Enzymatic Inhibitors of Aspartyl Protease EAP1 and Xylanase SRXL1 from Sporisorium reilianum Isolated from Corn Seeds
Abstract
1. Introduction
2. Results
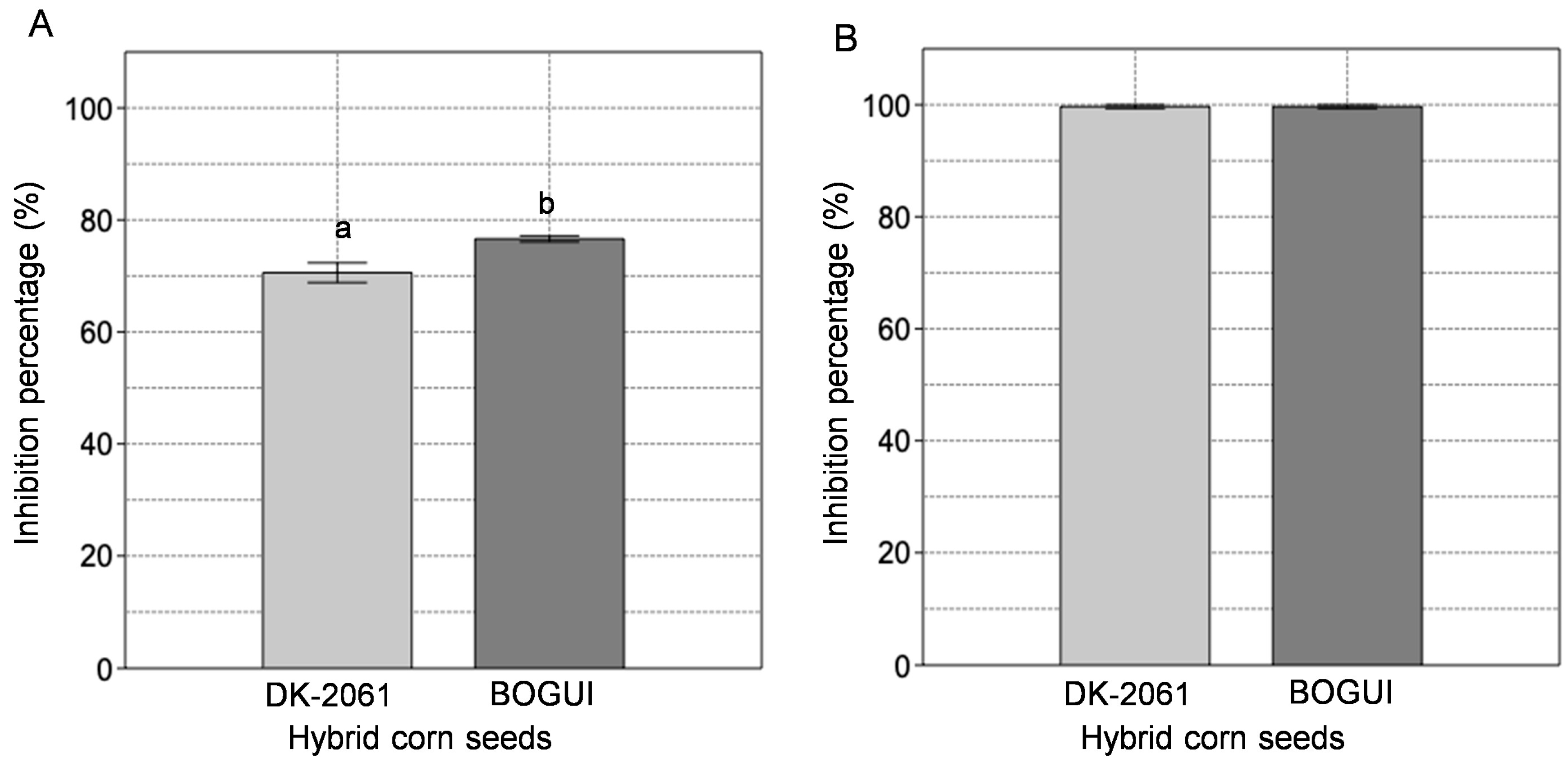
2.1. Enzyme Inhibitory Activity of the Aqueous Extracts Derived from Corn Seeds
2.2. Purification of the Enzyme Inhibitors
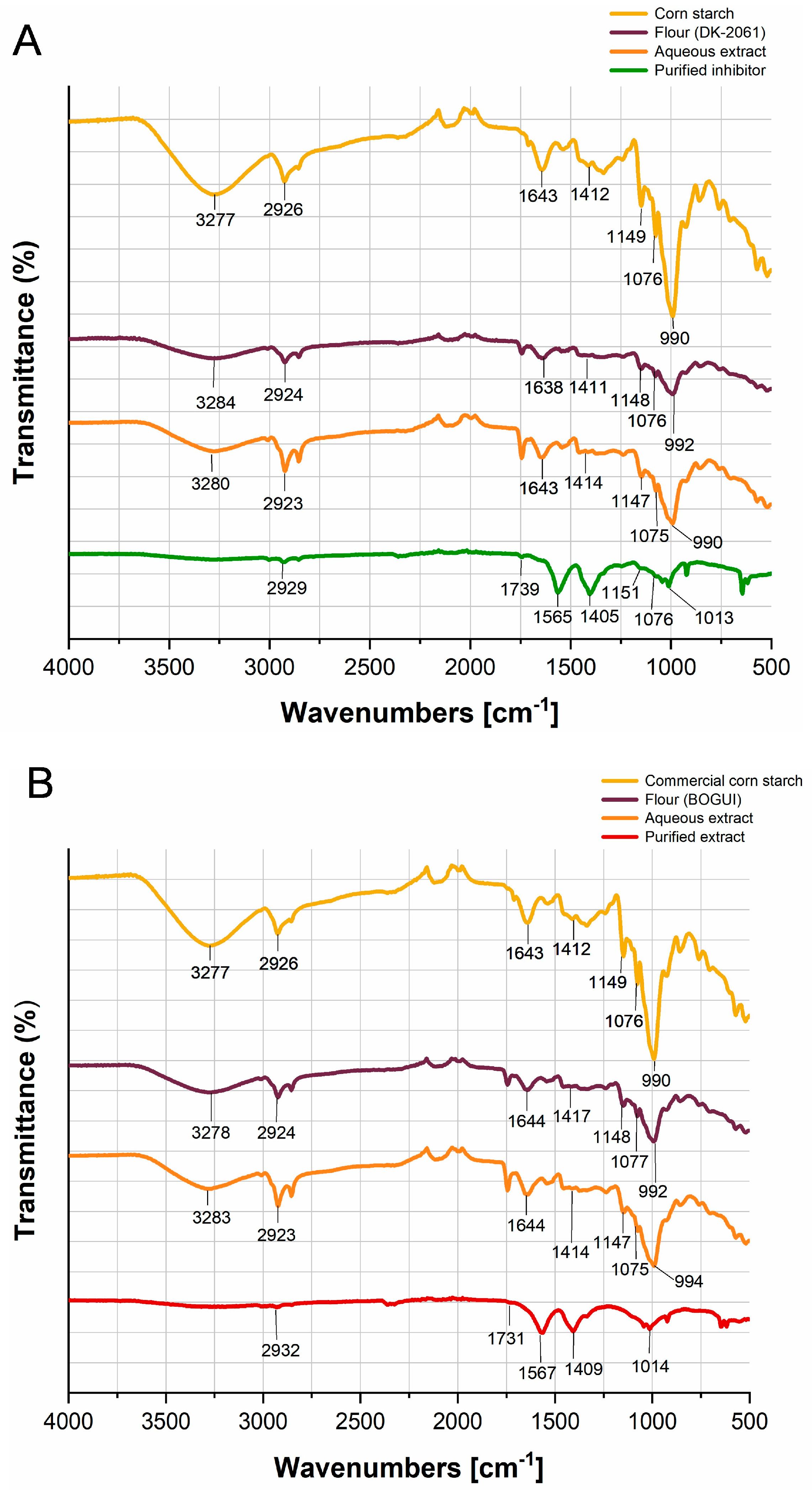
2.3. Fourier Transform Infrared Analysis of the Enzyme Inhibitors
2.4. Scanning Electron Microscopy of Enzyme Inhibitors
2.5. Energy Dispersive Spectroscopy Analysis of the Purified Inhibitors
2.6. Qualitative Analysis of the Starch
2.7. Determination of the Degree of Acetylation of the Purified Inhibitors
2.8. Activity of α-Amylase on Flours and Purified Inhibitors from Corn Hybrids DK-2061 and BOGUI
2.9. Effect of α-Amylase on the Stability of Inhibitors Obtained from Corn Hybrids DK-2061 and BOGUI
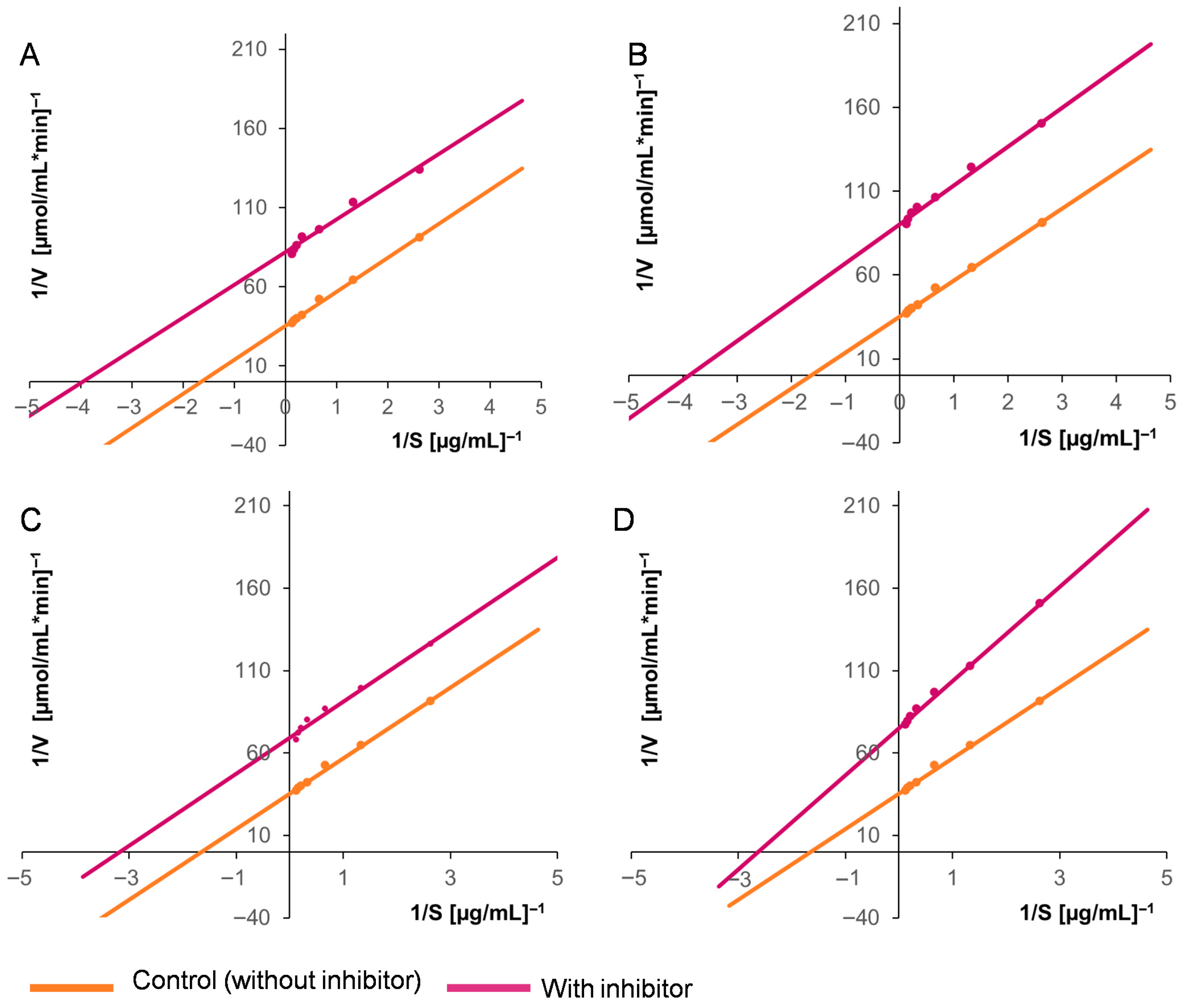
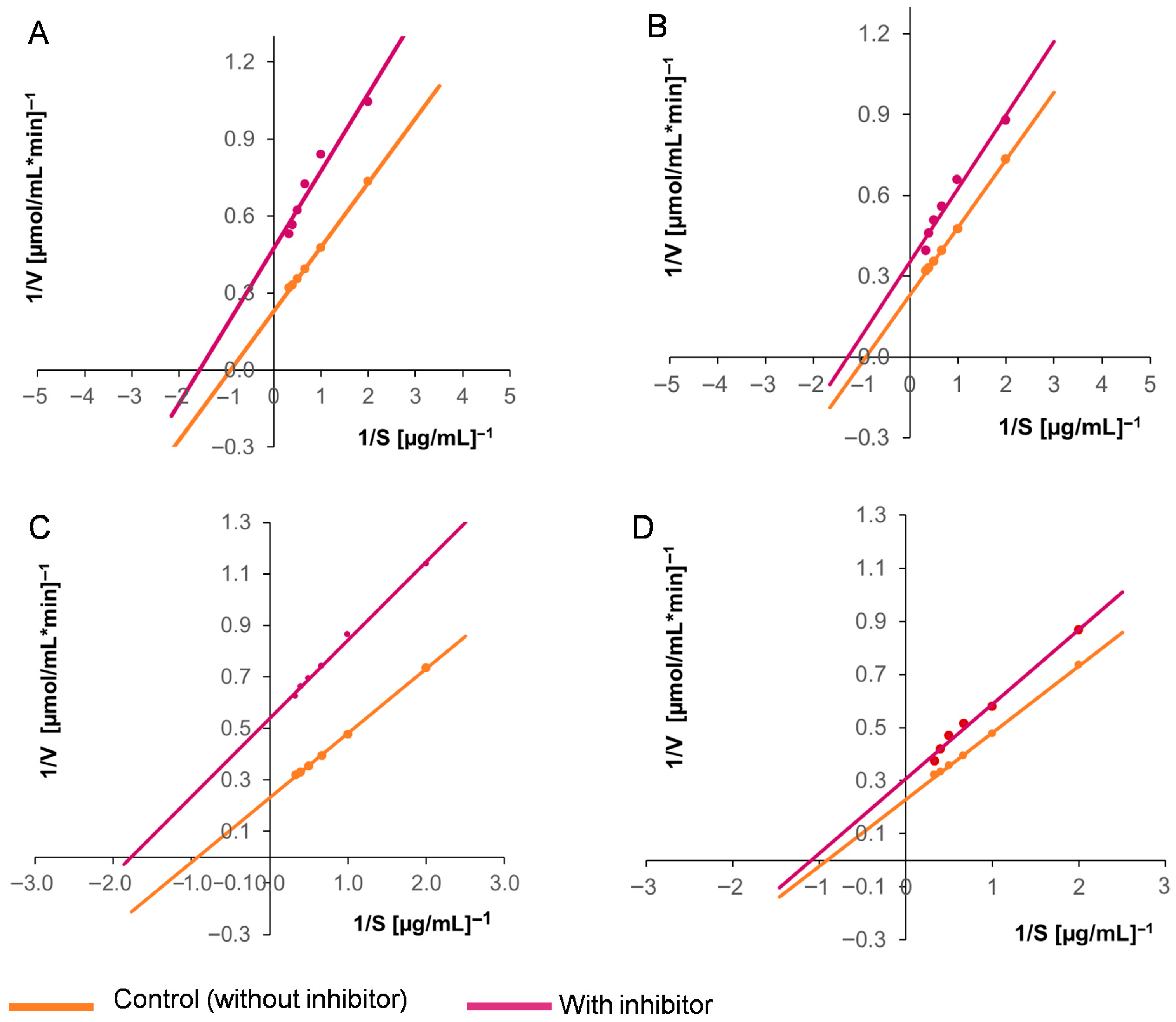
2.10. Effects of the Inhibitors on the Kinetic Parameters of EAP1 and SRXL1
2.11. Effect of the Purified Enzyme Inhibitors on the Development of S. reilianum
3. Discussion
4. Materials and Methods
4.1. Microorganisms and Conservation
4.2. Obtaining Aqueous Extracts from Corn Seeds
4.3. Obtaining the Crude Enzyme Extracts with Aspartyl Protease EAP1 and Xylanase SRXL1 Activity
4.4. Determination of the Activities of the Aspartyl Protease EAP1 and the Xylanase SRXL1
4.5. Enzyme Inhibition Assays
4.6. Purification of the Enzyme Inhibitor
4.7. Purification of the Aspartyl Protease EAP1 and Xylanase SRXL1 Enzymes
4.8. Fourier-Transform Infrared Spectroscopy (FT-IR) Analysis
4.9. Scanning Electron Microscopy (SEM) and Energy-Dispersive X-Ray Spectroscopy (EDS) Analyses
4.10. Qualitative Determination of Starch
4.11. Determination of the Degrees of Acetylation and Substitution of the Purified Inhibitors
4.12. Effects of α-Amylase on Flours and Purified Inhibitors from Corn Hybrids DK-2061 and BOGUI
4.13. Effects of α-Amylase on the Stability of Inhibitors Obtained from Corn Hybrids DK-2061 and BOGUI
4.14. Effects of the Inhibitors on the Kinetic Parameters of Aspartyl Proteases EAP1 and Xylanase SRXL1
4.15. Effects of the Purified Enzyme Inhibitors on the Development of S. reilianum
4.16. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiao, Y.; Chen, H.D.; Han, H.; Chang, Y. Development and utilization of corn processing by-products: A review. Foods 2022, 11, 3709. [Google Scholar] [CrossRef]
- Mohammed, A.A.B.A.; Hasan, Z.; Omran, A.A.B.; Kumar, V.V.; Elfaghi, A.M.; Ilyas, R.A.; Sapuan, S.M. Corn: Its structure, polymer, fiber, composite, properties, and applications. Polymers 2022, 14, 4396. [Google Scholar] [CrossRef]
- Matyac, C.A.; Kommedahl, T. Factors affecting the development of head smut caused by Sphacelotheca reiliana on corn. Phytopathology 1985, 75, 577–581. [Google Scholar] [CrossRef]
