Targeting Granulin Haploinsufficiency in Frontotemporal Dementia: From Genetic Mechanisms to Therapeutics
Abstract
1. Introduction: Structure and Functions of Granulin Gene and Protein
2. GRN Mutations, FTD, and Haploinsufficiency
3. Biomarkers of GRN Haploinsufficiency
4. Patient-Derived Cell Lines of GRN Haploinsufficiency
5. Animal Models of GRN Haploinsufficiency
6. Possible Therapeutic Targeting of GRN Haploinsufficiency
7. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Ferrari, R.; Hernandez, D.G.; Nalls, M.A.; Rohrer, J.D.; Ramasamy, A.; Kwok, J.B.; Dobson-Stone, C.; Brooks, W.S.; Schofield, P.R.; Halliday, G.M.; et al. Frontotemporal dementia and its subtypes: A genome-wide association study. Lancet Neurol. 2014, 13, 686–699. [Google Scholar] [CrossRef]
- Chu, M.; Jiang, D.; Li, D.; Yan, S.; Liu, L.; Nan, H.; Wang, Y.; Wang, Y.; Yue, A.; Ren, L.; et al. Atrophy network mapping of clinical subtypes and main symptoms in frontotemporal dementia. Brain 2024, 147, 3048–3058. [Google Scholar] [CrossRef]
- Finger, E.C. Frontotemporal Dementias. Contin. Lifelong Learn. Neurol. 2016, 22, 464–489. [Google Scholar] [CrossRef]
- Englund, B.; Brun, A.; Gustafson, L.; Passant, U.; Mann, D.; Neary, D.; Snowden, J.S. Clinical and neuropathological criteria for frontotemporal dementia. The Lund and Manchester Groups. J. Neurol. Neurosurg. Psychiatry 1994, 57, 416–418. [Google Scholar] [CrossRef] [PubMed]
- Abramzon, Y.A.; Fratta, P.; Traynor, B.J.; Chia, R. The Overlapping Genetics of Amyotrophic Lateral Sclerosis and Frontotemporal Dementia. Front. Neurosci. 2020, 14, 42. [Google Scholar] [CrossRef] [PubMed]
- Padovani, A.; Premi, E.; Pilotto, A.; Gazzina, S.; Cosseddu, M.; Archetti, S.; Cancelli, V.; Paghera, B.; Borroni, B. Overlap between frontotemporal dementia and Alzheimer’s disease: Cerebrospinal fluid pattern and neuroimaging study. J. Alzheimers Dis. 2013, 36, 49–55. [Google Scholar] [CrossRef]
- Greaves, C.V.; Rohrer, J.D. An update on genetic frontotemporal dementia. J. Neurol. 2019, 266, 2075–2086. [Google Scholar] [CrossRef]
- Kämäläinen, A.; Viswanathan, J.; Natunen, T.; Helisalmi, S.; Kauppinen, T.; Pikkarainen, M.; Pursiheimo, J.-P.; Alafuzoff, I.; Kivipelto, M.; Haapasalo, A.; et al. GRN Variant rs5848 Reduces Plasma and Brain Levels of Granulin in Alzheimer’s Disease Patients. J. Alzheimers Dis. 2012, 33, 23–27. [Google Scholar] [CrossRef]
- Morris, H.R.; Waite, A.J.; Williams, N.M.; Neal, J.W.; Blake, D.J. Recent advances in the genetics of the ALS-FTLD complex. Curr. Neurol. Neurosci. Rep. 2012, 12, 243–250. [Google Scholar] [CrossRef]
- Karamysheva, Z.N.; Tikhonova, E.B.; Karamyshev, A.L. Granulin in Frontotemporal Lobar Degeneration: Molecular Mechanisms of the Disease. Front. Neurosci. 2019, 13, 395. [Google Scholar] [CrossRef]
- Tolkatchev, D.; Malik, S.; Vinogradova, A.; Wang, P.; Chen, Z.; Xu, P.; Bennett, H.P.; Bateman, A.; Ni, F. Structure dissection of human progranulin identifies well-folded granulin/epithelin modules with unique functional activities. Protein Sci. 2008, 17, 711–724. [Google Scholar] [CrossRef] [PubMed]
- Gati, C.; Oberthuer, D.; Yefanov, O.; Bunker, R.D.; Stellato, F.; Chiu, E.; Yeh, S.M.; Aquila, A.; Basu, S.; Bean, R.; et al. Atomic structure of granulin determined from native nanocrystalline granulovirus using an X-ray free-electron laser. Proc. Natl. Acad. Sci. USA 2017, 114, 2247–2252. [Google Scholar] [CrossRef] [PubMed]
- Kao, A.W.; McKay, A.; Singh, P.P.; Brunet, A.; Huang, E.J. Progranulin, lysosomal regulation and neurodegenerative disease. Nat. Rev. Neurosci. 2017, 18, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Paushter, D.H.; Feng, T.; Sun, L.; Reinheckel, T.; Hu, F. Lysosomal processing of progranulin. Mol. Neurodegener. 2017, 12, 62. [Google Scholar] [CrossRef]
- Mohan, S.; Sampognaro, P.J.; Argouarch, A.R.; Maynard, J.C.; Welch, M.; Patwardhan, A.; Courtney, E.C.; Zhang, J.; Mason, A.; Li, K.H.; et al. Processing of progranulin into granulins involves multiple lysosomal proteases and is affected in frontotemporal lobar degeneration. Mol. Neurodegener. 2021, 16, 51. [Google Scholar] [CrossRef]
- Du, H.; Zhou, X.; Feng, T.; Hu, F. Regulation of lysosomal trafficking of progranulin by sortilin and prosaposin. Brain Commun. 2022, 4, fcab310. [Google Scholar] [CrossRef]
- Zheng, Y.; Brady, O.A.; Meng, P.S.; Mao, Y.; Hu, F. C-terminus of progranulin interacts with the beta-propeller region of sortilin to regulate progranulin trafficking. PLoS ONE 2011, 6, e21023. [Google Scholar] [CrossRef]
- Wauters, E.; Van Mossevelde, S.; Van der Zee, J.; Cruts, M.; Van Broeckhoven, C. Modifiers of GRN-Associated Frontotemporal Lobar Degeneration. Trends Mol. Med. 2017, 23, 962–979. [Google Scholar] [CrossRef]
- Pizarro, G.O.; Zhou, X.C.; Koch, A.; Gharib, M.; Raval, S.; Bible, K.; Jones, M.B. Prosurvival function of the granulin-epithelin precursor is important in tumor progression and chemoresponse. Int. J. Cancer 2007, 120, 2339–2343. [Google Scholar] [CrossRef]
- Arechavaleta-Velasco, F.; Perez-Juarez, C.E.; Gerton, G.L.; Diaz-Cueto, L. Progranulin and its biological effects in cancer. Med. Oncol. 2017, 34, 194. [Google Scholar] [CrossRef]
- Bhandari, V.; Palfree, R.G.; Bateman, A. Isolation and sequence of the granulin precursor cDNA from human bone marrow reveals tandem cysteine-rich granulin domains. Proc. Natl. Acad. Sci. USA 1992, 89, 1715–1719. [Google Scholar] [CrossRef]
- Horinokita, I.; Hayashi, H.; Oteki, R.; Mizumura, R.; Yamaguchi, T.; Usui, A.; Yuan, B.; Takagi, N. Involvement of Progranulin and Granulin Expression in Inflammatory Responses after Cerebral Ischemia. Int. J. Mol. Sci. 2019, 20, 5210. [Google Scholar] [CrossRef]
- Townley, R.A.; Boeve, B.F.; Benarroch, E.E. Progranulin: Functions and neurologic correlations. Neurology 2018, 90, 118–125, Erratum in Neurology 2018, 90, 1127. https://doi.org/10.1212/WNL.0000000000005191. [Google Scholar] [CrossRef]
- Du, H.; Wong, M.Y.; Zhang, T.; Santos, M.N.; Hsu, C.; Zhang, J.; Yu, H.; Luo, W.; Hu, F. A multifaceted role of progranulin in regulating amyloid-beta dynamics and responses. Life Sci. Alliance 2021, 4, e202000874. [Google Scholar] [CrossRef]
- Sun, L.; Eriksen, J.L. Recent insights into the involvement of progranulin in frontotemporal dementia. Curr. Neuropharmacol. 2011, 9, 632–642. [Google Scholar] [CrossRef]
- Gijselinck, I.; van der Zee, J.; Engelborghs, S.; Goossens, D.; Peeters, K.; Mattheijssens, M.; Corsmit, E.; Del-Favero, J.; De Deyn, P.P.; Van Broeckhoven, C.; et al. Progranulin locus deletion in frontotemporal dementia. Hum. Mutat. 2008, 29, 53–58. [Google Scholar] [CrossRef]
- Mao, Q.; Wang, D.; Li, Y.; Kohler, M.; Wilson, J.; Parton, Z.; Shmaltsuyeva, B.; Gursel, D.; Rademakers, R.; Weintraub, S.; et al. Disease and Region Specificity of Granulin Immunopositivities in Alzheimer Disease and Frontotemporal Lobar Degeneration. J. Neuropathol. Exp. Neurol. 2017, 76, 957–968. [Google Scholar] [CrossRef] [PubMed]
- Ryan, C.L.; Baranowski, D.C.; Chitramuthu, B.P.; Malik, S.; Li, Z.; Cao, M.; Minotti, S.; Durham, H.D.; Kay, D.G.; Shaw, C.A.; et al. Progranulin is expressed within motor neurons and promotes neuronal cell survival. BMC Neurosci. 2009, 10, 130. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Z.; Mackenzie, I.R.; Hutton, M.L.; Dickson, D.W. Progranulin in frontotemporal lobar degeneration and neuroinflammation. J. Neuroinflamm. 2007, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Suzuki, G.; Matsuwaki, T.; Hosokawa, M.; Serrano, G.; Beach, T.G.; Yamanouchi, K.; Hasegawa, M.; Nishihara, M. Progranulin regulates lysosomal function and biogenesis through acidification of lysosomes. Hum. Mol. Genet. 2017, 26, 969–988. [Google Scholar] [CrossRef]
- Bateman, A.; Bennett, H.P. The granulin gene family: From cancer to dementia. BioEssays 2009, 31, 1245–1254. [Google Scholar] [CrossRef]
- Gijselinck, I.; Van Broeckhoven, C.; Cruts, M. Granulin mutations associated with frontotemporal lobar degeneration and related disorders: An update. Hum. Mutat. 2008, 29, 1373–1386. [Google Scholar] [CrossRef]
- Chen-Plotkin, A.S.; Xiao, J.; Geser, F.; Martinez-Lage, M.; Grossman, M.; Unger, T.; Wood, E.M.; Van Deerlin, V.M.; Trojanowski, J.Q.; Lee, V.M.Y. Brain progranulin expression in GRN-associated frontotemporal lobar degeneration. Acta Neuropathol. 2010, 119, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Jahan, N.; Miller, Z.A.; Huang, E.J. Neuroimmune dysfunction in frontotemporal dementia: Insights from progranulin and C9orf72 deficiency. Curr. Opin. Neurobiol. 2022, 76, 102599. [Google Scholar] [CrossRef] [PubMed]
- Guven, G.; Bilgic, B.; Tufekcioglu, Z.; Erginel Unaltuna, N.; Hanagasi, H.; Gurvit, H.; Singleton, A.; Hardy, J.; Emre, M.; Gulec, C.; et al. Peripheral GRN mRNA and Serum Progranulin Levels as a Potential Indicator for Both the Presence of Splice Site Mutations and Individuals at Risk for Frontotemporal Dementia. J. Alzheimers Dis. 2019, 67, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Wauters, E.; Gossye, H.; Frydas, A.; Sieben, A.; Van Broeckhoven, C. Rare exonic variant affects GRN splicing and contributes to frontotemporal lobar degeneration. Neurobiol. Aging. 2023, 130, 61–69. [Google Scholar] [CrossRef]
