Simultaneous Accumulation of Holocellulose, Callose and Lignin: Cell Wall Markers for Resistance in Wheat Infested with Diuraphis noxia
Abstract
1. Introduction
2. Results
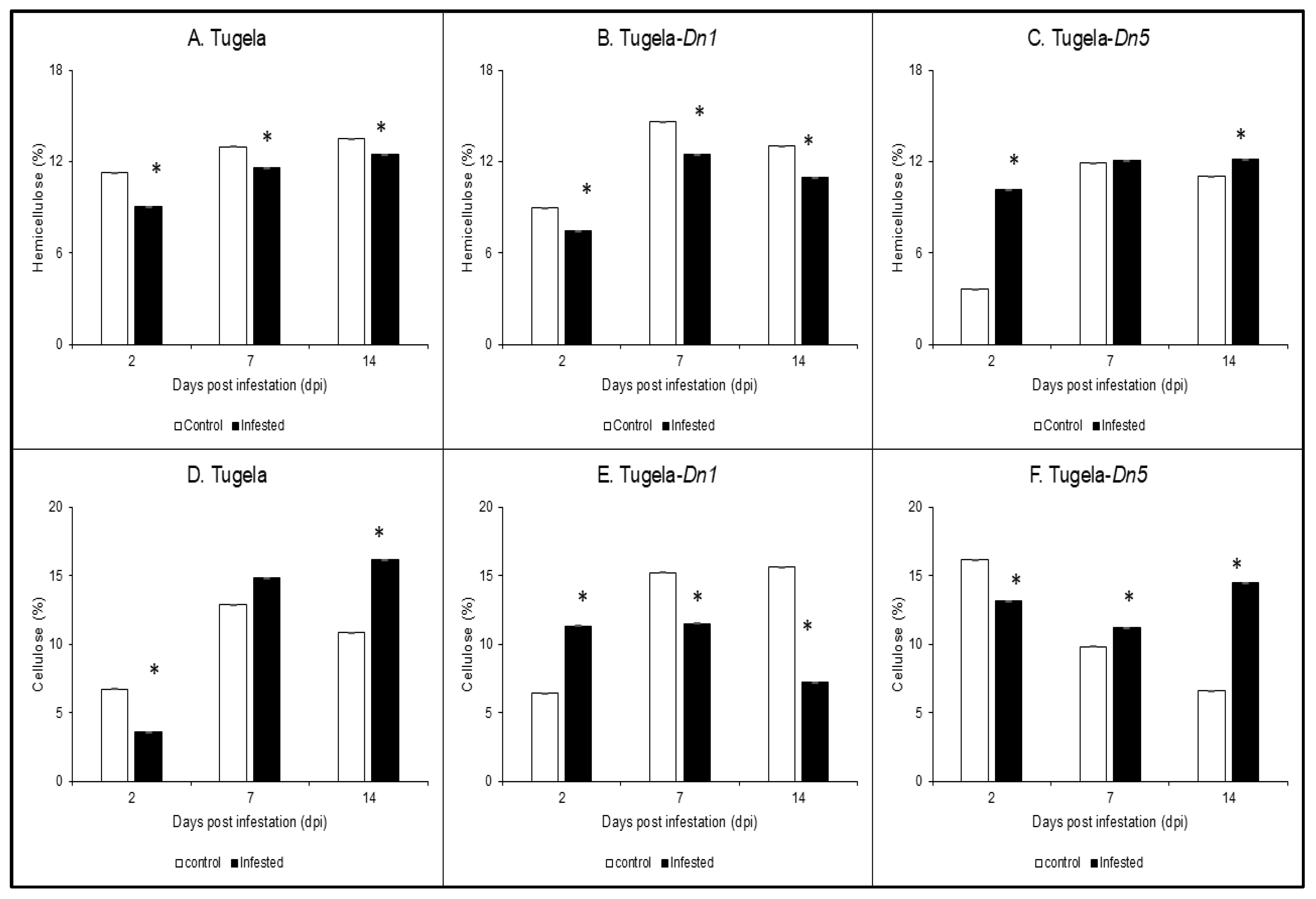
2.1. Hemicellulose and Cellulose Determination
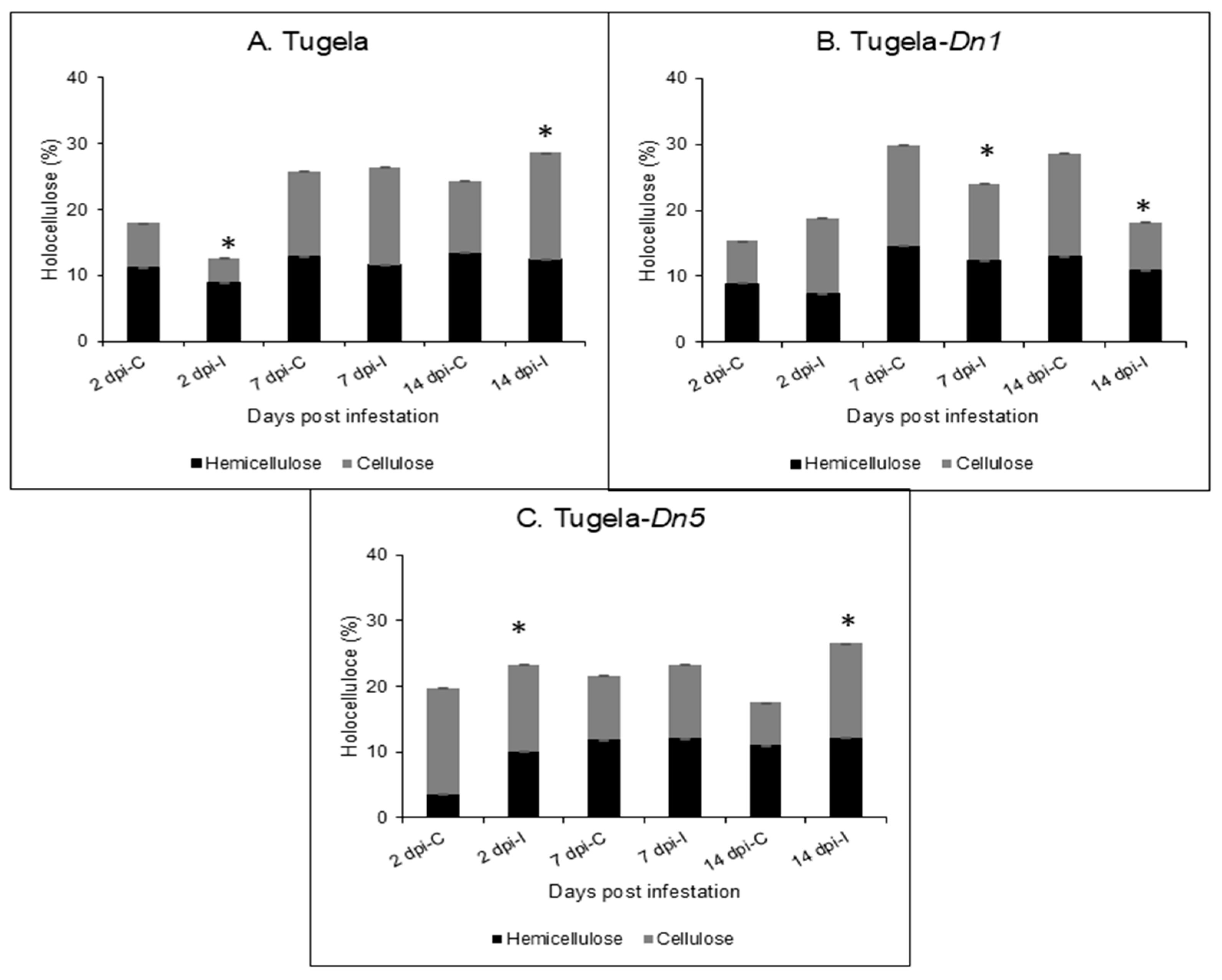
2.2. Total Holocellulose Determination
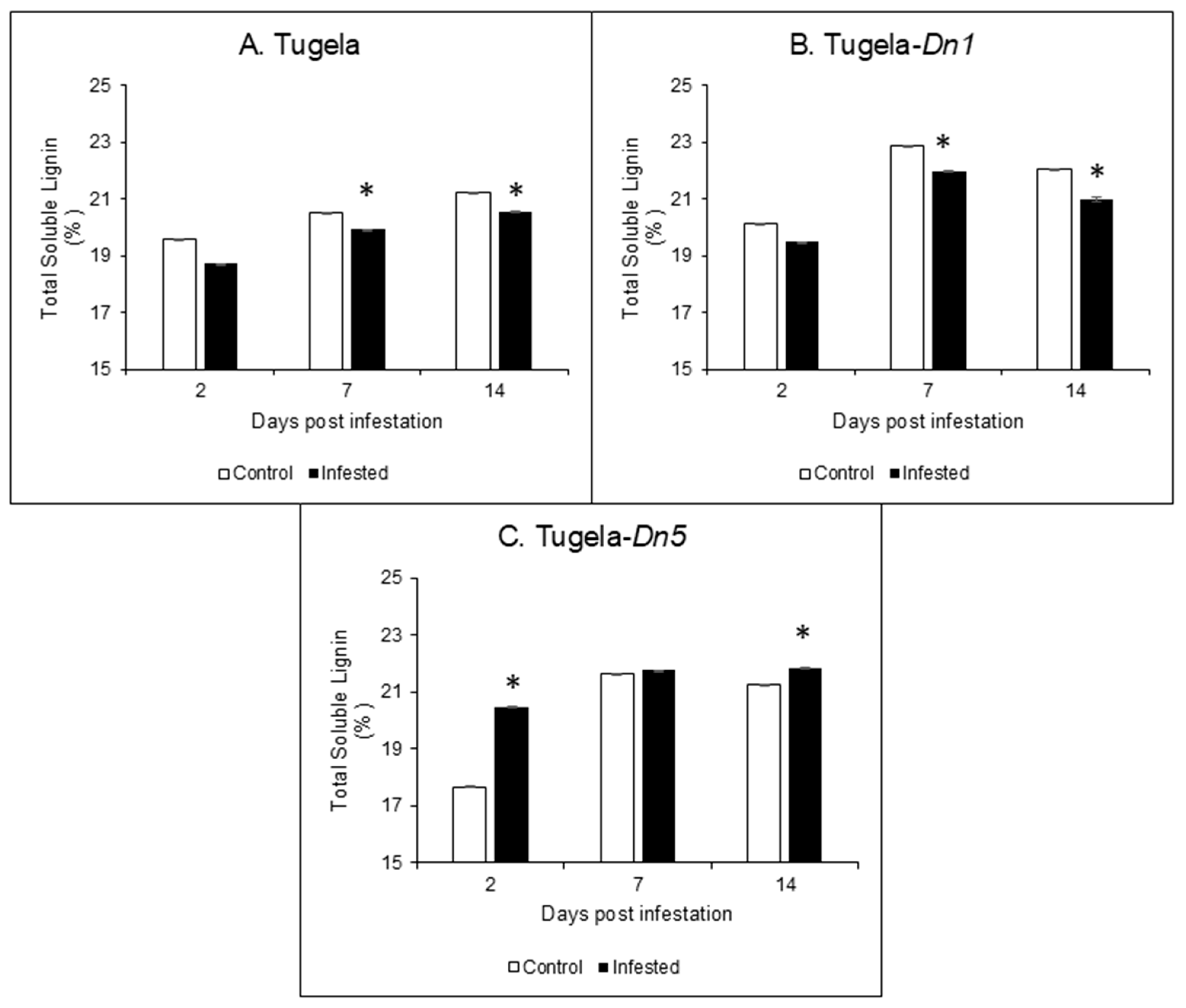
2.3. Acid-Soluble Lignin Content
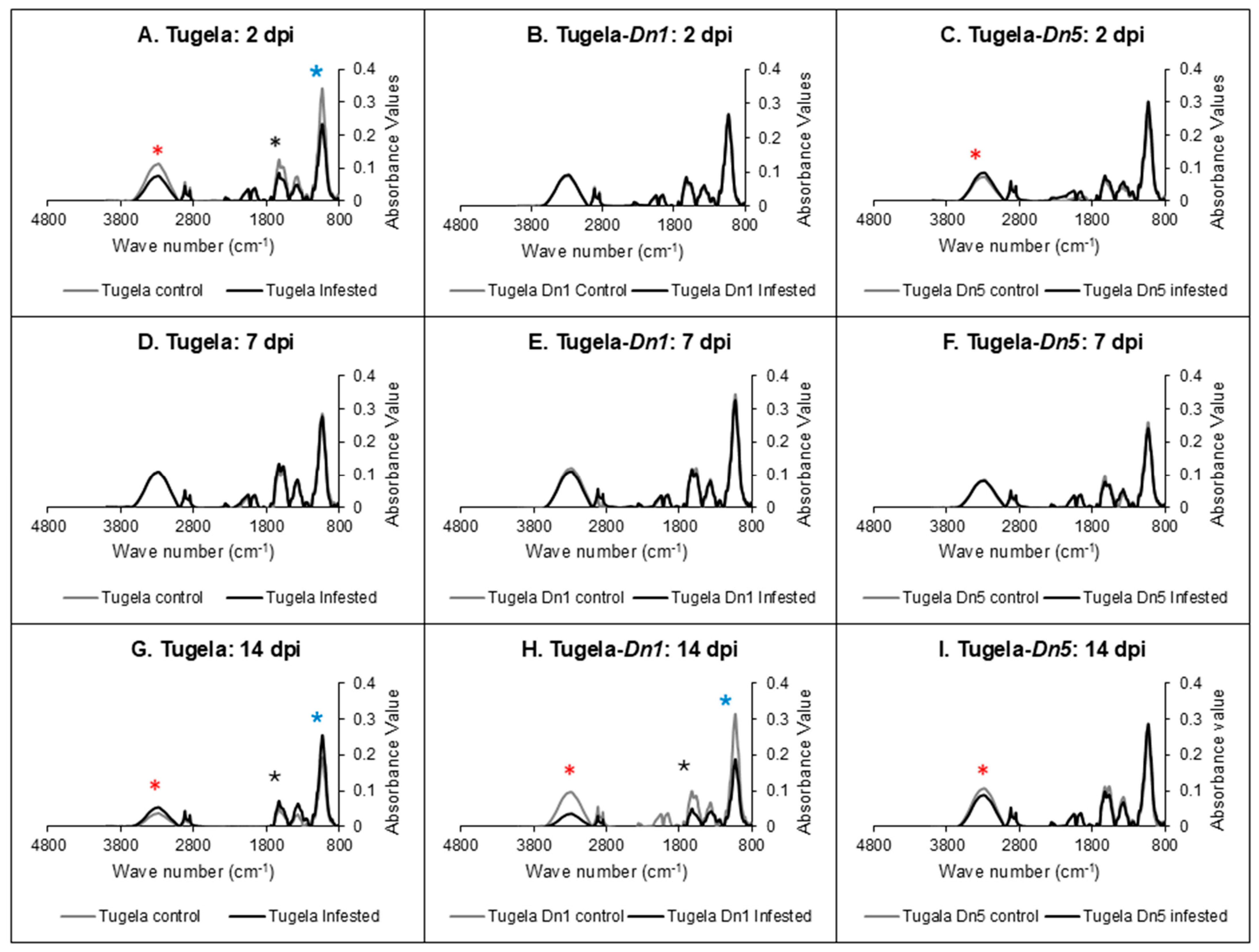
2.4. Fourier-Transform Infrared (FTIR) Spectroscopy Analysis
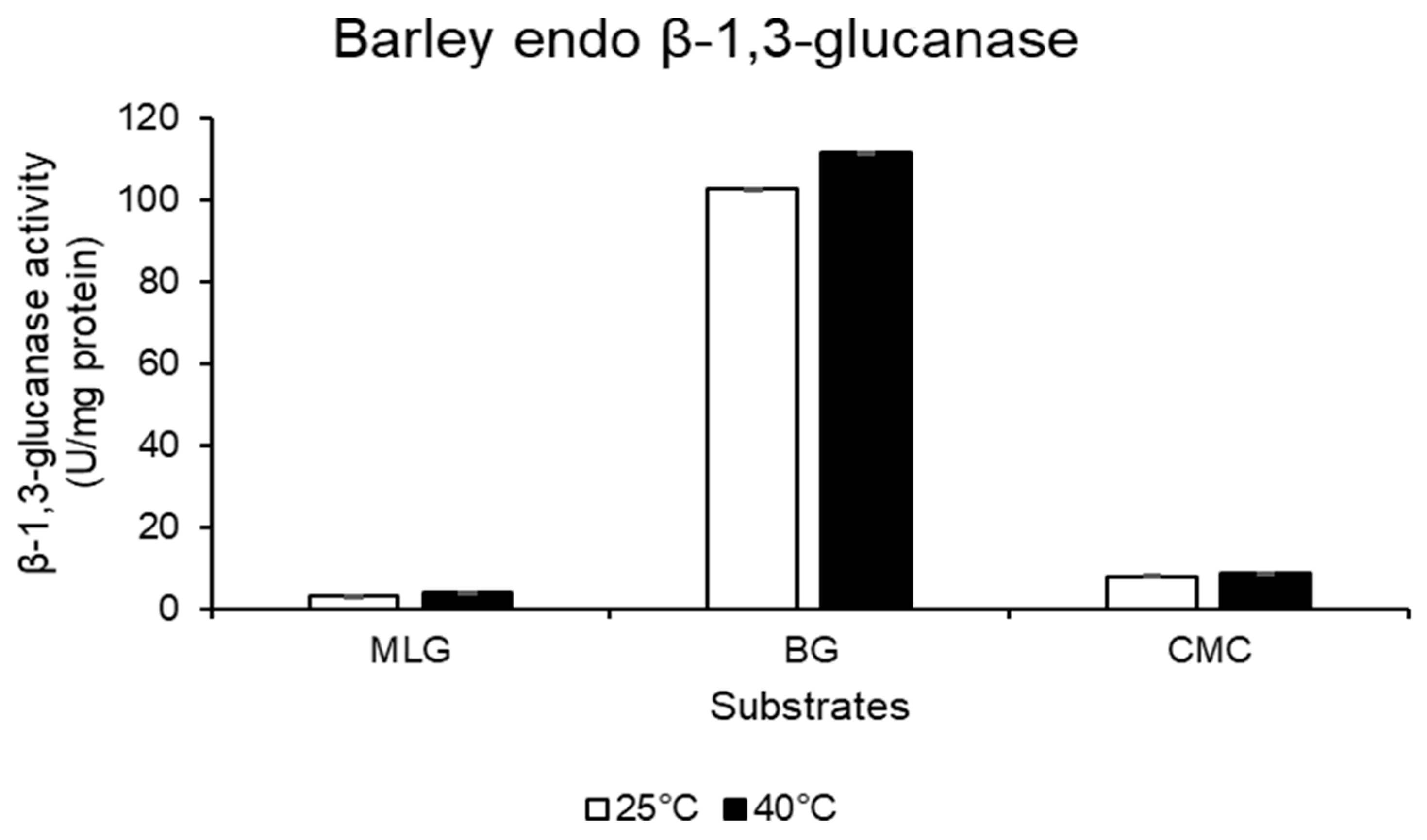
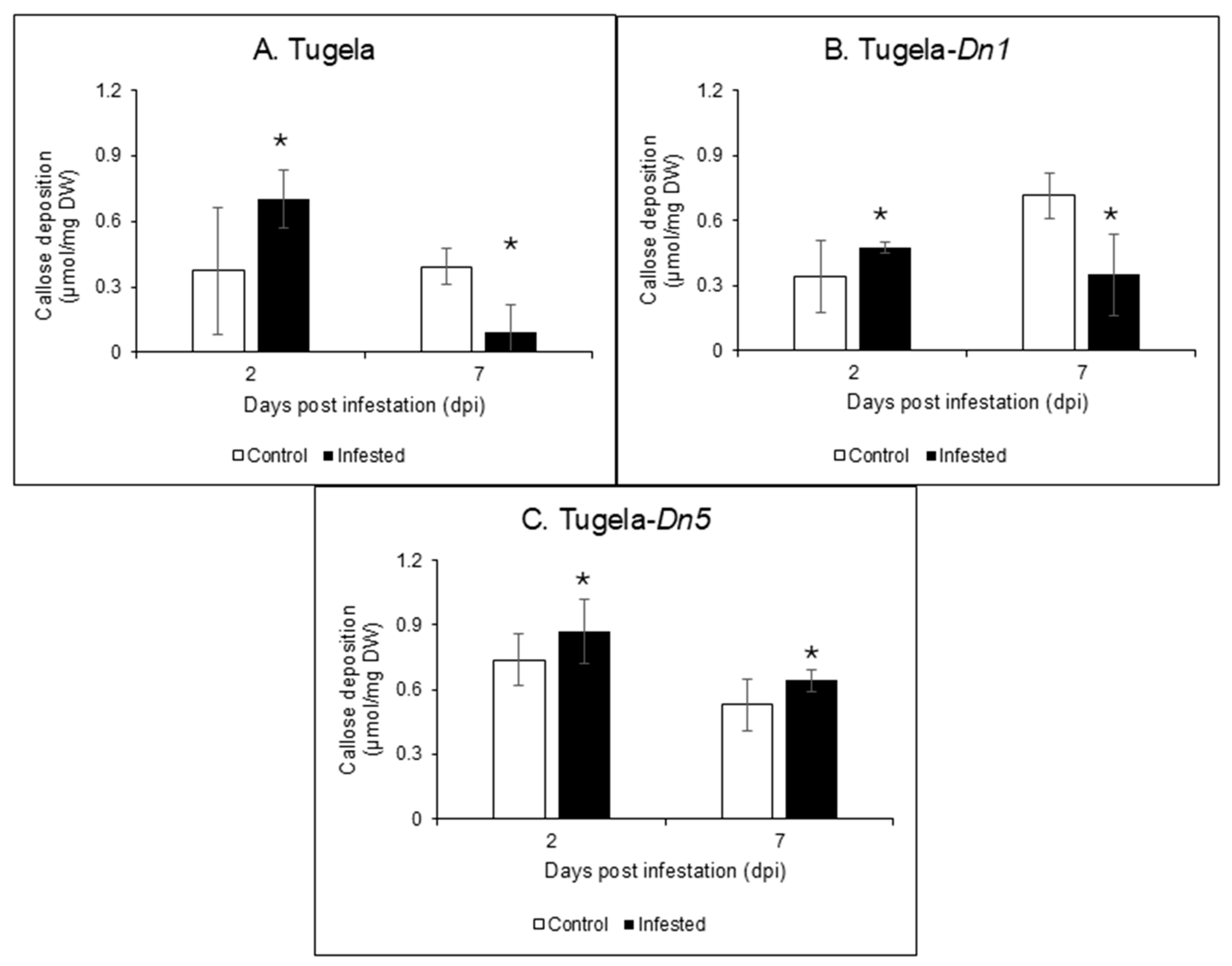
2.5. Callose Quantification in the RWASA2-Infested Wheat Cultivars
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Plant Growth Conditions
4.3. Russian Wheat Aphid (RWASA2) Infestation
4.4. Total Carbohydrate Determination
Hemicellulose, Cellulose and Lignin Content Quantification
4.5. Fourier-Transform Infrared (FTIR) Spectroscopy Analysis of the Cell Wall Samples
4.6. Callose Quantification in RWASA2-Infested Wheat Samples
4.7. Data Collection and Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| ARC-SG | Agricultural Research Council–Small Grain Institute |
| cm−1 | per centimetre |
| CWDE | Cell wall degrading enzymes |
| Dn | Diuraphis noxia resistance gene |
| DNS | 3,5-Dinitrosalicylic acid reagent |
| dpi | Days post infestation |
| FTIR | Fourier Transform Infrared Spectroscopy |
