Beyond Antioxidants: The Emerging Role of Nrf2 Activation in Amyotrophic Lateral Sclerosis (ALS)
Abstract
1. Introduction: ALS and the Search for Multi-Target Neuroprotection
2. Classical Nrf2 Pathway: Oxidative Stress and Redox Homeostasis
3. Astrocytic Nrf2 Activation: Glial Defence and Non-Cell Autonomous Protection
4. Nrf2 and Mitochondrial Quality Control in ALS
5. Nrf2 Meets RNA Metabolism: A New Frontier in ALS Mechanisms
5.1. Beyond Redox: Nrf2 as a Master Regulator of RNA-Binding Protein Homeostasis in ALS
5.2. Nrf2 in Stress Granule Clearance and Phase Separation Control
5.3. Nrf2 Crosstalk with Nucleocytoplasmic Transport and RNA Surveillance
5.4. Therapeutic Outlook: Integrating Nrf2 Activation into RNA-Targeted Strategies
6. Pharmacological Activation of Nrf2: From Repurposed Drugs to Novel Compounds
- Repurposed FDA-approved agents, such as DMF, Sulforaphane, and Curcumin, which exert partial Nrf2 induction through modification of KEAP1 cysteine residues or activation of upstream kinases.
- Second-generation Nrf2 activators, including bardoxolone methyl and omaveloxolone, are designed for improved potency, bioavailability, and BBB penetration.
- Dual-pathway compounds, such as hybrid molecules targeting both Nrf2 and NF-κB or Nrf2 and autophagy pathways, are potentially able to fine-tune the neuroinflammatory and proteostatic environment in ALS.
- Gene therapy and delivery strategies, including AAV-mediated Nrf2 expression and astrocyte-specific enhancers, offer targeted modulation with minimal off-target effects.
6.1. Repurposed Clinical Drugs with Nrf2-Activating Properties
6.1.1. Dimethyl Fumarate (DMF)
- Upregulation of Nrf2 target genes (HMOX1, NQO1, GCLC), enhancing glutathione synthesis and antioxidant buffering.
- Suppression of microglial activation and downregulation of NF-κB-mediated proinflammatory signaling, reducing neuroinflammatory burden.
- Improvement in mitochondrial bioenergetics, including increased membrane potential, ATP production, and Ca2+ buffering capacity in astrocytes and motor neurons.
6.1.2. Sulforaphane (SFN)
- Reduces endoplasmic reticulum (ER) stress and enhances the unfolded protein response (UPR), contributing to proteostasis restoration.
- Upregulates phase II detoxifying enzymes, including glutathione S-transferases (GSTs), UDP-glucuronosyltransferases (UGTs), and thioredoxin reductase 1 (TXNRD1), thereby improving redox buffering capacity [115].
- Restores glutathione homeostasis and prevents redox-mediated astrocyte dysfunction, ultimately preserving neuronal viability in neuron–astrocyte co-cultures under oxidative stress [116].
- Suppresses inflammatory gene expression via inhibiting NF-κB signaling and modulation of reactive glial phenotypes.
6.1.3. Curcumin
- Upregulates Nrf2 target genes, such as HMOX1, NQO1, and FTH1, promoting ROS detoxification and iron homeostasis [121].
- Reduces protein aggregation, including misfolded SOD1 and C-terminal TDP-43 fragments in ALS spinal cord tissue [121].
- Improves mitochondrial function, restoring membrane potential and ATP production in ALS-relevant cellular models [49].
6.2. Novel Synthetic Compounds and Targeted Nrf2 Activators
6.2.1. Non-Electrophilic Keap1–Nrf2 Protein–Protein Interaction (PPI) Inhibitors
- Reducing neuroinflammation through microglial reprogramming.
- Preserving motor neuron survival.
- Improving functional outcomes and prolonging lifespan [91].
6.2.2. M102: A Dual Nrf2–HSF1 Pathway Modulator
- Nrf2-dependent transcription of cytoprotective genes.
- HSF1-driven expression of molecular chaperones such as HSP70, HSP40, and HSPB8, essential for disaggregation and clearance of toxic protein aggregates.
6.3. RNA-Targeted Nrf2 Interventions
6.4. Delivery Strategies and Cell-Type Specific Modulation
- AAV9-based gene therapy platforms engineered to drive astrocyte-specific Nrf2 expression using glial fibrillary acidic protein (GFAP) promoters. In SOD1G93A ALS mouse models, systemic delivery of AAV9-GFAP-Nrf2 constructs:
- Delayed disease onset.
- Preserved motor neuron count in spinal cord regions.
- Prolonged survival compared to controls [45].
7. Conclusions
- Mitochondrial quality control is a key interface between oxidative stress, neurodegeneration, and ferroptosis regulation. Nrf2 activation promotes mitochondrial biogenesis, membrane potential stability, and calcium buffering, functions impaired in ALS astrocytes and motor neurons [136,137]. As mitochondrial dysfunction can influence ferroptotic susceptibility, degradation pathways play dual roles depending on whether they target anti- or pro-ferroptotic modulators. Selective modulation of these pathways could offer therapeutic potential in neurodegenerative diseases.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hardiman, O.; Al-Chalabi, A.; Chio, A.; Corr, E.M.; Logroscino, G.; Robberecht, W.; Shaw, P.J.; Simmons, Z.; van den Berg, L.H. Amyotrophic lateral sclerosis. Nat. Rev. Dis. Primers 2017, 3, 17071. [Google Scholar] [CrossRef] [PubMed]
- Renton, A.E.; Chiò, A.; Traynor, B.J. State of play in amyotrophic lateral sclerosis genetics. Nat. Neurosci. 2014, 17, 17–23. [Google Scholar] [CrossRef]
