Precision Profiling of Disease Progression in Murine Models of Sepsis and Septic Shock
Abstract
1. Introduction
2. Results
2.1. Survival Series
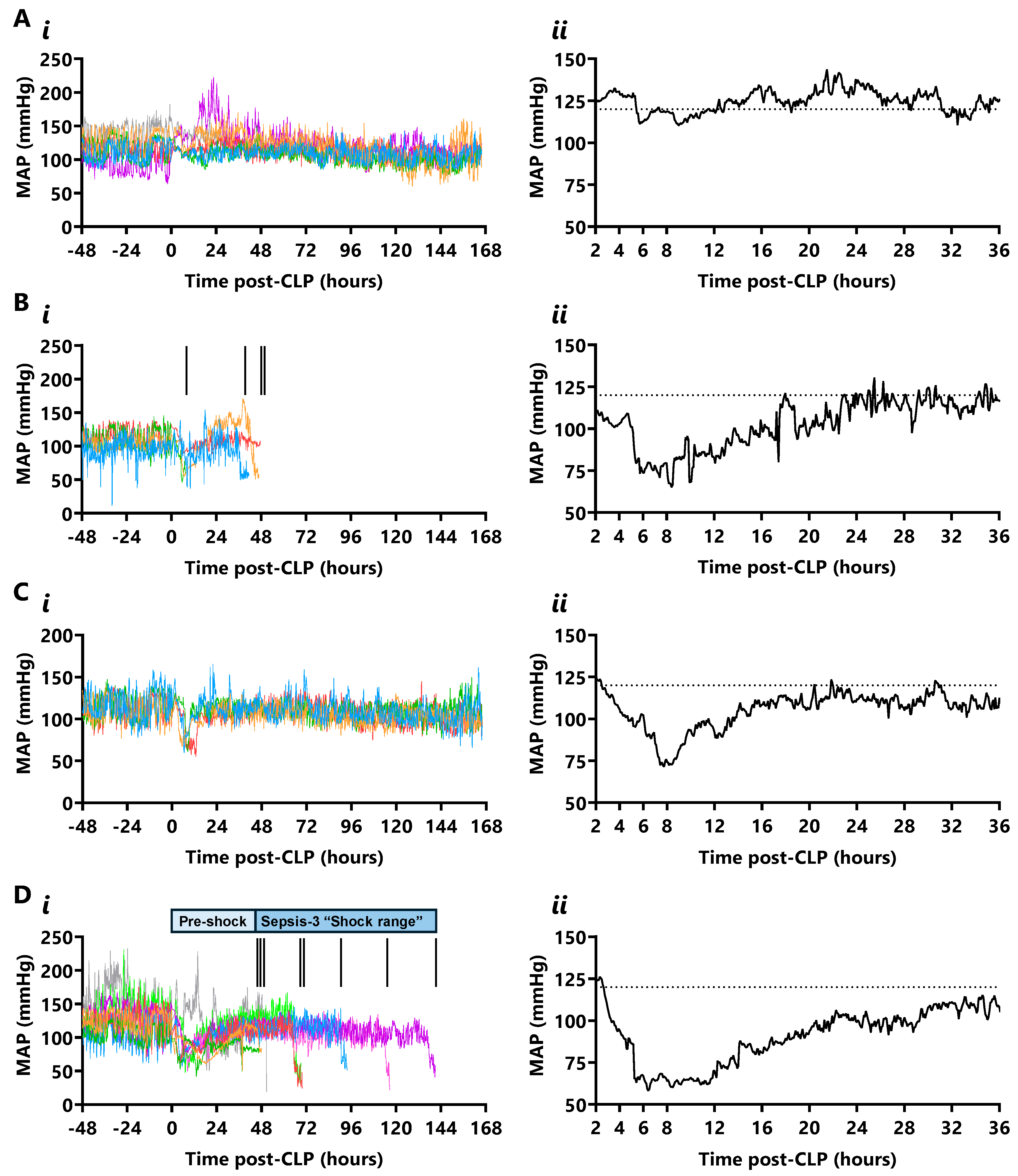
2.2. Mean Arterial Pressure (MAP)
2.3. Heart Rate
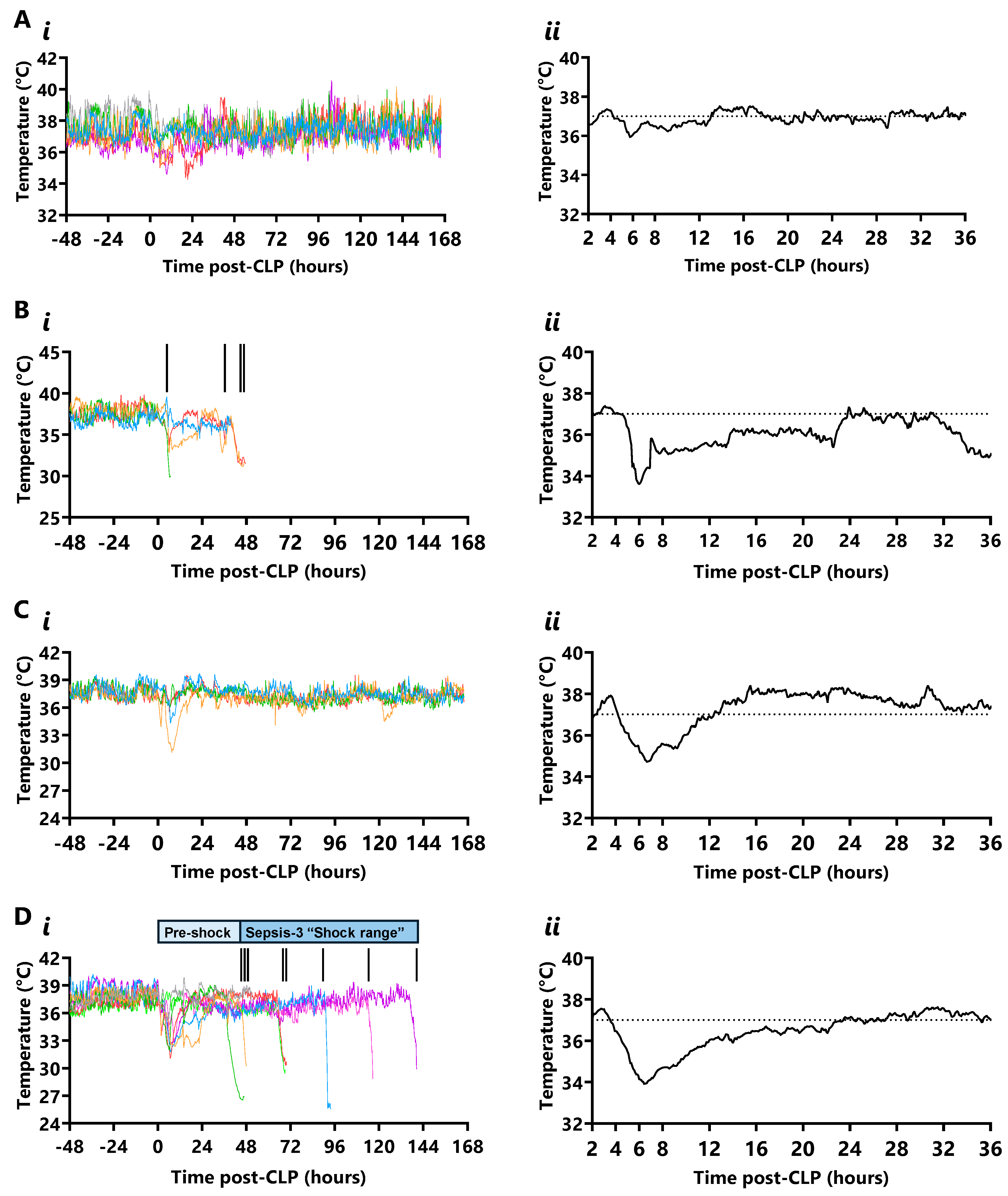
2.4. Temperature
2.5. Time Course Series
Plasma Damage and Stress Markers
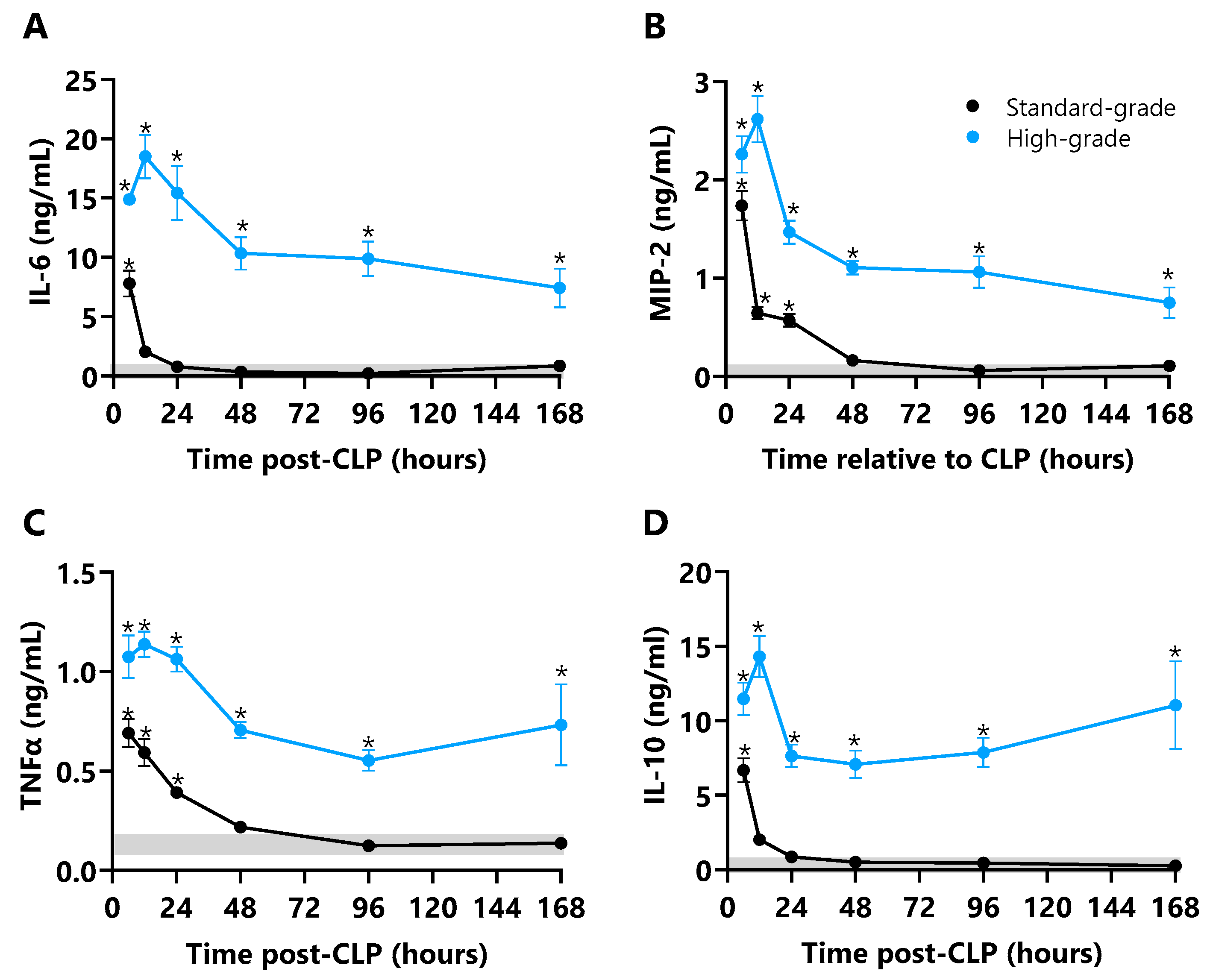
2.6. Plasma Cytokines
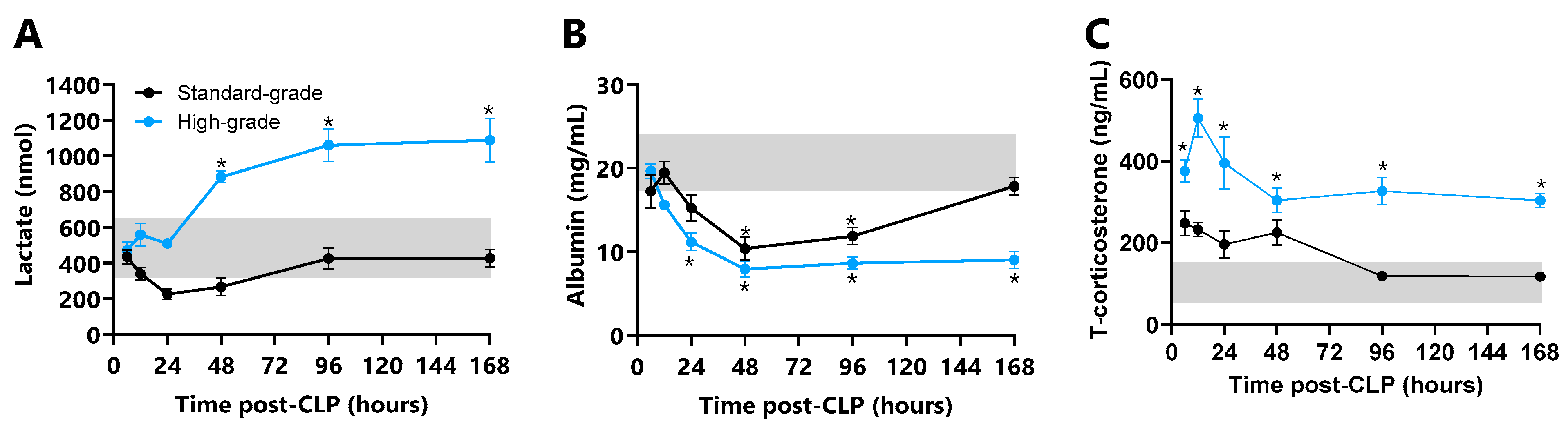
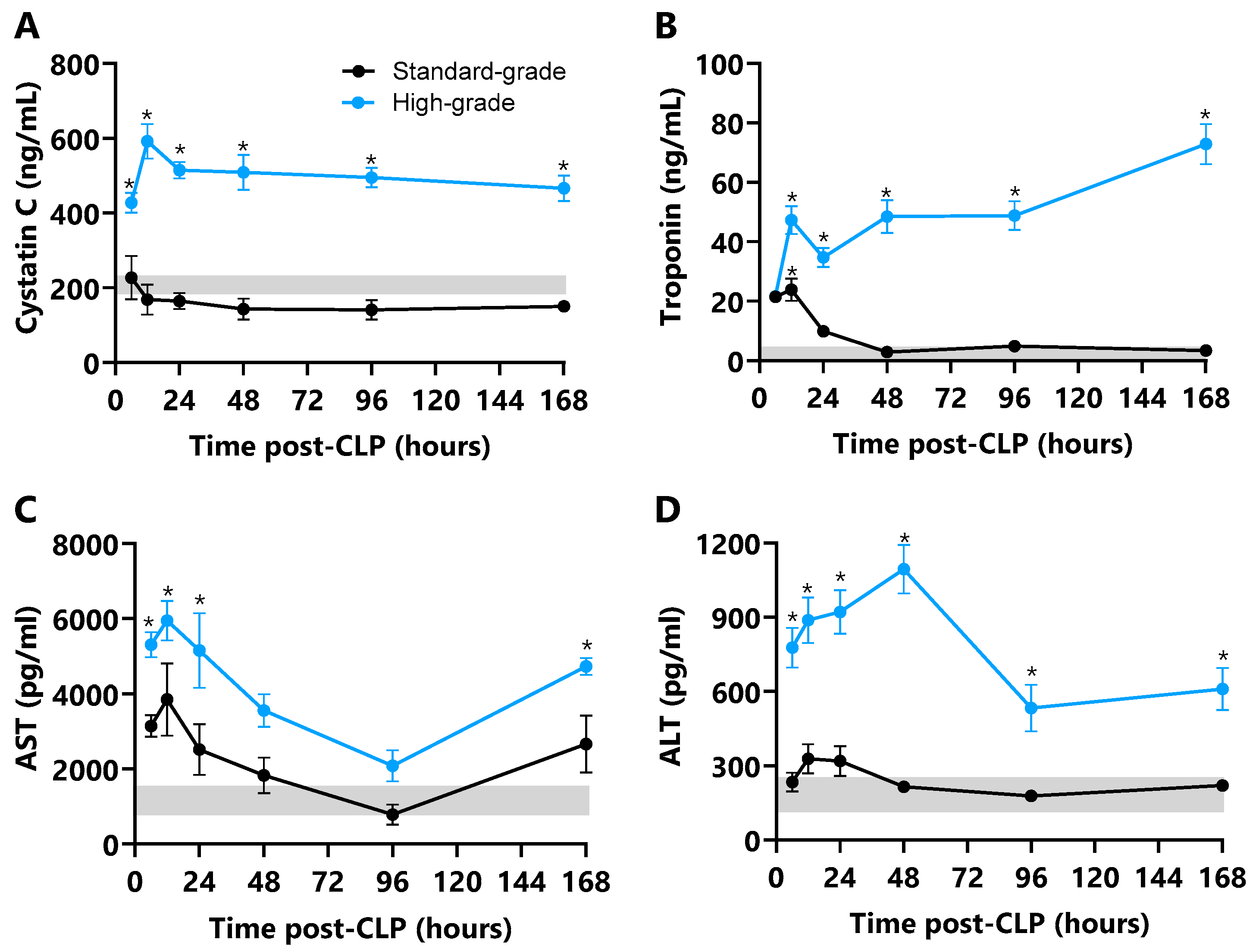
2.7. Plasma Markers of Organ Damage
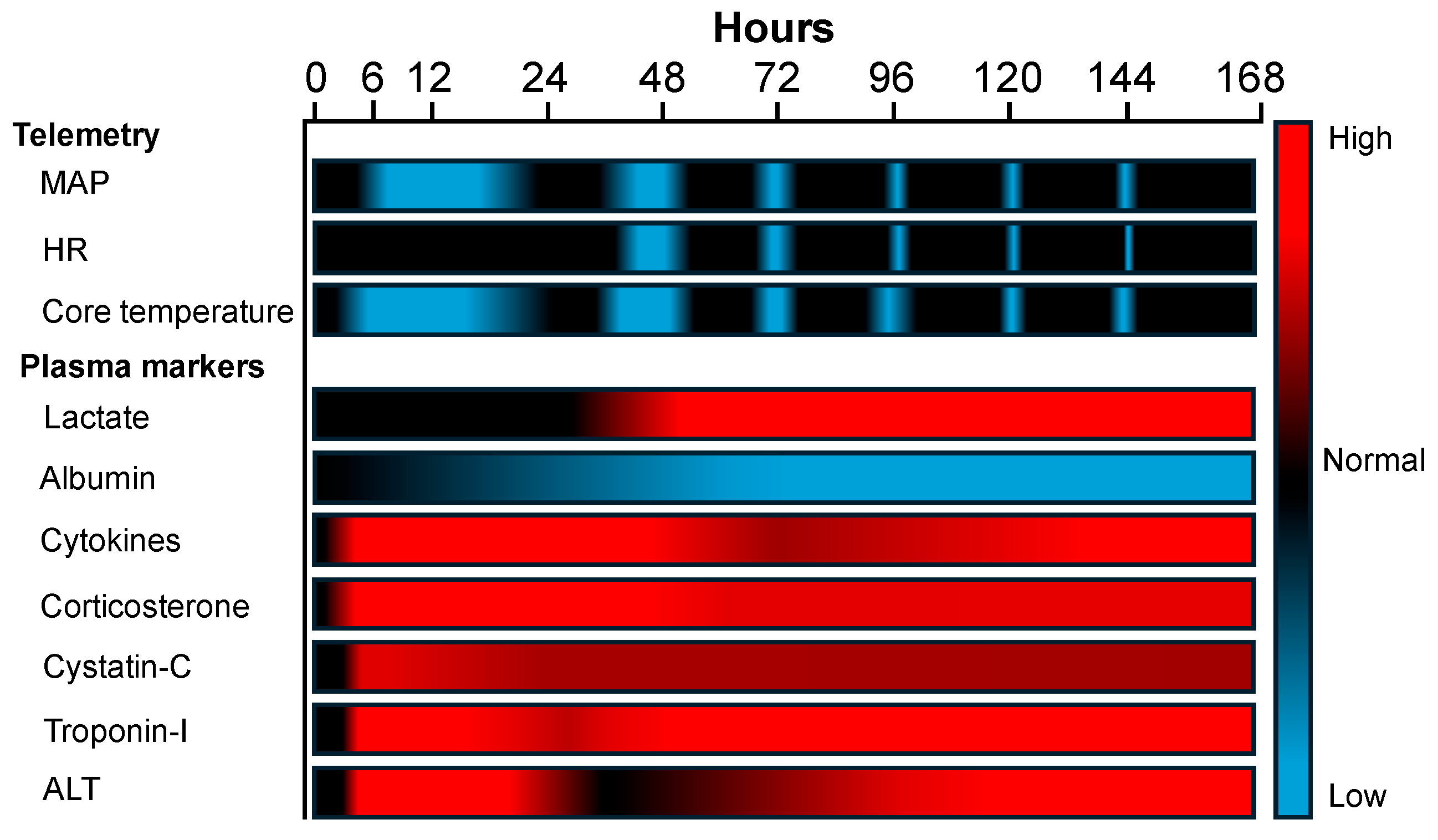
2.8. Summary Results
3. Discussion
3.1. Survival, Morbidity, and Weight
3.2. Mapping CLP Sequelae with Wireless Telemetry
3.3. Cytokines
3.4. Systemic Damage Markers
3.5. Corticosterone Response
3.6. Organ Damage Markers
4. Materials and Methods
4.1. Ethics
4.2. Animals
4.3. Surgical Procedures
4.3.1. Carotid Telemetry
4.3.2. Caecal Ligation and Puncture (CLP)
4.4. Post-Operative Care Regimen
4.5. Survival Series
4.6. Time Course Series
4.7. Plasma Bioassays
4.8. Biological Rhythms
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cecconi, M.E.L.; Levy, M.; Rhodes, A. Sepsis and septic shock. Lancet 2018, 7, 13. [Google Scholar] [CrossRef]
- Srzic, I.; Nesek Adam, V.; Tunjic Pejak, D. Sepsis Definition: What’s New in the Treatment Guidelines. Acta Clin. Croat. 2022, 61 (Suppl. S1), 67–72. [Google Scholar] [CrossRef] [PubMed]
- Chousterman, B.G.; Swirski, F.K.; Weber, G.F. Cytokine storm and sepsis disease pathogenesis. Semin. Immunopathol. 2017, 39, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Finfer, S.; Bellomo, R.; Lipman, J.; French, C.; Dobb, G.; Myburgh, J. Adult-population incidence of severe sepsis in Australian and New Zealand intensive care units. Intensive Care Med. 2004, 30, 589–596, Erratum in Intensive Care Med. 2004, 30, 1252. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, A.E.; Chapman, K.E. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol. Cell. Endocrinol. 2011, 335, 2–13. [Google Scholar] [CrossRef]
- Annane, D.; Bellissant, E.; Bollaert, P.-E.; Briegel, J.; Confalonieri, M.; De Gaudio, R.; Keh, D.; Kupfer, Y.; Oppert, M.; Meduri, G.U. Corticosteroids in the treatment of severe sepsis and septic shock in adults: A systematic review. JAMA 2009, 301, 2362–2375. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Moldawer, L.L.; Opal, S.M.; Reinhart, K.; Turnbull, I.R.; Vincent, J.L. Sepsis and septic shock. Nat. Rev. Dis. Primers 2016, 2, 16045. [Google Scholar] [CrossRef]
- Sligl, W.I.; Milner, D.A., Jr.; Sundar, S.; Mphatswe, W.; Majumdar, S.R. Safety and efficacy of corticosteroids for the treatment of septic shock: A systematic review and meta-analysis. Clin. Infect. Dis. 2009, 49, 93–101. [Google Scholar] [CrossRef]
