Inhibiting Fatty Acid Oxidation Suppresses Acquired Resistance to Standard Chemotherapy in Melanoma
Abstract
1. Introduction
2. Results
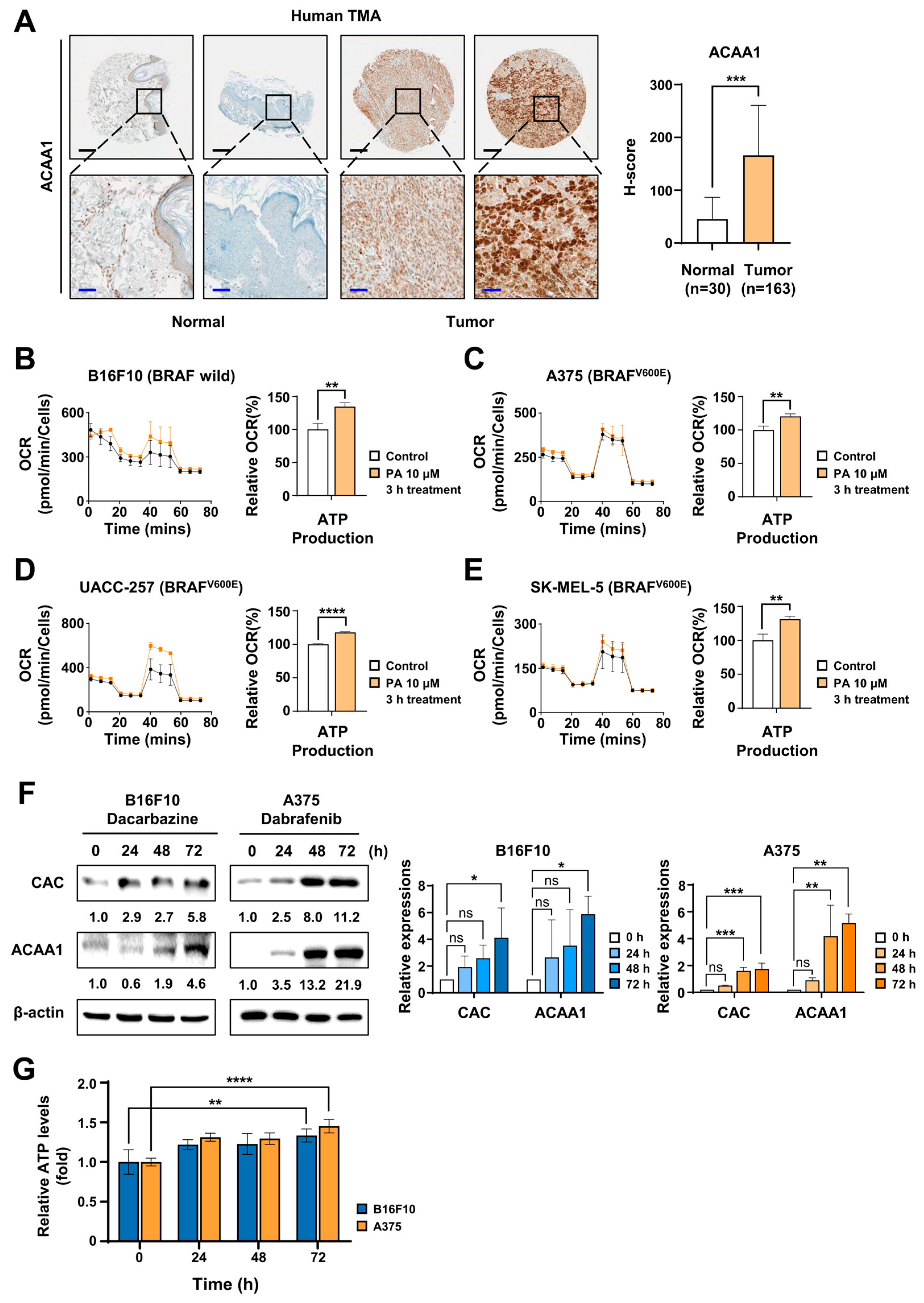
2.1. FAO Was Activated in Melanoma and Was Enhanced by Chemotherapy Treatment
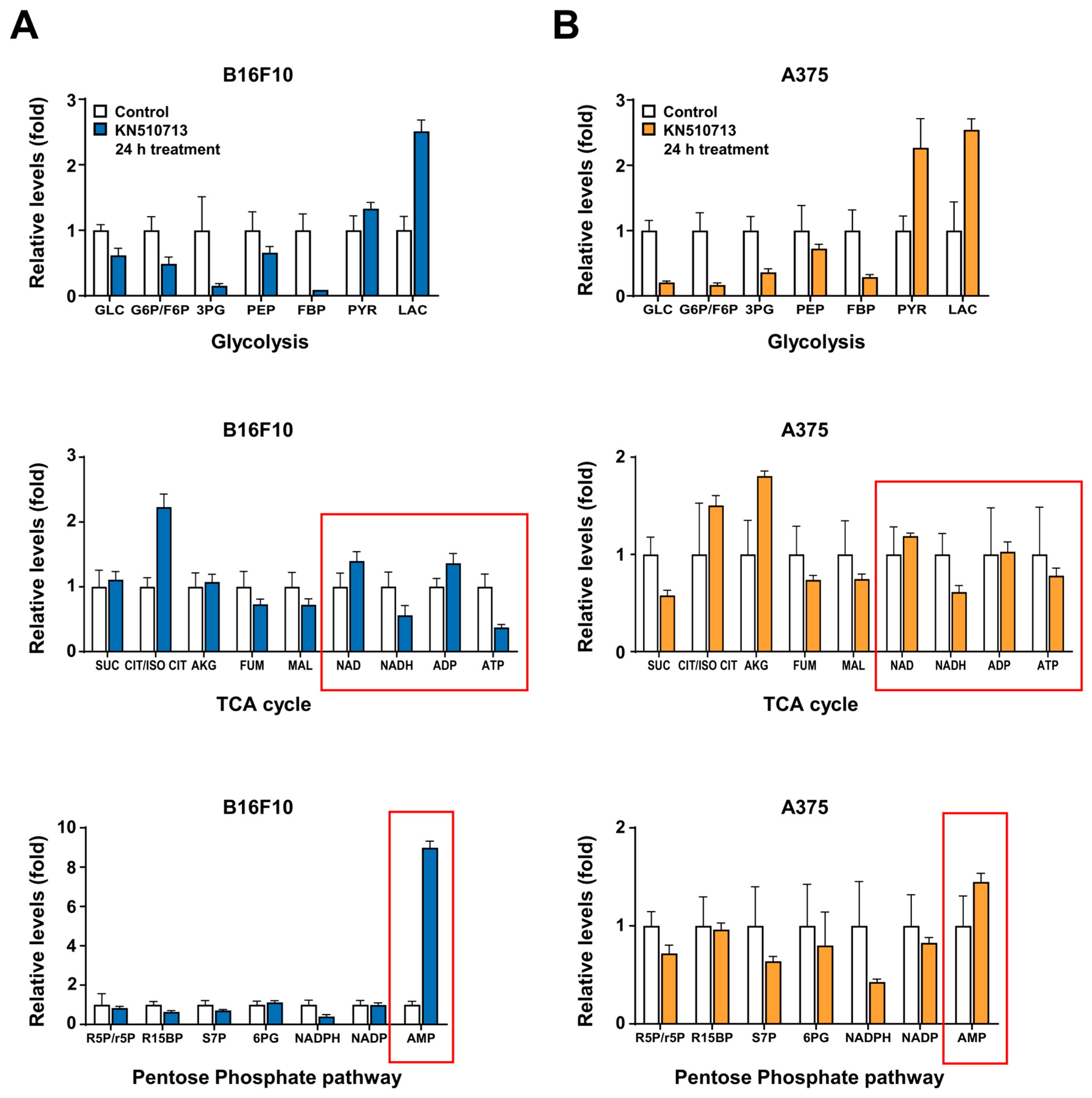
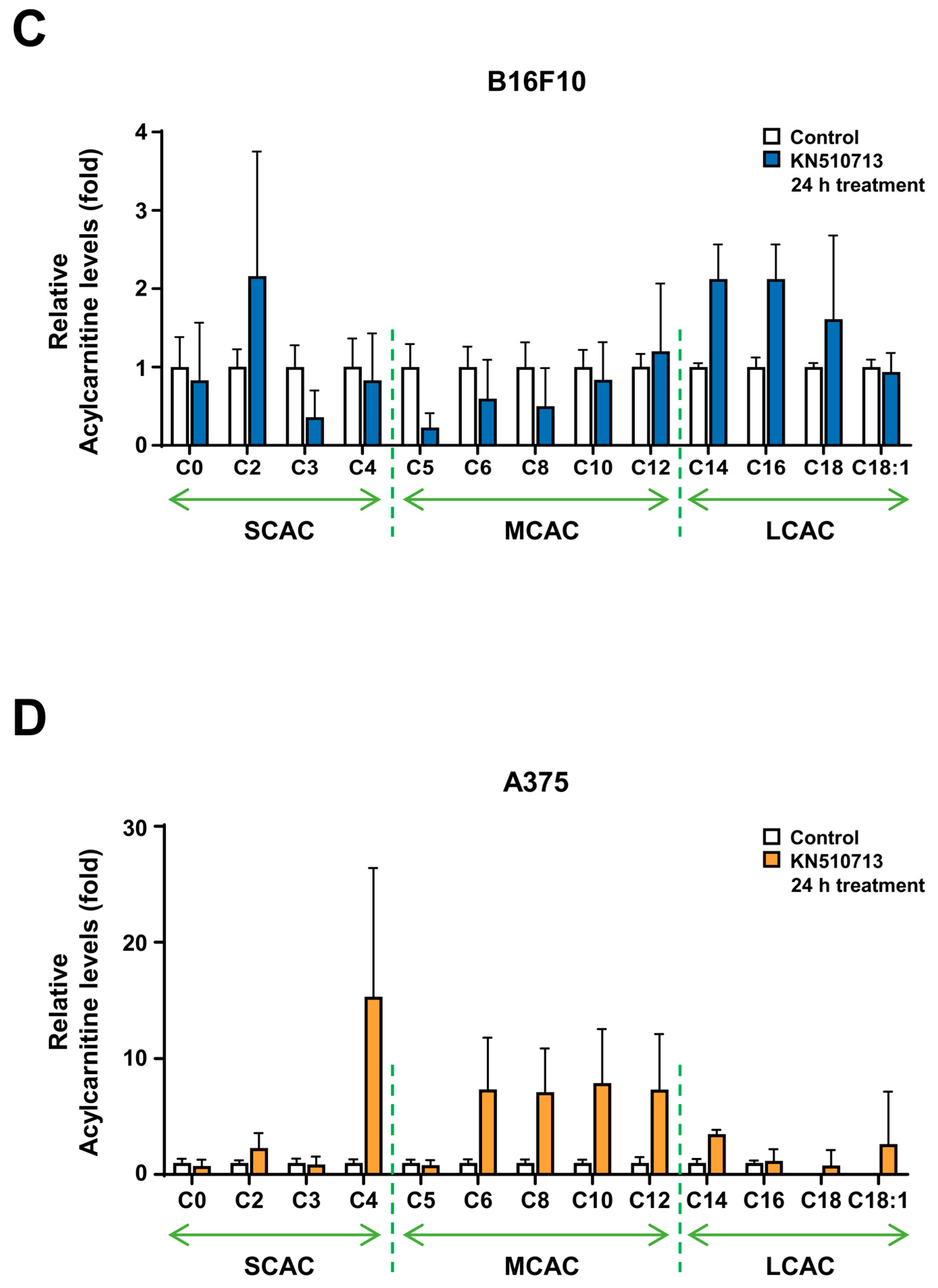
2.2. FAO Inhibition Reduced the Reduction of Energy Cofactors
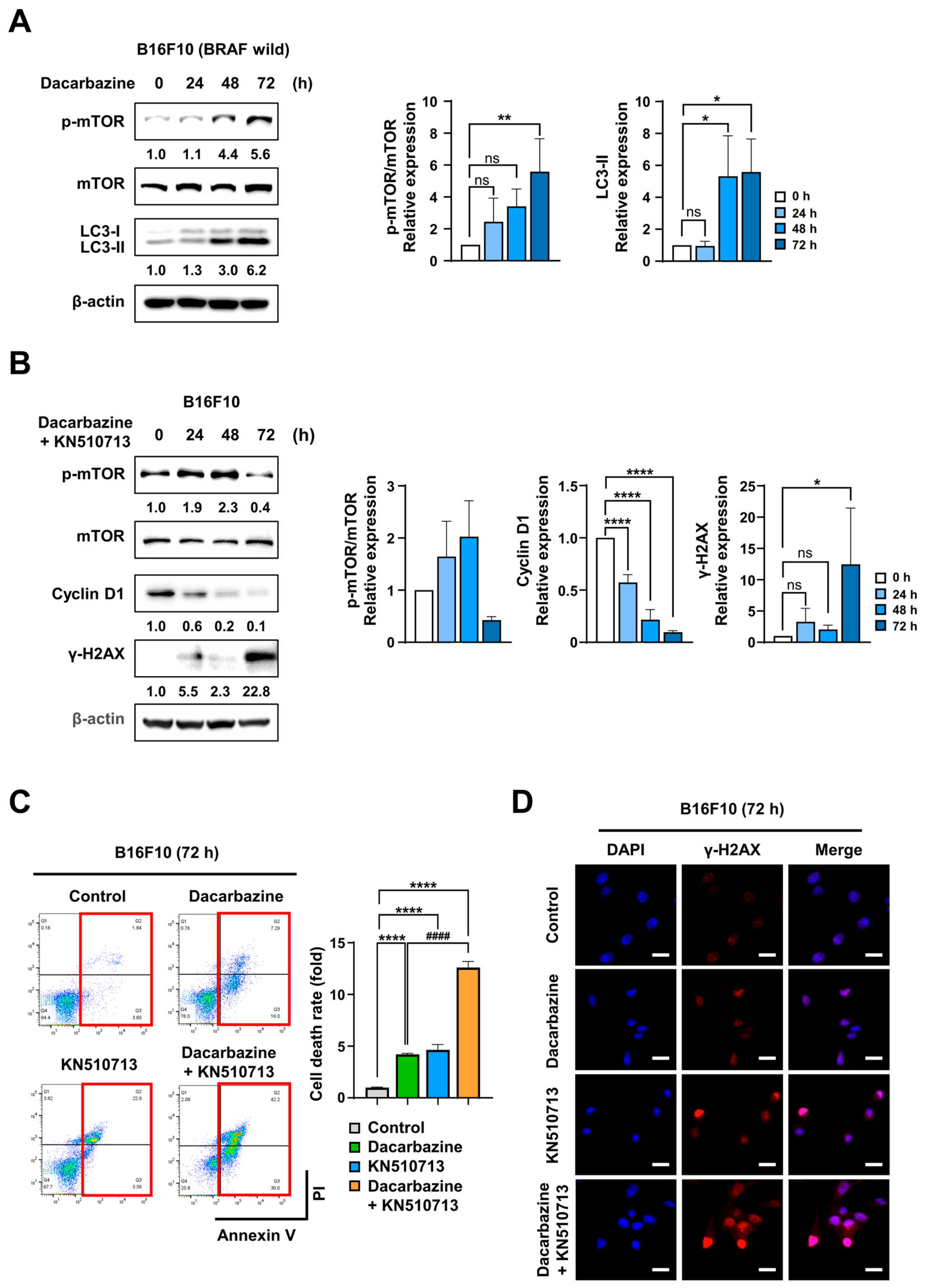
2.3. FAO Inhibition Induced Synergistic Cell Death with Dacarbazine in Melanoma with BRAF Wild Type
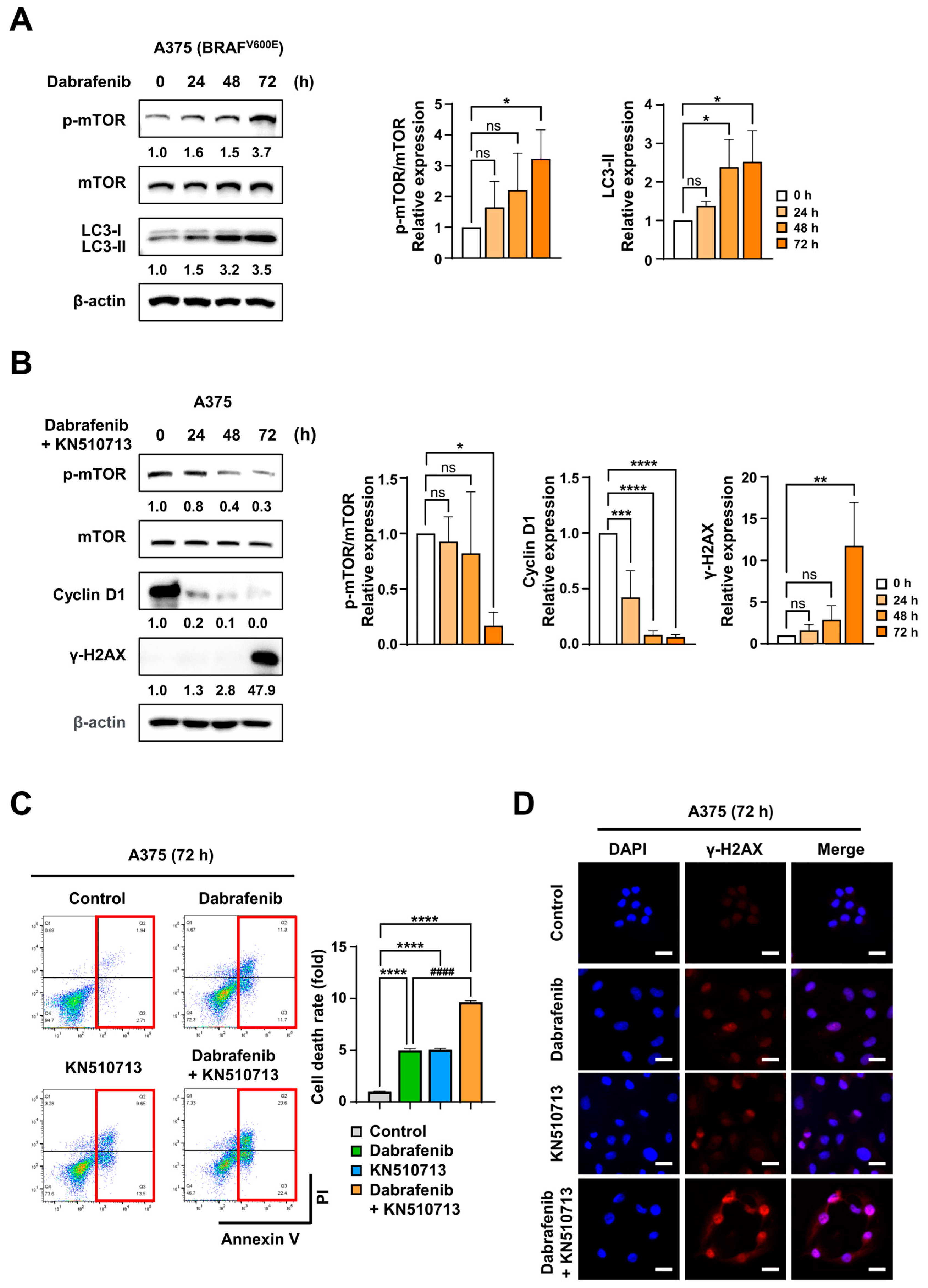
2.4. FAO Inhibition Induced Synergistic Cell Death with Dabrafenib in Melanoma with BRAFV600E
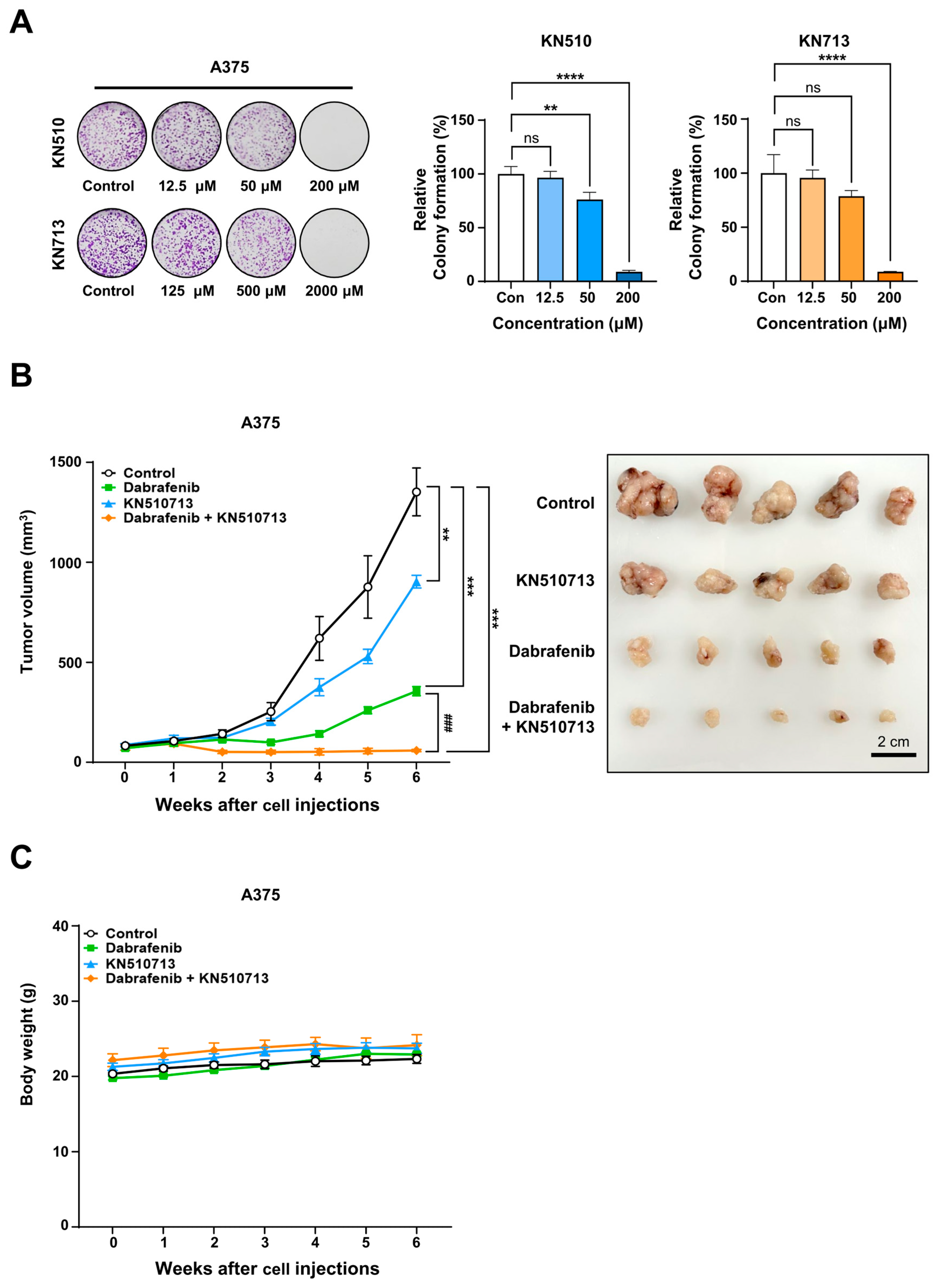
2.5. FAO Inhibition Induced Synergistic Cell Death with Dacarbazine in Xenograft Using Braf Wild-Type Melanoma
2.6. FAO Inhibition Induced Synergistic Cell Death with Dabrafenib in Xenograft Models Using Melanoma with BRAFV600E

3. Discussion
4. Materials and Methods
4.1. Immunohistochemical Staining
4.2. Image Acquisition and Analysis
4.3. Gene Expression Analysis (GEPIA2)
4.4. Cell Culture and Transfection
4.5. Oxygen Consumption Rate (OCR) Analysis
4.6. Preparation of BSA-Conjugated Palmitic Acid
4.7. Liquid Chromatography–Tandem Mass Spectrometry (LC-MS/MS)
4.8. Immunoblotting
4.9. Measurement of ATP Levels
4.10. Apoptosis Analysis
4.11. Immunofluorescence
4.12. Colony Formation Assay
4.13. Acetyl-CoA Assay
4.14. Allograft and Xenograft Models
4.15. Statistical Analysis
4.16. Ethics Statement
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FAO | fatty acid oxidation |
| ACAA1/2 | acetyl-Coenzyme A acyltransferase 1/2 |
| CAC | carnitine acyl-carnitine carrier, carnitine acyl-carnitine translocase |
| FACS | fluorescence-activated cell sorting |
