Roles of Tumor-Infiltrating Lymphocytes and Antitumor Immune Responses as Predictive and Prognostic Markers in Patients with Breast Cancer Receiving Neoadjuvant Chemotherapy
Abstract
1. Introduction
2. Therapeutic Effects of Anticancer Drug–Induced Antitumor Immunity
3. Modulation of the Tumor Microenvironment by Anticancer Drugs to Enhance the Therapeutic Effect
4. TILs and Immune Responses as Predictive and Prognostic Markers of Treatment Response
4.1. TN Breast Cancer
4.2. HER2+ Breast Cancer
4.3. Luminal Breast Cancer
4.4. Non-Subtype-Specified (NS) Breast Cancer
5. Overview of Predictive and Prognostic Factors Across Different Subtypes
6. Possible Causes of Conflicting Findings for TILs and Future Approaches
6.1. Correlation Between TIL, iTILs, sTILs and pCR and Prognosis
6.2. Predictive and Prognostic Values of PD-L1, FOXP3, and Systemic Immune Response
6.3. Evaluation of TILs
6.4. Comprehensive Understanding of Antitumor Immune Responses
6.5. Unresolved Issues and Strategies to Enhance Antitumor Immune Responses for Therapeutic Effects and Survival
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- An, J.; Peng, C.; Tang, H.; Liu, X.; Peng, F. New advances in the research of resistance to neoadjuvant chemotherapy in breast cancer. Int. J. Mol. Sci. 2021, 22, 9644. [Google Scholar] [CrossRef]
- Urueña, C.; Lasso, P.; Bernal-Estevez, D.; Rubio, D.; Salazar, A.J.; Olaya, M.; Barreto, A.; Tawil, M.; Torregrosa, L.; Fiorentino, S. The breast cancer immune microenvironment is modified by neoadjuvant chemotherapy. Sci. Rep. 2022, 12, 7981. [Google Scholar] [CrossRef]
- Park, Y.H.; Lal, S.; Lee, J.E.; Choi, Y.L.; Wen, J.; Ram, S.; Ding, Y.; Lee, S.H.; Powell, E.; Lee, S.K.; et al. Chemotherapy induces dynamic immune responses in breast cancers that impact treatment outcome. Nat. Commun. 2020, 11, 6175. [Google Scholar] [CrossRef]
- Kim, R.; Kawai, A.; Wakisaka, M.; Sawada, S.; Shimoyama, M.; Yasuda, N.; Hidaka, M.; Morita, Y.; Ohtani, S.; Arihiro, K. Immune correlates of the differing pathological and therapeutic effects of neoadjuvant chemotherapy in breast cancer. Eur. J. Surg. Oncol. 2020, 46, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Rui, W.; Fei, X.; Chen, X.; Shen, K. Association of tumor-infiltrating lymphocytes before and after neoadjuvant chemotherapy with pathological complete response and prognosis in patients with breast cancer. Cancer Med. 2021, 10, 7921–7933. [Google Scholar] [CrossRef]
- Wu, R.; Oshi, M.; Asaoka, M.; Yan, L.; Benesch, M.G.K.; Khoury, T.; Nagahashi, M.; Miyoshi, Y.; Endo, I.; Ishikawa, T.; et al. Intratumoral tumor infiltrating lymphocytes (TILs) are associated with cell proliferation and better survival but not always with chemotherapy response in breast cancer. Ann. Surg. 2023, 278, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Bense, R.D.; Sotiriou, C.; Piccart-Gebhart, M.J.; Haanen, J.B.A.G.; van Vugt, M.A.T.M.; de Vries, E.G.E.; Schröder, C.P.; Fehrmann, R.S.N. Relevance of tumor-infiltrating immune cell composition and functionality for disease outcome in breast cancer. J. Natl. Cancer Inst. 2016, 109, djw192. [Google Scholar] [CrossRef]
- Salgado, R.; Denkert, C.; Demaria, S.; Sirtaine, N.; Klauschen, F.; Pruneri, G.; Wienert, S.; Van den Eynden, G.; Baehner, F.L.; Penault-Llorca, F.; et al. International TILs Working Group 2014. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: Recommendations by an International TILs Working Group 2014. Ann. Oncol. 2015, 26, 259–2571. [Google Scholar] [CrossRef]
- Khoury, T.; Nagrale, V.; Opyrchal, M.; Peng, X.; Wang, D.; Yao, S. Prognostic significance of stromal versus intratumoral infiltrating lymphocytes in different subtypes of breast cancer treated with cytotoxic neoadjuvant chemotherapy. Appl. Immunohistochem. Mol. Morphol. 2018, 26, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Li, X.B.; Krishnamurti, U.; Bhattarai, S.; Klimov, S.; Reid, M.D.; O’Regan, R.; Aneja, R. Biomarkers Predicting Pathologic Complete Response to Neoadjuvant Chemotherapy in Breast Cancer. Am. J. Clin. Pathol. 2016, 145, 871–878. [Google Scholar] [CrossRef]
- Gao, Z.H.; Li, C.X.; Liu, M.; Jiang, J.Y. Predictive and prognostic role of tumour-infiltrating lymphocytes in breast cancer patients with different molecular subtypes: A meta-analysis. BMC Cancer 2020, 20, 1150. [Google Scholar] [CrossRef]
- Denkert, C.; von Minckwitz, G.; Darb-Esfahani, S.; Lederer, B.; Heppner, B.I.; Weber, K.E.; Budczies, J.; Huober, J.; Klauschen, F.; Furlanetto, J.; et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: A pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018, 19, 40–50. [Google Scholar] [CrossRef]
- Hamy, A.S.; Bonsang-Kitzis, H.; De Croze, D.; Laas, E.; Darrigues, L.; Topciu, L.; Menet, E.; Vincent-Salomon, A.; Lerebours, F.; Pierga, J.Y.; et al. Interaction between molecular subtypes and stromal immune infiltration before and after treatment in breast cancer patients treated with neoadjuvant chemotherapy. Clin. Cancer Res. 2019, 25, 6731–6741. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Hida, A.I.; Inoue, N.; Imamura, M.; Fujimoto, Y.; Akazawa, K.; Hirota, S.; Miyoshi, Y. Abundant tumor infiltrating lymphocytes after primary systemic chemotherapy predicts poor prognosis in estrogen receptor-positive/HER2-negative breast cancers. Breast Cancer Res. Treat. 2018, 168, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Miglietta, F.; Ragazzi, M.; Fernandes, B.; Griguolo, G.; Massa, D.; Girardi, F.; Bottosso, M.; Bisagni, A.; Zarrilli, G.; Porra, F.; et al. A prognostic model based on residual cancer burden and tumor-infiltrating lymphocytes on residual disease after neoadjuvant therapy in HER2+ breast cancer. Clin. Cancer Res. 2023, 29, 3429–3437. [Google Scholar] [CrossRef] [PubMed]
