Iron-Based Biochar for Efficient Persulfate Activation and Sulfamethoxazole Degradation
Abstract
1. Introduction
2. Results and Discussion
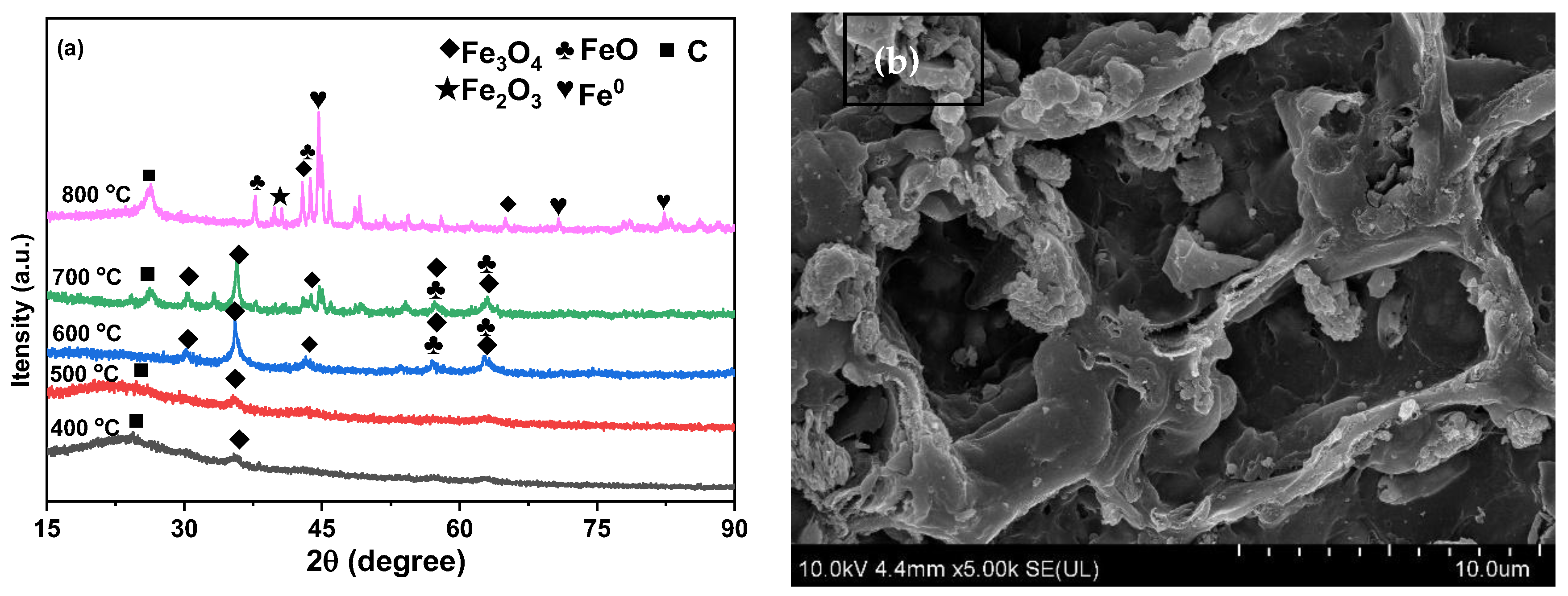
2.1. Characterizations
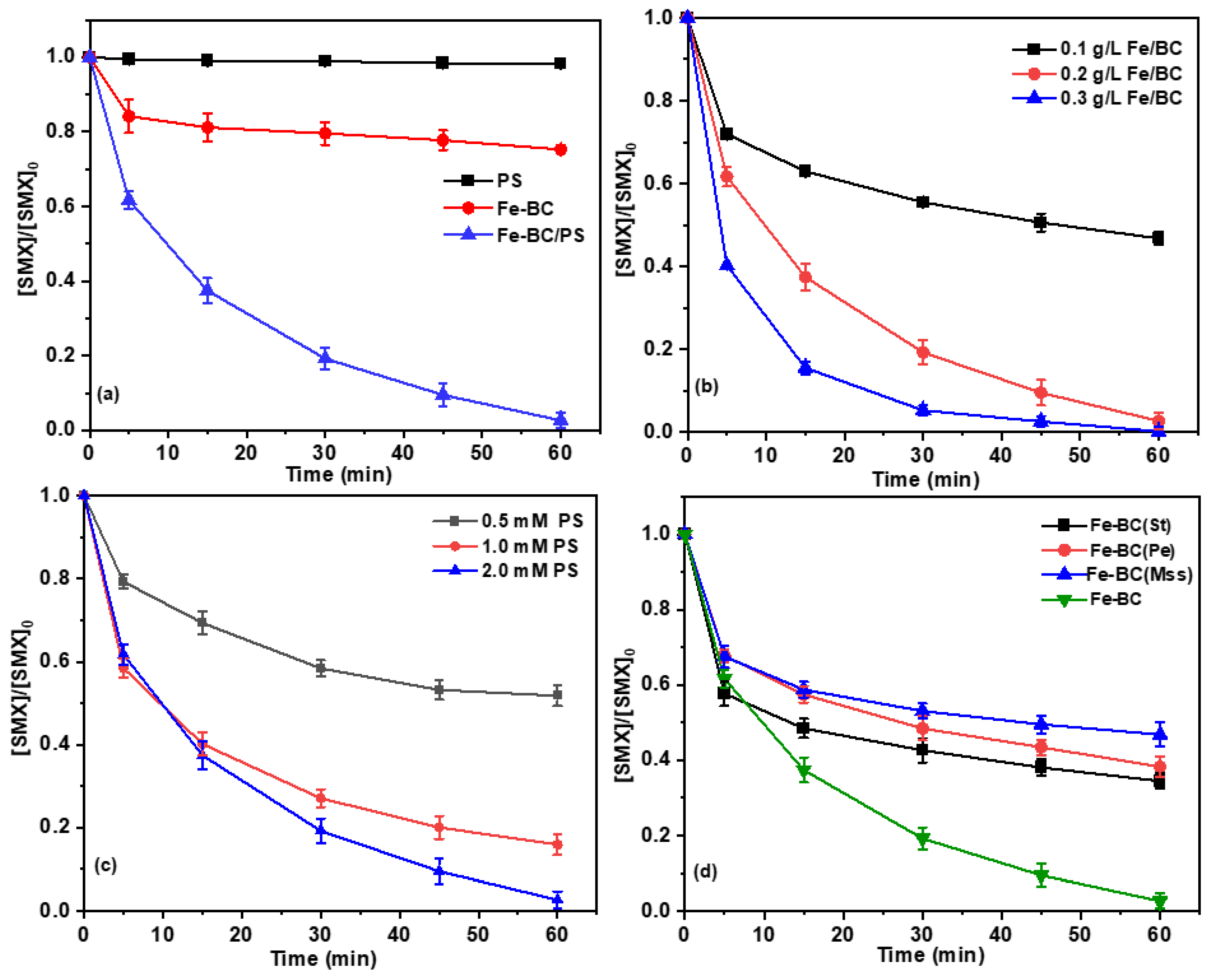
2.2. Catalytic Removal of SMX in Different Systems
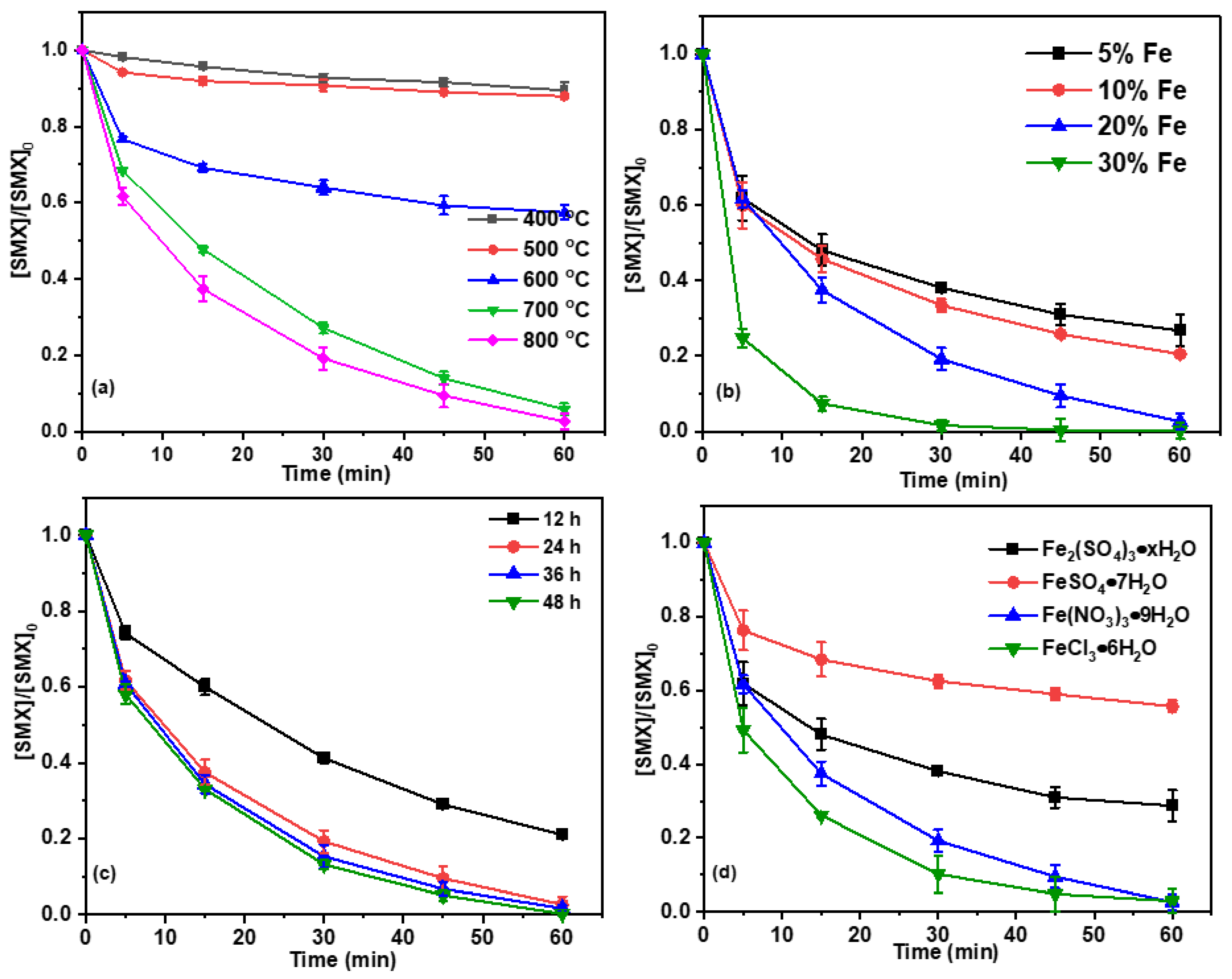
2.3. Effect of the Material Preparation Conditions on the Degradation of SMX
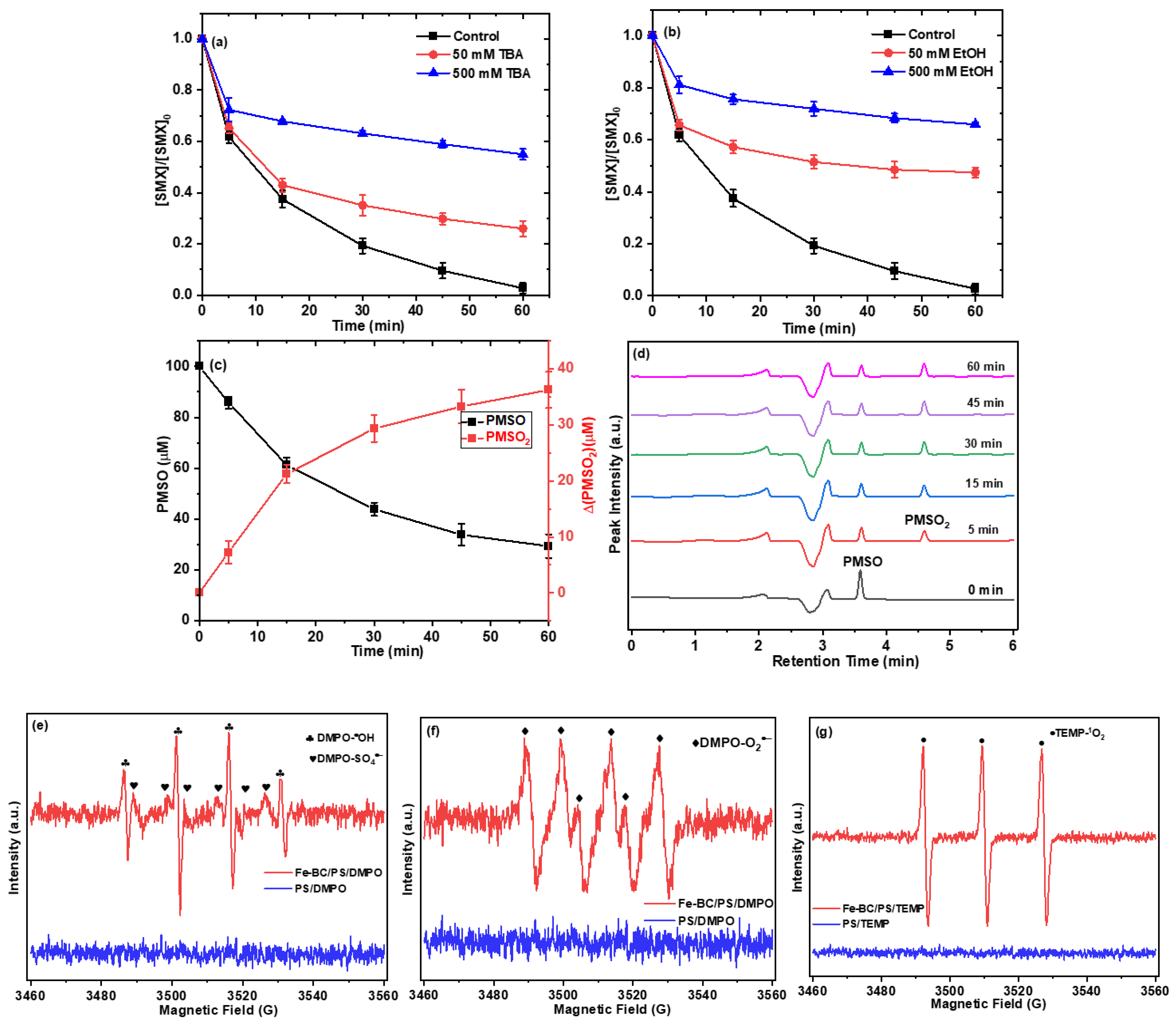
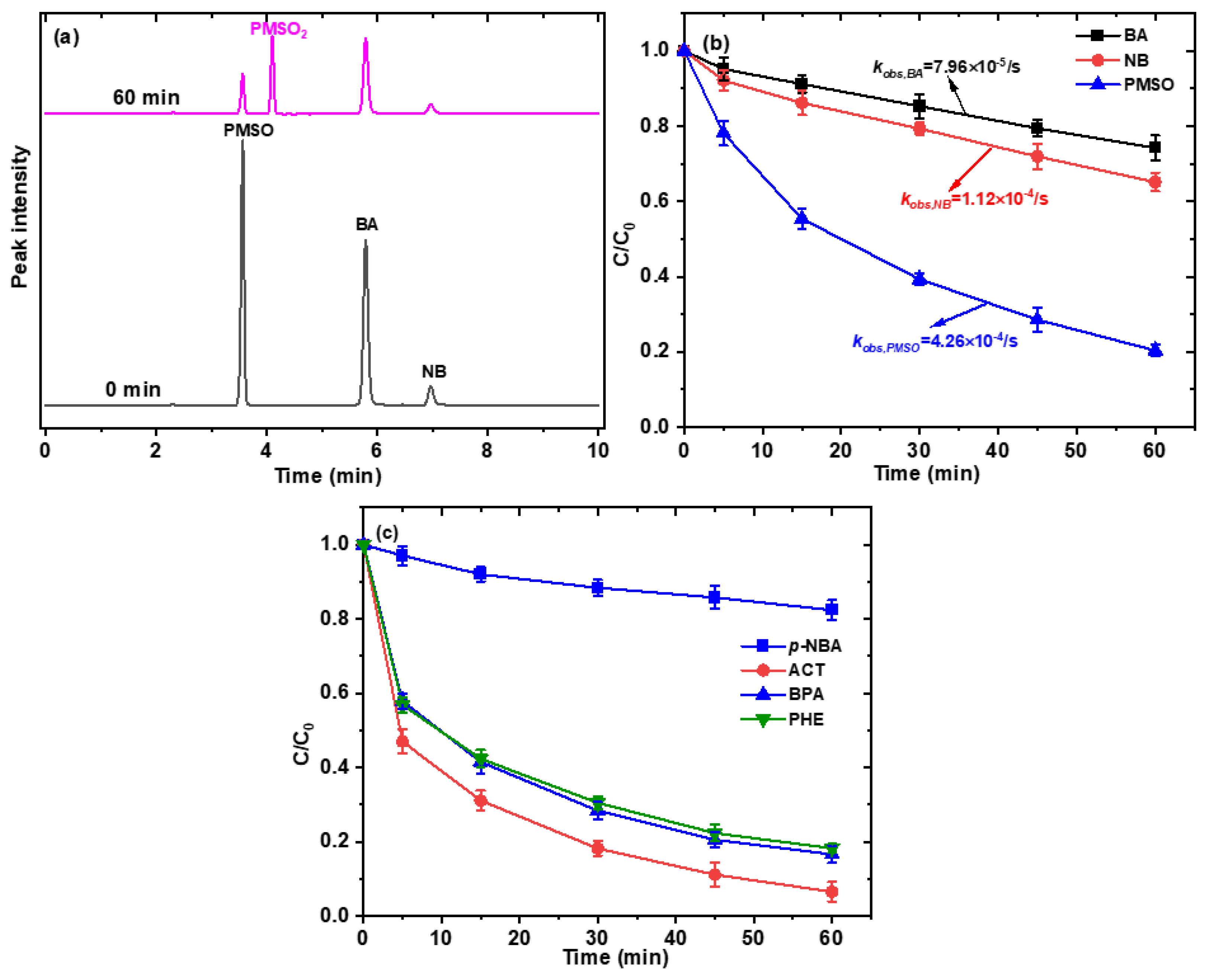
2.4. Identification of Reactive Species
2.5. Contribution of Different Reactive Species to SMX Degradation
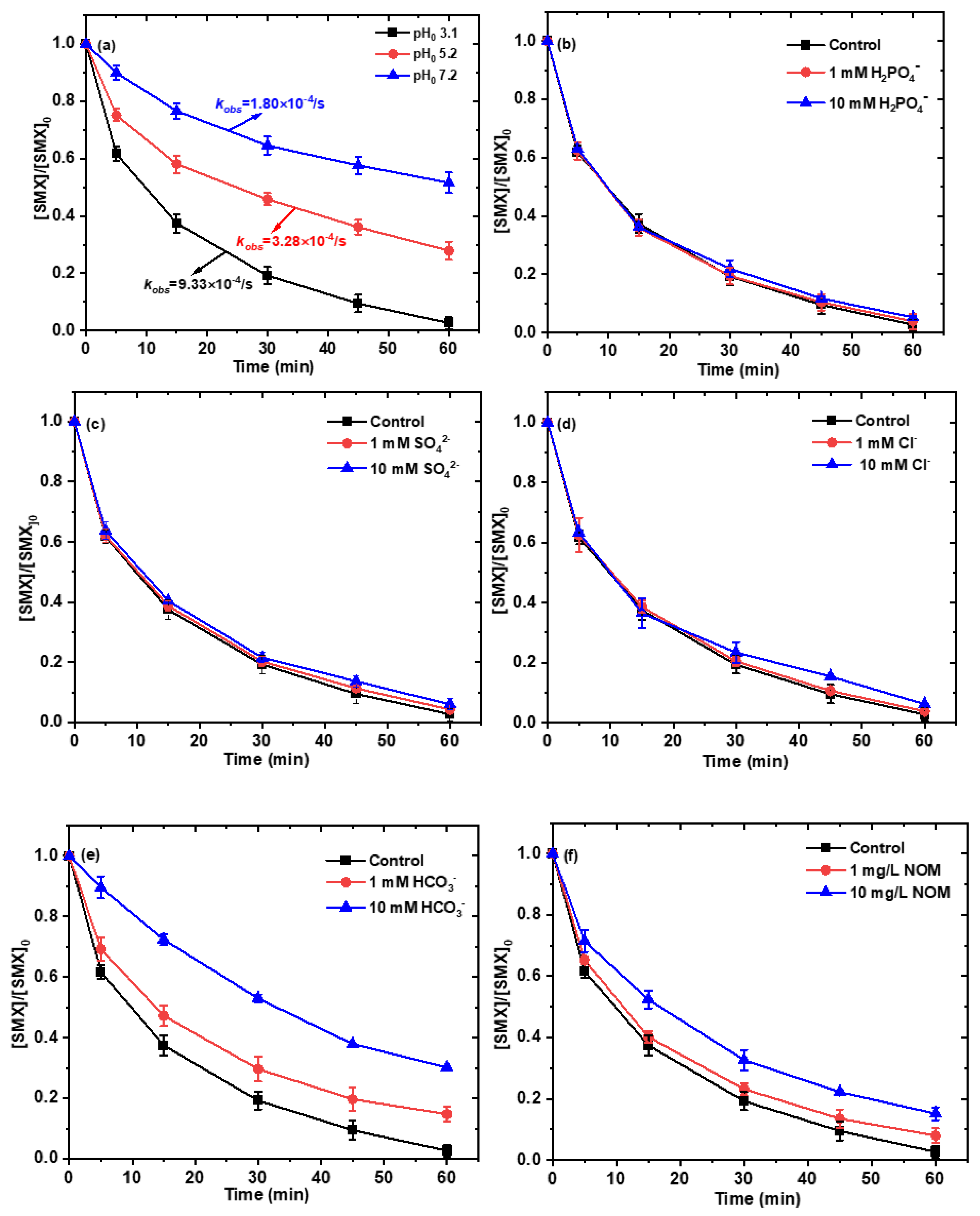
2.6. Influence of Water Quality Parameters on the Degradation of SMX
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Preparation of Fe-BC
3.3. Experimental Procedure
3.4. Analytical Methods
3.5. Steady-State Concentrations of Reactive Species and Their Relative Contribution to SMX Degradation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, B.; He, Y.; Shi, W.; Liu, L.; Li, L.; Liu, C.; Lens, P.N.L. Biotransformation of sulfamethoxazole (SMX) by aerobic granular sludge: Removal performance, degradation mechanism and microbial response. Sci. Total Environ. 2023, 858, 159771. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Tang, X.; Ng, H.Y.; Deng, S.; Shi, X.; Song, W.; Huang, S.; Li, G.; Liang, H. Biological sulfamethoxazole degradation along with anaerobically digested centrate treatment by immobilized microalgal-bacterial consortium: Performance, mechanism and shifts in bacterial and microalgal communities. Chem. Eng. J. 2020, 388, 124217. [Google Scholar] [CrossRef]
