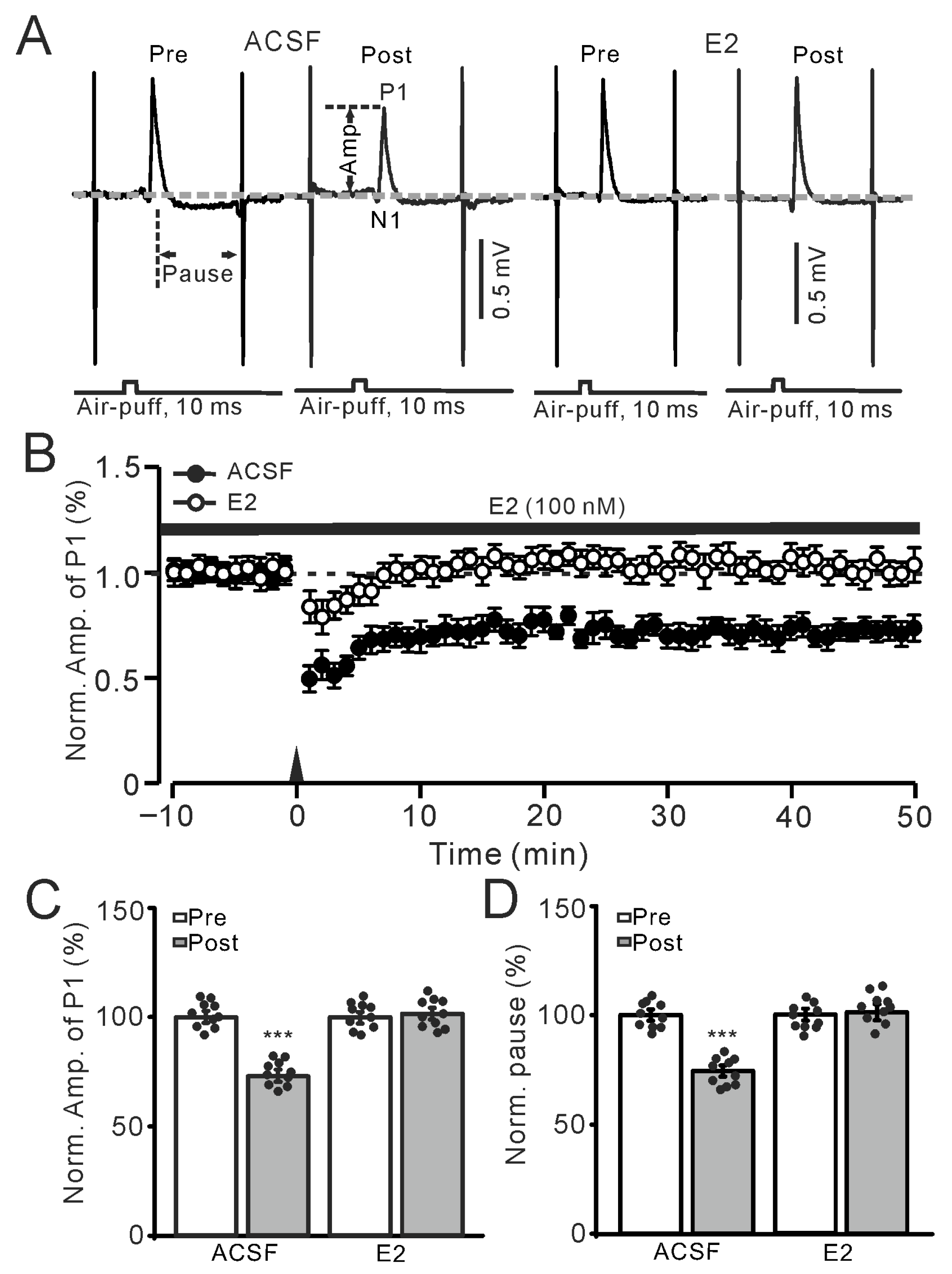
Figure 1.
Estradiol (E2) impaired the facial stimulation-induced molecular layer interneuron–Purkinje cell long-term depression (MLI-PC LTD) in the mouse cerebellar cortex. (A) Representative membrane potentials show the air-puff stimulation evoked-MLI-PC response (P1) before (Pre) and after (Post) the delivery of 1 Hz (240 pulses) stimulation during treatment with artificial cerebrospinal fluid (ACSF, left) and E2 (100 nM; right). The measurements of P1 amplitude (P1 Amp) and SS pause duration are shown in the figure. (B) A summary of data showing the normalized amplitude of P1 over time in the ACSF group (solid circles) and the E2 group (hollow circles) before and after the delivery of 1 Hz stimulation (black arrow). Note that facial stimulation induced MLI-PC LTD in ACSF, which was absent in the presence of E2. (C) The bar graph with individual data shows the normalized P1 amplitude before (Pre) and after (Post) 1 Hz stimulation during treatment with ACSF (Pre: 100.0 ± 1.5%, Post: 74.3 ± 1.2%, F (1,18) = 240.3, p < 0.001; n = 10 recordings/10 mice), and E2 (Pre: 100.0 ± 1.7%, Post: 101.5 ± 2.1%, F (1,18) = 0.48, p = 0.51; n = 10 recordings/10 mice). (D) Bar graph with individual data shows the normalized simple spike (SS) pause time before (Pre) and after (Post) 1 Hz stimulation during treatment with ACSF (Pre: 100.0 ± 1.4%, Post: 74.3 ± 1.2%, F (1,18) = 190.7, p < 0.001; n = 10 recordings/10 mice), and E2 (Pre: 100.0 ± 1.3%, Post: 101.2 ± 2.7%, F (1,18) = 0.45, p = 0.63; n = 10 recordings/10 mice). *** p < 0.001 versus Pre. Note that facial stimulation induced a decrease in P1 amplitude and SS pause duration in ACSF, but no such decrease was observed in the presence of E2.
Figure 1.
Estradiol (E2) impaired the facial stimulation-induced molecular layer interneuron–Purkinje cell long-term depression (MLI-PC LTD) in the mouse cerebellar cortex. (A) Representative membrane potentials show the air-puff stimulation evoked-MLI-PC response (P1) before (Pre) and after (Post) the delivery of 1 Hz (240 pulses) stimulation during treatment with artificial cerebrospinal fluid (ACSF, left) and E2 (100 nM; right). The measurements of P1 amplitude (P1 Amp) and SS pause duration are shown in the figure. (B) A summary of data showing the normalized amplitude of P1 over time in the ACSF group (solid circles) and the E2 group (hollow circles) before and after the delivery of 1 Hz stimulation (black arrow). Note that facial stimulation induced MLI-PC LTD in ACSF, which was absent in the presence of E2. (C) The bar graph with individual data shows the normalized P1 amplitude before (Pre) and after (Post) 1 Hz stimulation during treatment with ACSF (Pre: 100.0 ± 1.5%, Post: 74.3 ± 1.2%, F (1,18) = 240.3, p < 0.001; n = 10 recordings/10 mice), and E2 (Pre: 100.0 ± 1.7%, Post: 101.5 ± 2.1%, F (1,18) = 0.48, p = 0.51; n = 10 recordings/10 mice). (D) Bar graph with individual data shows the normalized simple spike (SS) pause time before (Pre) and after (Post) 1 Hz stimulation during treatment with ACSF (Pre: 100.0 ± 1.4%, Post: 74.3 ± 1.2%, F (1,18) = 190.7, p < 0.001; n = 10 recordings/10 mice), and E2 (Pre: 100.0 ± 1.3%, Post: 101.2 ± 2.7%, F (1,18) = 0.45, p = 0.63; n = 10 recordings/10 mice). *** p < 0.001 versus Pre. Note that facial stimulation induced a decrease in P1 amplitude and SS pause duration in ACSF, but no such decrease was observed in the presence of E2.