- Hernández, Á.P.; Juanes-Velasco, P.; Landeira-Viñuela, A.; Bareke, H.; Montalvillo, E.; Góngora, R.; Fuentes, M. Restoring the immunity in the tumor microenvironment: Insights into immunogenic cell death in onco-therapies. Cancers 2021, 13, 2821. [Google Scholar] [CrossRef]
- Fabian, K.P.; Wolfson, B.; Hodge, J.W. From immunogenic cell death to immunogenic modulation: Select chemotherapy regimens induce a spectrum of immune-enhancing activities in the tumor microenvironment. Front. Oncol. 2021, 11, 728018. [Google Scholar] [CrossRef]
- Kroemer, G.; Galassi, C.; Zitvogel, L.; Galluzzi, L. Immunogenic cell stress and death. Nat. Immunol. 2022, 23, 487–500. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, R.; Xie, Y.; Zhou, X.Q.; Yang, J.F.; Shi, Y.Y.; Liu, S. Comprehensive review of drug-mediated ICD inhibition of breast cancer: Mechanism, status, and prospects. Clin. Exp. Med. 2024, 24, 230. [Google Scholar] [CrossRef]
- Gao, W.; Wang, X.; Zhou, Y.; Wang, X.; Yu, Y. Autophagy, ferroptosis, pyroptosis, and necroptosis in tumor immunotherapy. Signal Transduct. Target. Ther. 2022, 7, 196. [Google Scholar] [CrossRef]
- Kim, R.; Kin, T. Current and future therapies for immunogenic cell death and related molecules to potentially cure primary breast cancer. Cancers 2021, 13, 4756. [Google Scholar] [CrossRef]
- Groth, C.; Hu, X.; Weber, R.; Fleming, V.; Altevogt, P.; Utikal, J.; Umansky, V. Immunosuppression mediated by myeloid-derived suppressor cells (MDSCs) during tumour progression. Br. J. Cancer 2019, 120, 16–25. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, T.; Shao, S.; Shi, B.; Zhao, Y. Phenotype, development, and biological function of myeloid-derived suppressor cells. Oncoimmunology 2015, 5, e1004983. [Google Scholar] [CrossRef] [PubMed]
- Ren, R.; Xiong, C.; Ma, R.; Wang, Y.; Yue, T.; Yu, J.; Shao, B. The recent progress of myeloid-derived suppressor cell and its targeted therapies in cancers. MedComm (2020) 2023, 4, e323. [Google Scholar] [CrossRef] [PubMed]
- Sarradin, V.; Lusque, A.; Filleron, T.; Dalenc, F.; Franchet, C. Immune microenvironment changes induced by neoadjuvant chemotherapy in triple-negative breast cancers: The MIMOSA-1 study. Breast Cancer Res. 2021, 23, 61. [Google Scholar] [CrossRef] [PubMed]
- Pelekanou, V.; Barlow, W.E.; Nahleh, Z.A.; Wasserman, B.; Lo, Y.C.; von Wahlde, M.K.; Hayes, D.; Hortobagyi, G.N.; Gralow, J.; Tripathy, D.; et al. Tumor-infiltrating lymphocytes and PD-L1 expression in pre- and posttreatment breast cancers in the SWOG S0800 phase II neoadjuvant chemotherapy trial. Mol. Cancer Ther. 2018, 17, 1324–1331. [Google Scholar] [CrossRef]
- Wood, S.J.; Gao, Y.; Lee, J.H.; Chen, J.; Wang, Q.; Meisel, J.L.; Li, X. High tumor infiltrating lymphocytes are significantly associated with pathological complete response in triple negative breast cancer treated with neoadjuvant KEYNOTE-522 chemoimmunotherapy. Breast Cancer Res. Treat. 2024, 205, 193–199. [Google Scholar] [CrossRef]
- Zhao, M.; Xing, H.; He, J.; Wang, X.; Liu, Y. Tumor infiltrating lymphocytes and neutrophil-to-lymphocyte ratio in relation to pathological complete remission to neoadjuvant therapy and prognosis in triple negative breast cancer. Pathol. Res. Pract. 2023, 248, 154687. [Google Scholar] [CrossRef]
- Ademuyiwa, F.O.; Gao, F.; Street, C.R.; Chen, I.; Northfelt, D.W.; Wesolowski, R.; Arora, M.; Brufsky, A.; Dees, E.C.; Santa-Maria, C.A.; et al. A randomized phase 2 study of neoadjuvant carboplatin and paclitaxel with or without atezolizumab in triple negative breast cancer (TNBC)—NCI 10013. NPJ Breast Cancer 2022, 8, 134. [Google Scholar] [CrossRef]
- Asano, Y.; Kashiwagi, S.; Goto, W.; Takada, K.; Takahashi, K.; Hatano, T.; Takashima, T.; Tomita, S.; Motomura, H.; Ohsawa, M.; et al. Prediction of treatment response to neoadjuvant chemotherapy in breast cancer by subtype using tumor-infiltrating lymphocytes. Anticancer Res. 2018, 38, 2311–2321. [Google Scholar]
- Castaneda, C.A.; Mittendorf, E.; Casavilca, S.; Wu, Y.; Castillo, M.; Arboleda, P.; Nunez, T.; Guerra, H.; Barrionuevo, C.; Dolores-Cerna, K.; et al. Tumor infiltrating lymphocytes in triple negative breast cancer receiving neoadjuvant chemotherapy. World J. Clin. Oncol. 2016, 7, 387–394. [Google Scholar] [CrossRef]
- Hida, A.I.; Watanabe, T.; Sagara, Y.; Kashiwaba, M.; Sagara, Y.; Aogi, K.; Ohi, Y.; Tanimoto, A. Diffuse distribution of tumor-infiltrating lymphocytes is a marker for better prognosis and chemotherapeutic effect in triple-negative breast cancer. Breast Cancer Res. Treat. 2019, 178, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Würfel, F.; Erber, R.; Huebner, H.; Hein, A.; Lux, M.P.; Jud, S.; Kremer, A.; Kranich, H.; Mackensen, A.; Häberle, L.; et al. TILGen: A program to investigate immune targets in breast cancer patients—First results on the influence of tumor-infiltrating lymphocytes. Breast Care 2018, 13, 8–14, Erratum in Breast Care 2020, 15, 322. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Tsuda, H.; Shimizu, C.; Yamamoto, S.; Shibata, T.; Yamamoto, H.; Hirata, T.; Yonemori, K.; Ando, M.; Tamura, K.; et al. Tumor-infiltrating lymphocytes are correlated with response to neoadjuvant chemotherapy in triple-negative breast cancer. Breast Cancer Res. Treat 2012, 132, 793–805. [Google Scholar] [CrossRef] [PubMed]
- Verdial, F.C.; Mamtani, A.; Pawloski, K.R.; Sevilimedu, V.; D’Alfonso, T.M.; Zhang, H.; Gemignani, M.L.; Barrio, A.V.; Morrow, M.; Tadros, A.B. The effect of age on outcomes after neoadjuvant chemotherapy for breast cancer. Ann. Surg. Oncol. 2022, 29, 3810–3819. [Google Scholar] [CrossRef]
- Herrero-Vicent, C.; Guerrero, A.; Gavilá, J.; Gozalbo, F.; Hernández, A.; Sandiego, S.; Algarra, M.A.; Calatrava, A.; Guillem-Porta, V.; Ruiz-Simón, A. Predictive and prognostic impact of tumour-infiltrating lymphocytes in triple-negative breast cancer treated with neoadjuvant chemotherapy. Ecancermedicalscience 2017, 11, 759. [Google Scholar] [CrossRef]