- Neumann, M.; Sampathu, D.M.; Kwong, L.K.; Truax, A.C.; Micsenyi, M.C.; Chou, T.T.; Bruce, J.; Schuck, T.; Grossman, M.; Clark, C.M.; et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 2006, 314, 130–133. [Google Scholar] [CrossRef]
- Giordana, M.T.; Piccinini, M.; Grifoni, S.; De Marco, G.; Vercellino, M.; Magistrello, M.; Pellerino, A.; Buccinnà, B.; Lupino, E.; Rinaudo, M.T. TDP-43 redistribution is an early event in sporadic amyotrophic lateral sclerosis. Brain Pathol. 2010, 20, 351–360. [Google Scholar] [CrossRef]
- Taylor, J.P.; Brown, R.H., Jr.; Cleveland, D.W. Decoding ALS: From genes to mechanism. Nature 2016, 539, 197–206. [Google Scholar] [CrossRef]
- Ling, S.C.; Polymenidou, M.; Cleveland, D.W. Converging mechanisms in ALS and FTD: Disrupted RNA and protein homeostasis. Neuron 2013, 79, 416–438. [Google Scholar] [CrossRef]
- Philips, T.; Robberecht, W. Neuroinflammation in amyotrophic lateral sclerosis: Role of glial activation in motor neuron disease. Lancet Neurol. 2011, 10, 253–263. [Google Scholar] [CrossRef]
- Smith, E.F.; Shaw, P.J.; De Vos, K.J. The role of mitochondria in amyotrophic lateral sclerosis. Neurosci. Lett. 2019, 710, 132933. [Google Scholar] [CrossRef] [PubMed]
- Kiskinis, E.; Sandoe, J.; Williams, L.A.; Boulting, G.L.; Moccia, R.; Wainger, B.J.; Han, S.; Peng, T.; Thams, S.; Mikkilineni, S.; et al. Pathways disrupted in human ALS motor neurons identified through genetic correction of mutant SOD1. Cell Stem Cell 2014, 14, 781–795. [Google Scholar] [CrossRef] [PubMed]
- Tam, O.H.; Rozhkov, N.V.; Shaw, R.; Kim, D.; Hubbard, I.; Fennessey, S.; Propp, N.; Fagegaltier, D.; Harris, B.T.; Ostrow, L.W.; et al. Postmortem Cortex Samples Identify Distinct Molecular Subtypes of ALS: Retrotransposon Activation, Oxidative Stress, and Activated Glia. Cell Rep. 2019, 29, 1164–1177.e5. [Google Scholar] [CrossRef]
- Van Damme, P.; Robberecht, W.; Van Den Bosch, L. Modelling amyotrophic lateral sclerosis: Progress and possibilities. Dis. Model. Mech. 2017, 10, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Le Gall, L.; Anakor, E.; Connolly, O.; Vijayakumar, U.G.; Duddy, W.J.; Duguez, S. Molecular and Cellular Mechanisms Affected in ALS. J. Pers. Med. 2020, 10, 101. [Google Scholar] [CrossRef]
- Prasad, A.; Bharathi, V.; Sivalingam, V.; Girdhar, A.; Patel, B.K. Molecular Mechanisms of TDP-43 Misfolding and Pathology in Amyotrophic Lateral Sclerosis. Front. Mol. Neurosci. 2019, 12, 25. [Google Scholar] [CrossRef]
- Wang, W.; Li, L.; Lin, W.L.; Dickson, D.W.; Petrucelli, L.; Zhang, T.; Wang, X. The ALS disease-associated mutant TDP-43 impairs mitochondrial dynamics and function in motor neurons. Hum. Mol. Genet. 2013, 22, 4706–4719. [Google Scholar] [CrossRef]
- Hinchcliffe, M.; Smith, A. Riluzole: Real-world evidence supports significant extension of median survival times in patients with amyotrophic lateral sclerosis. Degener. Neurol. Neuromuscul. Dis. 2017, 7, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Writing Group; Edaravone (MCI-186) ALS 19 Study Group. Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: A randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2017, 16, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.M.; Cudkowicz, M.E.; Genge, A.; Shaw, P.J.; Sobue, G.; Bucelli, R.C.; Chiò, A.; Van Damme, P.; Ludolph, A.C.; Glass, J.D.; et al. Trial of Antisense Oligonucleotide Tofersen for SOD1 ALS. N. Engl. J. Med. 2022, 387, 1099–1110. [Google Scholar] [CrossRef] [PubMed]
- Hollingworth, D.; Thomas, F.; Page, D.A.; Fouda, M.A.; De Castro, R.L.; Sula, A.; Mykhaylyk, V.B.; Kelly, G.; Ulmschneider, M.B.; Ruben, P.C.; et al. Structural basis for the rescue of hyperexcitable cells by the amyotrophic lateral sclerosis drug Riluzole. Nat. Commun. 2024, 15, 8426. [Google Scholar] [CrossRef]
- Cruz, M.P. Edaravone (Radicava): A Novel Neuroprotective Agent for the Treatment of Amyotrophic Lateral Sclerosis. Pharm. Ther. 2018, 43, 25–28. [Google Scholar]
- Takei, K.; Watanabe, K.; Yuki, S.; Akimoto, M.; Sakata, T.; Palumbo, J. Edaravone and its clinical development for amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Front. Degener. 2017, 18 (Suppl. S1), 5–10. [Google Scholar] [CrossRef]
- Mikuriya, S.; Takegawa-Araki, T.; Tamura, M. Edaravone mitigates TDP-43 mislocalization in human amyotrophic lateral sclerosis neurons with potential implication of the SIRT1-XBP1 pathway. Free Radic. Biol. Med. 2025, 230, 283–293. [Google Scholar] [CrossRef]
- Ciechanover, A.; Kwon, Y.T. Protein Quality Control by Molecular Chaperones in Neurodegeneration. Front. Neurosci. 2017, 11, 185. [Google Scholar] [CrossRef]
- Vomund, S.; Schäfer, A.; Parnham, M.J.; Brüne, B.; von Knethen, A. Nrf2, the Master Regulator of Anti-Oxidative Responses. Int. J. Mol. Sci. 2017, 18, 2772. [Google Scholar] [CrossRef]
- Holmström, K.M.; Kostov, R.V.; Dinkova-Kostova, A.T. The multifaceted role of Nrf2 in mitochondrial function. Curr. Opin. Toxicol. 2016, 1, 80–91. [Google Scholar] [CrossRef]
- Dodson, M.; Castro-Portuguez, R.; Zhang, D.D. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 2019, 23, 101107. [Google Scholar] [CrossRef] [PubMed]