References
- Wolchok, J.D.; Chiarion-Sileni, V.; Rutkowski, P.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Queirolo, P.; Dummer, R.; Butler, M.O.; Hill, A.G.; et al. Final, 10-Year Outcomes with Nivolumab plus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2025, 392, 11–22. [Google Scholar] [CrossRef]
- Robert, C.; Schachter, J.; Long, G.V.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2015, 372, 2521–2532. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Grob, J.J.; Stroyakovskiy, D.; Karaszewska, B.; Hauschild, A.; Levchenko, E.; Chiarion Sileni, V.; Schachter, J.; Garbe, C.; Bondarenko, I.; et al. Five-Year Outcomes with Dabrafenib plus Trametinib in Metastatic Melanoma. N. Engl. J. Med. 2019, 381, 626–636. [Google Scholar] [CrossRef]
- Robert, C.; Thomas, L.; Bondarenko, I.; O’Day, S.; Weber, J.; Garbe, C.; Lebbe, C.; Baurain, J.F.; Testori, A.; Grob, J.J.; et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N. Engl. J. Med. 2011, 364, 2517–2526. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Long, G.V.; Robert, C.; Brady, B.; Dutriaux, C.; Di Giacomo, A.M.; Mortier, L.; Hassel, J.C.; Rutkowski, P.; McNeil, C.; et al. Survival Outcomes in Patients with Previously Untreated BRAF Wild-Type Advanced Melanoma Treated with Nivolumab Therapy: Three-Year Follow-up of a Randomized Phase 3 Trial. JAMA Oncol. 2019, 5, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.Y.; Hsu, S.K.; Liu, T.Y.; Wu, C.Y.; Chiu, C.C. Melanoma biology and treatment: A review of novel regulated cell death-based approaches. Cancer Cell Int. 2024, 24, 63. [Google Scholar] [CrossRef]
