Molecular Landscape of Acute Myeloid Leukemia in Pediatric Patient-Age-Related Correlations: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Information Sources
2.3. Search Strategy
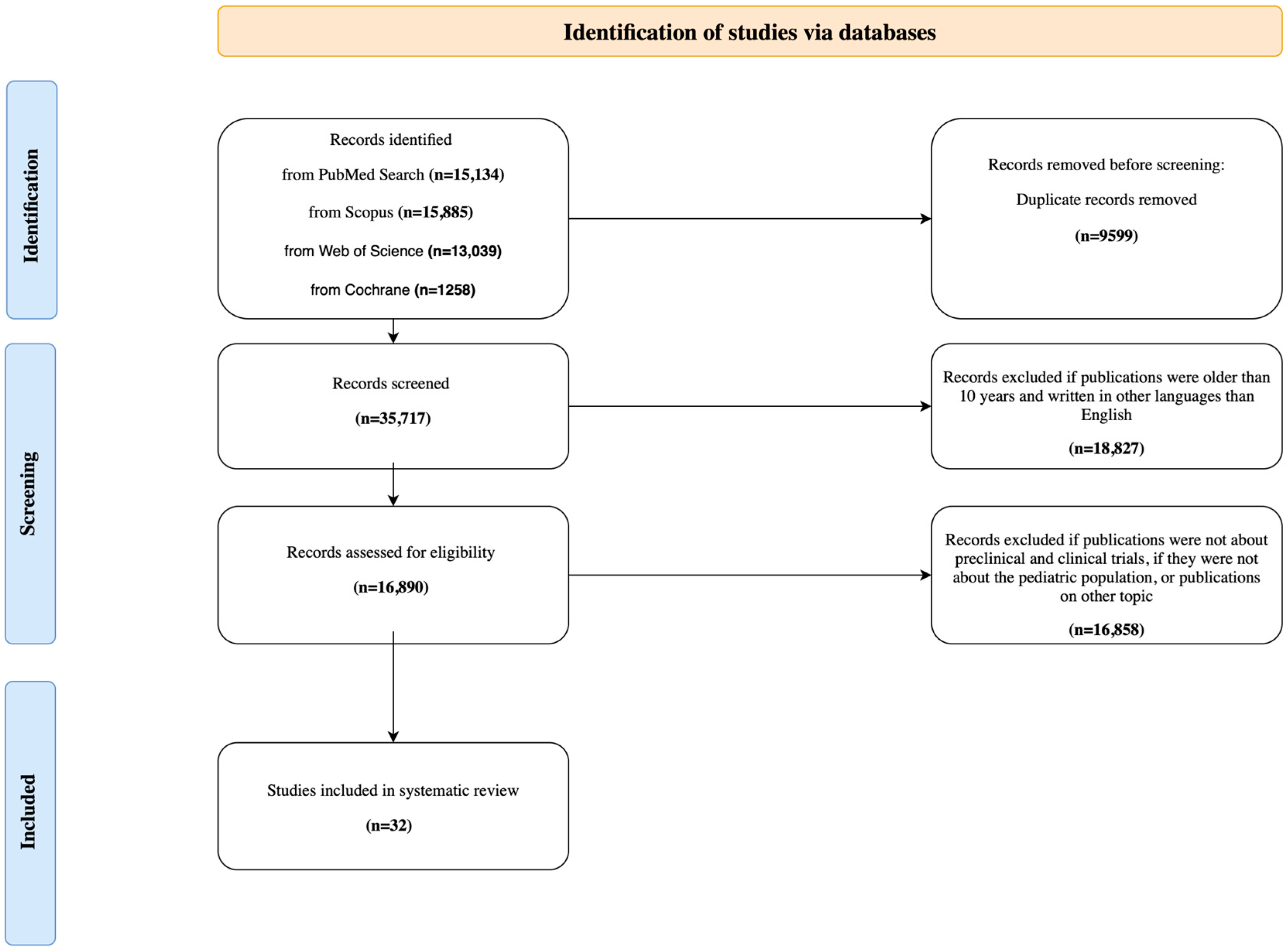
2.4. Study Selection and Results
2.5. Assessment of Risk of Bias and Methodological Quality
2.6. Limitations
3. Result
3.1. Differences in the Molecular Landscape of AML in Adult and Pediatric Patients
3.2. Genetic Alterations Specific to Pediatric Patients
3.2.1. Rearrangements and Mutations Correlating with Infancy (<3)
KMT2A Rearrangements
CBFA2T3::GLIS2 Fusions
| Genetic Alteration | Age Group | Prognostic Implications | References |
|---|---|---|---|
| KMT2A rearrangements | infants | Poor; 5-year OS 35–50%, EFS 30–40%; outcome depends on fusion partner. Requires intensive chemotherapy and strict Minimal Residual Disease (MRD) monitoring. | [7,9,10,11,36] |
| CBFA2T3::GLIS2 fusion | infants | Very poor; 5-year OS < 20%, EFS 15–20%, frequent relapses. Characteristic aggressive course of AMKL in infants. High resistance to standard chemotherapy schemes | [12,13,14,60,65] |
| t(7;12)/MNX1::ETV6 fusion | infants | Poor; 5-year OS < 30%, EFS 20–25%, high relapse in infancy. Standard chemotherapy regimen weakly effective. | [16,45,66] |
| RBM15::MKL1 fusion | infants | Intermediate-poor; 5-year OS 30–50%, EFS 35–40% Prognosis depends on response to induction. | [17,18,67,68] |
| CBF fusions (t(8;21), inv(16)) | children | favorable; OS 75–85%, EFS 60–75%. The prognosis is worsened by the presence of KIT, RAS, FLT3-ITD. | [19,47,69] |
| NUP98 rearrangements | children | Poor; OS < 40%, EFS < 30%, high risk of relapse. Co-occurrence of FLT3-ITD or WT1 worsens prognosis. | [22,23,24,70] |
| FLT3 mutations (ITD/TKD) | adolescents | Intermediate-poor; OS 40–50%, EFS 30–40%. High risk of relapse, especially ITD with high allelic ratio. | [25,47,71] |
| CEBPA mutations | adolescents | Favorable; 5-year OS 80–90%, EFS 70–80%. Co-occurrence of FLT3-ITD worsens prognosis. | [26,27] |
| NPM1 mutations | adolescents | Favorable; OS 75–85%, EFS 70–75%. The co-occurrence of FLT3-ITD worsens the prognosis. | [28,56] |
| Trisomy 8 | adolescents | Variable: neutral to moderately favorable; OS 60–70%, EFS 55–65%. Prognosis significantly dependent on additional aberrations. | [29,56] |
The t(7;12) Translocation and the MNX1::ETV6 Fusion Transcript
RBM15::MKL1 Fusion
3.2.2. Dominant Rearrangements and Mutations in Children (3–14)
CBF Fusions
NUP98 Rearrangements
3.2.3. Rearrangements and Mutations Correlating with Adolescence
FLT3 Mutations
CEBPA Mutations
NPM1 Mutations
Trisomy 8
3.3. Relatively Rare Genetic Manifestations in the Pediatric Population
3.3.1. DNMT3A Mutations
3.3.2. IDH Mutations
3.3.3. RUNX1 Alterations
3.3.4. TET2 Mutations
3.3.5. TP53 Lesions
3.4. Clinical Aspects
3.4.1. Therapeutic Strategies in the Younger Age Group
3.4.2. Therapeutic Strategies in the Older Age Group
3.4.3. Clinical Translation of Age-Related Molecular Profiles
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AML | Acute Myeloid Leukemia |
| OS | Overall Survival |
| ALL | Acute Lymphoblastic Leukemia |
| DNMT3A | DNA Methyltransferase 3 Alpha |
| ASXL1 | Additional Sex Combs-Like 1 |
| ASXL2 | Additional Sex Combs-Like 2 |
| TET2 | Ten-Eleven-Translocation 2 |
| TET1 | Ten-Eleven-Translocation 1 |
| TP53 | Tumor Protein 53 |
| KMT2A | Lysine (K)-Specific Methyltransferase 2A |
| RUNX1 | Runt Related Transcription Factor |
| RUNX1T1 | Runt-Related Transcription Factor 1, Translocated To 1 |
| NPM1 | Nucleophosmin 1 |
| IDH | Isocitrate Dehydrogenase |
| IDH1 | Isocitrate Dehydrogenase 1 |
| IDH2 | Isocitrate Dehydrogenase 2 |
| KRAS | Kirsten Rat Sarcoma Viral Oncogene Homolog |
| PTD | Partial Tandem Duplication |
| MLL | Mixed Lineage Leukemia |
| KIT | Receptor Tyrosine Kinase |
| CEBPA | CCAAT Enhancer Binding Protein Alpha |
| KMT | Lysine (K) Methyltransferase |
| FAB | French–American–British |
| HOX | Homeobox Genes |
| MLLT3 | Mixed-Lineage Leukemia; Translocated To 3 |
| MLLT10 | Mixed-Lineage Leukemia; Translocated To 10 |
| TARGET | Therapeutically Applicable Research to Generate Effective Treatments |
| RAS | Rat Sarcoma |
| NRAS | Neuroblastoma Rat Sarcoma Viral Oncogene Homolog |
| PTPN11 | Protein Tyrosine Phosphatase, Non-Receptor Type 11 |
| SETD2 | SET Domain Containing 2 |
| FLT3 | Fms-Like Tyrosine Kinase 3 |
| TKD | Tyrosine Kinase Domain |
| WT1 | Wilms Tumor 1 |
| ITD | Internal Tandem Duplication |
| EFS | Event-Free Survival |
| COG | Children’s Oncology Group |
| NF1 | Neurofibromin 1 |
| GATA2 | GATA Binding Protein 2 |
| MBNL1 | Muscleblind-Like Splicing Regulator 1 |
| ZEB2 | Zinc Finger E-Box Binding Homeobox 2 |
| AFDN | Mixed-Lineage Leukemia; Translocated To 4 |
| MLLT1 | Mixed-Lineage Leukemia; Translocated To 1 |
| CBF | Core-Binding Factor |
| CBFB | Core-Binding Factor Beta |
| MYH11 | Myosin Heavy Chain 11 |
| NUP98 | Nucleoporin 98 |
| NSD1 | Nuclear Receptor Binding SET Domain Protein 1 |
| CSPG4 | Chondroitin Sulfate Proteoglycan 4 |
| SEPT6 | Septin 6 |
| EPS15 | Epidermal Growth Factor Receptor Pathway Substrate 15 |
| CBFA2T3 | Core-Bindind Factor, Runt Domain, Alpha Subunit 2; Translocated To 3 |
| GLIS2 | GLI-Similar 2 |
| ETO | Eight-Twenty-One |
| AMKL | Acute Megakaryoblastic Leukemia |
| GATA1 | GATA Binding Protein 1 |
| NCAM1 | Neural Cell Adhesion Molecule 1 |
| CACNB2 | Calcium Voltage-Gated Channel Auxiliary Subunit Beta 2 |
| GABRE | Gamma-Aminobutyric Acid Type A Receptor Epsilon Subunit |
| RBM15 | RNA Binding Motif Protein 15 |
| MKL1 | Megakaryoblastic Leukemia 1 |
| KDM5A | Lysine Demethylase 5A |
| TNF | Tumor Necrosis Factor |
| TGFB | Transforming Growth Factor Beta |
| BMP | Bone Morphogenetic Proteins |
| PTCH1 | Patched 1 |
| HHIP | Hedgehog Interacting Protein |
| GLI1 | Glioma-Associated Oncogene Homolog 1 |
| CtBP1 | C-terminal Binding Protein 1 |
| JAK | Janus Kinase |
| STAT | Signal Transducer and Activator of Transcription |
| MNX1 | Motor Neuron and Pancreas Homeobox Protein 1 |
| ETS | E26 transformation-specific |
| ETV6 | ETS Variant Transcription Factor 6 |
