Comparative Effects of Trichoderma guizhouense NJAU4742 and Bacillus velezensis SQR9 on Growth and Pb Accumulation in Salix suchowensis
Abstract
1. Introduction
2. Results and Discussion
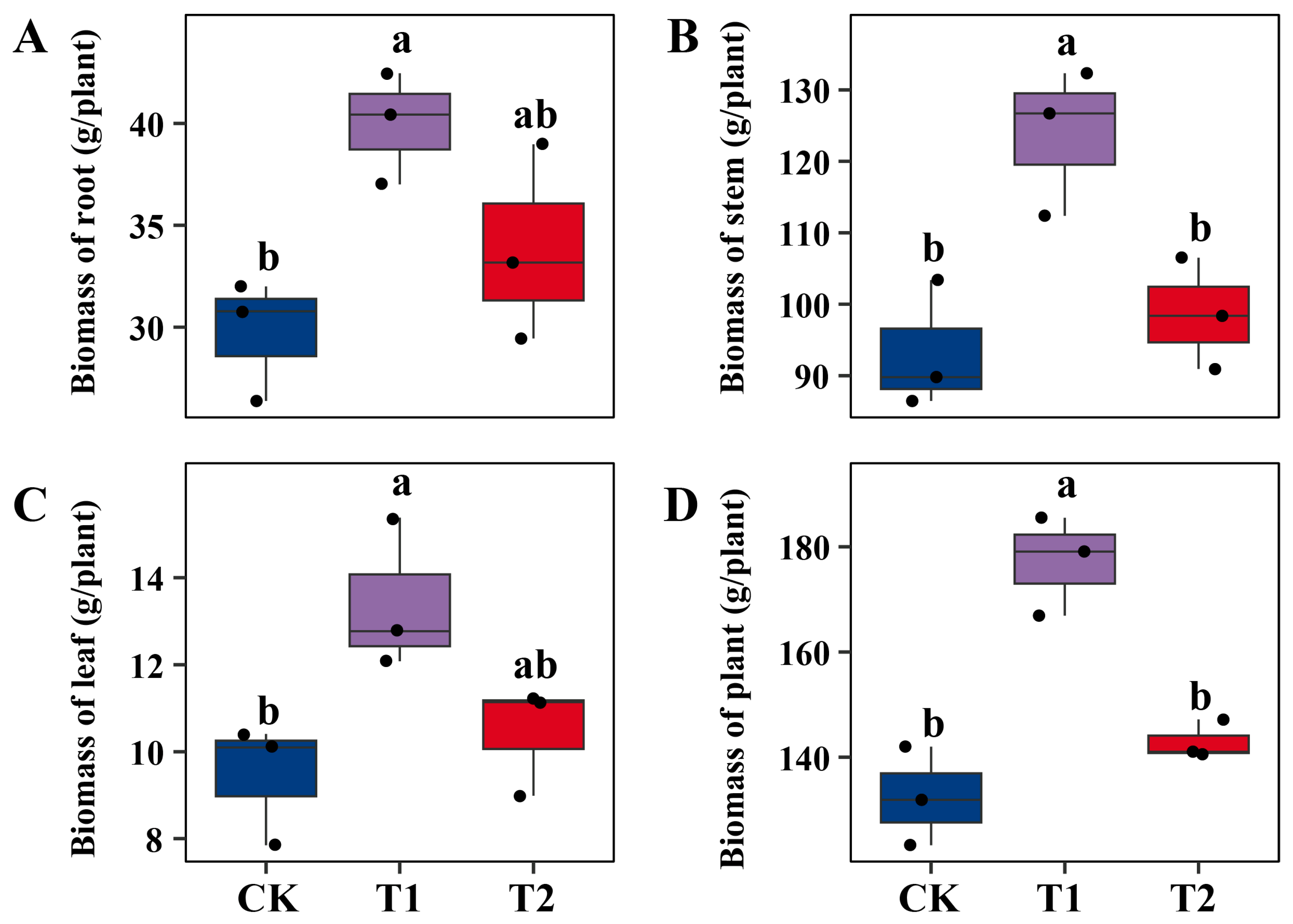
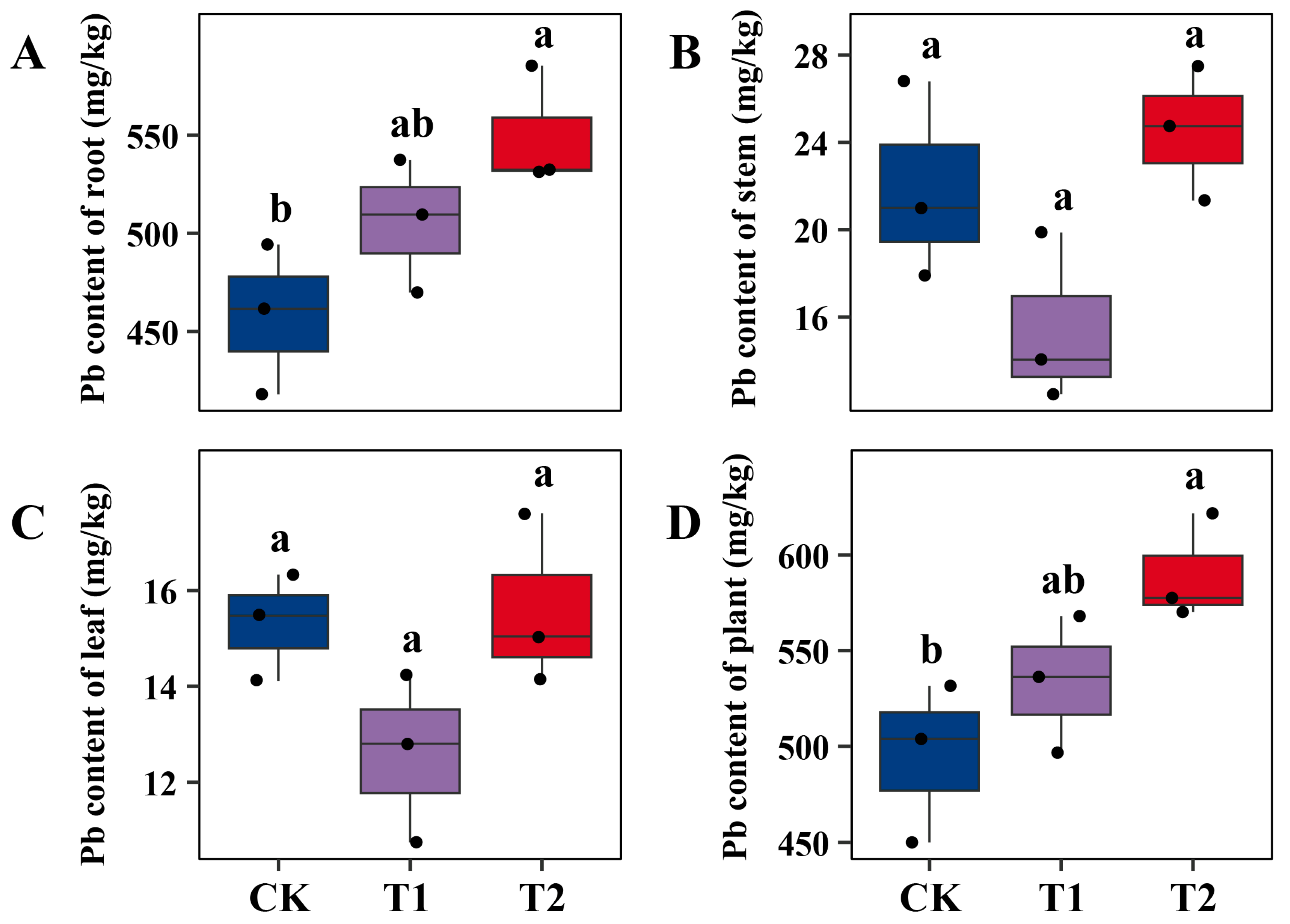
2.1. Differential Effects of Microbial Inoculation on Willow Growth and Pb Accumulation
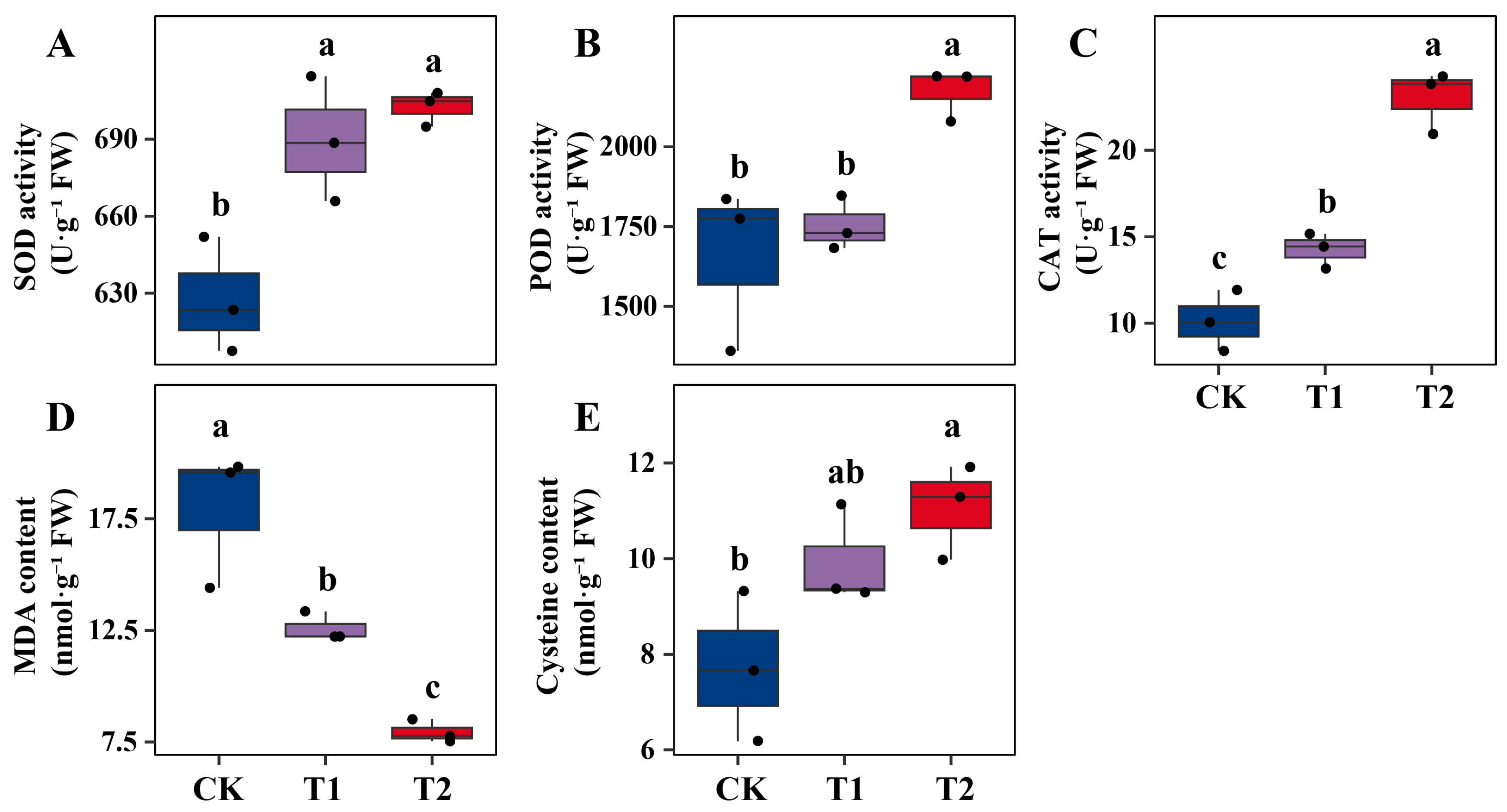
2.2. Enhancement of Antioxidant Defense and Detoxification Pathways by Microbial Inoculation Under Pb Stress
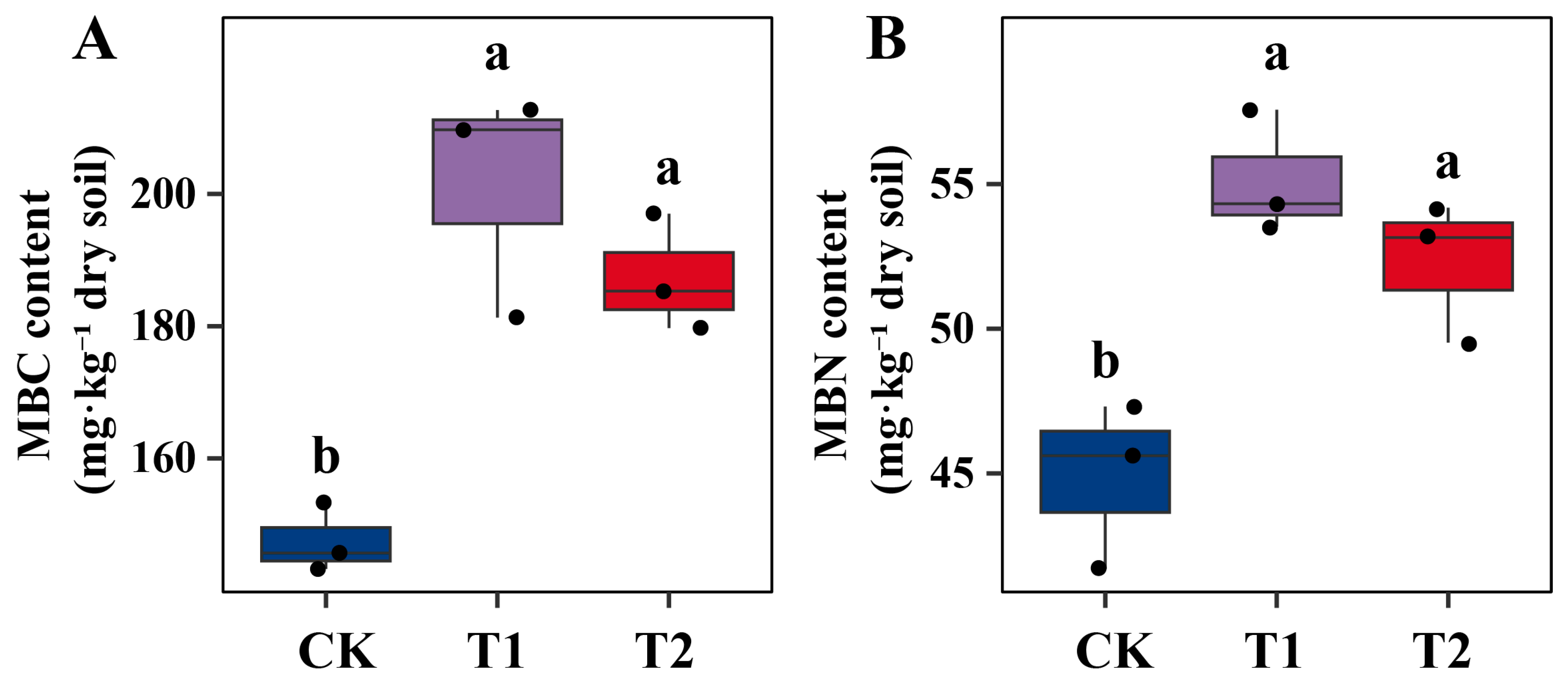
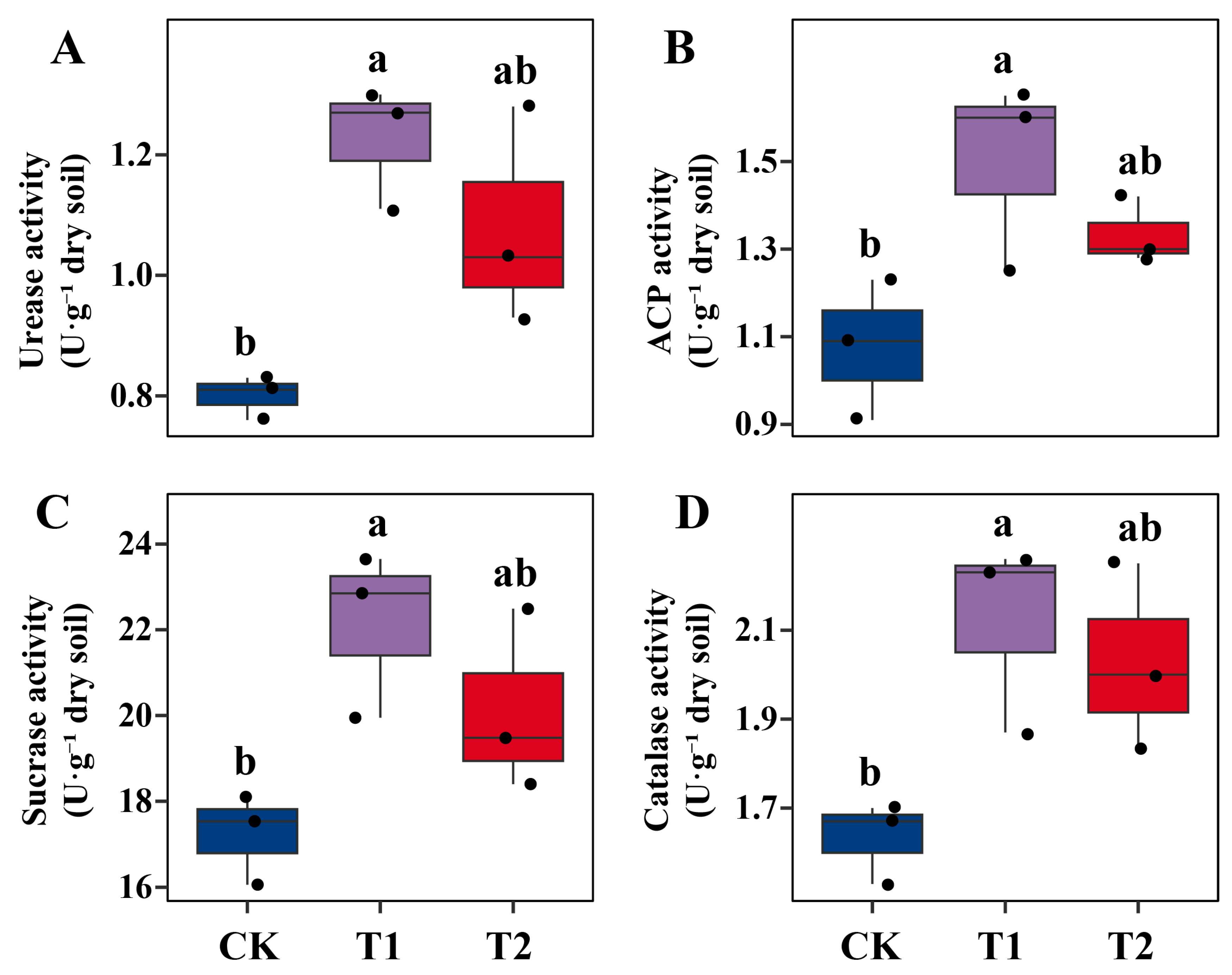
2.3. Stimulation of Soil Microbial Biomass and Enzymatic Activities by Microbial Inoculation Under Pb Stress
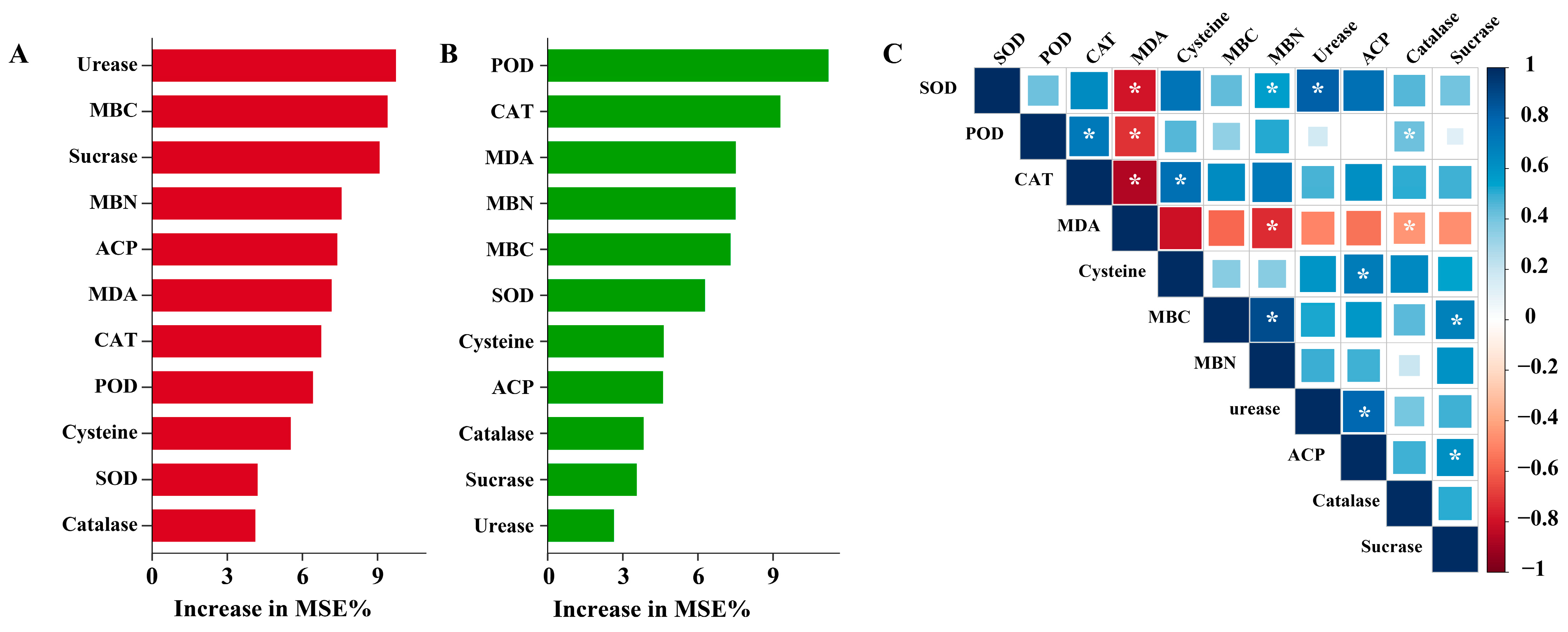
2.4. Integrative Analysis of Factors Driving Willow Biomass and Pb Accumulation
3. Materials and Methods
3.1. Sources of Plant, Microbial Inoculants, and Pb-Contaminated Soil
3.2. Experimental Design and Treatments
3.3. Determination of Plant Biomass and Pb Content
3.4. Determination of Physiological Parameters
3.5. Soil Sampling and Measurement of Microbial and Enzymatic Indices
3.6. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, X.; Li, B.; Liu, Y.; Xu, J. Rhizospheric Lactobacillus spp. contribute to the high Cd-accumulating characteristics of Phytolacca spp. in acidic Cd-contaminated soil. Environ. Res. 2023, 238, 117270. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, Y.; Gu, H.; Lam, S.S.; Chen, X.; Sonne, C.; Peng, W. A review of phytoremediation of environmental lead (pb) contamination. Chemosphere 2024, 362, 142691. [Google Scholar] [CrossRef]
- Chandwani, S.; Kayasth, R.; Naik, H.; Amaresan, N. Current status and future prospect of managing lead (Pb) stress through microbes for sustainable agriculture. Environ. Monit. Assess. 2023, 195, 479. [Google Scholar] [CrossRef] [PubMed]
- Kafle, A.; Timilsina, A.; Gautam, A.; Adhikari, K.; Bhattarai, A.; Aryal, N. Phytoremediation: Mechanisms, plant selection and enhancement by natural and synthetic agents. Environ. Adv. 2022, 8, 100203. [Google Scholar] [CrossRef]
