Boldo Restores Vascularization and Reduces Skeletal Muscle Inflammation in Symptomatic Mice with Dysferlinopathy
Abstract
1. Introduction
2. Results
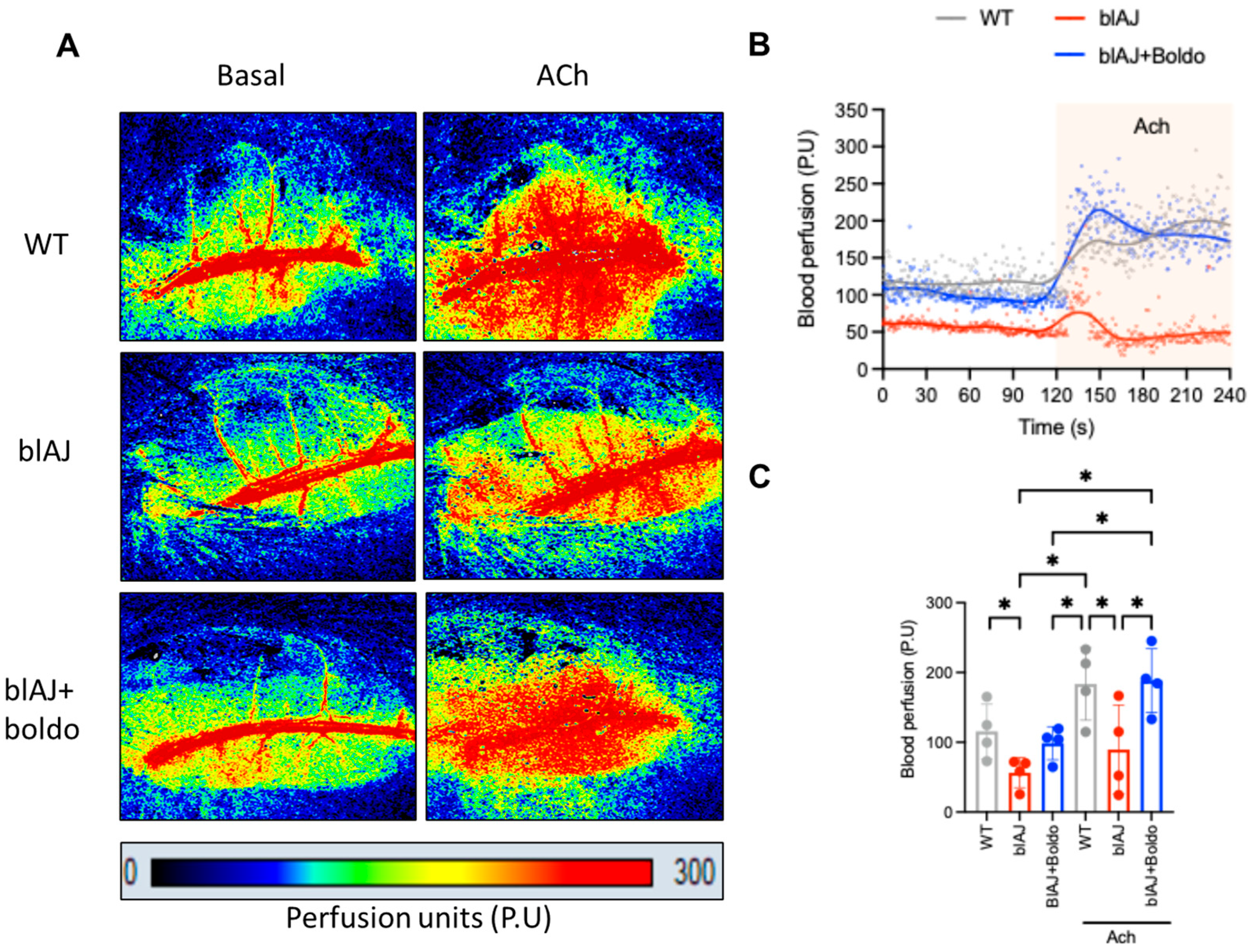
2.1. Boldo Improves Grip Strength and Blood Perfusion in Dysferlinopathy Mice
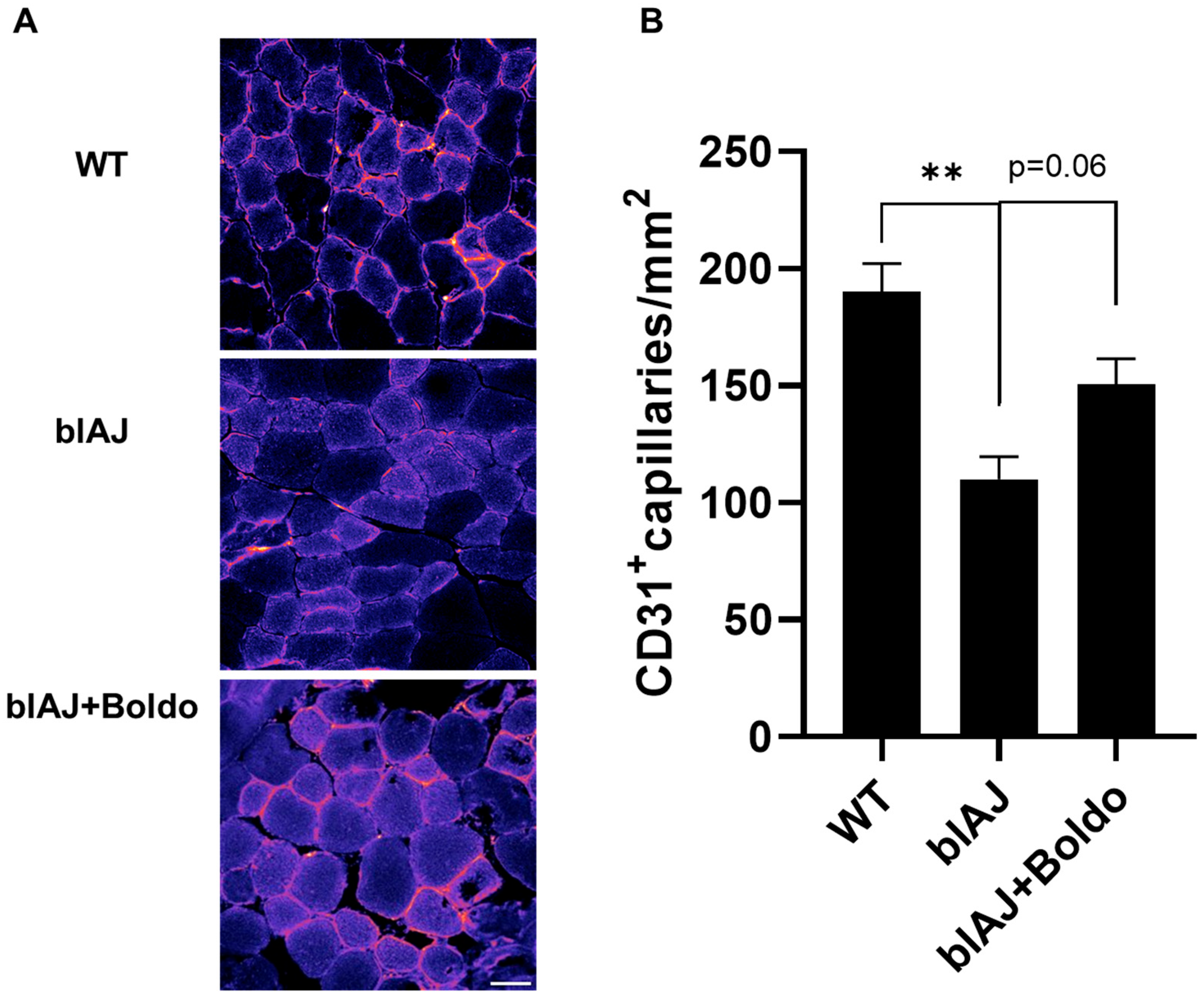
2.2. Boldo Increases Capillary Density in Skeletal Muscles from blAJ Mice
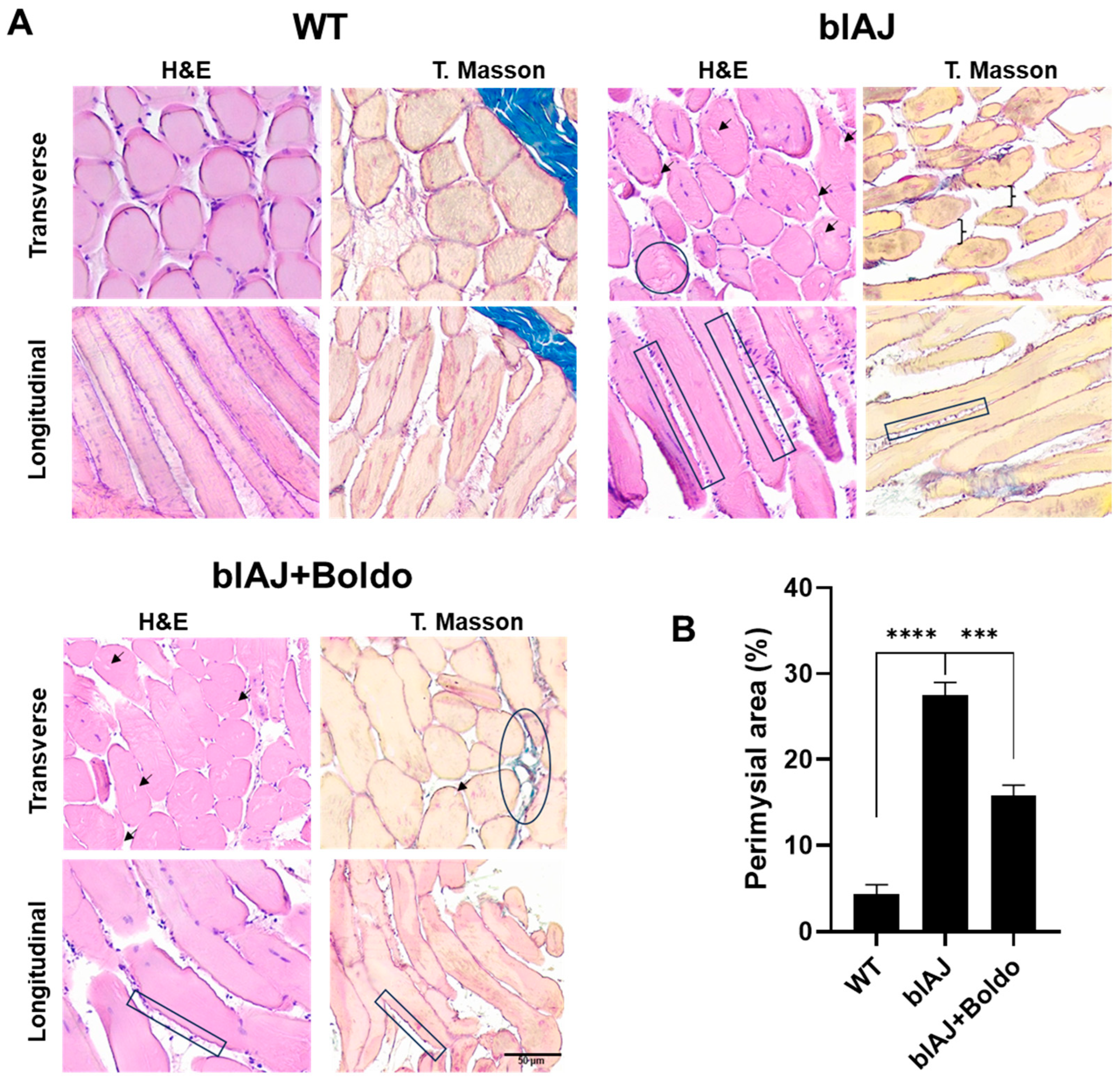
2.3. Boldo Improves the Muscular Architecture of blAJ Mice
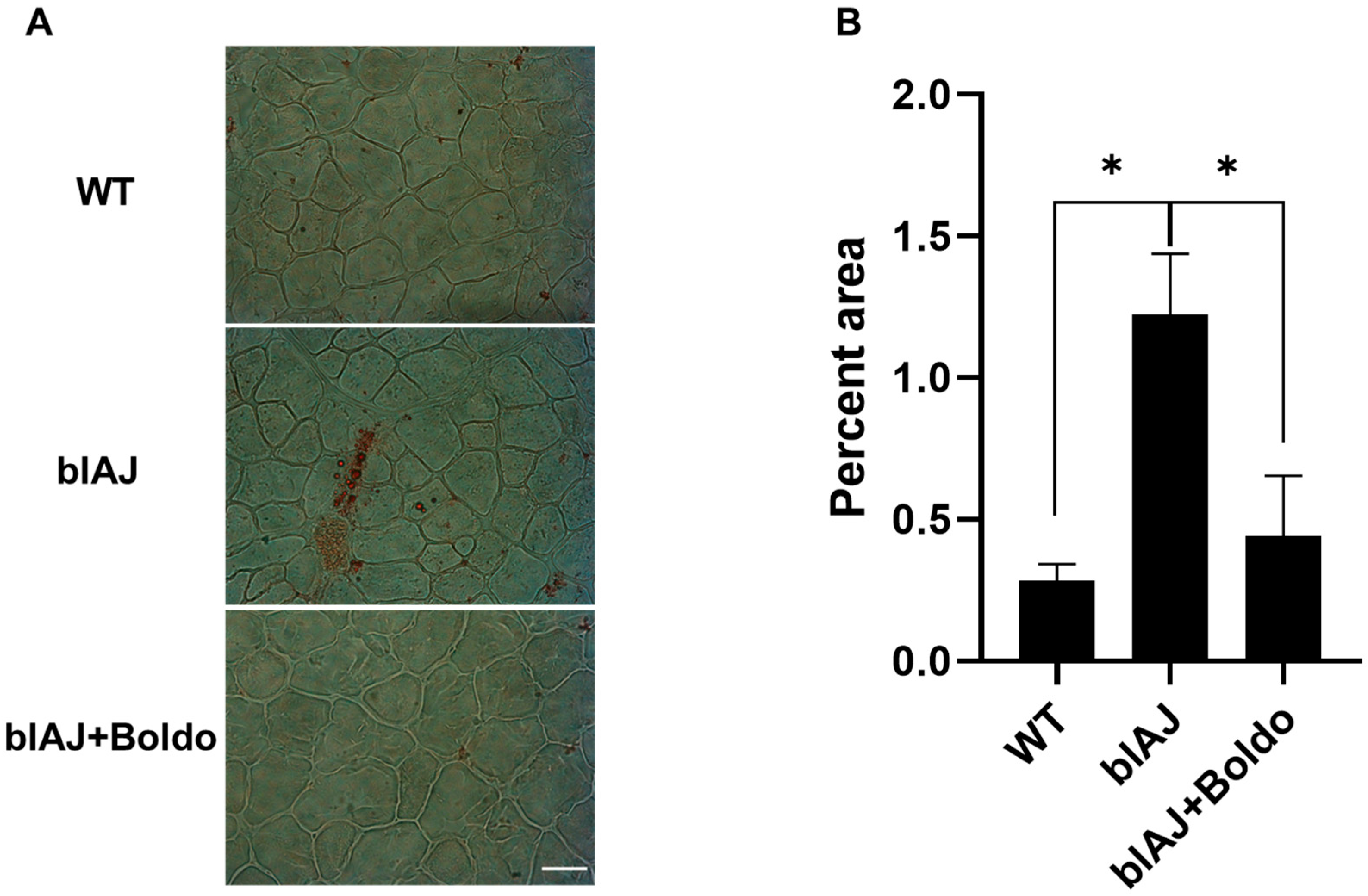
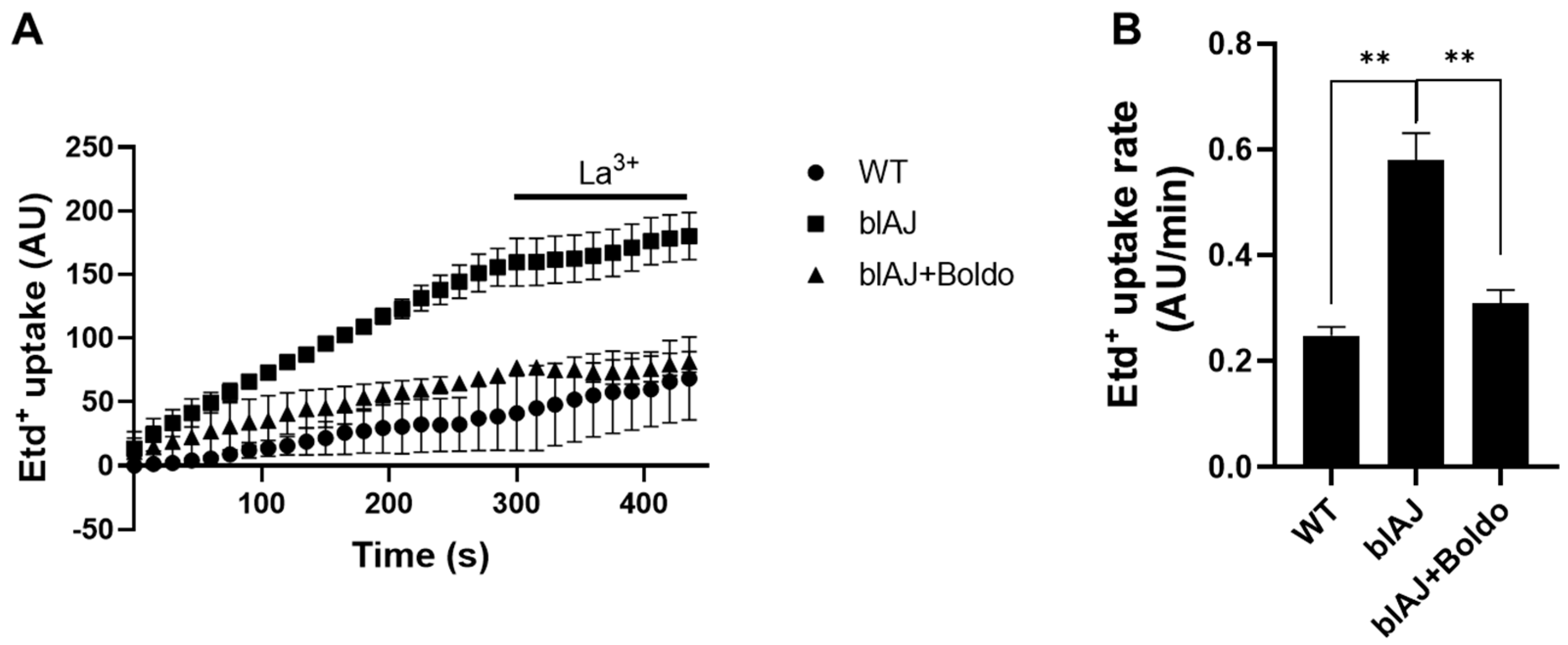
2.4. Boldo Reduces the Sarcolemma Permeability of Myofibers from blAJ Mice
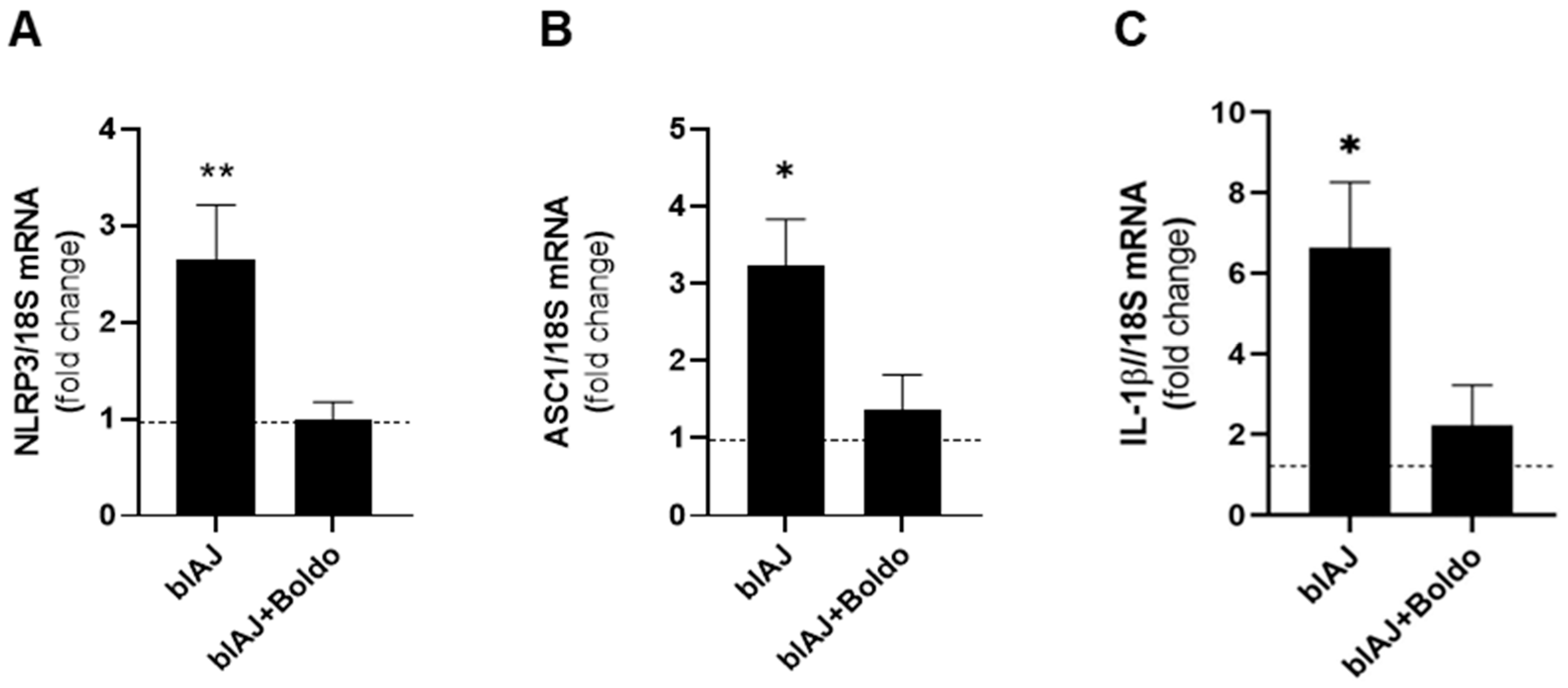
2.5. Boldo Reduces Levels of Inflammatory Markers in Skeletal Muscles from blAJ Mice
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Animals
4.3. Boldo Treatment
4.4. Blood Perfusion
4.5. Muscular Force Test
4.6. Histology
4.7. Cross-Sectional Area
4.8. Immunofluorescence Analysis
4.9. Oil Red O Staining
4.10. Isolation of Mouse Skeletal Myofibers
4.11. Time-Lapse Recording of Etd+ Uptake
4.12. Reverse Transcription Polymerase Chain Reaction (PCR)
4.13. The Oligos Used Were the Following
4.14. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Ach | Acetylcholine |
| ASC | Apoptosis-associated speck-like protein containing a CARD |
| ATP | Adenosine triphosphate |
| BSA | Bovine serum albumin |
| CSA | Cross-sectional area |
| Cx | Connexin |
| Cx HC | Connexin hemichannel |
| DAPI | 4′,6-diamidino-2-phenylindole |
| DMEM | Dulbecco’s modified Eagle medium |
| DYSF | Dysferlin gene |
| Etd+ | Ethidium bromide |
| FBS | Fetal bovine serum |
| FDB | Flexor digitorum brevis |
| H&E | Hematoxylin and eosin |
| IL-1β | Interleukin-1 beta |
| LD | Laser Doppler |
| LGMDR2 | Limb–girdle muscular dystrophy type 2B |
| NLRP3 | NOD-, LRR- and pyrin domain-containing protein 3 |
| Panx1 | Pannexin-1 |
| PBS | Phosphate-buffered saline |
| PPARγ | Peroxisome proliferator-activated receptor gamma |
| qPCR | Quantitative polymerase chain reaction |
References
