Spatial Raman Spectroscopy to Characterize (Sulfated) Glycosaminoglycans in Human Articular Cartilage
Abstract
1. Introduction
2. Results
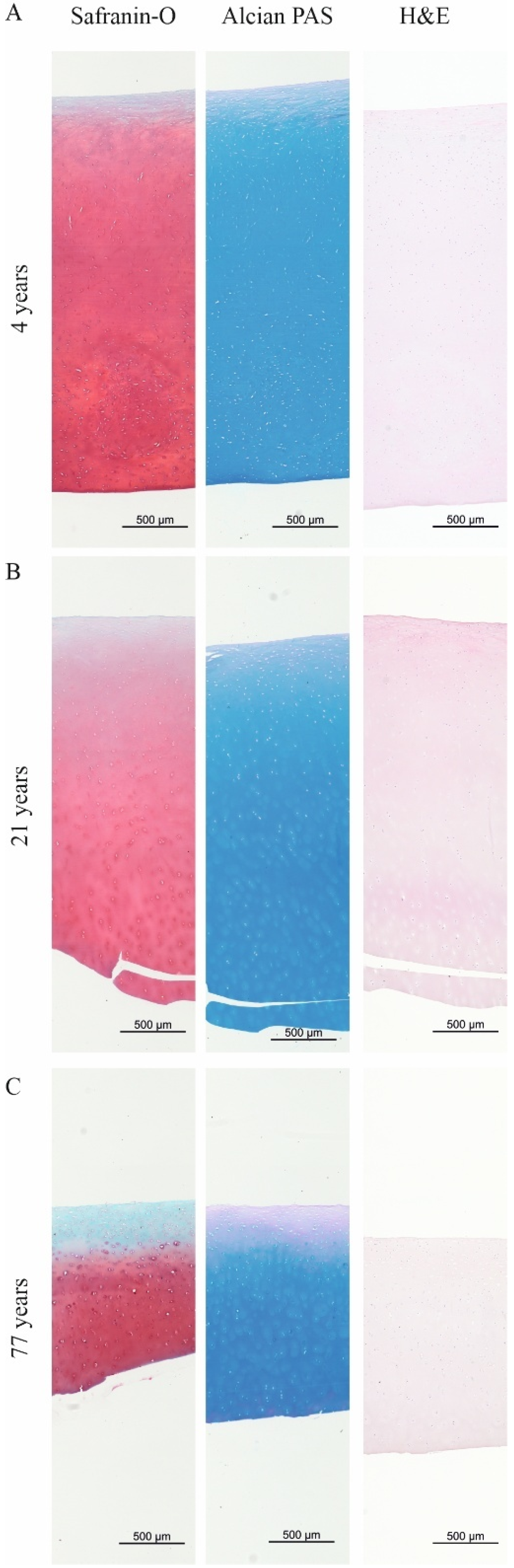
2.1. The Total GAG/CH2 Ratio over the Tissue Distance Is an Indicator to Estimate the Maturity of Human Articular Cartilage

2.2. The sGAG/CH2 Ratio over the Tissue Distance Is an Indicator to Estimate the Health of Human Articular Cartilage
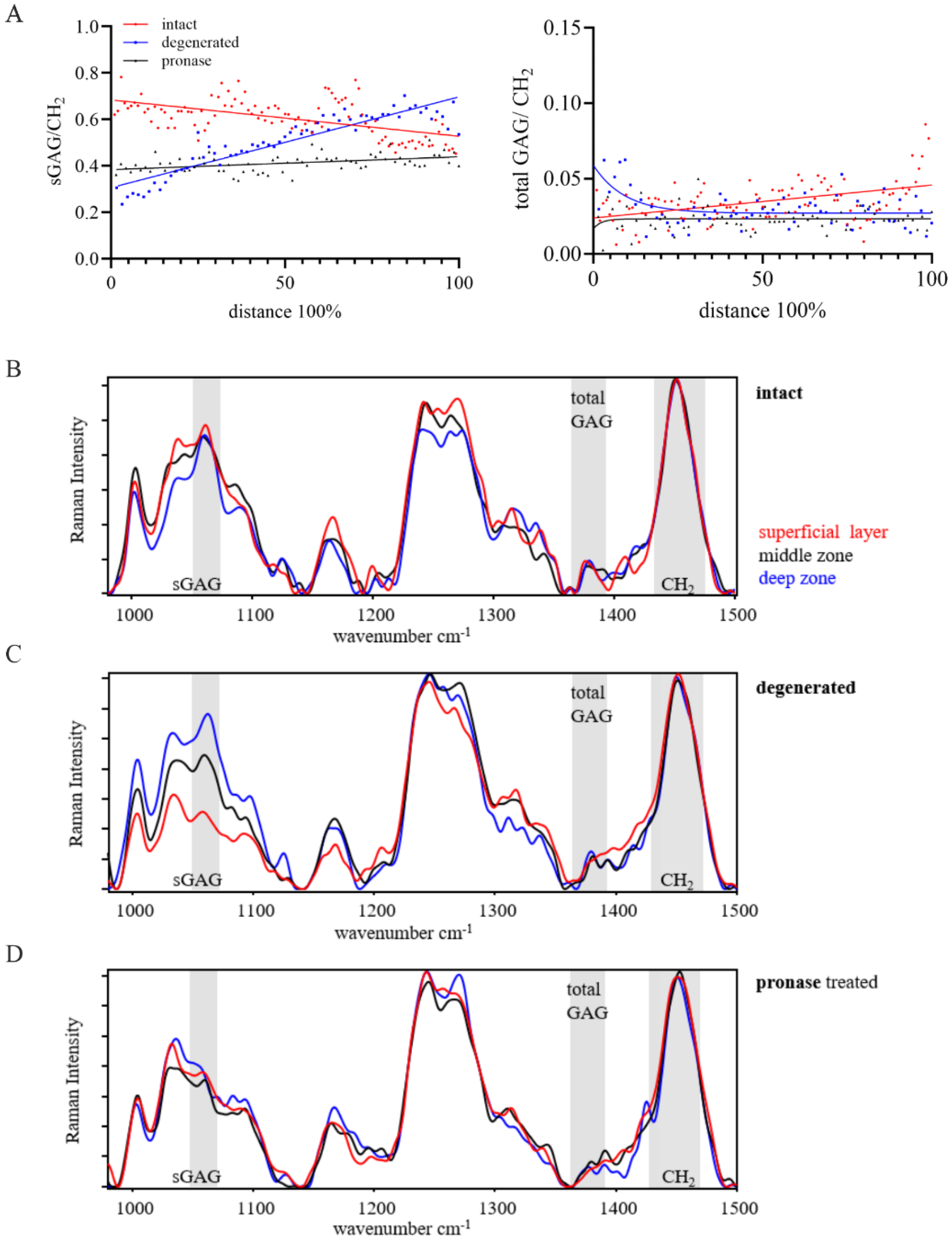
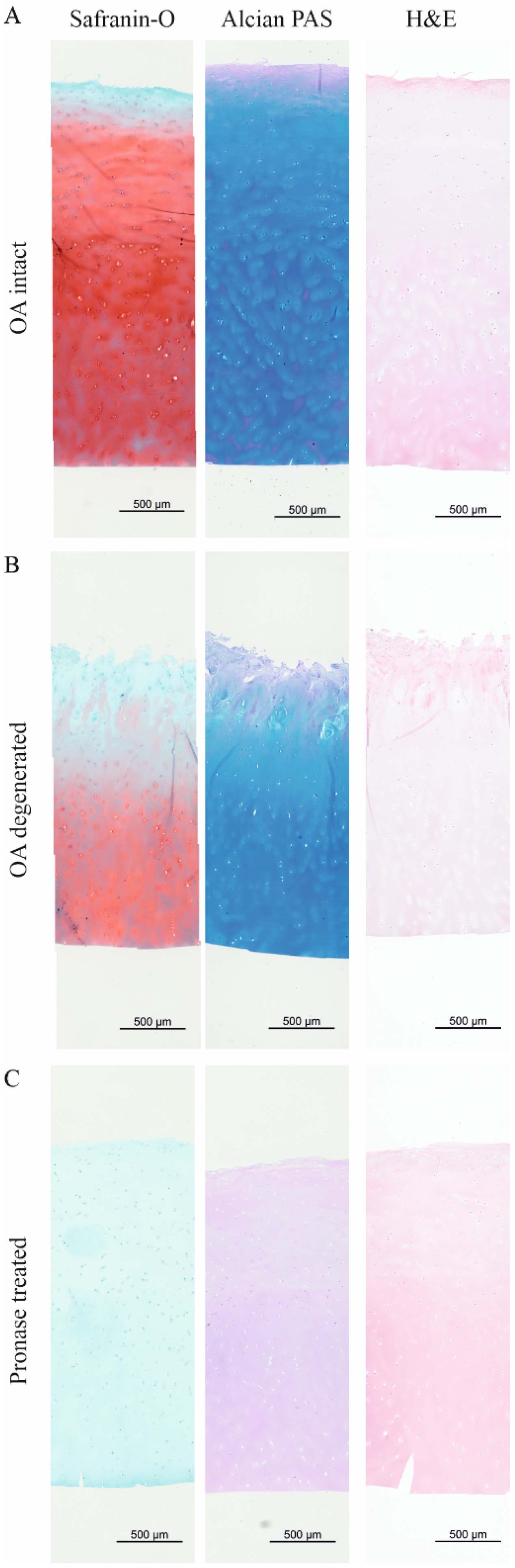
3. Discussion
4. Materials and Methods
4.1. Articular Cartilage Tissue Harvest
4.2. Histological Stainings
4.3. Raman Spectroscopic Line Scans
4.4. Raman Spectroscopic Data Analysis
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GAG | Glycosaminoglycan |
| sGAG | Sulfated glycosaminoglycan |
| OA | Osteoarthritis |
| ECM | Extracellular matrix |
| CH2 | Organic matrix band |
References
- Benninghoff, A. Form und Bau der Gelenkknorpel in ihren Beziehungen zur Funktion. Z. Zellforsch. Mikrosk. Anat. 1925, 2, 783–862. [Google Scholar] [CrossRef]
- Johnston, S.A. Osteoarthritis: Joint anatomy, physiology, and pathobiology. Vet. Clin. N. Am. Small Anim. Pract. 1997, 27, 699–723. [Google Scholar] [CrossRef]
- Redler, I.; Mow, V.C.; Zimny, M.L.; Mansell, J. The ultrastructure and biomechanical significance of the tidemark of articular cartilage. Clin. Orthop. Relat. Res. 1975, 112, 357–362. [Google Scholar] [CrossRef]
- Decker, R.S.; Koyama, E.; Pacifici, M. Articular Cartilage: Structural and Developmental Intricacies and Questions. Curr. Osteoporos. Rep. 2015, 13, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Eyre, D.R.; Wu, J.J. Collagen structure and cartilage matrix integrity. J. Rheumatol. Suppl. 1995, 43, 82–85. [Google Scholar] [PubMed]