- Shen, X.; Dai, M.; Yang, J.; Sun, L.; Tan, X.; Peng, C.; Ali, I.; Naz, I. A critical review on the phytoremediation of heavy metals from environment: Performance and challenges. Chemosphere 2022, 291, 132979. [Google Scholar] [CrossRef]
- Kristanti, R.A.; Tirtalistyani, R.; Tang, Y.Y.; Thao, N.T.T.; Kasongo, J.; Wijayanti, Y. Phytoremediation mechanism for emerging pollutants: A review. Trop. Aquat. Soil Pollut. 2023, 3, 88–108. [Google Scholar] [CrossRef]
- Shaffique, S.; Hussain, S.; Kang, S.-M.; Imran, M.; Kwon, E.-H.; Khan, M.A.; Lee, I.-J. Recent progress on the microbial mitigation of heavy metal stress in soybean: Overview and implications. Front. Plant Sci. 2023, 14, 1188856. [Google Scholar] [CrossRef]
- Narayanan, M.; Ma, Y. Mitigation of heavy metal stress in the soil through optimized interaction between plants and microbes. J. Environ. Manag. 2023, 345, 118732. [Google Scholar] [CrossRef]
- Devi, R.; Behera, B.; Raza, M.B.; Mangal, V.; Altaf, M.A.; Kumar, R.; Kumar, A.; Tiwari, R.K.; Lal, M.K.; Singh, B. An insight into microbes mediated heavy metal detoxification in plants: A review. J. Soil Sci. Plant Nutr. 2022, 22, 914–936. [Google Scholar] [CrossRef]
- Liu, C.; Lin, H.; Dong, Y.; Li, B. Increase of P and Cd bioavailability in the rhizosphere by endophytes promoted phytoremediation efficiency of Phytolacca acinosa. J. Hazard. Mater. 2022, 431, 128546. [Google Scholar] [CrossRef]
- Liu, L.; Xiao, C.; Gao, Y.; Jiang, T.; Xu, K.; Chen, J.; Lin, Z.; Chen, J.; Tian, S.; Lu, L. Inoculation of multi-metal-resistant Bacillus sp. to a hyperaccumulator plant Sedum alfredii for facilitating phytoextraction of heavy metals from contaminated soil. Chemosphere 2024, 366, 143464. [Google Scholar] [CrossRef]
- Mei, H.; Li, T.; Wu, H.; Xia, Y.; Huang, Q.; Liu, D.; Shen, Q. Transcriptomic Profiling Reveals Key Gene in Trichoderma guizhouense NJAU4742 Enhancing Tomato Tolerance Under Saline Conditions. Agriculture 2025, 15, 610. [Google Scholar] [CrossRef]