- Yam, C.; Yen, E.Y.; Chang, J.T.; Bassett, R.L.; Alatrash, G.; Garber, H.; Huo, L.; Yang, F.; Philips, A.V.; Ding, Q.Q.; et al. Immune phenotype and response to neoadjuvant therapy in triple-negative breast cancer. Clin. Cancer Res. 2021, 27, 5365–5375. [Google Scholar] [CrossRef]
- Giacchetti, S.; Faucheux, L.; Gardair, C.; Cuvier, C.; de Roquancourt, A.; Campedel, L.; Groheux, D.; de Bazelaire, C.; Lehmann-Che, J.; Miquel, C.; et al. Negative relationship between post-treatment stromal tumor-infiltrating lymphocyte (TIL) and survival in triple-negative breast cancer patients treated with dose-dense dose-intense neoadjuvant chemotherapy. Cancers 2022, 14, 1331. [Google Scholar] [CrossRef]
- Lopes, A.D.; Galdino, N.A.L.; Figueiredo, A.B.; Brianese, R.C.; Morais, K.L.P.; De Brot, M.; Osório, C.A.B.T.; Teixeira-Carvalho, A.; Calsavara, V.F.; Evangelista, G.F.B.; et al. Systemic immune mediators reflect tumour-infiltrating lymphocyte intensity and predict therapeutic response in triple-negative breast cancer. Immunology 2023, 169, 229–241. [Google Scholar] [CrossRef]
- Kimura, Y.; Sasada, S.; Emi, A.; Masumoto, N.; Kadoya, T.; Arihiro, K.; Okada, M. 18F-fluorodeoxyglucose positron emission tomography/Computed tomography predicts tumor immune microenvironment function in early triple-negative breast cancer. Anticancer Res. 2023, 43, 127–136. [Google Scholar] [CrossRef]
- Asano, Y.; Kashiwagi, S.; Goto, W.; Kurata, K.; Noda, S.; Takashima, T.; Onoda, N.; Tanaka, S.; Ohsawa, M.; Hirakawa, K. Tumour-infiltrating CD8 to FOXP3 lymphocyte ratio in predicting treatment responses to neoadjuvant chemotherapy of aggressive breast cancer. Br. J. Surg. 2016, 103, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Telli, M.L.; Chu, C.; Badve, S.S.; Vinayak, S.; Silver, D.P.; Isakoff, S.J.; Kaklamani, V.; Gradishar, W.; Stearns, V.; Connolly, R.M.; et al. Association of tumor-infiltrating lymphocytes with homologous recombination deficiency and BRCA1/2 status in patients with early triple-negative breast cancer: A pooled analysis. Clin. Cancer Res. 2020, 26, 2704–2710. [Google Scholar] [CrossRef] [PubMed]
- Luen, S.J.; Salgado, R.; Dieci, M.V.; Vingiani, A.; Curigliano, G.; Gould, R.E.; Castaneda, C.; D’Alfonso, T.; Sanchez, J.; Cheng, E.; et al. Prognostic implications of residual disease tumor-infiltrating lymphocytes and residual cancer burden in triple-negative breast cancer patients after neoadjuvant chemotherapy. Ann. Oncol. 2019, 30, 236–242. [Google Scholar] [CrossRef]
- Agarwal, G.; Vishvak Chanthar, K.M.M.; Katiyar, S.; Kumari, N.; Krishnani, N.; Sabaretnam, M.; Chand, G.; Mishra, A.; Lal, P. Predictive and prognostic role of tumor-infiltrating lymphocytes in patients with advanced breast cancer treated with primary systemic therapy. World J. Surg. 2023, 47, 1238–1246. [Google Scholar] [PubMed]
- Lusho, S.; Durando, X.; Mouret-Reynier, M.A.; Kossai, M.; Lacrampe, N.; Molnar, I.; Penault-Llorca, F.; Radosevic-Robin, N.; Abrial, C. Platelet-to-lymphocyte ratio is associated with favorable response to neoadjuvant chemotherapy in triple negative breast cancer: A study on 120 patients. Front. Oncol. 2021, 11, 678315. [Google Scholar] [CrossRef]
- Campedel, L.; Blanc-Durand, P.; Bin Asker, A.; Lehmann-Che, J.; Cuvier, C.; De Bazelaire, C.; Teixeira, L.; Becourt, S.; Ledoux, F.; Hocini, H.; et al. Prognostic impact of stromal immune infiltration before and after neoadjuvant chemotherapy (NAC) in triple negative inflammatory breast cancers (TNIBC) treated with dose-dense dose-intense NAC. Cancers 2020, 12, 2657. [Google Scholar] [CrossRef]
- Cao, B.; Zhang, Z.; Wang, C.; Lv, X. Prognostic relevance of tumor-infiltrating lymphocytes in residual tumor tissue from patients with triple-negative breast cancer following neoadjuvant chemotherapy: A systematic review and meta-analysis. Oncol. Lett. 2023, 26, 441. [Google Scholar] [CrossRef]
- Dieci, M.V.; Carbognin, L.; Miglietta, F.; Canino, F.; Giorgi, C.A.; Cumerlato, E.; Amato, O.; Massa, D.; Griguolo, G.; Genovesi, E.; et al. Incorporating weekly carboplatin in anthracycline and paclitaxel-containing neoadjuvant chemotherapy for triple-negative breast cancer: Propensity-score matching analysis and TIL evaluation. Br. J. Cancer 2023, 128, 266–274, Correction in Br. J. Cancer 2023, 128, 398. [Google Scholar] [CrossRef]
- Lee, H.; Lee, M.; Seo, J.H.; Gong, G.; Lee, H.J. Changes in tumor-infiltrating lymphocytes after neoadjuvant chemotherapy and clinical significance in triple negative breast cancer. Anticancer Res 2020, 40, 1883–1890. [Google Scholar] [CrossRef]
- Liefaard, M.C.; van der Voort, A.; van Seijen, M.; Thijssen, B.; Sanders, J.; Vonk, S.; Mittempergher, L.; Bhaskaran, R.; de Munck, L.; van Leeuwen-Stok, A.E.; et al. Tumor-infiltrating lymphocytes in HER2-positive breast cancer treated with neoadjuvant chemotherapy and dual HER2-blockade. NPJ Breast Cancer 2024, 10, 29. [Google Scholar] [CrossRef]
- De Angelis, C.; Nagi, C.; Hoyt, C.C.; Liu, L.; Roman, K.; Wang, C.; Zheng, Y.; Veeraraghavan, J.; Sethunath, V.; Nuciforo, P.; et al. Evaluation of the predictive role of tumor immune infiltrate in patients with HER2-positive breast cancer treated with neoadjuvant anti-HER2 therapy without chemotherapy. Clin. Cancer Res. 2020, 26, 738–745. [Google Scholar] [PubMed]
- Bae, S.J.; Kim, J.H.; Lee, M.J.; Baek, S.H.; Kook, Y.; Ahn, S.G.; Cha, Y.J.; Jeong, J. Predictive markers of treatment response to neoadjuvant systemic therapy with dual HER2-blockade. Cancers 2024, 16, 842. [Google Scholar] [CrossRef] [PubMed]
- Çetin, K.; Kökten, Ş.; Sarıkamış, B.; Yıldırım, S.; Gökçe, O.N.; Barışık, N.Ö.; Kılıç, Ü. The association of PD-L1 expression and CD8-positive T cell infiltration rate with the pathological complete response after neoadjuvant treatment in HER2-positive breast cancer. Breast Cancer Res. Treat. 2024, 205, 17–27. [Google Scholar] [CrossRef]
