Hemoglobin A1C: Intracellular Heterogeneity and Functional Implications in Prediabetic and T2 Diabetic Erythrocytes
Abstract
1. Introduction
2. Results
3. Discussion
3.1. Heterogeneous Intracellular Distribution of HbA1C and Interference with Other Hb Isoforms
3.2. Possible Involvement of HbA1C in RBC Physiology and Rheology
3.3. Limitations
4. Materials and Methods
4.1. Blood Samples
4.2. Buffers and Chemicals
4.3. Hemoglobin Variant Analysis
4.4. RBC Membrane Preparation
4.5. Glucose Consumption, Lactate Release, and Potassium (K+)-Leakage Studies
4.6. Determination of RBC Deformability
4.7. Measurement of Deprotonated Reduced Thiols
4.8. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roden, M.; Shulman, G.I. The Integrative Biology of Type 2 Diabetes. Nature 2019, 576, 51–60. [Google Scholar] [CrossRef]
- Sobel, B.E.; Schneider, D.J. Cardiovascular Complications in Diabetes Mellitus. Curr. Opin. Pharmacol. 2005, 5, 143–148. [Google Scholar] [CrossRef]
- Faselis, C.; Katsimardou, A.; Imprialos, K.; Deligkaris, P.; Kallistratos, M.; Dimitriadis, K. Microvascular Complications of Type 2 Diabetes Mellitus. Curr. Vasc. Pharmacol. 2020, 18, 117–124. [Google Scholar] [CrossRef]
- Avogaro, A.; Fadini, G.P. Microvascular Complications in Diabetes: A Growing Concern for Cardiologists. Int. J. Cardiol. 2019, 291, 29–35. [Google Scholar] [CrossRef]
- Brownlee, M. Biochemistry and Molecular Cell Biology of Diabetic Complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Genuth, S.M.; Palmer, J.P.; Nathan, D.M.; Cowie, C.C.; Casagrande, S.S.; Menke, A.; Cissell, M.A.; Eberhardt, M.S.; Meigs, J.B.; Gregg, E.W. Classification and Diagnosis of Diabetes. In Diabetes in America; National Institute of Diabetes and Digestive and Kidney Diseases (US): Bethesda, MD, USA, 2018; Chapter 1. [Google Scholar]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and Regional Diabetes Prevalence Estimates for 2019 and Projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th Edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef]
- Liddy, A.M.; Grundy, S.; Sreenan, S.; Tormey, W. Impact of Haemoglobin Variants on the Use of Haemoglobin A1c for the Diagnosis and Monitoring of Diabetes: A Contextualised Review. Ir. J. Med. Sci. 2023, 192, 169–176. [Google Scholar] [CrossRef]
- Raghothama, C.; Rao, P. Degradation of Glycated Hemoglobin. Clin. Chim. Acta 1997, 264, 13–25. [Google Scholar] [CrossRef]
- Sen, S.; Kar, M.; Roy, A.; Chakraborti, A.S. Effect of Nonenzymatic Glycation on Functional and Structural Properties of Hemoglobin. Biophys. Chem. 2005, 113, 289–298. [Google Scholar] [CrossRef]
- Kar, M.; Chakraborti, A.S. Release of Iron from Haemoglobin--a Possible Source of Free Radicals in Diabetes Mellitus. Indian J. Exp. Biol. 1999, 37, 190–192. [Google Scholar]
- de Orué Lucana, D.O.; Roscher, M.; Honigmann, A.; Schwarz, J. Iron-Mediated Oxidation Induces Conformational Changes within the Redox-Sensing Protein HbpS. J. Biol. Chem. 2010, 285, 28086–28096. [Google Scholar] [CrossRef] [PubMed]
- Shetty, J.K.; Prakash, M.; Ibrahim, M.S. Relationship between Free Iron and Glycated Hemoglobin in Uncontrolled Type 2 Diabetes Patients Associated with Complications. Indian J. Clin. Biochem. 2008, 23, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Atamna, H.; Ginsburg, H. Heme Degradation in the Presence of Glutathione. J. Biol. Chem. 1995, 270, 24876–24883. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Shao, L.; Jiang, M.; Ba, X.; Ma, B.; Zhou, T. Interpretation of HbA1c Lies at the Intersection of Analytical Methodology, Clinical Biochemistry and Hematology (Review). Exp. Ther. Med. 2022, 24, 707, Erratum in Exp. Ther. Med. 2023, 15, 186. [Google Scholar] [CrossRef]
