. Chin. Med. 2018, 13, 34. [Google Scholar] [CrossRef] [PubMed]
- Bhuia, M.S.; Chowdhury, R.; Ara, I.; Mamun, M.; Rouf, R.; Khan, M.A.; Uddin, S.J.; Shakil, M.A.K.; Habtemariam, S.; Ferdous, J.; et al. Bioactivities of morroniside: A comprehensive review of pharmacological properties and molecular mechanisms. Fitoterapia 2024, 175, 105896. [Google Scholar] [CrossRef]
- Choi, N.; Yang, G.; Jang, J.H.; Kang, H.C.; Cho, Y.Y.; Lee, H.S.; Lee, J.Y. Loganin alleviates gout inflammation by suppressing NLRP3 inflammasome activation and mitochondrial damage. Molecules 2021, 26, 1071. [Google Scholar] [CrossRef]
- Kim, O.K.; Yun, J.M.; Lee, M.; Kim, D.; Lee, J. Hypouricemic Effects of Chrysanthemum indicum L. and Cornus officinalis on Hyperuricemia-Induced HepG2 Cells, Renal Cells, and Mice. Plants 2021, 10, 1668. [Google Scholar] [CrossRef]
- Sung, Y.-Y.; Yuk, H.J.; Kim, D.-S. Saengmaeksan, a traditional herbal formulation consisting of Panax ginseng, ameliorates hyperuricemia by inhibiting xanthine oxidase activity and enhancing urate excretion in rats. J. Ginseng Res. 2021, 45, 565–574. [Google Scholar] [CrossRef]
- Fernández Rodríguez, C.M.; Aller, R.; Gutiérrez García, M.L.; Ampuero, J.; Gómez-Camarero, J.; Martín-Mateos, R.M.; Burgos-Santamaría, D.; Rosales, J.M.; Aspichueta, P.; Buque, X.; et al. Higher levels of serum uric acid influence hepatic damage in patients with non-alcoholic fatty liver disease (NAFLD). Rev. Esp. Enferm. Dig. 2019, 111, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Maiuolo, J.; Oppedisano, F.; Gratteri, S.; Muscoli, C.; Mollace, V. Regulation of uric acid metabolism and excretion. Int. J. Cardiol. 2016, 213, 8–14, Erratum in Int. J. Cardiol. 2023, 387, 131126. https://doi.org/10.1016/j.ijcard.2023.131126. [Google Scholar] [CrossRef] [PubMed]
- George, C.; Leslie, S.W.; Minter, D.A. Hyperuricemia. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Bortolotti, M.; Polito, L.; Battelli, M.G.; Bolognesi, A. Xanthine oxidoreductase: One enzyme for multiple physiological tasks. Redox Biol. 2021, 41, 101882. [Google Scholar] [CrossRef] [PubMed]
- Vickneson, K.; George, J. Xanthine oxidoreductase inhibitors. Handb. Exp. Pharmacol. 2021, 264, 205–228. [Google Scholar] [CrossRef]
- Lin, Z.; Gupta, J.K.; Maqbool, M.; Kumar, K.; Sharma, A.; Wahi, N. The therapeutic management of chemical and herbal medications on uric acid levels and gout: Modern and traditional wisdom. Pharmaceuticals 2024, 17, 1507. [Google Scholar] [CrossRef]
- Qin, Z.; Wang, S.; Lin, Y.; Zhao, Y.; Yang, S.; Song, J.; Xie, T.; Tian, J.; Wu, S.; Du, G. Antihyperuricemic effect of mangiferin aglycon derivative J99745 by inhibiting xanthine oxidase activity and urate transporter 1 expression in mice. Acta Pharm. Sin. B 2018, 8, 306–315. [Google Scholar] [CrossRef]
- Song, D.; Zhao, X.; Wang, F.; Wang, G. A brief review of urate transporter 1 (URAT1) inhibitors for the treatment of hyperuricemia and gout: Current therapeutic options and potential applications. Eur. J. Pharmacol. 2021, 907, 174291. [Google Scholar] [CrossRef]
- Yuan, Q.; Cheng, Y.; Sheng, R.; Yuan, Y.; Hu, M. A brief review of natural products with urate transporter 1 inhibition for the treatment of hyperuricemia. Evid. Based Complement. Altern. Med. 2022, 2022, 5419890. [Google Scholar] [CrossRef]
