Parity and NIS Expression in Atypical Cells of Triple-Negative Breast Cancer, and Prognosis
Abstract
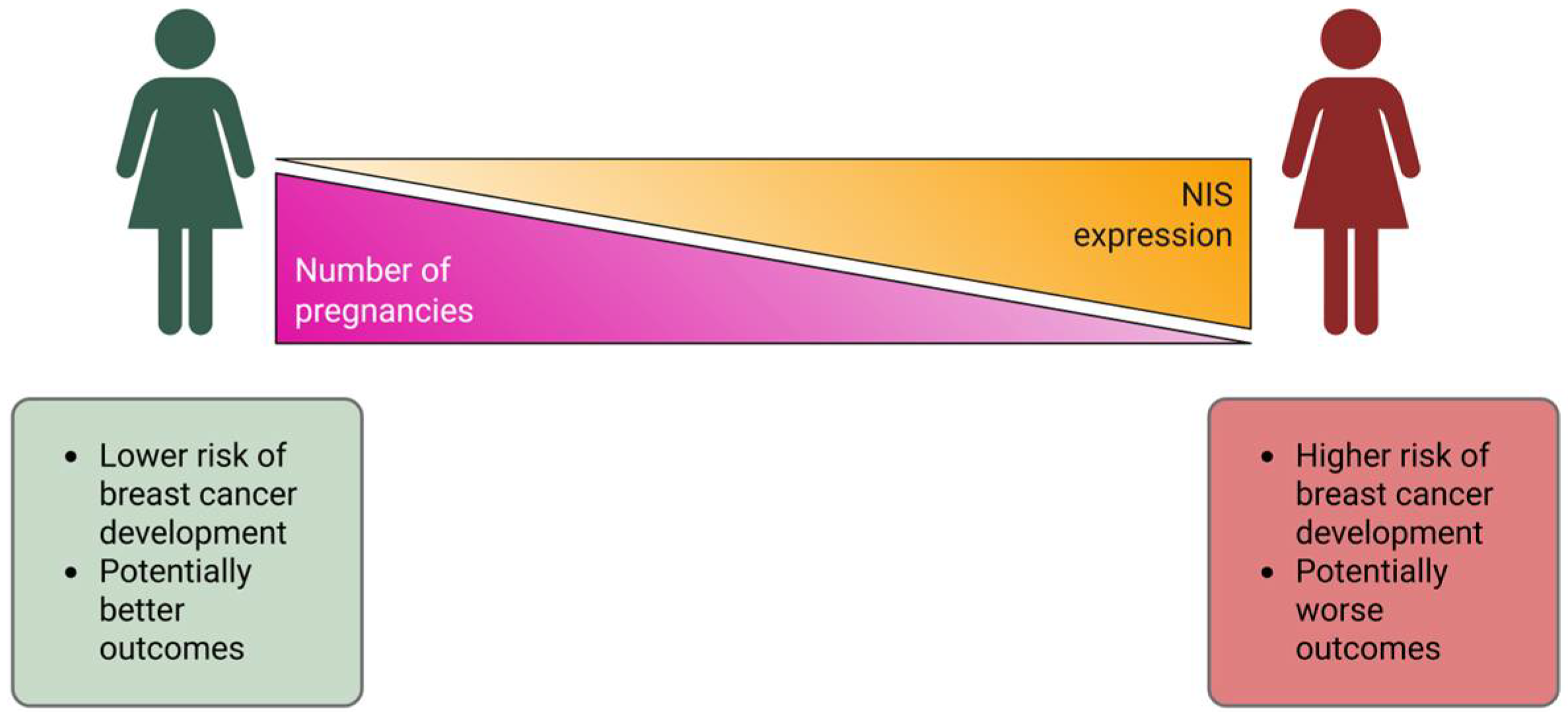
1. Introduction
Research Objective
2. Results
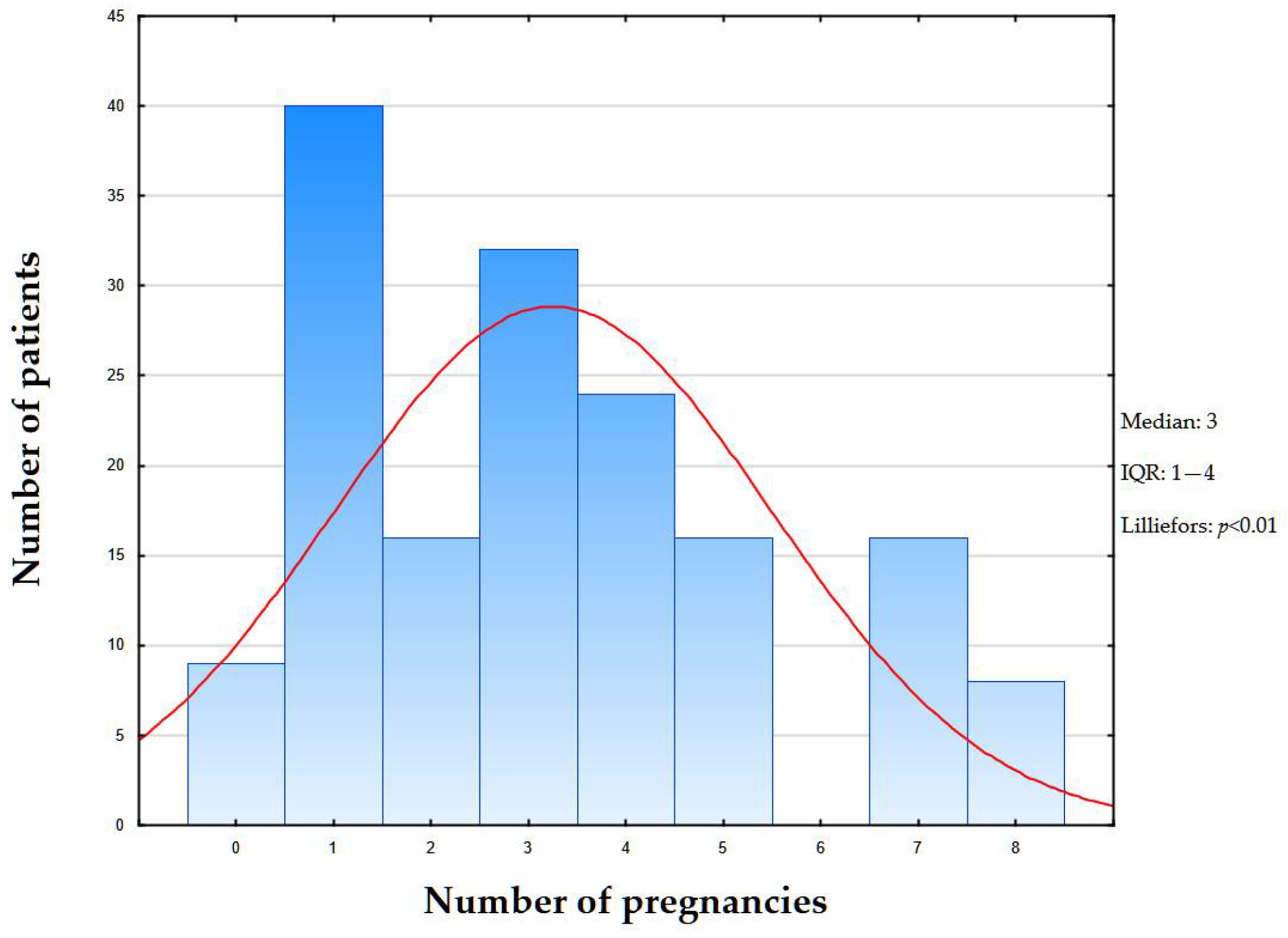
2.1. Clinical Data
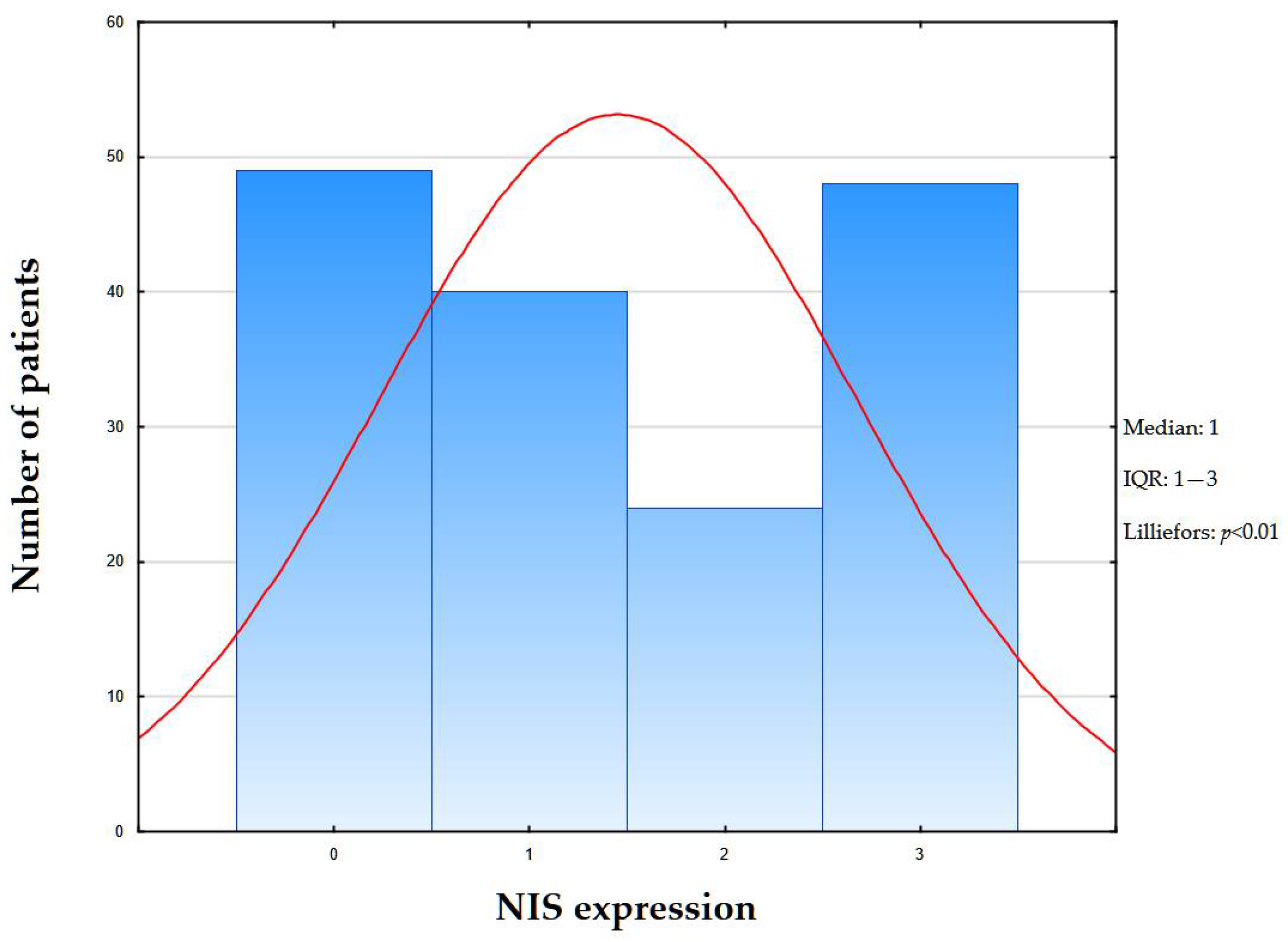
2.2. Genetic Testing
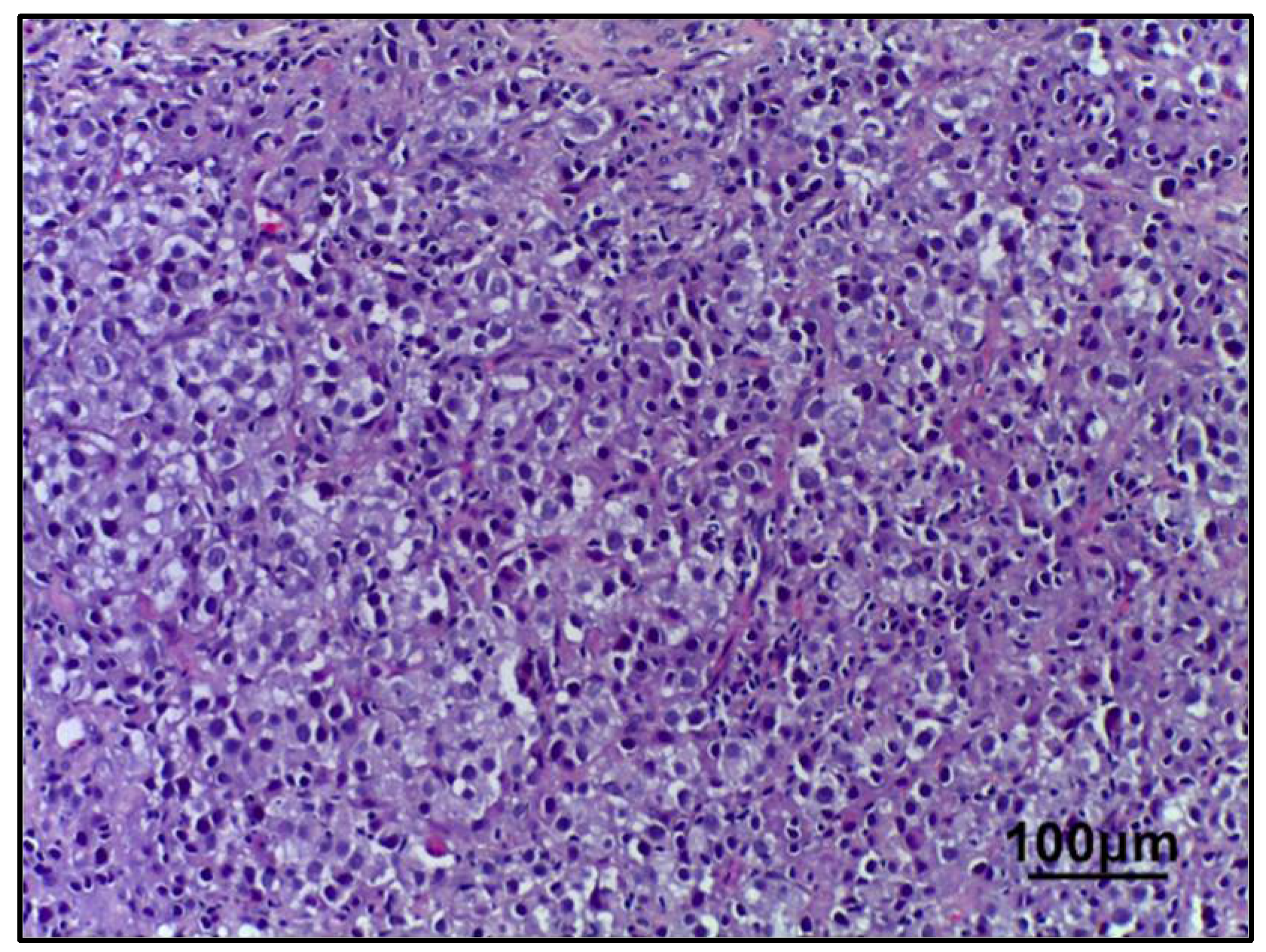
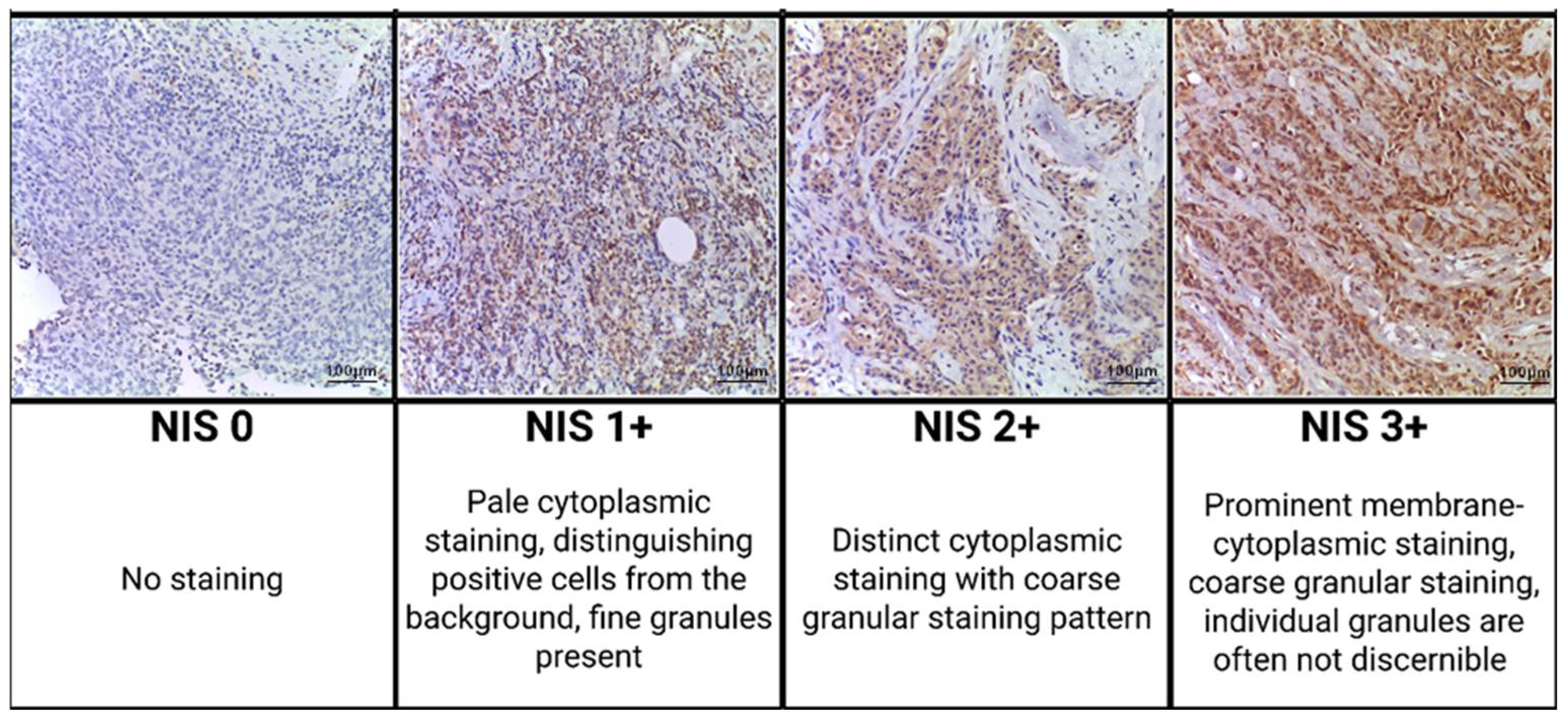
2.3. Morphological Examination
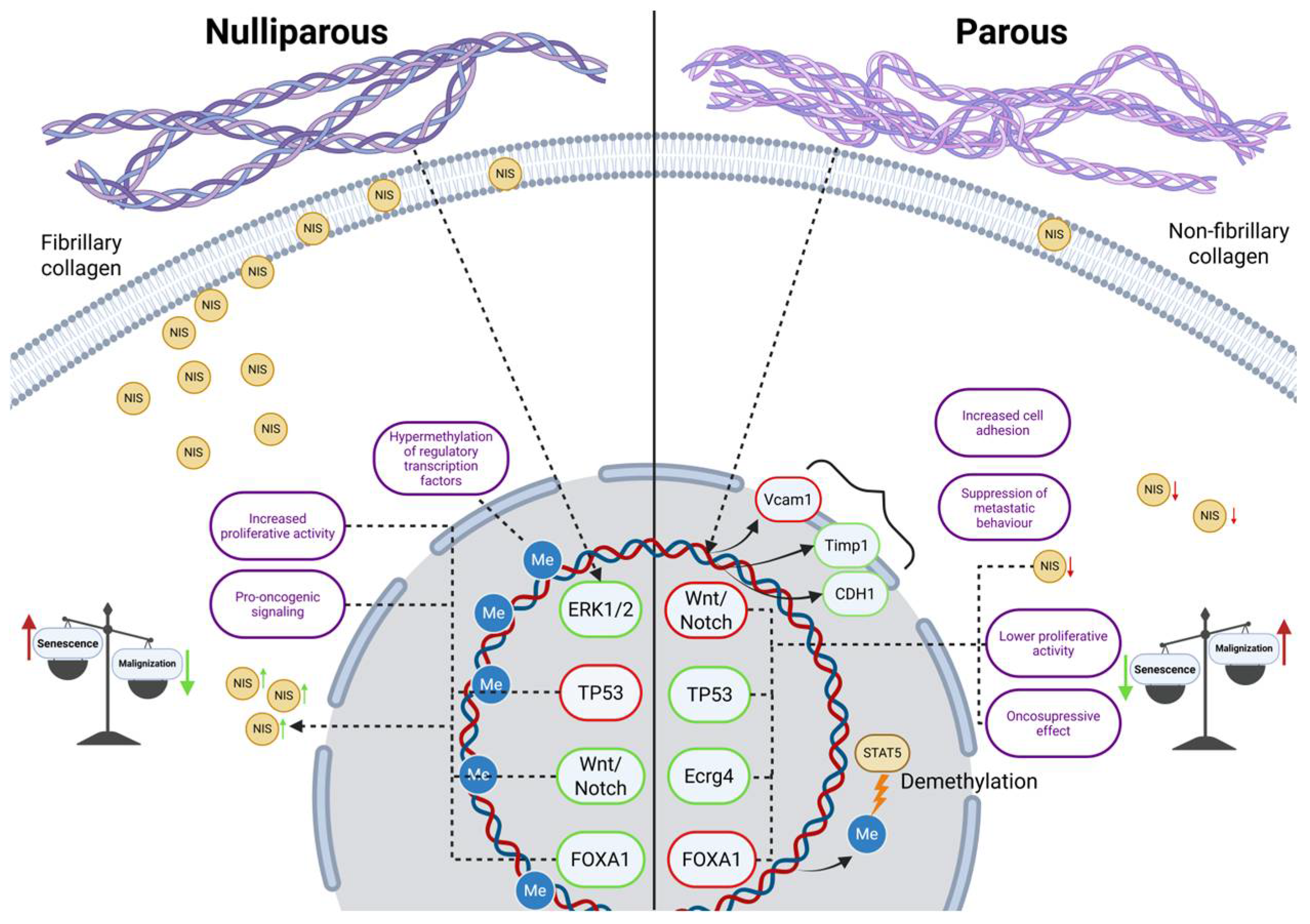
3. Discussion
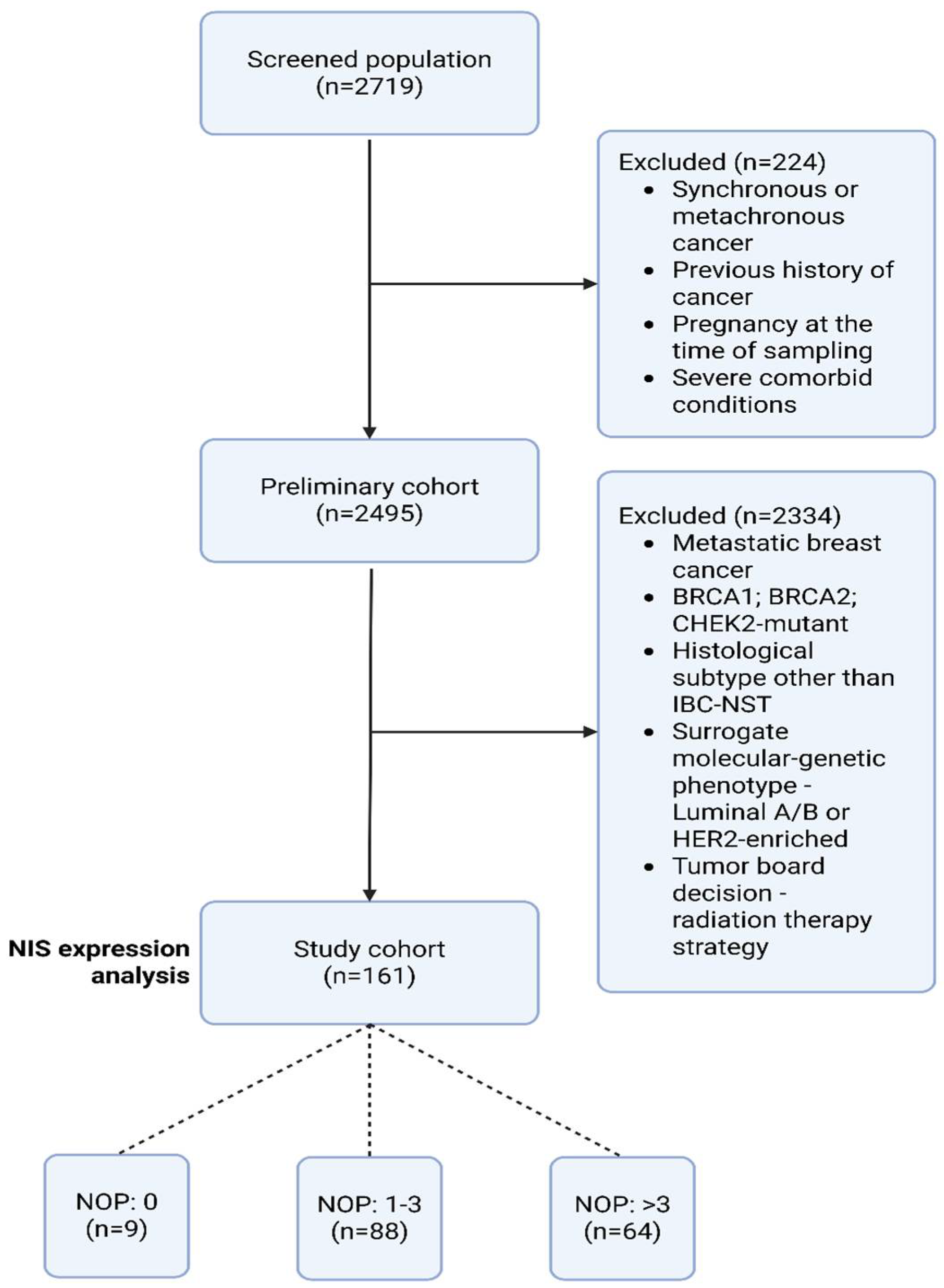
4. Materials and Methods
4.1. Patients and Clinico-Morphological Data
4.2. Real-Time Polymerase Chain Reaction BRCA1/2 and CHEK2 Mutation Analysis
4.3. Morphological Block
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
| BC | Breast Cancer |
| BRCA1 | Breast Cancer Gene 1 |
| BRCA2 | Breast Cancer Gene 2 |
| CHEK2 | Checkpoint Kinase 2 |
| CHF | Congestive Heart Failure |
| DAB | Diaminobenzidine (substrate for immunohistochemical detection) |
| ECOG | Eastern Cooperative Oncology Group (performance status scale) |
| ER | Estrogen Receptor |
| FOXA1 | Forkhead Box A1 (transcription factor) |
| HER2/neu | Human Epidermal Growth Factor Receptor 2 |
| HRP | Horseradish Peroxidase |
| IBC-NST | Invasive Breast Carcinoma of No Special Type |
| ICD-O | International Classification of Diseases for Oncology |
| ICMJE | International Committee of Medical Journal Editors |