- Liu, Y.; Li, T.; Zhu, H.; Zhou, Y.; Shen, Q.; Liu, D. Cysteine facilitates the lignocellulolytic response of Trichoderma guizhouense NJAU4742 by indirectly up-regulating membrane sugar transporters. Biotechnol. Biofuels Bioprod. 2023, 16, 159. [Google Scholar] [CrossRef]
- Huang, R.; Feng, H.; Xu, Z.; Zhang, N.; Liu, Y.; Shao, J.; Shen, Q.; Zhang, R. Identification of adhesins in plant beneficial rhizobacteria Bacillus velezensis SQR9 and their effect on root colonization. Mol. Plant-Microbe Interact. 2022, 35, 64–72. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, D.; Zhang, B.; Mao, H.; Wang, Z.; Yan, Z.; Tao, C.; Deng, X.; Shen, Q.; Li, R. Bacillus velezensis SQR9 promotes plant growth through colonization and rhizosphere–phyllosphere bacteria interaction. Environ. Microbiol. Rep. 2024, 16, e13250. [Google Scholar] [CrossRef]
- Bajraktari, D.; Zeneli, L.; Bauer, B. Salix alba phytoremediation potential of heavy metals. J. Environ. Prot. Ecol. 2022, 23, 1687–1696. [Google Scholar] [CrossRef]
- Tőzsér, D.; Magura, T.; Simon, E. Heavy metal uptake by plant parts of willow species: A meta-analysis. J. Hazard. Mater. 2017, 336, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Tang, S.; Meng, X.; Zhu, H.; Zhu, Y.; Liu, D.; Shen, Q. Proteomic analysis demonstrates a molecular dialog between Trichoderma guizhouense NJAU 4742 and cucumber (Cucumis sativus L.) roots: Role in promoting plant growth. Mol. Plant-Microbe Interact. 2021, 34, 631–644. [Google Scholar] [CrossRef]
- Meng, X.; Ma, L.; Li, T.; Zhu, H.; Guo, K.; Liu, D.; Ran, W.; Shen, Q. The functioning of a novel protein, Swollenin, in promoting the lignocellulose degradation capacity of Trichoderma guizhouense NJAU4742 from a proteomic perspective. Bioresour. Technol. 2020, 317, 123992. [Google Scholar] [CrossRef]
