Checkpoint Blockade Efficacy in Uveal Melanoma Is Linked to Tumor Immunity, CD28, and CCL8
Abstract
1. Introduction
2. Results
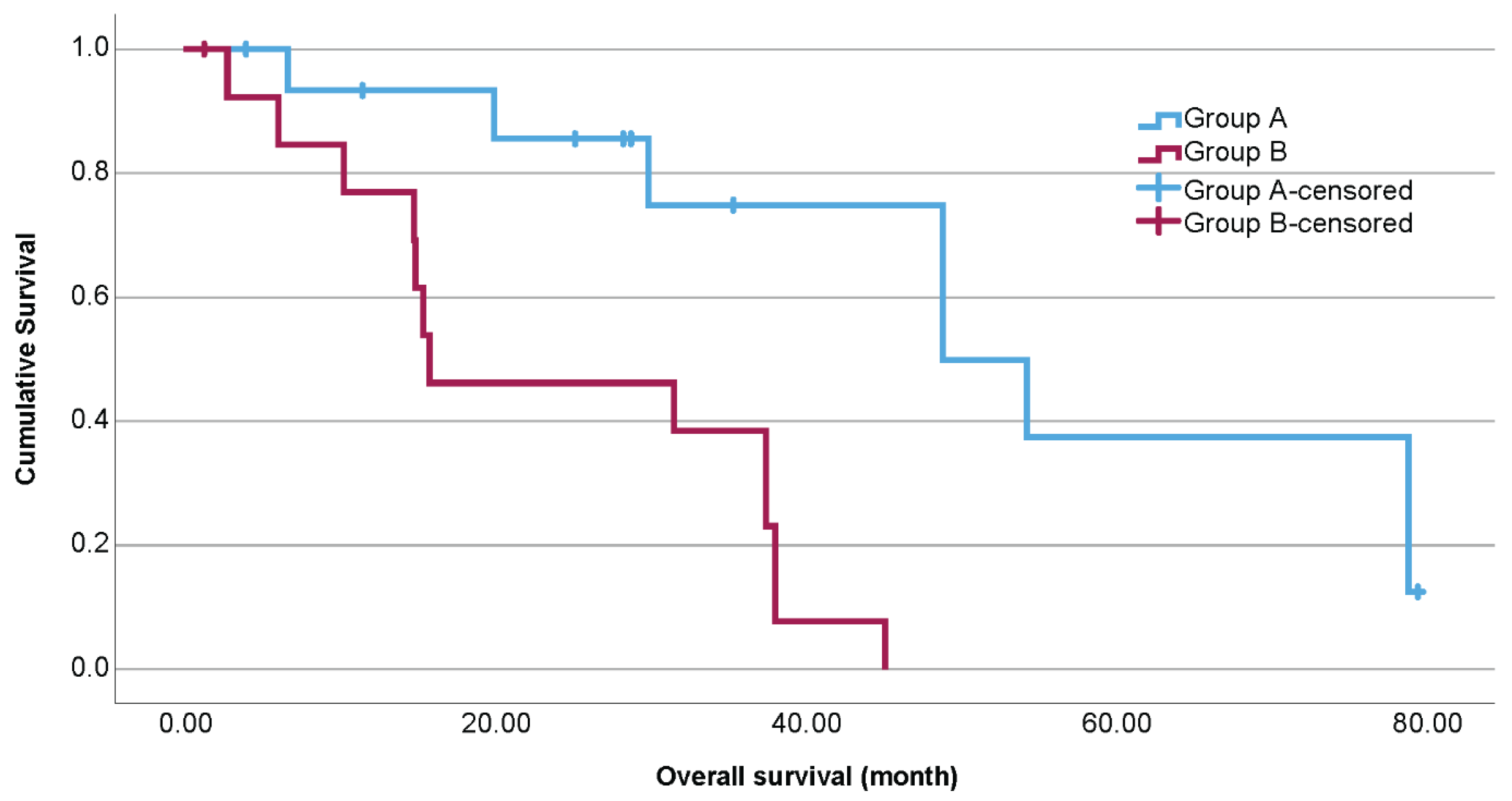
2.1. Baseline Characteristics
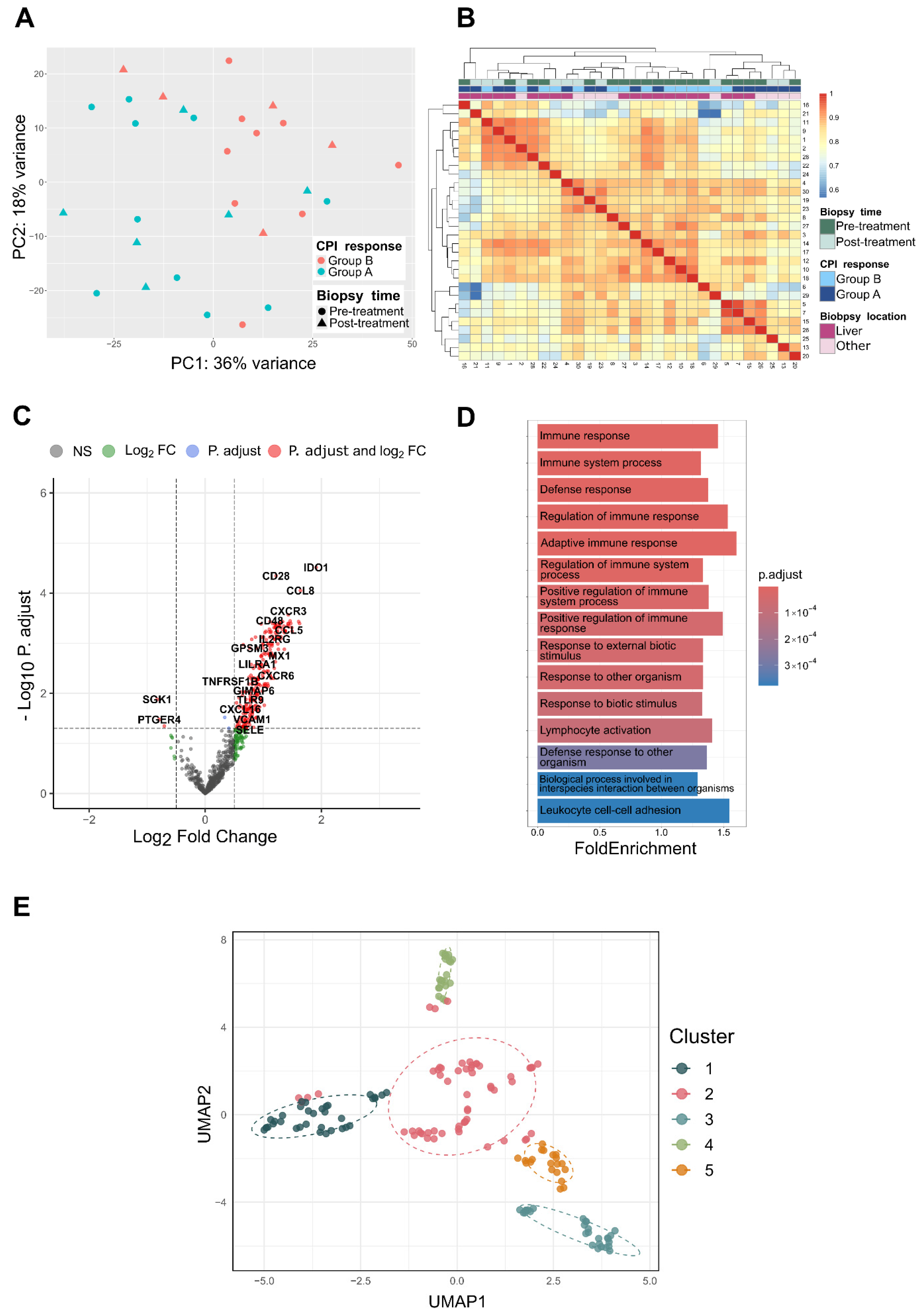
2.2. Differential Gene Expression
2.3. Enrichment Analysis
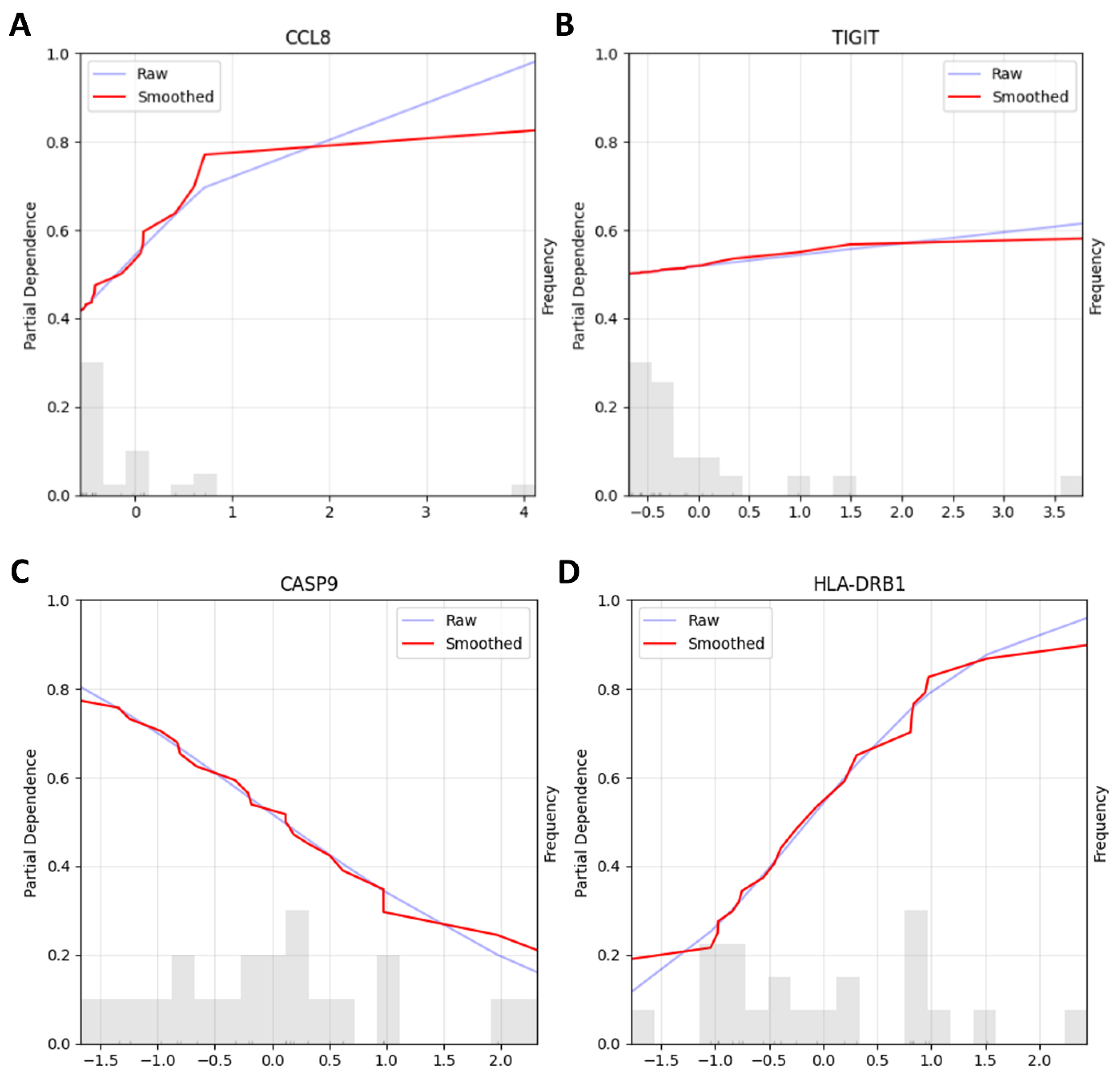
2.4. Machine Learning Pipelines
2.5. Immunohistochemistry
3. Discussion
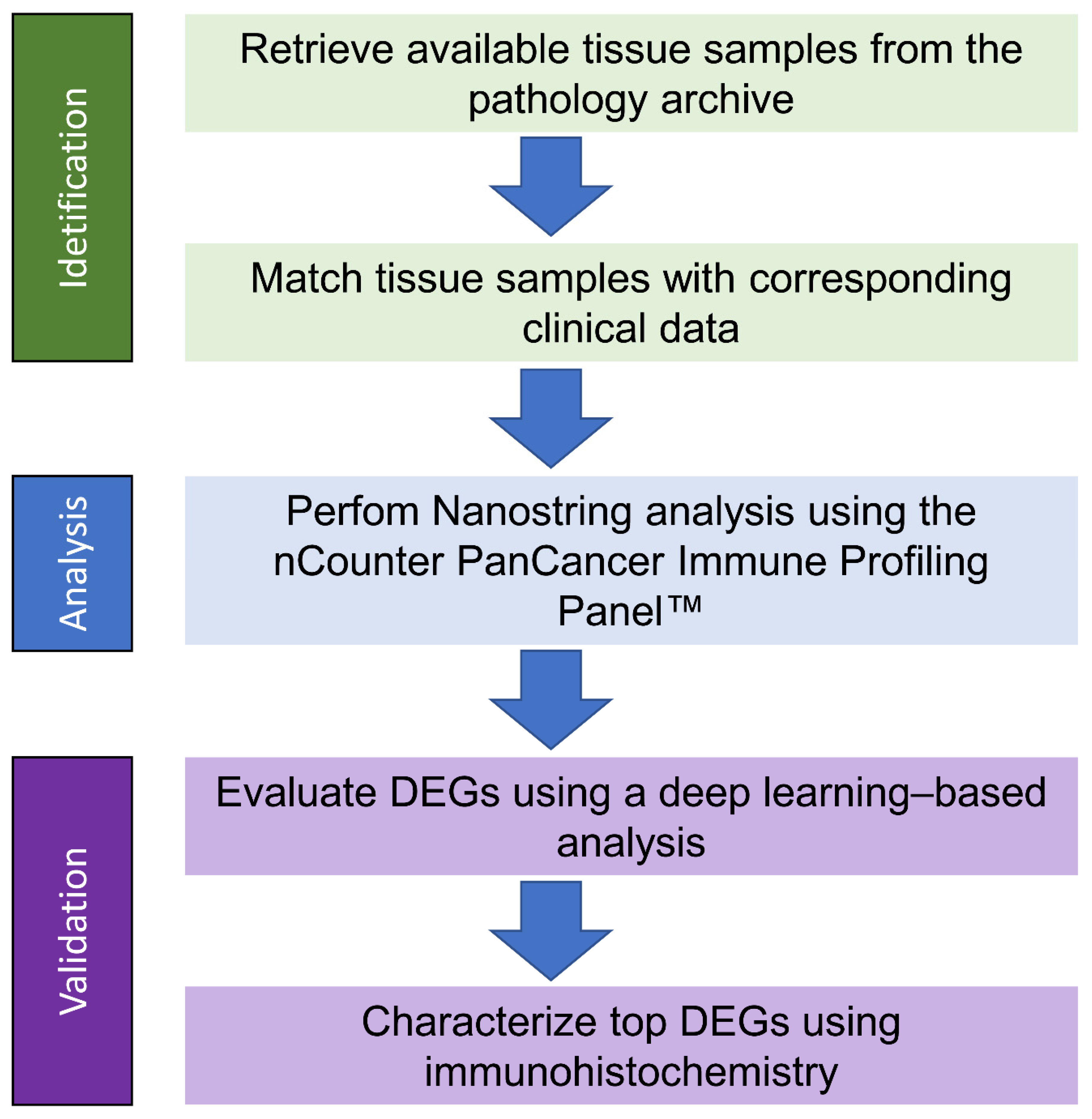
4. Materials and Methods
4.1. RNA Isolation
4.2. NanoString Analysis
4.3. Clinical Data
4.4. Machine Learning Pipelines
4.5. Immunohistochemistry
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CR | complete response |
| DCB | double checkpoint blockade |
| EMA | European Medicines Agency |
| FDA | Food and Drug Administration |
| FFPE | formalin-fixed, paraffin-embedded |
| GO | Gene Ontology |
| ICB | immune checkpoint blockade |
| IDO | Indolamin-2,3-Dioxygenase |
| IRS | immunoreactive score |
| LBCL | large B cell lymphoma |
| MR | mixed response |
| ORA | overrepresentation analysis |
| OS | overall survival |
| PCA | principal component analysis |
| PD | progressive disease |
| PR | partial response |
| QC | quality control |
| RFE | recursive feature elimination |
| SD | stable disease |
| TAM | tumor-associated macrophages |
| tebe | tebentafusp |
| TIGIT | T cell immunoreceptor with Ig and ITIM domains |
| TILs | tumor-infiltrating lymphocytes |
| UM | Uveal melanoma |
| VST | variance-stabilizing transformed |
References
- Mallone, S.; De Vries, E.; Guzzo, M.; Midena, E.; Verne, J.; Coebergh, J.W.; Marcos-Gragera, R.; Ardanaz, E.; Martinez, R.; Chirlaque, M.D.; et al. Descriptive epidemiology of malignant mucosal and uveal melanomas and adnexal skin carcinomas in Europe. Eur. J. Cancer 2012, 48, 1167–1175. [Google Scholar] [CrossRef] [PubMed]
- Collaborative Ocular Melanoma Study, G. Assessment of metastatic disease status at death in 435 patients with large choroidal melanoma in the Collaborative Ocular Melanoma Study (COMS): COMS report no. 15. Arch. Ophthalmol. 2001, 119, 670–676. [Google Scholar] [CrossRef]