![Ijms 26 09973 g001 Ijms 26 09973 g001]()
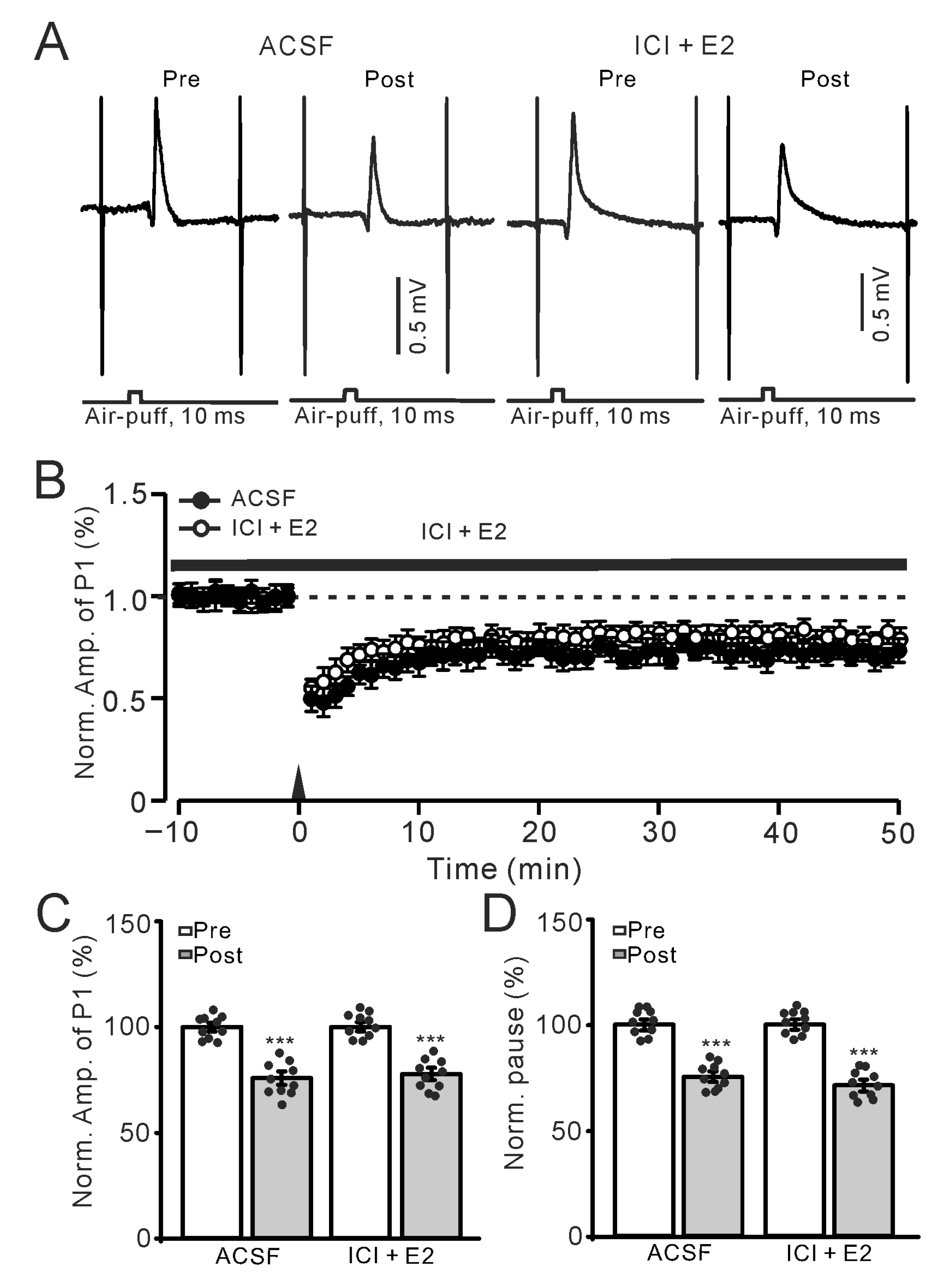
Figure 2.
Application of a nonselective ER antagonist, ICI (100 nM), eliminated the effect of E2 on the facial stimulation-evoked MLI-PC LTD. (A) Representative membrane potentials show air-puff stimulation-evoked MLI-PC response (P1) before (Pre) and after (Post) the delivery of 1 Hz stimulation during treatment with ACSF (left) and ICI182780 (ICI, 100 nM) + E2 (100 nM; right). (B) A summary of data showing the normalized amplitude of P1 over time in the ACSF group (solid circles) and the ICI + E2 group (hollow circles) before and after the delivery of an air-puff stimuli train (black arrow). (C) The bar graph with individual data shows the normalized P1 amplitude before (Pre) and after (Post) 1 Hz stimulation during treatment with ACSF (Pre: 100.0 ± 1.0%, Post: 74.7 ± 1.5%, F (1,18) = 210.7, p < 0.001; n = 10 recordings/10 mice) and ICI + E2 (Pre: 100.0 ± 0.8%, Post: 76.2 ± 1.4%, F (1,18) = 152.4, p < 0.0001; n = 10 recordings/10 mice). (D) The bar graph with individual data shows the normalized SS pause time before (Pre) and after (Post) 1 Hz stimulation during treatment with ACSF (Pre: 100.0 ± 1.2%, Post: 75.3 ± 1.5%, F (1,18) = 0.36, p = 0.77; n = 10 recordings/10 mice) and ICI + E2 (Pre: 100.0 ± 1.4%, Post: 75.3 ± 1.5%, F (1,18) = 196.1, p < 0.001; n = 10 recordings/10 mice). *** p < 0.001 versus Pre. Note that E2 failed to prevent the facial stimulation-evoked MLI-PC LTD in the presence of an ER antagonist.
Figure 2.
Application of a nonselective ER antagonist, ICI (100 nM), eliminated the effect of E2 on the facial stimulation-evoked MLI-PC LTD. (A) Representative membrane potentials show air-puff stimulation-evoked MLI-PC response (P1) before (Pre) and after (Post) the delivery of 1 Hz stimulation during treatment with ACSF (left) and ICI182780 (ICI, 100 nM) + E2 (100 nM; right). (B) A summary of data showing the normalized amplitude of P1 over time in the ACSF group (solid circles) and the ICI + E2 group (hollow circles) before and after the delivery of an air-puff stimuli train (black arrow). (C) The bar graph with individual data shows the normalized P1 amplitude before (Pre) and after (Post) 1 Hz stimulation during treatment with ACSF (Pre: 100.0 ± 1.0%, Post: 74.7 ± 1.5%, F (1,18) = 210.7, p < 0.001; n = 10 recordings/10 mice) and ICI + E2 (Pre: 100.0 ± 0.8%, Post: 76.2 ± 1.4%, F (1,18) = 152.4, p < 0.0001; n = 10 recordings/10 mice). (D) The bar graph with individual data shows the normalized SS pause time before (Pre) and after (Post) 1 Hz stimulation during treatment with ACSF (Pre: 100.0 ± 1.2%, Post: 75.3 ± 1.5%, F (1,18) = 0.36, p = 0.77; n = 10 recordings/10 mice) and ICI + E2 (Pre: 100.0 ± 1.4%, Post: 75.3 ± 1.5%, F (1,18) = 196.1, p < 0.001; n = 10 recordings/10 mice). *** p < 0.001 versus Pre. Note that E2 failed to prevent the facial stimulation-evoked MLI-PC LTD in the presence of an ER antagonist.
![Ijms 26 09973 g002 Ijms 26 09973 g002]()
Figure 3.