- Govarthanan, M.; Mythili, R.; Kamala-Kannan, S.; Selvankumar, T.; Srinivasan, P.; Kim, H. In-vitro bio-mineralization of arsenic and lead from aqueous solution and soil by wood rot fungus, Trichoderma sp. Ecotoxicol. Environ. Saf. 2019, 174, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Liu, Y.; Xu, Y.; Zhang, G.; Shen, Q.; Zhang, R. Exploring elicitors of the beneficial rhizobacterium Bacillus amyloliquefaciens SQR9 to induce plant systemic resistance and their interactions with plant signaling pathways. Mol. Plant-Microbe Interact. 2018, 31, 560–567. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Y.; Wu, G.; Dong, X.; Xiong, Q.; Chen, L.; Xu, Z.; Feng, H.; Zhang, R. Bacillus velezensis tolerance to the induced oxidative stress in root colonization contributed by the two-component regulatory system sensor ResE. Plant Cell Environ. 2021, 44, 3094–3102. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhao, Y.; Shao, J.; Wang, J.; Xun, W.; Sun, X.; Xu, Z.; Miao, Y.; Huang, G.; Liu, D. Rhizosphere domestication enhances root colonization and plant growth promotion performance of Bacillus velezensis SQR9. Front. Microbiol. 2025, 16, 1638130. [Google Scholar] [CrossRef]
- Xiong, Q.; Liu, D.; Zhang, H.; Dong, X.; Zhang, G.; Liu, Y.; Zhang, R. Quorum sensing signal autoinducer-2 promotes root colonization of Bacillus velezensis SQR9 by affecting biofilm formation and motility. Appl. Microbiol. Biotechnol. 2020, 104, 7177–7185. [Google Scholar] [CrossRef]
- Liu, Y.; Feng, H.; Fu, R.; Zhang, N.; Du, W.; Shen, Q.; Zhang, R. Induced root-secreted D-galactose functions as a chemoattractant and enhances the biofilm formation of Bacillus velezensis SQR9 in an McpA-dependent manner. Appl. Microbiol. Biotechnol. 2020, 104, 785–797. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Zhou, J.; Chen, G.C.; Su, B.; Wang, Q.; Huang, D.Z.; Niu, X.Y. Recruitment of beneficial microbes and differential metabolites promoted Pb transport in Salix integra Thunb. after inoculation with Bacillus megaterium B6. Plant Soil 2025, 510, 743–760. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, R.; Yan, X.; Fan, K. Superoxide dismutase nanozymes: An emerging star for anti-oxidation. J. Mater. Chem. B 2021, 9, 6939–6957. [Google Scholar] [CrossRef]
