Clinical Impact of LAG3 Single-Nucleotide Polymorphism in DLBCL Treated with CAR-T Cell Therapy
Abstract
1. Introduction
2. Results
2.1. Prevalence of the LAG3 Snp rs870849 in DLBCL Patients
2.2. Baseline Clinical Characteristics
2.3. Disease Features and CAR-T Cell Treatment
2.4. Treatment Outcome Associated with LAG3 Germline Variant
2.5. LAG3 and CTLA4 in CAR-T Cell Response
3. Discussion
4. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Su, M.; Zhang, Z.; Jiang, P.; Wang, X.; Tong, X.; Wu, G. CAR-T-Cell Therapy Based on Immune Checkpoint Modulation in the Treatment of Hematologic Malignancies. Cell Transplant. 2024, 33, 09636897241293964. [Google Scholar] [CrossRef]
- Grosser, R.; Cherkassky, L.; Chintala, N.; Adusumilli, P.S. Combination Immunotherapy with CAR T Cells and Checkpoint Blockade for the Treatment of Solid Tumors. Cancer Cell 2019, 36, 471–482. [Google Scholar] [CrossRef]
- Graydon, C.G.; Mohideen, S.; Fowke, K.R. LAG3’s Enigmatic Mechanism of Action. Front. Immunol. 2021, 11, 615317. [Google Scholar] [CrossRef]
- Mariuzza, R.A.; Shahid, S.; Karade, S.S. The Immune Checkpoint Receptor LAG3: Structure, Function, and Target for Cancer Immunotherapy. J. Biol. Chem. 2024, 300, 107241. [Google Scholar] [CrossRef]
- Kim, G.-R.; Choi, J.-M. Current Understanding of Cytotoxic T Lymphocyte Antigen-4 (CTLA-4) Signaling in T-Cell Biology and Disease Therapy. Mol. Cells 2022, 45, 513–521. [Google Scholar] [CrossRef]
- Ruffo, E.; Wu, R.C.; Bruno, T.C.; Workman, C.J.; Vignali, D.A.A. Lymphocyte-Activation Gene 3 (LAG3): The next Immune Checkpoint Receptor. Semin. Immunol. 2019, 42, 101305. [Google Scholar] [CrossRef]
- Pardoll, D.M. The Blockade of Immune Checkpoints in Cancer Immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Cruz, D.; Rodríguez-Romanos, R.; González-Bartulos, M.; García-Cadenas, I.; de la Cámara, R.; Heras, I.; Buño, I.; Santos, N.; Lloveras, N.; Velarde, P.; et al. LAG3 Genotype of the Donor and Clinical Outcome after Allogeneic Transplantation from HLA-Identical Sibling Donors. Front. Immunol. 2023, 14, 1066393. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.; Jasek, M.; Karabon, L. Immune Checkpoint Molecules-Inherited Variations as Markers for Cancer Risk. Front. Immunol. 2020, 11, 606721. [Google Scholar] [CrossRef] [PubMed]