- Pajares, M.; Jiménez-Moreno, N.; García-Yagüe, Á.J.; Escoll, M.; de Ceballos, M.L.; Van Leuven, F.; Rábano, A.; Yamamoto, M.; Rojo, A.I.; Cuadrado, A. Transcription factor NFE2L2/NRF2 is a regulator of macroautophagy genes. Autophagy 2016, 12, 1902–1916. [Google Scholar] [CrossRef]
- Ahmed, S.M.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef]
- Cui, Q.; Wang, W.; Namani, A.; Wang, H.; Hammad, A.; Huang, P.; Gao, Y.; Elshaer, M.; Wu, Y.; Wang, X.J.; et al. NRF2 has a splicing regulatory function involving the survival of motor neuron (SMN) in non-small cell lung cancer. Oncogene 2023, 42, 2751–2763. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Kerins, M.J.; Tian, W.; Neupane, D.; Zhang, D.D.; Ooi, A. Differential and overlapping targets of the transcriptional regulators NRF1, NRF2, and NRF3 in human cells. J. Biol. Chem. 2019, 294, 18131–18149. [Google Scholar] [CrossRef]
- Vargas, M.R.; Johnson, J.A. The Nrf2-ARE cytoprotective pathway in astrocytes. Expert. Rev. Mol. Med. 2009, 11, e17. [Google Scholar] [CrossRef] [PubMed]
- Lastres-Becker, I.; García-Yagüe, A.J.; Scannevin, R.H.; Casarejos, M.J.; Kügler, S.; Rábano, A.; Cuadrado, A. Repurposing the NRF2 Activator Dimethyl Fumarate as Therapy Against Synucleinopathy in Parkinson’s Disease. Antioxid. Redox Signal 2016, 25, 61–77. [Google Scholar] [CrossRef]
- Vargas, M.R.; Pehar, M.; Cassina, P.; Beckman, J.S.; Barbeito, L. Increased glutathione biosynthesis by Nrf2 activation in astrocytes prevents p75NTR-dependent motor neuron apoptosis. J. Neurochem. 2006, 97, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Kraft, A.D.; Johnson, D.A.; Johnson, J.A. Nuclear factor E2-related factor 2-dependent antioxidant response element activation by tert-butylhydroquinone and sulforaphane occurring preferentially in astrocytes conditions neurons against oxidative insult. J. Neurosci. 2004, 24, 1101–1112. [Google Scholar] [CrossRef]
- Au, W.H.; Miller-Fleming, L.; Sanchez-Martinez, A.; Lee, J.A.; Twyning, M.J.; Prag, H.A.; Raik, L.; Allen, S.P.; Shaw, P.J.; Ferraiuolo, L.; et al. Activation of the Keap1/Nrf2 pathway suppresses mitochondrial dysfunction, oxidative stress, and motor phenotypes in C9orf72 ALS/FTD models. Life Sci. Alliance 2024, 7, e202402853. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Amarilla, P.; Miquel, E.; Trostchansky, A.; Trias, E.; Ferreira, A.M.; Freeman, B.A.; Cassina, P.; Barbeito, L.; Vargas, M.R.; Rubbo, H. Electrophilic nitro-fatty acids prevent astrocyte-mediated toxicity to motor neurons in a cell model of familial amyotrophic lateral sclerosis via nuclear factor erythroid 2-related factor activation. Free Radic. Biol. Med. 2016, 95, 112–120. [Google Scholar] [CrossRef]
- Johnson, D.A.; Johnson, J.A. Nrf2—A therapeutic target for the treatment of neurodegenerative diseases. Free Radic. Biol. Med. 2015, 88 Pt B, 253–267. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef]
- Obrador, E.; Salvador-Palmer, R.; López-Blanch, R.; Jihad-Jebbar, A.; Vallés, S.L.; Estrela, J.M. The Link between Oxidative Stress, Redox Status, Bioenergetics and Mitochondria in the Pathophysiology of ALS. Int. J. Mol. Sci. 2021, 22, 6352. [Google Scholar] [CrossRef]
- Lv, B.; Xing, S.; Wang, Z.; Zhang, A.; Wang, Q.; Bian, Y.; Pei, Y.; Sun, H.; Chen, Y. NRF2 inhibitors: Recent progress, future design and therapeutic potential. Eur. J. Med. Chem. 2024, 279, 116822. [Google Scholar] [CrossRef]
- Tarot, P.; Lasbleiz, C.; Liévens, J.C. NRF2 signaling cascade in amyotrophic lateral sclerosis: Bridging the gap between promise and reality. Neural Regen. Res. 2024, 19, 1006–1012. [Google Scholar] [CrossRef]
- Zhang, D.D.; Hannink, M. Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol. Cell Biol. 2003, 23, 8137–8151. [Google Scholar] [CrossRef]
- Huang, H.C.; Nguyen, T.; Pickett, C.B. Regulation of the antioxidant response element by protein kinase C-mediated phosphorylation of NF-E2-related factor 2. Proc. Natl. Acad. Sci. USA 2000, 97, 12475–12480, Correction in Proc. Natl. Acad. Sci. USA 2001, 98, 379. [Google Scholar] [CrossRef]
- Chen, Q.M. Nrf2 for protection against oxidant generation and mitochondrial damage in cardiac injury. Free Radic. Biol. Med. 2022, 179, 133–143. [Google Scholar] [CrossRef]
- Shih, A.Y.; Johnson, D.A.; Wong, G.; Kraft, A.D.; Jiang, L.; Erb, H.; Johnson, J.A.; Murphy, T.H. Coordinate regulation of glutathione biosynthesis and release by Nrf2-expressing glia potently protects neurons from oxidative stress. J. Neurosci. 2003, 23, 3394–3406. [Google Scholar] [CrossRef] [PubMed]
- Vargas, M.R.; Johnson, D.A.; Sirkis, D.W.; Messing, A.; Johnson, J.A. Nrf2 activation in astrocytes protects against neurodegeneration in mouse models of familial amyotrophic lateral sclerosis. J. Neurosci. 2008, 28, 13574–13581. [Google Scholar] [CrossRef]
- Lim, J.H.; Kim, K.M.; Kim, S.W.; Hwang, O.; Choi, H.J. Bromocriptine activates NQO1 via Nrf2-PI3K/Akt signaling: Novel cytoprotective mechanism against oxidative damage. Pharmacol. Res. 2008, 57, 325–331. [Google Scholar] [CrossRef]
- Kanno, T.; Tanaka, K.; Yanagisawa, Y.; Yasutake, K.; Hadano, S.; Yoshii, F.; Hirayama, N.; Ikeda, J.E. A novel small molecule, N-(4-(2-pyridyl)(1,3-thiazol-2-yl))-2-(2,4,6-trimethylphenoxy) acetamide, selectively protects against oxidative stress-induced cell death by activating the Nrf2-ARE pathway: Therapeutic implications for ALS. Free Radic. Biol. Med. 2012, 53, 2028–2042. [Google Scholar] [CrossRef]