| AS | Antisense RNA |
| LOS | Loss of a Sex Chromosome |
| CR | Complete Remission |
| WBCs | White Blood Cells |
| RAD21 | Radiation Sensitive 21 |
| SMC1A | Structural Maintenance of Chromosomes 1A |
| VAF | Variant Allele Frequencies |
| BMI | Body Mass Index |
| NBPF14 | Neuroblastoma Breakpoint Family Member 14 |
| BCR | Breakpoint Cluster Region |
| ODF1 | Outer Dense Fiber of Sperm Tails 1 |
| SETBP1 | SET Binding Protein 1 |
| U2AF1 | U2 Small Nuclear RNA Auxiliary Factor 1 |
| RB1 | Retinoblastoma 1 |
| UBTF | Upstream Binding Transcription Factor |
| bZip | Basic Leucine Zipper |
| RR | Relative Risk |
| PML::RARA | Promyelocytic Leukemia—Retinoic Acid Receptor Alpha |
| CCR | Continuous Complete Remission |
| SNP | Single Nucleotide Polymorphism |
| CCG | Children’s Cancer Group |
| ELF1 | E74 Like ETS Transcription Factor 1 |
| PRPF8 | Pre-mRNA Processing Factor 8 |
| RP2D | Recommended Phase 2 Dose |
| PBSC | Peripheral Blood Stem Cell Transplantation |
| A/B TCD | ALFA/BETA T Cell Receptor Depletion |
| ORR | Overall Response Rate |
| MEN1 | Multiple Endocrine Neoplasia type 1 |
| DOT1L | Disruptor of Telomeric Silencing 1-Like |
| BCL-2 | B-cell Lymphoma 2 |
| AURKA | Aurora Kinase A |
| MRD | Minimal Residual Disease |
| NOPHO | Nordic Society for Pediatric Hematology and Oncology |
| allo-HSCT | Allogeneic Hematopoietic Stem Cell Transplantation |
| HSCT | Hematopoietic Stem Cell Transplantation |
| CAR-T | Chimeric Antigen Receptor T-cell |
| HDAC1 | Histone Deacetylase 1 |
| EZH | Enhancer of Zeste Homolog |
| KAT6A | Histone Acetyltransferase A/ lysine (K) acetyltransferase 6A |
| ELN | European Leukemia Net |
| GO | Gemtuzumab Ozogamicin |
| PRC2 | Polycomb Repressive Complex 2 |
| DFS | Disease-Free Survival |
| WHO | World Health Organization |
| NOTCH2 | Notch 2 Receptor 2 |
| NRM | Non-Relapse Mortality |
References
- Iyer, P. Pediatric AML: State of the Art and Future Directions. Pediatr. Hematol. Oncol. 2025, 42, 126–145. [Google Scholar] [CrossRef]
- Tseng, S.; Lee, M.-E.; Lin, P.-C. A Review of Childhood Acute Myeloid Leukemia: Diagnosis and Novel Treatment. Pharmaceuticals 2023, 16, 1614. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.E.; Schoonmade, L.J.; Kaspers, G.J.L. Pediatric Relapsed Acute Myeloid Leukemia: A Systematic Review. Expert Rev. Anticancer Ther. 2021, 21, 45–52. [Google Scholar] [CrossRef]
- Egan, G.; Tasian, S.K. Relapsed Pediatric Acute Myeloid Leukaemia: State-of-the-Art in 2023. Haematologica 2023, 108, 2275–2288. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Aromataris, E.; Lockwood, C.; Porritt, K.; Pilla, B.; Jordan, Z. JBI Manual for Evidence Synthesis. JBI 2024. Available online: https://synthesismanual.jbi.global (accessed on 8 October 2025). [CrossRef]
- Yuen, K.-Y.; Liu, Y.; Zhou, Y.-Z.; Wang, Y.; Zhou, D.-H.; Fang, J.-P.; Xu, L.-H. Mutational Landscape and Clinical Outcome of Pediatric Acute Myeloid Leukemia with 11q23/ KMT2A Rearrangements. Cancer Med. 2023, 12, 1418–1430. [Google Scholar] [CrossRef]
- Bolouri, H.; Farrar, J.E.; Triche, T.; Ries, R.E.; Lim, E.L.; Alonzo, T.A.; Ma, Y.; Moore, R.; Mungall, A.J.; Marra, M.A.; et al. The Molecular Landscape of Pediatric Acute Myeloid Leukemia Reveals Recurrent Structural Alterations and Age-Specific Mutational Interactions. Nat. Med. 2018, 24, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Meyer, C.; Larghero, P.; Lopes, B.A.; Burmeister, T.; Gröger, D.; Sutton, R.; Venn, N.C.; Cazzaniga, G.; Abascal, L.C.; Tsaur, G.; et al. The KMT2A Recombinome of Acute Leukemias in 2023. Leukemia 2023, 37, 988–1005. [Google Scholar] [CrossRef]
- Hoffmeister, L.M.; Orhan, E.; Walter, C.; Niktoreh, N.; Hanenberg, H.; von Neuhoff, N.; Reinhardt, D.; Schneider, M. Impact of KMT2A Rearrangement and CSPG4 Expression in Pediatric Acute Myeloid Leukemia. Cancers 2021, 13, 4817. [Google Scholar] [CrossRef]
- van Weelderen, R.E.; Harrison, C.J.; Klein, K.; Jiang, Y.; Abrahamsson, J.; Alonzo, T.; Aplenc, R.; Arad-Cohen, N.; Bart-Delabesse, E.; Buldini, B.; et al. Optimized Cytogenetic Risk-Group Stratification of KMT2A -Rearranged Pediatric Acute Myeloid Leukemia. Blood Adv. 2024, 8, 3200–3213. [Google Scholar] [CrossRef]
- Hara, Y.; Shiba, N.; Ohki, K.; Tabuchi, K.; Yamato, G.; Park, M.; Tomizawa, D.; Kinoshita, A.; Shimada, A.; Arakawa, H.; et al. Prognostic Impact of Specific Molecular Profiles in Pediatric Acute Megakaryoblastic Leukemia in non-Down Syndrome. Genes Chromosomes Cancer 2017, 56, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.L.; Ries, R.E.; Hylkema, T.; Alonzo, T.A.; Gerbing, R.B.; Santaguida, M.T.; Brodersen, L.E.; Pardo, L.; Cummings, C.L.; Loeb, K.R.; et al. Comprehensive Transcriptome Profiling of Cryptic CBFA2T3–GLIS2 Fusion–Positive AML Defines Novel Therapeutic Options: A COG and TARGET Pediatric AML Study. Clin. Cancer Res. 2020, 26, 726–737. [Google Scholar] [CrossRef] [PubMed]
- Zangrando, A.; Cavagnero, F.; Scarparo, P.; Varotto, E.; Francescato, S.; Tregnago, C.; Cuccurullo, R.; Fagioli, F.; Nigro, L.L.; Masetti, R.; et al. CD56, HLA-DR, and CD45 Recognize a Subtype of Childhood AML Harboring CBFA2T3-GLIS2 Fusion Transcript. Cytom. A 2021, 99, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Chisholm, K.M.; Smith, J.; Heerema-McKenney, A.E.; Choi, J.K.; Ries, R.E.; Hirsch, B.A.; Raimondi, S.C.; Wang, Y.-C.; Dang, A.; Alonzo, T.A.; et al. Pathologic, Cytogenetic, and Molecular Features of Acute Myeloid Leukemia with Megakaryocytic Differentiation: A Report from the Children’s Oncology Group. Pediatr. Blood Cancer 2023, 70, e30251. [Google Scholar] [CrossRef]
- Östlund, A.; Waraky, A.; Staffas, A.; Sjögren, H.; Moerloose, B.D.; Arad-Cohen, N.; Cheuk, D.; Navarro, J.M.F.; Jahnukainen, K.; Kaspers, G.J.L.; et al. Characterization of Pediatric Acute Myeloid Leukemia With t(7;12)(Q36;P13). Genes Chromosomes Cancer 2024, 63, e70003. [Google Scholar] [CrossRef]
- de Rooij, J.D.E.; Masetti, R.; van den Heuvel-Eibrink, M.M.; Cayuela, J.-M.; Trka, J.; Reinhardt, D.; Rasche, M.; Sonneveld, E.; Alonzo, T.A.; Fornerod, M.; et al. Recurrent Abnormalities Can Be Used for Risk Group Stratification in Pediatric AMKL: A Retrospective Intergroup Study. Blood 2016, 127, 3424–3430. [Google Scholar] [CrossRef]