- Mäurer, M.; Loserth, S.; Kolb-Mäurer, A.; Ponath, A.; Wiese, S.; Kruse, N.; Rieckmann, P. A Polymorphism in the Human Cytotoxic T-Lymphocyte Antigen 4 (CTLA4) Gene (Exon 1 +49) Alters T-Cell Activation. Immunogenetics 2002, 54, 1–8. [Google Scholar] [CrossRef]
- Seipel, K.; Shaforostova, I.; Nilius, H.; Bacher, U.; Pabst, T. Clinical Impact of CTLA-4 Single-Nucleotide Polymorphism in DLBCL Patients Treated with CAR-T Cell Therapy. Curr. Oncol. 2025, 32, 425. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, V.; Workman, C.J.; Vignali, D.A.A. LAG-3 as the Third Checkpoint Inhibitor. Nat. Immunol. 2023, 24, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Burnell, S.E.A.; Capitani, L.; MacLachlan, B.J.; Mason, G.H.; Gallimore, A.M.; Godkin, A. Seven Mysteries of LAG-3: A Multi-Faceted Immune Receptor of Increasing Complexity. Immunother. Adv. 2022, 2, ltab025. [Google Scholar] [CrossRef]
- Chocarro, L.; Blanco, E.; Zuazo, M.; Arasanz, H.; Bocanegra, A.; Fernández-Rubio, L.; Morente, P.; Fernández-Hinojal, G.; Echaide, M.; Garnica, M.; et al. Understanding LAG-3 Signaling. Int. J. Mol. Sci. 2021, 22, 5282. [Google Scholar] [CrossRef]
- Li, N.; Wang, Y.; Forbes, K.; Vignali, K.M.; Heale, B.S.; Saftig, P.; Hartmann, D.; Black, R.A.; Rossi, J.J.; Blobel, C.P.; et al. Metalloproteases Regulate T-Cell Proliferation and Effector Function via LAG-3. EMBO J. 2007, 26, 494–504. [Google Scholar] [CrossRef]
- Adam, K.; Lipatova, Z.; Abdul Ghafoor Raja, M.; Mishra, A.K.; Mariuzza, R.A.; Workman, C.J.; Vignali, D.A.A. Cutting Edge: LAG3 Dimerization Is Required for TCR/CD3 Interaction and Inhibition of Antitumor Immunity. J. Immunol. 2024, 213, 7–13. [Google Scholar] [CrossRef]