- Kujala, E.; Makitie, T.; Kivela, T. Very long-term prognosis of patients with malignant uveal melanoma. Investig. Ophthalmol. Vis. Sci. 2003, 44, 4651–4659. [Google Scholar] [CrossRef]
- Hassel, J.C.; Piperno-Neumann, S.; Rutkowski, P.; Baurain, J.F.; Schlaak, M.; Butler, M.O.; Sullivan, R.J.; Dummer, R.; Kirkwood, J.M.; Orloff, M.; et al. Three-Year Overall Survival with Tebentafusp in Metastatic Uveal Melanoma. N. Engl. J. Med. 2023, 389, 2256–2266. [Google Scholar] [CrossRef]
- Nathan, P.; Hassel, J.C.; Rutkowski, P.; Baurain, J.F.; Butler, M.O.; Schlaak, M.; Sullivan, R.J.; Ochsenreither, S.; Dummer, R.; Kirkwood, J.M.; et al. Overall Survival Benefit with Tebentafusp in Metastatic Uveal Melanoma. N. Engl. J. Med. 2021, 385, 1196–1206. [Google Scholar] [CrossRef]
- Piulats, J.M.; Espinosa, E.; de la Cruz Merino, L.; Varela, M.; Alonso Carrion, L.; Martin-Algarra, S.; Lopez Castro, R.; Curiel, T.; Rodriguez-Abreu, D.; Redrado, M.; et al. Nivolumab Plus Ipilimumab for Treatment-Naive Metastatic Uveal Melanoma: An Open-Label, Multicenter, Phase II Trial by the Spanish Multidisciplinary Melanoma Group (GEM-1402). J. Clin. Oncol. 2021, 39, 586–598. [Google Scholar] [CrossRef]
- Pelster, M.S.; Gruschkus, S.K.; Bassett, R.; Gombos, D.S.; Shephard, M.; Posada, L.; Glover, M.S.; Simien, R.; Diab, A.; Hwu, P.; et al. Nivolumab and Ipilimumab in Metastatic Uveal Melanoma: Results From a Single-Arm Phase II Study. J. Clin. Oncol. 2020, 39, 599–607. [Google Scholar] [CrossRef]
- Petzold, A.; Steeb, T.; Wessely, A.; Koch, E.A.T.; Vera, J.; Berking, C.; Heppt, M.V. Is tebentafusp superior to combined immune checkpoint blockade and other systemic treatments in metastatic uveal melanoma? A comparative efficacy analysis with population adjustment. Cancer Treat. Rev. 2023, 115, 102543. [Google Scholar] [CrossRef] [PubMed]
- Koch, E.A.T.; Petzold, A.; Wessely, A.; Dippel, E.; Erdmann, M.; Heinzerling, L.; Hohberger, B.; Knorr, H.; Leiter, U.; Meier, F.; et al. Clinical determinants of long-term survival in metastatic uveal melanoma. Cancer Immunol. Immunother. 2022, 71, 1467–1477. [Google Scholar] [CrossRef] [PubMed]