- Alharbi, K.; Alnusaire, T.S.; Alayafi, A.A.; Abdulmajeed, A.M.; ALrashidi, A.A.; Alghanem, S.; Al-Balawi, S.M.; Anazi, H.K.; Soliman, M.H. Synergistic Effects of Biochar and Methyl Jasmonate Nanoparticles in Enhancing Maize Resilience to Heat Stress. J. Plant Growth Regul. 2025, 1–21. [Google Scholar] [CrossRef]
- Xu, D.; Wu, L.; Yao, H.; Zhao, L. Catalase-like nanozymes: Classification, catalytic mechanisms, and their applications. Small 2022, 18, 2203400. [Google Scholar] [CrossRef]
- Sahile, A.A.; Khan, M.A.; Hamayun, M.; Imran, M.; Kang, S.-M.; Lee, I.-J. Novel Bacillus cereus strain, ALT1, enhance growth and strengthens the antioxidant system of soybean under cadmium stress. Agronomy 2021, 11, 404. [Google Scholar] [CrossRef]
- Mohideen, K.; Chandrasekar, K.; Ramsridhar, S.; Rajkumar, C.; Ghosh, S.; Dhungel, S. Assessment of oxidative stress by the estimation of lipid peroxidation marker malondialdehyde (MDA) in patients with chronic periodontitis: A systematic review and meta-analysis. Int. J. Dent. 2023, 2023, 6014706. [Google Scholar] [CrossRef] [PubMed]
- Ighodaro, O.; Akinloye, O. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, Y.; Xia, W.; Xu, H.; Su, B.; Jiang, G.; Ning, T. Responses of gas exchange, cellular membrane integrity, and antioxidant enzymes activities of salinity-stressed winter wheat to ozone pollution. Photosynthetica 2011, 49, 389–396. [Google Scholar] [CrossRef]
- Seregin, I.V.; Kozhevnikova, A.D. Phytochelatins: Sulfur-containing metal (loid)-chelating ligands in plants. Int. J. Mol. Sci. 2023, 24, 2430. [Google Scholar] [CrossRef]
- Sethi, S. Phytochelatins: Heavy metal detoxifiers in plants. In Advanced and Innovative Approaches of Environmental Biotechnology in Industrial Wastewater Treatment; Springer: Amsterdam, The Netherlands, 2023; pp. 361–379. [Google Scholar]