- Rahbar, S.; Blumenfeld, O.; Ranney, H.M. Studies of an Unusual Hemoglobin in Patients with Diabetes Mellitus. Biochem. Biophys. Res. Commun. 1969, 36, 838–843. [Google Scholar] [CrossRef]
- The American Diabetes Association, E.A. for the S. of D.I.F. of C.C. and L.M. and the I.D.F. Consensus Statement on the Worldwide Standardisation of the HbA1c Measurement. Diabetologia 2007, 50, 2042–2043. [Google Scholar] [CrossRef]
- Livshits, L.; Peretz, S.; Bogdanova, A.; Zoabi, H.; Eitam, H.; Barshtein, G.; Galindo, C.; Feldman, Y.; Pajic-Lijakovic, I.; Koren, A.; et al. The Impact of Ca2+ on Intracellular of Hemoglobin in Human Erythrocytes. Cells 2023, 12, 2280. [Google Scholar] [CrossRef]
- Low, P.S.; Allen, D.P.; Zioncheck, T.F.; Chari, P.; Willardson, B.M.; Geahlen, R.L.; Harrison, M.L. Tyrosine Phosphorylation of Band 3 Inhibits Peripheral Protein Binding. J. Biol. Chem. 1987, 262, 4592–4596. [Google Scholar] [CrossRef]
- Harrison, M.L.; Rathinavelu, P.; Arese, P.; Geahlen, R.L.; Low, P.S. Role of Band 3 Tyrosine Phosphorylation in the Regulation of Erythrocyte Glycolysis. J. Biol. Chem. 1991, 266, 4106–4111. [Google Scholar] [CrossRef]
- Passing, R.; Schubert, D. The Binding of Ca2+ to Solubilized Band 3 Protein of the Human Erythrocyte Membrane. Hoppe Seylers Z. Physiol. Chem. 1983, 364, 873–878. [Google Scholar] [CrossRef]
- Makhro, A.; Hänggi, P.; Goede, J.S.; Wang, J.; Brüggemann, A.; Gassmann, M.; Schmugge, M.; Kaestner, L.; Speer, O.; Bogdanova, A. N-Methyl-d-Aspartate Receptors in Human Erythroid Precursor Cells and in Circulating Red Blood Cells Contribute to the Intracellular Calcium Regulation. Am. J. Physiol.-Cell Physiol. 2013, 305, C1123–C1138. [Google Scholar] [CrossRef]
- Makhro, A.; Haider, T.; Wang, J.; Bogdanov, N.; Steffen, P.; Wagner, C.; Meyer, T.; Gassmann, M.; Hecksteden, A.; Kaestner, L.; et al. Comparing the Impact of an Acute Exercise Bout on Plasma Amino Acid Composition, Intraerythrocytic Ca2+ Handling, and Red Cell Function in Athletes and Untrained Subjects. Cell Calcium 2016, 60, 235–244. [Google Scholar] [CrossRef]
- Puchulu-Campanella, E.; Chu, H.; Anstee, D.J.; Galan, J.A.; Tao, W.A.; Low, P.S. Identification of the Components of a Glycolytic Enzyme Metabolon on the Human Red Blood Cell Membrane. J. Biol. Chem. 2013, 288, 848–858. [Google Scholar] [CrossRef]
- Issaian, A.; Hay, A.; Dzieciatkowska, M.; Roberti, D.; Perrotta, S.; Darula, Z.; Redzic, J.; Busch, M.P.; Page, G.P.; Rogers, S.C.; et al. The Interactome of the N-Terminus of Band 3 Regulates Red Blood Cell Metabolism and Storage Quality. Haematologica 2021, 106, 2971–2985. [Google Scholar] [CrossRef]
- Barshtein, G.; Livshits, L.; Gural, A.; Arbell, D.; Barkan, R.; Pajic-Lijakovic, I.; Yedgar, S. Hemoglobin Binding to the Red Blood Cell (RBC) Membrane Is Associated with Decreased Cell Deformability. Int. J. Mol. Sci. 2024, 25, 5814. [Google Scholar] [CrossRef] [PubMed]
- Walder, J.A.; Chatterjee, R.; Steck, T.L.; Low, P.S.; Musso, G.F.; Kaiser, E.T.; Rogers, P.H.; Arnone, A. The Interaction of Hemoglobin with the Cytoplasmic Domain of Band 3 of the Human Erythrocyte Membrane. J. Biol. Chem. 1984, 259, 10238–10246. [Google Scholar] [CrossRef] [PubMed]
- Castagnola, M.; Messana, I.; Sanna, M.T.; Giardina, B. Oxygen-Linked Modulation of Erythrocyte Metabolism: State of the Art. Blood Transfus. 2010, 8 (Suppl. 3), s53–s58. [Google Scholar] [CrossRef] [PubMed]
- Standl, E.; Khunti, K.; Hansen, T.B.; Schnell, O. The Global Epidemics of Diabetes in the 21st Century: Current Situation and Perspectives. Eur. J. Prev. Cardiol. 2019, 26, 7–14. [Google Scholar] [CrossRef]