- Tanaka, K.; Kanno, T.; Yanagisawa, Y.; Yasutake, K.; Inoue, S.; Hirayama, N.; Ikeda, J.E. A novel acylaminoimidazole derivative, WN1316, alleviates disease progression via suppression of glial inflammation in ALS mouse model. PLoS ONE 2014, 9, e87728. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Xu, L.; Wu, L.; Wang, X.; Duan, W.; Li, H.; Li, C. Curcumin abolishes mutant TDP-43 induced excitability in a motoneuron-like cellular model of ALS. Neuroscience 2014, 272, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Sato, H.; Okuda, M.; Wu, J.; Koyama, S.; Izumi, Y.; Waku, T.; Iino, M.; Aoki, M.; Arawaka, S.; et al. Therapeutic effect of a novel curcumin derivative GT863 on a mouse model of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Front. Degener. 2022, 23, 489–495. [Google Scholar] [CrossRef]
- Ahmadi, M.; Agah, E.; Nafissi, S.; Jaafari, M.R.; Harirchian, M.H.; Sarraf, P.; Faghihi-Kashani, S.; Hosseini, S.J.; Ghoreishi, A.; Aghamollaii, V.; et al. Safety and Efficacy of Nanocurcumin as Add-On Therapy to Riluzole in Patients with Amyotrophic Lateral Sclerosis: A Pilot Randomized Clinical Trial. Neurotherapeutics 2018, 15, 430–438. [Google Scholar] [CrossRef]
- Vucic, S.; Henderson, R.D.; Mathers, S.; Needham, M.; Schultz, D.; Kiernan, M.C. Safety and efficacy of dimethyl fumarate in ALS: Randomised controlled study. Ann. Clin. Transl. Neurol. 2021, 8, 1991–1999. [Google Scholar] [CrossRef]
- Pu, D.; Zhao, Y.; Chen, J.; Sun, Y.; Lv, A.; Zhu, S.; Luo, C.; Zhao, K.; Xiao, Q. Protective Effects of Sulforaphane on Cognitive Impairments and AD-like Lesions in Diabetic Mice are Associated with the Upregulation of Nrf2 Transcription Activity. Neuroscience 2018, 381, 35–45. [Google Scholar] [CrossRef]
- Van Harten, A.C.M.; Phatnani, H.; Przedborski, S. Non-cell-autonomous pathogenic mechanisms in amyotrophic lateral sclerosis. Trends Neurosci. 2021, 44, 658–668. [Google Scholar] [CrossRef] [PubMed]
- Sarlette, A.; Krampfl, K.; Grothe, C.; Neuhoff, N.; Dengler, R.; Petri, S. Nuclear erythroid 2-related factor 2-antioxidative response element signaling pathway in motor cortex and spinal cord in amyotrophic lateral sclerosis. J. Neuropathol. Exp. Neurol. 2008, 67, 1055–1062. [Google Scholar] [CrossRef]
- Gan, L.; Johnson, J.A. Oxidative damage and the Nrf2-ARE pathway in neurodegenerative diseases. Biochim. Biophys. Acta 2014, 1842, 1208–1218. [Google Scholar] [CrossRef]
- Johnson, J.A.; Johnson, D.A.; Kraft, A.D.; Calkins, M.J.; Jakel, R.J.; Vargas, M.R.; Chen, P.C. The Nrf2-ARE pathway: An indicator and modulator of oxidative stress in neurodegeneration. Ann. N. Y. Acad. Sci. 2008, 1147, 61–69. [Google Scholar] [CrossRef]
- Sagara, J.; Makino, N.; Bannai, S. Glutathione efflux from cultured astrocytes. J. Neurochem. 1996, 66, 1876–1881. [Google Scholar] [CrossRef] [PubMed]
- Dringen, R.; Gutterer, J.M.; Hirrlinger, J. Glutathione metabolism in brain metabolic interaction between astrocytes and neurons in the defense against reactive oxygen species. Eur. J. Biochem. 2000, 267, 4912–4916. [Google Scholar] [CrossRef]
- Drukarch, B.; Schepens, E.; Stoof, J.C.; Langeveld, C.H.; Van Muiswinkel, F.L. Astrocyte-enhanced neuronal survival is mediated by scavenging of extracellular reactive oxygen species. Free Radic. Biol. Med. 1998, 25, 217–220. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Hewett, S.J. Nrf2 Regulates Basal Glutathione Production in Astrocytes. Int. J. Mol. Sci. 2025, 26, 687. [Google Scholar] [CrossRef] [PubMed]
- Dringen, R. Metabolism and functions of glutathione in brain. Prog. Neurobiol. 2000, 62, 649–671. [Google Scholar] [CrossRef]
- Guo, N.; Shaw, C. Characterization and localization of glutathione binding sites on cultured astrocytes. Brain Res. Mol. Brain Res. 1992, 15, 207–215. [Google Scholar] [CrossRef]
- Lee, K.H.; Cha, M.; Lee, B.H. Crosstalk between Neuron and Glial Cells in Oxidative Injury and Neuroprotection. Int. J. Mol. Sci. 2021, 22, 13315. [Google Scholar] [CrossRef]
- Dash, U.C.; Bhol, N.K.; Swain, S.K.; Samal, R.R.; Nayak, P.K.; Raina, V.; Panda, S.K.; Kerry, R.G.; Duttaroy, A.K.; Jena, A.B. Oxidative stress and inflammation in the pathogenesis of neurological disorders: Mechanisms and implications. Acta Pharm. Sin. B 2025, 15, 15–34. [Google Scholar] [CrossRef]
- Jiménez-Villegas, J.; Ferraiuolo, L.; Mead, R.J.; Shaw, P.J.; Cuadrado, A.; Rojo, A.I. NRF2 as a therapeutic opportunity to impact in the molecular roadmap of ALS. Free Radic. Biol. Med. 2021, 173, 125–141. [Google Scholar] [CrossRef]
- Elsharkasi, M.M.O.; Villani, B.; Wells, G.; Kerr, F. Basal activation of astrocytic Nrf2 in neuronal culture media: Challenges and implications for neuron-astrocyte modelling. Brain Neurosci. Adv. 2025, 9, 23982128251351360. [Google Scholar] [CrossRef]
- Calkins, M.J.; Johnson, D.A.; Townsend, J.A.; Vargas, M.R.; Dowell, J.A.; Williamson, T.P.; Kraft, A.D.; Lee, J.M.; Li, J.; Johnson, J.A. The Nrf2/ARE pathway as a potential therapeutic target in neurodegenerative disease. Antioxid. Redox Signal 2009, 11, 497–508. [Google Scholar] [CrossRef]