| GH | Glycoside hydrolases |
| H | Hours |
| min | Minutes |
| NPP | Non-penetration papillae |
| NREL | National Renewable Energy Laboratory |
| OH | Hydroxylic group |
| PP | Penetration papillae |
| RWA | Russian wheat aphids |
| RWASA2 | Russian wheat aphid South African biotype |
References
- Vaahtera, L.; Schulz, J.; Hamann, T. Cell wall integrity maintenance during plant development and interaction with the environment. Nat. Plants 2019, 5, 924–932. [Google Scholar] [CrossRef] [PubMed]
- Moradi Tarnabi, Z.; Iranbakhsh, A.; Mehregan, I.; Ahmadvand, R. Impact of arbuscular mycorrhizal fungi (AMF) on gene expression of some cell wall and membrane elements of wheat (Triticum aestivum L.) under water deficit using transcriptome analysis. Physiol. Mol. Biol. Plants 2020, 26, 143–162. [Google Scholar] [CrossRef] [PubMed]
- Mafa, M.S.; Lebusa, N.; Gumani, T.F.; Kemp, G.; Visser, B.; Boshoff, W.H.; Castelyn, H.D. Accumulation of complex oligosaccharides and CAZymes activity under acid conditions constitute the Thatcher+Lr9 defence responses to Puccinia triticina. Biologia 2023, 78, 1929–1941. [Google Scholar] [CrossRef]
- Van Dyk, J.S.; Pletschke, B. A review of lignocellulose bioconversion using enzymatic hydrolysis and synergistic cooperation between enzymes-factors affecting enzymes, conversion and synergy. Biotechnol. Adv. 2012, 30, 1458–1480. [Google Scholar] [CrossRef]