- Hwang, H.W.; Jung, H.; Hyeon, J.; Park, Y.H.; Ahn, J.S.; Im, Y.H.; Nam, S.J.; Kim, S.W.; Lee, J.E.; Yu, J.H.; et al. A nomogram to predict pathologic complete response (pCR) and the value of tumor-infiltrating lymphocytes (TILs) for prediction of response to neoadjuvant chemotherapy (NAC) in breast cancer patients. Breast Cancer Res. Treat. 2019, 173, 255–266. [Google Scholar]
- Solinas, C.; Ceppi, M.; Lambertini, M.; Scartozzi, M.; Buisseret, L.; Garaud, S.; Fumagalli, D.; de Azambuja, E.; Salgado, R.; Sotiriou, C.; et al. Tumor-infiltrating lymphocytes in patients with HER2-positive breast cancer treated with neoadjuvant chemotherapy plus trastuzumab, lapatinib or their combination: A meta-analysis of randomized controlled trials. Cancer Treat. Rev. 2017, 57, 8–15. [Google Scholar]
- Ingold Heppner, B.; Untch, M.; Denkert, C.; Pfitzner, B.M.; Lederer, B.; Schmitt, W.; Eidtmann, H.; Fasching, P.A.; Tesch, H.; Solbach, C.; et al. Tumor-infiltrating lymphocytes: A predictive and prognostic biomarker in neoadjuvant-treated HER2-positive breast cancer. Clin. Cancer Res. 2016, 22, 5747–5754. [Google Scholar] [CrossRef]
- Eustace, A.J.; Madden, S.F.; Fay, J.; Collins, D.M.; Kay, E.W.; Sheehan, K.M.; Furney, S.; Moran, B.; Fagan, A.; Morris, P.G.; et al. The role of infiltrating lymphocytes in the neo-adjuvant treatment of women with HER2-positive breast cancer. Breast Cancer Res. Treat. 2021, 187, 635–645. [Google Scholar]
- Shang, M.; Chi, Y.; Zhang, J.; Chang, J.; Yang, H.; Yin, S.; Tan, Q.; Man, X.; Li, H. The therapeutic effectiveness of neoadjuvant trastuzumab plus chemotherapy for HER2-positive breast cancer can be predicted by tumor-infiltrating lymphocytes and PD-L1 expression. Front. Oncol. 2022, 11, 706606. [Google Scholar] [CrossRef]
- Zhao, J.; Meisel, J.; Guo, Y.; Nahta, R.; Hsieh, K.L.; Peng, L.; Wei, Z.; O’Regan, R.; Li, X. Evaluation of PD-L1, tumor-infiltrating lymphocytes, and CD8+ and FOXP3+ immune cells in HER2-positive breast cancer treated with neoadjuvant therapies. Breast Cancer Res. Treat. 2020, 183, 599–606. [Google Scholar] [CrossRef]
- Fernandez-Martinez, A.; Pascual, T.; Singh, B.; Nuciforo, P.; Rashid, N.U.; Ballman, K.V.; Campbell, J.D.; Hoadley, K.A.; Spears, P.A.; Pare, L.; et al. Prognostic and predictive value of immune-related gene expression signatures vs tumor-infiltrating lymphocytes in early-stage ERBB2/HER2-positive breast cancer: A Correlative Analysis of the CALGB 40601 and PAMELA Trials. JAMA Oncol. 2023, 9, 490–499. [Google Scholar] [PubMed]
- Fasching, P.A.; Szeto, C.; Denkert, C.; Benz, S.; Weber, K.; Spilman, P.; Budczies, J.; Schneeweiss, A.; Stickeler, E.; Schmatloch, S.; et al. Inferred immune-cell activity is an independent predictor of HER2-negative breast cancer prognosis and response to paclitaxel-based therapy in the GeparSepto trial. Clin. Cancer Res. 2023, 29, 2456–2465. [Google Scholar] [CrossRef]
- Vanguri, R.S.; Fenn, K.M.; Kearney, M.R.; Wang, Q.; Guo, H.; Marks, D.K.; Chin, C.; Alcus, C.F.; Thompson, J.B.; Leu, C.S.; et al. Tumor immune microenvironment and response to neoadjuvant chemotherapy in hormone receptor/HER2+ early stage breast cancer. Clin. Breast Cancer 2022, 22, 538–546. [Google Scholar] [CrossRef]
- Hamy, A.S.; Pierga, J.Y.; Sabaila, A.; Laas, E.; Bonsang-Kitzis, H.; Laurent, C.; Vincent-Salomon, A.; Cottu, P.; Lerebours, F.; Rouzier, R.; et al. Stromal lymphocyte infiltration after neoadjuvant chemotherapy is associated with aggressive residual disease and lower disease-free survival in HER2-positive breast cancer. Ann. Oncol. 2017, 28, 2233–2240. [Google Scholar] [CrossRef]
- Ignatiadis, M.; Van den Eynden, G.; Roberto, S.; Fornili, M.; Bareche, Y.; Desmedt, C.; Rothé, F.; Maetens, M.; Venet, D.; Holgado, E.; et al. Tumor-infiltrating lymphocytes in patients receiving trastuzumab/pertuzumab-based chemotherapy: A TRYPHAENA substudy. J. Natl. Cancer Inst. 2019, 111, 69–77. [Google Scholar] [CrossRef]
- Dieci, M.V.; Guarneri, V.; Tosi, A.; Bisagni, G.; Musolino, A.; Spazzapan, S.; Moretti, G.; Vernaci, G.M.; Griguolo, G.; Giarratano, T.; et al. Neoadjuvant Chemotherapy and Immunotherapy in Luminal B-like Breast Cancer: Results of the Phase II GIADA Trial. Clin Cancer Res. 2022, 28, 308–317. [Google Scholar] [CrossRef]
- Matikas, A.; Lövrot, J.; Ramberg, A.; Eriksson, M.; Lindsten, T.; Lekberg, T.; Hedenfalk, I.; Loman, N.; Bergh, J.; Hatschek, T.; et al. Dynamic evaluation of the immune infiltrate and immune function genes as predictive markers for neoadjuvant chemotherapy in hormone receptor positive, HER2 negative breast cancer. Oncoimmunology 2018, 7, e1466017. [Google Scholar] [CrossRef]
- Al-Saleh, K.; Abd El-Aziz, N.; Ali, A.; Abozeed, W.; Abd El-Warith, A.; Ibraheem, A.; Ansari, J.; Al-Rikabi, A.; Husain, S.; Nabholtz, J.M. Predictive and prognostic significance of CD8+ tumor-infiltrating lymphocytes in patients with luminal B/HER 2 negative breast cancer treated with neoadjuvant chemotherapy. Oncol. Lett. 2017, 14, 337–344. [Google Scholar] [CrossRef]
- Song, X.; Ma, J.; Zhang, H.; Zhang, Q. Prognostic significance of the primary tumor site and immune indexes in patients with estrogen receptor-positive, human epidermal growth factor receptor-2-negative breast cancer. Gland Surg. 2020, 9, 1450–1468. [Google Scholar] [CrossRef]
- Jeon, Y.; Lee, G.; Jeong, H.; Gong, G.; Kim, J.; Kim, K.; Jeong, J.H.; Lee, H.J. Proteomic analysis of breast cancer based on immune subtypes. Clin. Proteom. 2024, 21, 17. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Xu, J.; Zhang, T.; Xue, D. Tumor-infiltrating lymphocytes in breast cancer predict the response to chemotherapy and survival outcome: A meta-analysis. Oncotarget 2016, 7, 44288–44298. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Warren, S.; Pelekanou, V.; Wali, V.; Cesano, A.; Liu, M.; Danaher, P.; Elliott, N.; Nahleh, Z.A.; Hayes, D.F.; et al. Immune profiling of pre- and post-treatment breast cancer tissues from the SWOG S0800 neoadjuvant trial. J. Immunother. Cancer 2019, 7, 88. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, A.; Horimoto, Y.; Onagi, H.; Ikarashi, D.; Nakayama, T.; Nakatsura, T.; Shimizu, H.; Kojima, K.; Yao, T.; Matsumoto, T.; et al. Plasma cell infiltration and treatment effect in breast cancer patients treated with neoadjuvant chemotherapy. Breast Cancer Res. 2021, 23, 99. [Google Scholar] [CrossRef]