- Zuo, W.; Ökmen, B.; Depotter, J.R.L.; Ebert, M.K.; Redkar, A.; Misas Villamil, J.; Doehlemann, G. Molecular interactions between smut fungi and their host plants. Annu. Rev. Phytopathol. 2019, 57, 411–430. [Google Scholar] [CrossRef] [PubMed]
- Martinez, C.; Roux, C.; Jauneau, A.; Dargent, R. The biological cycle of Sporisorium reilianum f. sp. zeae: An overview using microscopy. Mycologia 2002, 94, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Schirawski, J.; Heinze, B.; Wagenknecht, M.; Kahmann, R. Mating type loci of Sporisorium reilianum: Novel pattern with three a and multiple b specificities. Eukaryot. Cell 2005, 4, 1317–1327. [Google Scholar] [CrossRef]
- Ghareeb, H.; Becker, A.; Iven, T.; Feussner, I.; Schirawski, J. Sporisorium reilianum infection changes inflorescence and branching architectures of maize. Plant Physiol. 2011, 156, 2037–2052. [Google Scholar] [CrossRef]
- Mack, H.J.; Baggett, J.R.; Koepsell, P.A. Effects of cultural practices on the incidence of head smut in sweet corn. HortScience 1984, 19, 77–78. [Google Scholar] [CrossRef]
- Wright, P.J.; Fullerton, R.A.; Koolaard, J.P. Fungicide control of head smut (Sporisorium reilianum) of sweetcorn (Zea mays). N. Z. J. Crop Hortic. Sci. 2006, 34, 23–26. [Google Scholar]
- Salinas, A.Q.; Velázquez, M.M.; De León, C.; Díaz, C.N.; Anguiano, A.M.H.; Licona, G.M. Sensibilidad del carbón de la espiga del maíz a diferentes fungicidas. CIENCIA Ergo-Sum 2023, 30, e200. [Google Scholar]
- Islam, T.; Danishuddin Tamanna, N.T.; Matin, M.N.; Barai, H.R.; Haque, M.A. Resistance mechanisms of plant pathogenic fungi to fungicide, environmental impacts of fungicides, and sustainable solutions. Plants 2024, 13, 2737. [Google Scholar] [CrossRef] [PubMed]
- Aquino-Martínez, J.G.; Sánchez-Flores, A.; González-Huerta, A.; Sánchez-Pale, J.R. Resistance of varieties and hybrids of corn (Zea mays) to Sporisorium reilianum and grain yield. Rev. Mex. Fitopatol. 2011, 29, 39–49. [Google Scholar]
- Quezada-Salinas, A.; Moreno-Velázquez, M.; De León-García de Alba, C.; Nava-Díaz, C.; Solano-Báez, A.R. Genetic resistance to Sporisorium reilianum f. sp. zeae in selected maize (Zea mays L.) lines with white and yellow endosperm. Rev. Mex. Fitopatol. 2017, 35, 534–548. [Google Scholar]
- Álvarez-Cervantes, J.; Hernández-Domínguez, E.M.; Tellez-Tellez, M.; Mandujano-González, V.; Mercado-Flores, Y.; Diaz-Godinez, G. Stenocarpella maydis and Sporisorium reilianum: Two pathogenic fungi of maize. In Fungal Pathogenicity; Sultan, S., Ed.; INTECH: London, UK, 2016; pp. 45–60. [Google Scholar]
- Mercado-Flores, Y.; Cárdenas-Álvarez, I.O.; Rojas-Olvera, A.V.; Pérez-Camarillo, J.P.; Leyva-Mir, S.G.; Anducho-Reyes, M.A. Application of Bacillus subtilis in the biological control of the phytopathogenic fungus Sporisorium reilianum. Biol. Control 2014, 76, 36–40. [Google Scholar] [CrossRef]
- González-León, Y.; De la Vega-Camarillo, E.; Ramírez-Vargas, R.; Anducho-Reyes, M.A.; Mercado-Flores, Y. Whole genome analysis of Bacillus velezensis 160, biological control agent of corn head smut. Microbiol. Spectr. 2024, 12, e0326423. [Google Scholar] [CrossRef]