- Roughley, P.J.; Lee, E.R. Cartilage proteoglycans: Structure and potential functions. Microsc. Res. Tech. 1994, 28, 385–397. [Google Scholar] [CrossRef]
- Merry, C.L.R.; Lindahl, U.; Couchman, J.; Esko, J.D. Proteoglycans and sulfated glycosaminoglycans. In Essentials of Glycobiology, 4th ed.; Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Mohnen, D., Kinoshita, T., Packer, N.H., Prestegard, J.H., et al., Eds.; Cold Springer Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2022. [Google Scholar]
- Hyttinen, M.M.; Holopainen, J.; van Weeren, P.R.; Firth, E.C.; Helminen, H.J.; Brama, P.A. Changes in collagen fibril network organization and proteoglycan distribution in equine articular cartilage during maturation and growth. J. Anat. 2009, 215, 584–591. [Google Scholar] [CrossRef]
- Calabro, A.; Midura, R.; Wang, A.; West, L.; Plaas, A.; Hascall, V.C. Fluorophore-assisted carbohydrate electrophoresis (FACE) of glycosaminoglycans. Osteoarthr. Cartil. 2001, 9 (Suppl. S1), S16–S22. [Google Scholar] [CrossRef]
- Hickery, M.S.; Bayliss, M.T.; Dudhia, J.; Lewthwaite, J.C.; Edwards, J.C.W.; Pitsillides, A.A. Age-related Changes in the Response of Human Articular Cartilage to IL-1α and Transforming Growth Factor-β (TGF-β): Chondrocytes Exhibit a Diminished Sensitivity to Tgf-β. J. Biol. Chem. 2003, 278, 53063–53071. [Google Scholar] [CrossRef]
- Pritzker, K.P.; Gay, S.; Jimenez, S.A.; Ostergaard, K.; Pelletier, J.P.; Revell, P.A.; Salter, D.; van den Berg, W.B. Osteoarthritis cartilage histopathology: Grading and staging. Osteoarthr. Cartil. 2006, 14, 13–29. [Google Scholar] [CrossRef]
- Goldring, S.R.; Goldring, M.B. Changes in the osteochondral unit during osteoarthritis: Structure, function and cartilage-bone crosstalk. Nat. Rev. Rheumatol. 2016, 12, 632–644. [Google Scholar] [CrossRef]