- Bansal, D.; Miyake, K.; Vogel, S.S.; Groh, S.; Chen, C.-C.; Williamson, R.; McNeil, P.L.; Campbell, K.P. Defective Membrane Repair in Dysferlin-Deficient Muscular Dystrophy. Nature 2003, 423, 168–172. [Google Scholar] [CrossRef]
- Matsuda, C.; Hayashi, Y.K.; Ogawa, M.; Aoki, M.; Murayama, K.; Nishino, I.; Nonaka, I.; Arahata, K.; Brown, R.H. The Sarcolemmal Proteins Dysferlin and Caveolin-3 Interact in Skeletal Muscle. Hum. Mol. Genet. 2001, 10, 1761–1766. [Google Scholar] [CrossRef]
- Anderson, L.V.; Davison, K.; Moss, J.A.; Young, C.; Cullen, M.J.; Walsh, J.; Johnson, M.A.; Bashir, R.; Britton, S.; Keers, S.; et al. Dysferlin Is a Plasma Membrane Protein and Is Expressed Early in Human Development. Hum. Mol. Genet. 1999, 8, 855–861. [Google Scholar] [CrossRef]
- McDade, J.R.; Archambeau, A.; Michele, D.E. Rapid Actin-Cytoskeleton–Dependent Recruitment of Plasma Membrane–Derived Dysferlin at Wounds Is Critical for Muscle Membrane Repair. FASEB J. 2014, 28, 3660–3670. [Google Scholar] [CrossRef]
- Leung, C.; Utokaparch, S.; Sharma, A.; Yu, C.; Abraham, T.; Borchers, C.; Bernatchez, P. Proteomic Identification of Dysferlin-Interacting Protein Complexes in Human Vascular Endothelium. Biochem. Biophys. Res. Commun. 2011, 415, 263–269. [Google Scholar] [CrossRef]
- Davenport, N.R.; Sonnemann, K.J.; Eliceiri, K.W.; Bement, W.M. Membrane Dynamics during Cellular Wound Repair. Mol. Biol. Cell 2016, 27, 2272–2285. [Google Scholar] [CrossRef]
- Muriel, J.; Lukyanenko, V.; Kwiatkowski, T.; Bhattacharya, S.; Garman, D.; Weisleder, N.; Bloch, R.J. The C2 Domains of Dysferlin: Roles in Membrane Localization, Ca2+ Signalling and Sarcolemmal Repair. J. Physiol. 2022, 600, 1953–1968. [Google Scholar] [CrossRef]
- Cárdenas, A.M.; González-Jamett, A.M.; Cea, L.A.; Bevilacqua, J.A.; Caviedes, P. Dysferlin Function in Skeletal Muscle: Possible Pathological Mechanisms and Therapeutical Targets in Dysferlinopathies. Exp. Neurol. 2016, 283, 246–254. [Google Scholar] [CrossRef]
- Sharma, A.; Yu, C.; Leung, C.; Trane, A.; Lau, M.; Utokaparch, S.; Shaheen, F.; Sheibani, N.; Bernatchez, P. A New Role for the Muscle Repair Protein Dysferlin in Endothelial Cell Adhesion and Angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2196–2204. [Google Scholar] [CrossRef]