- Inaba, H.; Zhou, Y.; Abla, O.; Adachi, S.; Auvrignon, A.; Beverloo, H.B.; de Bont, E.; Chang, T.-T.; Creutzig, U.; Dworzak, M.; et al. Heterogeneous Cytogenetic Subgroups and Outcomes in Childhood Acute Megakaryoblastic Leukemia: A Retrospective International Study. Blood 2015, 126, 1575–1584. [Google Scholar] [CrossRef]
- Duployez, N.; Marceau-Renaut, A.; Boissel, N.; Petit, A.; Bucci, M.; Geffroy, S.; Lapillonne, H.; Renneville, A.; Ragu, C.; Figeac, M.; et al. Comprehensive Mutational Profiling of Core Binding Factor Acute Myeloid Leukemia. Blood 2016, 127, 2451–2459. [Google Scholar] [CrossRef]
- Yamato, G.; Shiba, N.; Yoshida, K.; Shiraishi, Y.; Hara, Y.; Ohki, K.; Okubo, J.; Okuno, H.; Chiba, K.; Tanaka, H.; et al. ASXL2 Mutations Are Frequently Found in Pediatric AML Patients with t(8;21)/ RUNX1-RUNX1T1 and Associated with a Better Prognosis. Genes Chromosomes Cancer 2017, 56, 382–393. [Google Scholar] [CrossRef]
- Sendker, S.; Awada, A.; Domagalla, S.; Sendker, M.; Orhan, E.; Hoffmeister, L.M.; Antoniou, E.; Niktoreh, N.; Reinhardt, D.; von Neuhoff, N.; et al. RUNX1 Mutation Has No Prognostic Significance in Paediatric AML: A Retrospective Study of the AML-BFM Study Group. Leukemia 2023, 37, 1435–1443. [Google Scholar] [CrossRef] [PubMed]
- Niktoreh, N.; Walter, C.; Zimmermann, M.; von Neuhoff, C.; von Neuhoff, N.; Rasche, M.; Waack, K.; Creutzig, U.; Hanenberg, H.; Reinhardt, D. Mutated WT1, FLT3-ITD, and NUP98-NSD1 Fusion in Various Combinations Define a Poor Prognostic Group in Pediatric Acute Myeloid Leukemia. J. Oncol. 2019, 2019, 1609128. [Google Scholar] [CrossRef]
- Struski, S.; Lagarde, S.; Bories, P.; Puiseux, C.; Prade, N.; Cuccuini, W.; Pages, M.-P.; Bidet, A.; Gervais, C.; Lafage-Pochitaloff, M.; et al. NUP98 Is Rearranged in 3.8% of Pediatric AML Forming a Clinical and Molecular Homogenous Group with a Poor Prognosis. Leukemia 2017, 31, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Bertrums, E.J.M.; Smith, J.L.; Harmon, L.; Ries, R.E.; Wang, Y.-C.J.; Alonzo, T.A.; Menssen, A.J.; Chisholm, K.M.; Leonti, A.R.; Tarlock, K.; et al. Comprehensive Molecular and Clinical Characterization of NUP98 Fusions in Pediatric Acute Myeloid Leukemia. Haematologica 2023, 108, 2044–2058. [Google Scholar] [CrossRef]
- Tarlock, K.; Gerbing, R.B.; Ries, R.E.; Smith, J.L.; Leonti, A.; Huang, B.J.; Kirkey, D.; Robinson, L.; Peplinksi, J.H.; Lange, B.; et al. Prognostic Impact of Cooccurring Mutations in FLT3-ITD Pediatric Acute Myeloid Leukemia. Blood Adv. 2024, 8, 2094–2103. [Google Scholar] [CrossRef] [PubMed]
- Tarlock, K.; Lamble, A.J.; Wang, Y.-C.; Gerbing, R.B.; Ries, R.E.; Loken, M.R.; Brodersen, L.E.; Pardo, L.; Leonti, A.; Smith, J.L.; et al. CEBPA-bZip Mutations Are Associated with Favorable Prognosis in de Novo AML: A Report from the Children’s Oncology Group. Blood 2021, 138, 1137–1147. [Google Scholar] [CrossRef]
- Liao, X.; Fang, J.; Zhou, D.; Qiu, K. CEBPA Are Independent Good Prognostic Factors in Pediatric Acute Myeloid Leukemia. Hematol. Oncol. 2022, 40, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.-H.; Fang, J.-P.; Liu, Y.-C.; Jones, A.I.; Chai, L. Nucleophosmin Mutations Confer an Independent Favorable Prognostic Impact in 869 Pediatric Patients with Acute Myeloid Leukemia. Blood Cancer J. 2020, 10, 1. [Google Scholar] [CrossRef]
- Laursen, A.C.L.; Sandahl, J.D.; Kjeldsen, E.; Abrahamsson, J.; Asdahl, P.; Ha, S.-Y.; Heldrup, J.; Jahnukainen, K.; Jónsson, Ó.G.; Lausen, B.; et al. Trisomy 8 in Pediatric Acute Myeloid Leukemia: A NOPHO-AML Study. Genes Chromosomes Cancer 2016, 55, 719–726. [Google Scholar] [CrossRef]
- Li, W.; Cui, L.; Gao, C.; Liu, S.; Zhao, X.; Zhang, R.; Zheng, H.; Wu, M.; Li, Z. DNMT3A mutations in Chinese childhood acute myeloid leukemia. Medicine 2017, 96, e7620. [Google Scholar] [CrossRef]
- Zarnegar-Lumley, S.; Alonzo, T.A.; Gerbing, R.B.; Othus, M.; Sun, Z.; Ries, R.E.; Wang, J.; Leonti, A.; Kutny, M.A.; Ostronoff, F.; et al. Characteristics and Prognostic Impact of IDH Mutations in AML: A COG, SWOG, and ECOG Analysis. Blood Adv. 2023, 7, 5941–5953. [Google Scholar] [CrossRef]
- Yamato, G.; Shiba, N.; Yoshida, K.; Hara, Y.; Shiraishi, Y.; Ohki, K.; Okubo, J.; Park, M.; Sotomatsu, M.; Arakawa, H.; et al. RUNX1 Mutations in Pediatric Acute Myeloid Leukemia Are Associated with Distinct Genetic Features and an Inferior Prognosis. Blood 2018, 131, 2266–2270. [Google Scholar] [CrossRef]
- Li, M.-J.; Yang, Y.-L.; Lee, N.-C.; Jou, S.-T.; Lu, M.-Y.; Chang, H.-H.; Lin, K.-H.; Peng, C.-T.; Lin, D.-T. Tet Oncogene Family Member 2 Gene Alterations in Childhood Acute Myeloid Leukemia. J. Formos. Med. Assoc. 2016, 115, 801–806. [Google Scholar] [CrossRef]
- Kutny, M.A.; Alonzo, T.A.; Gamazon, E.R.; Gerbing, R.B.; Geraghty, D.; Lange, B.; Heerema, N.A.; Sung, L.; Aplenc, R.; Franklin, J.; et al. Ethnic Variation of TET2 SNP Rs2454206 and Association with Clinical Outcome in Childhood AML: A Report from the Children’s Oncology Group. Leukemia 2015, 29, 2424–2426. [Google Scholar] [CrossRef][Green Version]
- Cucchi, D.G.J.; Bachas, C.; Klein, K.; Huttenhuis, S.; Zwaan, C.M.; Ossenkoppele, G.J.; Janssen, J.M.W.M.; Kaspers, G.L.; Cloos, J. TP53 Mutations and Relevance of Expression of TP53 Pathway Genes in Paediatric Acute Myeloid Leukaemia. Br. J. Haematol. 2020, 188, 736–739. [Google Scholar] [CrossRef]
- Hara, Y.; Shiba, N.; Yoshida, K.; Yamato, G.; Kaburagi, T.; Shiraishi, Y.; Ohki, K.; Shiozawa, Y.; Kawamura, M.; Kawasaki, H.; et al. TP53 and RB1 Alterations Characterize Poor Prognostic Subgroups in Pediatric Acute Myeloid Leukemia. Genes Chromosomes Cancer 2023, 62, 412–422. [Google Scholar] [CrossRef]