- Wang, J.; Klein, C.; Cochran, J.R.; Sockolosky, J.; Lippow, S.M. Exploring New Frontiers in LAG-3 Biology and Therapeutics. Trends Pharmacol. Sci. 2025, 46, 638–652. [Google Scholar] [CrossRef]
- Al-Batran, S.-E.; Mueller, D.W.; Rafiyan, M.-R.; Kiselicki, D.; Atmaca, A.; Habibzada, T.; Mueller, C.; Brignone, C.; Triebel, F.; Loose, M.; et al. A Soluble LAG-3 Protein (Eftilagimod Alpha) and an Anti-PD-L1 Antibody (Avelumab) Tested in a Phase I Trial: A New Combination in Immuno-Oncology. ESMO Open 2023, 8, 101623. [Google Scholar] [CrossRef]
- Fougeray, S.; Brignone, C.; Triebel, F. A Soluble LAG-3 Protein as an Immunopotentiator for Therapeutic Vaccines: Preclinical Evaluation of IMP321. Vaccine 2006, 24, 5426–5433. [Google Scholar] [CrossRef] [PubMed]
- Gorgulho, J.; Roderburg, C.; Beier, F.; Bokemeyer, C.; Brümmendorf, T.H.; Loosen, S.H.; Luedde, T. Soluble Lymphocyte Activation Gene-3 (sLAG3) and CD4/CD8 Ratio Dynamics as Predictive Biomarkers in Patients Undergoing Immune Checkpoint Blockade for Solid Malignancies. Br. J. Cancer 2024, 130, 1013–1022. [Google Scholar] [CrossRef]
- Phan, L.; Zhang, H.; Wang, Q.; Villamarin, R.; Hefferon, T.; Ramanathan, A.; Kattman, B. The Evolution of dbSNP: 25 Years of Impact in Genomic Research. Nucleic Acids Res. 2025, 53, D925–D931. [Google Scholar] [CrossRef]
- Monne, M.; Piras, G.; Palmas, A.; Arru, L.; Murineddu, M.; Latte, G.; Noli, A.; Gabbas, A. Cytotoxic T-Lymphocyte Antigen-4 (CTLA-4) Gene Polymorphism and Susceptibility to Non-Hodgkin’s Lymphoma. Am. J. Hematol. 2004, 76, 14–18. [Google Scholar] [CrossRef]
- Cheng, T.-Y.; Lin, J.-T.; Chen, L.-T.; Shun, C.-T.; Wang, H.-P.; Lin, M.-T.; Wang, T.-E.; Cheng, A.-L.; Wu, M.-S. Association of T-Cell Regulatory Gene Polymorphisms with Susceptibility to Gastric Mucosa-Associated Lymphoid Tissue Lymphoma. J. Clin. Oncol. 2006, 24, 3483–3489. [Google Scholar] [CrossRef] [PubMed]
- Silberstein, J.L.; Du, J.; Chan, K.-W.; Frank, J.A.; Mathews, I.I.; Kim, Y.B.; You, J.; Lu, Q.; Liu, J.; Philips, E.A.; et al. Structural Insights Reveal Interplay between LAG-3 Homodimerization, Ligand Binding, and Function. Proc. Natl. Acad. Sci. USA 2024, 121, e2310866121. [Google Scholar] [CrossRef] [PubMed]