- Urtizberea, J.A.; Bassez, G.; Leturcq, F.; Nguyen, K.; Krahn, M.; Levy, N. Dysferlinopathies. Neurol. India 2008, 56, 289–297. [Google Scholar] [CrossRef]
- Han, R. Muscle Membrane Repair and Inflammatory Attack in Dysferlinopathy. Skelet. Muscle 2011, 1, 10. [Google Scholar] [CrossRef]
- Bashir, R.; Britton, S.; Strachan, T.; Keers, S.; Vafiadaki, E.; Lako, M.; Richard, I.; Marchand, S.; Bourg, N.; Argov, Z.; et al. A Gene Related to Caenorhabditis Elegans Spermatogenesis Factor Fer-1 Is Mutated in Limb-Girdle Muscular Dystrophy Type 2B. Nat. Genet. 1998, 20, 37–42. [Google Scholar] [CrossRef]
- Laval, S.H.; Bushby, K.M.D. Limb-Girdle Muscular Dystrophies--from Genetics to Molecular Pathology. Neuropathol. Appl. Neurobiol. 2004, 30, 91–105. [Google Scholar] [CrossRef]
- González-Jamett, A.; Vásquez, W.; Cifuentes-Riveros, G.; Martínez-Pando, R.; Sáez, J.C.; Cárdenas, A.M. Oxidative Stress, Inflammation and Connexin Hemichannels in Muscular Dystrophies. Biomedicines 2022, 10, 507. [Google Scholar] [CrossRef]
- Defour, A.; Medikayala, S.; Van der Meulen, J.H.; Hogarth, M.W.; Holdreith, N.; Malatras, A.; Duddy, W.; Boehler, J.; Nagaraju, K.; Jaiswal, J.K. Annexin A2 Links Poor Myofiber Repair with Inflammation and Adipogenic Replacement of the Injured Muscle. Hum. Mol. Genet. 2017, 26, 1979–1991. [Google Scholar] [CrossRef]
- Bouchard, C.; Tremblay, J.P. Portrait of Dysferlinopathy: Diagnosis and Development of Therapy. J. Clin. Med. 2023, 12, 6011. [Google Scholar] [CrossRef]
- Ivanova, A.; Smirnikhina, S.; Lavrov, A. Dysferlinopathies: Clinical and Genetic Variability. Clin. Genet. 2022, 102, 465–473. [Google Scholar] [CrossRef]
- Becker, N.; Moore, S.A.; Jones, K.A. The Inflammatory Pathology of Dysferlinopathy Is Distinct from Calpainopathy, Becker Muscular Dystrophy, and Inflammatory Myopathies. Acta Neuropathol. Commun. 2022, 10, 17. [Google Scholar] [CrossRef]