- Seo, A.N.; Lee, H.J.; Kim, E.J.; Kim, H.J.; Jang, M.H.; Lee, H.E.; Kim, Y.J.; Kim, J.H.; Park, S.Y. Tumour-infiltrating CD8+ lymphocytes as an independent predictive factor for pathological complete response to primary systemic therapy in breast cancer. Br. J. Cancer 2013, 109, 2705–2713. [Google Scholar] [CrossRef]
- Kitano, A.; Ono, M.; Yoshida, M.; Noguchi, E.; Shimomura, A.; Shimoi, T.; Kodaira, M.; Yunokawa, M.; Yonemori, K.; Shimizu, C.; et al. Tumour-infiltrating lymphocytes are correlated with higher expression levels of PD-1 and PD-L1 in early breast cancer. ESMO Open 2017, 2, e000150. [Google Scholar] [CrossRef]
- Alistar, A.; Chou, J.W.; Nagalla, S.; Black, M.A.; D’Agostino, R., Jr.; Miller, L.D. Dual roles for immune metagenes in breast cancer prognosis and therapy prediction. Genome Med. 2014, 6, 80. [Google Scholar] [CrossRef]
- Faur, I.F.; Dobrescu, A.; Clim, A.I.; Pasca, P.; Prodan-Barbulescu, C.; Gherle, B.D.; Tarta, C.; Isaic, A.; Brebu, D.; Duta, C.; et al. The value of tumor infiltrating lymphocytes (TIL) for predicting the response to neoadjuvant chemotherapy (NAC) in breast cancer according to the molecular subtypes. Biomedicines 2023, 11, 3037. [Google Scholar] [CrossRef] [PubMed]
- Landén, A.H.; Chin, K.; Kovács, A.; Holmberg, E.; Molnar, E.; Stenmark Tullberg, A.; Wärnberg, F.; Karlsson, P. Evaluation of tumor-infiltrating lymphocytes and mammographic density as predictors of response to neoadjuvant systemic therapy in breast cancer. Acta Oncol. 2023, 62, 1862–1872. [Google Scholar] [CrossRef]
- Erol, V.B.; Goktas Aydin, S.; Bilici, A.; Cakir, A.; Acikgoz, O.; Olmez, O.F.; Basim, P. Relationship between the change in tumour-infiltrating lymphocyte level and residual tumour after neoadjuvant chemotherapy in patients with locally advanced breast cancer. J. Chemother. 2023, 35, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Kusama, H.; Kittaka, N.; Soma, A.; Taniguchi, A.; Kanaoka, H.; Nakajima, S.; Oyama, Y.; Seto, Y.; Okuno, J.; Watanabe, N.; et al. Predictive factors for response to neoadjuvant chemotherapy: Inflammatory and immune markers in triple-negative breast cancer. Breast Cancer 2023, 30, 1085–1093. [Google Scholar] [CrossRef]
- Wimberly, H.; Brown, J.R.; Schalper, K.; Haack, H.; Silver, M.R.; Nixon, C.; Bossuyt, V.; Pusztai, L.; Lannin, D.R.; Rimm, D.L. PD-L1 expression correlates with tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy in breast cancer. Cancer Immunol. Res. 2015, 3, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tan, Q.; Li, H.; Yang, X. Predictive value of tumor-infiltrating lymphocytes for response to neoadjuvant chemotherapy and breast cancer prognosis. J. Surg. Oncol. 2021, 123, 89–95. [Google Scholar] [CrossRef]
- Goda, N.; Sasada, S.; Shigematsu, H.; Masumoto, N.; Arihiro, K.; Nishikawa, H.; Sakaguchi, S.; Okada, M.; Kadoya, T. The ratio of CD8 + lymphocytes to tumor-infiltrating suppressive FOXP3 + effector regulatory T cells is associated with treatment response in invasive breast cancer. Discov. Oncol. 2022, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.R.; Wimberly, H.; Lannin, D.R.; Nixon, C.; Rimm, D.L.; Bossuyt, V. Multiplexed quantitative analysis of CD3, CD8, and CD20 predicts response to neoadjuvant chemotherapy in breast cancer. Clin. Cancer Res. 2014, 20, 5995–6005. [Google Scholar] [CrossRef]
- Balkenhol, M.C.; Ciompi, F.; Świderska-Chadaj, Ż.; van de Loo, R.; Intezar, M.; Otte-Höller, I.; Geijs, D.; Lotz, J.; Weiss, N.; de Bel, T.; et al. Optimized tumour infiltrating lymphocyte assessment for triple negative breast cancer prognostics. Breast 2021, 56, 78–87. [Google Scholar] [CrossRef]
- Hendry, S.; Salgado, R.; Gevaert, T.; Russell, P.A.; John, T.; Thapa, B.; Christie, M.; van de Vijver, K.; Estrada, M.V.; Gonzalez-Ericsson, P.I.; et al. Assessing tumor-infiltrating lymphocytes in solid tumors: A practical review for pathologists and proposal for a standardized method from the international immunooncology biomarkers working group: Part 1: Assessing the host immune response, TILs in invasive breast carcinoma and ductal carcinoma in situ, metastatic tumor deposits and areas for further research. Adv. Anat. Pathol. 2017, 24, 235–251. [Google Scholar] [CrossRef]
- Swisher, S.K.; Wu, Y.; Castaneda, C.A.; Lyons, G.R.; Yang, F.; Tapia, C.; Wang, X.; Casavilca, S.A.; Bassett, R.; Castillo, M.; et al. Interobserver agreement between pathologists assessing tumor-infiltrating Lymphocytes (TILs) in breast cancer using methodology proposed by the international TILs working group. Ann. Surg. Oncol. 2016, 23, 2242–2248. [Google Scholar] [CrossRef]
- Tramm, T.; Di Caterino, T.; Jylling, A.B.; Lelkaitis, G.; Lænkholm, A.V.; Ragó, P.; Tabor, T.P.; Talman, M.M.; Vouza, E.; Scientific Committee of Pathology, Danish Breast Cancer Group (DBCG). Standardized assessment of tumor-infiltrating lymphocytes in breast cancer: An evaluation of inter-observer agreement between pathologists. Acta Oncol. 2018, 57, 90–94. [Google Scholar] [CrossRef]
- Jahangir, C.A.; Page, D.B.; Broeckx, G.; Gonzalez, C.A.; Burke, C.; Murphy, C.; Reis-Filho, J.S.; Ly, A.; Harms, P.W.; Gupta, R.R.; et al. Image-based multiplex immune profiling of cancer tissues: Translational implications. A report of the International Immuno-oncology Biomarker Working Group on Breast Cancer. J. Pathol. 2024, 262, 271–288. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.; Kawai, A.; Wakisaka, M.; Funaoka, Y.; Yasuda, N.; Hidaka, M.; Morita, Y.; Ohtani, S.; Ito, M.; Arihiro, K. A potential role for peripheral natural killer cell activity induced by preoperative chemotherapy in breast cancer patients. Cancer Immunol. Immunother. 2019, 68, 577–585. [Google Scholar] [CrossRef]
