Exploring the Mechanisms of n-Butanol Extract from Tibetan Medicine Biebersteinia heterostemon in Improving Type 2 Diabetes Based on Network Pharmacology and Cellular Experiments
Abstract
1. Introduction
2. Results
2.1. Chemical Composition Identification, Active Compound Screening, and Target Prediction of n-Butanol Extract from B. heterostemon
2.2. Network Pharmacology Analysis of BHBE in the Treatment of T2DM
2.2.1. Identification of Shared Targets Between Active Compounds of BHBE and T2DM, Network Construction, and Functional Pathway Enrichment
2.2.2. Network Analysis of “ BHBE-Active Ingredient-Common Targets-Pathway-T2DM”
2.3. Molecular Docking Between Active Compounds and Key Targets
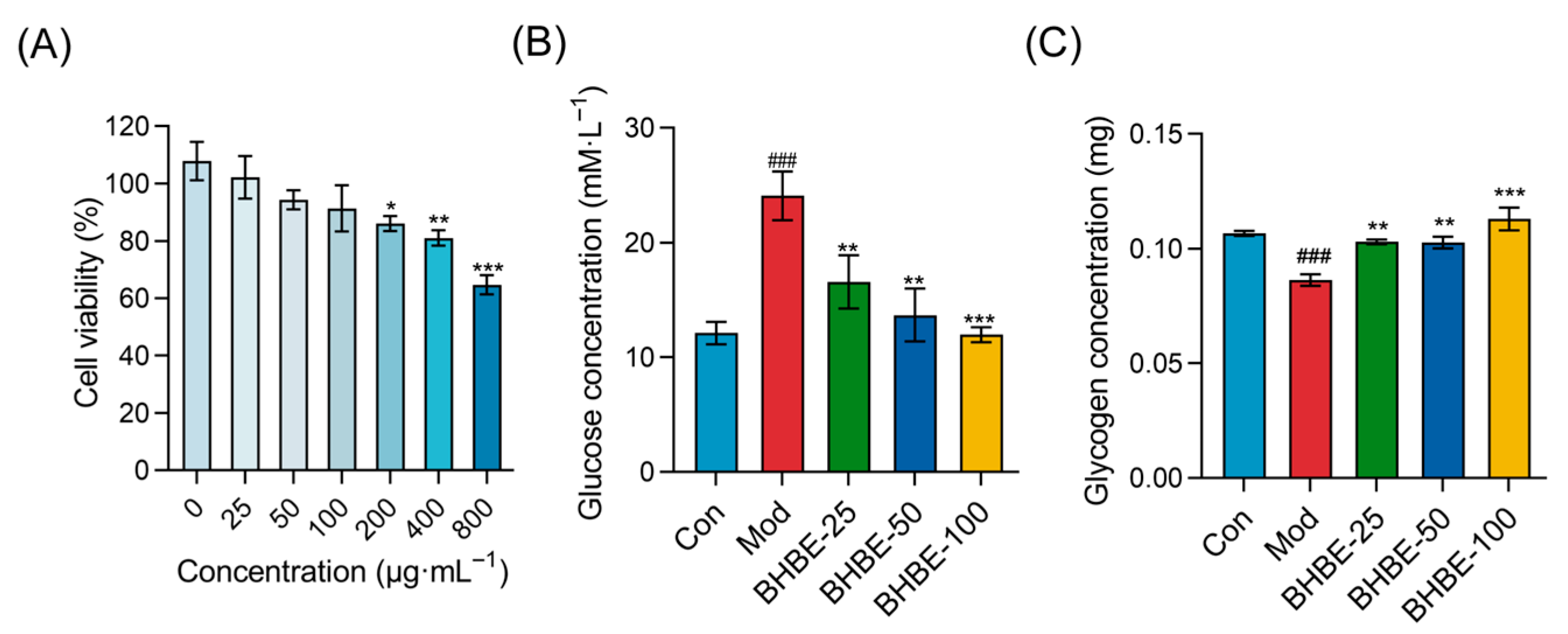
2.4. Improvement of Insulin Resistance in Insulin-Resistant (IR)—HepG2 Cells by BHBE
2.4.1. Glucose Uptake and Glycogen Content Assays
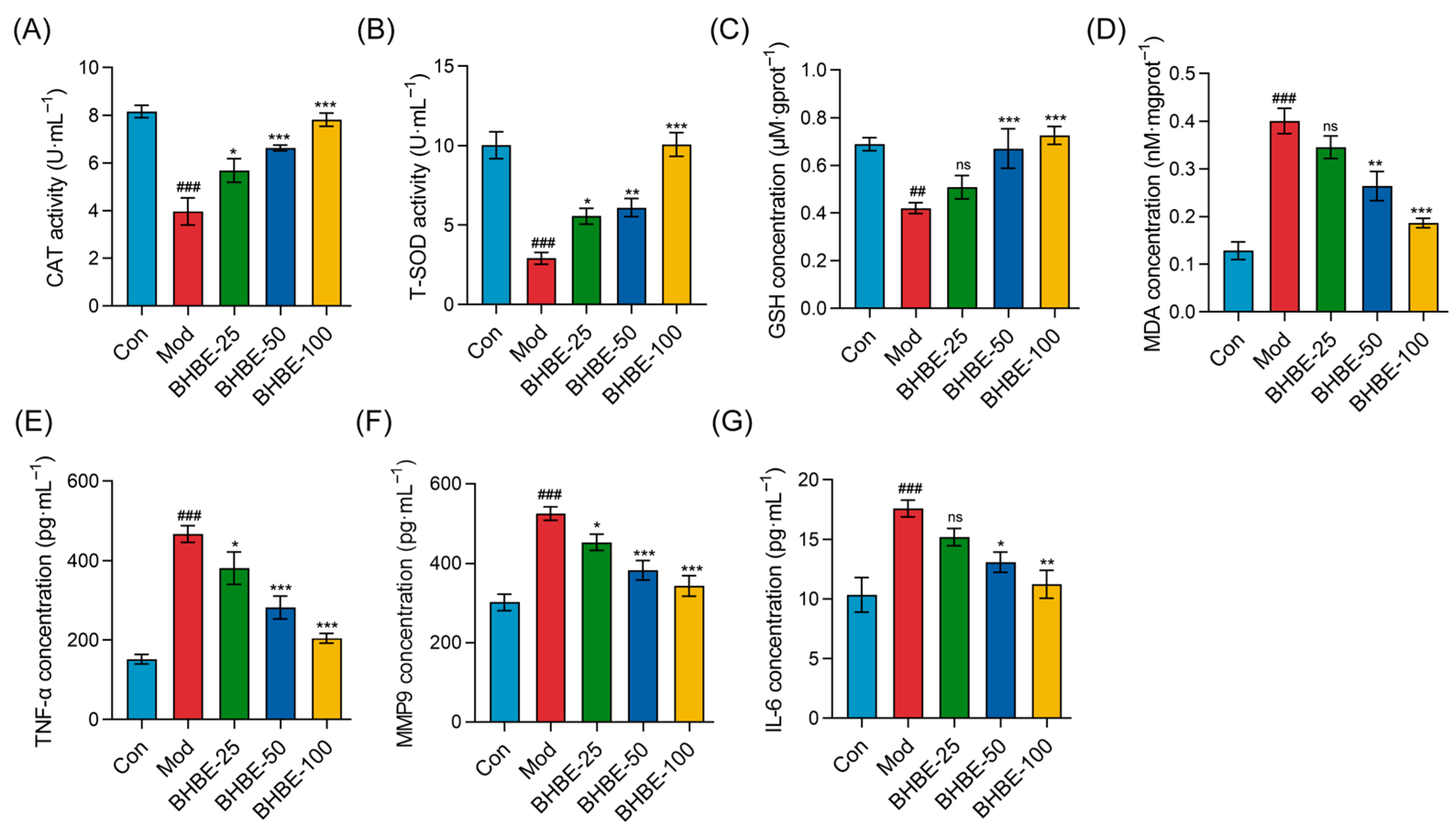
2.4.2. Regulation of Oxidative Stress and Inflammatory Markers in IR-HepG2 Cells by BHBE
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Preparation of the n-Butanol Extract
4.3. Qualitative Analysis of Chemical Constituents
4.4. Identification of Bioactive Compounds and Their Targets
4.5. Identification of T2DM-Associated Targets
4.6. Identification of Common Targets and PPI Network Construction
4.7. GO and KEGG Enrichment Analysis
4.8. Construction of the Compound-Target-Disease-Pathway Network
4.9. Molecular Docking
4.10. In Vitro Evaluation of Hypoglycemic Activity in IR-HepG2 Cells
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BP | Biological Process |
| CC | Cellular Component |
| DL | Drug-Likeness |
| DMEM | Dulbecco’ s Modified Eagle Medium |
| FBS | Fetal Bovine Serum |
| GO | Gene Ontology |
| IR | Insulin Resistance |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| LC-MS/MS | Liquid Chromatography-tandem Mass Spectrometry |
| MF | Molecular Function |