- Huet, O.; Chin-Dusting, J.P. Septic shock: Desperately seeking treatment. Clin. Sci. 2014, 126, 31–39. [Google Scholar] [CrossRef]
- Lin, H.; Ji, F.; Lin, K.-Q.; Zhu, Y.-T.; Yang, W.; Zhang, L.-H.; Zhao, J.-G.; Pei, Y.-H. LPS-aggravated Ferroptosis via Disrupting Circadian Rhythm by Bmal1/AKT/p53 in Sepsis-Induced Myocardial Injury. Inflammation 2023, 46, 1133–1143. [Google Scholar] [CrossRef]
- Mul Fedele, M.L.; Aiello, I.; Caldart, C.S.; Golombek, D.A.; Marpegan, L.; Paladino, N. Differential Thermoregulatory and Inflammatory Patterns in the Circadian Response to LPS-Induced Septic Shock. Front. Cell. Infect. Microbiol. 2020, 10, 100. [Google Scholar] [CrossRef]
- He, X.H.; Ouyang, D.Y.; Xu, L.H. Injection of Escherichia coli to Induce Sepsis. In Methods in Molecular Biology; Humana: New York, NY, USA, 2021; Volume 2321, pp. 43–51. [Google Scholar] [CrossRef]
- Dejager, L.; Pinheiro, I.; Dejonckheere, E.; Libert, C. Cecal ligation and puncture: The gold standard model for polymicrobial sepsis? Trends Microbiol. 2011, 19, 198–208. [Google Scholar] [CrossRef]
- Park, J.W.; Lee, S.J.; Kim, J.E.; Kang, M.J.; Bae, S.J.; Choi, Y.J.; Gong, J.E.; Kim, K.S.; Jung, Y.-S.; Cho, J.-Y.; et al. Comparison of response to LPS-induced sepsis in three DBA/2 stocks derived from different sources. Lab. Anim. Res. 2021, 37, 2. [Google Scholar] [CrossRef]
- Jianhui, L.; Rosenblatt-Velin, N.; Loukili, N.; Pacher, P.; Feihl, F.; Waeber, B.; Liaudet, L. Endotoxin impairs cardiac hemodynamics by affecting loading conditions but not by reducing cardiac inotropism. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H492–H501. [Google Scholar] [CrossRef]
- Brognara, F.; Castania, J.A.; Dias, D.P.M.; Kanashiro, A.; Salgado, H.C. Time Course of Hemodynamic Responses to Different Doses of Lipopolysaccharide in Unanesthetized Male Rats. Front. Physiol. 2019, 10, 771. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, S.; Vardon-Bounes, F.; Merlet-Dupuy, V.; Conil, J.-M.; Buléon, M.; Fourcade, O.; Tack, I.; Minville, V. Sepsis modeling in mice: Ligation length is a major severity factor in cecal ligation and puncture. Intensive Care Med. Exp. 2016, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Otero-Anton, E.; Gonzalez-Quintela, A.; Lopez-Soto, A.; Lopez-Ben, S.; Llovo, J.; Perez, L.F. Cecal ligation and puncture as a model of sepsis in the rat: Influence of the puncture size on mortality, bacteremia, endotoxemia and tumor necrosis factor alpha levels. Eur. Surg. Res. 2001, 33, 77–79. [Google Scholar] [CrossRef] [PubMed]
- Sjaastad, F.V.; Jensen, I.J.; Berton, R.R.; Badovinac, V.P.; Griffith, T.S. Inducing Experimental Polymicrobial Sepsis by Cecal Ligation and Puncture. Curr. Protoc. Immunol. 2020, 131, e110. [Google Scholar] [CrossRef]