- Selcen, D.; Stilling, G.; Engel, A.G. The Earliest Pathologic Alterations in Dysferlinopathy. Neurology 2001, 56, 1472–1481. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, J.; Liu, Y. Skeletal Muscle Regeneration in Cardiotoxin-Induced Muscle Injury Models. Int. J. Mol. Sci. 2022, 23, 13380. [Google Scholar] [CrossRef]
- Fernández-Eulate, G.; Querin, G.; Moore, U.; Behin, A.; Masingue, M.; Bassez, G.; Leonard-Louis, S.; Laforêt, P.; Maisonobe, T.; Merle, P.-E.; et al. Deep Phenotyping of an International Series of Patients with Late-Onset Dysferlinopathy. Eur. J. Neurol. 2021, 28, 2092–2102. [Google Scholar] [CrossRef]
- Fernández, J.; Lagos, P.; Rivera, P.; Zamorano-Ponce, E. Effect of Boldo (Peumus Boldus Molina) Infusion on Lipoperoxidation Induced by Cisplatin in Mice Liver. Phytother. Res. PTR 2009, 23, 1024–1027. [Google Scholar] [CrossRef]
- Ferrante, C.; Chiavaroli, A.; Angelini, P.; Venanzoni, R.; Angeles Flores, G.; Brunetti, L.; Petrucci, M.; Politi, M.; Menghini, L.; Leone, S.; et al. Phenolic Content and Antimicrobial and Anti-Inflammatory Effects of Solidago Virga-Aurea, Phyllanthus Niruri, Epilobium Angustifolium, Peumus Boldus, and Ononis Spinosa Extracts. Antibiotics 2020, 9, 783. [Google Scholar] [CrossRef]
- Schmeda-Hirschmann, G.; Rodriguez, J.A.; Theoduloz, C.; Astudillo, S.L.; Feresin, G.E.; Tapia, A. Free-Radical Scavengers and Antioxidants from Peumus Boldus Mol. (“Boldo”). Free Radic. Res. 2003, 37, 447–452. [Google Scholar] [CrossRef]
- Backhouse, N.; Delporte, C.; Givernau, M.; Cassels, B.K.; Valenzuela, A.; Speisky, H. Anti-Inflammatory and Antipyretic Effects of Boldine. Agents Actions 1994, 42, 114–117. [Google Scholar] [CrossRef]
- Shuker, E.; Farhood, M.; Al-Qudaihi, G.; Fouad, D. Potential Effects of Boldine on Oxidative Stress, Apoptosis, and Inflammatory Changes Induced by the Methylprednisolone Hepatotoxicity in Male Wistar Rats. Dose-Response Publ. Int. Hormesis Soc. 2022, 20, 15593258221082877. [Google Scholar] [CrossRef]
- Yang, X.; Gao, X.; Cao, Y.; Guo, Q.; Li, S.; Zhu, Z.; Zhao, Y.; Tu, P.; Chai, X. Anti-Inflammatory Effects of Boldine and Reticuline Isolated from Litsea Cubeba through JAK2/STAT3 and NF-κB Signaling Pathways. Planta Med. 2018, 84, 20–25. [Google Scholar] [CrossRef]