- Garbe, C.; Amaral, T.; Peris, K.; Hauschild, A.; Arenberger, P.; Basset-Seguin, N.; Bastholt, L.; Bataille, V.; Del Marmol, V.; Dreno, B.; et al. European consensus-based interdisciplinary guideline for melanoma. Part 2: Treatment-Update 2022. Eur. J. Cancer 2022, 170, 256–284. [Google Scholar] [CrossRef] [PubMed]
- McDermott, D.; Haanen, J.; Chen, T.T.; Lorigan, P.; O’Day, S.; MDX010-20 Investigators. Efficacy and safety of ipilimumab in metastatic melanoma patients surviving more than 2 years following treatment in a phase III trial (MDX010-20). Ann. Oncol. 2013, 24, 2694–2698. [Google Scholar] [CrossRef]
- Atkins, M.B.; Lee, S.J.; Chmielowski, B.; Tarhini, A.A.; Cohen, G.I.; Truong, T.G.; Moon, H.H.; Davar, D.; O’Rourke, M.; Stephenson, J.J.; et al. Combination Dabrafenib and Trametinib Versus Combination Nivolumab and Ipilimumab for Patients with Advanced BRAF-Mutant Melanoma: The DREAMseq Trial-ECOG-ACRIN EA6134. J. Clin. Oncol. 2023, 41, 186–197. [Google Scholar] [CrossRef]
- Sosman, J.A.; Kim, K.B.; Schuchter, L.; Gonzalez, R.; Pavlick, A.C.; Weber, J.S.; McArthur, G.A.; Hutson, T.E.; Moschos, S.J.; Flaherty, K.T.; et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N. Engl. J. Med. 2012, 366, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Karaszewska, B.; Schachter, J.; Rutkowski, P.; Mackiewicz, A.; Stroiakovski, D.; Lichinitser, M.; Dummer, R.; Grange, F.; Mortier, L.; et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N. Engl. J. Med. 2015, 372, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Menzies, A.M.; Long, G.V. Systemic treatment for BRAF-mutant melanoma: Where do we go next? Lancet Oncol. 2014, 15, e371–e381. [Google Scholar] [CrossRef] [PubMed]
- Chapman, P.B.; Robert, C.; Larkin, J.; Haanen, J.B.; Ribas, A.; Hogg, D.; Hamid, O.; Ascierto, P.A.; Testori, A.; Lorigan, P.C.; et al. Vemurafenib in patients with BRAFV600 mutation-positive metastatic melanoma: Final overall survival results of the randomized BRIM-3 study. Ann. Oncol. 2017, 28, 2581–2587. [Google Scholar] [CrossRef] [PubMed]