- Shang, K.; Morent, R.; Wang, N.; Wang, Y.; Peng, B.; Jiang, N.; Lu, N.; Li, J. Degradation of sulfamethoxazole (SMX) by water falling film DBD Plasma/Persulfate: Reactive species identification and their role in SMX degradation. Chem. Eng. J. 2022, 431, 133916. [Google Scholar] [CrossRef]
- Wang, R.; You, H.; Li, Z.; Zhao, J.; Li, M.; Zhu, J.; Zhang, G.; Wang, X.; Leng, H.; Qi, S.; et al. Non-radical mediated reduced graphene oxide/polypyrrole catalytic ceramic membrane-PDS system for source control of SMX. Chem. Eng. J. 2024, 479, 147769. [Google Scholar] [CrossRef]
- Milh, H.; Cabooter, D.; Dewil, R. Role of process parameters in the degradation of sulfamethoxazole by heat-activated peroxymonosulfate oxidation: Radical identification and elucidation of the degradation mechanism. Chem. Eng. J. 2021, 422, 130457. [Google Scholar] [CrossRef]
- Ghanbari, F.; Moradi, M. Application of peroxymonosulfate and its activation methods for degradation of environmental organic pollutants: Review. Chem. Eng. J. 2017, 310, 41–62. [Google Scholar] [CrossRef]
- Milh, H.; Schoenaers, B.; Stesmans, A.; Cabooter, D.; Dewil, R. Degradation of sulfamethoxazole by heat-activated persulfate oxidation: Elucidation of the degradation mechanism and influence of process parameters. Chem. Eng. J. 2020, 379, 122234. [Google Scholar] [CrossRef]
- Ding, X.; Song, X.; Chen, X.; Ding, D.; Xu, C.; Chen, H. Degradation and mechanism of hexafluoropropylene oxide dimer acid by thermally activated persulfate in aqueous solutions. Chemosphere 2022, 286, 131720. [Google Scholar] [CrossRef]