- Al-Mubarak, B.R.; Bell, K.F.S.; Chowdhry, S.; Meakin, P.J.; Baxter, P.S.; McKay, S.; Dando, O.; Ashford, M.L.J.; Gazaryan, I.; Hayes, J.D.; et al. Non-canonical Keap1-independent activation of Nrf2 in astrocytes by mild oxidative stress. Redox Biol. 2021, 47, 102158. [Google Scholar] [CrossRef]
- Mimoto, T.; Miyazaki, K.; Morimoto, N.; Kurata, T.; Satoh, K.; Ikeda, Y.; Abe, K. Impaired antioxydative Keap1/Nrf2 system and the downstream stress protein responses in the motor neuron of ALS model mice. Brain Res. 2012, 1446, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Silva-Islas, C.A.; Maldonado, P.D. Canonical and non-canonical mechanisms of Nrf2 activation. Pharmacol. Res. 2018, 134, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Brandes, M.S.; Gray, N.E. NRF2 as a Therapeutic Target in Neurodegenerative Diseases. ASN Neuro 2020, 12, 1759091419899782. [Google Scholar] [CrossRef]
- Nicholls, D.G.; Budd, S.L. Mitochondria and neuronal survival. Physiol. Rev. 2000, 80, 315–360. [Google Scholar] [CrossRef]
- Sasaki, S.; Iwata, M. Mitochondrial alterations in the spinal cord of patients with sporadic amyotrophic lateral sclerosis. J. Neuropathol. Exp. Neurol. 2007, 66, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Magrané, J.; Cortez, C.; Gan, W.B.; Manfredi, G. Abnormal mitochondrial transport and morphology are common pathological denominators in SOD1 and TDP43 ALS mouse models. Hum. Mol. Genet. 2014, 23, 1413–1424. [Google Scholar] [CrossRef] [PubMed]
- George, M.; Tharakan, M.; Culberson, J.; Reddy, A.P.; Reddy, P.H. Role of Nrf2 in aging, Alzheimer’s and other neurodegenerative diseases. Ageing Res. Rev. 2022, 82, 101756. [Google Scholar] [CrossRef]
- Bennett, C.F.; Latorre-Muro, P.; Puigserver, P. Mechanisms of mitochondrial respiratory adaptation. Nat. Rev. Mol. Cell Biol. 2022, 23, 817–835. [Google Scholar] [CrossRef]
- Bouchez, C.; Devin, A. Mitochondrial Biogenesis and Mitochondrial Reactive Oxygen Species (ROS): A Complex Relationship Regulated by the cAMP/PKA Signaling Pathway. Cells 2019, 8, 287. [Google Scholar] [CrossRef]
- Murata, H.; Takamatsu, H.; Liu, S.; Kataoka, K.; Huh, N.H.; Sakaguchi, M. NRF2 Regulates PINK1 Expression under Oxidative Stress Conditions. PLoS ONE 2015, 10, e0142438. [Google Scholar] [CrossRef]
- Cho, H.Y.; Miller-DeGraff, L.; Blankenship-Paris, T.; Wang, X.; Bell, D.A.; Lih, F.; Deterding, L.; Panduri, V.; Morgan, D.L.; Yamamoto, M.; et al. Sulforaphane enriched transcriptome of lung mitochondrial energy metabolism and provided pulmonary injury protection via Nrf2 in mice. Toxicol. Appl. Pharmacol. 2019, 364, 29–44. [Google Scholar] [CrossRef]
- Itoh, K.; Ye, P.; Matsumiya, T.; Tanji, K.; Ozaki, T. Emerging functional cross-talk between the Keap1-Nrf2 system and mitochondria. J. Clin. Biochem. Nutr. 2015, 56, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Briones-Herrera, A.; Ramírez-Camacho, I.; Zazueta, C.; Tapia, E.; Pedraza-Chaverri, J. Altered proximal tubule fatty acid utilization, mitophagy, fission and supercomplexes arrangement in experimental Fanconi syndrome are ameliorated by sulforaphane-induced mitochondrial biogenesis. Free Radic. Biol. Med. 2020, 153, 54–70. [Google Scholar]
- Renton, A.E.; Majounie, E.; Waite, A.; Simón-Sánchez, J.; Rollinson, S.; Gibbs, J.R.; Schymick, J.C.; Laaksovirta, H.; van Swieten, J.C.; Myllykangas, L.; et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron 2011, 72, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Crisman, E.; Duarte, P.; Dauden, E.; Cuadrado, A.; Rodríguez-Franco, M.I.; López, M.G.; León, R. KEAP1-NRF2 protein-protein interaction inhibitors: Design, pharmacological properties and therapeutic potential. Med. Res. Rev. 2023, 43, 237–287. [Google Scholar]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Zeng, J.; Zheng, S.; Chen, Y.; Qu, Y.; Xie, J.; Hong, E.; Lv, H.; Ding, R.; Feng, L.; Xie, Z. Puerarin attenuates intracerebral hemorrhage-induced early brain injury possibly by PI3K/Akt signal activation-mediated suppression of NF-κB pathway. J. Cell Mol. Med. 2021, 25, 7809–7824. [Google Scholar]
- Jiménez-Villegas, J.; Kirby, J.; Mata, A.; Cadenas, S.; Turner, M.R.; Malaspina, A.; Shaw, P.J.; Cuadrado, A.; Rojo, A.I. Dipeptide Repeat Pathology in C9orf72-ALS Is Associated with Redox, Mitochondrial and NRF2 Pathway Imbalance. Antioxidants 2022, 11, 1897. [Google Scholar] [CrossRef]
- Boeynaems, S.; Bogaert, E.; Van Damme, P.; Van Den Bosch, L. Inside out: The role of nucleocytoplasmic transport in ALS and FTLD. Acta Neuropathol. 2016, 132, 159–173. [Google Scholar] [CrossRef]
- Walker, C.; Herranz-Martin, S.; Karyka, E.; Liao, C.; Lewis, K.; Elsayed, W.; Lukashchuk, V.; Chiang, S.C.; Ray, S.; Mulcahy, P.J.; et al. C9orf72 expansion disrupts ATM-mediated chromosomal break repair. Nat. Neurosci. 2017, 20, 1225–1235. [Google Scholar] [PubMed]
- Arslanbaeva, L.; Bisaglia, M. Activation of the Nrf2 Pathway as a Therapeutic Strategy for ALS Treatment. Molecules 2022, 27, 1471. [Google Scholar] [CrossRef]
- Bono, S.; Feligioni, M.; Corbo, M. Impaired antioxidant KEAP1-NRF2 system in amyotrophic lateral sclerosis: NRF2 activation as a potential therapeutic strategy. Mol. Neurodegener. 2021, 16, 71. [Google Scholar] [CrossRef]