| IQR | Interquartile Range |
| NIS | Sodium/Iodide Symporter (syn. SLC5A5 protein) |
| NOP | Number of Pregnancies |
| NYHA | New York Heart Association (heart failure classification) |
| PI3K/AKT/mTOR | Phosphoinositide 3-Kinase/Protein Kinase B/Mammalian Target of Rapamycin (signaling pathway) |
| PR | Progesterone Receptor |
| SD | Standard Deviation |
| SLC5A5 | Solute Carrier Family 5 Member 5 (gene for NIS) |
| STAT5a | Signal Transducer and Activator of Transcription 5a |
| TNBC | Triple-Negative Breast Cancer |
| TNM | Tumor Node Metastasis (cancer staging system) |
| TP53 | Tumor Protein 53 (gene for p53) |
References
- Kim, J.; Harper, A.; McCormack, V.; Sung, H.; Houssami, N.; Morgan, E.; Mutebi, M.; Garvey, G.; Soerjomataram, I.; Fidler-Benaoudia, M.M. Global patterns and trends in breast cancer incidence and mortality across 185 countries. Nat. Med. 2025, 31, 1154–1162. [Google Scholar] [CrossRef]
- Sprague, B.L.; Trentham-Dietz, A.; Egan, K.M.; Titus-Ernstoff, L.; Hampton, J.M.; Newcomb, P.A. Proportion of invasive breast cancer attributable to risk factors modifiable after menopause. Am. J. Epidemiol. 2008, 168, 404–411. [Google Scholar] [CrossRef]
- Slepicka, P.F.; Cyrill, S.L.; Dos Santos, C.O. Pregnancy and Breast Cancer: Pathways to Understand Risk and Prevention. Trends Mol. Med. 2019, 25, 866–881. [Google Scholar] [CrossRef]