- Kubaski, F.; Osago, H.; Mason, R.W.; Yamaguchi, S.; Kobayashi, H.; Tsuchiya, M.; Orii, T.; Tomatsu, S. Glycosaminoglycans detection methods: Applications of mass spectrometry. Mol. Genet. Metab. 2017, 120, 67–77. [Google Scholar] [CrossRef]
- Bergholt, M.S.; St-Pierre, J.-P.; Offeddu, G.S.; Parmar, P.A.; Albro, M.B.; Puetzer, J.L.; Oyen, M.L.; Stevens, M.M. Raman Spectroscopy Reveals New Insights into the Zonal Organization of Native and Tissue-Engineered Articular Cartilage. ACS Cent. Sci. 2016, 2, 885–895. [Google Scholar] [CrossRef]
- Gamsjaeger, S.; Klaushofer, K.; Paschalis, E.P. Raman analysis of proteoglycans simultaneously in bone and cartilage. J. Raman Spectrosc. 2014, 45, 794–800. [Google Scholar] [CrossRef]
- Gao, T.; Boys, A.J.; Zhao, C.; Chan, K.; Estroff, L.A.; Bonassar, L.J. Non-Destructive Spatial Mapping of Glycosaminoglycan Loss in Native and Degraded Articular Cartilage Using Confocal Raman Microspectroscopy. Front. Bioeng. Biotechnol. 2021, 9, 744197. [Google Scholar] [CrossRef] [PubMed]
- Camacho, N.P.; West, P.; Torzilli, P.A.; Mendelsohn, R. FTIR microscopic imaging of collagen and proteoglycan in bovine cartilage. Biopolymers 2001, 62, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Das Gupta, S.; Finnilä, M.A.J.; Karhula, S.S.; Kauppinen, S.; Joukainen, A.; Kröger, H.; Korhonen, R.K.; Thambyah, A.; Rieppo, L.; Saarakkala, S. Raman microspectroscopic analysis of the tissue-specific composition of the human osteochondral junction in osteoarthritis: A pilot study. Acta Biomater. 2020, 106, 145–155. [Google Scholar] [CrossRef]
- Esmonde-White, K. Raman Spectroscopy of Soft Musculoskeletal Tissues. Appl. Spectrosc. 2014, 68, 1203–1218. [Google Scholar] [CrossRef]