- Mafa, M.S.; Rufetu, E.; Alexander, O.; Kemp, G.; Mohase, L. Cell-wall structural carbohydrates reinforcements are part of the defence mechanisms of wheat against Russian wheat aphid (Diuraphis noxia) infestation. Plant Physiol. Biochem. 2022, 179, 168–178. [Google Scholar] [CrossRef]
- Mafa, M.S.; Visser, B.; Boshoff, W.H.; Kemp, G.; Alexander, O.; Castelyn, H.D. Flagging defensive roles of carbohydrate-active enzymes (CAZymes) and carbohydrates during Puccinia triticina-wheat interactions. Physiol. Mol. Plant Pathol. 2023, 124, 101947. [Google Scholar] [CrossRef]
- Botha, C.E.; Matsiliza, B. Reduction in transport in wheat (Triticum aestivum) is caused by sustained phloem feeding by the Russian wheat aphid (Diuraphis noxia). S. Afr. J. Bot. 2004, 70, 249–254. [Google Scholar] [CrossRef]
- Saheed, S.A.; Cierlik, I.; Larsson, K.A.; Delp, G.; Bradley, G.; Jonsson, L.M.; Botha, C.E. Stronger induction of callose deposition in barley by Russian wheat aphid than bird cherry-oat aphid is not associated with differences in callose synthase or β-1,3-glucanase transcript abundance. Physiol. Plant 2009, 135, 150–161. [Google Scholar] [CrossRef]
- Lapitan, N.L.; Li, Y.C.; Peng, J.; Botha, A.M. Fractionated extracts of Russian wheat aphid eliciting defence responses in wheat. J. Econ. Entomol. 2007, 100, 990–999. [Google Scholar] [CrossRef]