- Ali, S.; Hamam, D.; Liu, X.; Lebrun, J.J. Terminal differentiation and anti-tumorigenic effects of prolactin in breast cancer. Front. Endocrinol. 2019, 13, 993570. [Google Scholar] [CrossRef]
- Ravera, S.; Reyna-Neyra, A.; Ferrandino, G.; Amzel, L.M.; Carrasco, N. The Sodium/Iodide Symporter (NIS): Molecular Physiology and Preclinical and Clinical Applications. Annu. Rev. Physiol. 2017, 79, 261–289. [Google Scholar] [CrossRef] [PubMed]
- Beyer, S.J.; Jimenez, R.E.; Shapiro, C.L.; Cho, J.Y.; Jhiang, S.M. Do cell surface trafficking impairments account for variable cell surface sodium iodide symporter levels in breast cancer? Breast Cancer Res. Treat. 2009, 115, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Tazebay, U.H.; Wapnir, I.L.; Levy, O.; Dohan, O.; Zuckier, L.S.; Zhao, Q.H.; Deng, H.F.; Amenta, P.S.; Fineberg, S.; Pestell, R.G.; et al. The mammary gland iodide transporter is expressed during lactation and in breast cancer. Nat. Med. 2000, 6, 871–878. [Google Scholar] [CrossRef]
- Rathod, M.; Kelkar, M.; Valvi, S.; Salve, G.; De, A. FOXA1 Regulation Turns Benzamide HDACi Treatment Effect-Specific in BC, Promoting NIS Gene-Mediated Targeted Radioiodine Therapy. Mol. Ther. Oncolytics 2020, 19, 93–104. [Google Scholar] [CrossRef]