- Fischer, S.; Nagel, R.L.; Bookchin, R.M.; Roth, E.F.; Tellez-Nagel, I. The Binding of Hemoglobin to Membranes of Normal and Sickle Erythrocytes. Biochim. Et Biophys. Acta (BBA)-Biomembr. 1975, 375, 422–433. [Google Scholar] [CrossRef]
- Steinberg, M.H.; Rodgers, G.P. HbA2: Biology, Clinical Relevance and a Possible Target for Ameliorating Sickle Cell Disease. Br. J. Haematol. 2015, 170, 781–786. [Google Scholar] [CrossRef]
- Yoshida, T.; Prudent, M.; D’alessandro, A. Red Blood Cell Storage Lesion: Causes and Potential Clinical Consequences. Blood Transfus. 2019, 17, 27–52. [Google Scholar] [CrossRef]
- Datta, P.; Chakrabarty, S.B.; Chakrabarty, A.; Chakrabarti, A. Interaction of Erythroid Spectrin with Hemoglobin Variants: Implications in β-Thalassemia. Blood Cells Mol. Dis. 2003, 30, 248–253. [Google Scholar] [CrossRef]
- Shviro, Y.; Zilber, I.; Shaklai, N. The Interaction of Hemoglobin with Phosphatidylserine Vesicles. Biochim. Et Biophys. Acta (BBA)-Biomembr. 1982, 687, 63–70. [Google Scholar] [CrossRef]
- Bucki, R.; Bachelot-Loza, C.; Zachowski, A.; Giraud, F.; Sulpice, J.-C. Calcium Induces Phospholipid Redistribution and Microvesicle Release in Human Erythrocyte Membranes by Independent Pathways. Biochemistry 1998, 37, 15383–15391. [Google Scholar] [CrossRef]
- Zhang, Q.; Tang, N.; Schepmoes, A.A.; Phillips, L.S.; Smith, R.D.; Metz, T.O. Proteomic Profiling of Nonenzymatically Glycated Proteins in Human Plasma and Erythrocyte Membranes. J. Proteome Res. 2008, 7, 2025–2032. [Google Scholar] [CrossRef]
- Zhang, Q.; Monroe, M.E.; Schepmoes, A.A.; Clauss, T.R.W.; Gritsenko, M.A.; Meng, D.; Petyuk, V.A.; Smith, R.D.; Metz, T.O. Comprehensive Identification of Glycated Peptides and Their Glycation Motifs in Plasma and Erythrocytes of Control and Diabetic Subjects. J. Proteome Res. 2011, 10, 3076–3088. [Google Scholar] [CrossRef]
- Chu, H.; Low, P.S. Mapping of Glycolytic Enzyme-Binding Sites on Human Erythrocyte Band 3. Biochem. J. 2006, 400, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Schuck, P.; Schubert, D. Band 3--hemoglobin Associations the Band 3 Tetramer Is the Oxyhemoglobin Binding Site. FEBS Lett. 1991, 293, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.; Breite, A.; Ciraolo, P.; Franco, R.S.; Low, P.S. Characterization of the Deoxyhemoglobin Binding Site on Human Erythrocyte Band 3: Implications for O2 Regulation of Erythrocyte Properties. Blood 2008, 111, 932–938. [Google Scholar] [CrossRef]
- Chu, H.; McKenna, M.M.; Krump, N.A.; Zheng, S.; Mendelsohn, L.; Thein, S.L.; Garrett, L.J.; Bodine, D.M.; Low, P.S. Reversible Binding of Hemoglobin to Band 3 Constitutes the Molecular Switch That Mediates O2 Regulation of Erythrocyte Properties. Blood 2016, 128, 2708–2716. [Google Scholar] [CrossRef] [PubMed]
- Marschner, J.P.; Rietbrock, N. Oxygen Release Kinetics in Healthy Subjects and Diabetic Patients. I: The Role of 2,3-Diphosphoglycerate. Int. J. Clin. Pharmacol. Ther. 1994, 32, 533–535. [Google Scholar] [PubMed]
- Elsheikh, E.; Aljohani, S.S.; Alshaikhmubarak, M.M.; Alhawl, M.A.; Alsubaie, A.W.; Alsultan, N.; Sharif, A.F.; Ibrahim Ali, S. Implications of Iron Deficiency Anaemia on Glycemic Dynamics in Diabetes Mellitus: A Critical Risk Factor in Cardiovascular Disease. Cureus 2023, 15, e49414. [Google Scholar] [CrossRef]
- Symeonidis, A.; Kouraklis-Symeonidis, A.; Psiroyiannis, A.; Leotsinidis, M.; Kyriazopoulou, V.; Vassilakos, P.; Vagenakis, A.; Zoumbos, N. Inappropriately Low Erythropoietin Response for the Degree of Anemia in Patients with Noninsulin-Dependent Diabetes Mellitus. Ann. Hematol. 2006, 85, 79–85. [Google Scholar] [CrossRef]