- Zhang, S.; Li, C.; Si, J.; Han, Z.; Chen, D. Action mechanisms of effectors in plant-pathogen interaction. Int. J. Mol. Sci. 2022, 23, 6758. [Google Scholar] [CrossRef]
- Misas-Villamil, J.C.; van der Hoorn, R.A. Enzyme-inhibitor interactions at the plant-pathogen interface. Curr. Opin. Plant Biol. 2008, 11, 380–388. [Google Scholar] [CrossRef]
- Rodríguez-Sifuentes, L.; Marszalek, J.E.; Chuck-Hernández, C.; Serna-Saldívar, S.O. Legumes protease inhibitors as biopesticides and their defense mechanisms against biotic factors. Int. J. Mol. Sci. 2020, 21, 3322. [Google Scholar] [CrossRef]
- Del Prete, S.; Pagano, M. Enzyme inhibitors as multifaceted tools in medicine and agriculture. Molecules 2024, 29, 4314. [Google Scholar] [CrossRef]
- Brutus, A.; Reca, I.B.; Herga, S.; Mattei, B.; Puigserver, A.; Chaix, J.C.; Juge, N.; Bellincampi, D.; Giardina, T. A family 11 xylanase from the pathogen Botrytis cinerea is inhibited by plant endoxylanase inhibitors XIP-I and TAXI-I. Biochem. Biophys. Res. Commun. 2005, 337, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Beliën, T.; Van Campenhout, S.; Van Acker, M.; Volckaert, G. Cloning and characterization of two endoxylanases from the cereal phytopathogen Fusarium graminearum and their inhibition profile against endoxylanase inhibitors from wheat. Biochem. Biophys. Res. Commun. 2005, 327, 407–414. [Google Scholar] [CrossRef]
- Fierens, E.; Rombouts, S.; Gebruers, K.; Goesaert, H.; Brijs, K.; Beaugrand, J.; Volckaert, G.; Van Campenhout, S.; Proost, P.; Courtin, C.M.; et al. TLXI, a novel type of xylanase inhibitor from wheat (Triticum aestivum) belonging to the thaumatin family. Biochem. J. 2007, 403, 583–591. [Google Scholar] [CrossRef]
- Moscetti, I.; Tundo, S.; Janni, M.; Sella, L.; Gazzetti, K.; Tauzin, A.; Giardina, T.; Masci, S.; Favaron, F.; D’Ovidio, R. Constitutive expression of the xylanase inhibitor TAXI-III delays Fusarium head blight symptoms in durum wheat transgenic plants. MPMI 2013, 26, 1464–1472. [Google Scholar] [CrossRef] [PubMed]
- Tundo, S.; Janni, M.; Moscetti, I.; Mandalà, G.; Savatin, D.; Blechl, A.; Favaron, F.; D’Ovidio, R. PvPGIP2 accumulation in specific floral tissues but not in the endosperm limits Fusarium graminearum infection in wheat. Mol. Plant Microbe Interact. 2016, 29, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Sella, L.; Castiglioni, C.; Roberti, S.; D’Ovidio, R.; Favaron, F. An endo-polygalacturonase (PG) of Fusarium moniliforme escaping inhibition by plant polygalacturonase-inhibiting proteins (PGIPs) provides new insights into the PG-PGIP interaction. FEMS Microbiol. Lett. 2004, 240, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Manfredini, C.; Sicilia, F.; Ferrari, S.; Pontiggia, D.; Salvi, G.; Caprari, C.; Lorito, M.; De Lorenzo, G. Polygalacturonase-inhibiting protein 2 of Phaseolus vulgaris inhibits BcPG1, a polygalacturonase of Botrytis cinerea important for pathogenicity, and protects transgenic plants from infection. Physiol. Mol. Plant Pathol. 2005, 67, 108–115. [Google Scholar] [CrossRef]