- Guyon, I.; Weston, J.; Barnhill, S.; Vapnik, V. Gene selection for cancer classification using support vector machines. Mach. Learn. 2002, 46, 389–422. [Google Scholar] [CrossRef]
- Berisha, V.; Krantsevich, C.; Hahn, P.R.; Hahn, S.; Dasarathy, G.; Turaga, P.; Liss, J. Digital medicine and the curse of dimensionality. npj Digit. Med. 2021, 4, 153. [Google Scholar] [CrossRef]
- Friedman, J.H. Greedy function approximation: A gradient boosting machine. Ann. Stat. 2001, 29, 1189–1232. [Google Scholar] [CrossRef]
- Koch, E.A.T.; Petzold, A.; Dippel, E.; Erdmann, M.; Gesierich, A.; Gutzmer, R.; Hassel, J.C.; Haferkamp, S.; Kähler, K.C.; Kreuzberg, N.; et al. Optimizing immune checkpoint blockade in metastatic uveal melanoma: Exploring the association of overall survival and the occurrence of adverse events. Front. Immunol. 2024, 15, 1395225. [Google Scholar] [CrossRef]
- Doroshow, D.B.; Bhalla, S.; Beasley, M.B.; Sholl, L.M.; Kerr, K.M.; Gnjatic, S.; Wistuba, I.I.; Rimm, D.L.; Tsao, M.S.; Hirsch, F.R. PD-L1 as a biomarker of response to immune-checkpoint inhibitors. Nat. Rev. Clin. Oncol. 2021, 18, 345–362. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Panet, F.; Barberi, V.; Salgado, R.; Oliveira, M.; Loi, S. Biomarkers of response and resistance to immune checkpoint inhibitors in breast cancer. Breast 2025, 83, 104545. [Google Scholar] [CrossRef] [PubMed]
- Havel, J.J.; Chowell, D.; Chan, T.A. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat. Rev. Cancer 2019, 19, 133–150. [Google Scholar] [CrossRef]
- Daud, A.I.; Wolchok, J.D.; Robert, C.; Hwu, W.J.; Weber, J.S.; Ribas, A.; Hodi, F.S.; Joshua, A.M.; Kefford, R.; Hersey, P.; et al. Programmed Death-Ligand 1 Expression and Response to the Anti-Programmed Death 1 Antibody Pembrolizumab in Melanoma. J. Clin. Oncol. 2016, 34, 4102–4109. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Bollin, K.; de Macedo, M.P.; Carapeto, F.; Kim, K.B.; Roszik, J.; Wani, K.M.; Reuben, A.; Reddy, S.T.; Williams, M.D.; et al. Immune profiling of uveal melanoma identifies a potential signature associated with response to immunotherapy. J. Immunother. Cancer 2020, 8, e000960, Erratum in J. Immunother. Cancer 2022, 10, e000960. [Google Scholar] [CrossRef]