- Walker, G.P. Sieve element occlusion: Interaction with phloem sap-feeding insects. A review. J. Plant Physiol. 2022, 269, 153582. [Google Scholar] [CrossRef]
- Mohase, L.; Van der Westhuizen, A.J. Glycoproteins from Russian wheat aphid infested wheat induce defence responses. Z. Naturforschung C. 2002, 57, 867–873. [Google Scholar]
- Botha, A.-M.; Li, Y.; Lapitan, N.L.V. Cereal host interactions with Russian wheat aphid: A review. J. Plant Interact. 2005, 1, 211–222. [Google Scholar] [CrossRef]
- Anderson, V.A.; Haley, S.D.; Peairs, F.B.; Van Eck, L.; Leach, J.E.; Lapitan, N.L. Virus-induced gene silencing suggests (1, 3; 1, 4)-β-glucanase is a susceptibility factor in the compatible Russian wheat aphid–wheat interaction. Mol. Plant-Microbe Interact. 2014, 27, 913–922. [Google Scholar] [CrossRef]
- Østergaard, L.; Teilum, K.; Mirza, O.; Mattsson, O.; Petersen, M.; Welinder, K.G.; Mundy, J.; Gajhede, M.; Henriksen, A. Arabidopsis ATP A2 peroxidase. Expression and high-resolution structure of a plant peroxidase with implications for lignification. Plant Mol. Biol. 2000, 44, 231–243. [Google Scholar] [CrossRef]
- Chowdhury, J.; Henderson, M.; Schweizer, P.; Burton, R.A.; Fincher, G.B.; Little, A. Different accumulation of callose, arabinoxylan and cellulose in nonpenetrated versus penetrated papillae on leaves of barley infected with Blumeria graminis f. sp. hordei. New Phytol. 2014, 204, 650–660. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Fan, B.; Zhu, C.; Chen, Z. Regulation and function of defense-related callose deposition in plants. Int. J. Mol. Sci. 2021, 22, 2393. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, L.; Khan, A.; Zhao, R.; Wei, S.; Jing, X. Fermented wheat bran by xylanase-producing Bacillus cereus boosts the intestinal microflora of broiler chickens. Poult. Sci. 2020, 99, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Mnich, E.; Bjarnholt, N.; Eudes, A.; Harholt, J.; Holland, C.; Jørgensen, B.; Larsen, F.H.; Liu, M.; Manat, R.; Meyer, A.S.; et al. Phenolic cross-links: Building and de-constructing the plant cell wall. Nat. Prod. Rep. 2020, 37, 919–961. [Google Scholar] [CrossRef]
- Meihls, L.N.; Handrick, V.; Glauser, G.; Barbier, H.; Kaur, H.; Haribal, M.M.; Lipka, A.E.; Gershenzon, J.; Buckler, E.S.; Erb, M.; et al. Natural variation in Maize aphid resistance is associated with 2,4-dihydroxy-7-methoxy-1,4-benzoxazin-3-one glucoside methyltransferase activity. Plant Cell 2013, 25, 2341–2355. [Google Scholar] [CrossRef] [PubMed]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D.L.A.P. Determination of structural carbohydrates and lignin in biomass. Lab. Anal. Proc. 2008, 1617, 1–16. [Google Scholar]
- Miedes, E.; Vanholme, R.; Boerjan, W.; Molina, A. The role of the secondary cell wall in plant resistance to pathogens. Front. Plant Sci. 2014, 5, 358. [Google Scholar] [CrossRef]