- Tao, W.; Enoh, V.T.; Lin, C.Y.; Johnston, W.E.; Li, P.; Sherwood, E.R. Cardiovascular dysfunction caused by cecal ligation and puncture is attenuated in CD8 knockout mice treated with anti-asialoGM1. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 289, R478–R485. [Google Scholar] [CrossRef][Green Version]
- Wen, H. Sepsis induced by cecal ligation and puncture. In Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2013; Volume 1031, pp. 117–124. [Google Scholar] [CrossRef]
- Coletta, C.; Módis, K.; Oláh, G.; Brunyánszki, A.; Herzig, D.S.; Sherwood, E.R.; Ungvári, Z.; Szabo, C. Endothelial dysfunction is a potential contributor to multiple organ failure and mortality in aged mice subjected to septic shock: Preclinical studies in a murine model of cecal ligation and puncture. Crit. Care 2014, 18, 511. [Google Scholar] [CrossRef] [PubMed]
- Niemeyer, J.E. Telemetry for small animal physiology. Lab Anim. 2016, 45, 255–257. [Google Scholar] [CrossRef] [PubMed]
- Aydin, P.; Bekmez, H. Experimental Sepsis Models: Advantages and Limitations. Eurasian J. Med. 2023, 55, 120–124. [Google Scholar] [CrossRef]
- Schabbauer, G. Polymicrobial sepsis models: CLP versus CASP. Drug Discov. Today Dis. Models 2012, 9, 5. [Google Scholar] [CrossRef]
- Godshall, C.J.; Scott, M.J.; Peyton, J.C.; Gardner, S.A.; Cheadle, W.G. Genetic background determines susceptibility during murine septic peritonitis. J. Surg. Res. 2002, 102, 45–49. [Google Scholar] [CrossRef]
- Diodato, M.D.; Knoferl, M.W.; Schwacha, M.G.; Bland, K.I.; Chaudry, I.H. Gender differences in the inflammatory response and survival following haemorrhage and subsequent sepsis. Cytokine 2001, 14, 162–169. [Google Scholar] [CrossRef]
- Turnbull, I.R.; Wlzorek, J.J.; Osborne, D.; Hotchkiss, R.S.; Coopersmith, C.M.; Buchman, T.G. Effects of age on mortality and antibiotic efficacy in cecal ligation and puncture. Shock 2003, 19, 310–313. [Google Scholar] [CrossRef]
- Wilson, M.A.; Chou, M.C.; Spain, D.A.; Downard, P.J.B.; Qian, Q.; Cheadle, W.G.; Garrison, R.N. Fluid resuscitation attenuates early cytokine mRNA expression after peritonitis. J. Trauma. 1996, 41, 622–627. [Google Scholar] [CrossRef]
- Toscano, M.G.; Ganea, D.; Gamero, A.M. Cecal ligation puncture procedure. J. Vis. Exp. 2011, 51, e2860. [Google Scholar] [CrossRef]
- Drechsler, S.; Osuchowski, M. Cecal Ligation and Puncture. In Methods in Molecular Biology; Humana: New York, NY, USA, 2021; Volume 2321, pp. 1–8. [Google Scholar] [CrossRef]
- Kim, G.O.; Kim, N.; Song, G.Y.; Bae, J.S. Inhibitory Activities of Rare Ginsenoside Rg4 on Cecal Ligation and Puncture-Induced Sepsis. Int. J. Mol. Sci. 2022, 23, 10836. [Google Scholar] [CrossRef]