- Singh, L.; Singh, M.K.; Kenney, M.C.; Jager, M.J.; Rizvi, M.A.; Meel, R.; Lomi, N.; Bakhshi, S.; Sen, S.; Kashyap, S. Prognostic significance of PD-1/PD-L1 expression in uveal melanoma: Correlation with tumor-infiltrating lymphocytes and clinicopathological parameters. Cancer Immunol. Immunother. 2021, 70, 1291–1303. [Google Scholar] [CrossRef]
- Moeckel, C.; Bakhl, K.; Georgakopoulos-Soares, I.; Zaravinos, A. The Efficacy of Tumor Mutation Burden as a Biomarker of Response to Immune Checkpoint Inhibitors. Int. J. Mol. Sci. 2023, 24, 6710. [Google Scholar] [CrossRef]
- Wu, H.X.; Wang, Z.X.; Zhao, Q.; Chen, D.L.; He, M.M.; Yang, L.P.; Wang, Y.N.; Jin, Y.; Ren, C.; Luo, H.Y.; et al. Tumor mutational and indel burden: A systematic pan-cancer evaluation as prognostic biomarkers. Ann. Transl. Med. 2019, 7, 640. [Google Scholar] [CrossRef] [PubMed]
- Lai, G.; Liu, H.; Deng, J.; Li, K.; Zhang, C.; Zhong, X.; Xie, B. The Characteristics of Tumor Microenvironment Predict Survival and Response to Immunotherapy in Adrenocortical Carcinomas. Cells 2023, 12, 755. [Google Scholar] [CrossRef] [PubMed]
- Lai, G.; Xie, B.; Zhang, C.; Zhong, X.; Deng, J.; Li, K.; Liu, H.; Zhang, Y.; Liu, A.; Liu, Y.; et al. Comprehensive analysis of immune subtype characterization on identification of potential cells and drugs to predict response to immune checkpoint inhibitors for hepatocellular carcinoma. Genes. Dis. 2025, 12, 101471. [Google Scholar] [CrossRef]
- Zhang, J.H.; Huang, D.; Saw, P.E.; Song, E.R. Turning cold tumors hot: From molecular mechanisms to clinical applications. Trends Immunol. 2022, 43, 523–545. [Google Scholar] [CrossRef]
- Scheper, W.; Kelderman, S.; Fanchi, L.F.; Linnemann, C.; Bendle, G.; de Rooij, M.A.J.; Hirt, C.; Mezzadra, R.; Slagter, M.; Dijkstra, K.; et al. Low and variable tumor reactivity of the intratumoral TCR repertoire in human cancers. Nat. Med. 2019, 25, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Li, Q.M.; Xu, G.J. Identification and validation of an immune-relevant risk signature predicting survival outcome and immune infiltration in uveal melanoma. Int. Ophthalmol. 2023, 43, 4689–4700. [Google Scholar] [CrossRef]