- Silva-Sanzana, C.; Estevez, J.M.; Blanco-Herrera, F. Influence of cell wall polymers and their modifying enzymes during plant–aphid interactions. J. Exp. Bot. 2020, 71, 3854–3864. [Google Scholar] [CrossRef] [PubMed]
- Tilsner, J.; Nicolas, W.; Rosado, A.; Bayer, E.M. Staying tight: Plasmodesmal membrane contact sites and the control of cell-to-cell connectivity in plants. Annu. Rev. Plant Biol. 2016, 67, 337–364. [Google Scholar] [CrossRef]
- Houston, K.; Tucker, M.R.; Chowdhury, J.; Shirley, N.; Little, A. The plant cell wall: A complex and dynamic structure as revealed by the responses of genes under stress conditions. Front. Plant Sci. 2016, 7, 205693. [Google Scholar] [CrossRef]
- Zondo, S.N.; Mohase, L.; Tolmay, V.L.; Mafa, M.S. Functional characterisation of cell wall-associated β-glucanases and peroxidase induced during wheat-Diuraphis noxia interactions. Biologia 2024, 79, 2873–2890. [Google Scholar] [CrossRef]
- Zhang, Y.H.P.; Lynd, L.R. Toward an aggregated understanding of enzymatic hydrolysis of cellulose: Noncomplexed cellulase systems. Biotechnol. Bioeng. 2004, 88, 797–824. [Google Scholar] [CrossRef]
- Moloi, M.J.; Van der Westhuizen, A.J. The reactive oxygen species are involved in resistance responses of wheat to the Russian wheat aphid. J. Plant Physiol. 2005, 163, 1118–1125. [Google Scholar] [CrossRef]