- Tripathi, A.S.; Awasthi, S.; Maurya, R.K.; Yasir, M.; Mohapatra, L.; Srivastav, V. Protective effect of vanillin on the management of cecal ligation and puncture induced sepsis rat model. Microb. Pathog. 2022, 165, 105493. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xing, M.; Wang, L.; Zhao, Y. Maresin 1 alleviates neuroinflammation and cognitive decline in a mouse model of cecal ligation and puncture. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2024, 49, 890–902. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Yang, Z.; Zheng, G.; Yu, T.; Wang, P.; Liu, X.; Ling, Q.; Jiang, L.; Tang, W. Lactate as a Potential Biomarker of Sepsis in a Rat Cecal Ligation and Puncture Model. Mediat. Inflamm. 2018, 2018, 8352727. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.J.; Yuan, D.; Zhang, X.; Angus, D.C.; Rosengart, M.R.; Seymour, C.W. Use of Biotelemetry to Define Physiology-Based Deterioration Thresholds in a Murine Cecal Ligation and Puncture Model of Sepsis. Crit. Care Med. 2016, 44, e420–e431. [Google Scholar] [CrossRef]
- Moreno-Navarrete, J.M.; Comas, F.; de Jager, V.; Fernandez-Real, J.M.; Bouma, H.R. Cecal Ligation and Puncture-Induced Sepsis Promotes Brown Adipose Tissue Inflammation Without Any Impact on Expression of Thermogenic-Related Genes. Front. Physiol. 2021, 12, 692618. [Google Scholar] [CrossRef]
- Mai, S.H.C.; Sharma, N.; Kwong, A.C.; Dwivedi, D.J.; Khan, M.; Grin, P.M.; Fox-Robichaud, A.E.; Liaw, P.C. Body temperature and mouse scoring systems as surrogate markers of death in cecal ligation and puncture sepsis. Intensive Care Med. Exp. 2018, 6, 20. [Google Scholar] [CrossRef]
- Steeland, S.; Van Ryckeghem, S.; Vandewalle, J.; Steeland, S.; Van Ryckeghem, S.; Vandewalle, J.; Ballegeer, M.; Van Wonterghem, E.; Eggermont, M.; Decruyenaere, J.; et al. Simultaneous Inhibition of Tumor Necrosis Factor Receptor 1 and Matrix Metalloproteinase 8 Completely Protects Against Acute Inflammation and Sepsis. Crit. Care Med. 2018, 46, e67–e75. [Google Scholar] [CrossRef]
- Vandewalle, J.; Steeland, S.; Van Ryckeghem, S.; Eggermont, M.; Van Wonterghem, E.; Vandenbroucke, R.E.; Libert, C. A Study of Cecal Ligation and Puncture-Induced Sepsis in Tissue-Specific Tumor Necrosis Factor Receptor 1-Deficient Mice. Front. Immunol. 2019, 10, 2574. [Google Scholar] [CrossRef]
- Shimazui, T.; Nakada, T.-A.; Walley, K.R.; Oshima, T.; Abe, T.; Ogura, H.; Shiraishi, A.; Kushimoto, S.; Saitoh, D.; Fujishima, S.; et al. Significance of body temperature in elderly patients with sepsis. Crit. Care 2020, 24, 387. [Google Scholar] [CrossRef]
- Kato, R.; Pinsky, M.R. Personalizing blood pressure management in septic shock. Ann. Intensive Care 2015, 5, 41. [Google Scholar] [CrossRef]
- Cecconi, M.; De Backer, D.; Antonelli, M.; Beale, R.; Bakker, J.; Hofer, C.; Jaeschke, R.; Mebazaa, A.; Pinsky, M.R.; Teboul, J.L.; et al. Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med. 2014, 40, 1795–1815. [Google Scholar] [CrossRef]