- Hara, Y.; Shiba, N.; Yamato, G.; Ohki, K.; Tabuchi, K.; Sotomatsu, M.; Tomizawa, D.; Kinoshita, A.; Arakawa, H.; Saito, A.M.; et al. Patients Aged Less than 3 Years with Acute Myeloid Leukaemia Characterize a Molecularly and Clinically Distinct Subgroup. Br. J. Haematol. 2020, 188, 528–539. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Li, J.; Li, W.; Zhao, X.; Xue, T.; Liu, S.; Zhang, R.; Zheng, H.; Gao, C. Clinical Features and Long-Term Outcomes of Pediatric Patients with de Novo Acute Myeloid Leukemia in China with or without Specific Gene Abnormalities: A Cohort Study of Patients Treated with BCH-AML 2005. Hematology 2024, 29, 2406596. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, F.; Zhang, Y.; Wang, M.; Tian, W.; Teng, W.; Ma, X.; Guo, L.; Fang, J.; Zhang, Y.; et al. Panoramic View of Common Fusion Genes in a Large Cohort of Chinese de Novo Acute Myeloid Leukemia Patients. Leuk. Lymphoma 2019, 60, 1071–1078. [Google Scholar] [CrossRef]
- Tarlock, K.; Zhong, S.; He, Y.; Ries, R.; Severson, E.; Bailey, M.; Morley, S.; Balasubramanian, S.; Erlich, R.; Lipson, D.; et al. Distinct Age-Associated Molecular Profiles in Acute Myeloid Leukemia Defined by Comprehensive Clinical Genomic Profiling. Oncotarget 2018, 9, 26417–26430. [Google Scholar] [CrossRef]
- Papaemmanuil, E.; Gerstung, M.; Bullinger, L.; Gaidzik, V.I.; Paschka, P.; Roberts, N.D.; Potter, N.E.; Heuser, M.; Thol, F.; Bolli, N.; et al. Genomic Classification and Prognosis in Acute Myeloid Leukemia. N. Engl. J. Med. 2016, 374, 2209–2221. [Google Scholar] [CrossRef] [PubMed]
- Aziz, H.; Ping, C.Y.; Alias, H.; Mutalib, N.-S.A.; Jamal, R. Gene Mutations as Emerging Biomarkers and Therapeutic Targets for Relapsed Acute Myeloid Leukemia. Front. Pharmacol. 2017, 8, 897. [Google Scholar] [CrossRef]
- Stratmann, S.; Yones, S.A.; Mayrhofer, M.; Norgren, N.; Skaftason, A.; Sun, J.; Smolinska, K.; Komorowski, J.; Herlin, M.K.; Sundström, C.; et al. Genomic Characterization of Relapsed Acute Myeloid Leukemia Reveals Novel Putative Therapeutic Targets. Blood Adv. 2021, 5, 900–912. [Google Scholar] [CrossRef]
- Conneely, S.E.; Rau, R.E. The genomics of acute myeloid leukemia in children. Cancer Metastasis Rev. 2020, 39, 189–209. [Google Scholar] [CrossRef]
- Weichenhan, D.; Riedel, A.; Sollier, E.; Toprak, U.H.; Hey, J.; Breuer, K.; Wierzbinska, J.A.; Touzart, A.; Lutsik, P.; Bähr, M.; et al. Altered Enhancer-Promoter Interaction Leads to MNX1 Expression in Pediatric Acute Myeloid Leukemia with t(7;12)(Q36;P13). Blood Adv. 2024, 8, 5100–5111. [Google Scholar] [CrossRef] [PubMed]
- Ho, P.A.; Alonzo, T.A.; Gerbing, R.B.; Pollard, J.; Stirewalt, D.L.; Hurwitz, C.; Heerema, N.A.; Hirsch, B.; Raimondi, S.C.; Lange, B.; et al. Prevalence and Prognostic Implications of CEBPA Mutations in Pediatric Acute Myeloid Leukemia: A Report from the Children’s Oncology Group. Blood 2009, 113, 6558–6566. [Google Scholar] [CrossRef]
- Qiu, K.-Y.; Liao, X.-Y.; Liu, Y.; Huang, K.; Li, Y.; Fang, J.-P.; Zhou, D.-H. Poor Outcome of Pediatric Patients with Acute Myeloid Leukemia Harboring High FLT3/ITD Allelic Ratios. Nat. Commun. 2022, 13, 3679. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.; Pieters, R.; Biondi, A. How I Treat Infant Leukemia. Blood 2019, 133, 205–214. [Google Scholar] [CrossRef]
- Alexander, T.B.; Mullighan, C.G. Molecular Biology of Childhood Leukemia. Annu. Rev. Cancer Biol. 2021, 5, 95–117. [Google Scholar] [CrossRef]
- Castiglioni, S.; Fede, E.D.; Bernardelli, C.; Lettieri, A.; Parodi, C.; Grazioli, P.; Colombo, E.; Ancona, S.; Milani, D.; Ottaviano, E.; et al. KMT2A: Umbrella Gene for Multiple Diseases. Genes 2022, 13, 514. [Google Scholar] [CrossRef] [PubMed]
- Guarnera, L.; D’Addona, M.; Bravo-Perez, C.; Visconte, V. KMT2A Rearrangements in Leukemias: Molecular Aspects and Therapeutic Perspectives. Int. J. Mol. Sci. 2024, 25, 9023. [Google Scholar] [CrossRef]
- Winters, A.C.; Bernt, K.M. MLL-Rearranged Leukemias—An Update on Science and Clinical Approaches. Front. Pediatr. 2017, 5, 4. [Google Scholar] [CrossRef]
- Strom, S.P.; Lozano, R.; Lee, H.; Dorrani, N.; Mann, J.; O’Lague, P.F.; Mans, N.; Deignan, J.L.; Vilain, E.; Nelson, S.F.; et al. De Novo Variants in the KMT2A (MLL) Gene Causing Atypical Wiedemann-Steiner Syndrome in Two Unrelated Individuals Identified by Clinical Exome Sequencing. BMC Med. Genet. 2014, 15, 49. [Google Scholar] [CrossRef] [PubMed]
- Milan, T.; Celton, M.; Lagacé, K.; Roques, É.; Safa-Tahar-Henni, S.; Bresson, E.; Bergeron, A.; Hebert, J.; Meshinchi, S.; Cellot, S.; et al. Epigenetic Changes in Human Model KMT2A Leukemias Highlight Early Events during Leukemogenesis. Haematologica 2020, 107, 86–99. [Google Scholar] [CrossRef]
- Ford, A.M.; Ridge, S.A.; Cabrera, M.E.; Mahmoud, H.; Steel, C.M.; Chan, L.C.; Greaves, M. In Utero Rearrangements in the Trithorax-Related Oncogene in Infant Leukaemias. Nature 1993, 363, 358–360. [Google Scholar] [CrossRef]
- Creutzig, U.; Zimmermann, M.; Reinhardt, D.; Rasche, M.; von Neuhoff, C.; Alpermann, T.; Dworzak, M.; Perglerová, K.; Zemanova, Z.; Tchinda, J.; et al. Changes in Cytogenetics and Molecular Genetics in Acute Myeloid Leukemia from Childhood to Adult Age Groups. Cancer 2016, 122, 3821–3830. [Google Scholar] [CrossRef] [PubMed]
- Andrade, F.G.; Noronha, E.P.; Brisson, G.D.; Bueno, F.d.S.V.; Cezar, I.S.; Terra-Granado, E.; Thuler, L.C.S.; Pombo-de-Oliveira, M.S.; Arancibia, A.M.; Basegio, R.M.; et al. Molecular Characterization of Pediatric Acute Myeloid Leukemia: Results of a Multicentric Study in Brazil. Arch. Med. Res. 2016, 47, 656–667. [Google Scholar] [CrossRef] [PubMed]
- Le, Q.; Hadland, B.; Smith, J.L.; Leonti, A.; Huang, B.J.; Ries, R.; Hylkema, T.A.; Castro, S.; Tang, T.T.; McKay, C.N.; et al. CBFA2T3-GLIS2 Model of Pediatric Acute Megakaryoblastic Leukemia Identifies FOLR1 as a CAR T Cell Target. J. Clin. Invest. 2022, 132, e157101, Erratum in J. Clin. Invest. 2024, 134, e184305. [Google Scholar] [CrossRef]
- Ishibashi, M.; Yokosuka, T.; Yanagimachi, M.; Iwasaki, F.; Tsujimoto, S.; Sasaki, K.; Takeuchi, M.; Tanoshima, R.; Kato, H.; Kajiwara, R.; et al. Clinical Courses of Two Pediatric Patients with Acute Megakaryoblastic Leukemia Harboring the CBFA2T3-GLIS2 Fusion Gene. Turk. J. Hematol. 2016, 33, 331–334. [Google Scholar] [CrossRef]
- Masetti, R.; Bertuccio, S.N.; Pession, A.; Locatelli, F. CBFA2T3-GLIS2-positive Acute Myeloid Leukaemia. A Peculiar Paediatric Entity. Br. J. Haematol. 2019, 184, 337–347. [Google Scholar] [CrossRef]