- Hernández-Salinas, R.; Vielma, A.Z.; Arismendi, M.N.; Boric, M.P.; Sáez, J.C.; Velarde, V. Boldine Prevents Renal Alterations in Diabetic Rats. J. Diabetes Res. 2013, 2013, 593672. [Google Scholar] [CrossRef]
- Yi, C.; Ezan, P.; Fernández, P.; Schmitt, J.; Sáez, J.C.; Giaume, C.; Koulakoff, A. Inhibition of Glial Hemichannels by Boldine Treatment Reduces Neuronal Suffering in a Murine Model of Alzheimer’s Disease. Glia 2017, 65, 1607–1625. [Google Scholar] [CrossRef]
- Cea, L.A.; Fernández, G.; Arias-Bravo, G.; Castillo-Ruiz, M.; Escamilla, R.; Brañes, M.C.; Sáez, J.C. Blockade of Hemichannels Normalizes the Differentiation Fate of Myoblasts and Features of Skeletal Muscles from Dysferlin-Deficient Mice. Int. J. Mol. Sci. 2020, 21, 6025. [Google Scholar] [CrossRef]
- Cea, L.A.; Bevilacqua, J.A.; Arriagada, C.; Cárdenas, A.M.; Bigot, A.; Mouly, V.; Sáez, J.C.; Caviedes, P. The Absence of Dysferlin Induces the Expression of Functional Connexin-Based Hemichannels in Human Myotubes. BMC Cell Biol. 2016, 17, 15. [Google Scholar] [CrossRef]
- Lloyd, E.M.; Crew, R.C.; Haynes, V.R.; White, R.B.; Mark, P.J.; Jackaman, C.; Papadimitriou, J.M.; Pinniger, G.J.; Murphy, R.M.; Watt, M.J.; et al. Pilot Investigations into the Mechanistic Basis for Adverse Effects of Glucocorticoids in Dysferlinopathy. Skelet. Muscle 2024, 14, 19. [Google Scholar] [CrossRef]
- Fernández, G.; Arias-Bravo, G.; Bevilacqua, J.A.; Castillo-Ruiz, M.; Caviedes, P.; Sáez, J.C.; Cea, L.A. Myofibers Deficient in Connexins 43 and 45 Expression Protect Mice from Skeletal Muscle and Systemic Dysfunction Promoted by a Dysferlin Mutation. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165800. [Google Scholar] [CrossRef]
- Grounds, M.D.; Terrill, J.R.; Radley-Crabb, H.G.; Robertson, T.; Papadimitriou, J.; Spuler, S.; Shavlakadze, T. Lipid Accumulation in Dysferlin-Deficient Muscles. Am. J. Pathol. 2014, 184, 1668–1676. [Google Scholar] [CrossRef]