- Bouchereau, S.; Chaplain, L.; Fort, M.; Beauchet, A.; Sidibe, T.; Chapalain, M.; Gonzalez-Lara, L.; Longvert, C.; Blom, A.; Saiag, P.; et al. Impact of prior treatment with immune checkpoint inhibitors on dacarbazine efficacy in metastatic melanoma. Br. J. Cancer 2021, 125, 948–954. [Google Scholar] [CrossRef]
- Marchesi, F.; Turriziani, M.; Tortorelli, G.; Avvisati, G.; Torino, F.; De Vecchis, L. Triazene compounds: Mechanism of action and related DNA repair systems. Pharmacol. Res. 2007, 56, 275–287. [Google Scholar] [CrossRef]
- Middleton, M.R.; Grob, J.J.; Aaronson, N.; Fierlbeck, G.; Tilgen, W.; Seiter, S.; Gore, M.; Aamdal, S.; Cebon, J.; Coates, A.; et al. Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J. Clin. Oncol. 2000, 18, 158–166. [Google Scholar] [CrossRef]
- Pham, J.P.; Joshua, A.M.; da Silva, I.P.; Dummer, R.; Goldinger, S.M. Chemotherapy in Cutaneous Melanoma: Is There Still a Role? Curr. Oncol. Rep. 2023, 25, 609–621. [Google Scholar] [CrossRef]
- Lee, J.; Chang, J.S.; Roh, M.R.; Jung, M.; Lee, C.K.; Oh, B.H.; Chung, K.Y.; Koom, W.S.; Shin, S.J. Clinical Outcomes of Immune Checkpoint Blocker Therapy for Malignant Melanoma in Korean Patients: Potential Clinical Implications for a Combination Strategy Involving Radiotherapy. Cancer Res. Treat. 2020, 52, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Debnath, J.; Gammoh, N.; Ryan, K.M. Autophagy and autophagy-related pathways in cancer. Nat. Rev. Mol. Cell Biol. 2023, 24, 560–575. [Google Scholar] [CrossRef]