- Yue, J.; Guo, W.; Liang, S.; Tillotson, M.R.; Zhu, Y.; Li, D.; Du, L.; Li, J.; Zhao, X. Kinetics, contributions, and pathways of the degradation of artificial sweeteners by primary and secondary radicals during UV/persulfate. Sep. Purif. Technol. 2025, 362, 131683. [Google Scholar] [CrossRef]
- Li, W.-Q.; Savateev, O.; Li, Y.-M.; Zheng, J.-K.; Wang, Y.-X.; Hou, N.; Liu, X.-C.; Ding, R.-R.; Zhou, X.-G.; Wang, Y.; et al. Boosting persulfate activation through efficient π → π* transition activated by Cu ion anchored porphyrin-based metal–organic framework. Chem. Eng. J. 2024, 490, 151802. [Google Scholar] [CrossRef]
- Liu, Z.; Pan, S.; Xu, F.; Wang, Z.; Zhao, C.; Xu, X.; Gao, B.; Li, Q. Revealing the fundamental role of MoO2 in promoting efficient and stable activation of persulfate by iron carbon based catalysts: Efficient Fe2+/Fe3+ cycling to generate reactive species. Water Res. 2022, 225, 119142. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, C.; Li, Y.; Zhou, L.; Lan, Y. Rapid degradation of aniline by peroxydisulfate activated with copper-nickel binary oxysulfide. Sep. Purif. Technol. 2019, 209, 1007–1015. [Google Scholar] [CrossRef]
- Luo, H.; Fu, H.; Yin, H.; Lin, Q. Carbon materials in persulfate-based advanced oxidation processes: The roles and construction of active sites. J. Hazard. Mater. 2022, 426, 128044. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Lin, Q.; Zhang, X.; Huang, Z.; Fu, H.; Xiao, R.; Liu, S.-S. Determining the key factors of nonradical pathway in activation of persulfate by metal-biochar nanocomposites for bisphenol A degradation. Chem. Eng. J. 2020, 391, 123555. [Google Scholar] [CrossRef]