- Rieppo, L.; Rieppo, J.; Jurvelin, J.S.; Saarakkala, S. Fourier transform infrared spectroscopic imaging and multivariate regression for prediction of proteoglycan content of articular cartilage. PLoS ONE 2012, 7, e32344. [Google Scholar] [CrossRef]
- Shaikh, R.; Nippolainen, E.; Virtanen, V.; Torniainen, J.; Rieppo, L.; Saarakkala, S.; Afara, I.O.; Töyräs, J. Raman spectroscopy is sensitive to biochemical changes related to various cartilage injuries. J. Raman Spectrosc. 2021, 52, 796–804. [Google Scholar] [CrossRef]
- Paschalis, E.P.; Gamsjaeger, S.; Klaushofer, K. Vibrational spectroscopic techniques to assess bone quality. Osteoporos. Int. 2017, 28, 2275–2291. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, A.; Stücker, S.; Koßlowski, F.; Lohmann, C.H.; Bertrand, J. High-Resolution Imaging Methods for Identification of Calcium Crystal Types in Osteoarthritis. Gout Urate Cryst. Depos. Dis. 2023, 1, 62–82. [Google Scholar] [CrossRef]
- Gaifulina, R.; Nunn, A.D.G.; Draper, E.R.C.; Strachan, R.K.; Blake, N.; Firth, S.; Thomas, G.M.H.; McMillan, P.F.; Dudhia, J. Intra-operative Raman spectroscopy and ex vivo Raman mapping for assessment of cartilage degradation. Clin. Spectrosc. 2021, 3, 100012. [Google Scholar] [CrossRef]
- Bansil, R.; Yannas, I.V.; Stanley, H.E. Raman Spectroscopy: A structural probe of glycosaminoglycans. Biochim. Biophys. Acta (BBA)-Gen. Subj. 1978, 541, 535–542. [Google Scholar] [CrossRef]
- Ellis, R.; Green, E.; Winlove, C.P. Structural Analysis of Glycosaminoglycans and Proteoglycans by Means of Raman Microspectrometry. Connect. Tissue Res. 2009, 50, 29–36. [Google Scholar] [CrossRef]
- Rieppo, L.; Töyräs, J.; Saarakkala, S. Vibrational spectroscopy of articular cartilage. Appl. Spectrosc. Rev. 2016, 52, 249–266. [Google Scholar] [CrossRef]
- Frushour, B.G.; Koenig, J.L. Raman scattering of collagen, gelatin, and elastin. Biopolymers 1975, 14, 379–391. [Google Scholar] [CrossRef]