- Brouwers, N.; Nuytemans, K.; van der Zee, J.; Gijselinck, I.; Engelborghs, S.; Theuns, J.; Kumar-Singh, S.; Pickut, B.A.; Pals, P.; Dermaut, B.; et al. Alzheimer and Parkinson diagnoses in progranulin null mutation carriers in an extended founder family. Arch. Neurol. 2007, 64, 1436–1446. [Google Scholar] [CrossRef]
- Menéndez-González, M.; García-Martínez, A.; Fernández-Vega, I.; Pitiot, A.; Álvarez, V. A variant in GRN of Spanish origin presenting with heterogeneous phenotypes. Neurologia 2022, 40, 57–65. [Google Scholar] [CrossRef]
- Yu, C.E.; Bird, T.D.; Bekris, L.M.; Montine, T.J.; Leverenz, J.B.; Steinbart, E.; Galloway, N.M.; Feldman, H.; Woltjer, R.; Miller, C.A.; et al. The spectrum of mutations in progranulin: A collaborative study screening 545 cases of neurodegeneration. Arch. Neurol. 2010, 67, 161–170. [Google Scholar] [CrossRef]
- Almeida, M.R.; Tábuas-Pereira, M.; Baldeiras, I.; Lima, M.; Durães, J.; Massano, J.; Pinto, M.; Cruto, C.; Santana, I. Characterization of Progranulin Gene Mutations in Portuguese Patients with Frontotemporal Dementia. Int. J. Mol. Sci. 2023, 25, 511. [Google Scholar] [CrossRef]
- Le Ber, I.; Camuzat, A.; Hannequin, D.; Pasquier, F.; Guedj, E.; Rovelet-Lecrux, A.; Hahn-Barma, V.; van der Zee, J.; Clot, F.; Bakchine, S.; et al. Phenotype variability in progranulin mutation carriers: A clinical, neuropsychological, imaging and genetic study. Brain 2008, 131 Pt 3, 732–746. [Google Scholar] [CrossRef] [PubMed]
- Capell, A.; Fellerer, K.; Haass, C. Progranulin transcripts with short and long 5′ untranslated regions (UTRs) are differentially expressed via posttranscriptional and translational repression. J. Biol. Chem. 2014, 289, 25879–25889. [Google Scholar] [CrossRef] [PubMed]
- Saracino, D.; Sellami, L.; Clot, F.; Camuzat, A.; Lamari, F.; Rucheton, B.; Benyounes, I.; Roué-Jagot, C.; Lagarde, J.; Sarazin, M.; et al. The missense p.Trp7Arg mutation in GRN gene leads to progranulin haploinsufficiency. Neurobiol. Aging 2020, 85, e9–e154. [Google Scholar] [CrossRef]
- Pinarbasi, E.S.; Karamyshev, A.L.; Tikhonova, E.B.; Wu, I.H.; Hudson, H.; Thomas, P.J. Pathogenic Signal Sequence Mutations in Progranulin Disrupt SRP Interactions Required for mRNA Stability. Cell Rep. 2018, 23, 2844–2851. [Google Scholar] [CrossRef]
- Hsiung, G.Y.; Fok, A.; Feldman, H.H.; Rademakers, R.; Mackenzie, I.R. rs5848 polymorphism and serum progranulin level. J. Neurol. Sci. 2011, 300, 28–32. [Google Scholar] [CrossRef]
- Eriksen, J.L.; Mackenzie, I.R. Progranulin: Normal function and role in neurodegeneration. J. Neurochem. 2008, 104, 287–297. [Google Scholar] [CrossRef]
- Woollacott, I.O.C.; Bocchetta, M.; Sudre, C.H.; Ridha, B.H.; Strand, C.; Courtney, R.; Ourselin, S.; Cardoso, M.J.; Warren, J.D.; Rossor, M.N.; et al. Pathological correlates of white matter hyperintensities in a case of progranulin mutation associated frontotemporal dementia. Neurocase 2018, 24, 166–174. [Google Scholar] [CrossRef]
- Cruts, M.; Kumar-Singh, S.; Van Broeckhoven, C. Progranulin mutations in ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Curr. Alzheimer Res. 2006, 3, 485–491. [Google Scholar] [CrossRef]
- Bonvicini, C.; Milanesi, E.; Pilotto, A.; Cattane, N.; Premi, E.; Archetti, S.; Padovani, A.; Gennarelli, M.; Borroni, B. Understanding phenotype variability in frontotemporal lobar degeneration due to granulin mutation. Neurobiol. Aging 2014, 35, 1206–1211. [Google Scholar] [CrossRef]
- Wauters, E.; Van Mossevelde, S.; Sleegers, K.; van der Zee, J.; Engelborghs, S.; Sieben, A.; Vandenberghe, R.; Philtjens, S.; Van den Broeck, M.; Peeters, K.; et al. Clinical variability and onset age modifiers in an extended Belgian GRN founder family. Neurobiol. Aging 2018, 67, 84–94. [Google Scholar] [CrossRef]
- Rademakers, R.; Baker, M.; Gass, J.; Adamson, J.; Huey, E.D.; Momeni, P.; Spina, S.; Coppola, G.; Karydas, A.M.; Stewart, H.; et al. Phenotypic variability associated with progranulin haploinsufficiency in patients with the common 1477C-->T (Arg493X) mutation: An international initiative. Lancet Neurol. 2007, 6, 857–868. [Google Scholar] [CrossRef] [PubMed]
- Puoti, G.; Lerza, M.C.; Ferretti, M.G.; Bugiani, O.; Tagliavini, F.; Rossi, G. A mutation in the 5′-UTR of GRN gene associated with frontotemporal lobar degeneration: Phenotypic variability and possible pathogenetic mechanisms. J. Alzheimers Dis. 2014, 42, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Sieben, A.; Van Mossevelde, S.; Wauters, E.; Engelborghs, S.; van der Zee, J.; Van Langenhove, T.; Santens, P.; Praet, M.; Boon, P.; Miatton, M.; et al. Extended FTLD pedigree segregating a Belgian GRN-null mutation: Neuropathological heterogeneity in one family. Alzheimers Res. Ther. 2018, 10, 7. [Google Scholar] [CrossRef] [PubMed]
- Cruts, M.; Gijselinck, I.; van der Zee, J.; Engelborghs, S.; Wils, H.; Pirici, D.; Rademakers, R.; Vandenberghe, R.; Dermaut, B.; Martin, J.J.; et al. Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature 2006, 442, 920–924. [Google Scholar] [CrossRef]
- Cioffi, S.M.; Galimberti, D.; Barocco, F.; Spallazzi, M.; Fenoglio, C.; Serpente, M.; Arcaro, M.; Gardini, S.; Scarpini, E.; Caffarra, P. Non Fluent Variant of Primary Progressive Aphasia Due to theel GRN g.9543delA(IVS3-2delA) Mutation. J. Alzheimers Dis. 2016, 54, 717–721. [Google Scholar] [CrossRef]
- Mukherjee, O.; Wang, J.; Gitcho, M.; Chakraverty, S.; Taylor-Reinwald, L.; Shears, S.; Kauwe, J.S.; Norton, J.; Levitch, D.; Bigio, E.H.; et al. Molecular characterization of novel progranulin (GRN) mutations in frontotemporal dementia. Hum. Mutat. 2008, 29, 512–521. [Google Scholar] [CrossRef]
- Marcon, G.; Rossi, G.; Giaccone, G.; Giovagnoli, A.R.; Piccoli, E.; Zanini, S.; Geatti, O.; Toso, V.; Grisoli, M.; Tagliavini, F. Variability of the clinical phenotype in an Italian family with dementia associated with an intronic deletion in the GRN gene. J. Alzheimers Dis. 2011, 26, 583–590. [Google Scholar] [CrossRef]
- Coppola, C.; Oliva, M.; Saracino, D.; Pappatà, S.; Zampella, E.; Cimini, S.; Ricci, M.; Giaccone, G.; Di Iorio, G.; Rossi, G. One novel GRN null mutation, two different aphasia phenotypes. Neurobiol. Aging 2020, 87, e9–e141. [Google Scholar] [CrossRef]
- Masellis, M.; Momeni, P.; Meschino, W.; Heffner, R., Jr.; Elder, J.; Sato, C.; Liang, Y.; St George-Hyslop, P.; Hardy, J.; Bilbao, J.; et al. Novel splicing mutation in the progranulin gene causing familial corticobasal syndrome. Brain 2006, 129 Pt 11, 3115–3123. [Google Scholar] [CrossRef]
- Moreno, F.; Indakoetxea, B.; Barandiaran, M.; Alzualde, A.; Gabilondo, A.; Estanga, A.; Ruiz, J.; Ruibal, M.; Bergareche, A.; Martí-Massó, J.F.; et al. “Frontotemporoparietal” dementia: Clinical phenotype associated with the c.709-1G>A PGRN mutation. Neurology 2009, 73, 1367–1374. [Google Scholar] [CrossRef]
- Alquezar, C.; Esteras, N.; Bartolomé, F.; Merino, J.J.; Alzualde, A.; López de Munain, A.; tín-Requero, Á. Alteration in cell cycle-related proteins in lymphoblasts from carriers of the c.709-1G>A PGRN mutation associated with FTLD-TDP dementia. Neurobiol. Aging 2012, 33, e7–e20. [Google Scholar] [CrossRef]
- Sassi, C.; Capozzo, R.; Gibbs, R.; Crews, C.; Zecca, C.; Arcuti, S.; Copetti, M.; Barulli, M.R.; Brescia, V.; Singleton, A.B.; et al. Ael Splice-Acceptor Site Mutation in GRN (c.709-2 A>T) Causes Frontotemporal Dementia Spectrum in a Large Family from Southern Italy. J. Alzheimers Dis. 2016, 53, 475–485. [Google Scholar] [CrossRef]
- Gaweda-Walerych, K.; Sitek, E.J.; Narożańska, E.; Wezyk, M.; Brockhuis, B.; Zekanowski, C.; Sławek, J. Functional characterization of a novel progranulin mutation in a patient with progressive nonfluent aphasia. Neurobiol. Aging 2018, 72, e9–e186. [Google Scholar] [CrossRef] [PubMed]
- Picillo, M.; Vitale, E.; Rendina, A.; Donizetti, A.; Aliperti, V.; Tepedino, M.F.; Dati, G.; Ginevrino, M.; Valente, E.M.; Barone, P. Clinical and Molecular Characterization of ael Progranulin Deletion Associated with Different Phenotypes. J. Alzheimers Dis. 2020, 76, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Calvi, A.; Cioffi, S.M.; Caffarra, P.; Fenoglio, C.; Serpente, M.; Pietroboni, A.M.; Arighi, A.; Ghezzi, L.; Gardini, S.; Scarpini, E.; et al. The novel GRN g.1159_1160delTG mutation is associated with behavioral variant frontotemporal dementia. J. Alzheimers Dis. 2015, 44, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Milan, G.; Napoletano, S.; Pappatà, S.; Gentile, M.T.; Colucci-D’Amato, L.; Della Rocca, G.; Maciag, A.; Rossetti, C.P.; Fucci, L.; Puca, A.; et al. GRN deletion in familial frontotemporal dementia showing association with clinical variability in 3 familial cases. Neurobiol. Aging 2017, 53, e9–e193. [Google Scholar] [CrossRef]
- Taghdiri, F.; Sato, C.; Ghani, M.; Moreno, D.; Rogaeva, E.; Tartaglia, M.C. Novel GRN Mutations in Patients with Corticobasal Syndrome. Sci. Rep. 2016, 6, 22913. [Google Scholar] [CrossRef]