- Mohase, L.; Taiwe, B. Saliva fractions from South African Russian wheat aphid biotypes induce differential defence responses in wheat. S. Afr. J. Plant Soil 2015, 32, 235–240. [Google Scholar] [CrossRef]
- Huckelhoven, R. The effective papillae hypothesis. New Phytol. 2014, 204, 438–440. [Google Scholar] [CrossRef]
- Voigt, C.A. Callose-mediated resistance to pathogenic intruders in plant defence-related papillae. Front. Plant Sci. 2014, 5, 86993. [Google Scholar] [CrossRef]
- Villada, E.S.; González, E.G.; López-Sesé, A.I.; Castiel, A.F.; Gómez-Guillamón, M.L. Hypersensitive response to Aphis gossypii Glover in melon genotypes carrying the Vat gene. J. Exp. Bot. 2009, 60, 3269–3277. [Google Scholar] [CrossRef] [PubMed]
- Jankielsohn, A. Changes in the Russian wheat aphid (Hemiptera: Aphididae) biotype complex in South Africa. J. Econ. Entomol. 2016, 109, 907–912. [Google Scholar] [CrossRef]
- Zhao, D.; MacKown, C.T.; Starks, P.J.; Kindiger, B.K. Rapid analysis of non-structural carbohydrate components in grass forage using microplate enzymatic assays. Crop Sci. 2010, 50, 1537–1545. [Google Scholar] [CrossRef]
- Gufe, C.; Thantsha, M.S.; Malgas, S. Recovery of xylan from Acacia mearnsii using ultrasound-assisted alkaline extraction. Biofuels Bioprod. Biorefining 2023, 17, 976–987. [Google Scholar] [CrossRef]