- Kim, R.; Kawai, A.; Wakisaka, M.; Sawada, S.; Shimoyama, M.; Yasuda, N.; Hidaka, M.; Morita, Y.; Ohtani, S.; Ito, M.; et al. Immune factors associated with the pathological and therapeutic effects of preoperative chemotherapy in patients with breast cancer. Transl. Oncol. 2021, 14, 100927. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Z. The history and advances in cancer immunotherapy: Understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol. Immunol. 2020, 17, 807–821. [Google Scholar] [CrossRef]
- Naimi, A.; Mohammed, R.N.; Raji, A.; Chupradit, S.; Yumashev, A.V.; Suksatan, W.; Shalaby, M.N.; Thangavelu, L.; Kamrava, S.; Shomali, N.; et al. Tumor immunotherapies by immune checkpoint inhibitors (ICIs); the pros and cons. Cell Commun. Signal. 2022, 20, 44. [Google Scholar] [CrossRef]
- Valenza, C.; Rizzo, G.; Passalacqua, M.I.; Boldrini, L.; Corti, C.; Trapani, D.; Curigliano, G. Evolving treatment landscape of immunotherapy in breast cancer: Current issues and future perspectives. Ther. Adv. Med. Oncol. 2023, 15, 17588359221146129. [Google Scholar] [CrossRef]
- Zhang, H.; Mi, J.; Xin, Q.; Cao, W.; Song, C.; Zhang, N.; Yuan, C. Recent research and clinical progress of CTLA-4-based immunotherapy for breast cancer. Front. Oncol. 2023, 13, 1256360. [Google Scholar] [CrossRef]
- Deng, H.; Wang, G.; Zhao, S.; Tao, Y.; Zhang, Z.; Yang, J.; Lei, Y. New hope for tumor immunotherapy: The macrophage-related “do not eat me” signaling pathway. Front. Pharmacol. 2023, 14, 1228962. [Google Scholar] [CrossRef]
- Advani, R.; Flinn, I.; Popplewell, L.; Forero, A.; Bartlett, N.L.; Ghosh, N.; Kline, J.; Roschewski, M.; LaCasce, A.; Collins, G.P.; et al. CD47 Blockade by Hu5F9-G4 and Rituximab in Non-Hodgkin’s Lymphoma. N. Engl. J. Med. 2018, 379, 1711–1721. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.Q.; Waaijer, S.J.H.; Zwager, M.C.; de Vries, E.G.E.; van der Vegt, B.; Schröder, C.P. Tumor-associated macrophages in breast cancer: Innocent bystander or important player? Cancer Treat. Rev. 2018, 70, 178–189. [Google Scholar] [CrossRef]
- Zwager, M.C.; Bense, R.; Waaijer, S.; Qiu, S.Q.; Timmer-Bosscha, H.; de Vries, E.G.E.; Schröder, C.P.; van der Vegt, B. Assessing the role of tumour-associated macrophage subsets in breast cancer subtypes using digital image analysis. Breast Cancer Res. Treat. 2023, 198, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wang, R.; Chen, X.; Hou, Y.; Jiang, J. Targeting CD47 as a Novel Immunotherapy for Breast Cancer. Front. Oncol. 2022, 12, 924740. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.M.; Brennan, M.J.; Elliott, J.A.; Abd Elwahab, S.; Barry, K.; Sweeney, K.; Malone, C.; Lowery, A.; Mclaughlin, R.; Kerin, M.J. Neoadjuvant chemotherapy for luminal a breast cancer: Factors predictive of histopathologic response and oncologic outcome. Am. J. Surg. 2021, 222, 368–376. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 2015, 517, 576–582. [Google Scholar]
- Zhang, Q.; Qin, J.; Zhong, L.; Gong, L.; Zhang, B.; Zhang, Y.; Gao, W.Q. CCL5-Mediated Th2 Immune Polarization Promotes Metastasis in Luminal Breast Cancer. Cancer Res. 2015, 75, 4312–4321. [Google Scholar] [CrossRef]
- Svensson, S.; Abrahamsson, A.; Rodriguez, G.V.; Olsson, A.K.; Jensen, L.; Cao, Y.; Dabrosin, C. CCL2 and CCL5 Are Novel Therapeutic Targets for Estrogen-Dependent Breast Cancer. Clin. Cancer Res. 2015, 21, 3794–3805. [Google Scholar] [CrossRef]
- Oner, G.; Altintas, S.; Canturk, Z.; Tjalma, W.; Van Berckelaer, C.; Broeckx, G.; Zwaenepoel, K.; Tholhuijsen, M.; Verhoeven, Y.; Berneman, Z.; et al. The immunologic aspects in hormone receptor positive breast cancer. Cancer Treat. Res. Commun. 2020, 25, 100207. [Google Scholar] [CrossRef] [PubMed]
- Haque, W.; Verma, V.; Hatch, S.; Suzanne Klimberg, V.; Brian Butler, E.; Teh, B.S. Response rates and pathologic complete response by breast cancer molecular subtype following neoadjuvant chemotherapy. Breast Cancer Res. Treat. 2018, 170, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Luen, S.; Virassamy, B.; Savas, P.; Salgado, R.; Loi, S. The genomic landscape of breast cancer and its interaction with host immunity. Breast 2016, 29, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Ciarka, A.; Piątek, M.; Pęksa, R.; Kunc, M.; Senkus, E. Tumor-infiltrating lymphocytes (TILs) in breast cancer: Prognostic and predictive significance across molecular subtypes. Biomedicines 2024, 12, 763. [Google Scholar] [CrossRef]
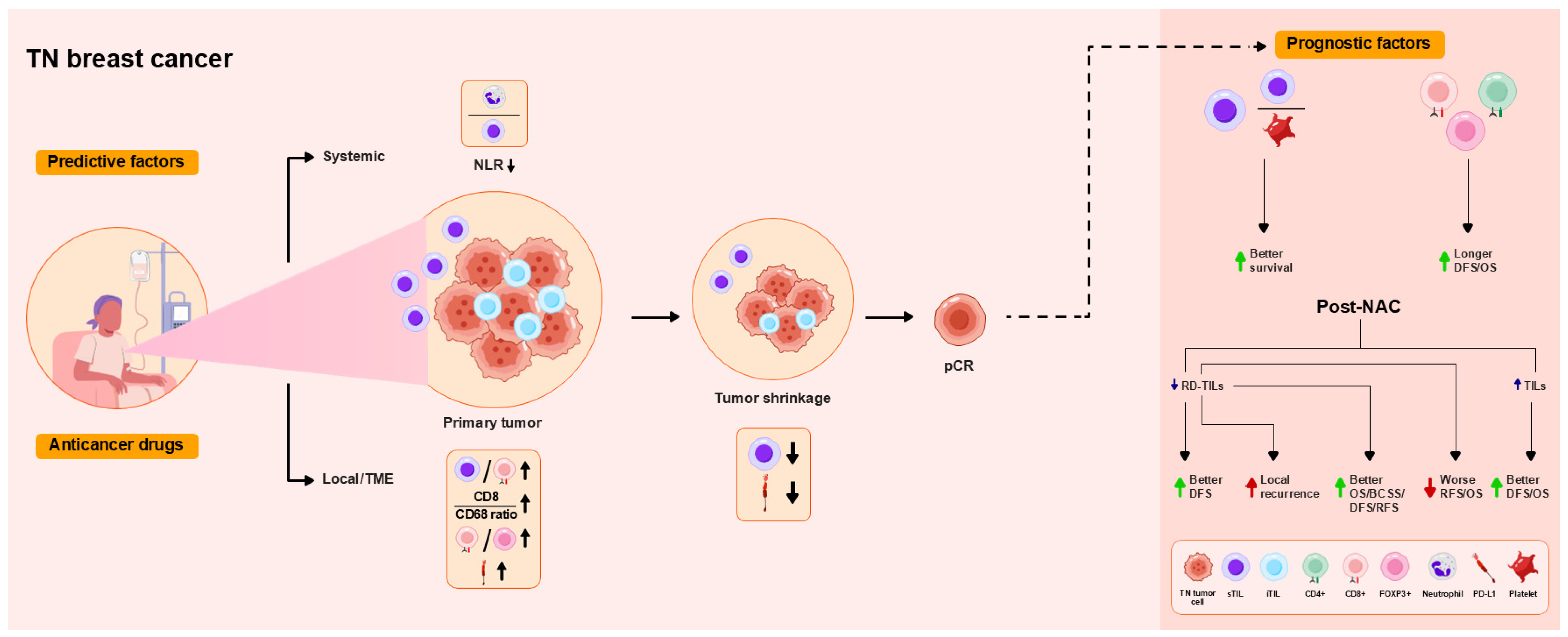
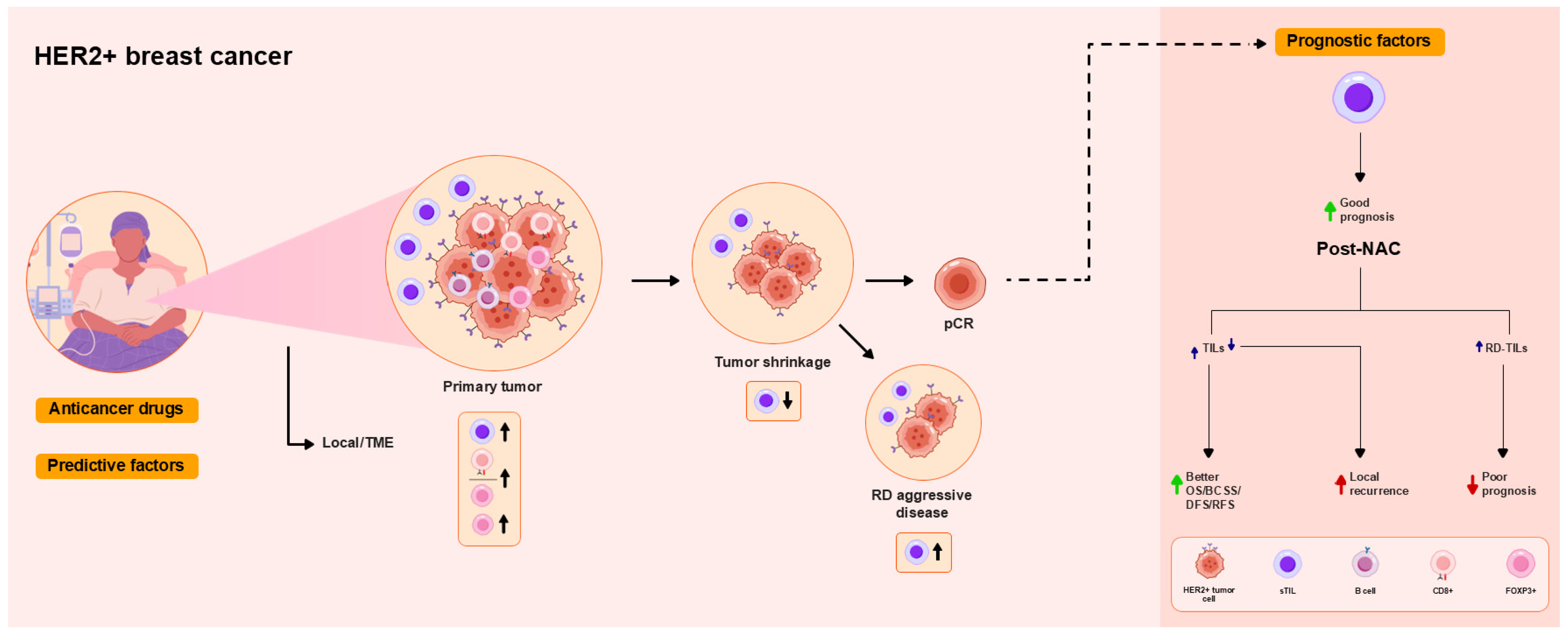
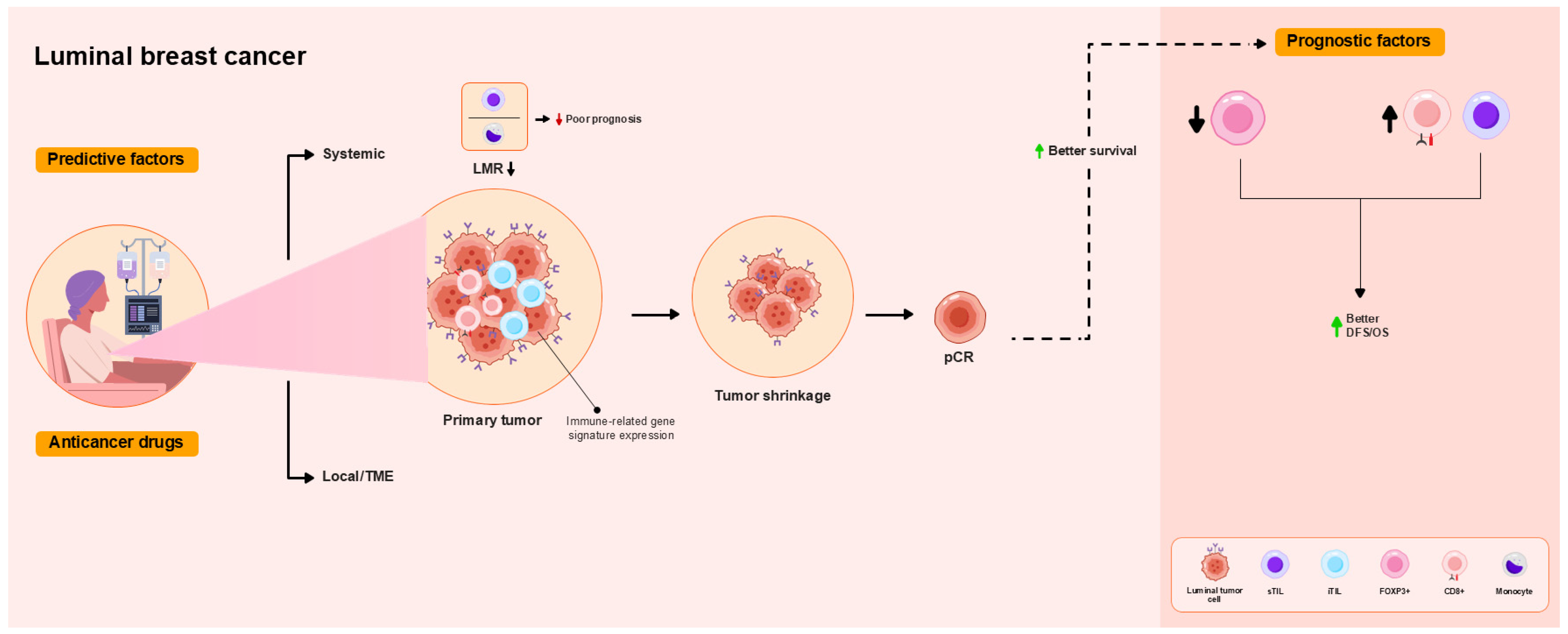


| Ref. (Year) | Subtype | TILs/Other | Immunophenotype/ Gene and Protein Expression | Predictive Factors | Prognostic Factors |
|---|---|---|---|---|---|
| [27] (2024) | TN | TILs | ND | TILs associated with pCR after NAC+ICI | ND |
| [47] (2023) | TN | RD TILs | CD4, CD8 | ND | Increased OS/BCSS with high TIL levels in RD after NAC |
| [28] (2023) | TN | TILs/NLR | CD8, FOXP3 | Low NLR/high TIL density associated with high pCR rate | Low NLR/high TIL levels associated with improved OS/BCSS |
| [39] (2023) | TN | sTILs/immune mediators | ND | Systemic immune mediators correlated with high TIL levels, leading to pCR | Circulating cytokines with TILs predict improved survival |
| [29] (2023) | TN | TILs | PD-L1 | High TIL levels associated with pCR; PD-L1+ associated with higher pCR rate after anticancer drug+ICI | ND |
| [40] (2023) | TN | TILs | CD8/FOXP3 ratio, PD-1, PD-L1 | CD8/FOXP3 ratio only independent predictor of pCR | ND |
| [48] (2023) | TN | sTILs | ND | Higher pCR rate with higher TIL levels, Carb incorporation into A-T | Increased TIL levels in RD associated with distant DFS for A-T, A-T/Carb |