- Leone, M.; Asfar, P.; Radermacher, P.; Vincent, J.L.; Martin, C. Optimizing mean arterial pressure in septic shock: A critical reappraisal of the literature. Crit. Care 2015, 19, 101. [Google Scholar] [CrossRef] [PubMed]
- Asfar, P.; Meziani, F.; Hamel, J.-F.; Grelon, F.; Megarbane, B.; Anguel, N.; Mira, J.-P.; Dequin, P.-F.; Gergaud, S.; Weiss, N.; et al. High versus low blood-pressure target in patients with septic shock. N. Engl. J. Med. 2014, 370, 1583–1593. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.X.; Yang, C.F.; Wang, H.Y.; Teng, Y.; He, H.Y. Should we initiate vasopressors earlier in patients with septic shock: A mini systemic review. World J. Crit. Care Med. 2023, 12, 204–216. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, H.; Zhou, J.; Zhong, Y.; Ali, M.M.; McGuire, F.; Nagarkatti, P.S.; Nagarkatti, M. Role of cytokines as a double-edged sword in sepsis. In Vivo 2013, 27, 669–684. [Google Scholar]
- Bown, M.J.; Nicholson, M.L.; Bell, P.R.; Sayers, R.D. Cytokines and inflammatory pathways in the pathogenesis of multiple organ failure following abdominal aortic aneurysm repair. Eur. J. Vasc. Endovasc. Surg. 2001, 22, 485–495. [Google Scholar] [CrossRef]
- Chiswick, E.L.; Mella, J.R.; Bernardo, J.; Remick, D.G. Acute-Phase Deaths from Murine Polymicrobial Sepsis Are Characterized by Innate Immune Suppression Rather Than Exhaustion. J. Immunol. 2015, 195, 3793–3802. [Google Scholar] [CrossRef]
- Walley, K.R.; Lukacs, N.W.; Standiford, T.J.; Strieter, R.M.; Kunkel, S.L. Balance of inflammatory cytokines related to severity and mortality of murine sepsis. Infect. Immun. 1996, 64, 4733–4738. [Google Scholar] [CrossRef]
- Piliponsky, A.M.; Chen, C.-C.; Grimbaldeston, M.A.; Burns-Guydish, S.M.; Hardy, J.; Kalesnikoff, J.; Contag, C.H.; Tsai, M.; Galli, S.J. Mast cell-derived TNF can exacerbate mortality during severe bacterial infections in C57BL/6-KitW-sh/W-sh mice. Am. J. Pathol. 2010, 176, 926–938. [Google Scholar] [CrossRef]
- Walley, K.R.; Lukacs, N.W.; Standiford, T.J.; Strieter, R.M.; Kunkel, S.L. Elevated levels of macrophage inflammatory protein 2 in severe murine peritonitis increase neutrophil recruitment and mortality. Infect. Immun. 1997, 65, 3847–3851. [Google Scholar] [CrossRef]
- Brogliato, A.R.; Antunes, C.A.; Carvalho, R.S.; Monteiro, A.P.T.; Tinoco, R.F.; Bozza, M.T.; Canetti, C.; Peters-Golden, M.; Kunkel, S.L.; Vianna-Jorge, R.; et al. Ketoprofen impairs immunosuppression induced by severe sepsis and reveals an important role for prostaglandin E2. Shock 2012, 38, 620–629. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.O.; Han, X.; Yu, Q. Interleukin-6 induces the generation of IL-10-producing Tr1 cells and suppresses autoimmune tissue inflammation. J. Autoimmun. 