- Jain, A.; Lamark, T.; Sjøttem, E.; Larsen, K.B.; Awuh, J.A.; Øvervatn, A.; McMahon, M.; Hayes, J.D.; Johansen, T. p62/SQSTM1 is a target gene for transcription factor NRF2 and creates a positive feedback loop by inducing antioxidant response element-driven gene transcription. J. Biol. Chem. 2010, 285, 22576–22591. [Google Scholar] [CrossRef]
- Zaepfel, B.L.; Zhang, Z.; Maulding, K.; Coyne, A.N.; Cheng, W.; Hayes, L.R.; Lloyd, T.E.; Sun, S.; Rothstein, J.D. UPF1 reduces C9orf72 HRE-induced neurotoxicity in the absence of nonsense-mediated decay dysfunction. Cell Rep. 2021, 34, 108925. [Google Scholar] [CrossRef]
- Moujalled, D.; Grubman, A.; Acevedo, K.; Yang, S.; Ke, Y.D.; Moujalled, D.M.; Duncan, C.; Caragounis, A.; Perera, N.D.; Turner, B.J.; et al. TDP-43 mutations causing amyotrophic lateral sclerosis are associated with altered expression of RNA-binding protein hnRNP K and affect the Nrf2 antioxidant pathway. Hum. Mol. Genet. 2017, 26, 1732–1746. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, Z.; Kang, Y.; Yi, Q.; Wang, T.; Bai, Y.; Liu, Y. Stress granule homeostasis is modulated by TRIM21-mediated ubiquitination of G3BP1 and autophagy-dependent elimination of stress granules. Autophagy 2023, 19, 1934–1951. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Gendron, T.F.; Xu, Y.F.; Ko, L.W.; Yen, S.H.; Petrucelli, L. Phosphorylation regulates proteasomal-mediated degradation and solubility of TAR DNA binding protein-43 C-terminal fragments. Mol. Neurodegener. 2010, 5, 33. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.C.; Zhang, Y.; Umoh, M.E.; Vaughan, S.W.; Lorenzini, I.; Liu, F.; Sayegh, M.; Donlin-Asp, P.G.; Chen, Y.H.; Duong, D.M.; et al. TDP-43 pathology disrupts nuclear pore complexes and nucleocytoplasmic transport in ALS/FTD. Nat. Neurosci. 2018, 21, 228–239. [Google Scholar] [CrossRef]
- Lee, P.J.; Yang, S.; Sun, Y.; Guo, J.U. Regulation of nonsense-mediated mRNA decay in neural development and disease. J. Mol. Cell Biol. 2021, 13, 269–281. [Google Scholar] [PubMed]
- Crossley, M.P.; Bocek, M.; Cimprich, K.A. R-Loops as Cellular Regulators and Genomic Threats. Mol. Cell 2019, 73, 398–411. [Google Scholar] [CrossRef]
- Sharbafshaaer, M.; Pepe, R.; Notariale, R.; Canale, F.; Tessitore, A.; Tedeschi, G.; Trojsi, F. Neuroaxonal Degeneration as a Converging Mechanism in Motor Neuron Diseases (MNDs): Molecular Insights into RNA Dysregulation and Emerging Therapeutic Targets. Int. J. Mol. Sci. 2025, 26, 7644. [Google Scholar] [CrossRef]
- Balendra, R.; Isaacs, A.M. C9orf72-mediated ALS and FTD: Multiple pathways to disease. Nat. Rev. Neurol. 2018, 14, 544–558. [Google Scholar] [CrossRef]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef]
- Desai, M.; Gulati, K.; Agrawal, M.; Ghumra, S.; Sahoo, P.K. Stress granules: Guardians of cellular health and triggers of disease. Neural Regen. Res. 2026, 21, 588–597. [Google Scholar] [CrossRef]
- Haeusler, A.R.; Donnelly, C.J.; Rothstein, J.D. The expanding biology of the C9orf72 nucleotide repeat expansion in neurodegenerative disease. Nat. Rev. Neurosci. 2016, 17, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.-H.; Chen, Y.-H.; Huang, T.-Y. Ubiquitin-mediated regulation of autophagy. J. Biomed. Sci. 2019, 26, 80. [Google Scholar] [CrossRef] [PubMed]
- Oliviero, S. SINEUPs to boost translation. Mol. Ther. Nucleic Acids 2023, 33, 47–48. [Google Scholar] [CrossRef] [PubMed]
- Ngo, V.; Duennwald, M.L. Nrf2 and Oxidative Stress: A General Overview of Mechanisms and Implications in Human Disease. Antioxidants 2022, 11, 2345. [Google Scholar] [CrossRef]
- Linker, R.A.; Lee, D.H.; Ryan, S.; van Dam, A.M.; Conrad, R.; Bista, P.; Zeng, W.; Hronowsky, X.; Buko, A.; Chollate, S.; et al. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain 2011, 134 Pt 3, 678–692. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Scannevin, R.H.; Chollate, S.; Jung, M.Y.; Shackett, M.; Patel, H.; Bista, P.; Zeng, W.; Ryan, S.; Yamamoto, M.; Lukashev, M.; et al. Fumarates promote cytoprotection of central nervous system cells against oxidative stress via the nuclear factor (erythroid-derived 2)-like 2 pathway. J. Pharmacol. Exp. Ther. 2012, 341, 274–284. [Google Scholar] [CrossRef]
- Boas, S.M.; Joyce, K.L.; Cowell, R.M. The NRF2-Dependent Transcriptional Regulation of Antioxidant Defense Pathways: Relevance for Cell Type-Specific Vulnerability to Neurodegeneration and Therapeutic Intervention. Antioxidants 2021, 11, 8. [Google Scholar] [CrossRef]
- Guo, T.Z.; Shi, X.; Li, W.; Wei, T.; Kingery, W.S.; Clark, J.D. Dimethyl Fumarate Reduces Oxidative Stress and Pronociceptive Immune Responses in a Murine Model of Complex Regional Pain Syndrome. Anesth. Analg. 2021, 132, 1475–1485. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Holtzclaw, W.D.; Kensler, T.W. The role of Keap1 in cellular protective responses. Chem. Res. Toxicol. 2005, 18, 1779–1791. [Google Scholar] [CrossRef]
- Petri, S.; Körner, S.; Kiaei, M. Nrf2/ARE Signaling Pathway: Key Mediator in Oxidative Stress and Potential Therapeutic Target in ALS. Neurol. Res. Int. 2012, 2012, 878030. [Google Scholar] [CrossRef]
- Uddin, M.S.; Mamun, A.A.; Jakaria, M.; Thangapandiyan, S.; Ahmad, J.; Rahman, M.A.; Mathew, B.; Abdel-Daim, M.M.; Aleya, L. Emerging promise of sulforaphane-mediated Nrf2 signaling cascade against neurological disorders. Sci. Total Env. 2020, 707, 135624. [Google Scholar]