- Sicilia, F.; Fernandez-Recio, J.; Caprari, C.; De Lorenzo, G.; Tsernoglou, D.; Cervone, F.; Federici, L. The polygalacturonase-inhibiting protein PGIP2 of Phaseolus vulgaris has evolved a mixed mode of inhibition of endopolygalacturonase PG1 of Botrytis cinerea. Plant Physiol. 2005, 139, 1380–1388. [Google Scholar] [CrossRef]
- Lin, P.; Wong, J.H.; Ng, T.B.; Ho, V.S.; Xia, L. A sorghum xylanase inhibitor-like protein with highly potent antifungal, antitumor and HIV-1 reverse transcriptase inhibitory activities. Food Chem. 2013, 141, 2916–2922. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, N.; Mishra, B.; Dube, D.; Sinha, M.; Singh, S.B.; Dey, S.; Kaur, P.; Sharma, S.; Singh, T.P. Modulation of inhibitory activity of xylanase-α-amylase inhibitor protein (XAIP): Binding studies and crystal structure determination of XAIP-II from Scadoxus multiflorus at 1.2 Å resolution. BMC Struct. Biol. 2010, 10, 41. [Google Scholar] [CrossRef]
- Hou, C.; Lv, T.; Zhan, Y.; Peng, Y.; Huang, Y.; Jiang, D.; Weng, X. Overexpression of the RIXI xylanase inhibitor improves disease resistance to the fungal pathogen, Magnaporthe oryzae, in rice. PCTOC 2015, 120, 167–177. [Google Scholar]
- Hellinger, R.; Gruber, C.W. Peptide-based protease inhibitors from plants. Drug Discov. Today 2019, 24, 1877–1889. [Google Scholar] [CrossRef]
- Gutierrez-Gongora, D.; Geddes-McAlister, J. From naturally-sourced protease inhibitors to new treatments for fungal infections. J. Fungi 2021, 7, 1016. [Google Scholar] [CrossRef]
- Parisi, M.G.; Ozón, B.; Vera, G.S.M.; García-Pardo, J.; Obregón, W.D. Plant protease inhibitors as emerging antimicrobial peptide agents: A comprehensive review. Pharmaceutics 2024, 16, 582. [Google Scholar] [CrossRef] [PubMed]
- Kunitz, M. Crystallization of a trypsin inhibitor from soybean. Science 1945, 101, 668–669. [Google Scholar] [CrossRef]
- Khan, S.; Arshad, S.; Arif, A.; Tanveer, R.; Amin, Z.S.; Abbas, S.; Maqsood, A.; Raza, M.; Munir, A.; Latif, A.; et al. Trypsin inhibitor isolated from Glycine max (soya bean) extraction, purification, and characterization. Dose-Response 2022, 20, 15593258221131462. [Google Scholar] [CrossRef]
- Christeller, J.T.; Farley, P.; Ramsay, R.; Sullivan, P.; Laing, W. Purification, characterization and cloning of an aspartic proteinase inhibitor from squash phloem exudate. Eur. J. Biochem. 1998, 254, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Nair, M.; Sandhu, S. A Kunitz trypsin inhibitor from chickpea (Cicer arietinum L.) that exerts an antimicrobial effect on Fusarium oxysporum f.sp. ciceris. Agric. Sci. 2013, 4, 585–594. [Google Scholar] [CrossRef]
- Wang, S.; Shao, B.; Lu, W.; Hong, J.; Rao, P. Isolation of a trypsin-chymotrypsin inhibitor and its functional properties. Prep. Biochem. Biotechnol. 2014, 44, 545–557. [Google Scholar]