In the presence of AM-251, E2 triggered the facial stimulation-induced MLI-PC LTP. (A) Representative membrane potentials showing air-puff stimulation evoked MLI-PC response before (Pre) and after (Post) delivery of the 1 Hz (240 pulses) stimulation during treatment with AM-251 (5 μM control; left) and AM-251 (5 μM) + E2 (100 nM; right). (B) A summary of data showing the normalized amplitude of P1 over time in the AM-251 group (solid circles) and the AM-251 + E2 group (open circles) before and after the air-puff stimuli train (black arrow). (C) The bar graph with individual data shows the normalized P1 amplitude before (Pre) and after (Post) 1 Hz stimulation during treatment with AM-251 (Pre: 100.0 ± 1.2%, Post: 101.4 ± 1.4%, F (1,18) = 0.41, p = 0.53; n = 10 recordings/10 mice) and AM-251 + E2 (Pre: 100.0 ± 1.2%, Post: 122.4 ± 1.3%, F (1,18) = 295.7, p < 0.001; n = 10 recordings/10 mice). (D) The bar graph with individual data shows the normalized SS pause time before (Pre) and after (Post) 1 Hz stimulation during treatment with AM-251 (Pre: 100.0 ± 1.2%, Post: 101.1 ± 1.3%, F (1,18) = 0.36, p = 0.65; n = 10 recordings/10 mice) and AM-251 + E2 (Pre: 100.0 ± 1.3%, Post: 123.4 ± 1.6%, F (1,18) = 305.1, p < 0.001; n = 10 recordings/10 mice). *** p < 0.001 versus Pre; ### p < 0.001 versus Post of AM-251. Note that E2 triggered the facial stimulation-induced MLI-PC LTP in the presence of a CB1R antagonist.
Figure 3.
In the presence of AM-251, E2 triggered the facial stimulation-induced MLI-PC LTP. (A) Representative membrane potentials showing air-puff stimulation evoked MLI-PC response before (Pre) and after (Post) delivery of the 1 Hz (240 pulses) stimulation during treatment with AM-251 (5 μM control; left) and AM-251 (5 μM) + E2 (100 nM; right). (B) A summary of data showing the normalized amplitude of P1 over time in the AM-251 group (solid circles) and the AM-251 + E2 group (open circles) before and after the air-puff stimuli train (black arrow). (C) The bar graph with individual data shows the normalized P1 amplitude before (Pre) and after (Post) 1 Hz stimulation during treatment with AM-251 (Pre: 100.0 ± 1.2%, Post: 101.4 ± 1.4%, F (1,18) = 0.41, p = 0.53; n = 10 recordings/10 mice) and AM-251 + E2 (Pre: 100.0 ± 1.2%, Post: 122.4 ± 1.3%, F (1,18) = 295.7, p < 0.001; n = 10 recordings/10 mice). (D) The bar graph with individual data shows the normalized SS pause time before (Pre) and after (Post) 1 Hz stimulation during treatment with AM-251 (Pre: 100.0 ± 1.2%, Post: 101.1 ± 1.3%, F (1,18) = 0.36, p = 0.65; n = 10 recordings/10 mice) and AM-251 + E2 (Pre: 100.0 ± 1.3%, Post: 123.4 ± 1.6%, F (1,18) = 305.1, p < 0.001; n = 10 recordings/10 mice). *** p < 0.001 versus Pre; ### p < 0.001 versus Post of AM-251. Note that E2 triggered the facial stimulation-induced MLI-PC LTP in the presence of a CB1R antagonist.
![Ijms 26 09973 g003 Ijms 26 09973 g003]()
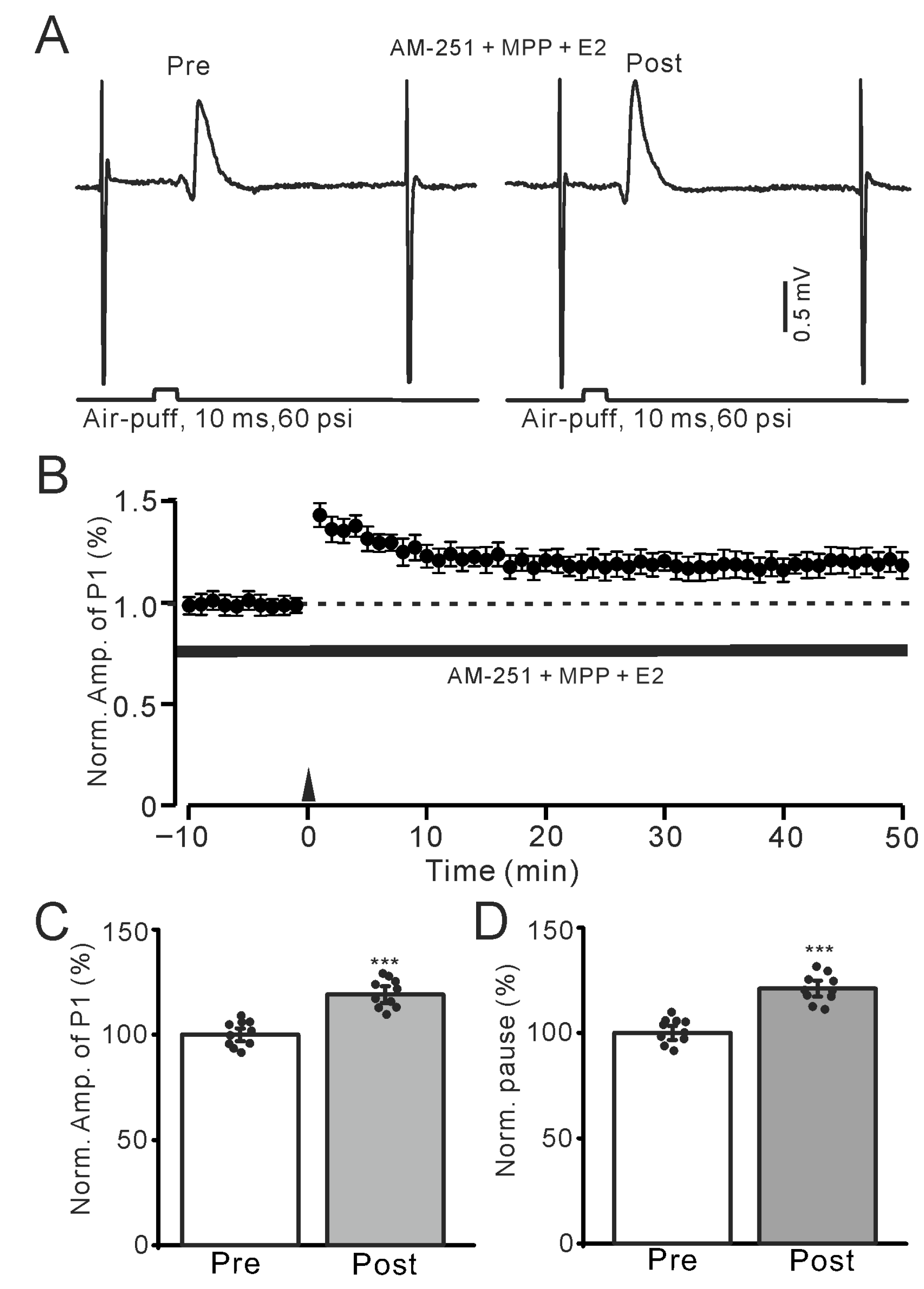
Figure 4.
In the presence of an ERα antagonist and AM-251, E2 triggered the facial stimulation-induced MLI-PC LTP. (A) Representative membrane potentials showing air-puff stimulation evoked MLI-PC response before (Pre) and after (Post) delivery of 1 Hz (240 pulses) facial stimulation during treatment AM-251 (5 μM) + MPP (100 nM) + E2 (100 nM). (B) A summary of data showing the normalized amplitude of P1 in the presence of AM-251 + MPP + E2 before and after the air-puff stimuli train (black arrow). (C) The bar graph with individual data shows the normalized P1 amplitude before (Pre) and after (Post) 1 Hz stimulation in the presence of AM-251 + MPP + E2 (Pre: 100.0 ± 2.9%, Post: 119.1 ± 3.8%, F (1,18) = 236.4, p < 0.001; n = 10 recordings/10 mice). (D) The bar graph with individual data shows the normalized SS pause time before (Pre) and after (Post) 1 Hz stimulation in the presence of AM-251 + MPP + E2 (Pre: 100.0 ± 3.2%, Post: 121.1 ± 3.7%, F (1,18) = 275.6, p < 0.001; n = 10 recordings/10 mice). *** p < 0.001 versus Pre. Note that the E2-triggered MLI-PC LTP persisted in the presence of an ERα antagonist.