- Roughley, P.J.; White, R.J. Age-related changes in the structure of the proteoglycan subunits from human articular cartilage. J. Biol. Chem. 1980, 255, 217–224. [Google Scholar] [CrossRef]
- Hardingham, T.; Bayliss, M. Proteoglycans of articular cartilage: Changes in aging and in joint disease. Semin. Arthritis Rheum. 1990, 20, 12–33. [Google Scholar] [CrossRef]
- Bergholt, M.S.; Albro, M.B.; Stevens, M.M. Online quantitative monitoring of live cell engineered cartilage growth using diffuse fiber-optic Raman spectroscopy. Biomaterials 2017, 140, 128–137. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwab, A.; Jahn, J.; Sitte, K.; Lohmann, C.H.; Bertrand, J.; Gamsjaeger, S. Spatial Raman Spectroscopy to Characterize (Sulfated) Glycosaminoglycans in Human Articular Cartilage. Int. J. Mol. Sci. 2025, 26, 9875. https://doi.org/10.3390/ijms26209875
Schwab A, Jahn J, Sitte K, Lohmann CH, Bertrand J, Gamsjaeger S. Spatial Raman Spectroscopy to Characterize (Sulfated) Glycosaminoglycans in Human Articular Cartilage. International Journal of Molecular Sciences. 2025; 26(20):9875. https://doi.org/10.3390/ijms26209875
Chicago/Turabian StyleSchwab, Andrea, Jannik Jahn, Kerstin Sitte, Christoph H. Lohmann, Jessica Bertrand, and Sonja Gamsjaeger. 2025. "Spatial Raman Spectroscopy to Characterize (Sulfated) Glycosaminoglycans in Human Articular Cartilage" International Journal of Molecular Sciences 26, no. 20: 9875. https://doi.org/10.3390/ijms26209875
APA StyleSchwab, A., Jahn, J., Sitte, K., Lohmann, C. H., Bertrand, J., & Gamsjaeger, S. (2025). Spatial Raman Spectroscopy to Characterize (Sulfated) Glycosaminoglycans in Human Articular Cartilage. International Journal of Molecular Sciences, 26(20), 9875. https://doi.org/10.3390/ijms26209875