- Kim, M.H.; Park, S.C.; Kim, J.Y.; Lee, S.Y.; Lim, H.T.; Cheong, H.; Hahm, K.S.; Park, Y. Purification and characterization of a heat-stable serine protease inhibitor from the tubers of new potato variety “Golden Valley”. Biochem. Biophys. Res. Commun. 2006, 346, 681–686. [Google Scholar] [CrossRef]
- Mandujano-González, V.; Arana-Cuenca, A.; Anducho-Reyes, M.A.; Téllez-Jurado, A.; González-Becerra, A.E.; Mercado-Flores, Y. Biochemical study of the extracellular aspartyl protease Eap1 from the phytopathogen fungus Sporisorium reilianum. Protein Expr. Purif. 2013, 92, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Cervantes, J.; Hernández-Domínguez, E.M.; Arana-Cuenca, A.; Díaz-Godínez, G.; Mercado-Flores, Y. Purification and characterization of xylanase SRXL1 from Sporisorium reilianum grown in submerged and solid-state fermentation. BioRes 2013, 8, 5309–5318. [Google Scholar] [CrossRef]
- Han, G.Z. Origin and evolution of the plant immune system. New Phytol. 2019, 222, 70–83. [Google Scholar] [CrossRef]
- Han, Z.; Schneiter, R. Dual functionality of pathogenesis-related proteins: Defensive role in plants versus immunosuppressive role in pathogens. Front. Plant Sci. 2024, 15, 1368467. [Google Scholar] [CrossRef] [PubMed]
- Delaunois, B.; Jeandet, P.; Clément, C.; Baillieul, F.; Dorey, S.; Cordelier, S. Uncovering plant-pathogen crosstalk through apoplastic proteomic studies. Front. Plant Sci. 2014, 5, 249. [Google Scholar] [CrossRef]
- Smirnova, O.G.; Kochetov, A.V. Plant cell wall and mechanisms of resistance to pathogens. Russ. J. Genet. Appl. Res. 2016, 6, 622–631. [Google Scholar] [CrossRef]
- Li, P.; Lu, Y.J.; Chen, H.; Day, B. The lifecycle of the plant immune system. Crit. Rev. Plant Sci. 2020, 39, 72–100. [Google Scholar] [CrossRef] [PubMed]
- Dash, C.; Ahmad, A.; Nath, D.; Rao, M. Novel bifunctional inhibitor of xylanase and aspartic protease: Implications for inhibition of fungal growth. Antimicrob. Agents Chemother. 2001, 45, 2008–2017. [Google Scholar] [CrossRef]
- Tundo, S.; Mandalà, G.; Sella, L.; Favaron, F.; Bedre, R.; Kalunke, R.M. Xylanase inhibitors: Defense players in plant immunity with implications in agro-industrial processing. Int. J. Mol. Sci. 2022, 23, 14994. [Google Scholar] [CrossRef]
- Cotabarren, J.; Lufrano, D.; Parisi, M.G.; Obregón, W.D. Biotechnological, biomedical, and agronomical applications of plant protease inhibitors with high stability: A systematic review. Int. J. Exp. Plant Biol. 2020, 292, 110398. [Google Scholar] [CrossRef]
- Qu, L.J.; Chen, J.; Liu, M.; Pan, N.; Okamoto, H.; Lin, Z.; Li, C.; Li, D.; Wang, J.; Zhu, G.; et al. Molecular cloning and functional analysis of a novel type of Bowman-Birk inhibitor gene family in rice. Plant Physiol. 2003, 133, 560–570. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Mazumdar, S.; Leighton, S.M.; Babu, C.R. A Kunitz proteinase inhibitor from Archidendron ellipticum seeds: Purification, characterization, and kinetic properties. Phytochemistry 2006, 67, 232–241. [Google Scholar] [CrossRef]