- Dalbeth, N.; Gosling, A.L.; Gaffo, A.; Abhishek, A. Gout. Lancet 2021, 397, 1808. [Google Scholar] [CrossRef]
- Chung, S.; Kim, G.H. Urate transporters in the kidney: What clinicians need to know. Electrolyte Blood Press. 2021, 19, 1–9. [Google Scholar] [CrossRef]
- Ndrepepa, G. Uric acid and cardiovascular disease. Clin. Chim. Acta 2018, 484, 150–163. [Google Scholar] [CrossRef]
- Yuk, H.J.; Ryu, H.W.; Kim, D.-S. Potent Xanthine Oxidase Inhibitory Activity of Constituents of Agastache rugosa (Fisch. and C.A.Mey.) Kuntze. Foods 2023, 12, 573. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kim, S.H.; Yuk, H.J.; Kim, D.S. DKB114, a mixture of Chrysanthemum indicum Linne flower and Cinnamomum cassia (L.) J. Presl bark extracts, improves hyperuricemia through inhibition of xanthine oxidase activity and increasing urine excretion. Nutrients 2018, 10, 1381. [Google Scholar] [CrossRef]
- Enomoto, A.; Kimura, H.; Chairoungdua, A.; Shigeta, Y.; Jutabha, P.; Cha, S.H.; Hosoyamada, M.; Takeda, M.; Sekine, T.; Igarashi, T.; et al. Molecular identification of a renal urate–anion exchanger that regulates blood urate levels. Nature 2002, 417, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Guggino, S.E.; Aronson, P.S. Paradoxical effects of pyrazinoate and nicotinate on urate transport in dog renal microvillus membranes. J. Clin. Invest. 1985, 76, 543–547. [Google Scholar] [CrossRef]
- Toyoda, Y.; Manolescu, A.R.; Takeda, M.; Endou, H.; Fujita, T.; Hosoyamada, M. OAT10/SLC22A13 acts as a renal urate re-absorber: Clinico-genetic and functional analyses with pharmacological impacts. Front. Pharmacol. 2022, 13, 842717. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Nakanishi, T.; Tamai, I. Functional cooperation of SMCTs and URAT1 for renal reabsorption transport of urate. Drug Metab. Pharmacokinet. 2013, 28, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.K.; Mercado, A.; Foster, A.; Zandi-Nejad, K.; Mount, D.B. Uricosuric targets of tranilast. Pharmacol. Res. Perspect. 2017, 5, e00291. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Lee, C.-H. Transport mechanism and structural pharmacology of human urate transporter URAT1. Cell Res. 2024, 34, 776–787. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sung, Y.-Y.; Kim, D.-S.; Kim, S.-H.; Yuk, H.J. Antihyperuricemic Effects of Cornus officinalis Extract via URAT1 Regulation and Renoprotective Mechanisms. Int. J. Mol. Sci. 2025, 26, 9980. https://doi.org/10.3390/ijms26209980
Sung Y-Y, Kim D-S, Kim S-H, Yuk HJ. Antihyperuricemic Effects of Cornus officinalis Extract via URAT1 Regulation and Renoprotective Mechanisms. International Journal of Molecular Sciences. 2025; 26(20):9980. https://doi.org/10.3390/ijms26209980
Chicago/Turabian StyleSung, Yoon-Young, Dong-Seon Kim, Seung-Hyung Kim, and Heung Joo Yuk. 2025. "Antihyperuricemic Effects of Cornus officinalis Extract via URAT1 Regulation and Renoprotective Mechanisms" International Journal of Molecular Sciences 26, no. 20: 9980. https://doi.org/10.3390/ijms26209980
APA StyleSung, Y.-Y., Kim, D.-S., Kim, S.-H., & Yuk, H. J. (2025). Antihyperuricemic Effects of Cornus officinalis Extract via URAT1 Regulation and Renoprotective Mechanisms. International Journal of Molecular Sciences, 26(20), 9980. https://doi.org/10.3390/ijms26209980