- Borroni, B.; Alberici, A.; Cercignani, M.; Premi, E.; Serra, L.; Cerini, C.; Cosseddu, M.; Pettenati, C.; Turla, M.; Archetti, S.; et al. Granulin mutation drives brain damage and reorganization from preclinical to symptomatic FTLD. Neurobiol. Aging 2012, 33, 2506–2520. [Google Scholar] [CrossRef]
- Guerreiro, R.J.; Santana, I.; Bras, J.M.; Revesz, T.; Rebelo, O.; Ribeiro, M.H.; Santiago, B.; Oliveira, C.R.; Singleton, A.; Hardy, J. Novel progranulin mutation: Screening for PGRN mutations in a Portuguese series of FTD/CBS cases. Mov. Disord. 2008, 23, 1269–1273. [Google Scholar] [CrossRef]
- Dominguez, J.; Ng, A.; Yu, J.; Guevarra, A.C.; Daroy, M.L.; Alfon, A.; Catindig, J.A.; Dizon, M.; Santiago, J.; Del Moral, M.C.; et al. Autosomal Dominant Frontotemporal Lobar Degeneration in a Filipino Family with Progranulin Mutation. Dement. Geriatr. Cogn. Disord. 2020, 49, 557–564. [Google Scholar] [CrossRef]
- Kuuluvainen, L.; Pöyhönen, M.; Pasanen, P.; Siitonen, M.; Rummukainen, J.; Tienari, P.J.; Paetau, A.; Myllykangas, L. Ael Loss-of-Function GRN Mutation p.(Tyr229*): Clinical and Neuropathological Features. J. Alzheimers Dis. 2017, 55, 1167–1174. [Google Scholar] [CrossRef]
- Natarajan, K.; Eisfeldt, J.; Hammond, M.; Laffita-Mesa, J.M.; Patra, K.; Khoshnood, B.; Öijerstedt, L.; Graff, C. Single-cell multimodal analysis in a case with reduced penetrance of Progranulin-Frontotemporal Dementia. Acta Neuropathol. Commun. 2021, 9, 132. [Google Scholar] [CrossRef]
- Chiang, H.H.; Forsell, C.; Lilius, L.; Öijerstedt, L.; Thordardottir, S.; Shanmugarajan, K.; Westerlund, M.; Nennesmo, I.; Thonberg, H.; Graff, C. el progranulin mutations with reduced serum-progranulin levels in frontotemporal lobar degeneration. Eur. J. Hum. Genet. 2013, 21, 1260–1265. [Google Scholar] [CrossRef] [PubMed]
- Benussi, A.; Padovani, A.; Borroni, B. Phenotypic Heterogeneity of Monogenic Frontotemporal Dementia. Front. Aging Neurosci. 2015, 7, 171. [Google Scholar] [CrossRef] [PubMed]
- Aswathy, P.M.; Jairani, P.S.; Raghavan, S.K.; Verghese, J.; Gopala, S.; Srinivas, P.; Mathuranath, P.S. Progranulin mutation analysis: Identification of one novel mutation in exon 12 associated with frontotemporal dementia. Neurobiol. Aging 2016, 39, e1–e3. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, O.; Pastor, P.; Cairns, N.J.; Chakraverty, S.; Kauwe, J.S.; Shears, S.; Behrens, M.I.; Budde, J.; Hinrichs, A.L.; Norton, J.; et al. HDDD2 is a familial frontotemporal lobar degeneration with ubiquitin-positive, tau-negative inclusions caused by a missense mutation in the signal peptide of progranulin. Ann. Neurol. 2006, 60, 314–322. [Google Scholar] [CrossRef]
- Karch, C.M.; Ezerskiy, L.; Redaelli, V.; Giovagnoli, A.R.; Tiraboschi, P.; Pelliccioni, G.; Pelliccioni, P.; Kapetis, D.; D’Amato, I.; Piccoli, E.; et al. Missense mutations in progranulin gene associated with frontotemporal lobar degeneration: Study of pathogenetic features. Neurobiol. Aging 2016, 38, e1–e215. [Google Scholar] [CrossRef]
- Luzzi, S.; Colleoni, L.; Corbetta, P.; Baldinelli, S.; Fiori, C.; Girelli, F.; Silvestrini, M.; Caroppo, P.; Giaccone, G.; Tagliavini, F.; et al. Missense mutation in GRN gene affecting RNA splicing and plasma progranulin level in a family affected by frontotemporal lobar degeneration. Neurobiol. Aging 2017, 54, e1–e214. [Google Scholar] [CrossRef]
- Meda, F.; Simrén, J.; Borroni, B.; Cantoni, V.; Archetti, S.; Biasiotto, G.; Andreasson, U.; Blennow, K.; Kvartsberg, H.; Zetterberg, H. Analytical and clinical validation of a blood progranulin ELISA in frontotemporal dementias. Clin. Chem. Lab. Med. 2023, 61, 2195–2204. [Google Scholar] [CrossRef]
- Finch, N.; Baker, M.; Crook, R.; Swanson, K.; Kuntz, K.; Surtees, R.; Bisceglio, G.; Rovelet-Lecrux, A.; Boeve, B.; Petersen, R.C.; et al. Plasma progranulin levels predict progranulin mutation status in frontotemporal dementia patients and asymptomatic family members. Brain 2009, 132 Pt 3, 583–591. [Google Scholar] [CrossRef]
- Almeida, M.R.; Baldeiras, I.; Ribeiro, M.H.; Santiago, B.; Machado, C.; Massano, J.; Guimarães, J.; Resende Oliveira, C.; Santana, I. Progranulin peripheral levels as a screening tool for the identification of subjects with progranulin mutations in a Portuguese cohort. Neurodegener. Dis. 2014, 13, 214–223. [Google Scholar] [CrossRef]
- Ghidoni, R.; Paterlini, A.; Benussi, L. Circulating progranulin as a biomarker for neurodegenerative diseases. Am. J. Neurodegener. Dis. 2012, 1, 180–190. [Google Scholar]
- Caroppo, P.; Le Ber, I.; Camuzat, A.; Clot, F.; Naccache, L.; Lamari, F.; Detenville, A.; Bertrand, A.; Belliard, S.; Hannequin, D.; et al. Extensive white matter involvement in patients with frontotemporal lobar degeneration: Think progranulin. JAMA Neurol. 2014, 71, 1562–1566. [Google Scholar] [CrossRef] [PubMed]
- Sung, W.; Noh, M.Y.; Nahm, M.; Kim, Y.S.; Ki, C.S.; Kim, Y.E.; Kim, H.J.; Kim, S.H. Progranulin haploinsufficiency mediates cytoplasmic TDP-43 aggregation with lysosomal abnormalities in human microglia. J. Neuroinflamm. 2024, 21, 47. [Google Scholar] [CrossRef] [PubMed]
- Ljubenkov, P.A.; Edwards, L.; Iaccarino, L.; La Joie, R.; Rojas, J.C.; Koestler, M.; Harris, B.; Boeve, B.F.; Borroni, B.; van Swieten, J.C.; et al. Effect of the Histone Deacetylase Inhibitor FRM-0334 on Progranulin Levels in Patients With Progranulin Gene Haploinsufficiency: A Randomized Clinical Trial. JAMA Netw. Open 2021, 4, e2125584. [Google Scholar] [CrossRef] [PubMed]
- Ljubenkov, P.A.; Miller, Z.; Mumford, P.; Zhang, J.; Allen, I.E.; Mitic, L.; Staffaroni, A.; Heuer, H.; Rojas, J.C.; Cobigo, Y.; et al. Peripheral Innate Immune Activation Correlates With Disease Severity in GRN Haploinsufficiency. Front. Neurol. 2019, 10, 1004. [Google Scholar] [CrossRef]
- Zanardini, R.; Benussi, L.; Fostinelli, S.; Saraceno, C.; Ciani, M.; Borroni, B.; Padovani, A.; Binetti, G.; Ghidoni, R. Serum C-Peptide, Visfatin, Resistin, and Ghrelin are Altered in Sporadic and GRN-Associated Frontotemporal Lobar Degeneration. J. Alzheimers Dis. 2018, 61, 1053–1060. [Google Scholar] [CrossRef]
- Arrant, A.E.; Roth, J.R.; Boyle, N.R.; Kashyap, S.N.; Hoffmann, M.Q.; Murchison, C.F.; Ramos, E.M.; Nana, A.L.; Spina, S.; Grinberg, L.T.; et al. Impaired β-glucocerebrosidase activity and processing in frontotemporal dementia due to progranulin mutations. Acta Neuropathol. Commun. 2019, 7, 218. [Google Scholar] [CrossRef]
- Valdez, C.; Wong, Y.C.; Schwake, M.; Bu, G.; Wszolek, Z.K.; Krainc, D. Progranulin-mediated deficiency of cathepsin D results in FTD and NCL-like phenotypes in neurons derived from FTD patients. Hum. Mol. Genet. 2017, 26, 4861–4872. [Google Scholar] [CrossRef]
- Zhou, X.; Sun, L.; Bracko, O.; Choi, J.W.; Jia, Y.; Nana, A.L.; Brady, O.A.; Hernandez, J.C.C.; Nishimura, N.; Seeley, W.W.; et al. Impaired prosaposin lysosomal trafficking in frontotemporal lobar degeneration due to progranulin mutations. Nat. Commun. 2017, 8, 15277. [Google Scholar] [CrossRef]
- Raitano, S.; Ordovàs, L.; De Muynck, L.; Guo, W.; Espuny-Camacho, I.; Geraerts, M.; Khurana, S.; Vanuytsel, K.; Tóth, B.I.; Voets, T.; et al. Restoration of progranulin expression rescues cortical neuron generation in an induced pluripotent stem cell model of frontotemporal dementia. Stem Cell Rep. 2015, 4, 16–24. [Google Scholar] [CrossRef]
- Almeida, S.; Zhang, Z.; Coppola, G.; Mao, W.; Futai, K.; Karydas, A.; Geschwind, M.D.; Tartaglia, M.C.; Gao, F.; Gianni, D.; et al. Induced pluripotent stem cell models of progranulin-deficient frontotemporal dementia uncover specific reversible neuronal defects. Cell Rep. 2012, 2, 789–798. [Google Scholar] [CrossRef]
- Cenik, B.; Sephton, C.F.; Dewey, C.M.; Xian, X.; Wei, S.; Yu, K.; Niu, W.; Coppola, G.; Coughlin, S.E.; Lee, S.E.; et al. Suberoylanilide hydroxamic acid (vorinostat) up-regulates progranulin transcription: Rational therapeutic approach to frontotemporal dementia. J. Biol. Chem. 2011, 286, 16101–16108. [Google Scholar] [CrossRef]
- Almeida, S.; Gao, F.; Coppola, G.; Gao, F.B. Suberoylanilide hydroxamic acid increases progranulin production in iPSC-derived cortical neurons of frontotemporal dementia patients. Neurobiol. Aging 2016, 42, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.R.; Martins, S.; Cammarata, G.; Martins, M.; Cardoso, A.M.; Almeida, M.R.; do Carmo Macário, M.; Santana, I.; Peça, J.; Cardoso, A.L. Generation and Characterization ofel iPSC Lines from a Portuguese Family Bearing Heterozygous and Homozygous GRN Mutations. Biomedicines 2022, 10, 1905. [Google Scholar] [CrossRef] [PubMed]
- Holler, C.J.; Taylor, G.; Deng, Q.; Kukar, T. Intracellular Proteolysis of Progranulin Generates Stable, Lysosomal Granulins that Are Haploinsufficient in Patients with Frontotemporal Dementia Caused by GRN Mutations. eNeuro 2017, 4. [Google Scholar] [CrossRef]
- Ward, M.E.; Chen, R.; Huang, H.Y.; Ludwig, C.; Telpoukhovskaia, M.; Taubes, A.; Boudin, H.; Minami, S.S.; Reichert, M.; Albrecht, P.; et al. Individuals with progranulin haploinsufficiency exhibit features of neuronal ceroid lipofuscinosis. Sci. Transl. Med. 2017, 9, eaah5642. [Google Scholar] [CrossRef]