- Gopal, A.K.; Armand, P.; Neelapu, S.S.; Bartlett, N.L.; Spurgeon, S.E.; Kuruvilla, J.; Savage, K.J.; Leonard, J.P.; Gelb, A.B.; Ahmed, N.; et al. Nivolumab plus Relatlimab for Patients with Relapsed or Progressed B-Cell Malignancies in RELATIVITY-022. Blood Adv. 2025, 9, 3383–3394. [Google Scholar] [CrossRef]
- Luke, J.J.; Patel, M.R.; Blumenschein, G.R.; Hamilton, E.; Chmielowski, B.; Ulahannan, S.V.; Connolly, R.M.; Santa-Maria, C.A.; Wang, J.; Bahadur, S.W.; et al. The PD-1- and LAG-3-Targeting Bispecific Molecule Tebotelimab in Solid Tumors and Hematologic Cancers: A Phase 1 Trial. Nat. Med. 2023, 29, 2814–2824. [Google Scholar] [CrossRef]
- Luo, Y.; Cai, X.; Yang, B.; Lu, F.; Yi, C.; Wu, G. Advances in Understanding the Role of Immune Checkpoint LAG-3 in Tumor Immunity: A Comprehensive Review. Front. Oncol. 2024, 14, 1402837. [Google Scholar] [CrossRef]
- Wu, L.; Seung, E.; Xu, L.; Rao, E.; Lord, D.M.; Wei, R.R.; Cortez-Retamozo, V.; Ospina, B.; Posternak, V.; Ulinski, G.; et al. Trispecific Antibodies Enhance the Therapeutic Efficacy of Tumor-Directed T Cells through T Cell Receptor Co-Stimulation. Nat. Cancer 2020, 1, 86–98. [Google Scholar] [CrossRef]
- Cianciotti, B.C.; Magnani, Z.I.; Ugolini, A.; Camisa, B.; Merelli, I.; Vavassori, V.; Potenza, A.; Imparato, A.; Manfredi, F.; Abbati, D.; et al. TIM-3, LAG-3, or 2B4 Gene Disruptions Increase the Anti-Tumor Response of Engineered T Cells. Front. Immunol. 2024, 15, 1315283. [Google Scholar] [CrossRef] [PubMed]

| Parameter | All Patients | LAG3 I455hom | LAG3 I455Thet | LAG3 T455hom | p-Value |
|---|---|---|---|---|---|
| Patients n (%) | 112 (100) | 25 (22) | 60 (54) | 27 (24) | |
| M:F (ratio) | 64:48 (1.3) | 16:9 (1.8) | 32:28 (1.2) | 15:12 (1.2) | 0.66 1 |
| Age at ID, years median (range) | 62 (25–79) | 62 (36–78) | 62 (29–79) | 59 (25–77) | 0.47 2 |
| Age at CAR-T, years median (range) | 66 (26–82) | 65 (37–82) | 69 (30–80) | 65 (26–79) | 0.69 2 |
| Initial diagnosis | 112 (100) | 0.84 1 | |||