- D’Angelo, G. Role of Hepcidin in the Pathophysiology and Diagnosis of Anemia. Blood Res. 2013, 48, 10–15. [Google Scholar] [CrossRef]
- Camaschella, C.; Nai, A.; Silvestri, L. Iron Metabolism and Iron Disorders Revisited in the Hepcidin Era. Haematologica 2020, 105, 260–272. [Google Scholar] [CrossRef]
- Sahin, M.; Tutuncu, N.B.; Ertugrul, D.; Tanaci, N.; Guvener, N.D. Effects of Metformin or Rosiglitazone on Serum Concentrations of Homocysteine, Folate, and Vitamin B12 in Patients with Type 2 Diabetes Mellitus. J. Diabetes Complicat. 2007, 21, 118–123. [Google Scholar] [CrossRef]
- Sayedali, E.; Yalin, A.E.; Yalin, S. Association between Metformin and Vitamin B12 Deficiency in Patients with Type 2 Diabetes. World J. Diabetes 2023, 14, 585–593. [Google Scholar] [CrossRef]
- Wang, R.; Liu, Y.; Thabane, L.; Olier, I.; Li, L.; Ortega-Martorell, S.; Lip, G.Y.H.; Li, G. Relationship between Trajectories of Dietary Iron Intake and Risk of Type 2 Diabetes Mellitus: Evidence from a Prospective Cohort Study. Nutr. J. 2024, 23, 15. [Google Scholar] [CrossRef] [PubMed]
- Harthoorn-Lasthuizen, E.J.; Lindemans, J.; Langenhuijsen, M.M.A.C. Influence of Iron Deficiency Anaemia on Haemoglobin A 2 Levels: Possible Consequences for ß?Thalassaemia Screening. Scand. J. Clin. Lab. Investig. 1999, 59, 65–70. [Google Scholar] [CrossRef]
- Tyczyńska, M.; Hunek, G.; Kawecka, W.; Brachet, A.; Gędek, M.; Kulczycka, K.; Czarnek, K.; Flieger, J.; Baj, J. Association Between Serum Concentrations of (Certain) Metals and Type 2 Diabetes Mellitus. J. Clin. Med. 2024, 13, 7443. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.; Stern, Z.; Gutman, A.; Naparstek, Y.; Gavin, J.R.; Avioli, L.V. Plasma Calcium and Phosphate Levels in an Adult Noninsulin-Dependent Diabetic Population. Calcif. Tissue Int. 1986, 39, 316–318. [Google Scholar] [CrossRef]
- Becerra-Tomás, N.; Estruch, R.; Bulló, M.; Casas, R.; Díaz-López, A.; Basora, J.; Fitó, M.; Serra-Majem, L.; Salas-Salvadó, J. Increased Serum Calcium Levels and Risk of Type 2 Diabetes in Individuals at High Cardiovascular Risk. Diabetes Care 2014, 37, 3084–3091. [Google Scholar] [CrossRef]
- Lorenzo, C.; Hanley, A.J.; Rewers, M.J.; Haffner, S.M. Calcium and Phosphate Concentrations and Future Development of Type 2 Diabetes: The Insulin Resistance Atherosclerosis Study. Diabetologia 2014, 57, 1366–1374. [Google Scholar] [CrossRef]
- Diaz, D.; Fonseca, V.; Aude, Y.W.; Lamas, G.A. Chelation Therapy to Prevent Diabetes-Associated Cardiovascular Events. Curr. Opin. Endocrinol. Diabetes Obes. 2018, 25, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.; Nagengast, A.K.; Knapp, C.; Behrens, B.; Dewey, E.N.; Goodman, A.; Bommiasamy, A.; Schreiber, M. Massive Transfusions and Severe Hypocalcemia: An Opportunity for Monitoring and Supplementation Guidelines. Transfusion 2021, 61, S188–S194. [Google Scholar] [CrossRef]
- Aleem, A.; Al-Momen, A.-K.; Al-Harakati, M.S.; Hassan, A.; Al-Fawaz, I. Hypocalcemia Due to Hypoparathyroidism in β-Thalassemia Major Patients. Ann. Saudi Med. 2000, 20, 364–366. [Google Scholar] [CrossRef]
- Jankowski, S.; Vincent, J.-L. Calcium Administration for Cardiovascular Support in Critically Ill Patients: When Is It Indicated? J. Intensive Care Med. 1995, 10, 91–100. [Google Scholar] [CrossRef] [PubMed]
- McMahon, M.J.; Woodhead, J.S.; Hayward, R.D. The Nature of Hypocalcaemia in Acute Pancreatitis. Br. J. Surg. 2005, 65, 216–218. [Google Scholar] [CrossRef] [PubMed]
- Decaux, G.; Hallemans, R.; Mockel, J.; Naeije, R. Chronic Alcoholism: A Predisposing Factor for Hypocalcemia in Acute Pancreatitis. Digestion 1980, 20, 175–179. [Google Scholar] [CrossRef]