| OB | Oral Bioavailability |
| PPI | Protein–Protein Interaction |
| TIC | Total Ion Chromatogram |
| T2DM | Type 2 Diabetes Mellitus |
References
- Zhou, B.; Rayner, A.W.; Gregg, E.W.; Sheffer, K.E.; Carrillo-Larco, R.M.; Bennett, J.E.; Shaw, J.E.; Paciorek, C.J.; Singleton, R.K.; Barradas Pires, A.; et al. Worldwide trends in diabetes prevalence and treatment from 1990 to 2022: A pooled analysis of 1108 population-representative studies with 141 million participants. Lancet 2024, 404, 2077–2093. [Google Scholar] [CrossRef] [PubMed]
- Duncan, B.B.; Magliano, D.J.; Boyko, E.J. IDF diabetes atlas 11th edition 2025: Global prevalence and projections for 2050. Nephrol. Dial. Transplant. 2025, gfaf177. [Google Scholar] [CrossRef]
- Tayyab, S.L.; Seher, W.; Hussain, K.; Murtaza, I. Diabetes: A Global Health Concern and Potential Strategies to Reduce Its Prevalence. In Integrated Science for Sustainable Development Goal 3: Empowering Global Wellness Initiatives; Rezaei, N., Ed.; Springer Nature: Cham, Switzerland, 2024; pp. 329–348. [Google Scholar]
- Kalyani, R.R.; Neumiller, J.J.; Maruthur, N.M.; Wexler, D.J. Diagnosis and Treatment of Type 2 Diabetes in Adults: A Review. JAMA 2025, 334, 984–1002. [Google Scholar] [CrossRef]
- Lu, X.; Xie, Q.; Pan, X.; Zhang, R.; Zhang, X.; Peng, G.; Zhang, Y.; Shen, S.; Tong, N. Type 2 diabetes mellitus in adults: Pathogenesis, prevention and therapy. Signal Transduct. Target. Ther. 2024, 9, 262. [Google Scholar] [CrossRef]
- Shiwen, Y.; Ying, L.; Shengzhao, Z.; Fengbo, W.; Dan, L.; Qingfang, W.; Hanrui, Z.; Ping, F.; Na, S. Risk of diabetic ketoacidosis of SGLT2 inhibitors in patients with type 2 diabetes: A systematic review and network meta-analysis of randomized controlled trials. Front. Pharmacol. 2023, 14, 1145587. [Google Scholar] [CrossRef] [PubMed]