- Fratta, E.; Giurato, G.; Guerrieri, R.; Colizzi, F.; Dal Col, J.; Weisz, A.; Steffan, A.; Montico, B. Autophagy in BRAF-mutant cutaneous melanoma: Recent advances and therapeutic perspective. Cell Death Discov. 2023, 9, 202. [Google Scholar] [CrossRef]
- Ma, X.H.; Piao, S.; Wang, D.; McAfee, Q.W.; Nathanson, K.L.; Lum, J.J.; Li, L.Z.; Amaravadi, R.K. Measurements of tumor cell autophagy predict invasiveness, resistance to chemotherapy, and survival in melanoma. Clin. Cancer Res. 2011, 17, 3478–3489. [Google Scholar] [CrossRef] [PubMed]
- Lazova, R.; Camp, R.L.; Klump, V.; Siddiqui, S.F.; Amaravadi, R.K.; Pawelek, J.M. Punctate LC3B expression is a common feature of solid tumors and associated with proliferation, metastasis, and poor outcome. Clin. Cancer Res. 2012, 18, 370–379. [Google Scholar] [CrossRef]
- Ma, X.H.; Piao, S.F.; Dey, S.; McAfee, Q.; Karakousis, G.; Villanueva, J.; Hart, L.S.; Levi, S.; Hu, J.; Zhang, G.; et al. Targeting ER stress-induced autophagy overcomes BRAF inhibitor resistance in melanoma. J. Clin. Investig. 2014, 124, 1406–1417. [Google Scholar] [CrossRef] [PubMed]
- Amaravadi, R.K. Clinical trial results show promise of targeting autophagy BRAF mutant melanoma. Autophagy 2022, 18, 1470–1471. [Google Scholar] [CrossRef]
- Mehnert, J.M.; Mitchell, T.C.; Huang, A.C.; Aleman, T.S.; Kim, B.J.; Schuchter, L.M.; Linette, G.P.; Karakousis, G.C.; Mitnick, S.; Giles, L.; et al. BAMM (BRAF Autophagy and MEK Inhibition in Melanoma): A Phase I/II Trial of Dabrafenib, Trametinib, and Hydroxychloroquine in Advanced BRAFV600-mutant Melanoma. Clin. Cancer Res. 2022, 28, 1098–1106. [Google Scholar] [CrossRef]
- Ghanbari, A.; Jalili, C.; Rashidi, I.; Toranj, H.; Raissi, F.; Mazini, F.; Akhshi, N. Pogostone Effect on Dacarbazine-Induced Autophagy and Apoptosis in Human Melanoma Cells. World Cancer Res. J. 2023, 10, e2677. [Google Scholar]