- Palliprath, S.; Poolakkalody, N.J.; Ramesh, K.; Manisseri, C. Lignocellulosic content and biofuel potential of post-harvest sugarcane leaves from commonly cultivated Indian varieties. Sci. Technol. J. 2020, 8, 15–23. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zondo, S.N.N.; Mohase, L.; Tolmay, V.; Mafa, M. Simultaneous Accumulation of Holocellulose, Callose and Lignin: Cell Wall Markers for Resistance in Wheat Infested with Diuraphis noxia. Int. J. Mol. Sci. 2025, 26, 9874. https://doi.org/10.3390/ijms26209874
Zondo SNN, Mohase L, Tolmay V, Mafa M. Simultaneous Accumulation of Holocellulose, Callose and Lignin: Cell Wall Markers for Resistance in Wheat Infested with Diuraphis noxia. International Journal of Molecular Sciences. 2025; 26(20):9874. https://doi.org/10.3390/ijms26209874
Chicago/Turabian StyleZondo, Siphephelo N. N., Lintle Mohase, Vicki Tolmay, and Mpho Mafa. 2025. "Simultaneous Accumulation of Holocellulose, Callose and Lignin: Cell Wall Markers for Resistance in Wheat Infested with Diuraphis noxia" International Journal of Molecular Sciences 26, no. 20: 9874. https://doi.org/10.3390/ijms26209874
APA StyleZondo, S. N. N., Mohase, L., Tolmay, V., & Mafa, M. (2025). Simultaneous Accumulation of Holocellulose, Callose and Lignin: Cell Wall Markers for Resistance in Wheat Infested with Diuraphis noxia. International Journal of Molecular Sciences, 26(20), 9874. https://doi.org/10.3390/ijms26209874