- Thiollier, C.; Lopez, C.K.; Gerby, B.; Ignacimouttou, C.; Poglio, S.; Duffourd, Y.; Guégan, J.; Rivera-Munoz, P.; Bluteau, O.; Mabialah, V.; et al. Characterization of Novel Genomic Alterations and Therapeutic Approaches Using Acute Megakaryoblastic Leukemia Xenograft Models. J. Exp. Med. 2012, 209, 2017–2031. [Google Scholar] [CrossRef]
- Gruber, T.A.; Gedman, A.L.; Zhang, J.; Koss, C.S.; Marada, S.; Ta, H.Q.; Chen, S.-C.; Su, X.; Ogden, S.K.; Dang, J.; et al. An Inv(16)(P13.3q24.3)-Encoded CBFA2T3-GLIS2 Fusion Protein Defines an Aggressive Subtype of Pediatric Acute Megakaryoblastic Leukemia. Cancer Cell 2012, 22, 683–697. [Google Scholar] [CrossRef]
- Gruber, T.A.; Downing, J.R. The Biology of Pediatric Acute Megakaryoblastic Leukemia. Blood 2015, 126, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Conneely, S.E.; Stevens, A.M. Acute Myeloid Leukemia in Children: Emerging Paradigms in Genetics and New Approaches to Therapy. Curr. Oncol. Rep. 2021, 23, 16. [Google Scholar] [CrossRef]
- Masetti, R.; Guidi, V.; Ronchini, L.; Bertuccio, N.S.; Locatelli, F.; Pession, A. The Changing Scenario of Non-Down Syndrome Acute Megakaryoblastic Leukemia in Children. Crit. Rev. Oncol. Hematol. 2019, 138, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Espersen, A.D.L.; Noren-Nyström, U.; Abrahamsson, J.; Ha, S.-Y.; Pronk, C.J.; Jahnukainen, K.; Jónsson, Ó.G.; Lausen, B.; Palle, J.; Zeller, B.; et al. Acute Myeloid Leukemia (AML) with t(7;12)(Q36;P13) Is Associated with Infancy and Trisomy 19: Data from Nordic Society for Pediatric Hematology and Oncology (NOPHO-AML) and Review of the Literature. Genes Chromosomes Cancer 2018, 57, 359–365. [Google Scholar] [CrossRef]
- Nakano, Y.; Yamasaki, K.; Otsuka, Y.; Ujiro, A.; Kawakita, R.; Tamagawa, N.; Okada, K.; Fujisaki, H.; Yorifuji, T.; Hara, J. Acute Myeloid Leukemia With RBM15-MKL1 Presenting as Severe Hepatic Failure. Glob. Pediatr. Health 2017, 4, 2333794X16689011. [Google Scholar] [CrossRef] [PubMed]
- Marques-Piubelli, M.L.; Cordeiro, M.G.; Cristofani, L.; Barroso, R.d.S.; Paes, V.R.; Castelli, J.B.; Velloso, E.D.R.P. Acute Megakaryoblastic Leukemia with t(1;22)(P13.3;Q13.1); RBM15-MKL1 Mimicking Hepatoblastoma in an Infant: The Role of Karyotype in Differential Diagnosis. Pediatr. Blood Cancer 2020, 67, e28111. [Google Scholar] [CrossRef] [PubMed]
- Klein, K.; Kaspers, G.; Harrison, C.J.; Beverloo, H.B.; Reedijk, A.; Bongers, M.; Cloos, J.; Pession, A.; Reinhardt, D.; Zimmerman, M.; et al. Clinical Impact of Additional Cytogenetic Aberrations, cKIT and RAS Mutations, and Treatment Elements in Pediatric t(8;21)-AML: Results From an International Retrospective Study by the International Berlin-Frankfurt-Münster Study Group. J. Clin. Oncol. 2015, 33, 4247–4258. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Yang, X.-G.; Xu, L.-H. NUP98::NSD1 and FLT3/ITD Co-Expression Is an Independent Predictor of Poor Prognosis in Pediatric AML Patients. BMC Pediatr. 2024, 24, 547. [Google Scholar] [CrossRef]
- Buelow, D.R.; Pounds, S.B.; Wang, Y.-D.; Shi, L.; Li, Y.; Finkelstein, D.; Shurtleff, S.; Neale, G.; Inaba, H.; Ribeiro, R.C.; et al. Uncovering the Genomic Landscape in Newly Diagnosed and Relapsed Pediatric Cytogenetically Normal FLT 3- ITD AML. Clin. Transl. Sci. 2019, 12, 641–647. [Google Scholar] [CrossRef]
- Ragusa, D.; Dijkhuis, L.; Pina, C.; Tosi, S. Mechanisms Associated with t(7;12) Acute Myeloid Leukaemia: From Genetics to Potential Treatment Targets. Biosci. Rep. 2023, 43, BSR20220489. [Google Scholar] [CrossRef]
- Waraky, A.; Östlund, A.; Nilsson, T.; Weichenhan, D.; Lutsik, P.; Bähr, M.; Hey, J.; Tunali, G.; Adamsson, J.; Jacobsson, S.; et al. Aberrant MNX1 Expression Associated with t(7;12)(Q36;P13) Pediatric Acute Myeloid Leukemia Induces the Disease through Altering Histone Methylation. Haematologica 2023, 109, 725–739. [Google Scholar] [CrossRef]
- Saito, Y.; Makita, S.; Chinen, S.; Kito, M.; Fujino, T.; Ida, H.; Hosoba, R.; Tanaka, T.; Fukuhara, S.; Munakata, W.; et al. Acute Megakaryoblastic Leukaemia with t(1;22)(P13·3;Q13·1)/ RBM15-MKL1 in an Adult Patient Following a Non-mediastinal Germ Cell Tumour. Br. J. Haematol. 2020, 190, e329–e332. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, Y.; Makimoto, M.; Nomura, K.; Hoshino, A.; Hamashima, T.; Hiwatari, M.; Nakazawa, A.; Takita, J.; Yoshida, T.; Kanegane, H. Neonatal Acute Megakaryoblastic Leukemia Mimicking Congenital Neuroblastoma. Clin. Case Rep. 2015, 3, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Morris, S.W.; Valentine, V.; Martin, L.; Herbrick, J.-A.; Cui, X.; Bouman, D.; Li, Y.; Mehta, P.K.; Nizetic, D.; et al. Fusion of Two Novel Genes, RBM15 and MKL1, in the t(1;22)(P13;Q13) of Acute Megakaryoblastic Leukemia. Nat. Genet. 2001, 28, 220–221. [Google Scholar] [CrossRef] [PubMed]
- Roberts, I.; Fordham, N.J.; Rao, A.; Bain, B.J. Neonatal Leukaemia. Br. J. Haematol. 2018, 182, 170–184, Erratum in Br. J. Haematol. 2019, 186, 196. [Google Scholar] [CrossRef] [PubMed]
- Mercher, T.; Raffel, G.D.; Moore, S.A.; Cornejo, M.G.; Baudry-Bluteau, D.; Cagnard, N.; Jesneck, J.L.; Pikman, Y.; Cullen, D.; Williams, I.R.; et al. The OTT-MAL Fusion Oncogene Activates RBPJ-Mediated Transcription and Induces Acute Megakaryoblastic Leukemia in a Knockin Mouse Model. J. Clin. Invest. 2009, 119, 852–864. [Google Scholar] [CrossRef]
- Quessada, J.; Cuccuini, W.; Saultier, P.; Loosveld, M.; Harrison, C.J.; Lafage-Pochitaloff, M. Cytogenetics of Pediatric Acute Myeloid Leukemia: A Review of the Current Knowledge. Genes 2021, 12, 924. [Google Scholar] [CrossRef]
- Qiu, K.; Liao, X.; Li, Y.; Huang, K.; Xu, H.; Fang, J.; Zhou, D. Outcome and Prognostic Factors of CBF Pediatric AML Patients with t(8;21) Differ from Patients with Inv(16). BMC Cancer 2023, 23, 476. [Google Scholar] [CrossRef]
- Chen, W.; Xie, H.; Wang, H.; Chen, L.; Sun, Y.; Chen, Z.; Li, Q. Prognostic Significance of KIT Mutations in Core-Binding Factor Acute Myeloid Leukemia: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0146614. [Google Scholar] [CrossRef]