- Zhu, F.; Wu, Y.; Liang, Y.; Li, H.; Liang, W. Degradation mechanism of norfloxacin in water using persulfate activated by BC@nZVI/Ni. Chem. Eng. J. 2020, 389, 124276. [Google Scholar] [CrossRef]
- Yan, J.; Guo, Z.; Sun, Y.; Yan, Z.; Liu, R.; Chen, Y.; Song, J. Mechanism insight into sulfidated nano zero-valent iron/biochar activated persulfate for highly efficient degradation of p-chloroaniline. Chemosphere 2025, 375, 144229. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Li, J.; Xu, J.; Qi, W.; Hao, R.; Lin, D.; Li, D.; Zhang, J. Magnetic biochar based on platanus leaves and iron sludge for persulfate activation and catalytic degradation of tetracycline. J. Clean. Prod. 2022, 370, 133336. [Google Scholar] [CrossRef]
- Hu, H.; Liu, J.; Zheng, X.; Zhao, K.; Lin, Y.; Xu, X.; Long, H.; Zhang, Y.; Wang, X.; Chen, D.; et al. Recent advances in iron-based catalyst-driven persulfate activation for organic pollutant degradation. J. Water Process Eng. 2025, 71, 107423. [Google Scholar] [CrossRef]
- Liang, J.; Duan, X.; Xu, X.; Chen, K.; Zhang, Y.; Zhao, L.; Qiu, H.; Wang, S.; Cao, X. Persulfate Oxidation of Sulfamethoxazole by Magnetic Iron-Char Composites via Nonradical Pathways: Fe(IV) Versus Surface-Mediated Electron Transfer. Environ. Sci. Technol. 2021, 55, 10077–10086. [Google Scholar] [CrossRef]
- Rong, X.; Xie, M.; Kong, L.; Natarajan, V.; Ma, L.; Zhan, J. The magnetic biochar derived from banana peels as a persulfate activator for organic contaminants degradation. Chem. Eng. J. 2019, 372, 294–303. [Google Scholar] [CrossRef]