- Lacoste, C.; Hervé, J.; Nader, M.B.; Dos Santos, A.; Moniaux, N.; Valogne, Y.; Montjean, R.; Dorseuil, O.; Samuel, D.; Cassio, D.; et al. Iodide Transporter NIS Regulates Cancer Cell Motility and Invasiveness by Interacting with the Rho Guanine Nucleotide Exchange Factor LARG. Cancer Res. 2012, 72, 5505–5515. [Google Scholar] [CrossRef]
- Micali, S.; Bulotta, S.; Puppin, C.; Territo, A.; Navarra, M.; Bianchi, G.; Damante, G.; Filetti, S.; Russo, D. Sodium iodide symporter (NIS) in extrathyroidal malignancies: Focus on breast and urological cancer. BMC Cancer 2014, 14, 303. [Google Scholar] [CrossRef] [PubMed]
- Feigman, M.J.; Moss, M.A.; Chen, C.; Cyrill, S.L.; Ciccone, M.F.; Trousdell, M.C.; Yang, S.-T.; Frey, W.D.; Wilkinson, J.E.; dos Santos, C.O. Pregnancy reprograms the epigenome of mammary epithelial cells and blocks the development of premalignant lesions. Nat. Commun. 2020, 11, 2649. [Google Scholar] [CrossRef]
- Maller, O.; Hansen, K.C.; Lyons, T.R.; Acerbi, I.; Weaver, V.M.; Prekeris, R.; Tan, A.-C.; Schedin, P. Collagen architecture in pregnancy-induced protection from breast cancer. J. Cell Sci. 2013, 126, 4108–4110. [Google Scholar] [CrossRef] [PubMed]
- Kelkar, M.G.; Thakur, B.; Derle, A.; Chatterjee, S.; Ray, P.; De, A. Tumor suppressor protein p53 exerts negative transcriptional regulation on human sodium iodide symporter gene expression in breast cancer. Breast Cancer Res. Treat. 2017, 164, 603–615. [Google Scholar] [CrossRef]