- Lee, H.; Kang, M.; Sim, S.H.; Kang, J.H.; Choi, W.; Chun, J.W.; Hong, W.; Kim, C.; Ham, W.; Park, J.H.; et al. ACAA1 knockout increases the survival rate of KPC mice by activating autophagy. Mol. Metab. 2025, 100, 102237. [Google Scholar] [CrossRef]
- Woo, S.M.; Lee, H.; Kang, J.H.; Kang, M.; Choi, W.; Sim, S.H.; Chun, J.W.; Han, N.; Kim, K.H.; Ham, W.; et al. Loss of SLC25A20 in Pancreatic Adenocarcinoma Reversed the Tumor-Promoting Effects of a High-Fat Diet. Theranostics 2025, 15, 6516–6533. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y. Fatty acid addiction in cancer: A high-fat diet directly promotes tumor growth through fatty acid oxidation. Biochim. Biophys. Acta Rev. Cancer 2025, 1880, 189428. [Google Scholar]
- Tawbi, H.A.; Schadendorf, D.; Lipson, E.J.; Ascierto, P.A.; Matamala, L.; Castillo Gutierrez, E.; Rutkowski, P.; Gogas, H.J.; Lao, C.D.; De Menezes, J.J.; et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N. Engl. J. Med. 2022, 386, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Lasheras-Otero, I.; Feliu, I.; Maillo, A.; Moreno, H.; Redondo-Munoz, M.; Aldaz, P.; Bocanegra, A.; Olias-Arjona, A.; Lecanda, F.; Fernandez-Irigoyen, J.; et al. The Regulators of Peroxisomal Acyl-Carnitine Shuttle CROT and CRAT Promote Metastasis in Melanoma. J. Investig. Dermatol. 2023, 143, 305–316.e5. [Google Scholar]
- Shen, S.; Faouzi, S.; Souquere, S.; Roy, S.; Routier, E.; Libenciuc, C.; Andre, F.; Pierron, G.; Scoazec, J.Y.; Robert, C. Melanoma Persister Cells Are Tolerant to BRAF/MEK Inhibitors via ACOX1-Mediated Fatty Acid Oxidation. Cell Rep. 2020, 33, 108421. [Google Scholar]