- Boland, S.; Swarup, S.; Ambaw, Y.A.; Malia, P.C.; Richards, R.C.; Fischer, A.W.; Singh, S.; Aggarwal, G.; Spina, S.; Nana, A.L.; et al. Deficiency of the frontotemporal dementia gene GRN results in gangliosidosis. Nat. Commun. 2022, 13, 5924. [Google Scholar] [CrossRef]
- Alquézar, C.; Esteras, N.; de la Encarnación, A.; Alzualde, A.; Moreno, F.; López de Munain, A.; Martín-Requero, A. PGRN haploinsufficiency increased Wnt5a signaling in peripheral cells from frontotemporal lobar degeneration-progranulin mutation carriers. Neurobiol. Aging 2014, 35, 886–898. [Google Scholar] [CrossRef]
- Alquézar, C.; de la Encarnación, A.; Moreno, F.; López de Munain, A.; Martín-Requero, Á. Progranulin deficiency induces overactivation of WNT5A expression via TNF-α/NF-κB pathway in peripheral cells from frontotemporal dementia-linked granulin mutation carriers. J. Psychiatry Neurosci. 2016, 41, 225–239. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, N.; Li, X.; Zheng, X.; Zhao, J.; Xia, H.; Mao, Q. Suppression of Progranulin Expression Leads to Formation of Intranuclear TDP-43 Inclusions In Vitro: A Cell Model of Frontotemporal Lobar Degeneration. J. Neuropathol. Exp. Neurol. 2019, 78, 1124–1129. [Google Scholar] [CrossRef]
- Filiano, A.J.; Martens, L.H.; Young, A.H.; Warmus, B.A.; Zhou, P.; Diaz-Ramirez, G.; Jiao, J.; Zhang, Z.; Huang, E.J.; Gao, F.B.; et al. Dissociation of frontotemporal dementia-related deficits and neuroinflammation in progranulin haploinsufficient mice. J. Neurosci. 2013, 33, 5352–5361. [Google Scholar] [CrossRef]
- Arrant, A.E.; Filiano, A.J.; Warmus, B.A.; Hall, A.M.; Roberson, E.D. Progranulin haploinsufficiency causes biphasic social dominance abnormalities in the tube test. Genes. Brain Behav. 2016, 15, 588–603. [Google Scholar] [CrossRef]
- Cook, A.K.; Greathouse, K.M.; Manuel, P.N.; Cooper, N.H.; Eberhardt, J.M.; Freeman, C.D.; Weber, A.J.; Herskowitz, J.H.; Arrant, A.E. Dendritic spine head diameter is reduced in the prefrontal cortex of progranulin haploinsufficient mice. Mol. Brain 2024, 17, 33. [Google Scholar] [CrossRef]
- Life, B.; Petkau, T.L.; Cruz, G.N.F.; Navarro-Delgado, E.I.; Shen, N.; Korthauer, K.; Leavitt, B.R. FTD-associated behavioural and transcriptomic abnormalities in ‘humanized’ progranulin-deficient mice: A novel model for progranulin-associated FTD. Neurobiol. Dis. 2023, 182, 106138. [Google Scholar] [CrossRef]
- Frew, J.; Nygaard, H.B. Neuropathological and behavioral characterization of aged Grn R493X progranulin-deficient frontotemporal dementia knockin mice. Acta Neuropathol. Commun. 2021, 9, 57. [Google Scholar] [CrossRef]
- Smith, D.M.; Aggarwal, G.; Niehoff, M.L.; Jones, S.A.; Banerjee, S.; Farr, S.A.; Nguyen, A.D. Biochemical, Biomarker, and Behavioral Characterization of the GrnR493X Mouse Model of Frontotemporal Dementia. Mol. Neurobiol. 2024, 61, 9708–9722. [Google Scholar] [CrossRef]
- Nguyen, A.D.; Nguyen, T.A.; Zhang, J.; Devireddy, S.; Zhou, P.; Karydas, A.M.; Xu, X.; Miller, B.L.; Rigo, F.; Ferguson, S.M.; et al. Murine knockin model for progranulin-deficient frontotemporal dementia with nonsense-mediated mRNA decay. Proc. Natl. Acad. Sci. USA 2018, 115, E2849–E2858. [Google Scholar] [CrossRef]
- Kaplelach, A.K.; Fox, S.N.; Cook, A.K.; Hall, J.A.; Dannemiller, R.S.; Jaunarajs, K.L.; Arrant, A.E. Regulation of extracellular progranulin in medial prefrontal cortex. Neurobiol. Dis. 2023, 188, 106326. [Google Scholar] [CrossRef]
- Minami, S.S.; Min, S.W.; Krabbe, G.; Wang, C.; Zhou, Y.; Asgarov, R.; Li, Y.; Martens, L.H.; Elia, L.P.; Ward, M.E.; et al. Progranulin protects against amyloid β deposition and toxicity in Alzheimer’s disease mouse models. Nat. Med. 2014, 20, 1157–1164. [Google Scholar] [CrossRef]
- Mendsaikhan, A.; Tooyama, I.; Walker, D.G. Microglial Progranulin: Involvement in Alzheimer’s Disease and Neurodegenerative Diseases. Cells 2019, 8, 230. [Google Scholar] [CrossRef]
- Hosokawa, M.; Tanaka, Y.; Arai, T.; Kondo, H.; Akiyama, H.; Hasegawa, M. Progranulin haploinsufficiency reduces amyloid beta deposition in Alzheimer’s disease model mice. Exp. Anim. 2018, 67, 63–70. [Google Scholar] [CrossRef]
- Arrant, A.E.; Nicholson, A.M.; Zhou, X.; Rademakers, R.; Roberson, E.D. Partial Tmem106b reduction does not correct abnormalities due to progranulin haploinsufficiency. Mol. Neurodegener. 2018, 13, 32. [Google Scholar] [CrossRef]
- Hosokawa, M.; Arai, T.; Masuda-Suzukake, M.; Kondo, H.; Matsuwaki, T.; Nishihara, M.; Hasegawa, M.; Akiyama, H. Progranulin reduction is associated with increased tau phosphorylation in P301L tau transgenic mice. J. Neuropathol. Exp. Neurol. 2015, 74, 158–165. [Google Scholar] [CrossRef]
- Reifschneider, A.; Robinson, S.; van Lengerich, B.; Gnörich, J.; Logan, T.; Heindl, S.; Vogt, M.A.; Weidinger, E.; Riedl, L.; Wind, K.; et al. Loss of TREM2 rescues hyperactivation of microglia, but not lysosomal deficits and neurotoxicity in models of progranulin deficiency. EMBO J. 2022, 41, e109108. [Google Scholar] [CrossRef]
- Tapia, L.; Milnerwood, A.; Guo, A.; Mills, F.; Yoshida, E.; Vasuta, C.; Mackenzie, I.R.; Raymond, L.; Cynader, M.; Jia, W.; et al. Progranulin deficiency decreases gross neural connectivity but enhances transmission at individual synapses. J. Neurosci. 2011, 31, 11126–11132. [Google Scholar] [CrossRef]
- Longhena, F.; Zaltieri, M.; Grigoletto, J.; Faustini, G.; La Via, L.; Ghidoni, R.; Benussi, L.; Missale, C.; Spano, P.; Bellucci, A. Depletion of Progranulin Reduces GluN2B-Containing NMDA Receptor Density, Tau Phosphorylation, and Dendritic Arborization in Mouse Primary Cortical Neurons. J. Pharmacol. Exp. Ther. 2017, 363, 164–175. [Google Scholar] [CrossRef]
- Galimberti, D.; Fenoglio, C.; Scarpini, E. Progranulin as a therapeutic target for dementia. Expert. Opin. Ther. Targets 2018, 22, 579–585. [Google Scholar] [CrossRef]
- Hu, F.; Padukkavidana, T.; Vægter, C.B.; Brady, O.A.; Zheng, Y.; Mackenzie, I.R.; Feldman, H.H.; Nykjaer, A.; Strittmatter, S.M. Sortilin-mediated endocytosis determines levels of the frontotemporal dementia protein, progranulin. Neuron 2010, 68, 654–667. [Google Scholar] [CrossRef]
- Terryn, J.; Verfaillie, C.M.; Van Damme, P. Tweaking Progranulin Expression: Therapeutic Avenues and Opportunities. Front. Mol. Neurosci. 2021, 14, 713031. [Google Scholar] [CrossRef]
- Chitramuthu, B.P.; Bennett, H.P.J.; Bateman, A. Progranulin: A new avenue towards the understanding and treatment of neurodegenerative disease. Brain 2017, 140, 3081–3104. [Google Scholar] [CrossRef]
- Arrant, A.E.; Filiano, A.J.; Unger, D.E.; Young, A.H.; Roberson, E.D. Restoring neuronal progranulin reverses deficits in a mouse model of frontotemporal dementia. Brain 2017, 140, 1447–1465. [Google Scholar] [CrossRef]
- Nicholson, A.M.; Gass, J.; Petrucelli, L.; Rademakers, R. Progranulin axis and recent developments in frontotemporal lobar degeneration. Alzheimers Res. Ther. 2012, 4, 4. [Google Scholar] [CrossRef]
- Sevigny, J.; Uspenskaya, O.; Heckman, L.D.; Wong, L.C.; Hatch, D.A.; Tewari, A.; Vandenberghe, R.; Irwin, D.J.; Saracino, D.; Le Ber, I.; et al. Progranulin AAV gene therapy for frontotemporal dementia: Translational studies and phase 1/2 trial interim results. Nat. Med. 2024, 30, 1406–1415. [Google Scholar] [CrossRef]
- Arrant, A.E.; Onyilo, V.C.; Unger, D.E.; Roberson, E.D. Progranulin Gene Therapy Improves Lysosomal Dysfunction and Microglial Pathology Associated with Frontotemporal Dementia and Neuronal Ceroid Lipofuscinosis. J. Neurosci. 2018, 38, 2341–2358. [Google Scholar] [CrossRef]
- Banzhaf-Strathmann, J.; Claus, R.; Mücke, O.; Rentzsch, K.; van der Zee, J.; Engelborghs, S.; De Deyn, P.P.; Cruts, M.; van Broeckhoven, C.; Plass, C.; et al. Promoter DNA methylation regulates progranulin expression and is altered in FTLD. Acta Neuropathol. Commun. 2013, 1, 16. [Google Scholar] [CrossRef]
- Moreno-Yruela, C.; Fass, D.M.; Cheng, C.; Herz, J.; Olsen, C.A.; Haggarty, S.J. Kinetic Tuning of HDAC Inhibitors Affords Potent Inducers of Progranulin Expression. ACS Chem. Neurosci. 2019, 10, 3769–3777. [Google Scholar] [CrossRef]
- Rosenthal, Z.C.; Fass, D.M.; Payne, N.C.; She, A.; Patnaik, D.; Hennig, K.M.; Tesla, R.; Werthmann, G.C.; Guhl, C.; Reis, S.A.; et al. Epigenetic modulation through BET bromodomain inhibitors as a novel therapeutic strategy for progranulin-deficient frontotemporal dementia. Sci. Rep. 2024, 14, 9064. [Google Scholar] [CrossRef]
- Dabrowski, M.; Bukowy-Bieryllo, Z.; Zietkiewicz, E. Advances in therapeutic use of a drug-stimulated translational readthrough of premature termination codons. Mol. Med. 2018, 24, 25. [Google Scholar] [CrossRef]
- Martins-Dias, P.; Romão, L. Nonsense suppression therapies in human genetic diseases. Cell Mol. Life Sci. 2021, 78, 4677–4701. [Google Scholar] [CrossRef]
- Tsai, R.M.; Boxer, A.L. Therapy and clinical trials in frontotemporal dementia: Past, present, and future. J. Neurochem. 2016, 138 (Suppl. S1), 211–221. [Google Scholar] [CrossRef]
- Alberici, A.; Archetti, S.; Pilotto, A.; Premi, E.; Cosseddu, M.; Bianchetti, A.; Semeraro, F.; Salvetti, M.; Muiesan, M.L.; Padovani, A.; et al. Results from a pilot study on amiodarone administration in monogenic frontotemporal dementia with granulin mutation. Neurol. Sci. 2014, 35, 1215–1219. [Google Scholar] [CrossRef]