- Espinal, A.C.; Buas, M.F.; Wang, D.; Cheng, D.T.-Y.; Sucheston-Campbell, L.; Hu, Q.; Yan, L.; Payne-Ondracek, R.; Cortes, E.; Tang, L.; et al. FOXA1 hypermethylation: Link between parity and ER-negative breast cancer in African American women? Breast Cancer Res. Treat. 2017, 166, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Kogai, T.; Schultz, J.J.; Johnson, L.S.; Huang, M.; Brent, G.A. Retinoic acid induces sodium/iodide symporter gene expression and radioiodide uptake in the MCF-7 breast cancer cell line. Proc. Natl. Acad. Sci. USA 2000, 97, 8519–8524. [Google Scholar] [CrossRef]
- Lavudi, K.; Nuguri, S.M.; Olverson, Z.; Dhanabalan, A.K.; Patnaik, S.; Kokkanti, R.R. Targeting the retinoic acid signaling pathway as a modern precision therapy against cancers. Front. Cell Dev. Biol. 2023, 11, 1254612. [Google Scholar] [CrossRef]
- Cheng, T.-Y.D.; Yao, S.; Omilian, A.R.; Khoury, T.; Buas, M.F.; Payne-Ondracek, R.; Sribenja, S.; Bshara, W.; Hong, C.-C.; Bandera, E.V.; et al. FOXA1 Protein Expression in ER+ and ER- Breast Cancer in Relation to Parity and Breastfeeding in Black and White Women. Cancer Epidemiol. Biomark. Prev. 2020, 29, 379–385. [Google Scholar] [CrossRef]
- Chen, Q.-X.; Yang, Y.-Z.; Liang, Z.-Z.; Chen, J.-L.; Li, Y.-L.; Huang, Z.-Y.; Weng, Z.-J.; Zhang, X.-F.; Guan, J.-X.; Tang, L.-Y.; et al. Time-varying effects of FOXA1 on breast cancer prognosis. Breast Cancer Res. Treat. 2021, 187, 867–875. [Google Scholar] [CrossRef]