- Danilov, C.A.; Chandrasekaran, K.; Racz, J.; Soane, L.; Zielke, C.; Fiskum, G. Sulforaphane protects astrocytes against oxidative stress and delayed death caused by oxygen and glucose deprivation. Glia 2009, 57, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Santín-Márquez, R.; Alarcón-Aguilar, A.; López-Diazguerrero, N.E.; Chondrogianni, N.; Königsberg, M. Sulforaphane—Role in aging and neurodegeneration. Geroscience 2019, 41, 655–670. [Google Scholar] [CrossRef]
- Sun, Z.; Huang, Z.; Zhang, D.D. Phosphorylation of Nrf2 at multiple sites by MAP kinases has a limited contribution in modulating the Nrf2-dependent antioxidant response. PLoS ONE 2009, 4, e6588. [Google Scholar] [CrossRef]
- Dalla Bella, E.; Bersano, E.; Antonini, G.; Borghero, G.; Capasso, M.; Caponnetto, C.; Chiò, A.; Corbo, M.; Filosto, M.; Giannini, F.; et al. The unfolded protein response in amyotrophic later sclerosis: Results of a phase 2 trial. Brain 2021, 144, 2635–2647. [Google Scholar] [CrossRef]
- Abdalkader, M.; Lampinen, R.; Kanninen, K.M.; Malm, T.M.; Liddell, J.R. Targeting Nrf2 to Suppress Ferroptosis and Mitochondrial Dysfunction in Neurodegeneration. Front. Neurosci. 2018, 12, 466. [Google Scholar] [CrossRef] [PubMed]
- Hadadi, M.; Farazi, M.M.; Mehrabani, M.; Tashakori-Miyanroudi, M.; Behroozi, Z. Curcumin reduces pain after spinal cord injury in rats by decreasing oxidative stress and increasing GABAA receptor and GAD65 levels. Sci. Rep. 2025, 15, 12910. [Google Scholar] [CrossRef] [PubMed]
- Belcaro, G.; Cesarone, M.R.; Dugall, M.; Pellegrini, L.; Ledda, A.; Grossi, M.G.; Togni, S.; Appendino, G. Efficacy and safety of Meriva®, a curcumin-phosphatidylcholine complex, during extended administration in osteoarthritis patients. Altern. Med. Rev. 2010, 15, 337–344. [Google Scholar]
- Wang, J.; Cao, Y.; Lu, Y.; Zhu, H.; Zhang, J.; Che, J.; Zhuang, R.; Shao, J. Recent progress and applications of small molecule inhibitors of Keap1-Nrf2 axis for neurodegenerative diseases. Eur. J. Med. Chem. 2024, 264, 115998. [Google Scholar] [CrossRef]
- Keerie, A.F.; Martins, R.R.; Allen, C.F.; Bowden, K.; Al Mashhadi, S.; Marlow, T.; Myszczynska, M.; Thakur, N.; Beal, S.N.; Shaw, A.; et al. M102, a combined NRF2 and HSF-1 activator for neuroprotection in amyotrophic lateral sclerosis. bioRxiv 2024. [Google Scholar] [CrossRef]
- Kim, H.J.; Taylor, J.P. Lost in Transportation: Nucleocytoplasmic Transport Defects in ALS and Other Neurodegenerative Diseases. Neuron 2017, 96, 285–297. [Google Scholar] [CrossRef]
- Theodore, M.; Kawai, Y.; Yang, J.; Kleshchenko, Y.; Reddy, S.P.; Villalta, F.; Arinze, I.J. Multiple nuclear localization signals function in the nuclear import of the transcription factor Nrf2. J. Biol. Chem. 2008, 283, 8984–8994. [Google Scholar] [CrossRef]
- Xiao, J.L.; Liu, H.Y.; Sun, C.C.; Tang, C.F. Regulation of Keap1-Nrf2 signaling in health and diseases. Mol. Biol. Rep. 2024, 51, 809. [Google Scholar] [CrossRef]
- Mayer, C.; Riera-Ponsati, L.; Kauppinen, S.; Klitgaard, H.; Erler, J.T.; Hansen, S.N. Targeting the NRF2 pathway for disease modification in neurodegenerative diseases: Mechanisms and therapeutic implications. Front. Pharmacol. 2024, 15, 1437939. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Chen, Q.; Chen, X.; Han, F.; Chen, Z.; Wang, Y. The blood–brain barrier: Structure, regulation and drug delivery. Signal Transduct. Target. Ther. 2023, 8, 217. [Google Scholar] [CrossRef]
- Yamamoto, M.; Kensler, T.W.; Motohashi, H. The KEAP1-NRF2 System: A Thiol-Based Sensor-Effector Apparatus for Maintaining Redox Homeostasis. Physiol. Rev. 2018, 98, 1169–1203. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tavana, O.; Gu, W. p53 modifications: Exquisite decorations of the powerful guardian. J. Mol. Cell Biol. 2019, 11, 564–577. [Google Scholar] [CrossRef]
- Cuadrado, A.; Rojo, A.I.; Wells, G.; Hayes, J.D.; Cousin, S.P.; Rumsey, W.L.; Attucks, O.C.; Franklin, S.; Levonen, A.L.; Kensler, T.W.; et al. Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases. Nat. Rev. Drug Discov. 2019, 18, 295–317. [Google Scholar] [CrossRef]
- Freibaum, B.D.; Taylor, J.P. The Role of Dipeptide Repeats in C9ORF72-Related ALS-FTD. Front. Mol. Neurosci. 2017, 10, 35. [Google Scholar] [CrossRef]
- Dormann, D.; Haass, C. TDP-43 and FUS: A nuclear affair. Trends Neurosci. 2011, 34, 339–348. [Google Scholar] [CrossRef]
- Sebghatollahi, Z.; Yogesh, R.; Mahato, N.; Kumar, V.; Mohanta, Y.K.; Baek, K.-H.; Mishra, A.K. Signaling Pathways in Oxidative Stress-Induced Neurodegenerative Diseases: A Review of Phytochemical Therapeutic Interventions. Antioxidants 2025, 14, 457. [Google Scholar] [CrossRef] [PubMed]
- Holmström, K.M.; Baird, L.; Zhang, Y.; Hargreaves, I.; Chalasani, A.; Land, J.M.; Stanyer, L.; Yamamoto, M.; Dinkova-Kostova, A.T.; Abramov, A.Y. Nrf2 impacts cellular bioenergetics by controlling substrate availability for mitochondrial respiration. Biol. Open 2013, 2, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yu, C.; Kang, R.; Kroemer, G.; Tang, D. Cellular degradation systems in ferroptosis. Cell Death Differ. 2021, 28, 1135–1148. [Google Scholar] [CrossRef] [PubMed]