Figure 4.
In the presence of an ERα antagonist and AM-251, E2 triggered the facial stimulation-induced MLI-PC LTP. (A) Representative membrane potentials showing air-puff stimulation evoked MLI-PC response before (Pre) and after (Post) delivery of 1 Hz (240 pulses) facial stimulation during treatment AM-251 (5 μM) + MPP (100 nM) + E2 (100 nM). (B) A summary of data showing the normalized amplitude of P1 in the presence of AM-251 + MPP + E2 before and after the air-puff stimuli train (black arrow). (C) The bar graph with individual data shows the normalized P1 amplitude before (Pre) and after (Post) 1 Hz stimulation in the presence of AM-251 + MPP + E2 (Pre: 100.0 ± 2.9%, Post: 119.1 ± 3.8%, F (1,18) = 236.4, p < 0.001; n = 10 recordings/10 mice). (D) The bar graph with individual data shows the normalized SS pause time before (Pre) and after (Post) 1 Hz stimulation in the presence of AM-251 + MPP + E2 (Pre: 100.0 ± 3.2%, Post: 121.1 ± 3.7%, F (1,18) = 275.6, p < 0.001; n = 10 recordings/10 mice). *** p < 0.001 versus Pre. Note that the E2-triggered MLI-PC LTP persisted in the presence of an ERα antagonist.
![Ijms 26 09973 g004 Ijms 26 09973 g004]()
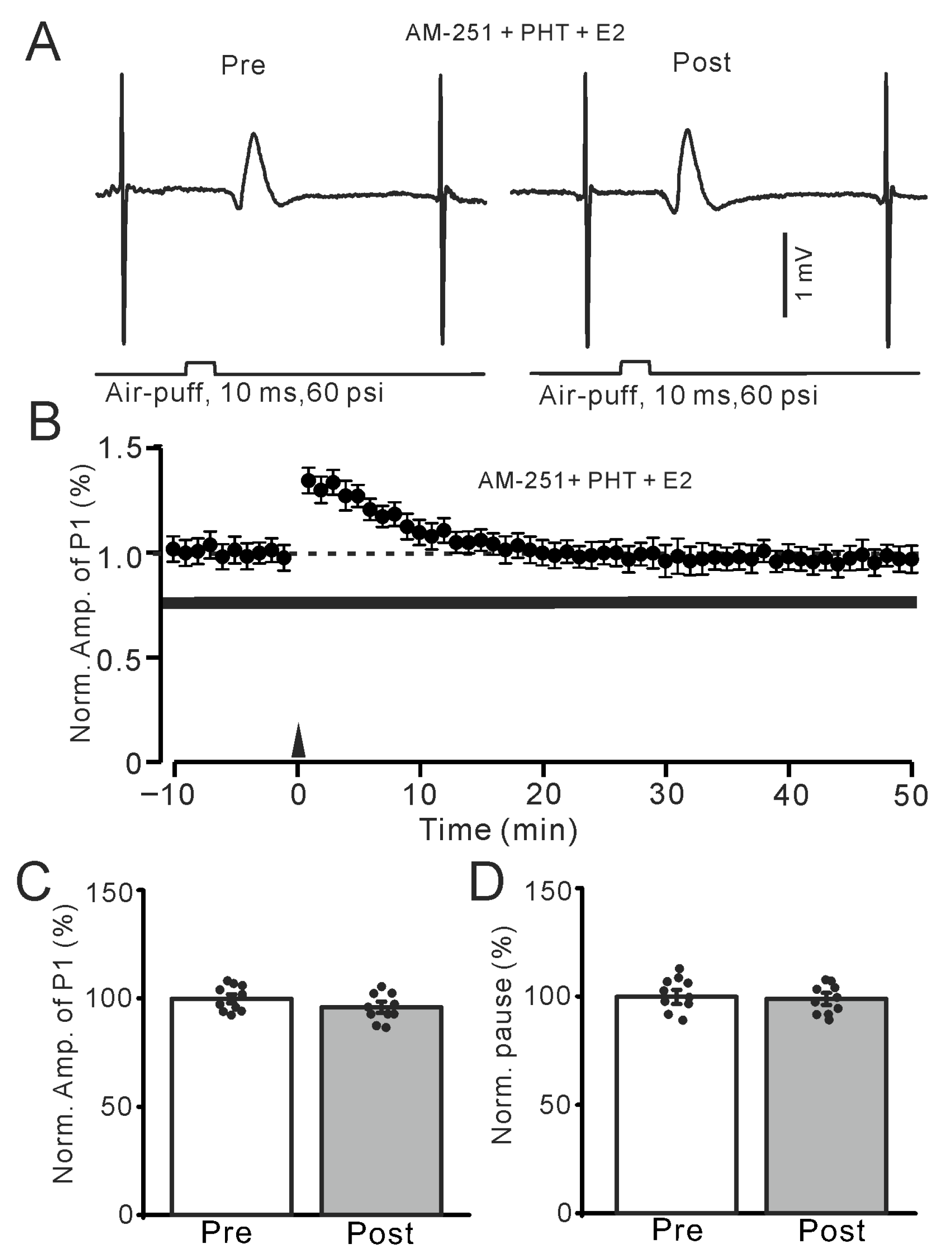
Figure 5.
In the presence of an ERβ antagonist and AM-251, E2 failed to trigger the facial stimulation-induced MLI-PC LTP. (A) Representative membrane potentials showing air-puff stimulation evoked MLI-PC response before (Pre) and after (Post) delivery of the 1 Hz (240 pulses) stimulation during treatment with AM-251 (5 μM) + PHT (100 nM) + E2 (100 nM). (B) A summary of data showing the normalized amplitude of P1 in the presence of AM-251 + PHT + E2 before and after the air-puff stimuli train (black arrow). (C) Bar graph with individual data shows the normalized P1 amplitude before (Pre) and after (Post) 1 Hz stimulation during treatment with a mixture of AM-251 + PHT + E2 (Pre: 100.0 ± 2.0%, Post: 97.1 ± 2.6%, F (1,18) = 1.65, p = 0.36; n = 10 recordings/10 mice). (D) Bar graph with individual data shows the normalized SS pause time before (Pre) and after (Post) 1 Hz stimulation during treatment with a mixture of AM-251 + PHT + E2 (Pre: 100.0 ± 3.2%, Post: 99.1 ± 2.7%, F (1,18) = 1.26, p = 0.67; n = 10 recordings/10 mice). Note that the E2-triggered MLI-PC LTP was abolished by the blockade of ERβ.
Figure 5.
In the presence of an ERβ antagonist and AM-251, E2 failed to trigger the facial stimulation-induced MLI-PC LTP. (A) Representative membrane potentials showing air-puff stimulation evoked MLI-PC response before (Pre) and after (Post) delivery of the 1 Hz (240 pulses) stimulation during treatment with AM-251 (5 μM) + PHT (100 nM) + E2 (100 nM). (B) A summary of data showing the normalized amplitude of P1 in the presence of AM-251 + PHT + E2 before and after the air-puff stimuli train (black arrow). (C) Bar graph with individual data shows the normalized P1 amplitude before (Pre) and after (Post) 1 Hz stimulation during treatment with a mixture of AM-251 + PHT + E2 (Pre: 100.0 ± 2.0%, Post: 97.1 ± 2.6%, F (1,18) = 1.65, p = 0.36; n = 10 recordings/10 mice). (D) Bar graph with individual data shows the normalized SS pause time before (Pre) and after (Post) 1 Hz stimulation during treatment with a mixture of AM-251 + PHT + E2 (Pre: 100.0 ± 3.2%, Post: 99.1 ± 2.7%, F (1,18) = 1.26, p = 0.67; n = 10 recordings/10 mice). Note that the E2-triggered MLI-PC LTP was abolished by the blockade of ERβ.