- Li, B.; Zhang, Y.; Xu, J.; Fan, S.; Xu, H. Facile preparation of magnetic porous biochars from tea waste for the removal of tetracycline from aqueous solutions: Effect of pyrolysis temperature. Chemosphere 2022, 291, 132713. [Google Scholar] [CrossRef]
- Li, W.; Liu, B.; Wang, Z.; Wang, K.; Lan, Y.; Zhou, L. Efficient activation of peroxydisulfate (PDS) by rice straw biochar modified by copper oxide (RSBC-CuO) for the degradation of phenacetin (PNT). Chem. Eng. J. 2020, 395, 125094. [Google Scholar] [CrossRef]
- He, L.; Liu, Z.; Hu, J.; Qin, C.; Yao, L.; Zhang, Y.; Piao, Y. Sugarcane biochar as novel catalyst for highly efficient oxidative removal of organic compounds in water. Chem. Eng. J. 2021, 405, 126895. [Google Scholar] [CrossRef]
- Wang, X.; Gong, W.; Zhu, J.; Peng, G. New insights into the quantiffcation of Fe(IV) using methyl phenyl sulfoxide (PMSO) as probe in the iron-based heterogeneous catalyst activated persulfate process. Environ. Pollut. 2024, 362, 124924. [Google Scholar] [CrossRef]
- Wang, R.; Yu, Y.; Zhang, R.; Ren, X.; Guo, W. Vacancy-rich structure inducing efficient persulfate activation for tetracycline degradation over Ni-Fe layered double hydroxide nanosheets. Sep. Purif. Technol. 2022, 289, 120663. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, X.; Zhang, P.; Ling, Z.; Qiu, H.; Cao, X. Pyrolysis-temperature depended quinone and carbonyl groups as the electron accepting sites in barley grass derived biochar. Chemosphere 2019, 232, 273–280. [Google Scholar] [CrossRef]
- Lai, M.; Li, J.; Li, H.; Gui, Y.; Lü, J. Adsorption-reduction of Fe(III) by different biochars and their co-activation of H2O2 for oxidation of refractory pollutants. Catal. Commun. 2023, 176, 106626. [Google Scholar] [CrossRef]