- Johnson, R.G.; Le, H.C.; Evenson, K.; Loberg, S.W.; Myslajek, T.M.; Prabhu, A.; Manley, A.-M.; O’Shea, C.; Grunenwald, H.; Haddican, M.; et al. Connexin Hemichannels: Methods for Dye Uptake and Leakage. J. Membr. Biol. 2016, 249, 713–741. [Google Scholar] [CrossRef] [PubMed]
- Rawat, R.; Cohen, T.V.; Ampong, B.; Francia, D.; Henriques-Pons, A.; Hoffman, E.P.; Nagaraju, K. Inflammasome Up-Regulation and Activation in Dysferlin-Deficient Skeletal Muscle. Am. J. Pathol. 2010, 176, 2891–2900. [Google Scholar] [CrossRef]
- Russo, A.; Cardile, V.; Caggia, S.; Gunther, G.; Troncoso, N.; Garbarino, J. Boldo Prevents UV Light and Nitric Oxide-Mediated Plasmid DNA Damage and Reduces the Expression of Hsp70 Protein in Melanoma Cancer Cells. J. Pharm. Pharmacol. 2011, 63, 1219–1229. [Google Scholar] [CrossRef]
- Speisky, H.; Cassels, B.K. Boldo and Boldine: An Emerging Case of Natural Drug Development. Pharmacol. Res. 1994, 29, I994. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Barros, G.; Echeverría, J.; Mattar, C.; Liberona, L.; Giordano, A.; Suárez-Rozas, C.; Salas-Norambuena, J.; González-Cooper, A.; Cassels, B.K.; Castro-Saavedra, S. Phytochemical Variation of Wild and Farmed Populations of Boldo (Peumus boldus Molina). J. Appl. Res. Med. Aromat. Plants 2023, 35, 100502. [Google Scholar] [CrossRef]
- Cea, L.A.; Vásquez, W.; Hernández-Salinas, R.; Vielma, A.Z.; Castillo-Ruiz, M.; Velarde, V.; Salgado, M.; Sáez, J.C. Skeletal Muscle Atrophy Induced by Diabetes Is Mediated by Non-Selective Channels and Prevented by Boldine. Biomolecules 2023, 13, 708. [Google Scholar] [CrossRef] [PubMed]
- Toro, C.A.; Johnson, K.; Hansen, J.; Siddiq, M.M.; Vásquez, W.; Zhao, W.; Graham, Z.A.; Sáez, J.C.; Iyengar, R.; Cardozo, C.P. Boldine Modulates Glial Transcription and Functional Recovery in a Murine Model of Contusion Spinal Cord Injury. Front. Cell. Neurosci. 2023, 17, 1163436. [Google Scholar] [CrossRef]
- Loufrani, L.; Levy, B.I.; Henrion, D. Defect in Microvascular Adaptation to Chronic Changes in Blood Flow in Mice Lacking the Gene Encoding for Dystrophin. Circ. Res. 2002, 91, 1183–1189. [Google Scholar] [CrossRef]
- Fanin, M.; Nascimbeni, A.C.; Angelini, C. Muscle Atrophy, Ubiquitin–Proteasome, and Autophagic Pathways in Dysferlinopathy. Muscle Nerve 2014, 50, 340–347. [Google Scholar] [CrossRef]