![Ijms 26 09973 g005 Ijms 26 09973 g005]()
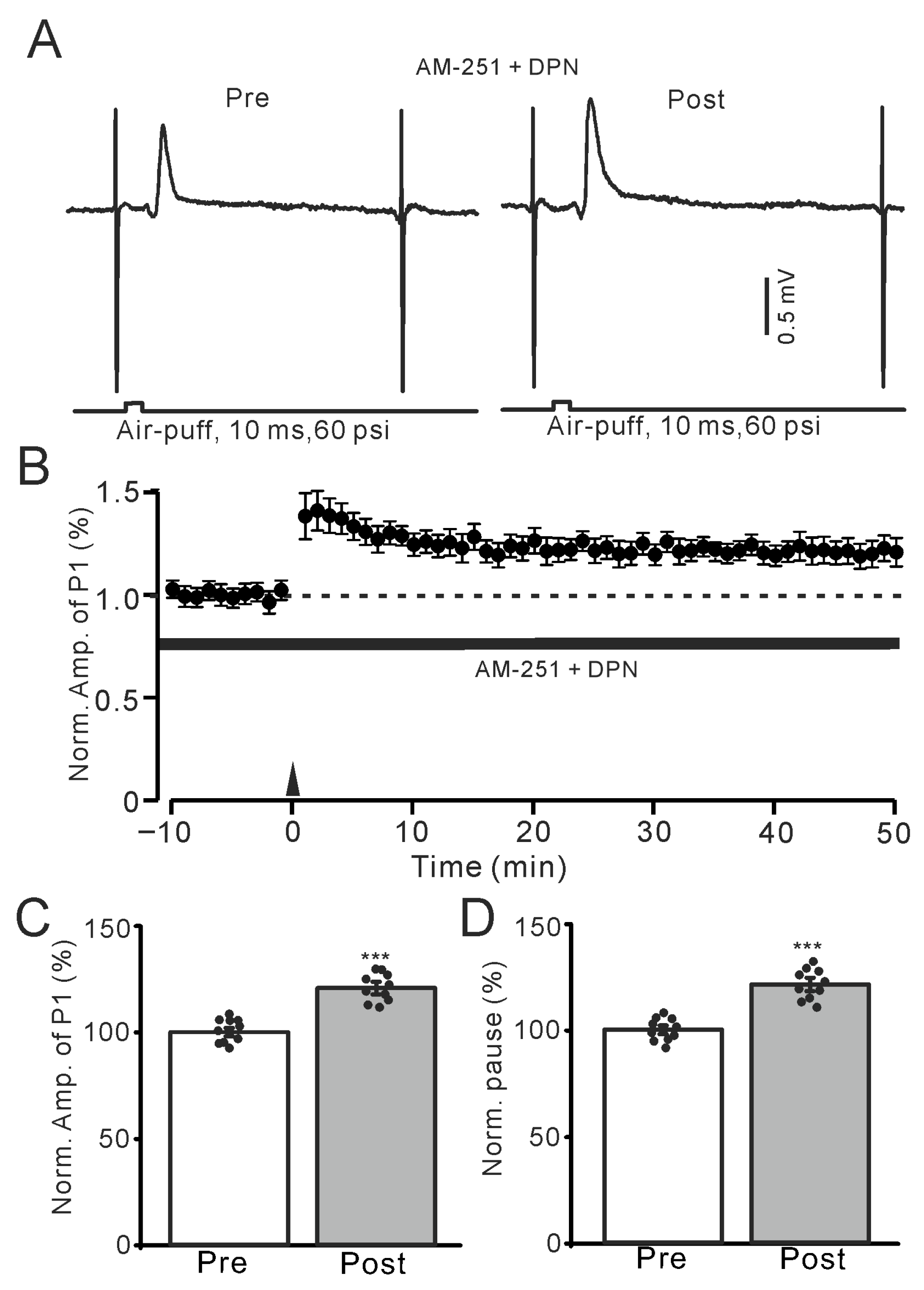
Figure 6.
Application of an ERβ agonist, DPN, triggered the facial stimulation-induced MLI-PC LTP. (A) Representative membrane potentials showing air-puff stimulation evoked MLI-PC response before (Pre) and after (Post) delivery of the 1 Hz (240 pulses) stimulation during treatment AM-251 (5 μM) + DPN (100 nM). (B) A summary of data showing the normalized amplitude of P1 in the presence of AM-251 + DPN before and after the air-puff stimuli train (black arrow). (C) The bar graph with individual data shows the normalized P1 amplitude before (Pre) and after (Post) 1 Hz stimulation during treatment with AM-251 + DPN (Pre: 100.0 ± 1.9%, Post: 120.1 ± 2.9%, F (1,18) = 231.6, p < 0.001; n = 10 recordings/10 mice). (D) The bar graph with individual data shows the normalized SS pause time before (Pre) and after (Post) 1 Hz stimulation during treatment with AM-251 + PHT + E2 (Pre: 100.0 ± 2.1%, Post: 121.3 ± 3.1%, F (1,18) = 253.5, p < 0.001; n = 10 recordings/10 mice). *** p < 0.001 versus Pre. Note that pharmacological activation of ERβ triggered MLI-PC LTP.
Figure 6.
Application of an ERβ agonist, DPN, triggered the facial stimulation-induced MLI-PC LTP. (A) Representative membrane potentials showing air-puff stimulation evoked MLI-PC response before (Pre) and after (Post) delivery of the 1 Hz (240 pulses) stimulation during treatment AM-251 (5 μM) + DPN (100 nM). (B) A summary of data showing the normalized amplitude of P1 in the presence of AM-251 + DPN before and after the air-puff stimuli train (black arrow). (C) The bar graph with individual data shows the normalized P1 amplitude before (Pre) and after (Post) 1 Hz stimulation during treatment with AM-251 + DPN (Pre: 100.0 ± 1.9%, Post: 120.1 ± 2.9%, F (1,18) = 231.6, p < 0.001; n = 10 recordings/10 mice). (D) The bar graph with individual data shows the normalized SS pause time before (Pre) and after (Post) 1 Hz stimulation during treatment with AM-251 + PHT + E2 (Pre: 100.0 ± 2.1%, Post: 121.3 ± 3.1%, F (1,18) = 253.5, p < 0.001; n = 10 recordings/10 mice). *** p < 0.001 versus Pre. Note that pharmacological activation of ERβ triggered MLI-PC LTP.