- Li, L.; Yin, Z.; Cheng, M.; Qin, L.; Liu, S.; Yi, H.; Zhang, M.; Fu, Y.; Yang, X.; Zhou, X.; et al. Insights into reactive species generation and organics selective degradation in Fe-based heterogeneous Fenton-like systems: A critical review. Chem. Eng. J. 2023, 454, 140126. [Google Scholar] [CrossRef]
- Gao, L.; Guo, Y.; Zhan, J.; Yu, G.; Wang, Y. Assessment of the validity of the quenching method for evaluating the role of reactive species in pollutant abatement during the persulfate-based process. Water Res. 2022, 221, 118730. [Google Scholar] [CrossRef]
- Meng, X.; Peng, G.; Yan, Y.; Wang, X.; Zhu, J.; Belver, C.; Gong, W.; Blaney, L. Analysis of the steady-state concentrations of reactive species and their role in contaminant degradation by the iron-biochar/persulfate advanced oxidation process: Comparison of probe compound and quenching agent methods. Sep. Purif. Technol. 2025, 354, 128502. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, M.; Cao, Q.; Sun, P.; Chen, Y.; Meng, F. The superoxide radicals’ production via persulfate activated with CuFe2O4@Biochar composites to promote the redox pairs cycling for efficient degradation of o-nitrochlorobenzene in soil. J. Hazard. Mater. 2020, 400, 122887. [Google Scholar] [CrossRef]
- Ouyang, D.; Chen, Y.; Yan, J.; Qian, L.; Han, L.; Chen, M. Activation mechanism of peroxymonosulfate by biochar for catalytic degradation of 1,4-dioxane: Important role of biochar defect structures. Chem. Eng. J. 2019, 370, 614–624. [Google Scholar] [CrossRef]