- Capell, A.; Liebscher, S.; Fellerer, K.; Brouwers, N.; Willem, M.; Lammich, S.; Gijselinck, I.; Bittner, T.; Carlson, A.M.; Sasse, F.; et al. Rescue of progranulin deficiency associated with frontotemporal lobar degeneration by alkalizing reagents and inhibition of vacuolar ATPase. J. Neurosci. 2011, 31, 1885–1894. [Google Scholar] [CrossRef]
- Holler, C.J.; Taylor, G.; McEachin, Z.T.; Deng, Q.; Watkins, W.J.; Hudson, K.; Easley, C.A.; Hu, W.T.; Hales, C.M.; Rossoll, W.; et al. Trehalose upregulates progranulin expression in human and mouse models of GRN haploinsufficiency: A novel therapeutic lead to treat frontotemporal dementia. Mol. Neurodegener. 2016, 11, 46. [Google Scholar] [CrossRef]
- Chen, A.; Gibney, P.A. Dietary Trehalose as a Bioactive Nutrient. Nutrients 2023, 15, 1393. [Google Scholar] [CrossRef]
- Lee, W.C.; Almeida, S.; Prudencio, M.; Caulfield, T.R.; Zhang, Y.J.; Tay, W.M.; Bauer, P.O.; Chew, J.; Sasaguri, H.; Jansen-West, K.R.; et al. Targeted manipulation of the sortilin-progranulin axis rescues progranulin haploinsufficiency. Hum. Mol. Genet. 2014, 23, 1467–1478. [Google Scholar] [CrossRef]
- Miyakawa, S.; Sakuma, H.; Warude, D.; Asanuma, S.; Arimura, N.; Yoshihara, T.; Tavares, D.; Hata, A.; Ida, K.; Hori, Y.; et al. Anti-sortilin1 Antibody Up-Regulates Progranulin via Sortilin1 Down-Regulation. Front. Neurosci. 2020, 14, 586107. [Google Scholar] [CrossRef]
- Götzl, J.K.; Brendel, M.; Werner, G.; Parhizkar, S.; Monasor, L.S.; Kleinberger, G.; Colombo, A.; Deussing, M.; Wagner, M.; Winkelmann, J.; et al. Opposite microglial activation stages upon loss of PGRN or TREM 2 result in reduced cerebral glucose metabolism. EMBO Mol. Med. 2019, 11, e9711. [Google Scholar] [CrossRef]
- Aggarwal, G.; Banerjee, S.; Jones, S.A.; Benchaar, Y.; Bélanger, J.; Sévigny, M.; Smith, D.M.; Niehoff, M.L.; Pavlack, M.; de Vera, I.M.S.; et al. Antisense oligonucleotides targeting the miR-29b binding site in the GRN mRNA increase progranulin translation. J. Biol. Chem. 2023, 299, 105475. [Google Scholar] [CrossRef]
- Cao, Z.; He, W.; Hu, R.; Chen, Y.; Xue, J.; Gao, F.; Lan, Y. Honokiol rescues progranulin deficiency in pathogen-induced and genetically driven neurodegeneration: Bridging veterinary models with therapeutic development. Vet. Microbiol. 2025, 307, 110616. [Google Scholar] [CrossRef]
- Fried, L.E.; Arbiser, J.L. Honokiol, a multifunctional antiangiogenic and antitumor agent. Antioxid. Redox Signal. 2009, 11, 1139–1148. [Google Scholar] [CrossRef]
- Yao, X.; Qin, R.; Cui, Z.; He, D.; Sun, X.; Sun, Y.; He, X. Effect of overexpression of GRN on the proliferation and osteogenic capacity of human periodontal cells. Exp. Ther. Med. 2024, 29, 33. [Google Scholar] [CrossRef]
- Kusakari, S.; Suzuki, H.; Nawa, M.; Sudo, K.; Yamazaki, R.; Miyagi, T.; Ohara, T.; Matsuoka, M.; Kanekura, K. Excessive expression of progranulin leads to neurotoxicity rather than neuroprotection. Neurobiol. Dis. 2025, 209, 106895. [Google Scholar] [CrossRef]
- Huang, M.; Modeste, E.; Dammer, E.; Merino, P.; Taylor, G.; Duong, D.M.; Deng, Q.; Holler, C.J.; Gearing, M.; Dickson, D.; et al. Network analysis of the progranulin-deficient mouse brain proteome reveals pathogenic mechanisms shared in human frontotemporal dementia caused by GRN mutations. Acta Neuropathol. Commun. 2020, 8, 163. [Google Scholar] [CrossRef]
- Carecchio, M.; Fenoglio, C.; De Riz, M.; Guidi, I.; Comi, C.; Cortini, F.; Venturelli, E.; Restelli, I.; Cantoni, C.; Bresolin, N.; et al. Progranulin plasma levels as potential biomarker for the identification of GRN deletion carriers. A case with atypical onset as clinical amnestic Mild Cognitive Impairment converted to Alzheimer’s disease. J. Neurol. Sci. 2009, 287, 291–293. [Google Scholar] [CrossRef]
- Meeter, L.H.; Patzke, H.; Loewen, G.; Dopper, E.G.; Pijnenburg, Y.A.; van Minkelen, R.; van Swieten, J.C. Progranulin Levels in Plasma and Cerebrospinal Fluid in Granulin Mutation Carriers. Dement. Geriatr. Cogn. Dis. Extra 2016, 6, 330–340. [Google Scholar] [CrossRef]
- Paushter, D.H.; Du, H.; Feng, T.; Hu, F. The lysosomal function of progranulin, a guardian against neurodegeneration. Acta Neuropathol. 2018, 136, 1–17. [Google Scholar] [CrossRef]
- Gaweda-Walerych, K.; Aragona, V.; Lodato, S.; Sitek, E.J.; Narożańska, E.; Buratti, E. Progranulin deficiency in the brain: The interplay between neuronal and non-neuronal cells. Transl. Neurodegener. 2025, 14, 18. [Google Scholar] [CrossRef]
- Shi, Y.; Hou, W.; Li, B.; Zhu, C. PGRN as an emerging regulator of lipid metabolism in neurodegenerative diseases. Commun. Biol. 2025, 8, 844. [Google Scholar] [CrossRef]
- Kayasuga, Y.; Chiba, S.; Suzuki, M.; Kikusui, T.; Matsuwaki, T.; Yamanouchi, K.; Kotaki, H.; Horai, R.; Iwakura, Y.; Nishihara, M. Alteration of behavioural phenotype in mice by targeted disruption of the progranulin gene. Behav. Brain Res. 2007, 185, 110–118. [Google Scholar] [CrossRef]
- Yin, F.; Banerjee, R.; Thomas, B.; Zhou, P.; Qian, L.; Jia, T.; Ma, X.; Ma, Y.; Iadecola, C.; Beal, M.F.; et al. Exaggerated inflammation, impaired host defense, and neuropathology in progranulin-deficient mice. J. Exp. Med. 2010, 207, 117–128. [Google Scholar] [CrossRef]
- Yin, F.; Dumont, M.; Banerjee, R.; Ma, Y.; Li, H.; Lin, M.T.; Beal, M.F.; Nathan, C.; Thomas, B.; Ding, A. Behavioral deficits and progressive neuropathology in progranulin-deficient mice: A mouse model of frontotemporal dementia. FASEB J. 2010, 24, 4639–4647. [Google Scholar] [CrossRef] [PubMed]
- Kleinberger, G.; Capell, A.; Haass, C.; Van Broeckhoven, C. Mechanisms of granulin deficiency: Lessons from cellular and animal models. Mol. Neurobiol. 2013, 47, 337–360. [Google Scholar] [CrossRef]
- Reich, M.; Simon, M.J.; Polke, B.; Paris, I.; Werner, G.; Schrader, C.; Spieth, L.; Davis, S.S.; Robinson, S.; de Melo, G.L.; et al. Peripheral expression of brain-penetrant progranulin rescues pathologies in mouse models of frontotemporal lobar degeneration. Sci. Transl. Med. 2024, 16, eadj7308. [Google Scholar] [CrossRef]
- Kashyap, S.N.; Boyle, N.R.; Roberson, E.D. Preclinical Interventions in Mouse Models of Frontotemporal Dementia Due to Progranulin Mutations. Neurotherapeutics 2023, 20, 140–153. [Google Scholar] [CrossRef]
- Boddaert, J.; Wils, H.; Kumar-Singh, S. Methods to Investigate the Molecular Basis of Progranulin Actions on Brain and Behavior In Vivo Using Knockout Mice. Methods Mol. Biol. 2018, 1806, 233–253. [Google Scholar] [CrossRef]
- Rhinn, H.; Tatton, N.; McCaughey, S.; Kurnellas, M.; Rosenthal, A. Progranulin as a therapeutic target in neurodegenerative diseases. Trends Pharmacol. Sci. 2022, 43, 641–652. [Google Scholar] [CrossRef]
- Cui, Y.; Hettinghouse, A.; Liu, C.J. Progranulin: A conductor of receptors orchestra, a chaperone of lysosomal enzymes and a therapeutic target for multiple diseases. Cytokine Growth Factor Rev. 2019, 45, 53–64. [Google Scholar] [CrossRef]
- Boylan, M.A.; Pincetic, A.; Romano, G.; Tatton, N.; Kenkare-Mitra, S.; Rosenthal, A. Targeting Progranulin as an Immuno-Neurology Therapeutic Approach. Int. J. Mol. Sci. 2023, 24, 15946. [Google Scholar] [CrossRef]
- Gass, J.; Prudencio, M.; Stetler, C.; Petrucelli, L. Progranulin: An emerging target for FTLD therapies. Brain Res. 2012, 1462, 118–128. [Google Scholar] [CrossRef]
- Cenik, B.; Sephton, C.F.; Cenik, B.K.; Herz, J.; Yu, G. Progranulin: A proteolytically processed protein at the crossroads of inflammation and neurodegeneration. J. Biol. Chem. 2012, 287, 32298–32306. [Google Scholar] [CrossRef]
- Elia, L.P.; Reisine, T.; Alijagic, A.; Finkbeiner, S. Approaches to develop therapeutics to treat frontotemporal dementia. Neuropharmacology 2020, 166, 107948. [Google Scholar] [CrossRef] [PubMed]
- Boxer, A.L.; Gold, M.; Huey, E.; Gao, F.; Burton, E.A.; Chow, T.; Kao, A.; Leavitt, B.R.; Lamb, B.; Grether, M.; et al. Frontotemporal degeneration, the next therapeutic frontier: Molecules and animal models for frontotemporal degeneration drug development. Alzheimer’s Dement. 2012, 9, 176–188. [Google Scholar] [CrossRef] [PubMed]
- Root, J.; Mendsaikhan, A.; Taylor, G.; Merino, P.; Nandy, S.; Wang, M.; Araujo, L.T.; Ryu, D.; Holler, C.; Thompson, B.M.; et al. Granulins rescue inflammation, lysosome dysfunction, lipofuscin, and neuropathology in a mouse model of progranulin deficiency. Cell Rep. 2024, 43, 114985. [Google Scholar] [CrossRef]
- Buccellato, F.R.; D’anca, M.; Tartaglia, G.M.; Del Fabbro, M.; Galimberti, D. Frontotemporal dementia: From genetics to therapeutic approaches. Expert Opin. Investig. Drugs 2024, 33, 561–573. [Google Scholar] [CrossRef]
- Simon, M.J.; Logan, T.; DeVos, S.L.; Di Paolo, G. Lysosomal functions of progranulin and implications for treatment of frontotemporal dementia. Trends Cell Biol. 2022, 33, 324–339. [Google Scholar] [CrossRef]
- Available online: http://clinicaltrials.eu/trial/study-on-avb-101-for-patients-with-frontotemporal-dementia-with-progranulin-mutations/ (accessed on 30 September 2025).