2013, 40, 28–44. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.A.; Keelan, J.A.; Mitchell, M.D. Critical paracrine interactions between TNF-alpha and IL-10 regulate lipopolysaccharide-stimulated human choriodecidual cytokine and prostaglandin E2 production. J. Immunol. 2003, 170, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Friedman, G.; Jankowski, S.; Marchant, A.; Goldman, M.; Kahn, R.J.; Vincent, J.L. Blood interleukin 10 levels parallel the severity of septic shock. J. Crit. Care 1997, 12, 183–187. [Google Scholar] [CrossRef]
- Turcato, G.; Zaboli, A.; Sibilio, S.; Rella, E.; Bonora, A.; Brigo, F. Albumin as a prognostic marker of 30-day mortality in septic patients admitted to the emergency department. Intern. Emerg. Med. 2023, 18, 2407–2417. [Google Scholar] [CrossRef]
- Liu, V.; Morehouse, J.W.; Soule, J.; Whippy, A.; Escobar, G.J. Fluid volume, lactate values, and mortality in sepsis patients with intermediate lactate values. Ann. Am. Thorac. Soc. 2013, 10, 466–473. [Google Scholar] [CrossRef]
- Sugimoto, M.; Takayama, W.; Murata, K.; Otomo, Y. The impact of lactate clearance on outcomes according to infection sites in patients with sepsis: A retrospective observational study. Sci. Rep. 2021, 11, 22394. [Google Scholar] [CrossRef]
- Chang, R.; Holcomb, J.B.; Johansson, P.I.; Pati, S.; Schreiber, M.A.; Wade, C.E. Plasma Resuscitation Improved Survival in a Cecal Ligation and Puncture Rat Model of Sepsis. Shock 2018, 49, 53–61. [Google Scholar] [CrossRef]
- Martins, J.T.; Li, D.J.; Baskin, L.B.; Jialal, I.; Keffer, J.H. Comparison of cardiac troponin I and lactate dehydrogenase isoenzymes for the late diagnosis of myocardial injury. Am. J. Clin. Pathol. 1996, 106, 705–708. [Google Scholar] [CrossRef]
- Zymliński, R.; Biegus, J.; Sokolski, M.; Siwołowski, P.; Nawrocka-Millward, S.; Todd, J.; Jankowska, E.A.; Banasiak, W.; Cotter, G.; Cleland, J.G.; et al. Increased blood lactate is prevalent and identifies poor prognosis in patients with acute heart failure without overt peripheral hypoperfusion. Eur. J. Heart Fail. 2018, 20, 1011–1018. [Google Scholar] [CrossRef]
- Herrmann, I.K.; Castellon, M.M.; Schwartz, D.E.; Hasler, M.; Urner, M.; Hu, G.; Minshall, R.D.; Beck-Schimmer, B. Volatile anesthetics improve survival after cecal ligation and puncture. Anesthesiology 2013, 119, 901–906. [Google Scholar] [CrossRef]
- Ali, M.A.; Raza, M.T.; Majeed, S.; Tahir, U.; Ahmad, W.; Bin Tahir, M.; Ali, R.S.; Afzal, A.; Hasan, M.Q.; Hassan, M.; et al. Correlation of Serum Albumin Levels with the Severity of Sepsis Among Intensive Care Unit Patients. Cureus 2024, 16, e71411. [Google Scholar] [CrossRef] [PubMed]