| DLBCL, n (%) de novo, n (%) transformed, n (%) PMBCL CLL/SLL FL, n (%) | 103 (92) 68 (61) 35 (31) 2 (2) 1 (1) 6 (5) | 24 (96) 17 (68) 8 (33) 0 0 1 (4) | 53 (88) 35 (58) 18 (30) 1 (2) 1 (2) 5 (8) | 26 (96) 16 (59) 10 (37) 1 (4) 0 0 | |
| Initial stage | 88 (100) | 21 (24) | 46 (53) | 21 (23) | 0.85 1 |
| One, n (%) Two, n (%) Three, n (%) Four, n (%) | 5 (6) 16 (18) 18 (20) 49 (56) | 0 5 (24) 5 (24) 11 (52) | 3 (6) 6 (13) 9 (20) 28 (61) | 2 (10) 5 (24) 4 (19) 10 (48) | |
| Chemotx lines | 112 (100) | 0.35 1 | |||
| 1 2 3 4–6 | 5 (4) 41 (36) 58 (52) 7 (6) | 2 (8) 5 (19) 17 (65) 1 (4) | 2 (3) 24 (40) 30 (50) 4 (7) | 1 (4) 12 (46) 11 (42) 2 (8) | |
| Radiotherapy | 47 (42) | 11 (48) | 21 (38) | 15 (55) | 0.57 1 |
| Prior ASCT | 41 (40) | 11 (48) | 19 (34) | 11 (48) | 0.49 1 |
| Parameter | All Patients | LAG3 I455hom | LAG3 I455Thet | LAG3 T455hom | p-Value |
|---|---|---|---|---|---|
| Group size, n (%) | 112 (100) | 25 (22) | 60 (54) | 27 (24) | |
| IPI | 0.31 1 | ||||
| 1, n (%) | 3 (2) | 0 | 2 (3) | 1 (4) | |
| 2, n (%) | 8 (7) | 4 (17) | 2 (3) | 2 (9) | |
| 3, n (%) | 35 (31) | 10 (40) | 17 (28) | 8 (30) | |
| 4, n (%) | 34 (31) | 4 (17) | 21 (35) | 9 (35) | |
| na, n (%) | 32 (29) | 7 (27) | 18 (30) | 7 (27) | |
| Remission Status at CAR-T infusion | 0.56 1 | ||||
| CR, n (%) | 7 (8) | 2 (8) | 5 (9) | 0 | |
| PR, n (%) | 26 (29) | 10 (38) | 17 (32) | 9 (39) | |
| SD, n (%) | 4 (4) | 1 (4) | 3 (5) | 0 | |
| PD, n (%) | 58 (52) | 12 (48) | 28 (52) | 18 (67) | |
| Bridging chemotx | 42 (37) | 11 (44) | 23 (43) | 10 (37) | 0.88 1 |
| Bridging radiotx | 17 (15) | 7 (30) | 5 (9) | 5 (22) | 0.07 1 |
| LDH pre CAR-T (U/L) median (range) | 337 (134–3949) | 329 (145–3949) | 324 (135–2355) | 342 (134–1171) | 0.91 1 |
| CAR-T-cell product | 0.51 1 | ||||
| Tisa-cel | 64 (58) | 16 (64) | 33 (61) | 15 (55) | |
| Axi-cel | 42 (37) | 7 (27) | 23 (43) | 12 (46) | |
| Liso-cel | 6 (5) | 2 (8) | 4 (7) | 0 | |
| CRS | 86 (76) | 0.59 1 | |||
| Grade 1 | 55 (49) | 15 (58) | 30 (49) | 10 (38) | |