- Hoon, C.Y.; KyungDo, H.; Rae, C.I.; Seok, L.I.; Kon, R.J.; YongTae, K.; Hyun, C.K.; Hyub, L.S. Underweight Is Associated with a Higher Risk of Acute Pancreatitis in Type 2 Diabetes: A Nationwide Cohort Study. J. Clin. Med. 2022, 11, 5641. [Google Scholar] [CrossRef]
- H, L.C.; Dtw, L.; Ksl, L. Non-alcoholic fatty liver disease and type 2 diabetes—An Update. J. Diabetes Investig. 2022, 13, 930–940. [Google Scholar]
- Yang, D.R.; Wang, M.Y.; Zhang, C.L.; Wang, Y. Endothelial dysfunction in vascular complications of diabetes: A comprehensive review of mechanisms and implications. Front. Endocrinol. 2024, 15, 1359255. [Google Scholar] [CrossRef] [PubMed]
- Uma, A.; Shanmugapriyan, S.; Rajesh, M.; Panneerselvam, P. Diabetic Kidney Disease in Type 2 Diabetes: A Comprehensive Review of Epidemiology, Pathophysiology, and Therapeutic Advances. J. Pharm. Bioallied Sci. 2025, 17, 33–35. [Google Scholar] [CrossRef]
- Rafał, F.; Mateusz, K.; Andrzej, W.; Monika, R.; Tadeusz, P.; Kasper, S.; Marcin, K. Type 2 Diabetes Mellitus, Non-Alcoholic Fatty Liver Disease, and Metabolic Repercussions: The Vicious Cycle and Its Interplay with Inflammation. Int. J. Mol. Sci. 2023, 24, 9677. [Google Scholar] [CrossRef] [PubMed]
- Egbuna, C.; Awuchi, C.G.; Kushwaha, G.; Rudrapal, M.; Patrick-Iwuanyanwu, K.C.; Singh, O.; Odoh, U.E.; Khan, J.; Jeevanandam, J.; Kumarasamy, S.; et al. Bioactive Compounds Effective Against Type 2 Diabetes Mellitus: A Systematic Review. Curr. Top Med. Chem. 2021, 21, 1067–1095. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J. Non-Insulin-Based Drug Entities Used to Treat Diabetes Type 2 Disease (T2DM), Based on Natural Products from All Sources. J Nat Prod 2024, 87, 629–637. [Google Scholar] [CrossRef]
- Ni, Y.; Wu, X.; Yao, W.; Zhang, Y.; Chen, J.; Ding, X. Evidence of traditional Chinese medicine for treating type 2 diabetes mellitus: From molecular mechanisms to clinical efficacy. Pharm. Biol. 2024, 62, 592–606. [Google Scholar] [CrossRef] [PubMed]