![Ijms 26 09973 g006 Ijms 26 09973 g006]()
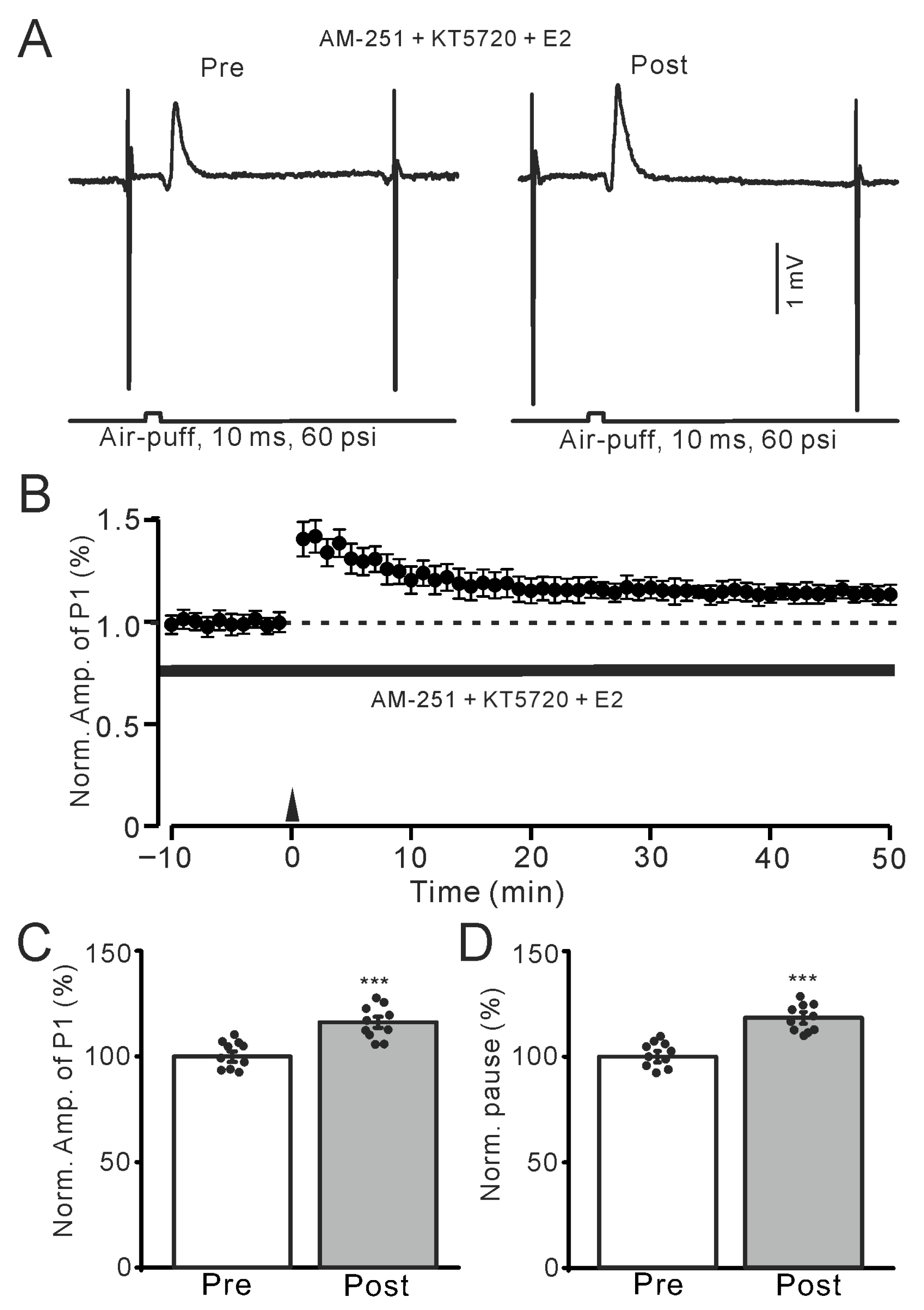
Figure 7.
In the presence of KT-5720 and AM-251, E2 still triggered the facial stimulation-induced MLI-PC LTP. (A) Representative membrane potentials showing air-puff stimulation evoked MLI-PC response before (Pre) and after (Post) delivery of the 1 Hz (240 pulses) stimulation during treatment with a mixture of KT5720 (100 nM) + AM-251 (5 μM) + E2 (100 nM). (B) A summary of data showing the normalized amplitude of P1 in the presence of KT5720 + AM-251 + E2 before and after the air-puff stimuli train (black arrow). (C) The bar graph with individual data shows the normalized P1 amplitude before (Pre) and after (Post) 1 Hz stimulation during treatment with KT5720 + AM-251 + E2 (Pre: 100.0 ± 2.5%, Post: 116.3 ± 2.6%, F (1,18) = 97.8, p < 0.001; n = 10 recordings/10 mice). (D) The bar graph with individual data shows the normalized SS pause time before (Pre) and after (Post) 1 Hz stimulation during treatment with a mixture of KT5720 + AM-251 + E2 (Pre: 100.0 ± 2.6%, Post: 118.4 ± 2.7%, F (1,18) = 112.6, p < 0.001; n = 10 recordings/10 mice). *** p < 0.001 versus Pre. Note that inhibition of PKA failed to prevent the E2-triggered MLI-PC LTD in mouse cerebellar cortex in vivo.
Figure 7.
In the presence of KT-5720 and AM-251, E2 still triggered the facial stimulation-induced MLI-PC LTP. (A) Representative membrane potentials showing air-puff stimulation evoked MLI-PC response before (Pre) and after (Post) delivery of the 1 Hz (240 pulses) stimulation during treatment with a mixture of KT5720 (100 nM) + AM-251 (5 μM) + E2 (100 nM). (B) A summary of data showing the normalized amplitude of P1 in the presence of KT5720 + AM-251 + E2 before and after the air-puff stimuli train (black arrow). (C) The bar graph with individual data shows the normalized P1 amplitude before (Pre) and after (Post) 1 Hz stimulation during treatment with KT5720 + AM-251 + E2 (Pre: 100.0 ± 2.5%, Post: 116.3 ± 2.6%, F (1,18) = 97.8, p < 0.001; n = 10 recordings/10 mice). (D) The bar graph with individual data shows the normalized SS pause time before (Pre) and after (Post) 1 Hz stimulation during treatment with a mixture of KT5720 + AM-251 + E2 (Pre: 100.0 ± 2.6%, Post: 118.4 ± 2.7%, F (1,18) = 112.6, p < 0.001; n = 10 recordings/10 mice). *** p < 0.001 versus Pre. Note that inhibition of PKA failed to prevent the E2-triggered MLI-PC LTD in mouse cerebellar cortex in vivo.
![Ijms 26 09973 g007 Ijms 26 09973 g007]()
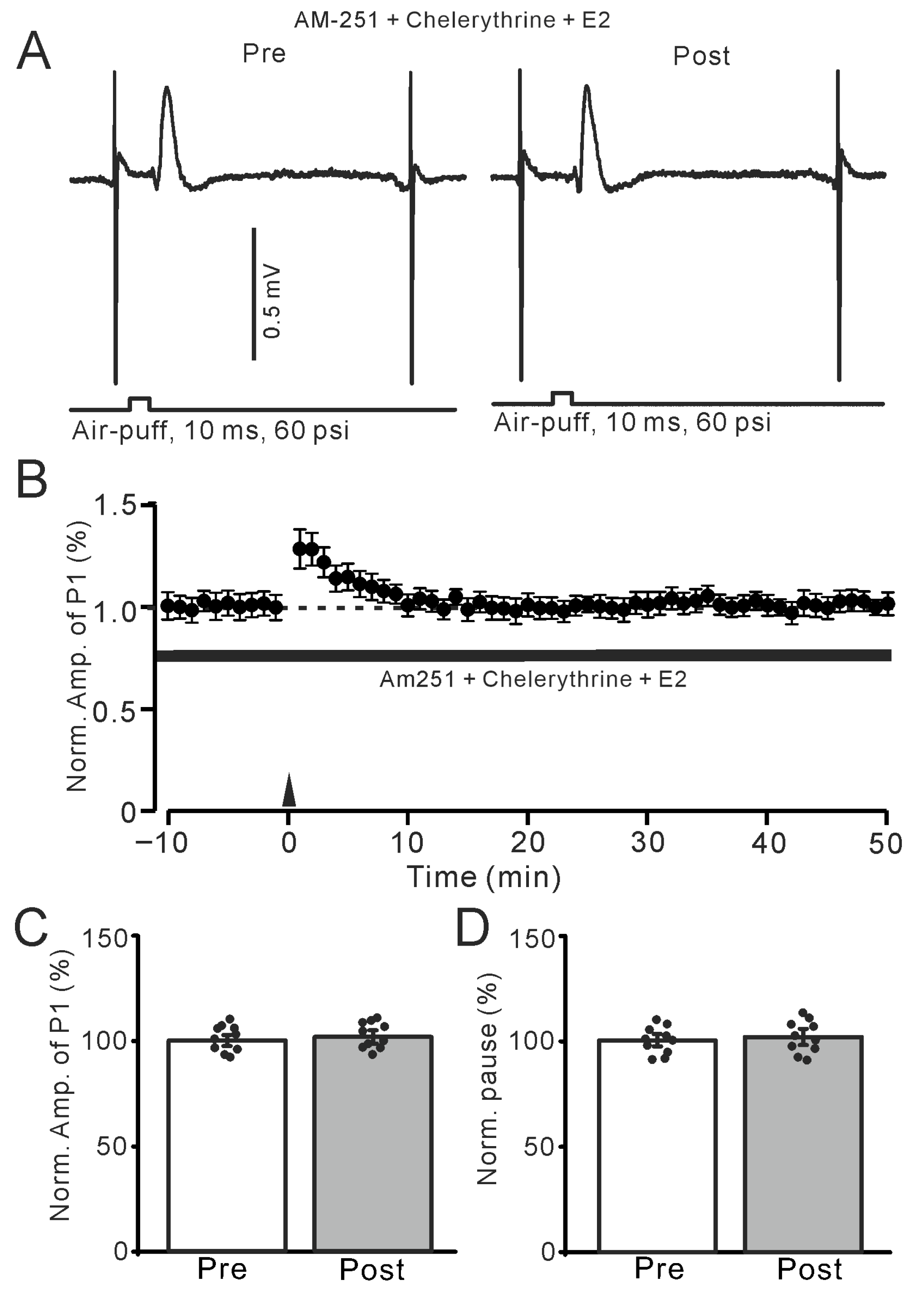
Figure 8.