- Tundo, S.; Paccanaro, M.C.; Elmaghraby, I.; Moscetti, I.; D’Ovidio, R.; Favaron, F.; Sella, L. The xylanase inhibitor TAXI-I increases plant resistance to Botrytis cinerea by Inhibiting the BcXyn11a xylanase necrotizing activity. Plants 2020, 9, 601. [Google Scholar] [CrossRef]
- Valueva, T.A.; Revina, T.A.; Kladnitskaya, G.V.; Mosolov, V.V. Kunitz-type proteinase inhibitors from intact and Phytophthora-infected potato tubers. FEBS Lett. 1998, 426, 131–134. [Google Scholar] [CrossRef]
- Ye, X.Y.; Ng, T.B.; Rao, P.F. A Bowman-Birk-type trypsin-chymotrypsin inhibitor from broad beans. Biochem. Biophys. Res. Commun. 2001, 289, 91–96. [Google Scholar] [CrossRef]
- Wang, S.; Lin, J.; Ye, M.; Ng, T.B.; Rao, P.; Ye, X. Isolation and characterization of a novel mung bean protease inhibitor with antipathogenic and anti-proliferative activities. Peptides 2006, 27, 3129–3136. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, J.; Wang, X.; Fang, W.; Bidochka, M.J.; She, R.; Xiao, Y.; Pei, Y. Psc-AFP, an antifungal protein with trypsin inhibitor activity from Psoralea corylifolia seeds. Peptides 2006, 27, 1726–1731. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, E.A.; Santana, C.G.; Godoy, C.V.; Seixas, C.D.; Silva, M.S.; Moreira, L.R.; Oliveira-Neto, O.B.; Price, D.; Fitches, E.; Filho, E.X.; et al. A new chitinase-like xylanase inhibitor protein (XIP) from coffee (Coffea arabica) affects Soybean Asian rust (Phakopsora pachyrhizi) spore germination. BMC Biotechnol. 2011, 11, 14. [Google Scholar] [CrossRef]
- Müller, V.; Bonacci, G.; Batthyany, C.; Amé, M.V.; Carrari, F.; Gieco, J.; Asis, R. Peanut seed cultivars with contrasting resistance to Aspergillus parasiticus colonization display differential temporal response of protease inhibitors. Phytopathology 2017, 107, 474–482. [Google Scholar] [CrossRef]
- Malehorn, D.E.; Borgmeyer, J.R.; Smith, C.E.; Shah, D.M. Characterization and expression of an antifungal zeamatin-like protein (Zlp) gene from Zea mays. Plant Physiol. 1994, 106, 1471–1481. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Labra, A.; Chagolla-Lopez, A.; Marínez-Gallardo, N.; Valdes-Rodriguez, S. Further characterization of the 12 kda protease/alpha amylase inhibitor present in maize seeds. J. Food Biochem. 1995, 19, 27–41. [Google Scholar] [CrossRef]
- Apriyanto, A.; Compart, J.; Fettke, J. A review of starch, a unique biopolymer—Structure, metabolism and in planta modifications. Int. J. Exp. Plant Biol. 2022, 318, 111223. [Google Scholar] [CrossRef]
- Patadiya, N.; Panchal, N.; Vaghela, V. A review on enzyme inhibitors. Int. Res. J. Pharm. 2021, 12, 60–66. [Google Scholar] [CrossRef]
- Daza, O.S.M.; Parra, A.G.P. Espectroscopia de infrarrojo con transformada de fourier (FT-IR) para análisis de muestras de harina de trigo, fécula de maíz y almidón de yuca. Cienc. Tecnol. Aliment. 2021, 19, 5–16. [Google Scholar]