- Venâncio, J.P.F.; Rodrigues, C.S.D.; Nunes, O.C.; Madeira, L.M. Application of iron-activated persulfate for municipal wastewater disinfection. J. Hazard. Mater. 2022, 426, 127989. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Qin, Y.; Song, H.; Zou, W.; Cao, Z.; Ding, L.; Pan, Y.; Zhou, M. Efficient removal of bisphenol A by a novel biochar-based Fe/C granule via persulfate activation: Performance, mechanism, and toxicity assessment. Process Saf. Environ. Prot. 2023, 169, 48–60. [Google Scholar] [CrossRef]
- Peng, G.; Yan, Y.; Qi, C.; Chen, J.; Meng, X.; Blaney, L.; Gong, W. Mechanistic insights into peracetic acid activation by iron-biochar composites prepared at low and high temperature for enhanced contaminant degradation: Selective reactive species generation. Chem. Eng. J. 2025, 512, 162165. [Google Scholar] [CrossRef]
- Wu, Q.-Y.; Yang, Z.-W.; Wang, Z.-W.; Wang, W.-L. Oxygen doping of cobalt-single-atom coordination enhances peroxymonosulfate activation and high-valent cobalt–oxo species formation. Proc. Natl. Acad. Sci. USA 2023, 120, e2219923120. [Google Scholar] [CrossRef]
- Zong, Y.; Guan, X.; Xu, J.; Feng, Y.; Mao, Y.; Xu, L.; Chu, H.; Wu, D. Unraveling the Overlooked Involvement of High-Valent Cobalt-Oxo Species Generated from the Cobalt(II)-Activated Peroxymonosulfate Process. Environ. Sci. Technol. 2020, 54, 16231–16239. [Google Scholar] [CrossRef]
- Dong, H.; Li, Y.; Wang, S.; Liu, W.; Zhou, G.; Xie, Y.; Guan, X. Both Fe(IV) and Radicals Are Active Oxidants in the Fe(II)/Peroxydisulfate Process. Environ. Sci. Technol. Lett. 2020, 7, 219–224. [Google Scholar] [CrossRef]