- Shiba, N.; Yoshida, K.; Shiraishi, Y.; Okuno, Y.; Yamato, G.; Hara, Y.; Nagata, Y.; Chiba, K.; Tanaka, H.; Terui, K.; et al. Whole-exome Sequencing Reveals the Spectrum of Gene Mutations and the Clonal Evolution Patterns in Paediatric Acute Myeloid Leukaemia. Br. J. Haematol. 2016, 175, 476–489. [Google Scholar] [CrossRef]
- Song, G.; Wang, L.; Bi, K.; Jiang, G. Regulation of the C/EBPα Signaling Pathway in Acute Myeloid Leukemia (Review). Oncol. Rep. 2015, 33, 2099–2106. [Google Scholar] [CrossRef]
- Garay, C.M.; Sánchez, K.C.; Lagunes, L.L.F.; Olivares, M.J.; Rivas, A.M.; Torres, B.E.V.; Aguilar, H.F.; Enríquez, J.C.N.; Hernández, E.J.; Méndez, V.C.B.; et al. Mutational Landscape of CEBPA in Mexican Pediatric Acute Myeloid Leukemia Patients: Prognostic Implications. Front. Pediatr. 2022, 10, 899742. [Google Scholar] [CrossRef]
- Ley, T.J.; Ding, L.; Walter, M.J.; McLellan, M.D.; Lamprecht, T.; Larson, D.E.; Kandoth, C.; Payton, J.E.; Baty, J.; Welch, J.; et al. DNMT3A Mutations in Acute Myeloid Leukemia. N. Engl. J. Med. 2010, 363, 2424–2433. [Google Scholar] [CrossRef]
- Genovese, G.; Kähler, A.K.; Handsaker, R.E.; Lindberg, J.; Rose, S.A.; Bakhoum, S.F.; Chambert, K.; Mick, E.; Neale, B.M.; Fromer, M.; et al. Clonal Hematopoiesis and Blood-Cancer Risk Inferred from Blood DNA Sequence. N. Engl. J. Med. 2014, 371, 2477–2487. [Google Scholar] [CrossRef] [PubMed]
- Guryanova, O.A.; Shank, K.; Spitzer, B.; Luciani, L.; Koche, R.P.; Garrett-Bakelman, F.E.; Ganzel, C.; Durham, B.H.; Mohanty, A.; Hoermann, G.; et al. DNMT3A Mutations Promote Anthracycline Resistance in Acute Myeloid Leukemia via Impaired Nucleosome Remodeling. Nat. Med. 2016, 22, 1488–1495. [Google Scholar] [CrossRef]
- Ho, P.A.; Kutny, M.A.; Alonzo, T.A.; Gerbing, R.B.; Joaquin, J.; Raimondi, S.C.; Gamis, A.S.; Meshinchi, S. Leukemic Mutations in the Methylation-associated Genes DNMT3A and IDH2 Are Rare Events in Pediatric AML: A Report from the Children’s Oncology Group. Pediatr. Blood Cancer 2011, 57, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Friedman, A.D. Cell Cycle and Developmental Control of Hematopoiesis by Runx1. J. Cell. Physiol. 2009, 219, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.-L.; Hou, H.-A.; Chen, C.-Y.; Liu, C.-Y.; Chou, W.-C.; Tseng, M.-H.; Huang, C.-F.; Lee, F.-Y.; Liu, M.-C.; Yao, M.; et al. AML1/RUNX1 Mutations in 470 Adult Patients with de Novo Acute Myeloid Leukemia: Prognostic Implication and Interaction with Other Gene Alterations. Blood 2009, 114, 5352–5361. [Google Scholar] [CrossRef]
- Langemeijer, S.M.C.; Jansen, J.H.; Hooijer, J.; van Hoogen, P.; Stevens-Linders, E.; Massop, M.; Waanders, E.; van Reijmersdal, S.V.; Stevens-Kroef, M.J.P.L.; Zwaan, C.M.; et al. TET2 Mutations in Childhood Leukemia. Leukemia 2011, 25, 189–192. [Google Scholar] [CrossRef]
- Ko, M.; Huang, Y.; Jankowska, A.M.; Pape, U.J.; Tahiliani, M.; Bandukwala, H.S.; An, J.; Lamperti, E.D.; Koh, K.P.; Ganetzky, R.; et al. Impaired Hydroxylation of 5-Methylcytosine in Myeloid Cancers with Mutant TET2. Nature 2010, 468, 839–843. [Google Scholar] [CrossRef]
- Kadia, T.M.; Jain, P.; Ravandi, F.; Garcia-Manero, G.; Andreef, M.; Takahashi, K.; Borthakur, G.; Jabbour, E.; Konopleva, M.; Daver, N.G.; et al. TP53 Mutations in Newly Diagnosed Acute Myeloid Leukemia: Clinicomolecular Characteristics, Response to Therapy, and Outcomes. Cancer 2016, 122, 3484–3491. [Google Scholar] [CrossRef]
- U.S. National Library of Medicine. ClinicalTrials.gov. A Global Study of Midostaurin in Combination with Chemotherapy to Evaluate Safety, Efficacy and Pharmacokinetics in Newly Diagnosed Pediatric Patients with FLT3 Mutated AML (NCT03591510). Available online: https://clinicaltrials.gov/study/NCT03591510 (accessed on 28 September 2025).
- U.S. National Library of Medicine. ClinicalTrials.gov. An Open-Label Feasibility Study to Assess the Safety and Pharmacokinetics of Enasidenib in Pediatric Patients With Relapsed/Refractory Acute Myeloid Leukemia (IDH2-Mutant) (NCT04203316). Available online: https://clinicaltrials.gov/study/NCT04203316 (accessed on 28 September 2025).
- U.S. National Library of Medicine. ClinicalTrials.gov. International Randomised Phase III Clinical Trial in Children with Acute Myeloid Leukaemia—Incorporating an Embedded Dose Finding Study for Gemtuzumab Ozogamicin in Combination With Induction Chemotherapy (MyeChild01, NCT02724163). Available online: https://clinicaltrials.gov/study/NCT02724163 (accessed on 28 September 2025).
- U.S. National Library of Medicine. ClinicalTrials.gov. A Phase I Study Investigating the Combination of the Menin Inhibitor SNDX-5613 and Gemtuzumab Ozogamicin in Pediatric Patients with Relapsed or Refractory Acute Myeloid Leukemia (NCT06448013). Available online: https://clinicaltrials.gov/study/NCT06448013 (accessed on 28 September 2025).
- U.S. National Library of Medicine. ClinicalTrials.gov. Luveltamab Tazevibulin (STRO-002) in Patients with Acute Myeloid Leukemia Harboring a Specific Genetic Abnormality (NCT06679582). Available online: https://clinicaltrials.gov/study/NCT06679582 (accessed on 28 September 2025).
- U.S. National Library of Medicine. ClinicalTrials.gov. Safety and Efficacy of Avapritinib in Relapsed or Refractory Pediatric CBF-AML with KIT Mutation (NCT06316960). Available online: https://clinicaltrials.gov/study/NCT06316960 (accessed on 28 September 2025).
- U.S. National Library of Medicine. ClinicalTrials.gov. A Randomized Phase 3 Trial of Fludarabine/Cytarabine/Gemtuzumab Ozogamicin With or Without Venetoclax in Children with Relapsed Acute Myeloid Leukemia (NCT05183035). Available online: https://clinicaltrials.gov/study/NCT05183035 (accessed on 28 September 2025).
- U.S. National Library of Medicine. ClinicalTrials.gov. CHIP-AML22/Quizartinib: A Phase 2 Study of Quizartinib in Combination with Chemotherapy in Newly Diagnosed Pediatric FLT3-ITD Acute Myeloid Leukemia (NCT06262438). Available online: https://clinicaltrials.gov/study/NCT06262438 (accessed on 28 September 2025).
- U.S. National Library of Medicine. ClinicalTrials.gov. A Study of Revumenib in Combination with Chemotherapy in Pediatric Patients with Relapsed or Refractory Acute Myeloid Leukemia (NCT05761171). Available online: https://clinicaltrials.gov/study/NCT05761171 (accessed on 28 September 2025).