| Grade 2 | 27 (24) | 5 (19) | 14 (23) | 8 (31) | |
| Grade 3 | 4 (4) | 0 | 2 (4) | 2 (8) | |
| ICANS | 37 (33) | 0.65 1 | |||
| Grade 1 | 11 (10) | 2 (9) | 9 (15) | 0 | |
| Grade 2 | 7 (6) | 2 (9) | 3 (5) | 2 (9) | |
| Grade 3 | 13 (11) | 4 (17) | 6 (10) | 3 (12) | |
| Grade 4 | 6 (5) | 1 (4) | 4 (7) | 1 (4) | |
| CRP peak, mg/L, median (range) | 42 (2–328) | 41 (3–328) | 36 (2–288) | 54 (4–323) | 0.76 2 |
| IL-6 peak, pg/mL, median (range) | 558 (4–157,117) | 791 (22–49,990) | 409 (4–157,117) | 842 (9–8767) | 0.63 2 |
| Ferritin peak, µg/L, median (range) | 1323 (99–13,393) | 1055 (161–13,393) | 1511 (190–8690) | 901 (99–4168) | 0.18 2 |
| CAR-T peak (copies/µg cfDNA), median (range) | 4720 (30–218,384) | 4121 (320–139,656) | 4641 (30–218,384) | 6567 (37–35,298) | 0.95 2 |
| Time to CAR-T peak, days, median (range) | 9 (4–83) | 8 (4–26) | 9 (4–83) | 9 (7–37) | 0.39 2 |
| CAR-T persistence, six months, median (range) | 95 (0–4061) | 130 (0–2317) | 74 (0–4061) | 222 (0–2191) | 0.18 2 |
| Parameter | All Patients | LAG3 I455hom | LAG3 I455Thet | LAG3 T455hom | p-Value |
|---|---|---|---|---|---|
| Group size, n (%) | 112 (100) | 25 (22) | 60 (54) | 27 (24) | |
| Best response after CAR-T cell therapy | 0.049 1 | ||||
| OR, n (%) | 81 (72) | 17 (68) | 55 (90) | 22 (82) | |
| CR, n (%) | 56 (58) | 11 (42) | 43 (71) | 14 (54) | |
| PR, n (%) | 25 (26) | 6 (24) | 12 (22) | 8 (27) | |
| SD, n (%) | 6 (6) | 4 (16) | 5 (9) | 3 (11) | |
| PD, n (%) | 9 (9) | 4 (16) | 1 (4) | 4 (15) | |
| Relapse, n | 46 (41) | 18 (69) | 25 (46) | 9 (35) | 0.08 1 |
| Early relapse, n | 33 (29) | 14 (54) | 17 (28) | 4 (15) | 0.014 1 |
| Death, n | 47 (42) | 17 (65) | 27 (44) | 12 (46) | 0.18 1 |
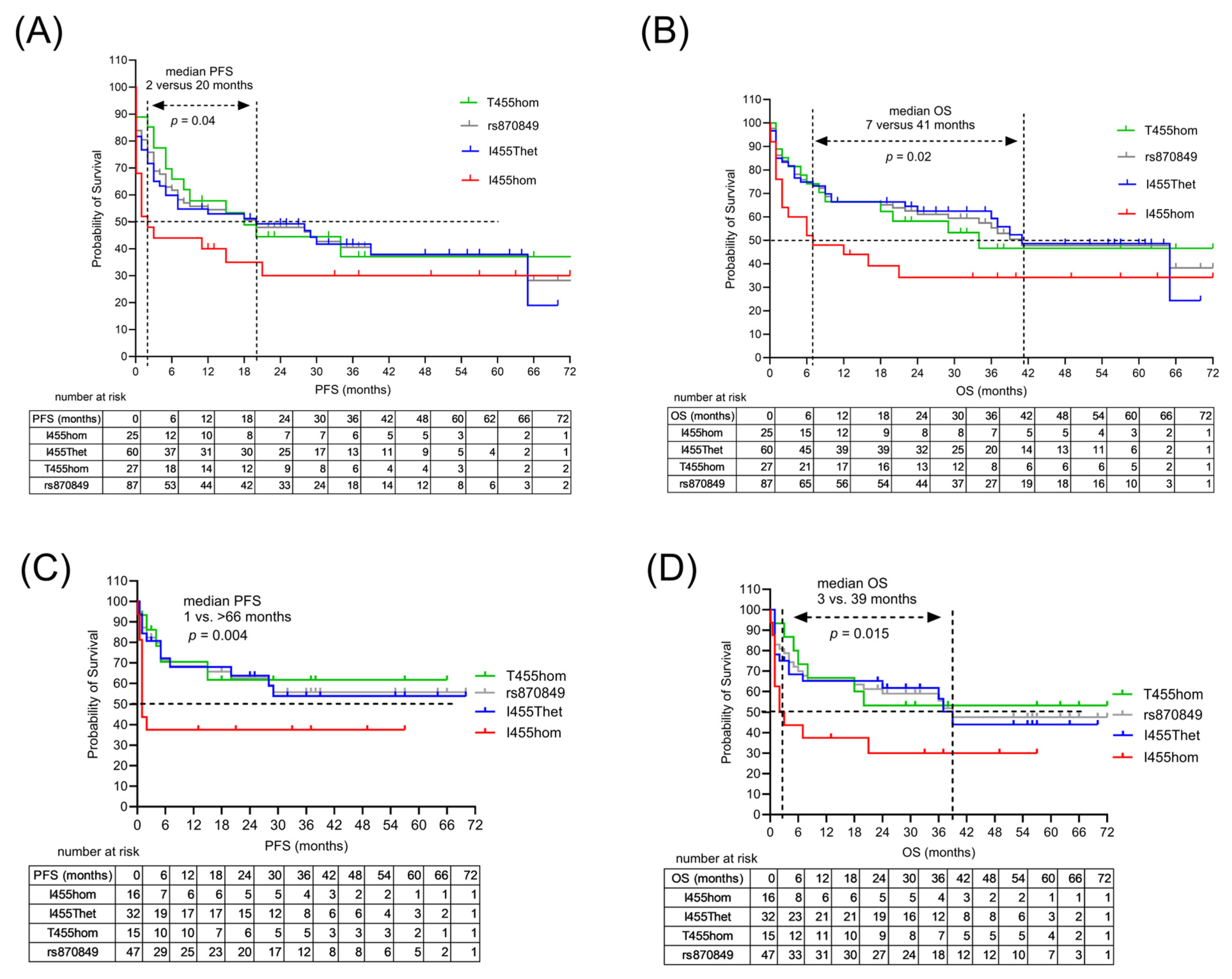
| PFS (mo), median | 12 | 2 | 20 | 18 | 0.025 2 |
| OS (mo), median | 37 | 6 | 41 | 34 | 0.007 2 |
| PFS | OS | |||
|---|---|---|---|---|
| Predictors | HR | p-Value | HR | p-Value |
| LAG3 I455 hom vs. T455hom | 2.95 | 0.04 | 3.72 | 0.008 |
| IPI 4 vs. IPI 3 | 1.89 | 0.27 | 1.09 | 0.88 |
| Prior chemotherapy lines: 3 vs. 2 | 2.7 | 0.07 | 1.33 | 0.56 |