- Yadav, S.K. Heavy metals toxicity in plants: An overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S. Afr. J. Bot. 2010, 76, 167–179. [Google Scholar] [CrossRef]
- Bargali, S.S. Soil microbial biomass: A crucial indicator of soil health. Curr. Agric. Res. J. 2024, 12, 01–06. [Google Scholar] [CrossRef]
- Broadbent, A.A.; Newbold, L.K.; Pritchard, W.J.; Michas, A.; Goodall, T.; Cordero, I.; Giunta, A.; Snell, H.S.; Pepper, V.V.; Grant, H.K. Climate change disrupts the seasonal coupling of plant and soil microbial nutrient cycling in an alpine ecosystem. Glob. Change Biol. 2024, 30, e17245. [Google Scholar] [CrossRef]
- Arif, S.; Munis, M.F.H.; Liaquat, F.; Gulzar, S.; Haroon, U.; Zhao, L.; Zhang, Y. Trichoderma viride establishes biodefense against clubroot (Plasmodiophora brassicae) and fosters plant growth via colonizing root hairs in pak choi (Brassica campestris spp. chinesnsis). Biol. Control 2023, 183, 105265. [Google Scholar] [CrossRef]
- Gooruee, R.; Hojjati, M.; Behbahani, B.A.; Shahbazi, S.; Askari, H. Extracellular enzyme production by different species of Trichoderma fungus for lemon peel waste bioconversion. Biomass Convers. Biorefinery 2024, 14, 2777–2786. [Google Scholar]
- Chang, D.; Song, Y.; Liang, H.; Liu, R.; Cai, C.; Lv, S.; Liao, Y.; Nie, J.; Duan, T.; Cao, W. Planting Chinese milk vetch with phosphate-solubilizing bacteria inoculation enhances phosphorus turnover by altering the structure of the phoD-harboring bacteria community. Eur. J. Soil Biol. 2024, 123, 103678. [Google Scholar]
- Wang, S.; Long, H.; Hu, X.; Wang, H.; Wang, Y.; Guo, J.; Zheng, X.; Ye, Y.; Shao, R.; Yang, Q. The co-inoculation of Trichoderma viridis and Bacillus subtilis improved the aerobic composting efficiency and degradation of lignocellulose. Bioresour. Technol. 2024, 394, 130285. [Google Scholar] [CrossRef] [PubMed]