| [38] (2022) | TN | TILs | ND | Change in TIL levels with treatment correlated with metabolic response (SUV), pCR | High post-NAC TIL levels predicted poor RFS/OS |
| [35] (2022) | TN | TILs | ND | Patients aged <40 years had higher TIL levels, pCR rate | ND |
| [45] (2021) | TN | TILs/PLR | ND | TIL levels retained pCR predictive value | PLR better predictor of less distant recurrence or longer distant RFS with pCR or small RD |
| [37] (2021) | TN | TILs | TCRs, PD-L1, CD3/CD68 ratio | Higher TCR clonality, PD-L1+, higher CD3/CD68 ratios, closer T/tumor cell proximity associated with pCR; T cell proximity, PD-L1 expression enhanced pCR prediction | ND |
| [25] (2021) | TN | TILs | PD-L1, TIM-3, LAG-3 | TIL levels, PD-L1 expression decreased after NAC in pCR; TIM-3+ more frequent in non-pCR | High TIL levels associated with better OS; PD-L1 expression, TIM-3+ associated with worse OS |
| [46] (2020) | TNI | TILs | ND | ND | TIL levels associated negatively with EFS |
| [42] (2020) | TN | sTILs | ND | sTIL levels associated with RCB 0/I status, not pCR; HRD associated with pCR, RCB 0/I status | ND |
| [49] (2020) | TN | TILs | ND | ND | Decrease in TIL levels improved DFS |
| [32] (2019) | TN | TILs | ND | High TIL levels associated with diffuse TIL pattern, high pCR rate | Higher TIL density, diffuse TIL pattern correlated with improved RFS/OS |
| [43] (2019) | TN | TILs | ND | RD TIL levels correlated with CD8 T cell density | Higher RD TIL levels associated with improved RFS/OS, especially in RCB II |
| [33] (2018) | TN/HER2+ | TILs | ND | TILs as strong predictors of pCR | ND |
| [30] (2018) | TN/HER2+ | TILs | ND | High TIL levels associated with pCR | High TIL levels predict good prognosis; decreased TIL levels potentially associated with local recurrence |
| [36] (2017) | TN | TILs | ND | pCR rate higher for LPBC than non-LPBC | DFS better for LPBC than non-LPBC |
| [31] (2016) | TN | TILs | CD3, CD4, CD8, FOXP3 | Higher pre-NAC TIL levels, CD8/CD4 ratio, post-NAC CD4 level associated with pCR | Longer DFS associated with higher pre-NAC CD3, CD4, CD8, FOXP3 levels, CD4/FOXP3 ratio; longer OS associated with higher pre-NAC CD3 level |
| [34] (2012) | TN | TILs/apoptosis | ND | Higher TIL levels, apoptosis scores associated with pCR | ND |
| Ref. (Year) | Subtype | TILs/Other | Immunophenotype/ Gene and Protein Expression | Predictive Factors | Prognostic Factors |
|---|---|---|---|---|---|
| [50] (2024) | HER2+ | TILs | ND | TIL levels not associated with pCR; trend of association for HR+ | Excellent 3-year invasive DFS, regardless of pCR |
| [52] (2024) | HER2+ | TILs | ND | TIL levels associated with pCR, numerical or high levels predict pCR | ND |
| [53] (2024) | HER2+ | TILs | CD8 | High TIL levels, CD8 infiltration predict pCR | ND |
| [15] (2023) | HER2+ | RD TILs | ND | ND | Higher RD TIL levels associated with poor prognosis |
| [61] (2023) | HER2− | TILs | iICA of 23 immune cell types | TIL levels correlated with iICA cluster, better predicted pCR | iICA cluster predicted better DFS/OS |
| [60] (2023) | HER2+ | TILs/immune-related signatures | 36 immune signatures | Multiple B cell-related signatures more associated with pCR than TIL levels | Multiple B cell-related signatures more associated with EFS than TIL levels |
| [62] (2022) | HR+/HER2+ | sTILs | CD3+, CD3+CD8−FOXP3−, CD8+, FOXP3+ | sTILs were associated with pCR; increased CD3+, CD3+CD8−FOXP3−, CD8+, FOXP3+ sTIL levels, immune cell aggregates associated with pCR in TME | ND |
| [58] (2022) | HER2+ | TILs | PD-L1 | High TIL, PD-L1+ TIL levels predicted pCR; PD-L1+ tumor cells reduced by Tz + chemotherapy | ND |
| [57] (2021) | HER2+ | TILs | TILs, sTILs, CD4 | pCR associated with higher TIL (not sTIL) levels pre-treatment, higher sTIL (not TIL) levels during treatment; infiltrative lymphocyte levels increased in non-pCR, CD8 levels decreased in pCR cases during treatment | ND |
| [59] (2020) | HER2+ | TILs, Ki-67 | CD8, FOXP3 | High Ki-67 levels strongly predict pCR; TIL, and FOXP3+ T cell levels may play roles in tumor response | ND |
| [51] (2020) | HER2+ | sTILs | CD4, CD8, CD20, CD68, FOXP3 | High CD4+, CD8+, CD20+ sTIL, CD20+ iTIL levels associated with higher pCR rates; pCR associated with more baseline sCD4, iCD4, iCD20+ TIL infiltration | ND |
| [54] (2019) | HER2+ | TILs | ND | High pre-NAC TIL levels predict pCR | Increased post-NAC TIL levels associated with improved BCSS/DFS |
| [64] (2019) | HER2+ | TILs | ND | Baseline TIL levels not associated with pCR | Increased baseline TIL levels associated with improved EFS |
| [33] (2018) | TN/HER2+ | TILs | ND | TIL levels strongly predict pCR | ND |
| [30] (2018) | TN/HER2+ | TILs | ND | High TIL levels associated with pCR | High TIL levels predicted good prognosis; decreased TIL levels potentially associated with local recurrence |
| [63] (2017) | HER2+ | sTILs | ND | Magnitude of TIL level decrease associated with pCR; High post-NAC TIL levels in RD associated with aggressive disease | High post-NAC TIL levels in RD associated with worse outcomes |
| [55] (2017) | HER2+ | TILs | ND | High baseline TIL levels associated with increased pCR | ND |
| [56] (2016) | HER2+ | TILs | ND | LPBC increased, predicted pCR | TILs more prognostically relevant than pCR in triple-positive breast cancer |
| Ref. (Year) | Subtype | TILs/Other | Immunophenotype/ Gene and Protein Expression | Predictive Factors | Prognostic Factors |
|---|---|---|---|---|---|
| [65] (2022) | L-B | TILs/immune-related gene signatures, immune cell subpopulations | ND | TIL levels, immune-related gene signatures, immune cell subpopulations associated with pCR; sequential anthracyclines/anti-PD-1 may activate antitumor immune response in basal molecular subtype | ND |
| [68] (2020) | ER+/HER2− | TILs/LMR | ND | ND | TILs > 10%, LMR < 5.2 correlated with poor prognosis |