- Cuenca, P.S.; Ferrero, S.; Albani, O.A. Preparation and characterization of cassava starch acetate with high substitution degree. Food Hydrocoll. 2020, 100, 105430. [Google Scholar] [CrossRef]
- Zięba, T.; Wilczak, A.; Kobryń, J.; Musiał, W.; Kapelko-Żeberska, M.; Gryszkin, A.; Meisel, M. The annealing of acetylated potato starch with various substitution degrees. Molecules 2021, 26, 2096. [Google Scholar] [CrossRef]
- Chi, H.; Xu, K.; Wu, X.; Chen, Q.; Xue, D.; Song, C.; Zhang, W.; Wang, P. Effect of acetylation on the properties of corn starch. Food Chem. 2008, 106, 923–928. [Google Scholar] [CrossRef]
- Chi, C.; Lian, S.; Zou, Y.; Chen, B.; He, Y.; Zheng, M.; Zhao, Y.; Wang, H. Preparation, multi-scale structures, and functionalities of acetylated starch: An updated review. Int. J. Biol. Macromol. 2023, 249, 126142. [Google Scholar] [CrossRef]
- Romainor, A.N.; Chin, S.F.; Lihan, S. Antimicrobial starch-based film for food packaging application. Starch-Stärke 2022, 74, 2100207. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Bahdanovich, P.; Axelrod, K.; Khlystov, A.Y.; Samburova, V. Optimized spectrophotometry method for starch quantification. Analytica 2022, 3, 394–405. [Google Scholar] [CrossRef]
- Zhang, C.; Du, M.; Cao, T.; Xu, W. The effect of acetylation on the physicochemical properties of chickpea starch. Foods 2023, 12, 2462. [Google Scholar] [CrossRef] [PubMed]
- Wurzburg, O.B. Acetylation. In Methods in Carbohydrate Chemistry; Whistler, R.L., Ed.; Academic Press: New York, NY, USA, 1964; pp. 286–288. [Google Scholar]
- Aledo, J.C. Enzyme kinetic parameters estimation: A tricky task? Biochem. Mol. Biol. Educ. 2021, 49, 633–638. [Google Scholar] [CrossRef] [PubMed]


| Substrate | α-Amylase Activity (U/mL) |
|---|---|
| Potato starch | 47.4 ± 1.2 A |
| Flour from hybrid DK-2061 | 31.8 ± 0.3 D |
| Flour from hybrid BOGUI | 39.5 ± 0.6 B |
| Purified inhibitor from DK-2061 | 22.7 ± 0.2 E |
| Purified inhibitor from BOGUI | 32.1 ± 4.3 C |
| Substrate | % Inhibition | |||||
|---|---|---|---|---|---|---|
| Treatment | Control 1 | Control 2 | ||||
| EAP1 | SRXLI | EAP1 | SRXLI | EAP1 | SRXLI | |
| Flour from DK-2061 | 0 ± 0 | 0 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 |
| Flour from BOGUI | 0 ± 0 | 0 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 |
| Purified inhibitor from DK-2061 | 0 ± 0 | 0 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 |
| Purified inhibitor from BOGUI | 0 ± 0 | 0 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 |
| Aspartyl Protease EAP1 | Xylanase SRXL1 | ||||||
|---|---|---|---|---|---|---|---|
| DK-2061 | BOGUI | DK-2061 | BOGUI | ||||
| µg | % Inhibition | µg | % Inhibition | µg | % Inhibition | µg | % Inhibition |
| 0.0 | 0.0 ± 0.0 A | 0.0 | 0.0 ± 0.0 A | 0.0 | 0.0 ± 0.0 A | 0.0 | 0.0 ± 0.0 A |
| 5.0 | 11.9 ± 0.6 B | 10.0 | 19.9 ± 0.8 B | 2.5 | 46.4 ± 0.9 B | 2.5 | 42.4 ± 1.0 B |