- Available online: https://www.clinicaltrials.gov/study/NCT04747431?tab=table#trial-description (accessed on 30 September 2025).
- Available online: https://www.clinicaltrials.gov/study/NCT04747431 (accessed on 30 September 2025).
- Voss, T.; Triglia, P.; Ni, Y.G.; Browne, S.E.; Chou, W.; Ducharme, S.; Irwin, D.J.; Santana, I. Interim Safety and Biomarker Data From upliFT-D Trial of PBFT02 in FTD with GRN Mutations, Alzheimer’s Association International Conference (AAIC). Available online: https://s203.q4cdn.com/877117837/files/doc_events/2025/Jul/30/Passage-Bio_AAIC-2025_UPLIFT-D-Poster-114x114cm-S03-DIGITAL.pdf (accessed on 30 September 2025).
- Wang, C.; Pan, C.; Yong, H.; Wang, F.; Bo, T.; Zhao, Y.; Ma, B.; He, W.; Li, M. Emerging non-viral vectors for gene delivery. J. Nanobiotechnol. 2023, 21, 272. [Google Scholar] [CrossRef]
- Wilar, G.; Suhandi, C.; Wathoni, N.; Fukunaga, K.; Kawahata, I. Nanoparticle-Based Drug Delivery Systems Enhance Treatment of Cognitive Defects. Int. J. Nanomed. 2024, 19, 11357–11378. [Google Scholar] [CrossRef]
- Yan, D.; Ouyang, W.; Lin, J.; Liu, Z. Smart coating by thermo-sensitive Pluronic F-127 for enhanced corneal healing via delivery of biological macromolecule progranulin. Int. J. Biol. Macromol. 2023, 253 Pt 8, 127586. [Google Scholar] [CrossRef]
- Tesla, R.; Guhl, C.; Werthmann, G.C.; Dixon, D.; Cenik, B.; Addepalli, Y.; Liang, J.; Fass, D.M.; Rosenthal, Z.; Haggarty, S.J.; et al. Benzoxazole-derivatives enhance progranulin expression and reverse the aberrant lysosomal proteome caused by GRN haploinsufficiency. Nat. Commun. 2024, 15, 6125. [Google Scholar] [CrossRef]
- Verwaerde, P.; Estrella, C.; Burlet, S.; Barrier, M.; Marotte, A.A.; Clincke, G. First-In-Human Safety, Tolerability, and Pharmacokinetics of Single and Multiple Doses of AZP2006, A Synthetic Compound for the Treatment of Alzheimer’s Disease and Related Diseases. J. Alzheimers Dis. 2024, 98, 715–727. [Google Scholar] [CrossRef]
- Callizot, N.; Estrella, C.; Burlet, S.; Henriques, A.; Brantis, C.; Barrier, M.; Campanari, M.L.; Verwaerde, P. AZP2006, a new promising treatment for Alzheimer’s and related diseases. Sci. Rep. 2021, 11, 16806. [Google Scholar] [CrossRef]
- Kurnellas, M.; Mitra, A.; Schwabe, T.; Paul, R.; Arrant, A.E.; Roberson, E.D.; Ward, M.; Yeh, F.; Long, H.; Rosenthal, A. Latozinemab, a novel progranulin-elevating therapy for frontotemporal dementia. J. Transl. Med. 2023, 21, 387. [Google Scholar] [CrossRef] [PubMed]
- Allemailem, K.S.; Almatroudi, A.; Rahmani, A.H.; Alrumaihi, F.; Alradhi, A.E.; Alsubaiyel, A.M.; Algahtani, M.; Almousa, R.M.; Mahzari, A.; Sindi, A.A.A.; et al. Recent Updates of the CRISPR/Cas9 Genome Editing System:el Approaches to Regulate Its Spatiotemporal Control by Genetic and Physicochemical Strategies. Int. J. Nanomed. 2024, 19, 5335–5363. [Google Scholar] [CrossRef]
- Garrow, D.H.; Crocker, D.R. The effects of sex, birth order and admission to a special care baby unit on the fear of strangers reaction of infants. Dev. Med. Child. Neurol. 1985, 27, 628–634. [Google Scholar] [CrossRef]
- Ali, A.; Rahman, M.Y.; Sheikh, D. The Role of CRISPR/Cas9 in Revolutionizing Duchenne’s Muscular Dystrophy Treatment: Opportunities and Obstacles. Glob. Med. Genet. 2024, 11, 349–357. [Google Scholar] [CrossRef]
- Yun, Y.; Ha, Y. CRISPR/Cas9-Mediated Gene Correction to Understand ALS. Int. J. Mol. Sci. 2020, 21, 3801. [Google Scholar] [CrossRef]
- Kim, H.; Han, J.H.; Kim, H.; Kim, M.; Jo, S.I.; Lee, N.; Cha, S.; Oh, M.J.; Choi, G.; Kim, H.S. CRISPR/Cas9 targeting of passenger single nucleotide variants in haploinsufficient or essential genes expands cancer therapy prospects. Sci. Rep. 2024, 14, 7436. [Google Scholar] [CrossRef]
- Matharu, N.; Rattanasopha, S.; Tamura, S.; Maliskova, L.; Wang, Y.; Bernard, A.; Hardin, A.; Eckalbar, W.L.; Vaisse, C.; Ahituv, N. CRISPR-mediated activation of a promoter or enhancer rescues obesity caused by haploinsufficiency. Science 2019, 363, eaau0629. [Google Scholar] [CrossRef]
- Sen, T.; Thummer, R.P. CRISPR and iPSCs: Recent Developments and Future Perspectives in Neurodegenerative Disease Modelling, Research, and Therapeutics. Neurotox. Res. 2022, 40, 1597–1623. [Google Scholar] [CrossRef]
- Raffaele, I.; Cipriano, G.L.; Anchesi, I.; Oddo, S.; Silvestro, S. CRISPR/Cas9 and iPSC-Based Therapeutic Approaches in Alzheimer’s Disease. Antioxidants 2025, 14, 781. [Google Scholar] [CrossRef]
- Boxer, A.L.; Gold, M.; Feldman, H.; Boeve, B.F.; Dickinson, S.L.; Fillit, H.; Ho, C.; Paul, R.; Pearlman, R.; Sutherland, M.; et al. New directions in clinical trials for frontotemporal lobar degeneration: Methods and outcome measures. Alzheimers Dement. 2020, 16, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Galimberti, D.; Fumagalli, G.G.; Fenoglio, C.; Cioffi, S.M.G.; Arighi, A.; Serpente, M.; Borroni, B.; Padovani, A.; Tagliavini, F.; Masellis, M.; et al. Progranulin plasma levels predict the presence of GRN mutations in asymptomatic subjects and do not correlate with brain atrophy: Results from the GENFI study. Neurobiol. Aging 2018, 62, e9–e245. [Google Scholar] [CrossRef] [PubMed]
- Licata, A.; Grimmer, T.; Winkelmann, J.; Wagner, M.; Goldhardt, O.; Riedl, L.; Roßmeier, C.; Yakushev, I.; Diehl-Schmid, J. Variability of clinical syndromes and cerebral glucose metabolism in symptomatic frontotemporal lobar degeneration associated with progranulin mutations. Amyotroph. Lateral Scler. Front. Degener. 2020, 21, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, R.; Forabosco, P.; Vandrovcova, J.; Botía, J.A.; Guelfi, S.; Warren, J.D.; UK Brain Expression Consortium (UKBEC); Momeni, P.; Weale, M.E.; Ryten, M.; et al. Frontotemporal dementia: Insights into the biological underpinnings of disease through gene co-expression network analysis. Mol. Neurodegener. 2016, 11, 21. [Google Scholar] [CrossRef]
- Pottier, C.; Zhou, X.; Perkerson, R.B., 3rd; Baker, M.; Jenkins, G.D.; Serie, D.J.; Ghidoni, R.; Benussi, L.; Binetti, G.; López de Munain, A.; et al. Potential genetic modifiers of disease risk and age at onset in patients with frontotemporal lobar degeneration and GRN mutations: A genome-wide association study. Lancet Neurol. 2018, 17, 548–558. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Committee on Assessing Genetic Risks. In Assessing Genetic Risks: Implications for Health and Social Policy; Social, Legal, and Ethical Implications of Genetic Testing. Motulsky, A.G., Holtzman, N.A., Fullarton, J.E., Andrews, L.B., Eds.; National Academies Press (US): Washington, DC, USA, 1994; Volume 8. Available online: https://www.ncbi.nlm.nih.gov/books/NBK236044/ (accessed on 30 September 2025).