- Willcox, M.L.; Elugbaju, C.; Al-Anbaki, M.; Lown, M.; Graz, B. Effectiveness of Medicinal Plants for Glycaemic Control in Type 2 Diabetes: An Overview of Meta-Analyses of Clinical Trials. Front. Pharmacol. 2021, 12, 777561. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, L.; Yan, Y.; Hu, X.; Li, Q.; Li, L.; Wang, Y.; Tao, X.; Yang, L.; Peng, M.; et al. Investigation of the Pharmacodynamic Components of Gastrodia elata Blume for Treatment of Type 2 Diabetes Mellitus through HPLC, Bioactivity, Network Pharmacology and Molecular Docking. Int. J. Mol. Sci. 2024, 25, 498. [Google Scholar] [CrossRef]
- Kukavica, B.; Škondrić, S.; Trifković, T.; Mišić, D.; Gašić, U.; Topalić-Trivunović, L.; Savić, A.; Velemir, A.; Davidović-Plavšić, B.; Šešić, M.; et al. Comparative polyphenolic profiling of five ethnomedicinal plants and their applicative potential in the treatment of type 2 diabetes. J. Ethnopharmacol. 2024, 320, 117377. [Google Scholar] [CrossRef] [PubMed]
- Ying, W.; Zhi, Z.S.; Xia, L.F. Recent Advances in the Bioactive Constituents of Tibetan Medicinal Plant Biebersteinia heterostemon Maxim. J. Tradit. Chin. Vet. Med. 2022, 41, 92–96. [Google Scholar] [CrossRef]
- Liu, Z.; Silva, J.; Shao, A.S.; Liang, J.; Wallner, M.; Shao, X.M.; Li, M.; Olsen, R.W. Flavonoid compounds isolated from Tibetan herbs, binding to GABA(A) receptor with anxiolytic property. J. Ethnopharmacol. 2021, 267, 113630. [Google Scholar] [CrossRef]
- Zhang, B.; Jin, X.; Yin, H.; Zhang, D.; Zhou, H.; Zhang, X.; Tran, L.P. Natural Products, Traditional Uses and Pharmacological Activities of the Genus Biebersteinia (Biebersteiniaceae). Plants 2020, 9, 595. [Google Scholar] [CrossRef]
- Ali, M.; Hassan, M.; Ansari, S.A.; Alkahtani, H.M.; Al-Rasheed, L.S.; Ansari, S.A. Quercetin and Kaempferol as Multi-Targeting Antidiabetic Agents against Mouse Model of Chemically Induced Type 2 Diabetes. Pharmaceuticals 2024, 17, 757. [Google Scholar] [CrossRef] [PubMed]