Inhibition of PKC abolished the E2-triggered facial stimulation-induced MLI-PC LTP. (A) Representative membrane potentials showing air-puff stimulation evoked MLI-PC response before (Pre) and after (Post) delivery of the 1 Hz (240 pulses) stimulation during treatment with a mixture of chelerythrine (5 μM) + AM-251 (5 μM) + E2 (100 nM). (B) A summary of data showing the normalized amplitude of P1 in the presence of chelerythrine + AM-251 + E2 before and after the air-puff stimuli train (black arrow). (C) The bar graph with individual data shows the normalized P1 amplitude before (Pre) and after (Post) 1 Hz stimulation during treatment with chelerythrine + AM-251 + E2 (Pre: 100.0 ± 2.5%, Post: 101.1 ± 3.1%, F (1,18) = 2.11, p = 0.22; n = 10 recordings/10 mice). (D) The bar graph with individual data shows the normalized SS pause time before (Pre) and after (Post) 1 Hz stimulation during treatment with chelerythrine + AM-251 + E2 (Pre: 100.0 ± 2.7%, Post: 101.6 ± 3.3%, F (1,18) = 3.12, p = 0.11; n = 10 recordings/10 mice). Note that inhibition of PKC abolished the E2-triggered MLI-PC LTD in the mouse cerebellar cortex in vivo.
Figure 8.
Inhibition of PKC abolished the E2-triggered facial stimulation-induced MLI-PC LTP. (A) Representative membrane potentials showing air-puff stimulation evoked MLI-PC response before (Pre) and after (Post) delivery of the 1 Hz (240 pulses) stimulation during treatment with a mixture of chelerythrine (5 μM) + AM-251 (5 μM) + E2 (100 nM). (B) A summary of data showing the normalized amplitude of P1 in the presence of chelerythrine + AM-251 + E2 before and after the air-puff stimuli train (black arrow). (C) The bar graph with individual data shows the normalized P1 amplitude before (Pre) and after (Post) 1 Hz stimulation during treatment with chelerythrine + AM-251 + E2 (Pre: 100.0 ± 2.5%, Post: 101.1 ± 3.1%, F (1,18) = 2.11, p = 0.22; n = 10 recordings/10 mice). (D) The bar graph with individual data shows the normalized SS pause time before (Pre) and after (Post) 1 Hz stimulation during treatment with chelerythrine + AM-251 + E2 (Pre: 100.0 ± 2.7%, Post: 101.6 ± 3.3%, F (1,18) = 3.12, p = 0.11; n = 10 recordings/10 mice). Note that inhibition of PKC abolished the E2-triggered MLI-PC LTD in the mouse cerebellar cortex in vivo.
![Ijms 26 09973 g008 Ijms 26 09973 g008]()
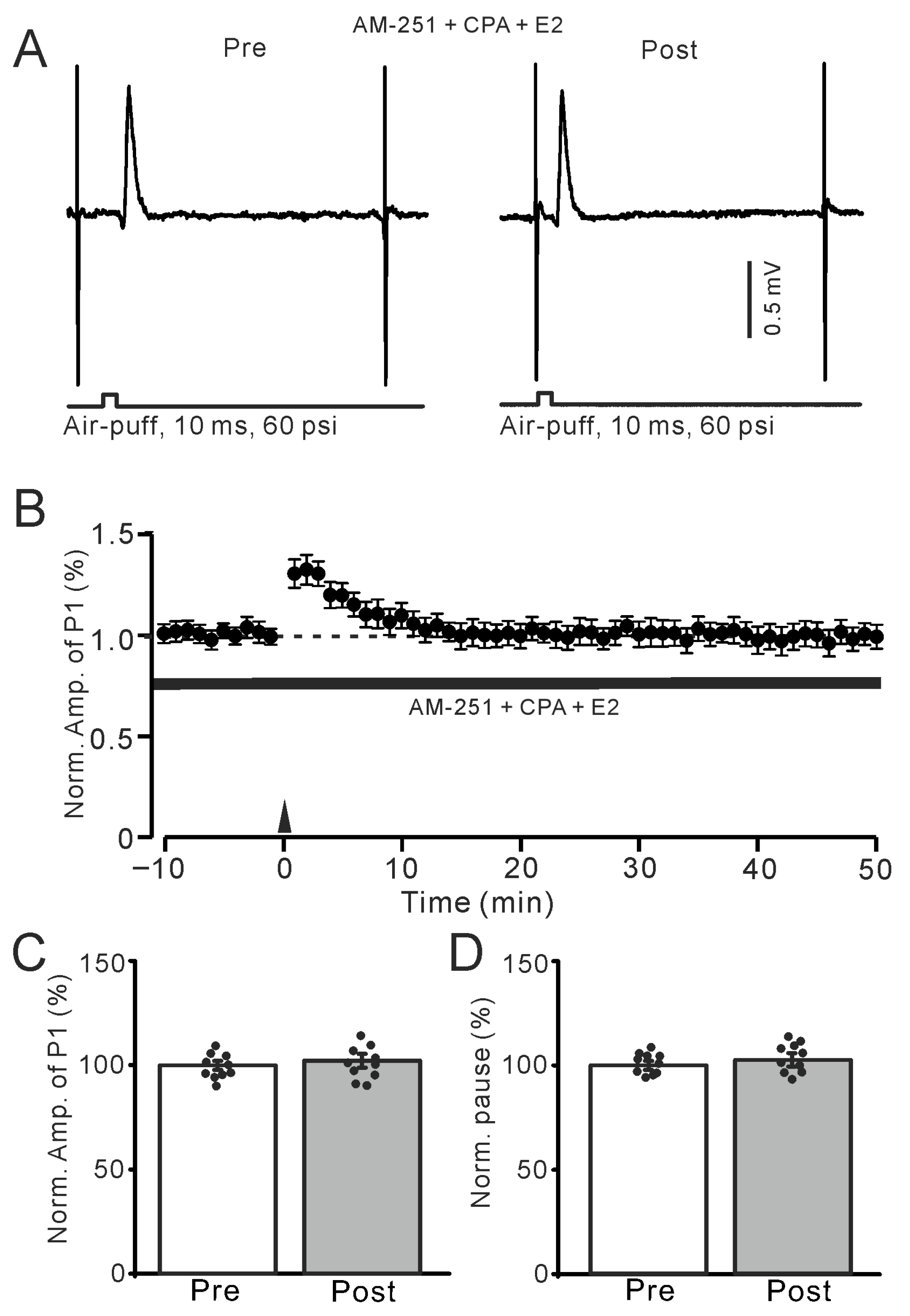
Figure 9.