- Zhang, S.; Peng, X.; Yang, S.; Li, X.; Huang, M.; Wei, S.; Liu, J.; He, G.; Zheng, H.; Yang, L.; et al. The regulation, function, and role of lipophagy, a form of selective autophagy, in metabolic disorders. Cell Death Dis. 2022, 13, 132. [Google Scholar] [CrossRef]
- Talley, J.T.; Mohiuddin, S.S. Biochemistry, Fatty Acid Oxidation. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Lee, H.; Woo, S.M.; Jang, H.; Kang, M.; Kim, S.Y. Cancer depends on fatty acids for ATP production: A possible link between cancer and obesity. Semin. Cancer Biol. 2022, 86, 347–357. [Google Scholar] [CrossRef]
- Tang, Z.; Kang, B.; Li, C.; Chen, T.; Zhang, Z. GEPIA2: An enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 2019, 47, W556–W560. [Google Scholar] [CrossRef]
- Lee, J.S.; Lee, H.; Woo, S.M.; Jang, H.; Jeon, Y.; Kim, H.Y.; Song, J.; Lee, W.J.; Hong, E.K.; Park, S.J.; et al. Overall survival of pancreatic ductal adenocarcinoma is doubled by Aldh7a1 deletion in the KPC mouse. Theranostics 2021, 11, 3472–3488. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Kang, J.H.; Lee, S.H.; Hong, D.; Son, J.; Hong, K.M.; Song, J.; Kim, S.Y. Dual targeting of glutaminase 1 and thymidylate synthase elicits death synergistically in NSCLC. Cell Death Dis. 2016, 7, e2511. [Google Scholar] [CrossRef]
- Indiveri, C.; Giangregorio, N.; Iacobazzi, V.; Palmieri, F. Site-directed mutagenesis and chemical modification of the six native cysteine residues of the rat mitochondrial carnitine carrier: Implications for the role of cysteine-136. Biochemistry 2002, 41, 8649–8656. [Google Scholar] [CrossRef] [PubMed]
- Tonazzi, A.; Eberini, I.; Indiveri, C. Molecular mechanism of inhibition of the mitochondrial carnitine/acylcarnitine transporter by omeprazole revealed by proteoliposome assay, mutagenesis and bioinformatics. PLoS ONE 2013, 8, e82286. [Google Scholar] [CrossRef]