- U.S. National Library of Medicine. ClinicalTrials.gov. Treatment of Newly Diagnosed Acute Monocytic Leukemia in Children (NCT05313958). Available online: https://clinicaltrials.gov/study/NCT05313958 (accessed on 28 September 2025).
- U.S. National Library of Medicine. ClinicalTrials.gov. A Phase 3 Randomized Trial for Patients with De Novo AML Comparing Standard Therapy Including Gemtuzumab Ozogamicin (GO) to CPX-351 with GO, and the Addition of the FLT3 Inhibitor Gilteritinib for Patients With FLT3 Mutations (NCT04293562). Available online: https://clinicaltrials.gov/study/NCT04293562 (accessed on 28 September 2025).
- U.S. National Library of Medicine. ClinicalTrials.gov. Base Edited CAR T Cells Against AML: Deep Conditioning Ahead of Allogeneic Stem Cell Transplantation (CARAML). Available online: https://clinicaltrials.gov/study/NCT05942599 (accessed on 28 September 2025).
- U.S. National Library of Medicine. ClinicalTrials.gov. Clinical Study of Induction Therapy Options Based on Molecular Subtyping and MRD in Children and Adolescents with AML (GMCAII). Available online: https://clinicaltrials.gov/study/NCT06221683 (accessed on 28 September 2025).
- U.S. National Library of Medicine. ClinicalTrials.gov. MT2021-08T Cell Receptor Alpha/ Beta Depletion PBSC Transplantation for Heme Malignancies. Available online: https://clinicaltrials.gov/study/NCT05735717 (accessed on 28 September 2025).
- U.S. National Library of Medicine. ClinicalTrials.gov. Safety and Preliminary Efficacy of JK500 Cell Injection in Relapsed/ Refractory Pediatric Acute Myeloid Leukemia. Available online: https://clinicaltrials.gov/study/NCT05519384 (accessed on 28 September 2025).
- U.S. National Library of Medicine. ClinicalTrials.gov. Safety and Efficacy of NK520 to Treat Pediatric Relapsed/ Refractory Acute Myeloid Leukemia. Available online: https://clinicaltrials.gov/study/NCT06541405 (accessed on 28 September 2025).
- Gutiérrez-Jimeno, M.; Panizo-Morgado, E.; Calvo-Imirizaldu, M.; Galán-Gómez, V.; Escudero-López, A.; Patiño-García, A. Case Report: The Value of Genomic Analysis in a Case of Megakaryoblastic Leukemia With Atypical Initial Manifestation. Front. Pediatr. 2022, 10, 875510. [Google Scholar] [CrossRef]
- Liu, T.; Rao, J.; Hu, W.; Cui, B.; Cai, J.; Liu, Y.; Sun, H.; Chen, X.; Tang, Y.; Chen, J.; et al. Distinct Genomic Landscape of Chinese Pediatric Acute Myeloid Leukemia Impacts Clinical Risk Classification. Nat. Commun. 2022, 13, 1640. [Google Scholar] [CrossRef]
- Jones, L.M.; Tarlock, K.; Cooper, T. Targeted Therapy in Pediatric AML: An Evolving Landscape. Pediatr. Drugs 2021, 23, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Egan, G.; Tasian, S.K. Precision Medicine for High-Risk Gene Fusions in Pediatric AML: A Focus on KMT2A, NUP98, and GLIS2 Rearrangements. Blood 2025, 145, 2574–2586. [Google Scholar] [CrossRef] [PubMed]
- Murphy, L.A.; Winters, A.C. Emerging and Future Targeted Therapies for Pediatric Acute Myeloid Leukemia: Targeting the Leukemia Stem Cells. Biomedicines 2023, 11, 3248. [Google Scholar] [CrossRef]
- Choi, E.-J.; Lee, J.-H.; Kim, H.; Choi, Y.; Lee, W.-S.; Lee, S.-M.; Park, J.-H.; Park, H.-S.; Lee, J.-H.; CoOperative Study Group A for Hematology (COSAH); et al. Autologous Hematopoietic Cell Transplantation Following High-Dose Cytarabine Consolidation for Core-Binding Factor–Acute Myeloid Leukemia in First Complete Remission: A Phase 2 Prospective Trial. Int. J. Hematol. 2021, 113, 851–860. [Google Scholar] [CrossRef]
- Marcucci, G.; Geyer, S.; Laumann, K.; Zhao, W.; Bucci, D.; Uy, G.L.; Blum, W.; Eisfeld, A.K.; Pardee, T.S.; Wang, E.S.; et al. Combination of Dasatinib with Chemotherapy in Previously Untreated Core Binding Factor Acute Myeloid Leukemia: CALGB 10801. Blood Adv. 2020, 4, 696–705. [Google Scholar] [CrossRef]
- Xiong, R.-J.; Tang, H.-X.; Yin, T.-T.; Pan, H.-Y.; Jin, R.-M. From Low Remission to Hope: The Efficacy of Targeted Therapies in NUP98-R Positive Pediatric Acute Myeloid Leukemia. World J. Pediatr. 2025, 21, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Ni, L.; Chen, T.; Wu, Y.; Lai, X.; Liu, L.; Xu, Z.; Xu, Y.; Yang, T.; Lu, Y.; et al. NUP98 Rearrangement Dynamics Predict Outcomes in Adult Patients with Acute Myeloid Leukemia Undergoing Allogeneic Hematopoietic Stem Cell Transplantation: A Multicenter Real-World Study. Transplant. Cell. Ther. 2025, S2666, 6367. [Google Scholar] [CrossRef] [PubMed]
- Hollink, I.H.I.M.; van den Heuvel-Eibrink, M.M.; Arentsen-Peters, S.T.C.J.M.; Zimmermann, M.; Peeters, J.K.; Valk, P.J.; Balgobind, B.V.; Sonneveld, E.; Kaspers, G.J.; de Bont, E.S.; et al. Characterization of CEBPA Mutations and Promoter Hypermethylation in Pediatric Acute Myeloid Leukemia. Haematologica 2011, 96, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, H.; Kajihara, M.; Tomizawa, D.; Watanabe, T.; Saito, A.M.; Fujimoto, J.; Horibe, K.; Kodama, K.; Tokumasu, M.; Itoh, H.; et al. Prognostic Implications of CEBPA Mutations in Pediatric Acute Myeloid Leukemia: A Report from the Japanese Pediatric Leukemia/Lymphoma Study Group. Blood Cancer J. 2014, 4, e226. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, V.M.; Othus, M.; Ries, R.E.; Naru, J.; Pogosova-Agadjanyan, E.L.; Appelbaum, F.R.; Beppu, L.W.; Chauncey, T.R.; Erba, H.P.; Godwin, J.E.; et al. Evaluation of ELN2022 Risk Stratification in NPM1 Mutated Acute Myeloid Leukemia: A Study from the Fred Hutch and SWOG. Blood 2024, 144, S4309. [Google Scholar] [CrossRef]
- Seval, G.C.; Ozcan, M. Treatment of Acute Myeloid Leukemia in Adolescent and Young Adult Patients. J. Clin. Med. 2015, 4, 441–459. [Google Scholar] [CrossRef]
| Question | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | Quallity |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yuen et al. [7] | Y | Y | Y | Y | Y | NA | Y | Y | U | Y | Y | high |
| Bolouri et al. [8] | Y | NA | NA | Y | Y | Y | Y | Y | Y | Y | Y | high |
| Meyer et al. [9] | Y | Y | Y | NA | NA | NA | Y | NA | NA | NA | Y | high |
| Hoffmeister et al. [10] | Y | Y | Y | Y | N | NA | Y | Y | U | Y | Y | high |
| Weelderen et al. [11] | Y | Y | Y | Y | N | NA | Y | Y | Y | Y | Y | high |
| Hara et al. [12] | Y | Y | Y | Y | Y | NA | Y | Y | Y | Y | Y | high |
| Smith et al. [13] | Y | Y | Y | Y | Y | NA | Y | Y | Y | Y | Y | high |
| Zangrando et al. [14] | Y | Y | Y | NA | NA | NA | Y | Y | NA | NA | Y | high |
| Chisholm et al. [15] | Y | Y | Y | Y | Y | NA | Y | U | Y | Y | Y | high |
| Östlund et al. [16] | Y | Y | Y | NA | NA | NA | Y | Y | Y | Y | Y | high |
| Rooij et al. [17] | Y | Y | Y | Y | Y | NA | Y | Y | Y | Y | Y | high |
| Inaba et al. [18] | Y | Y | Y | Y | Y | NA | Y | Y | Y | Y | Y | high |
| Duployez et al. [19] | Y | Y | Y | Y | Y | NA | Y | Y | Y | Y | Y | high |
| Yamato et al. [20] | Y | NA | NA | Y | U | NA | Y | Y | U | N | Y | high |
| Sendker et al. [21] | Y | Y | Y | Y | Y | NA | Y | Y | Y | Y | Y | high |
| Niktoreh et al. [22] | Y | Y | Y | Y | Y | NA | Y | Y | Y | Y | Y | high |
| Struski et al. [23] | Y | Y | Y | Y | Y | Y | Y | Y | U | N | Y | high |