| [66] (2018) | HR+/HER2− | TILs/GE | FOXP3 T cells, CD163 macrophages | GE associated with higher pCR rates, tumor shrinkage. GE, TIL levels predicted tumor shrinkage | Low FOXP3 levels associated with improved DFS |
| [67] (2017) | L-B/HER2− | TILs | CD8 | iCD8+ TIL level correlated with pCR | CD8+ TIL level correlated with DFS/OS; strong iCD8+ TIL expression associated with OS |
| Ref. (Year) | Subtype | TILs/Other | Immunophenotype/ Gene and Protein Expression | Predictive Factors | Prognostic Factors |
|---|---|---|---|---|---|
| [69] (2024) | NS | TILs | Titin | Titin expression elevated in pCR | ND |
| [76] (2024) | NS | sTILs/iTILs | ND | Pre-NAC sTIL levels predict pCR regardless of tumor subtype | ND |
| [77] (2024) | NS | TILs | ND | High TIL levels associated independently with pCR | ND |
| [79] (2023) | NS | TILs/NLR, PLR | ND | TIL levels, NLR, PLR predict pCR | ND |
| [78] (2023) | NS | TILs | ND | Higher pre-NAC TIL levels predict pCR | Lower post-NAC TIL levels and pCR associated with DFS |
| [44] (2023) | NS | sTILs | ND | High TIL levels predict pCR regardless of subtype | pCR associated with improved DFS/OS in TN, HER2+ (not luminal) subtypes |
| [82] (2022) | NS | TILs | eTregs, CD4+FOXP3highCD45RA−, other FOXP3+ Treg subsets, CD8 | CD8/eTreg ratio independently predicts pCR | ND |
| [5] (2021) | NS | TILs | ND | High pre-NAC TIL levels predict higher pCR rates | Pre-NAC TIL levels associated with DFS |
| [81] (2021) | NS | TILs/LNR | ND | TIL levels may predict pCR rate, postoperative residual lymph node involvement | TIL levels may predict DFS |
| [72] (2021) | NS | PCs, CD8, CD4, CD8 FOXP3, B cells | ND | PC and B cell infiltration correlated with pCR | PC infiltration correlated with longer DFS in HR− patients |
| [11] (2020) | NS | TILs | ND | High TIL levels predict pCR in TN, HER2-enriched (not luminal) subtypes | High TIL levels associated with better DFS/OS for TN, HER2-enriched subtypes, worse survival for luminal subtype |
| [13] (2019) | NS | sTILs | ND | Pre-NAC TIL levels higher in pCR cases for luminal, TN (not HER2+) subtypes; mean TIL levels decreased after NAC in association with pCR | Pre-NAC TIL levels associated with DFS; High post-NAC TIL levels associated with impaired DFS in HER2 (not luminal, TN) subtype |
| [71] (2019) | NS | TILs/CCL21, CCL19 | CTL | Higher baseline TIL, chemoattractant cytokine (CCL21, CCL19), CTL marker levels associated with higher pCR rates; stromal functions associated with RD; TIL levels, most immune gene expression decreased after NAC in pCR cases | ND |
| [26] (2018) | NS | TILs/PD-L1 | ND | Higher baseline TIL levels, PD-L1+ rate associated with higher pCR rate; TIL levels (not PD-L1 expression) decreased after NAC | ND |
| [12] (2018) | NS | TILs | ND | Increased TIL levels predicted response to NAC in all subtypes | Increased TIL levels associated with improved DFS in TN, HER2+ (not luminal) subtypes, improved OS in TN (not HER2+) subtype, shorter OS in luminal subtype |
| [14] (2018) | NS | TILs | ND | Higher TIL levels associated with higher pCR rates in TN subtype, tended to be associated with pCR in luminal subtype | Low post-NAC TIL levels associated with better RFS for luminal subtype |
| [9] (2018) | NS | sTILs/iTILs | ND | pCR predicted by iTIL (not sTIL) levels in luminal subtype, iTIL and sTIL levels in TN subtype, neither in HER2+ subtype | ND |
| [74] (2017) | NS | TILs/PD-L1, PD-1 | ND | PD-L1, PD-1 expression correlated with increased TIL levels, pCR | PD-L1, PD-1 expression correlated with poor prognosis |
| [70] (2016) | NS | TILs | CD4, CD8, FOXP3 | High TIL levels associated with improved pCR rates, especially in TN subtype; CD8 (not CD4, FOXP3) infiltration associated with higher pCR rates | High TIL levels associated with longer DFS/OS |
| [10] (2016) | NS | iTILs/sTILs | ND | iTIL, sTIL levels correlated with pCR in HER2+, TN subtypes; no biomarker correlated with pCR in luminal subtype | ND |
| [41] (2016) | NS | TILs | CFR | High CFRs associated with higher pCR rates in TN and HER2+ subtypes | High CFRs predict good prognosis |
| [80] (2015) | NS | iTILs/sTILs/PD-L1 | ND | iTIL levels, sTIL levels, strong PD-L1 expression predict pCR | ND |
| [75] (2014) | NS | ND | B/P, T/NK, M/D metagenes | B/P, M/D, to lesser extent T/NK metagenes associated with favorable pathological responses | ND |
| [83] (2014) | NS | sTILs | CD3, CD8, CD20 | TIL levels, stromal AQUA scores for CD3, CD8, CD20 predicted pCR | ND |
| Subtype | Predictive Factors | Prognostic Factors |
|---|---|---|
| TN |
|
|
| HER2 |
|
|
| Luminal |
|
|
| NS |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, R.; Kin, T.; Arihiro, K. Roles of Tumor-Infiltrating Lymphocytes and Antitumor Immune Responses as Predictive and Prognostic Markers in Patients with Breast Cancer Receiving Neoadjuvant Chemotherapy. Int. J. Mol. Sci. 2025, 26, 9959. https://doi.org/10.3390/ijms26209959
Kim R, Kin T, Arihiro K. Roles of Tumor-Infiltrating Lymphocytes and Antitumor Immune Responses as Predictive and Prognostic Markers in Patients with Breast Cancer Receiving Neoadjuvant Chemotherapy. International Journal of Molecular Sciences. 2025; 26(20):9959. https://doi.org/10.3390/ijms26209959
Chicago/Turabian StyleKim, Ryungsa, Takanori Kin, and Koji Arihiro. 2025. "Roles of Tumor-Infiltrating Lymphocytes and Antitumor Immune Responses as Predictive and Prognostic Markers in Patients with Breast Cancer Receiving Neoadjuvant Chemotherapy" International Journal of Molecular Sciences 26, no. 20: 9959. https://doi.org/10.3390/ijms26209959
APA StyleKim, R., Kin, T., & Arihiro, K. (2025). Roles of Tumor-Infiltrating Lymphocytes and Antitumor Immune Responses as Predictive and Prognostic Markers in Patients with Breast Cancer Receiving Neoadjuvant Chemotherapy. International Journal of Molecular Sciences, 26(20), 9959. https://doi.org/10.3390/ijms26209959