- Shah, S.K.; Hull, S.C.; Spinner, M.A.; Berkman, B.E.; Sanchez, L.A.; Abdul-Karim, R.; Hsu, A.P.; Claypool, R.; Holland, S.M. What does the duty to warn require? Am. J. Bioeth. 2013, 13, 62–63. [Google Scholar] [CrossRef]
- Coustasse, A.; Pekar, A.; Sikula, A.; Lurie, S. Ethical considerations of genetic presymptomatic testing for Huntington’s disease. J. Hosp. Mark. Public Relations 2009, 19, 129–141. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Uchida, E.; Okada, T.; Ozawa, K.; Onodera, M.; Kume, A.; Shimada, T.; Takahashi, S.; Tani, K.; Nasu, Y.; et al. Aspects of Gene Therapy Products Using Current Genome-Editing Technology in Japan. Hum. Gene Ther. 2020, 31, 1043–1053. [Google Scholar] [CrossRef]
- Benussi, A.; Gazzina, S.; Premi, E.; Cosseddu, M.; Archetti, S.; Dell’Era, V.; Cantoni, V.; Cotelli, M.S.; Alberici, A.; Micheli, A.; et al. Clinical and biomarker changes in presymptomatic genetic frontotemporal dementia. Neurobiol. Aging 2019, 76, 133–140. [Google Scholar] [CrossRef]
- Wertz, D.C. Human Genetics: Choice and Responsibility. Am. J. Hum. Genet. 1998, 62, 474–483. [Google Scholar]
- Gee, M.S.; Kwon, E.; Song, M.H.; Jeon, S.H.; Kim, N.; Lee, J.K.; Koo, T. CRISPR base editing-mediated correction of a tau mutation rescues cognitive decline in a mouse model of tauopathy. Transl. Neurodegener. 2024, 13, 21. [Google Scholar] [CrossRef]
- Khan, H.; Riaz, H.; Ahmed, A.; Kiyani, M.M.; Jawad, S.M.; Ud Din Shah, S.S.; Abualait, T.; Al-Hussain, F.; Li, H.T.; Bashir, S. CRISPR/Cas9 a genomic engineering technology for treatment in ALS mouse models. Regen. Ther. 2025, 30, 575–583. [Google Scholar] [CrossRef]
- Kempthorne, L.; Vaizoglu, D.; Cammack, A.J.; Carcolé, M.; Roberts, M.J.; Mikheenko, A.; Fisher, A.; Suklai, P.; Muralidharan, B.; Kroll, F.; et al. Dual-targeting CRISPR-CasRx reduces C9orf72 ALS/FTD sense and antisense repeat RNAs in vitro and in vivo. Nat. Commun. 2025, 16, 459. [Google Scholar] [CrossRef]

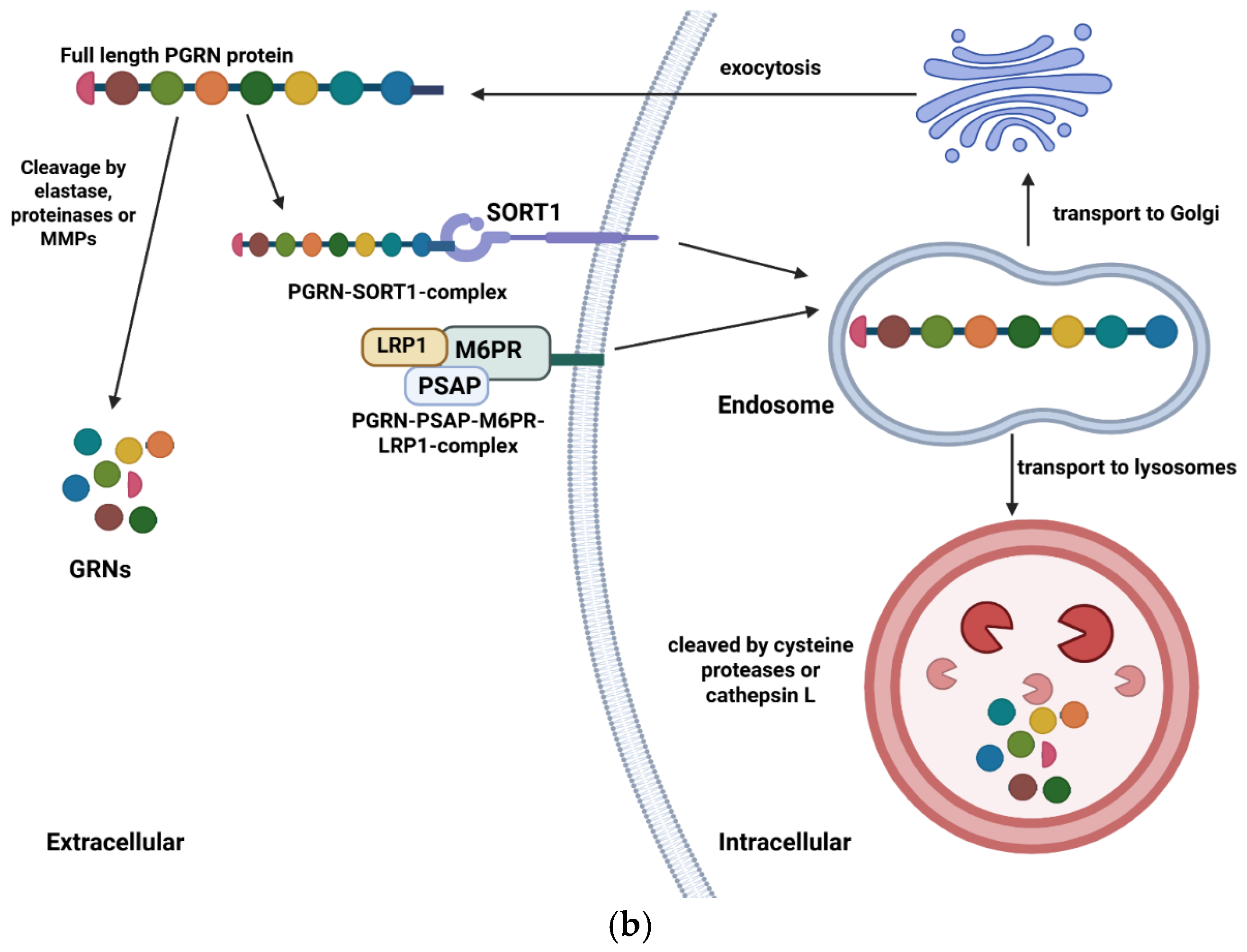
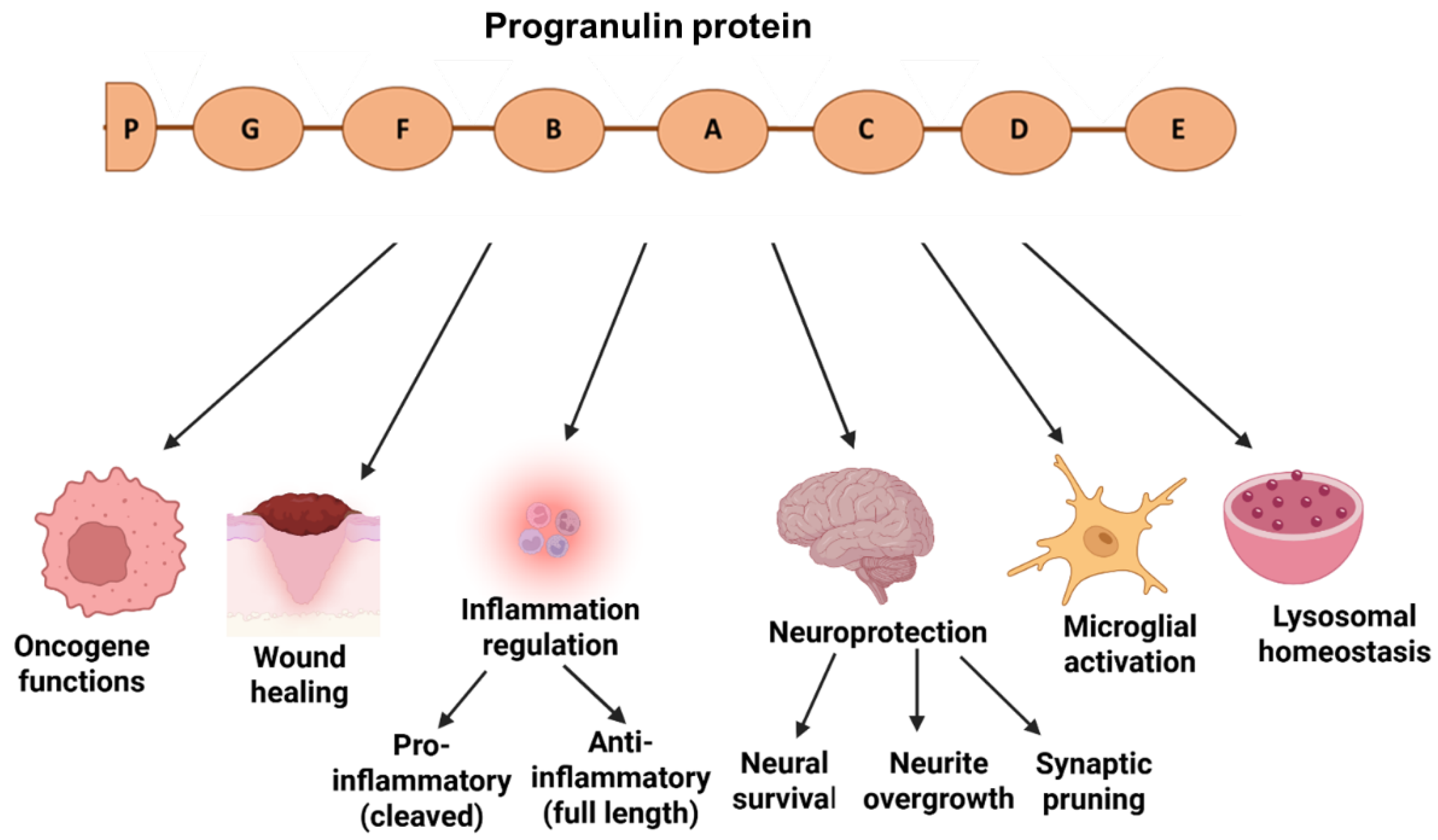
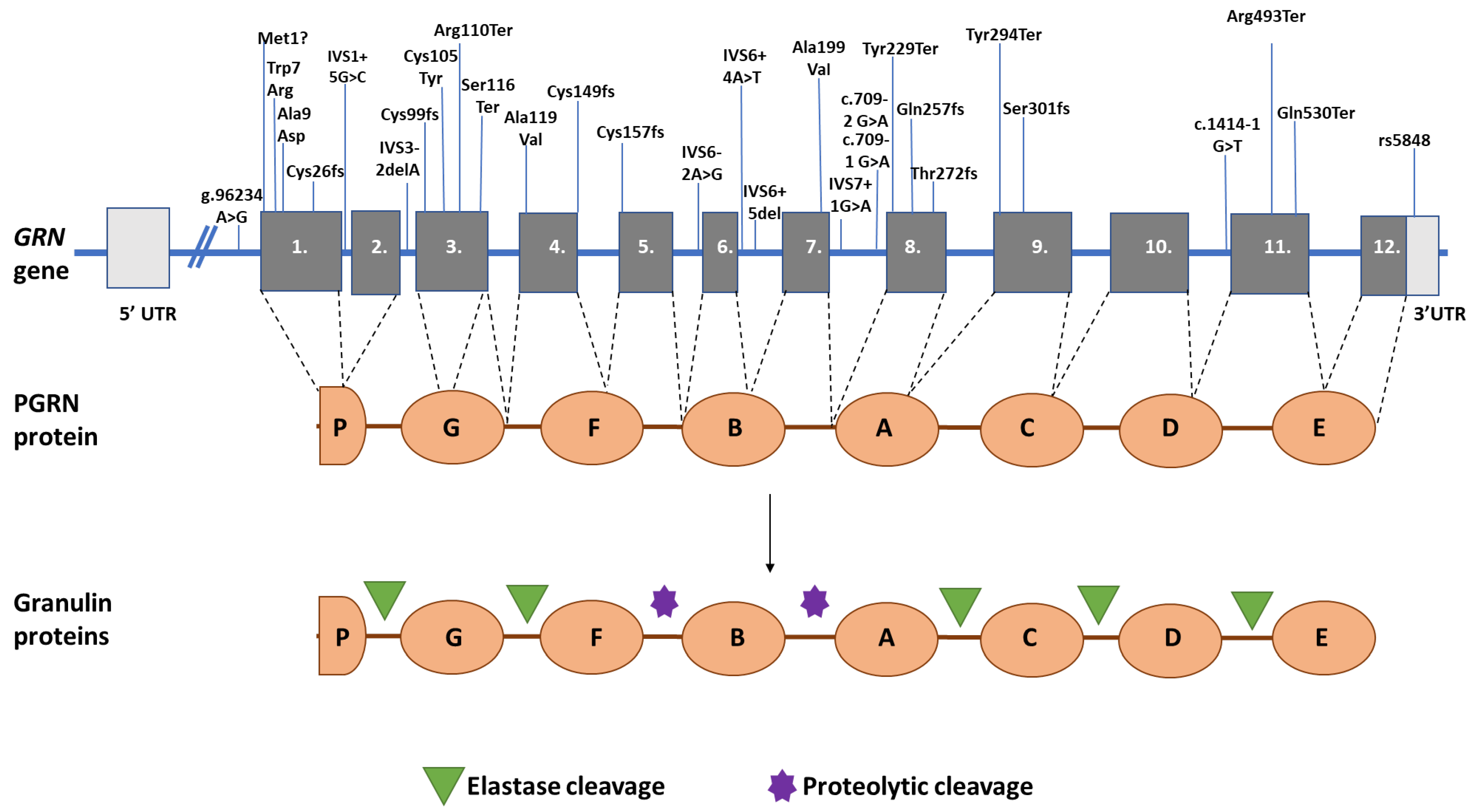
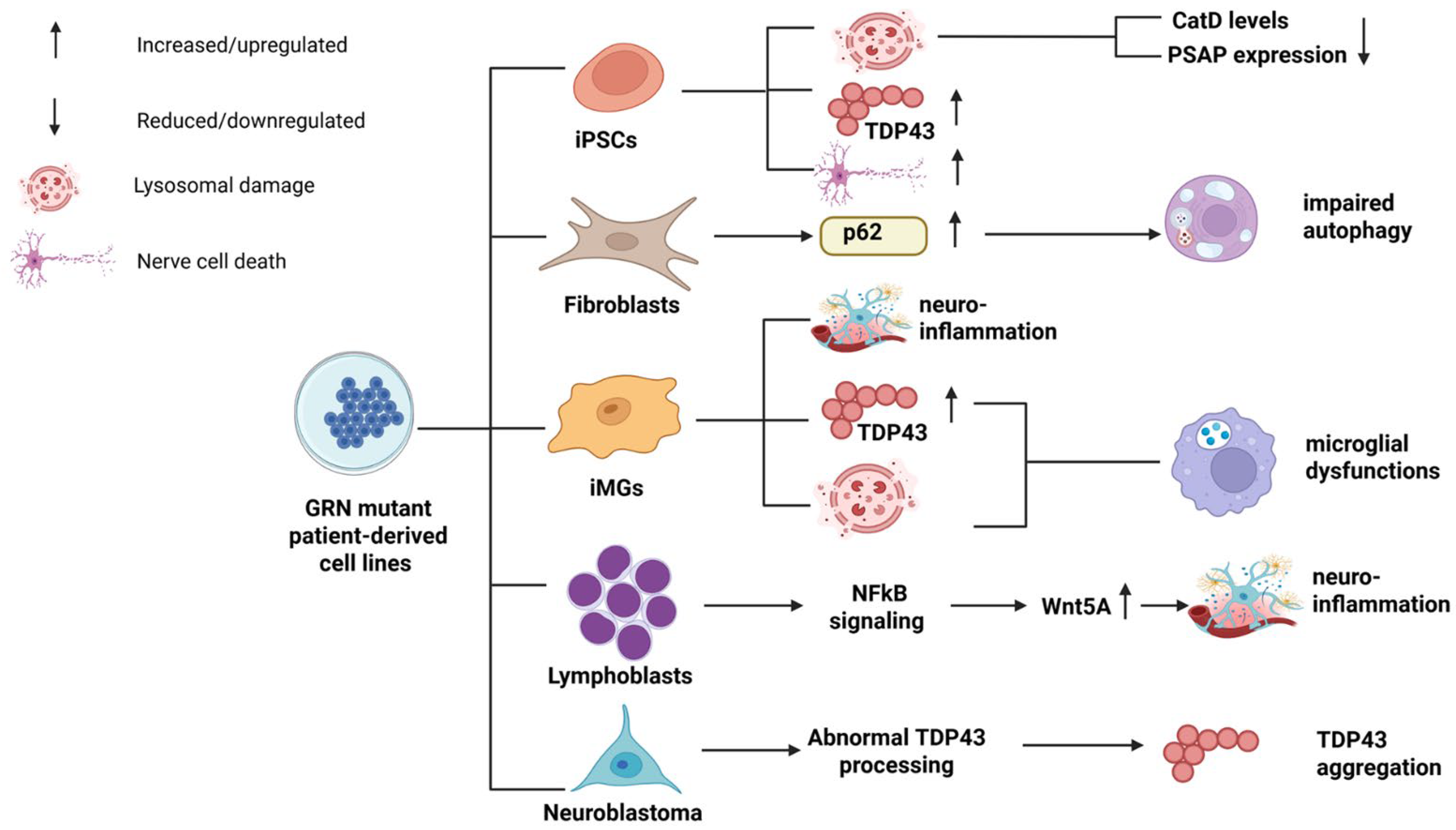
| Type of Mutation | Mutation | FTD Phenotypes | AOO | Family History | Imaging | Reference |
|---|---|---|---|---|---|---|
| Start codon loss | c.1A > G, p.Met1? | Language or behavioral disorders, depression, bulimia | 57 | + | NA | [41] |
| Splice site | g. 96234 A > G | apathy, loss of interests, delusions, attention deficits, and language impairment | 69 | + | Asymmetric cerebral atrophy in the right hemisphere, frontal temporal lobe | [52] |
| IVS1 + 5G > C | Primary progressive aphasia or behavioral FTD | 45–70 | +/− | TDP43 positivity | [53,54] | |
| IVS3−2delA | Language and memory dysfunctions | 63 | + | Brain glucose hypometabolism in several brain areas | [55] | |
| IVS6−2A > G | Language, behavioral, and memory impairment | 50–68 | + | Ubiquitin-positive FTD | [56] | |
| IVS6 + 5_8delGTGA | Apathy, social withdrawal, depression, and language impairment | 54 | + | Mild diffuse cortical atrophy | [57] | |
| 708 + 4A > T | Apathy, language impairment, and executive dysfunctions | 69 | + | Diffuse cortical atrophy in the left hemisphere hypometabolism involving the frontal, parietal, and temporal cortices | [58] | |
| IVS7 + 1G- > A | Corticobasal syndrome | 62 | + | Asymmetric hemispheric cortical atrophy and ventricular dilatation | [59] | |
| c.709−1 G > A | bvFTD, PNFA, AD-or PD-like symptoms | 42–71 | +/− | Gray matter loss in the frontal and parietal lobes, parietal atrophy | [60,61] | |
| c.709−2 A > T | Behavioral changes, apathy, disinhibition, aggression, disinhibition, ADS | 43–80 | + | Bilateral frontal atrophy | [62] | |
| c.1414−1G > T | bvFTD, apathy, perseverative behavior, hyperorality, aphasia, AD-like symptoms | 59–69 | −/+ | Hypoperfusion in the frontotemporal lobes/ parietal cortices | [38] | |
| Frameshift | Cys26Serfs*28 | Language impairment, minor motor impairment | 63 | NA | Left-sided frontotemporal atrophy | [63] |
| Cys99Profs*15 | Motor, language, and cognitive dysfunctions, behavioral issues | 57–63 | + | Cortical atrophy | [64] | |
| Cys149fs*10 | Language deficits, apathy, emotional lability, anhedonia, depressive mood, and anosognosia | 60s | + | Asymmetric frontal 114 and temporal cortical atrophy | [65] | |
| Cys157Lysfs*97 | bvFTD nonfluent/agrammatic variant of primary progressive aphasia | 50s | +/− | Hypometabolism in the left medial temporal cortex, frontal cortex, and posterior cingulate | [66] | |
| Gln257fs | Memory dysfunctions, language impairment | 67 | − | Asymmetrical, right-dominant frontoparietal atrophy | [67] | |