- Folker, E.; Baylies, M. Nuclear Positioning in Muscle Development and Disease. Front. Physiol. 2013, 4, 363. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Du, J.; Wang, Z.; Zhang, W.; Lv, H.; Meng, L.; Xiao, J.; Yuan, Y. Heterogeneous Characteristics of MRI Changes of Thigh Muscles in Patients with Dysferlinopathy. Muscle Nerve 2016, 54, 1072–1079. [Google Scholar] [CrossRef] [PubMed]
- Seror, P.; Krahn, M.; Laforet, P.; Leturcq, F.; Maisonobe, T. Complete Fatty Degeneration of Lumbar Erector Spinae Muscles Caused by a Primary Dysferlinopathy. Muscle Nerve 2008, 37, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Price, F.; Rudnicki, M.A. Satellite Cells and the Muscle Stem Cell Niche. Physiol. Rev. 2013, 93, 23–67. [Google Scholar] [CrossRef]
- Schalper, K.A.; Sánchez, H.A.; Lee, S.C.; Altenberg, G.A.; Nathanson, M.H.; Sáez, J.C. Connexin 43 Hemichannels Mediate the Ca2+ Influx Induced by Extracellular Alkalinization. Am. J. Physiol.-Cell Physiol. 2010, 299, C1504–C1515. [Google Scholar] [CrossRef]
- Cea, L.A.; Cisterna, B.A.; Puebla, C.; Frank, M.; Figueroa, X.F.; Cardozo, C.; Willecke, K.; Latorre, R.; Sáez, J.C. De Novo Expression of Connexin Hemichannels in Denervated Fast Skeletal Muscles Leads to Atrophy. Proc. Natl. Acad. Sci. USA 2013, 110, 16229–16234. [Google Scholar] [CrossRef]
- Samways, D.S.K.; Wolf, K.; Bowles, E.A.; Richards, J.P.; Bruno, J.; Dutertre, S.; DiPaolo, R.J.; Egan, T.M. Quantifying Ca2+ Current and Permeability in ATP-Gated P2X7 Receptors. J. Biol. Chem. 2015, 290, 7930–7942. [Google Scholar] [CrossRef]
- Kang, J.; Kang, N.; Lovatt, D.; Torres, A.; Zhao, Z.; Lin, J.; Nedergaard, M. Connexin 43 Hemichannels Are Permeable to ATP. J. Neurosci. 2008, 28, 4702–4711. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef]
- Confalonieri, P.; Oliva, L.; Andreetta, F.; Lorenzoni, R.; Dassi, P.; Mariani, E.; Morandi, L.; Mora, M.; Cornelio, F.; Mantegazza, R. Muscle Inflammation and MHC Class I Up-Regulation in Muscular Dystrophy with Lack of Dysferlin: An Immunopathological Study. J. Neuroimmunol. 2003, 142, 130–136. [Google Scholar] [CrossRef]