- Calì, T.; Ottolini, D.; Brini, M. Calcium and endoplasmic reticulum-mitochondria tethering in neurodegeneration. DNA Cell Biol. 2013, 32, 140–146. [Google Scholar] [CrossRef]
- Diskin, C.; Day, E.A.; Henry, Ó.C.; Toller-Kawahisa, J.E.; O’Neill, L.A.J. 4-Octyl Itaconate and Dimethyl Fumarate Induce Secretion of the Anti-Inflammatory Protein Annexin A1 via NRF2. J. Immunol. 2023, 211, 1032–1041. [Google Scholar] [CrossRef]
- Tarozzi, A.; Angeloni, C.; Malaguti, M.; Morroni, F.; Hrelia, S.; Hrelia, P. Sulforaphane as a potential protective phytochemical against neurodegenerative diseases. Oxid. Med. Cell Longev. 2013, 2013, 415078. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim Fouad, G.; El-Sayed, S.A.M.; Mabrouk, M.; Ahmed, K.A.; Beherei, H.H. Neuroprotective Potential of Intranasally Delivered Sulforaphane-Loaded Iron Oxide Nanoparticles Against Cisplatin-Induced Neurotoxicity. Neurotox. Res. 2022, 40, 1479–1498. [Google Scholar] [CrossRef] [PubMed]
- Satoh, T.; Okamoto, S.-i.; Cui, J.; Watanabe, Y.; Furuta, K.; Suzuki, M.; Tohyama, K.; Lipton, S.A. Activation of the Keap1/Nrf2 pathway for neuroprotection by electrophillic phase II inducers. Proc. Natl. Acad. Sci. USA 2006, 103, 768–773. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Long, A.; Hao, L.; Shi, Z.; Zhang, M. Astrocyte in Neurological Disease: Pathogenesis and Therapy. MedComm (2020) 2025, 6, e70299. [Google Scholar] [CrossRef] [PubMed]
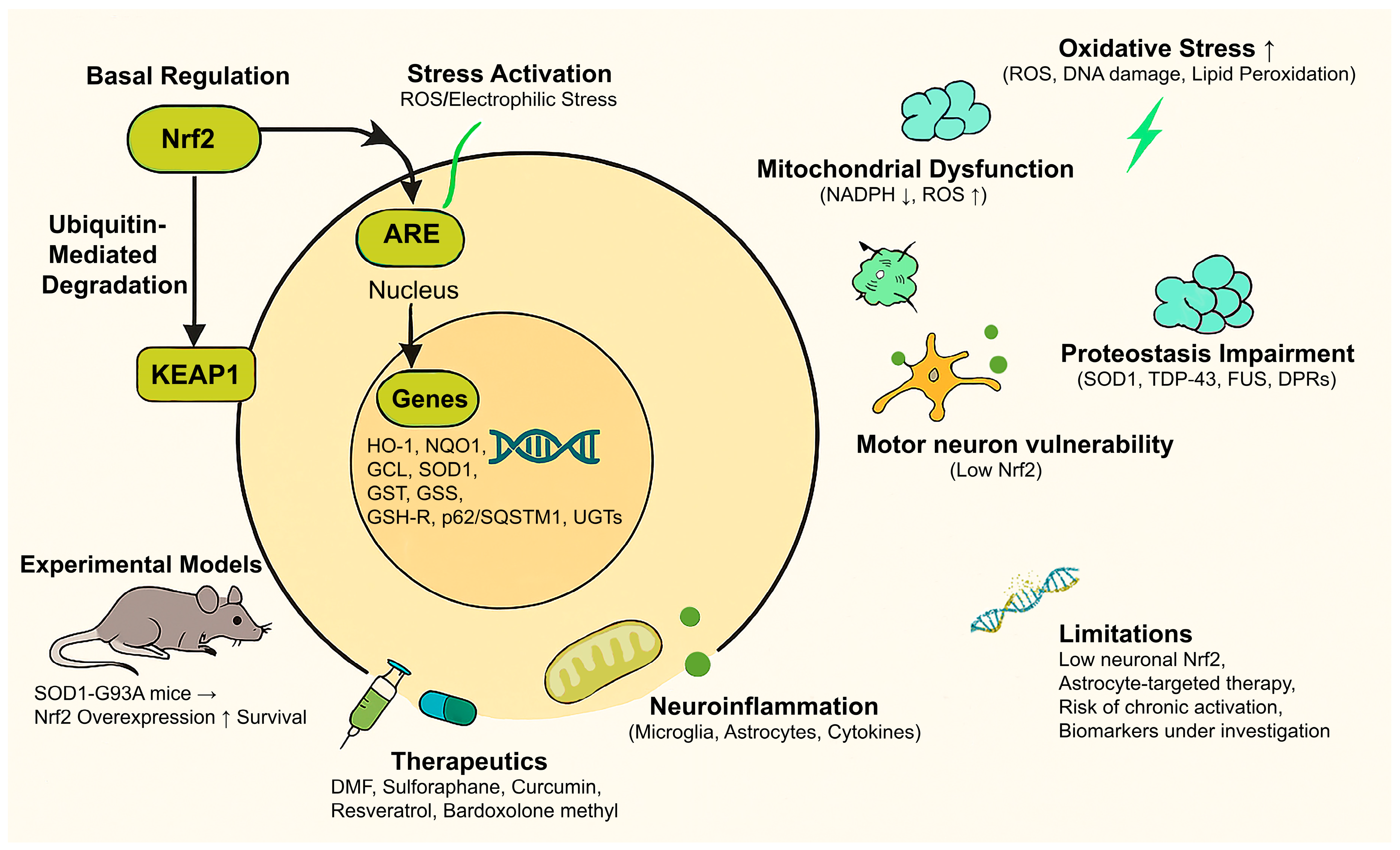
| Category | Description |
|---|---|
| Nrf2 Overview | Nrf2 is a transcription factor that regulates the expression of antioxidants and cytoprotective genes via the ARE pathway. |
| Basal Regulation | In unstressed conditions, Nrf2 is bound to KEAP1 in the cytoplasm and targeted for ubiquitin-mediated degradation. |
| Stress Activation | Upon oxidative/electrophilic stress, KEAP1 is modified, allowing newly synthesized Nrf2 proteins to a) escape Keap1-mediated ubiquitination b) translocate to the nucleus, c) bind to AREs, and activate gene transcription. |
| Main Target Genes | E.g., encoding HO-1, NQO1, GCL, SOD1, GST, GSS, GSH-R, p62/SQSTM1, proteasomal subunits, UGTs, involved in antioxidant defense, detoxification, and proteostasis. |
| Oxidative Stress in ALS | Elevated ROS, lipid peroxidation, and oxidative DNA/protein damage are prominent in both familial and sporadic ALS. |
| Nrf2 Role in Redox Balance | Induces antioxidant enzymes and glutathione biosynthesis pathways; reduces ROS accumulation. |
| Mitochondrial Dysfunction | ALS shows impaired mitochondrial function; Nrf2 supports mitochondrial health through NADPH production and mitochondrial gene regulation. |
| Proteostasis Impairment | Misfolded protein aggregation (SOD1, TDP-43, FUS, and DPRs in C9orf72-ALS); Nrf2 enhances autophagy and UPS via p62/SQSTM1 to promote aggregate clearance. |
| Neuroinflammation | Microglial and astrocytic activation drive neurodegeneration; Nrf2 suppresses inflammatory cytokines and supports anti-inflammatory glial phenotypes. |
| Motor Neuron Vulnerability | Motor neurons have intrinsically low Nrf2 levels; glial-specific Nrf2 activation provides non-cell-autonomous protection. |
| Experimental Models | In ALS models (e.g., SOD1-G93A mice), Nrf2 overexpression delays onset and prolongs survival; astrocyte-targeted Nrf2 shows stronger effects. |
| Therapeutic Candidates | DMF, Sulforaphane, Curcumin, Resveratrol, and Bardoxolone Methyl, preclinical evidence supports Nrf2 activation as a therapeutic strategy. |
| Limitations & Gaps | Neuronal Nrf2 expression is low; targeted delivery to astrocytes may be needed. Long-term activation may carry metabolic or oncogenic risks. |
| Clinical Translation | Early-phase trials with Nrf2 activators are ongoing; biomarkers of Nrf2 activation in ALS patients are still under investigation. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharbafshaaer, M.; Pepe, R.; Notariale, R.; Canale, F.; Tedeschi, G.; Tessitore, A.; Bergamo, P.; Trojsi, F. Beyond Antioxidants: The Emerging Role of Nrf2 Activation in Amyotrophic Lateral Sclerosis (ALS). Int. J. Mol. Sci. 2025, 26, 9872. https://doi.org/10.3390/ijms26209872
Sharbafshaaer M, Pepe R, Notariale R, Canale F, Tedeschi G, Tessitore A, Bergamo P, Trojsi F. Beyond Antioxidants: The Emerging Role of Nrf2 Activation in Amyotrophic Lateral Sclerosis (ALS). International Journal of Molecular Sciences. 2025; 26(20):9872. https://doi.org/10.3390/ijms26209872
Chicago/Turabian StyleSharbafshaaer, Minoo, Roberta Pepe, Rosaria Notariale, Fabrizio Canale, Gioacchino Tedeschi, Alessandro Tessitore, Paolo Bergamo, and Francesca Trojsi. 2025. "Beyond Antioxidants: The Emerging Role of Nrf2 Activation in Amyotrophic Lateral Sclerosis (ALS)" International Journal of Molecular Sciences 26, no. 20: 9872. https://doi.org/10.3390/ijms26209872
APA StyleSharbafshaaer, M., Pepe, R., Notariale, R., Canale, F., Tedeschi, G., Tessitore, A., Bergamo, P., & Trojsi, F. (2025). Beyond Antioxidants: The Emerging Role of Nrf2 Activation in Amyotrophic Lateral Sclerosis (ALS). International Journal of Molecular Sciences, 26(20), 9872. https://doi.org/10.3390/ijms26209872