- Qi, X.; Kong, H.; Ding, W.; Wu, C.; Ji, N.; Huang, M.; Li, T.; Wang, X.; Wen, J.; Wu, W.; et al. Abnormal Coagulation Function of Patients With COVID-19 Is Significantly Related to Hypocalcemia and Severe Inflammation. Front. Med. 2021, 8, 638194. [Google Scholar] [CrossRef]
- Zaloga, G.P.; Chernow, B. The Multifactorial Basis for Hypocalcemia During Sepsis. Ann. Intern. Med. 1987, 107, 36–41. [Google Scholar] [CrossRef]
- Chatzinikolaou, P.N.; Margaritelis, N.V.; Paschalis, V.; Theodorou, A.A.; Vrabas, I.S.; Kyparos, A.; D’Alessandro, A.; Nikolaidis, M.G. Erythrocyte Metabolism. Acta Physiol. 2024, 240, e14081. [Google Scholar] [CrossRef] [PubMed]
- Montel-Hagen, A.; Kinet, S.; Manel, N.; Mongellaz, C.; Prohaska, R.; Battini, J.-L.; Delaunay, J.; Sitbon, M.; Taylor, N. Erythrocyte Glut1 Triggers Dehydroascorbic Acid Uptake in Mammals Unable to Synthesize Vitamin C. Cell 2008, 132, 1039–1048. [Google Scholar] [CrossRef]
- Carruthers, A.; DeZutter, J.; Ganguly, A.; Devaskar, S.U. Will the Original Glucose Transporter Isoform Please Stand Up! Am. J. Physiol.-Endocrinol. Metab. 2009, 297, E836–E848. [Google Scholar] [CrossRef] [PubMed]
- Galochkina, T.; Ng Fuk Chong, M.; Challali, L.; Abbar, S.; Etchebest, C. New Insights into GluT1 Mechanics during Glucose Transfer. Sci. Rep. 2019, 9, 998. [Google Scholar] [CrossRef] [PubMed]
- Guizouarn, H.; Allegrini, B. Erythroid Glucose Transport in Health and Disease. Pflug. Arch. 2020, 472, 1371–1383. [Google Scholar] [CrossRef]
- Domene, C.; Wiley, B.; Gonzalez-Resines, S.; Naftalin, R.J. Insight into the Mechanism of d-Glucose Accelerated Exchange in GLUT1 from Molecular Dynamics Simulations. Biochemistry 2025, 64, 928–939. [Google Scholar] [CrossRef]
- Zheng, H.; Wu, J.; Jin, Z.; Yan, L.-J. Protein Modifications as Manifestations of Hyperglycemic Glucotoxicity in Diabetes and Its Complications. Biochem. Insights 2016, 9, 1–9. [Google Scholar] [CrossRef]
- Nielsen, M.F.; Basu, R.; Wise, S.; Caumo, A.; Cobelli, C.; Rizza, R.A. Normal Glucose-Induced Suppression of Glucose Production but Impaired Stimulation of Glucose Disposal in Type 2 Diabetes: Evidence for a Concentration-Dependent Defect in Uptake. Diabetes 1998, 47, 1735–1747. [Google Scholar] [CrossRef]
- Porter-Turner, M.M.; Skidmore, J.C.; Khokher, M.A.; Singh, B.M.; Rea, C.A. Relationship between Erythrocyte GLUT1 Function and Membrane Glycation in Type 2 Diabetes. Br. J. Biomed. Sci. 2011, 68, 203–207. [Google Scholar] [CrossRef]
- Hu, X.; Peng, F.; Zhou, H.; Zhang, Z.; Cheng, W.; Feng, H. The Abnormality of Glucose Transporter in the Erythrocyte Membrane of Chinese Type 2 Diabetic Patients. Biochim. Et Biophys. Acta (BBA)-Biomembr. 2000, 1466, 306–314. [Google Scholar] [CrossRef]
- Martins Freire, C.; King, N.R.; Dzieciatkowska, M.; Stephenson, D.; Moura, P.L.; Dobbe, J.; Streekstra, G.; D’Alessandro, A.; Toye, A.M.; Satchwell, T.J. Complete Absence of GLUT1 Does Not Impair Human Terminal Erythroid Differentiation. Blood Adv. 2024, 8, 5166–5178. [Google Scholar] [CrossRef]
- Li, X.; Yang, Y.; Zhang, B.; Lin, X.; Fu, X.; An, Y.; Zou, Y.; Wang, J.-X.; Wang, Z.; Yu, T. Lactate Metabolism in Human Health and Disease. Signal Transduct. Target. Ther. 2022, 7, 305, Erratum in Signal Transduct. Target. Ther. 2022, 7, 372. [Google Scholar] [CrossRef] [PubMed]
- Pattillo, R.E.; Gladden, L.B. Red Blood Cell Lactate Transport in Sickle Disease and Sickle Cell Trait. J. Appl. Physiol. 2005, 99, 822–827. [Google Scholar] [CrossRef]
- Mahmoud, A.A.; Nor El-Din, A.K.A. Glucose-6-Phosphate Dehydrogenase Activity and Protein Oxidative Modification in Patients with Type 2 Diabetes Mellitus. J. Biomark. 2013, 2013, 430813. [Google Scholar] [CrossRef]