- Yeboah, J.O. Effect of heavy metal contamination on soil enzymes activities. J. Geosci. Environ. Prot. 2021, 9, 135–154. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Wu, F.; Zhang, J.; Ai, S.; Liu, Z. Microbial community composition and degradation potential of petroleum-contaminated sites under heavy metal stress. J. Hazard. Mater. 2023, 457, 131814. [Google Scholar] [CrossRef]
- Alam, J.; Olofintila, O.E.; Moen, F.; Noel, Z.; Liles, M.R.; Goodwin, D.C. Broad antibiosis activity of Bacillus velezensis and Bacillus subtilis is accounted for by a conserved capacity for lipopeptide biosynthesis. Front. Microbiol. 2025, 16, 1636481. [Google Scholar] [CrossRef] [PubMed]
- Organo, N.D.; Granada, S.M.J.M.; Pineda, H.G.S.; Sandro, J.M.; Nguyen, V.H.; Gummert, M. Assessing the potential of a Trichoderma-based compost activator to hasten the decomposition of incorporated rice straw. Sci. Rep. 2022, 12, 448, Correction in Sci. Rep. 2022, 12, 1647. [Google Scholar] [PubMed]
- Schekaleva, O.; Luneva, O.; Klimenko, E.; Shaliukhina, S.; Breygina, M. Dynamics of ROS production, SOD, POD and CAT activity during stigma maturation and pollination in Nicotiana tabacum and Lilium longiflorum. Plant Biol. 2024, 26, 1240–1246. [Google Scholar] [CrossRef]
- Wu, H.; Kong, Y.; Zhao, W.; Wang, F. Measurement of cellular MDA content through MTBE-extraction based TBA assay by eliminating cellular interferences. J. Pharm. Biomed. Anal. 2024, 248, 116332. [Google Scholar] [CrossRef]
- Stauß, A.C.; Fuchs, C.; Jansen, P.; Repert, S.; Alcock, K.; Ludewig, S.; Rozhon, W. The ninhydrin reaction revisited: Optimisation and application for quantification of free amino acids. Molecules 2024, 29, 3262. [Google Scholar] [CrossRef]
- Chen, Z.; Xiao, Y.; Dong, X.; Deng, Z.; Zhou, X.; Yan, G.; Zhang, J.; Han, S. Nitrogen addition promotes soil organic phosphorus accumulation through increasing microbial biomass phosphorus in a temperate forest. Plant Soil 2025, 511, 1433–1448. [Google Scholar] [CrossRef]
- Vitolo, M. Notes on urea hydrolysis by urease. J. Pharm. Pharm. Sci. 2022, 11, 96–135. [Google Scholar]