- Eng, W.W.; Yuan, Z.W. The Effect of the Active Alkaloid Fraction from Biebersteinia heterostemon on Blood Glucose in Streptozotocin-Induced Diabetic Mice. Chin. Tradit. Pat. Med. 2011, 33, 1584–1586. [Google Scholar]
- Jin, L.; Wei, R.; Ping, c.G.; Ke, L.; Miao, X.; Ming, Y.J.; Ming, L.; Yuan, Q.X. Effects of soybean oligopeptides and pea oligopeptides on the glucose metabolism and relating mechanisms of insulin-resistant HepG2. Food Ferment. Ind. 2024, 50, 67–72. [Google Scholar] [CrossRef]
- Hou, X.; Snarski, P.; Higashi, Y.; Yoshida, T.; Jurkevich, A.; Delafontaine, P.; Sukhanov, S. Nuclear complex of glyceraldehyde-3-phosphate dehydrogenase and DNA repair enzyme apurinic/apyrimidinic endonuclease I protect smooth muscle cells against oxidant-induced cell death. FASEB J. 2017, 31, 3179–3192. [Google Scholar] [CrossRef]
- Elizabeth, H.; Matthew, L.; John, W.T.; Tarasov, A.I.; Jonas, S.; Idoia, P.; Terron, E.R.; Gregor, S.; Malgorzata, C.; Maria, R.; et al. Altered glycolysis triggers impaired mitochondrial metabolism and mTORC1 activation in diabetic β-cells. Nat. Commun. 2022, 13, 6754. [Google Scholar] [CrossRef]
- Kalinina, E.V.; Novichkova, M.D. S-Glutathionylation and S-Nitrosylation as Modulators of Redox-Dependent Processes in Cancer Cell. Biochemistry 2023, 88, 924–943. [Google Scholar]
- Jinhee, H.; Thurmond, D.C. Exocytosis Proteins: Typical and Atypical Mechanisms of Action in Skeletal Muscle. Front. Endocrinol. 2022, 13, 915509. [Google Scholar] [CrossRef]
- Won, L.Y.; Hee, P.Y. Monascus-fermented grain vinegar enhances glucose homeostasis through the IRS-1/PI3K/Akt and AMPK signaling pathways in HepG2 cell and db/db mice. Food Sci. Biotechnol. 2022, 31, 1583–1591. [Google Scholar]