- Zeng, J.; Davies, M.J. Protein and Low Molecular Mass Thiols as Targets and Inhibitors of Glycation Reactions. Chem. Res. Toxicol. 2006, 19, 1668–1676. [Google Scholar] [CrossRef]
- Uceda, A.B.; Mariño, L.; Casasnovas, R.; Adrover, M. An Overview on Glycation: Molecular Mechanisms, Impact on Proteins, Pathogenesis, and Inhibition. Biophys. Rev. 2024, 16, 189–218. [Google Scholar] [CrossRef]
- Livshits, L.; Barshtein, G.; Arbell, D.; Gural, A.; Levin, C.; Guizouarn, H. Do We Store Packed Red Blood Cells under “Quasi-Diabetic” Conditions? Biomolecules 2021, 11, 992. [Google Scholar] [CrossRef]
- Al-Abed, Y.; VanPatten, S.; Li, H.; Lawson, J.A.; FitzGerald, G.A.; Manogue, K.R.; Bucala, R. Characterization of a Novel Hemoglobin-Glutathione Adduct That Is Elevated in Diabetic Patients. Mol. Med. 2001, 7, 619–623. [Google Scholar] [CrossRef]
- Kosower, E.M.; Kosower, N.S. Bromobimane Probes for Thiols. Methods Enzymol. 1995, 251, 133–148. [Google Scholar] [PubMed]
- Gould, B.J.; Davie, S.J.; Yudkin, J.S. Investigation of the Mechanism Underlying the Variability of Glycated Haemoglobin in Non-Diabetic Subjects Not Related to Glycaemia. Clin. Chim. Acta 1997, 260, 49–64. [Google Scholar] [CrossRef]
- Warkentin, T.; Barr, R.; Ali, M.; Mohandas, N. Recurrent Acute Splenic Sequestration Crisis Due to Interacting Genetic Defects: Hemoglobin SC Disease and Hereditary Spherocytosis. Blood 1990, 75, 266–270. [Google Scholar] [CrossRef]
- Mohandas, N.; Chasis, J.A. Red Blood Cell Deformability, Membrane Material Properties and Shape: Regulation by Transmembrane, Skeletal and Cytosolic Proteins and Lipids. Semin. Hematol. 1993, 30, 171–192. [Google Scholar]
- Parthasarathi, K.; Lipowsky, H.H. Capillary Recruitment in Response to Tissue Hypoxia and Its Dependence on Red Blood Cell Deformability. Am. J. Physiol.-Heart Circ. Physiol. 1999, 277, H2145–H2157. [Google Scholar] [CrossRef]
- Sakr, Y.; Chierego, M.; Piagnerelli, M.; Verdant, C.; Dubois, M.-J.; Koch, M.; Creteur, J.; Gullo, A.; Vincent, J.-L.; De Backer, D. Microvascular Response to Red Blood Cell Transfusion in Patients with Severe Sepsis. Crit. Care Med. 2007, 35, 1639–1644. [Google Scholar] [CrossRef] [PubMed]
- Friederichs, E.; Farley, R.A.; Meiselman, H.J. Influence of Calcium Permeabilization and Membrane-attached Hemoglobin on Erythrocyte Deformability. Am. J. Hematol. 1992, 41, 170–177. [Google Scholar] [CrossRef]
- Jin, X.; Zhang, Y.; Wang, D.; Zhang, X.; Li, Y.; Wang, D.; Liang, Y.; Wang, J.; Zheng, L.; Song, H.; et al. Metabolite and Protein Shifts in Mature Erythrocyte under Hypoxia. iScience 2024, 27, 109315. [Google Scholar] [CrossRef] [PubMed]
- McMillan, D.E.; Utterback, N.G.; Puma, J. La Reduced Erythrocyte Deformability in Diabetes. Diabetes 1978, 27, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Bareford, D.; Jennings, P.E.; Stone, P.C.; Baar, S.; Barnett, A.H.; Stuart, J. Effects of Hyperglycaemia and Sorbitol Accumulation on Erythrocyte Deformability in Diabetes Mellitus. J. Clin. Pathol. 1986, 39, 722–727. [Google Scholar] [CrossRef]
- Fujita, J.; Tsuda, K.; Takeda, T.; Yu, L.; Fujimoto, S.; Kajikawa, M.; Nishimura, M.; Mizuno, N.; Hamamoto, Y.; Mukai, E.; et al. Nisoldipine Improves the Impaired Erythrocyte Deformability Correlating with Elevated Intracellular Free Calciumion Concentration and Poor Glycaemic Control in NIDDM. Br. J. Clin. Pharmacol. 1999, 47, 499–506. [Google Scholar] [CrossRef]