- Gallardo, E.; Rojas-García, R.; de Luna, N.; Pou, A.; Brown, R.H.; Illa, I. Inflammation in Dysferlin Myopathy: Immunohistochemical Characterization of 13 Patients. Neurology 2001, 57, 2136–2138. [Google Scholar] [CrossRef]
- McNally, E.M.; Ly, C.T.; Rosenmann, H.; Mitrani Rosenbaum, S.; Jiang, W.; Anderson, L.V.B.; Soffer, D.; Argov, Z. Splicing Mutation in Dysferlin Produces Limb-Girdle Muscular Dystrophy with Inflammation. Am. J. Med. Genet. 2000, 91, 305–312. [Google Scholar] [CrossRef]
- Dell, R.B.; Holleran, S.; Ramakrishnan, R. Sample Size Determination. ILAR J. 2002, 43, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Koopman, R.; Schaart, G.; Hesselink, M.K. Optimisation of Oil Red O Staining Permits Combination with Immunofluorescence and Automated Quantification of Lipids. Histochem. Cell Biol. 2001, 116, 63–68. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vásquez, W.; Troncoso, F.; Lira, A.; Escudero, C.; Sáez, J.C. Boldo Restores Vascularization and Reduces Skeletal Muscle Inflammation in Symptomatic Mice with Dysferlinopathy. Int. J. Mol. Sci. 2025, 26, 9945. https://doi.org/10.3390/ijms26209945
Vásquez W, Troncoso F, Lira A, Escudero C, Sáez JC. Boldo Restores Vascularization and Reduces Skeletal Muscle Inflammation in Symptomatic Mice with Dysferlinopathy. International Journal of Molecular Sciences. 2025; 26(20):9945. https://doi.org/10.3390/ijms26209945
Chicago/Turabian StyleVásquez, Walter, Felipe Troncoso, Andrea Lira, Carlos Escudero, and Juan C. Sáez. 2025. "Boldo Restores Vascularization and Reduces Skeletal Muscle Inflammation in Symptomatic Mice with Dysferlinopathy" International Journal of Molecular Sciences 26, no. 20: 9945. https://doi.org/10.3390/ijms26209945
APA StyleVásquez, W., Troncoso, F., Lira, A., Escudero, C., & Sáez, J. C. (2025). Boldo Restores Vascularization and Reduces Skeletal Muscle Inflammation in Symptomatic Mice with Dysferlinopathy. International Journal of Molecular Sciences, 26(20), 9945. https://doi.org/10.3390/ijms26209945