- Zhao, H.; Zhai, B.W.; Zhang, M.Y.; Huang, H.; Zhu, H.L.; Yang, H.; Ni, H.Y.; Fu, Y.J. Phlorizin from Lithocarpus litseifolius [Hance] Chun ameliorates FFA-induced insulin resistance by regulating AMPK/PI3K/AKT signaling pathway. Phytomedicine 2024, 130, 155743. [Google Scholar] [CrossRef]
- Yan, J.; Wang, C.; Jin, Y.; Meng, Q.; Liu, Q.; Liu, Z.; Liu, K.; Sun, H. Catalpol ameliorates hepatic insulin resistance in type 2 diabetes through acting on AMPK/NOX4/PI3K/AKT pathway. Pharmacol. Res. 2018, 130, 466–480. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liu, G.; Guo, J.; Su, Z. The PI3K/AKT pathway in obesity and type 2 diabetes. Int. J. Biol. Sci. 2018, 14, 1483–1496. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.E.; Hoon, L.D.; SaeSeul, I.; Jeong, Y.; Sun, G.H. Correlation between PPARG Pro12Ala Polymorphism and Therapeutic Responses to Thiazolidinediones in Patients with Type 2 Diabetes: A Meta-Analysis. Pharmaceutics 2023, 15, 1778. [Google Scholar] [CrossRef]
- Jurjus, A.; Eid, A.; Kattar, S.A.; Zeenny, M.N.; Gerges-Geagea, A.; Haydar, H.; Hilal, A.; Oueidat, D.; Matar, M.; Tawilah, J.; et al. Inflammatory bowel disease, colorectal cancer and type 2 diabetes mellitus: The links. BBA Clin. 2016, 5, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Fan, J.; Du, L.; Ren, G. Prenylated flavonoid fractions from Glycyrrhiza glabra alleviate insulin resistance in HepG2 cells by regulating the ERK/IRS-1 and PI3K/Akt signaling pathways. Arch. Pharmacal. Res. 2024, 47, 127–145. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, Y.; Xiao, L.; Zhu, G.; Li, J.; Song, X.; Xu, L.; Hu, J. Acetylcorynoline inhibits microglia activation by regulating EGFR/MAPK signaling to promote functional recovery of injured mouse spinal cord. Nan Fang Yi Ke Da Xue Xue Bao 2023, 43, 915–923. [Google Scholar] [CrossRef]
- Lee, Y.H.; Seo, E.K.; Lee, S.T. Skullcapflavone II Inhibits Degradation of Type I Collagen by Suppressing MMP-1 Transcription in Human Skin Fibroblasts. Int. J. Mol. Sci. 2019, 20, 2734. [Google Scholar] [CrossRef]