Depletion of intracellular Ca2+ abolished the E2-triggered MLI-PC LTP. (A) Representative membrane potentials show air-puff stimulation evoked MLI-PC synaptic response before (Pre) and after (Post) the delivery of 1 Hz stimulation during treatment with cyclopiazonic acid (CPA, 100 μM) + AM-251 (5 μM) + E2 (100 nM). (B) A summary of data showing the normalized amplitude of P1 in the presence of CPA + AM-251 + E2 before and after the air-puff stimuli train (black arrow). (C) The bar graph with individual data shows the normalized P1 amplitude before (Pre) and after (Post) 1 Hz stimulation during treatment with CPA + AM-251 + E2 (Pre: 100.0 ± 2.1%, Post: 100.8 ± 3.3%, F (1,18) = 2.06, p = 0.34; n = 10 recordings/10 mice). (D) The bar graph with individual data shows the normalized SS pause time before (Pre) and after (Post) 1 Hz stimulation during treatment with CPA + AM-251 + E2 (Pre: 100.0 ± 2.2%, Post: 101.3 ± 3.2%, F (1,18) = 3.22, p = 0.15; n = 10 recordings/10 mice). Note that depletion of intracellular Ca2+ completely prevented the E2-triggered MLI-PC LTD in the mouse cerebellar cortex in vivo.
Figure 9.
Depletion of intracellular Ca2+ abolished the E2-triggered MLI-PC LTP. (A) Representative membrane potentials show air-puff stimulation evoked MLI-PC synaptic response before (Pre) and after (Post) the delivery of 1 Hz stimulation during treatment with cyclopiazonic acid (CPA, 100 μM) + AM-251 (5 μM) + E2 (100 nM). (B) A summary of data showing the normalized amplitude of P1 in the presence of CPA + AM-251 + E2 before and after the air-puff stimuli train (black arrow). (C) The bar graph with individual data shows the normalized P1 amplitude before (Pre) and after (Post) 1 Hz stimulation during treatment with CPA + AM-251 + E2 (Pre: 100.0 ± 2.1%, Post: 100.8 ± 3.3%, F (1,18) = 2.06, p = 0.34; n = 10 recordings/10 mice). (D) The bar graph with individual data shows the normalized SS pause time before (Pre) and after (Post) 1 Hz stimulation during treatment with CPA + AM-251 + E2 (Pre: 100.0 ± 2.2%, Post: 101.3 ± 3.2%, F (1,18) = 3.22, p = 0.15; n = 10 recordings/10 mice). Note that depletion of intracellular Ca2+ completely prevented the E2-triggered MLI-PC LTD in the mouse cerebellar cortex in vivo.
![Ijms 26 09973 g009 Ijms 26 09973 g009]()
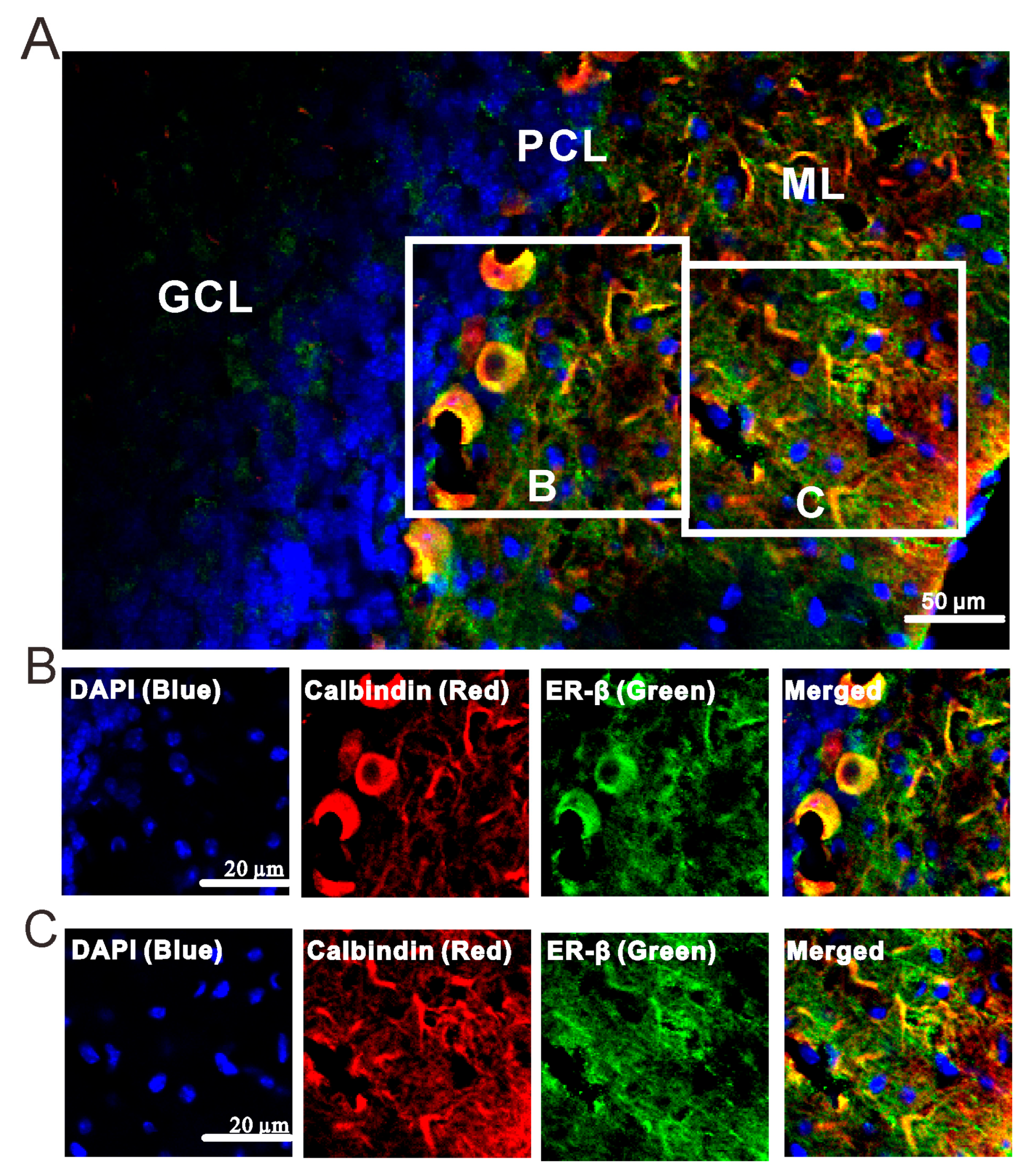
Figure 10.
Expression of ERβ in the mouse cerebellar cortical Crus II. (A) The digital micrograph shows the confocal merged image of DAPI (blue), ERβ (green), and calbindin (red) in the mouse cerebellar Crus II. DAPI is a blue nucleic acid dye that preferentially dyes the dsDNA of cells. Calbindin is a marker for labeling PCs. (B) Higher magnifications of the boxed area (B) in (A), showing DAPI (blue), ERβ (green), calbindin (red), and merged immunofluorescence in the soma of PC. (C) Higher magnifications of the boxed area (C) in (A), showing DAPI (blue), ERβ (green), calbindin (red), and merged immunofluorescence in ML. The green ERβ immunoreactivity is expressed in dendrites and somas of PCs. ML, molecular layer; PCL, Purkinje cell layer; GCL, granular layer. Note that ERβ immunoreactivity was mostly expressed around the dendrites and somas of PCs in the cerebellar cortical Crus II.
Figure 10.
Expression of ERβ in the mouse cerebellar cortical Crus II. (A) The digital micrograph shows the confocal merged image of DAPI (blue), ERβ (green), and calbindin (red) in the mouse cerebellar Crus II. DAPI is a blue nucleic acid dye that preferentially dyes the dsDNA of cells. Calbindin is a marker for labeling PCs. (B) Higher magnifications of the boxed area (B) in (A), showing DAPI (blue), ERβ (green), calbindin (red), and merged immunofluorescence in the soma of PC. (C) Higher magnifications of the boxed area (C) in (A), showing DAPI (blue), ERβ (green), calbindin (red), and merged immunofluorescence in ML. The green ERβ immunoreactivity is expressed in dendrites and somas of PCs. ML, molecular layer; PCL, Purkinje cell layer; GCL, granular layer. Note that ERβ immunoreactivity was mostly expressed around the dendrites and somas of PCs in the cerebellar cortical Crus II.