| 10.0 | 14.5 ± 1.5 B | 15.0 | 26.1 ± 0.6 C | 5.0 | 50.5 ± 0.9 C | 5.0 | 54.4 ± 0.8 C |
| 15.0 | 17.1 ± 1.7 B | 20.0 | 29.2 ± 0.5 D | 10.0 | 54.8 ± 0.6 D | 7.5 | 64.3 ± 0.7 D |
| 20.0 | 51.7 ± 0.2 C | 25.0 | 34.5 ± 1.1 E | 15.0 | 57.5 ± 0.6 E | 10.0 | 96.7 ± 0.1 E |
| 25.0 | 92.6 ± 0.2 D | 30.0 | 52.0 ± 1.8 F | 20.0 | 65.6 ± 0.4 F | - | - |
| - | - | 35.0 | 92.1 ± 0.2 G | 25.0 | 96.6 ± 0.1 G | - | - |
| Inhibitor | Inhibitor Concentration (µg/mL) | Vmax | Km | Vmax/Km | R2 Value | Type of Inhibition |
|---|---|---|---|---|---|---|
| Aspartyl protease EAP1 | ||||||
| DK-2061 | No inhibitor | 0.028 ± 0.001 A | 0.61 ± 0.028 A | 0.050 | 0.996 | Uncompetitive |
| 15 | 0.012 ± 0.001 B | 0.25 ± 0.013 B | 0.048 | 0.979 | ||
| 20 | 0.011 ± 0.001 C | 0.26 ± 0.020 B | 0.044 | 0.989 | ||
| BOGUI | No inhibitor | 0.028 ± 0.001 A | 0.61 ± 0.028 A | 0.050 | 0.996 | Uncompetitive |
| 25 | 0.014 ± 0.001 B | 0.32 ± 0.021 B | 0.046 | 0.984 | ||
| 30 | 0.013 ± 0.001 C | 0.38 ± 0.013 C | 0.035 | 0.997 | ||
| Xylanase SRXL1 | ||||||
| DK-2061 | No inhibitor | 4.34 ± 0.101 A | 1.090 ± 0.190 A | 3.984 | 0.999 | Uncompetitive |
| 2.5 | 2.97 ± 0.109 B | 0.771 ± 0.040 B | 3.857 | 0.944 | ||
| 5.0 | 2.00 ± 0.049 C | 0.635 ± 0.039 C | 3.153 | 0.970 | ||
| BOGUI | No inhibitor | 4.343 ± 0.101 A | 1.090 ± 0.190 A | 3.984 | 0.999 | Uncompetitive |
| 2.5 | 3.368 ± 0.192 B | 0.944 ± 0.048 B | 3.567 | 0.996 | ||
| 5.0 | 1.857 ± 0.060 C | 0.056 ± 0.059 C | 3.281 | 0.988 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velázquez-Juárez, Y.; Téllez-Jurado, A.; Hernández-Chávez, M.; Villa-Tanaca, L.; Falcón-León, M.P.; Mercado-Flores, Y. Enzymatic Inhibitors of Aspartyl Protease EAP1 and Xylanase SRXL1 from Sporisorium reilianum Isolated from Corn Seeds. Int. J. Mol. Sci. 2025, 26, 9974. https://doi.org/10.3390/ijms26209974
Velázquez-Juárez Y, Téllez-Jurado A, Hernández-Chávez M, Villa-Tanaca L, Falcón-León MP, Mercado-Flores Y. Enzymatic Inhibitors of Aspartyl Protease EAP1 and Xylanase SRXL1 from Sporisorium reilianum Isolated from Corn Seeds. International Journal of Molecular Sciences. 2025; 26(20):9974. https://doi.org/10.3390/ijms26209974
Chicago/Turabian StyleVelázquez-Juárez, Yusiri, Alejandro Téllez-Jurado, Macaria Hernández-Chávez, Lourdes Villa-Tanaca, Martha Patricia Falcón-León, and Yuridia Mercado-Flores. 2025. "Enzymatic Inhibitors of Aspartyl Protease EAP1 and Xylanase SRXL1 from Sporisorium reilianum Isolated from Corn Seeds" International Journal of Molecular Sciences 26, no. 20: 9974. https://doi.org/10.3390/ijms26209974
APA StyleVelázquez-Juárez, Y., Téllez-Jurado, A., Hernández-Chávez, M., Villa-Tanaca, L., Falcón-León, M. P., & Mercado-Flores, Y. (2025). Enzymatic Inhibitors of Aspartyl Protease EAP1 and Xylanase SRXL1 from Sporisorium reilianum Isolated from Corn Seeds. International Journal of Molecular Sciences, 26(20), 9974. https://doi.org/10.3390/ijms26209974