- Guiu, S.; Mollevi, C.; Charon-Barra, C.; Boissière, F.; Crapez, E.; Chartron, E.; Lamy, P.-J.; Gutowski, M.; Bourgier, C.; Romieu, G.; et al. Prognostic value of androgen receptor and FOXA1 co-expression in non-metastatic triple negative breast cancer and correlation with other biomarkers. Br. J. Cancer 2018, 119, 76–79. [Google Scholar] [CrossRef]
- Derakhshan, F.; Reis-Filho, J.S. Pathogenesis of Triple-Negative Breast Cancer. Annu. Rev. Pathol. Mech. Dis. 2022, 17, 181–204. [Google Scholar] [CrossRef] [PubMed]
- Gainor, D.L.; Chute, D.J.; Lorenz, R.R. Sodium Iodide Symporter Expression in Adenoid Cystic Carcinoma of the Head and Neck. Arch. Otolaryngol. Neck Surg. 2015, 141, 739. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.R.; Bergert, E.R.; Beito, T.G.; McIver, B.; Goellner, J.R.; Morris, J.C. Development of monoclonal antibodies against the human sodium iodide symporter: Immunohistochemical characterization of this protein in thyroid cells. J. Clin. Endocrinol. Metab. 1999, 84, 2957–2962. [Google Scholar] [CrossRef] [PubMed]

| TNM Stage | Number of Patients (%) |
|---|---|
| Stage IA | 8 (4.97%) |
| Stage IB | 1 (0.62%) |
| Stage IIA | 88 (54.66%) |
| Stage IIB | 16 (9.94%) |
| Stage IIIA | 8 (4.97%) |
| Stage IIIB | 16 (9.94%) |
| Stage IIIC | 24 (14.9%) |
| Stage IV | 0 |
| Total: 161 (100%) | |
| Variables | TNBC Samples (Total n = 161) |