- Marques, R.F.; Moreno, D.A.; da Silva, L.; Leal, L.F.; de Paula, F.E.; Santana, I.; Teixeira, G.; Saggioro, F.; Neder, L.; Junior, C.A.; et al. Digital expression profile of immune checkpoint genes in medulloblastomas identifies CD24 and CD276 as putative immunotherapy targets. Front. Immunol. 2023, 14, 1062856. [Google Scholar] [CrossRef]
- Rudd, C.E.; Taylor, A.; Schneider, H. CD28 and CTLA-4 coreceptor expression and signal transduction. Immunol. Rev. 2009, 229, 12–26. [Google Scholar] [CrossRef]
- Munn, D.H.; Sharma, M.D.; Mellor, A.L. Ligation of B7-1/B7-2 by human CD4+ T cells triggers indoleamine 2,3-dioxygenase activity in dendritic cells. J. Immunol. 2004, 172, 4100–4110. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, X.; Zhu, J.; Chai, Y.; Cong, B.; Li, B.; Gao, W.; Hu, Y.; Wen, M.; Liu, Y.; et al. Inhibiting intracellular CD28 in cancer cells enhances antitumor immunity and overcomes anti-PD-1 resistance via targeting PD-L1. Cancer Cell 2025, 43, 86–102.e110. [Google Scholar] [CrossRef]
- Suntharalingam, G.; Perry, M.R.; Ward, S.; Brett, S.J.; Castello-Cortes, A.; Brunner, M.D.; Panoskaltsis, N. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N. Engl. J. Med. 2006, 355, 1018–1028. [Google Scholar] [CrossRef] [PubMed]
- Ecker, M.; Schregle, R.; Kapoor-Kaushik, N.; Rossatti, P.; Betzler, V.M.; Kempe, D.; Biro, M.; Ariotti, N.; Redpath, G.M.; Rossy, J. SNX9-induced membrane tubulation regulates CD28 cluster stability and signalling. eLife 2022, 11, e67550. [Google Scholar] [CrossRef]
- Zhai, L.J.; Ladomersky, E.; Lenzen, A.; Nguyen, B.; Patel, R.; Lauing, K.L.; Wu, M.J.; Wainwright, D.A. IDO1 in cancer: A Gemini of immune checkpoints. Cell Mol. Immunol. 2018, 15, 447–457. [Google Scholar] [CrossRef]
- Zhai, L.J.; Ladomersky, E.; Lauing, K.L.; Wu, M.J.; Genet, M.; Gritsina, G.; Gyorffy, B.; Brastianos, P.K.; Binder, D.C.; Sosman, J.A.; et al. Infiltrating T Cells Increase IDO1 Expression in Glioblastoma and Contribute to Decreased Patient Survival. Clin. Cancer Res. 2017, 23, 6650–6660. [Google Scholar] [CrossRef]