- Pestovsky, O.; Bakac, A. Aqueous Ferryl(IV) Ion: Kinetics of Oxygen Atom Transfer to Substrates and Oxo Exchange with Solvent Water. Inorg. Chem. 2006, 45, 814–820. [Google Scholar] [CrossRef]
- Liu, B.; Guo, W.; Wang, H.; Zheng, S.; Si, Q.; Zhao, Q.; Luo, H.; Ren, N. Peroxymonosulfate activation by cobalt(II) for degradation of organic contaminants via high-valent cobalt-oxo and radical species. J. Hazard. Mater. 2021, 416, 125679. [Google Scholar] [CrossRef]
- Zong, Y.; Shao, Y.; Zeng, Y.; Shao, B.; Xu, L.; Zhao, Z.; Liu, W.; Wu, D. Enhanced Oxidation of Organic Contaminants by Iron(II)-Activated Periodate: The Significance of High-Valent Iron–Oxo Species. Environ. Sci. Technol. 2021, 55, 7634–7642. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.; Qi, C.; Peng, G.; Gao, Y.; Zhang, R. Iron-Based Biochar for Efficient Persulfate Activation and Sulfamethoxazole Degradation. Int. J. Mol. Sci. 2025, 26, 9971. https://doi.org/10.3390/ijms26209971
Lu Y, Qi C, Peng G, Gao Y, Zhang R. Iron-Based Biochar for Efficient Persulfate Activation and Sulfamethoxazole Degradation. International Journal of Molecular Sciences. 2025; 26(20):9971. https://doi.org/10.3390/ijms26209971
Chicago/Turabian StyleLu, Ying, Chengdu Qi, Guilong Peng, Yi Gao, and Ronglong Zhang. 2025. "Iron-Based Biochar for Efficient Persulfate Activation and Sulfamethoxazole Degradation" International Journal of Molecular Sciences 26, no. 20: 9971. https://doi.org/10.3390/ijms26209971
APA StyleLu, Y., Qi, C., Peng, G., Gao, Y., & Zhang, R. (2025). Iron-Based Biochar for Efficient Persulfate Activation and Sulfamethoxazole Degradation. International Journal of Molecular Sciences, 26(20), 9971. https://doi.org/10.3390/ijms26209971