- Mohn, C.E.; Fernandez-Solari, J.; De Laurentiis, A.; Prestifilippo, J.P.; de la Cal, C.; Funk, R.; Bornstein, S.R.; McCann, S.M.; Rettori, V. The rapid release of corticosterone from the adrenal induced by ACTH is mediated by nitric oxide acting by prostaglandin E2. Proc. Natl. Acad. Sci. USA 2005, 102, 6213–6218. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.; McQueen, A.; Chen, T.C.; Wang, J.C. Regulation of Glucose Homeostasis by Glucocorticoids. Adv. Exp. Med. Biol. 2015, 872, 99–126. [Google Scholar] [CrossRef] [PubMed]
- Mishima, Y.; Shinoda, Y.; Sadakata, T.; Kojima, M.; Wakana, S.; Furuichi, T. Lack of stress responses to long-term effects of corticosterone in Caps2 knockout mice. Sci. Rep. 2015, 5, 8932. [Google Scholar] [CrossRef]
- Guo, L.; Zheng, Z.; Ai, J.; Howatt, D.A.; Mittelstadt, P.R.; Thacker, S.; Daugherty, A.; Ashwell, J.D.; Remaley, A.T.; Li, X.-A. Scavenger receptor BI and high-density lipoprotein regulate thymocyte apoptosis in sepsis. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 966–975. [Google Scholar] [CrossRef]
- Miyao, M.; Hirotsu, A.; Tatsumi, K.; Tanaka, T. Prior exposure to stress exacerbates neuroinflammation and causes long-term behavior changes in sepsis. Heliyon 2023, 9, e16904. [Google Scholar] [CrossRef]
- Sam, S.; Corbridge, T.C.; Mokhlesi, B.; Comellas, A.P.; Molitch, M.E. Cortisol levels and mortality in severe sepsis. Clin. Endocrinol. 2004, 60, 29–35. [Google Scholar] [CrossRef]
- Ho, J.T.; Al-Musalhi, H.; Chapman, M.J.; Quach, T.; Thomas, P.D.; Bagley, C.J.; Lewis, J.G.; Torpy, D.J. Septic shock and sepsis: A comparison of total and free plasma cortisol levels. J. Clin. Endocrinol. Metab. 2006, 91, 105–114. [Google Scholar] [CrossRef]
- Bendel, S.; Karlsson, S.; Pettilä, V.; Loisa, P.; Varpula, M.; Ruokonen, E. Free cortisol in sepsis and septic shock. Anesth. Analg. 2008, 106, 1813–1819. [Google Scholar] [CrossRef]
- Caraballo, C.; Jaimes, F. Organ Dysfunction in Sepsis: An Ominous Trajectory from Infection to Death. Yale J. Biol. Med. 2019, 92, 629–640. [Google Scholar]
- Landry, D.W.; Oliver, J.A. The pathogenesis of vasodilatory shock. N. Engl. J. Med. 2001, 345, 588–595. [Google Scholar] [CrossRef]
- Trzeciak, S.; Cinel, I.; Dellinger, R.P.; Shapiro, N.I.; Arnold, R.C.; Parrillo, J.E.; Hollenberg, S.M.; on behalf of the Microcirculatory Alterations in Resuscitation and Shock (MARS) Investigators. Resuscitating the microcirculation in sepsis: The central role of nitric oxide, emerging concepts for novel therapies, and challenges for clinical trials. Acad. Emerg. Med. 2008, 15, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Cuenca, A.G.; Delano, M.J.; Kelly-Scumpia, K.M.; Moldawer, L.L.; Efron, P.A. Cecal ligation and puncture. Curr. Protoc. Immunol. 2010, 91, 19.13.1–19.13.11. [Google Scholar] [CrossRef]