- Siu, L.L.; Gelmon, K.; Chu, Q.; Pachynski, R.; Alese, O.; Basciano, P.; Walker, J.; Mitra, P.; Zhu, L.; Phillips, P.; et al. BMS-986205, an optimized indoleamine 2,3-dioxygenase 1 (IDO1) inhibitor, is well tolerated with potent pharmacodynamic (PD) activity, alone and in combination with nivolumab (nivo) in advanced cancers in a phase 1/2a trial. Cancer Res. 2017, 77, CT116. [Google Scholar] [CrossRef]
- Koch, E.A.T.; Schaft, N.; Kummer, M.; Berking, C.; Schuler, G.; Hasumi, K.; Dorrie, J.; Schuler-Thurner, B. A One-Armed Phase I Dose Escalation Trial Design: Personalized Vaccination with IKKbeta-Matured, RNA-Loaded Dendritic Cells for Metastatic Uveal Melanoma. Front. Immunol. 2022, 13, 785231. [Google Scholar] [CrossRef]
- Gangadhar, T.C.; Hamid, O.; Smith, D.C.; Bauer, T.M.; Wasser, J.S.; Luke, J.J.; Balmanoukian, A.S.; Kaufman, D.R.; Zhao, Y.; Maleski, J.; et al. Preliminary results from a Phase I/II study of epacadostat (incb024360) in combination with pembrolizumab in patients with selected advanced cancers. J. Immunother. Cancer 2015, 3, O7. [Google Scholar] [CrossRef]
- Spira, A.I.; Hamid, O.; Bauer, T.M.; Borges, V.F.; Wasser, J.S.; Smith, D.C.; Clark, A.S.; Schmidt, E.V.; Zhao, Y.F.; Maleski, J.E.; et al. Efficacy/safety of epacadostat plus pembrolizumab in triple-negative breast cancer and ovarian cancer: Phase I/II ECHO-202 study. J. Clin. Oncol. 2017, 35, 1103. [Google Scholar] [CrossRef]
- Yang, P.; Chen, W.; Xu, H.; Yang, J.; Jiang, J.; Jiang, Y.; Xu, G. Correlation of CCL8 expression with immune cell infiltration of skin cutaneous melanoma: Potential as a prognostic indicator and therapeutic pathway. Cancer Cell Int. 2021, 21, 635. [Google Scholar] [CrossRef]
- Chen, S.; Huang, C.; Li, K.; Cheng, M.; Zhang, C.; Xiong, J.; Tian, G.; Zhou, R.; Ling, R.; Wang, X.; et al. Tumor-initiating cells escape tumor immunity via CCL8 from tumor-associated macrophages in mice. J. Clin. Investig. 2025, 135, e180893. [Google Scholar] [CrossRef] [PubMed]
- Lou, X.L.; Zhao, K.; Xu, J.Z.; Shuai, L.X.; Niu, H.; Cao, Z.F.; Wang, J.; Zhang, Y.S. CCL8 as a promising prognostic factor in diffuse large B-cell lymphoma M2 macrophage interactions: A bioinformatic analysis of the tumor microenvironment. Front. Immunol. 2022, 13, 950213. [Google Scholar] [CrossRef]