|---|---|
| Age (years) | |
| Mean ± SD | 53.6 ± 11.97 |
| Range | 25–75 |
| Sex (n, %) | |
| Male | 0 |
| Female | 161 (100%) |
| Histologic type (n, %) | |
| IBC-NST | 161 (100%) |
| Tumor size (cm) | |
| Median [IQR] | 2.5 [2.2–3.5] |
| Range | 0.7–5.6 |
| Variables | TNBC Samples (Total n = 161) | ||||
|---|---|---|---|---|---|
| NIS Expression | |||||
| NIS 0 | NIS 1+ | NIS 2+ | NIS 3+ | Kendall Tau Correlation p-Value | |
| Age (years) (n, %) | |||||
| <45 | 17 (10.56%) | 6 (3.73%) | 0 | 16 (9.94%) | 0.073 |
| ≥45 | 32 (19.88%) | 34 (21.12%) | 24 (14.91%) | 32 (19.88%) | |
| cT stage (n, %) | |||||
| 1 | 1 (0.62%) | 0 | 8 (4.97%) | 0 | 0.507 |
| 2 | 40 (24.84%) | 40 (24.84%) | 8 (4.97%) | 40 (24.84%) | |
| 3 | 8 (4.97%) | 0 | 0 | 0 | |
| 4 | 0 | 0 | 8 (4.97%) | 8 (4.97%) | |
| cN stage (n, %) | |||||
| 0 | 40 (24.84%) | 16 (9.94%) | 16 (9.94%) | 40 (24.84%) | 0.240 |
| 1 | 0 | 8 (4.97%) | 8 (4.97%) | 0 | |
| 2 | 0 | 8 (4.97%) | 0 | 0 | |
| 3 | 9 (5.59%) | 8 (4.97%) | 0 | 8 (4.97%) | |
| Lymphovascular invasion (n, %) | |||||
| Not identified | 40 (24.84%) | 16 (9.94%) | 16 (9.94%) | 40 (24.84%) | 0.390 |
| Present | 9 (5.59%) | 24 (14.9%) | 8 (4.97%) | 8 (4.97%) | |
| Number of pregnancies (n, %) | |||||
| 0 | 9 (5.5%) | 0 | 0 | 0 | <0.05 |
| 1–3 | 8 (4.97%) | 16 (9.94%) | 24 (14.9%) | 40 (24.8%) | |
| 3+ | 32 (19.87%) | 24 (14.9%) | 0 | 8 (4.97%) | |