- Schwartz, R.S.; Madsen, J.W.; Rybicki, A.C.; Nagel, R.L. Oxidation of Spectrin and Deformability Defects in Diabetic Erythrocytes. Diabetes 1991, 40, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Babu, N. Influence of Hypercholesterolemia on Deformability and Shape Parameters of Erythrocytes in Hyperglycemic Subjects. Clin. Hemorheol. Microcirc. 2009, 41, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Cahn, A.; Livshits, L.; Srulevich, A.; Raz, I.; Yedgar, S.; Barshtein, G. Diabetic Foot Disease Is Associated with Reduced Erythrocyte Deformability. Int. Wound J. 2016, 13, 500–504. [Google Scholar] [CrossRef]
- Li, Q.; Yang, L.Z. Hemoglobin A1c Level Higher than 9.05% Causes a Significant Impairment of Erythrocyte Deformability in Diabetes Mellitus. Acta Endocrinol. 2018, 14, 66–75. [Google Scholar] [CrossRef]
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef]
- Hamley, S.; Kloosterman, D.; Duthie, T.; Dalla Man, C.; Visentin, R.; Mason, S.A.; Ang, T.; Selathurai, A.; Kaur, G.; Morales-Scholz, M.G.; et al. Mechanisms of Hyperinsulinaemia in Apparently Healthy Non-Obese Young Adults: Role of Insulin Secretion, Clearance and Action and Associations with Plasma Amino Acids. Diabetologia 2019, 62, 2310–2324. [Google Scholar] [CrossRef]
- Janssen, J.A.M.J.L. Hyperinsulinemia and Its Pivotal Role in Aging, Obesity, Type 2 Diabetes, Cardiovascular Disease and Cancer. Int. J. Mol. Sci. 2021, 22, 7797. [Google Scholar] [CrossRef]
- Brun, J.-F.; Aloulou, I.; Varlet-Marie, E. Hemorheological Aspects of the Metabolic Syndrome: Markers of Insulin Resistance, Obesity or Hyperinsulinemia? Clin. Hemorheol. Microcirc. 2004, 30, 203–209. [Google Scholar]
- Farber, P.L.; Dias, A.; Freitas, T.; Pinho, A.C.; Viggiano, D.; Saldanha, C.; Silva-Herdade, A.S. Evaluation of Hemorheological Parameters as Biomarkers of Calcium Metabolism and Insulin Resistance in Postmenopausal Women. Clin. Hemorheol. Microcirc. 2021, 77, 395–410. [Google Scholar] [CrossRef]
- Barshtein, G.; Gural, A.; Zelig, O.; Arbell, D.; Yedgar, S. Unit-to-Unit Variability in the Deformability of Red Blood Cells. Transfus. Apher. Sci. 2020, 59, 102876. [Google Scholar] [CrossRef] [PubMed]
- Barshtein, G.; Rasmusen, T.L.; Zelig, O.; Arbell, D.; Yedgar, S. Interdonor Variability in Deformability of Red Blood Cells in Blood Units. Transfus. Med. 2020, 30, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Barshtein, G.; Pries, A.R.; Goldschmidt, N.; Zukerman, A.; Orbach, A.; Zelig, O.; Arbell, D.; Yedgar, S. Deformability of Transfused Red Blood Cells Is a Potent Determinant of Transfusion-Induced Change in Recipient’s Blood Flow. Microcirculation 2016, 23, 479–486. [Google Scholar] [CrossRef] [PubMed]

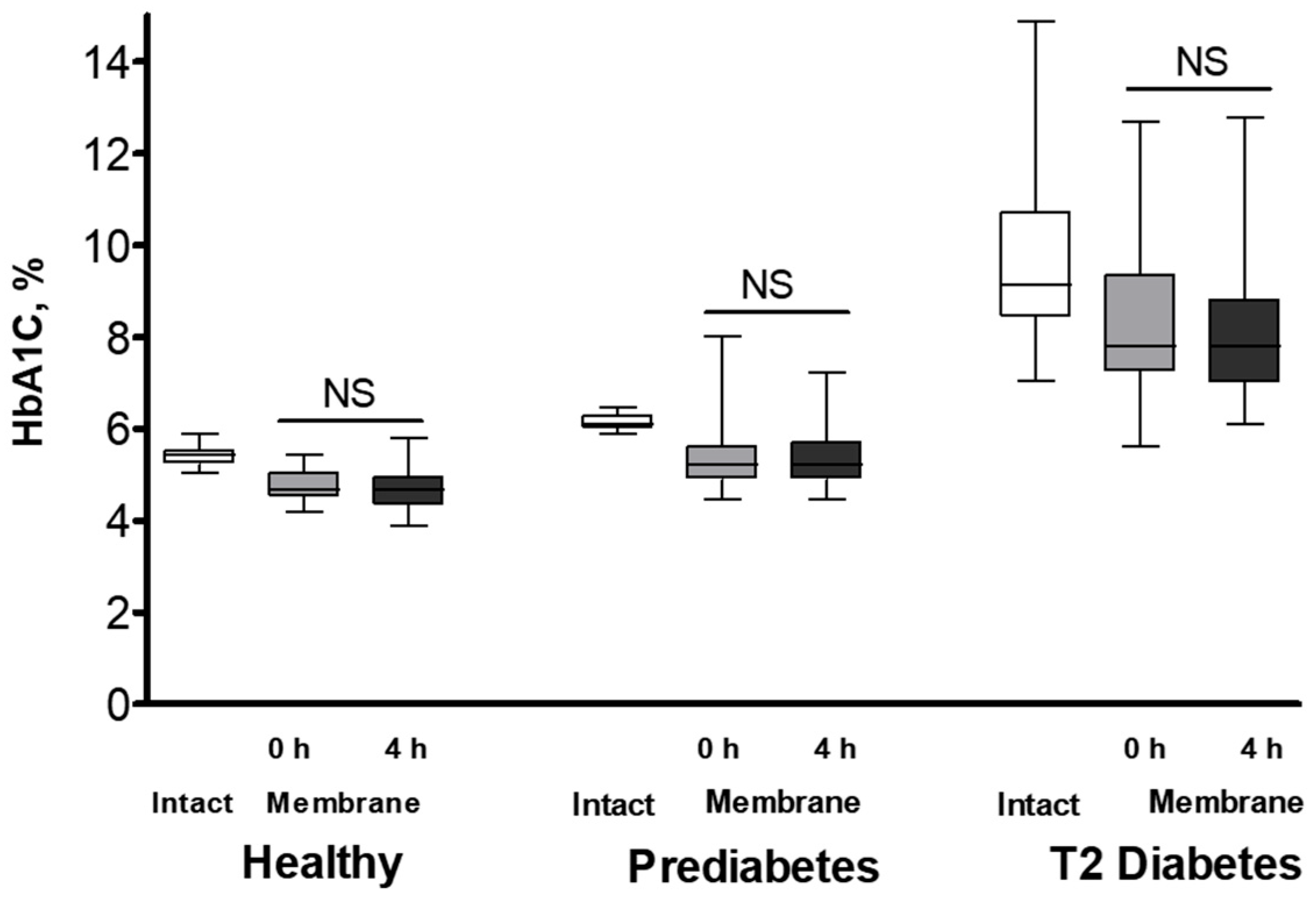
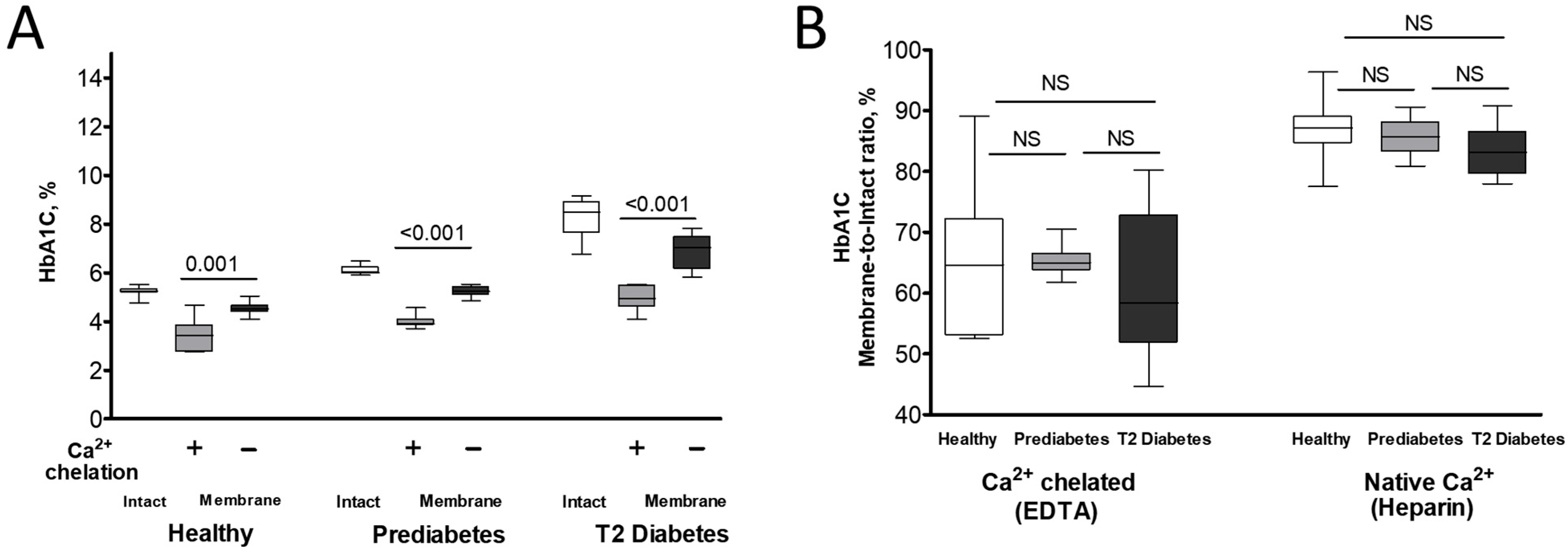
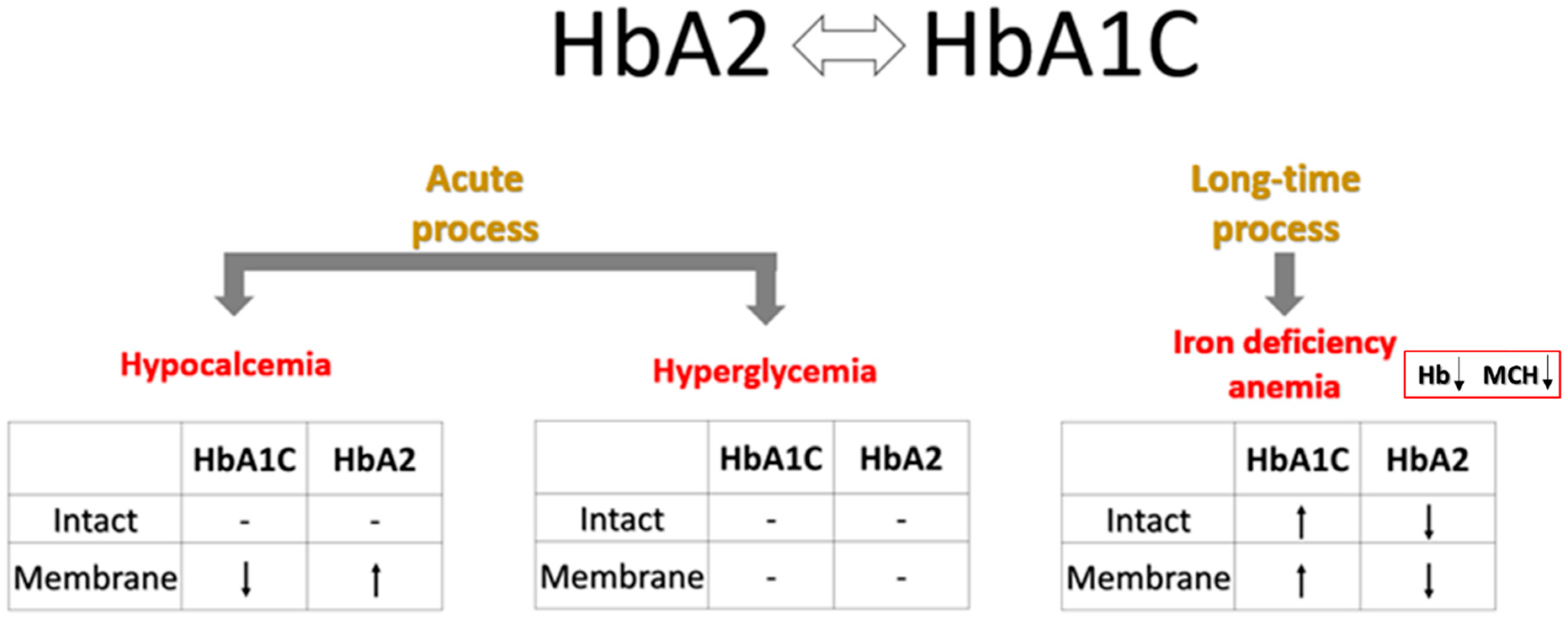

| Reference Ranges | Healthy | Prediabetes | Type 2 (T2) Diabetes | |
|---|---|---|---|---|
| n | 56 | 53 | 56 | |
| Age (years) | 58.3 ± 14.0 | 65.3 ± 11.3 | 62.6 ± 14.3 | |
| M/F | 24/32 | 29/24 | 30/26 | |
| HbA1C (%) | <5.7 | 5.24 ± 0.26 | 6.01 ± 0.16 | 8.88 ± 1.67 |
| RBC (106/µL) | Female: 4.0–5.0 Male: 4.5–5.5 | 4.71 ± 0.55 | 4.77 ± 0.51 NS | 5.03 ± 0.63 0.005/0.021 |
| HCT (%) | Female: 37.0–47.0 Male: 40.0–54.0 | 41.1 ± 4.6 | 41.8 ± 3.8 NS | 42.6 ± 4.9 NS/NS |
| Hb (g/dL) | Female: 12.0–15.0 Male: 14.0–17.0 | 13.6 ± 1.6 | 13.8 ± 1.2 NS | 13.9 ± 1.8 NS/NS |
| MCV (fL) | Female: 80.0–94.0 Male: 80.0–95.0 | 87.1 ± 8.2 | 88.2 ± 5.8 NS | 84.8 ± 6.6 NS/0.007 |
| MCH (pg) | 27.0–31.0 | 29.0 ± 2.9 | 29.1 ± 2.0 NS | 27.7 ± 2.4 0.008/0.002 |
| MCHC (g/dL) | 32.0–35.0 | 33.1 ± 1.3 | 33.0 ± 1.2 NS | 32.5 ± 1.4 0.020/NS |
| RDW (%) | 11.5–14.5 | 13.9 ± 1.7 | 13.9 ± 0.9 NS | 14.3 ± 1.4 NS/0.046 |
| HbF (%) | 0.5–1.3 | 0.41 ± 0.34 | 0.33 ± 0.13 NS | 0.40 ± 0.26 NS/NS |
| HbA2 (%) | 1.5–3.6 | 2.83 ± 0.34 | 2.81 ± 0.32 NS | 2.64 ± 0.35 0.005/0.009 |
| HbA0 (%) | >95.9 | 96.8 ± 0.5 | 96.9 ± 0.3 NS | 97.0 ± 0.5 0.027/NS |