- Pu, Y.Z.; Ji, Q. Tumor-Associated Macrophages Regulate PD-1/PD-L1 Immunosuppression. Front. Immunol. 2022, 13, 874589. [Google Scholar] [CrossRef]
- Chavez, B.; Kiaris, H. Insights on the role of the chemokine CCL8 in pathology. Cell Signal 2025, 134, 111951. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Fedchenko, N.; Reifenrath, J. Different approaches for interpretation and reporting of immunohistochemistry analysis results in the bone tissue—A review. Diagn. Pathol. 2014, 9, 221. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Stephens, M. False discovery rates: A new deal. Biostatistics 2017, 18, 275–294. [Google Scholar] [CrossRef]
- Blighe, K.; Rana, S.; Lewis, M. EnhancedVolcano: Publication-Ready Volcano Plots with Enhanced Colouring and Labeling; Bioconductor: Buffalo, NY, USA, 2024. [Google Scholar]
- Yu, G.C.; Wang, L.G.; Han, Y.Y.; He, Q.Y. clusterProfiler: An R Package for Comparing Biological Themes Among Gene Clusters. Omics 2012, 16, 284–287. [Google Scholar] [CrossRef]
| Categories | Group A (n = 16) | Group B (n = 14) | p Value | |
|---|---|---|---|---|
| Therapy response to ICB | CR | 0 (0%) | N/A | N/A |
| PR | 5 (31.25%) | N/A | ||
| SD | 10 (62.5%) | N/A | ||
| MR | 1 (6.25%) | N/A | ||
| PD | N/A | 14 (100%) | ||
| Age (date of diagnosis of Stage IV) | Mean in years | 62.8 | 60.5 years | 0.64 |
| Std. dev. = 14.1 | Std. dev. = 12 | |||
| Sex | Male | 7 (43.75%) | 11 (78.6%) | 0.052 |
| Female | 9 (56.25%) | 3 (21.4%) | ||
| LDH (date of diagnosis of Stage IV) | Mean in U/l | 239.4 | 271.5 | 0.37 |
| Std. dev. = 68.32 | Std. dev. = 116.24 | |||
| N/A | 1 (6.25%) | 0 (0%) | ||
| ECOG status (date of diagnosis of Stage IV) | 0 | 13 (81.25%) | 13 (92.9%) | 0.53 |
| 1 | 2 (12.5%) | 1 (7.1%) | ||
| N/A | 1 (6.25%) | 0 (0%) | ||
| Sites of metastases (n) | Liver only | 2 (12.5%) | 2 (14.3%) | 0.33 |
| Liver and Extrahepatic | 10 (62.5%) | 11 (78.6%) | ||
| Extrahepatic only | 4 (25%) | 1 (7.1%) | ||
| Biopsy timing (n) | Before systemic therapy | 10 (62.5%) | 9 (64.3%) | 0.91 |
| After systemic therapy | 6 (37.5%) | 5 (35.7%) | ||