| Bertrums et al. [24] | Y | Y | Y | Y | Y | NA | Y | Y | U | N | Y | high |
| Tarloch et al. [25] | Y | Y | Y | Y | Y | NA | Y | Y | Y | Y | Y | high |
| Tarloch et al. [26] | Y | Y | Y | Y | Y | NA | Y | Y | Y | Y | Y | high |
| Liao et al. [27] | Y | Y | Y | Y | Y | NA | Y | Y | Y | Y | Y | high |
| Xu et al. [28] | Y | Y | Y | Y | Y | NA | Y | Y | Y | Y | Y | high |
| Laursen et al. [29] | Y | Y | Y | Y | Y | NA | Y | Y | Y | Y | Y | high |
| Li et al. [30] | Y | Y | Y | Y | Y | NA | Y | Y | Y | Y | Y | high |
| Zarnegar-Lumley et al. [31] | Y | Y | Y | Y | Y | NA | Y | Y | Y | Y | Y | high |
| Yamato et al. [32] | Y | Y | Y | Y | Y | NA | Y | Y | Y | Y | Y | high |
| Li et al. [33] | Y | Y | Y | Y | Y | NA | Y | Y | Y | Y | Y | high |
| Kutny et al. [34] | Y | Y | Y | Y | Y | NA | Y | Y | Y | Y | Y | high |
| Cucchi et al. [35] | Y | Y | Y | Y | U | NA | Y | Y | Y | Y | Y | high |
| Hara et al. [36] | Y | Y | Y | Y | Y | NA | Y | Y | Y | Y | Y | high |
| Hara et al. [37] | Y | Y | Y | Y | Y | NA | Y | Y | Y | Y | Y | high |
| He et al. [38] | Y | Y | Y | Y | Y | NA | Y | Y | Y | Y | Y | high |
| Mutations | Occurrence in Children (%) | Occurrence in Adults (%) |
|---|---|---|
| NPM1 | 10 | 30 |
| DNMT3A | 1 | 25 |
| IDH1 | 1 | 6 |
| IDH2 | 2 | 9 |
| TET2 | 5 | 10 |
| FLT3 | 32 | 36 |
| NRAS | 30 | 10 |
| KRAS | 11 | 2 |
| KIT | 12 | 5 |
| CEBPA | 9 | 10 |
| RUNX1 | 2 | 10 |
| TP53 | 1 | 4 |
| WT1 | 13 | 9 |
| Genetic Alteration | Age Group (Years) | References |
|---|---|---|
| KMT2A rearrangements | infants (<3) | [8] |
| CBFA2T3::GLIS2 fusion | infants (<3) | [8] |
| t(7;12)/MNX1::ETV6 fusion | infants (<2) | [45] |
| RBM15::MKL1 fusion | infants (median age = 0.7) | [17] |
| CBF fusions (t(8;21), inv(16)) | children (3–14) | [8] |
| NUP98 rearrangements | children (3–14) | [8] |
| CEBPA mutations | adolescents (median age = 13.5) | [46] |
| Trisomy 8 | adolescents (median age = 10.1) | [29] |
| NPM1 mutations | adolescents (>14) | [8] |
| FLT3 mutations (ITD/TKD) | adolescents (median age = 11.9 FLT3/ITD) | [47] |
| Targeted Therapy | Mechanism | Study Purpose | Phase of Clinical Study | ClinicalTrials.gov Identifier | References |
|---|---|---|---|---|---|
| Midostaurin | FLT3 tyrosine kinase inhibitor | Evaluation of the safety, pharmacokinetics, and efficacy of midostaurin in combination with standard chemotherapy | Phase 2 | NCT03591510 | [94] |
| Enasidenib | IDH2 inhibitor | Evaluation of the safety, pharmacokinetics, and clinical activity of enasidenib in children and adolescents with IDH2-mutated AML. | Phase 2 | NCT04203316 | [95] |
| Gemtuzumab ozogamicin (GO) | Antibody-drug conjugate targeting CD33 | Determination of the optimal dose of gemtuzumab ozogamicin (up to 3 doses) in combination with induction chemotherapy, safety assessment | Phase 3 | NCT02724163 | [96] |
| Ziftomenib | Menin inhibitor, blocks interaction with KMT2A | Determination of safety, tolerability, and recommended dose of ziftomenib in combination with gemtuzumab ozogamicin and venetoclax | Phase 1 | NCT06448013 | [97] |
| Luveltamab tazevibulin | Tubulin inhibitor, targeting CBFA2T3::GLIS2 | Evaluation of the safety, efficacy, and pharmacokinetics of luveltamab tazevibulin in children with CBFA2T3::GLIS2 gene fusion. | Phase 1, Phase 2 | NCT06679582 | [98] |
| Avapritinib | Tyrosine kinase inhibitor | Assessment of the safety and efficacy of avapritinib in the treatment of CBF-AML with KIT mutation | Phase 2 | NCT06316960 | [99] |
| Venetoclax | BCL-2 inhibitor | Evaluation if randomised addition of venetoclax to the chemotherapy regimen (fludarabine/cytarabine/gemtuzumab ozogamicin) improves survival | Phase 3 | NCT05183035 | [100] |
| Quizartinib | FLT3-ITD inhibitor | Evaluation of the safety, efficacy, pharmacokinetics, and pharmacodynamics of quizartinib added to standard chemotherapy in patients with FLT3-ITD-positive and NPM1-positive wild-type AML | Phase 2 | NCT06262438 | [101] |
| Revumenib | Menin inhibitor | Evaluation of the safety and determination of the optimal dose of revumenib in combination with chemotherapy, and assessment of whether this treatment improves outcomes in pediatric patients with KMT2A-positive AML | Phase 2 | NCT05761171 | [102] |
| Sorafenib | Multi-kinase inhibitor | Assessment of the safety and efficacy of combining targeted therapy with sorafenib and CLAG chemotherapy. | Phase 2, Phase 3 | NCT05313958 | [103] |
| Gilteritinib | Tyrosine kinase inhibitor | Comparison of the efficacy and safety of standard chemotherapy with CPX-351 therapy and/or gilteritinib | Phase 3 | NCT04293562 | [104] |
| Study Purpose | ClinicalTrials.gov Identifier | Phase of Clinical Study | Estimated Numbers of Patients | Age Criteria for the Study Population | References |
|---|---|---|---|---|---|
| Safety evaluation of “BE CAR-33” therapy with CAR-T lymphocytes before planned bone marrow transplantation | NCT05942599 | Phase 1 | 10 | 6 months–16 years | [105] |
| Molecular subtyping in association with MRD-based remission induction regimen | NCT06221683 | Phase 2 | 500 | up to 18 years | [106] |
| Peripheral blood stem cell transplantation (PBSC) with ALFA/BETA T cell receptor depletion (A/B TCD) in children and adults with hematological malignancies | NCT05735717 | Phase 2 | 150 | up to 60 years | [107] |
| Evaluation of the clinical infusion safety and initial efficacy of JK500 cell injection in the treatment of children with relapsed/refractory AML | NCT05519384 | Phase 1 | 12 | up to 18 years | [108] |
| Evaluation of the safety and efficacy of allogeneic NK cells (NK520) administered by infusion in pediatric patients with relapsed/refractory AML | NCT06541405 | Phase 1 | 9 | 6 years–18 years | [109] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cencelewicz, K.; Pieniążek, B.; Chajec, J.; Buziak, J.; Ozygała, A.; Sochaczewska, J.; Lejman, M.; Zawitkowska, J. Molecular Landscape of Acute Myeloid Leukemia in Pediatric Patient-Age-Related Correlations: A Systematic Review. Int. J. Mol. Sci. 2025, 26, 9893. https://doi.org/10.3390/ijms26209893
Cencelewicz K, Pieniążek B, Chajec J, Buziak J, Ozygała A, Sochaczewska J, Lejman M, Zawitkowska J. Molecular Landscape of Acute Myeloid Leukemia in Pediatric Patient-Age-Related Correlations: A Systematic Review. International Journal of Molecular Sciences. 2025; 26(20):9893. https://doi.org/10.3390/ijms26209893
Chicago/Turabian StyleCencelewicz, Katarzyna, Barbara Pieniążek, Joanna Chajec, Jakub Buziak, Aleksandra Ozygała, Julia Sochaczewska, Monika Lejman, and Joanna Zawitkowska. 2025. "Molecular Landscape of Acute Myeloid Leukemia in Pediatric Patient-Age-Related Correlations: A Systematic Review" International Journal of Molecular Sciences 26, no. 20: 9893. https://doi.org/10.3390/ijms26209893
APA StyleCencelewicz, K., Pieniążek, B., Chajec, J., Buziak, J., Ozygała, A., Sochaczewska, J., Lejman, M., & Zawitkowska, J. (2025). Molecular Landscape of Acute Myeloid Leukemia in Pediatric Patient-Age-Related Correlations: A Systematic Review. International Journal of Molecular Sciences, 26(20), 9893. https://doi.org/10.3390/ijms26209893