- Kantor, P.F.; Lucien, A.; Kozak, R.; Lopaschuk, G.D. The antianginal drug trimetazidine shifts cardiac energy metabolism from fatty acid oxidation to glucose oxidation by inhibiting mitochondrial long-chain 3-ketoacyl coenzyme A thiolase. Circ. Res. 2000, 86, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Sebaugh, J.L. Guidelines for accurate EC50/IC50 estimation. Pharm. Stat. 2011, 10, 128–134. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, W.; Ham, W.; Park, J.H.; Sim, S.H.; Chun, J.W.; Kang, M.; Kim, C.; Hong, W.; Koh, E.-B.; Kang, J.H.; et al. Inhibiting Fatty Acid Oxidation Suppresses Acquired Resistance to Standard Chemotherapy in Melanoma. Int. J. Mol. Sci. 2025, 26, 9873. https://doi.org/10.3390/ijms26209873
Choi W, Ham W, Park JH, Sim SH, Chun JW, Kang M, Kim C, Hong W, Koh E-B, Kang JH, et al. Inhibiting Fatty Acid Oxidation Suppresses Acquired Resistance to Standard Chemotherapy in Melanoma. International Journal of Molecular Sciences. 2025; 26(20):9873. https://doi.org/10.3390/ijms26209873
Chicago/Turabian StyleChoi, Wonyoung, Woojin Ham, Jeong Hwan Park, Sung Hoon Sim, Jung Won Chun, Mingyu Kang, Chaeyoung Kim, Woosol Hong, Eun-Byeol Koh, Joon Hee Kang, and et al. 2025. "Inhibiting Fatty Acid Oxidation Suppresses Acquired Resistance to Standard Chemotherapy in Melanoma" International Journal of Molecular Sciences 26, no. 20: 9873. https://doi.org/10.3390/ijms26209873
APA StyleChoi, W., Ham, W., Park, J. H., Sim, S. H., Chun, J. W., Kang, M., Kim, C., Hong, W., Koh, E.-B., Kang, J. H., Woo, S. M., & Kim, S.-Y. (2025). Inhibiting Fatty Acid Oxidation Suppresses Acquired Resistance to Standard Chemotherapy in Melanoma. International Journal of Molecular Sciences, 26(20), 9873. https://doi.org/10.3390/ijms26209873