- Kunqi, Z.; Chang, G.; Zhutong, S.; Ce, S.; Dali, M. Hepatoprotective Effect Associated with Alkaloids from Corydalis tomentella Franch. based on Network Pharmacology, Molecular Docking and in Vitro Experiment. Chem. Biodivers. 2022, 19, e202200542. [Google Scholar] [CrossRef]
- Wu, F.; Li, S.; Zhang, N.; Huang, W.; Li, X.; Wang, M.; Bai, D.; Han, B. Hispidulin alleviates high-glucose-induced podocyte injury by regulating protective autophagy. Biomed. Pharmacother. 2018, 104, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, L.; Chen, C.; Zou, M.; Wang, K.; Liu, X.; Kang, T.; Li, M.; Wu, D.; Jiang, Z.; et al. Investigation of the efficacy and potential pharmacological mechanism of Yupingfeng in treating chronic obstructive pulmonary disease: A meta-analysis and in silico study. J. Ethnopharmacol. 2025, 343, 119441. [Google Scholar] [CrossRef]
- Yang, X.; Lai, K.; Zhang, J.; Chen, Z.; Ding, W.; Jiang, Y.; Liu, Y. Glabridin Alleviates Metabolic Disorders in Diet-Induced Diabetic Mice. Phytother. Res. 2025. [Google Scholar] [CrossRef] [PubMed]
- Chawsheen, M. Predicting the efficacy of Akt inhibitors using AutoDock Vina software. J. Garmian Univ. 2018, 5, 1–10. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Sathyapalan, T.; Atkin, S.L.; Sahebkar, A. Molecular Mechanisms Linking Oxidative Stress and Diabetes Mellitus. Oxid. Med. Cell Longev. 2020, 2020, 8609213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Sun, B.; Wang, L.; Korla, P.K.; Liu, C. Micheliolide mitigates diabetic nephropathy by modulating oxidative stress and inflammation in rats. Phytomedicine 2025, 145, 157025. [Google Scholar] [CrossRef] [PubMed]
- Ru, J.; Li, P.; Wang, J.; Zhou, W.; Li, B.; Huang, C.; Li, P.; Guo, Z.; Tao, W.; Yang, Y.; et al. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform. 2014, 6, 1–6. [Google Scholar] [CrossRef] [PubMed]