- Calvo-Marzal, P.; Rosatto, S.S.; Granjeiro, P.A.; Aoyama, H.; Kubota, L.T. Electroanalytical determination of acid phosphatase activity by monitoring p-nitrophenol. Anal. Chim. Acta 2001, 441, 207–214. [Google Scholar] [CrossRef]
- Mueller, S.; Riedel, H.-D.; Stremmel, W. Determination of catalase activity at physiological hydrogen peroxide concentrations. Anal. Biochem. 1997, 245, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Hämäläinen, M.; Karjalainen, S.; Söderling, E. A simple test for the determination of salivary sucrase activity. Caries Res. 1988, 22, 4–6. [Google Scholar] [CrossRef]
- Weng, M.; Zhang, X.; Li, P.; Liu, H.; Liu, Q.; Wang, Y. Exploring the impact of land use scales on water quality based on the random forest model: A case study of the Shaying River basin, China. Water 2024, 16, 420. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, R.; Wang, B.; Xu, M.; Sui, D.; He, X. Comparative Effects of Trichoderma guizhouense NJAU4742 and Bacillus velezensis SQR9 on Growth and Pb Accumulation in Salix suchowensis. Int. J. Mol. Sci. 2025, 26, 9961. https://doi.org/10.3390/ijms26209961
Huang R, Wang B, Xu M, Sui D, He X. Comparative Effects of Trichoderma guizhouense NJAU4742 and Bacillus velezensis SQR9 on Growth and Pb Accumulation in Salix suchowensis. International Journal of Molecular Sciences. 2025; 26(20):9961. https://doi.org/10.3390/ijms26209961
Chicago/Turabian StyleHuang, Ruifang, Baosong Wang, Ming Xu, Dezong Sui, and Xudong He. 2025. "Comparative Effects of Trichoderma guizhouense NJAU4742 and Bacillus velezensis SQR9 on Growth and Pb Accumulation in Salix suchowensis" International Journal of Molecular Sciences 26, no. 20: 9961. https://doi.org/10.3390/ijms26209961
APA StyleHuang, R., Wang, B., Xu, M., Sui, D., & He, X. (2025). Comparative Effects of Trichoderma guizhouense NJAU4742 and Bacillus velezensis SQR9 on Growth and Pb Accumulation in Salix suchowensis. International Journal of Molecular Sciences, 26(20), 9961. https://doi.org/10.3390/ijms26209961