- Hill, A.; Khalil, H.; Laborc, K.; Kounelis-Wuillaume, S.; Gavade, S.; Johnston, C.; Singer, B.H.; Spencer-Segal, J.L. Corticosteroid Treatment During Sepsis Alters Hippocampal Function in Male and Female Survivors. Biol. Psychiatry Glob. Open Sci. 2024, 4, 336–345. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. BMJ Open Sci. 2020, 4, e100115. [Google Scholar] [CrossRef]
- Refinetti, R. Circadian Rhythm Laboratory Software. 2025. Available online: https://www.circadian.org/softwar.html (accessed on 2 March 2025).



| Biomarker | Standard-Grade CLP | High-Grade CLP | ||||
|---|---|---|---|---|---|---|
| Survivors | Non-Survivors | p-Value | Survivors | Non-Survivors | p-Value | |
| IL-6 (ng/mL) | 0.9 ± 0.1 | 5.6 ± 2.2 | 0.001 | 8.9 ± 0.8 | 13.9 ± 1.4 | 0.001 |
| MIP-2 (ng/mL) | 0.1 ± 0.02 | 0.3 ± 0.2 | 0.532 | 0.9 ± 0.04 | 1.2 ± 0.07 | 0.219 |
| TNFα (ng/mL) | 0.7 ± 0.2 | 0.7 ± 0.6 | 0.992 | 0.5 ± 0.05 | 0.9 ± 0.06 | 0.022 |
| IL-10 (ng/mL) | 0.3 ± 0.04 | 0.5 ± 0.1 | 0.617 | 11.1 ± 2.95 | 8.8 ± 1.7 | 0.251 |
| Lactate (nmol) | 426 ± 49 | 317 ± 67 | 0.203 | 1089 ± 123 | 1381 ± 73 | 0.021 |
| Albumin (mg/mL) | 17.8 ± 1.1 | 5.3 ± 0.1 | <0.001 | 9.1 ± 1.2 | 3.7 ± 0.9 | 0.002 |
| Corticosterone (ng/mL) | 118 ± 8.0 | 346 ± 26 | 0.023 | 304 ± 17 | 627 ± 38 | <0.001 |
| Cystatin C | 150 ± 6 | 122 ± 18 | 0.293 | 466 ± 34 | 460 ± 27 | 0.554 |
| Troponin-I | 3.3 ± 0.4 | 4.9 ± 0.9 | 0.057 | 72.9 ± 6.7 | 57.6 ± 9.5 | 0.335 |
| ALT (pg/mL) | 220 ± 17 | 209 ± 43 | 0.562 | 610 ± 86 | 625 ± 138 | 0.916 |
| AST (pg/mL) | 2666 ± 762 | 2377 ± 814 | 0.897 | 4734 ± 231 | 3652 ± 637 | 0.842 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramsay, S.D.; Kilgariff, D.E.; Young, B.J.; Plummer, M.P.; Nenke, M.A.; Meyer, E.J.; Torpy, D.J.; Young, R.L. Precision Profiling of Disease Progression in Murine Models of Sepsis and Septic Shock. Int. J. Mol. Sci. 2025, 26, 9954. https://doi.org/10.3390/ijms26209954
Ramsay SD, Kilgariff DE, Young BJ, Plummer MP, Nenke MA, Meyer EJ, Torpy DJ, Young RL. Precision Profiling of Disease Progression in Murine Models of Sepsis and Septic Shock. International Journal of Molecular Sciences. 2025; 26(20):9954. https://doi.org/10.3390/ijms26209954
Chicago/Turabian StyleRamsay, Stewart D., Declan E. Kilgariff, Benjamin J. Young, Mark P. Plummer, Marni A. Nenke, Emily J. Meyer, David J. Torpy, and Richard L. Young. 2025. "Precision Profiling of Disease Progression in Murine Models of Sepsis and Septic Shock" International Journal of Molecular Sciences 26, no. 20: 9954. https://doi.org/10.3390/ijms26209954
APA StyleRamsay, S. D., Kilgariff, D. E., Young, B. J., Plummer, M. P., Nenke, M. A., Meyer, E. J., Torpy, D. J., & Young, R. L. (2025). Precision Profiling of Disease Progression in Murine Models of Sepsis and Septic Shock. International Journal of Molecular Sciences, 26(20), 9954. https://doi.org/10.3390/ijms26209954