| NanoString cartridges (n) | Batch 1 | 4 (25%) | 3 (21.4%) | 0.33 |
| Batch 2 | 7 (43.75%) | 3 (21.4%) | ||
| Batch 3 | 1 (6.25%) | 4 (28.6%) | ||
| Batch 4 | 4 (25%) | 4 (28.6%) | ||
| Biopsy location | Liver | 10 (62.5%) | 10 (71.4%) | 0.71 |
| Extrahepatic | 6 (37.5%) | 4 (28.6%) | ||
| Overall survival | Median in month | 48.9 | 15.8 | <0.001 Log Rank (Mantel–Cox) |
| 95% Confidence Interval | 27.3–70.4 | 0–35.3 |
| Group | N | Mean | Std. Deviation | t Test (One-Sided) | |
|---|---|---|---|---|---|
| TIGIT | A | 15 | 1.6 | 0.95 | p = 0.4 |
| B | 13 | 1.5 | 1.39 | ||
| CD28 | A | 14 | 2.6 | 2.09 | p = 0.039 |
| B | 14 | 1.1 | 1.81 | ||
| IDO-1 | A | 16 | 0.75 | 1 | p = 0.175 |
| B | 12 | 0.42 | 0.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koch, E.A.T.; Liguori, R.; Castro, A.A.; Schliep, S.; Petzold, A.; Wessely, A.; Fröhlich, W.; Ferrazzi, F.; Vera, J.; Eckstein, M.; et al. Checkpoint Blockade Efficacy in Uveal Melanoma Is Linked to Tumor Immunity, CD28, and CCL8. Int. J. Mol. Sci. 2025, 26, 9964. https://doi.org/10.3390/ijms26209964
Koch EAT, Liguori R, Castro AA, Schliep S, Petzold A, Wessely A, Fröhlich W, Ferrazzi F, Vera J, Eckstein M, et al. Checkpoint Blockade Efficacy in Uveal Melanoma Is Linked to Tumor Immunity, CD28, and CCL8. International Journal of Molecular Sciences. 2025; 26(20):9964. https://doi.org/10.3390/ijms26209964
Chicago/Turabian StyleKoch, Elias A. T., Renato Liguori, Alejandro Afonso Castro, Stefan Schliep, Anne Petzold, Anja Wessely, Waltraud Fröhlich, Fulvia Ferrazzi, Julio Vera, Markus Eckstein, and et al. 2025. "Checkpoint Blockade Efficacy in Uveal Melanoma Is Linked to Tumor Immunity, CD28, and CCL8" International Journal of Molecular Sciences 26, no. 20: 9964. https://doi.org/10.3390/ijms26209964
APA StyleKoch, E. A. T., Liguori, R., Castro, A. A., Schliep, S., Petzold, A., Wessely, A., Fröhlich, W., Ferrazzi, F., Vera, J., Eckstein, M., Berking, C., & Heppt, M. V. (2025). Checkpoint Blockade Efficacy in Uveal Melanoma Is Linked to Tumor Immunity, CD28, and CCL8. International Journal of Molecular Sciences, 26(20), 9964. https://doi.org/10.3390/ijms26209964