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demyashkin, G.; Kogan, E.; Demura, T.; Guzik, A.; Belokopytov, D.; Batov, M.; Shchekin, V.; Bicherova, I.; Shegai, P.; Kaprin, A. Parity and NIS Expression in Atypical Cells of Triple-Negative Breast Cancer, and Prognosis. Int. J. Mol. Sci. 2025, 26, 9947. https://doi.org/10.3390/ijms26209947
Demyashkin G, Kogan E, Demura T, Guzik A, Belokopytov D, Batov M, Shchekin V, Bicherova I, Shegai P, Kaprin A. Parity and NIS Expression in Atypical Cells of Triple-Negative Breast Cancer, and Prognosis. International Journal of Molecular Sciences. 2025; 26(20):9947. https://doi.org/10.3390/ijms26209947
Chicago/Turabian StyleDemyashkin, Grigory, Eugenia Kogan, Tatiana Demura, Anastasia Guzik, Dmitriy Belokopytov, Maxim Batov, Vladimir Shchekin, Irina Bicherova, Petr Shegai, and Andrei Kaprin. 2025. "Parity and NIS Expression in Atypical Cells of Triple-Negative Breast Cancer, and Prognosis" International Journal of Molecular Sciences 26, no. 20: 9947. https://doi.org/10.3390/ijms26209947
APA StyleDemyashkin, G., Kogan, E., Demura, T., Guzik, A., Belokopytov, D., Batov, M., Shchekin, V., Bicherova, I., Shegai, P., & Kaprin, A. (2025). Parity and NIS Expression in Atypical Cells of Triple-Negative Breast Cancer, and Prognosis. International Journal of Molecular Sciences, 26(20), 9947. https://doi.org/10.3390/ijms26209947