| Thr272fs | Behavioral variant FTD | 53–63 | +/− | Asymmetric atrophy, frontal lobes | [68] | |
| Ser301Cysfs*61 | bvFTD, corticobasal syndrome, PNFA | 53–60 | + | NA | [40,69] | |
| Stopgain | Arg110Ter | Motor and language impairment, or bvFTD | 50–60s | + | Atrophy and hypometabolism in frontal temporal areas | [70] |
| Ser116Ter | Typical FTD | 57 | + | NA | [39] | |
| Tyr229Ter | Dyspraxia, dysgraphia, dysphasia, hemiparesis, and depression | 60 | + | Mild atrophy predominantly in parietal and frontal regions, TDP43-positive inclusions | [71] | |
| Tyr294Ter | PNFA or bvFTD | 54–70 | + | TDP43 positivity possible, frontal lobes atrophy on the left side | [72,73] | |
| Arg493Ter | Bv FTD, PPA, memory-and executive impairment | 44–69 | +/− | Atrophy, hypometabolism, or hypoperfusion in the frontal/frontotemporal lobes | [51,74] | |
| Gln530Ter | bvFTD | 70s | + | NA | [75] | |
| Missense | Trp7Arg | bvFTD, apathy, diet change, impaired attention, language, and memory | 53 | + | Asymmetrical frontal, temporal, and parietal atrophy and hypometabolism | [43,44] |
| Ala9Asp | FTD with behavior and language deficits | 52–77 | + | Ubiquitin positive, Tau negative, atrophic hippocampus may be possible | [44,76] | |
| Cys105Tyr | Psychomotor agitation, motor, and memory impairment | 70s | + | Hypometabolism in the left frontotemporal lobe | [77] | |
| Ala119Val | Language and motor impairment, behavioral issues | 61–66 | + | Bilateral frontal and parietal cortical atrophy | [78] | |
| Ala199Val | Typical FTD | 62 | + | NA | [77] |
| Therapeutic Approach | Mechanisms | Benefits | Limitations | References |
|---|---|---|---|---|
| Gene Therapy (PR006, AVB-101, PBFT02) | Delivery of functional GRN via AAV vectors | Long-term expression of GRN Tolerable in patients | Invasive methods needed (e.g., intracranial injection), risk for off-target or abnormal immune response | [122,123,124,125,166,167,168,169,170] |
| Non-viral Delivery (e.g., lipid nanoparticles, hydrogels) | Non-viral GRN gene/protein delivery | Non-invasive methods, easier to release | Not tested for GRN yet; delivery efficiency uncertain | [173] |
| Epigenetic Modulation (HDAC inhibitor | Reactivate GRN expression by altering chromatin state | Small molecule-based, reversible mechanisms | Limited efficacy (FRM-0334 trial failed); bioavailability issues | [127,128] |
| Small Molecules (e.g., Amiodarone, Bafilomycin A1) | Enhance GRN expression or lysosomal function | Oral/small-molecule delivery; targets secondary pathways | Off-target effects, variable potency, microbiome, or acidosis issues | [116,133,134,135,136,140,141] |
| PTC Readthrough Compounds (e.g., G418, gentamicin) | Promote translation through premature stop codons | May restore GRN expression and PGRN protein levels | Toxicity, low bioavailability, and specificity to nonsense mutations | [129,130] |
| SORT1 Pathway Modulation (e.g., AL001) | Prevent PGRN degradation via SORT1 blockade | PGRN elevation in CSF/plasma; well-tolerated in early trials | Long-term efficacy unknown; possible compensatory effects | [113,177] |
| TREM2 Modulation (Anti-TREM2 antibodies) | Reduce microglial hyperactivation | Potential neuroprotection, reduced synaptic loss | No improvement in lysosomal dysfunction; possible neurotoxicity | [115] |
| ASOs (e.g., anti–miR-29b) | Block microRNA-related repression of GRN | Allele-independent; adaptable | Requires repeated dosing; off-target risk | [140] |
| CRISPR-Cas Systems (Cas9, Cas13) | Promising future study in correcting GRN mutation or expression | Potential for permanent correction | Ethical, delivery, and safety challenges; no study on GRN correction yet | [178,179] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bagyinszky, E.; An, S.S.A. Targeting Granulin Haploinsufficiency in Frontotemporal Dementia: From Genetic Mechanisms to Therapeutics. Int. J. Mol. Sci. 2025, 26, 9960. https://doi.org/10.3390/ijms26209960
Bagyinszky E, An SSA. Targeting Granulin Haploinsufficiency in Frontotemporal Dementia: From Genetic Mechanisms to Therapeutics. International Journal of Molecular Sciences. 2025; 26(20):9960. https://doi.org/10.3390/ijms26209960
Chicago/Turabian StyleBagyinszky, Eva, and Seong Soo A. An. 2025. "Targeting Granulin Haploinsufficiency in Frontotemporal Dementia: From Genetic Mechanisms to Therapeutics" International Journal of Molecular Sciences 26, no. 20: 9960. https://doi.org/10.3390/ijms26209960
APA StyleBagyinszky, E., & An, S. S. A. (2025). Targeting Granulin Haploinsufficiency in Frontotemporal Dementia: From Genetic Mechanisms to Therapeutics. International Journal of Molecular Sciences, 26(20), 9960. https://doi.org/10.3390/ijms26209960