| Axi-cel vs. Tisa-cel | 1.35 | 0.58 | 1.37 | 0.55 |
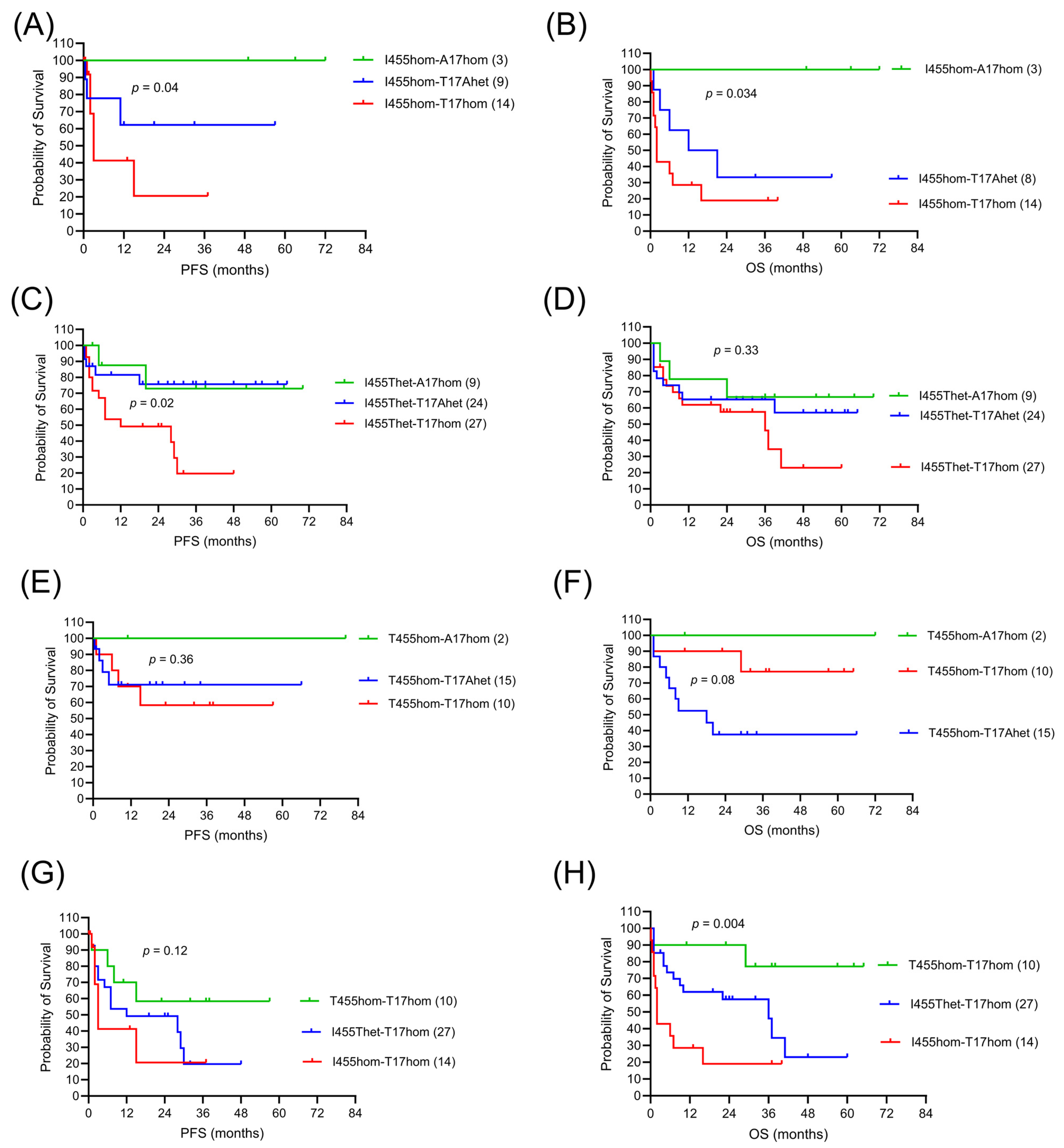
| Germline Variants | Group Size | ORR | Best Response, n (%) | p-Value | ||
|---|---|---|---|---|---|---|
| LAG3-CTLA4 | n (%) | n (%) | CR | PR | SD/PD | 0.08 1 |
| I455hom-A17hom | 3 (3) | 3 (100) | 3 (100) | 0 | 0 | |
| I455hom-T17Ahet | 8 (7) | 6 (67) | 5 (62) | 1 (12) | 2 (25) | |
| I455hom-T17hom | 14 (13) | 8 (57) | 3 (21) | 5 (36) | 6 (43) | |
| I455Thet-A17hom | 9 (8) | 8 (89) | 7 (78) | 1 (11) | 1 (11) | |
| I455Thet-T17Ahet | 24 (21) | 17 (79) | 16 (67) | 3 (12) | 5 (21) | |
| I455Thet-T17hom | 27 (24) | 22 (72) | 14 (52) | 8 (20) | 5 (19) | |
| T455hom-A17hom | 2 (2) | 2 (100) | 2 (100) | 0 | 0 | |
| T455hom-T17Ahet | 15 (13) | 13 (87) | 6 (40) | 7 (47) | 2 (13) | |
| T455hom-T17hom | 10 (9) | 9 (90) | 8 (80) | 1 (10) | 1 (10) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seipel, K.; Spahr, S.M.; Shaforostova, I.; Bacher, U.; Nilius, H.; Pabst, T. Clinical Impact of LAG3 Single-Nucleotide Polymorphism in DLBCL Treated with CAR-T Cell Therapy. Int. J. Mol. Sci. 2025, 26, 9905. https://doi.org/10.3390/ijms26209905
Seipel K, Spahr SM, Shaforostova I, Bacher U, Nilius H, Pabst T. Clinical Impact of LAG3 Single-Nucleotide Polymorphism in DLBCL Treated with CAR-T Cell Therapy. International Journal of Molecular Sciences. 2025; 26(20):9905. https://doi.org/10.3390/ijms26209905
Chicago/Turabian StyleSeipel, Katja, Sophia Maria Spahr, Inna Shaforostova, Ulrike Bacher, Henning Nilius, and Thomas Pabst. 2025. "Clinical Impact of LAG3 Single-Nucleotide Polymorphism in DLBCL Treated with CAR-T Cell Therapy" International Journal of Molecular Sciences 26, no. 20: 9905. https://doi.org/10.3390/ijms26209905
APA StyleSeipel, K., Spahr, S. M., Shaforostova, I., Bacher, U., Nilius, H., & Pabst, T. (2025). Clinical Impact of LAG3 Single-Nucleotide Polymorphism in DLBCL Treated with CAR-T Cell Therapy. International Journal of Molecular Sciences, 26(20), 9905. https://doi.org/10.3390/ijms26209905