| p2 (related to HbA1C) (%) | <4.5 | 4.06 ± 0.26 | 4.79 ± 0.22 <0.001 | 7.92 ± 1.87 <0.001/<0.001 |
| Healthy (n = 56) | Prediabetes (n = 53) | T2 Diabetes (n = 56) | ||||
|---|---|---|---|---|---|---|
| Intact | Membrane | Intact | Membrane | Intact | Membrane | |
| HbF | 0.30 ± 0.9 | 0.30 ± 0.11 | 0.30 ± 0.04 NS | 0.30 ± 0.06 NS | 0.30 ± 0.07 NS/NS | 0.30 ± 0.08 NS/0.043 |
| HbA2 | 2.80 ± 0.09 | 4.50 ± 0.17 | 2.80 ± 0.09 NS | 4.30 ± 0.19 NS | 2.60 ± 0.09 0.001/0.003 | 4.20 ± 0.24 NS/NS |
| HbA0 | 96.8 ± 0.1 | 95.2 ± 0.2 | 96.8 ± 0.09 NS | 95.4 ± 0.2 NS | 97.0 ± 0.1 0.021/0.049 | 95.2 ± 0.3 NS/NS |
| HbA1C | 5.33 ± 0.07 | 4.61 ± 0.08 | 6.00 ± 0.06 <0.001 | 5.14 ± 0.08 <0.001 | 8.58 ± 0.47 <0.001/<0.001 | 6.91 ± 0.43 <0.001/<0.001 |
| Whole Cohort (n = 165) | Healthy (n = 56) | Prediabetes (n = 53) | T2 Diabetes (n = 56) | |||||
|---|---|---|---|---|---|---|---|---|
| Intact | Membrane | Intact | Membrane | Intact | Membrane | Intact | Membrane | |
| HbF | 0.013 (NS) | −0.018 (NS) | 0.146 (NS) | −0.011 (NS) | 0.107 (NS) | −0.013 (NS) | −0.063 (NS) | −0.186 (NS) |
| HbA2 | −0.334 (<0.001) | −0.318 (<0.001) | −0.263 (NS) | −0.525 (<0.001) | 0.176 (NS) | −0.449 (<0.001) | −0.391 (0.003) | −0.630 (<0.001) |
| HbA0 | +0.259 (<0.001) | +0.281 (<0.001) | 0.082 (NS) | +0.415 (0.001) | −0.213 (NS) | +0.406 (0.003) | +0.342 (0.010) | +0.629 (<0.001) |
| Healthy | Prediabetes | T2 Diabetes | ||||
|---|---|---|---|---|---|---|
| Intact HbA1C | Membrane HbA1C | Intact HbA1C | Membrane HbA1C | Intact HbA1C | Membrane HbA1C | |
| K+ loss (mM/h per Hb) | −0.040 (NS) | +0.043 (NS) | +0.010 (NS) | +0.009 (NS) | −0.037 (NS) | +0.166 (NS) |
| (n = 15) | (n = 14) | (n = 14) | ||||
| Glucose consumption (mg/dL per hour per Hb) | +0.083 (NS) | −0.193 (NS) | +0.011 (NS) | −0.561 (0.037) | −0.222 (NS) | −0.206 (NS) |
| (n = 15) | (n = 14) | (n = 14) | ||||
| Lactate release (mM/h per Hb) | −0.515 (0.049) | +0.469 (NS) | −0.489 (NS) | −0.034 (NS) | +0.318 (NS) | +0.264 (NS) |
| (n = 15) | (n = 14) | (n = 14) | ||||
| Intact deprotonated reduced thiol (A.U., normalized) | +0.629 (0.012) | +0.403 (NS) | +0.497 (NS) | +0.252 (NS) | −0.144 (NS) | −0.284 (NS) |
| (n = 15) | (n = 15) | (n = 17) | ||||
| Median elongation rate | +0.013 (NS) | +0.175 (NS) | −0.839 (0.005) | −0.256 (NS) | −0.345 (NS) | −0.222 (NS) |
| (n = 9) | (n = 9) | (n = 9) | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petukhova, G.; Wani, A.; Barshtein, G.; Bogdanova, A.; Koren, A.; Levin, C.; Livshits, L. Hemoglobin A1C: Intracellular Heterogeneity and Functional Implications in Prediabetic and T2 Diabetic Erythrocytes. Int. J. Mol. Sci. 2025, 26, 9890. https://doi.org/10.3390/ijms26209890
Petukhova G, Wani A, Barshtein G, Bogdanova A, Koren A, Levin C, Livshits L. Hemoglobin A1C: Intracellular Heterogeneity and Functional Implications in Prediabetic and T2 Diabetic Erythrocytes. International Journal of Molecular Sciences. 2025; 26(20):9890. https://doi.org/10.3390/ijms26209890
Chicago/Turabian StylePetukhova, Galina, Areen Wani, Gregory Barshtein, Anna Bogdanova, Ariel Koren, Carina Levin, and Leonid Livshits. 2025. "Hemoglobin A1C: Intracellular Heterogeneity and Functional Implications in Prediabetic and T2 Diabetic Erythrocytes" International Journal of Molecular Sciences 26, no. 20: 9890. https://doi.org/10.3390/ijms26209890
APA StylePetukhova, G., Wani, A., Barshtein, G., Bogdanova, A., Koren, A., Levin, C., & Livshits, L. (2025). Hemoglobin A1C: Intracellular Heterogeneity and Functional Implications in Prediabetic and T2 Diabetic Erythrocytes. International Journal of Molecular Sciences, 26(20), 9890. https://doi.org/10.3390/ijms26209890