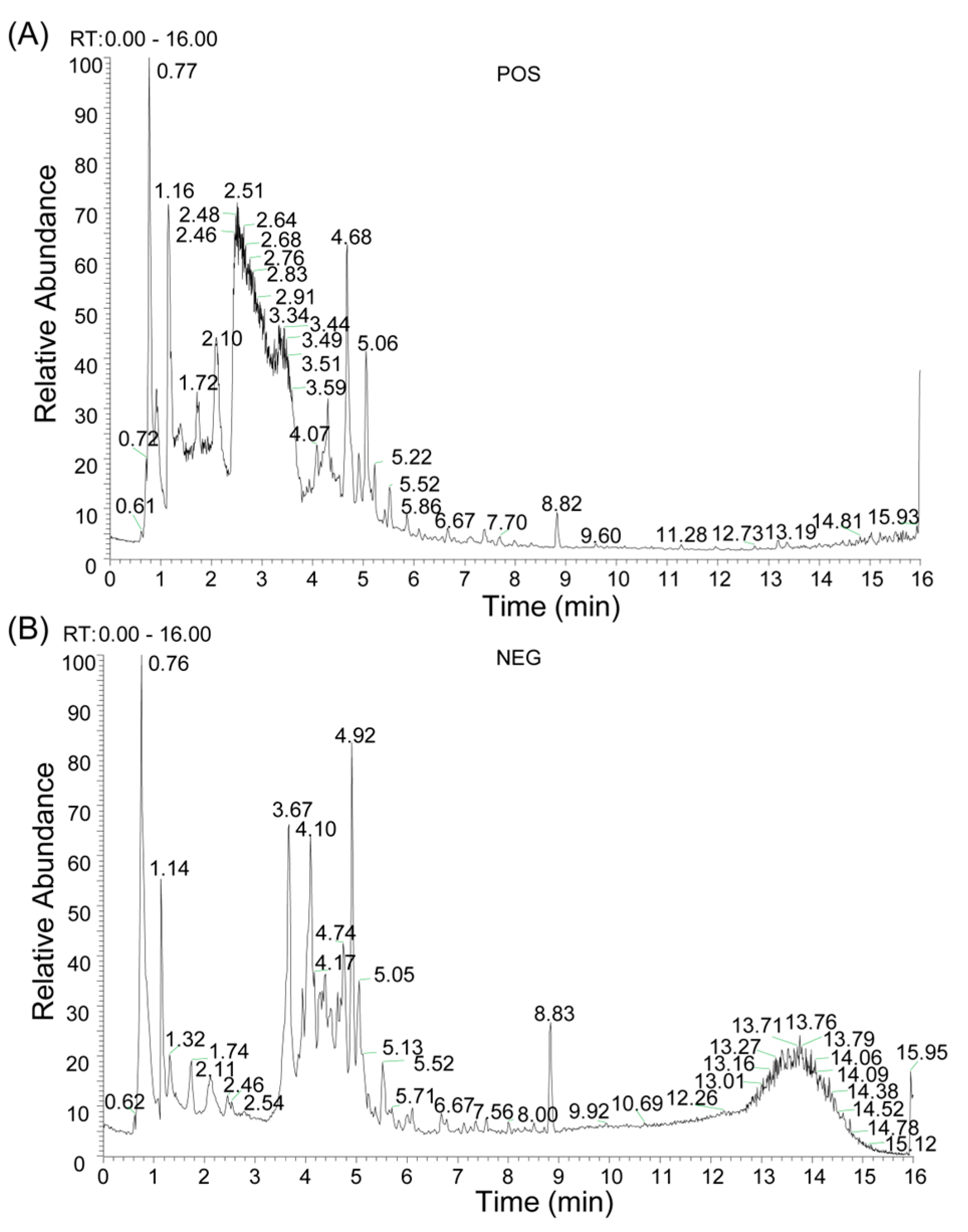
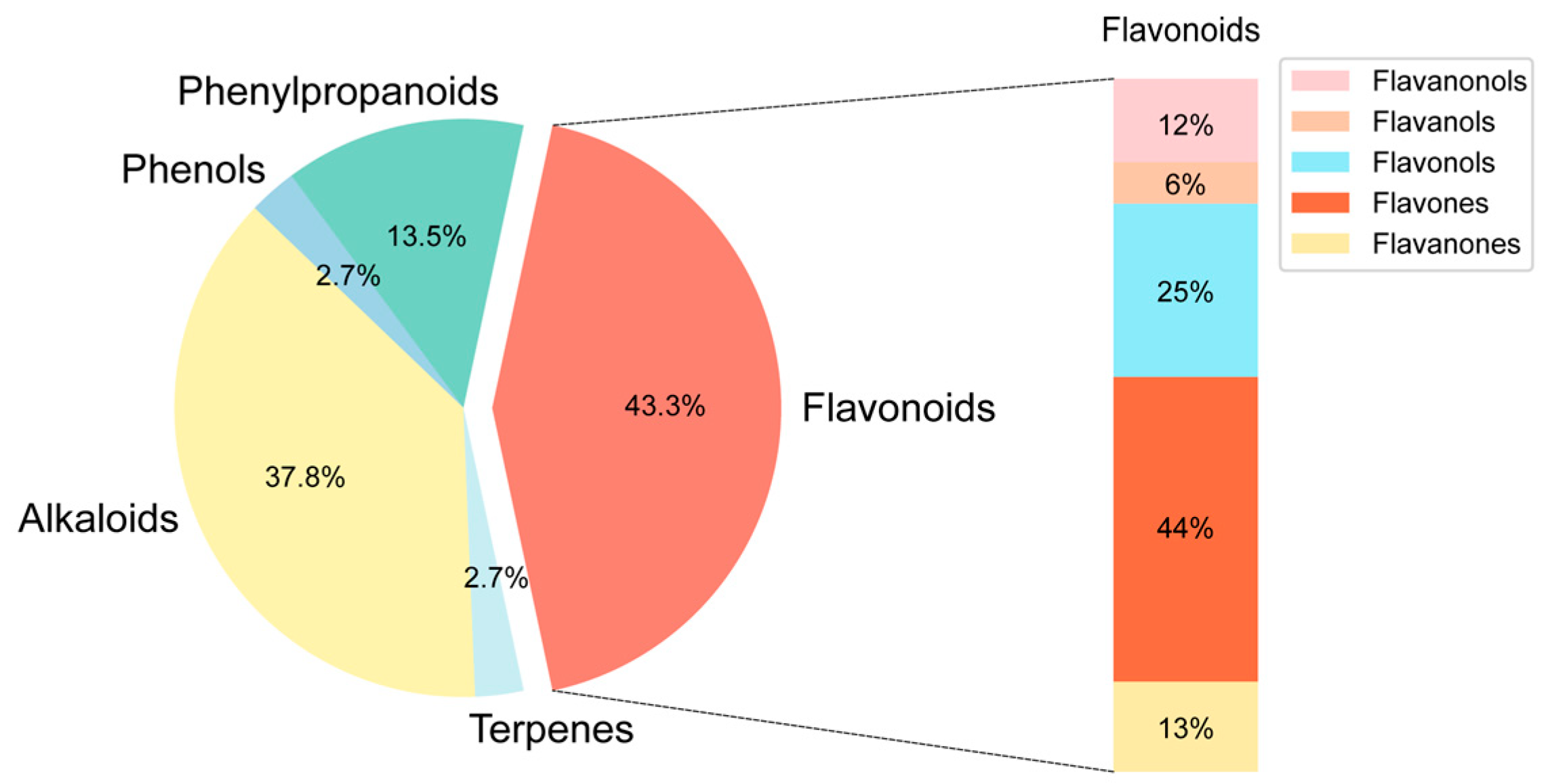
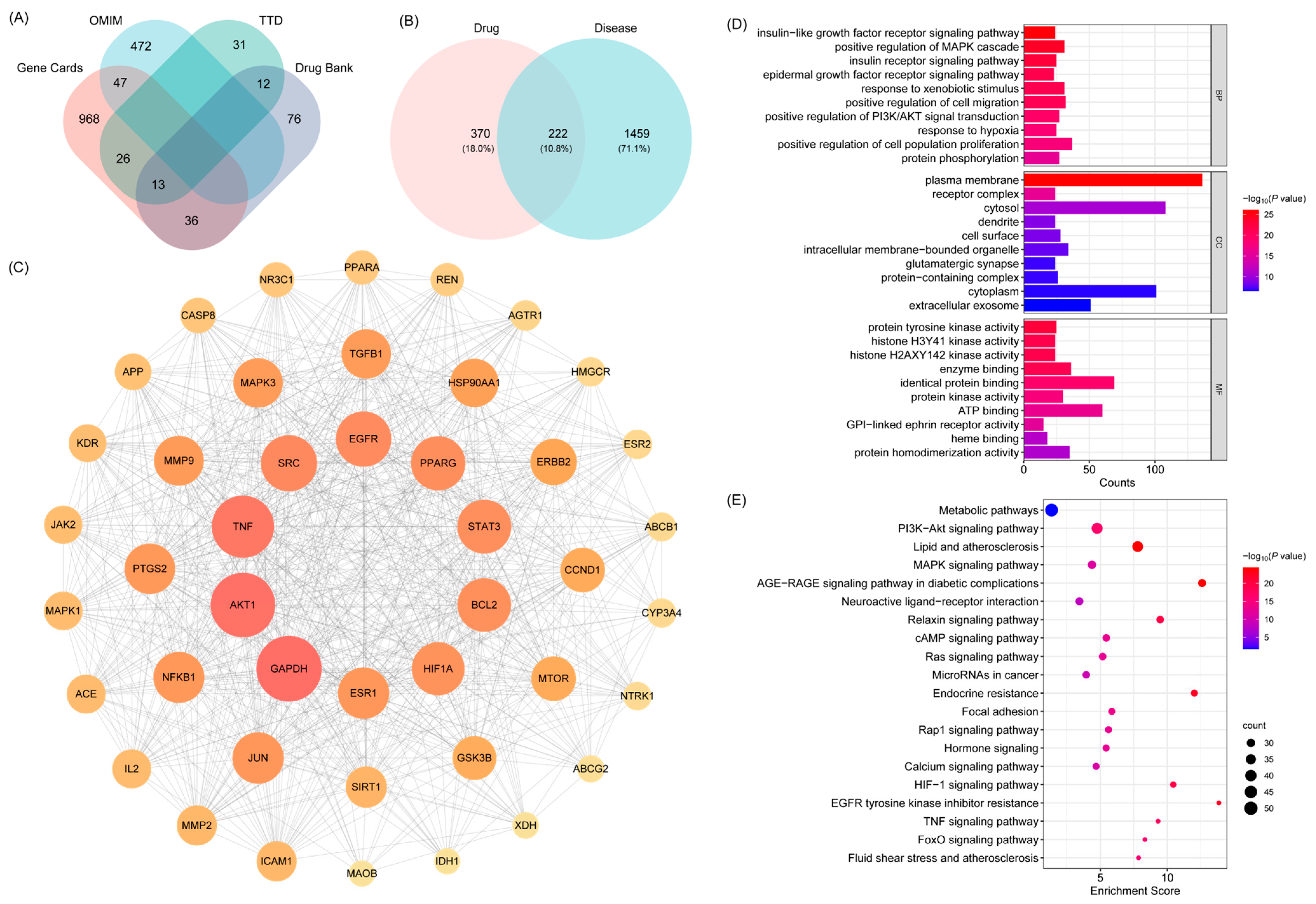
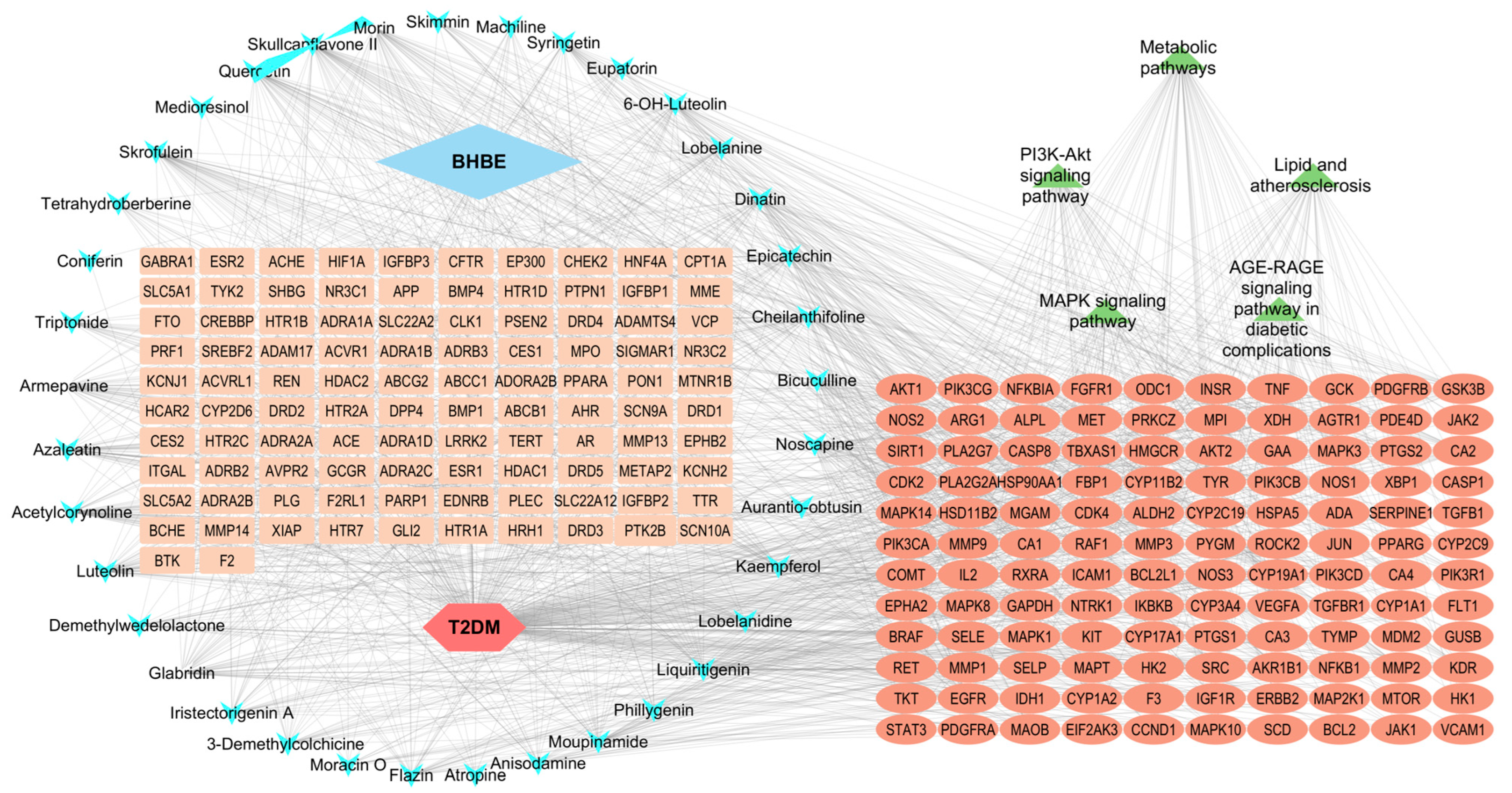

| Number | Molecule ID | Molecule Name | OB (≥30%) | DL (≥0.18) |
|---|---|---|---|---|
| 1 | MOL007274 | skrofulein | 30.35 | 0.3 |
| 2 | MOL000073 | epicatechin | 48.96 | 0.24 |
| 3 | MOL013083 | skimmin | 38.35 | 0.32 |
| 4 | MOL000006 | luteolin | 36.16 | 0.25 |
| 5 | MOL001733 | eupatorin | 30.23 | 0.37 |
| 6 | MOL001735 | dinatin | 30.97 | 0.27 |
| 7 | MOL003759 | iristectorigenin A | 63.36 | 0.34 |
| 8 | MOL009295 | flazin | 94.28 | 0.39 |
| 9 | MOL011604 | syringetin | 36.82 | 0.37 |
| 10 | MOL002219 | atropine | 34.53 | 0.21 |
| 11 | MOL004004 | 6-OH-luteolin | 46.93 | 0.28 |
| 12 | MOL009009 | medioresinol | 87.19 | 0.62 |
| 13 | MOL000737 | morin | 46.23 | 0.27 |
| 14 | MOL012719 | moracin O | 62.33 | 0.44 |
| 15 | MOL000422 | kaempferol | 41.88 | 0.24 |
| 16 | MOL012208 | lobelanine | 54.13 | 0.32 |
| 17 | MOL007207 | machiline | 79.64 | 0.24 |
| 18 | MOL001792 | liquiritigenin | 32.76 | 0.18 |
| 19 | MOL002927 | skullcapflavone II | 69.51 | 0.44 |
| 20 | MOL001455 | tetrahydroberberine | 30.35 | 0.3 |
| 21 | MOL003244 | triptonide | 53.83 | 0.77 |
| 22 | MOL008647 | moupinamide | 68.45 | 0.68 |
| 23 | MOL003330 | phillygenin | 86.71 | 0.26 |
| 24 | MOL009330 | noscapine | 95.04 | 0.57 |
| 25 | MOL000098 | quercetin | 53.29 | 0.88 |
| 26 | MOL005409 | anisodamine | 46.43 | 0.28 |
| 27 | MOL007206 | armepavine | 34.87 | 0.23 |
| 28 | MOL008648 | acetylcorynoline | 69.31 | 0.29 |
| 29 | MOL012207 | lobelanidine | 43.72 | 0.83 |
| 30 | MOL009458 | 3-demethylcolchicine | 60.53 | 0.32 |
| 31 | MOL003402 | demethylwedelolactone | 39.34 | 0.57 |
| 32 | MOL004908 | glabridin | 72.13 | 0.43 |
| 33 | MOL006472 | aurantio-obtusin | 52.51 | 0.5 |
| 34 | MOL004093 | azaleatin | 31.55 | 0.37 |
| 35 | MOL000791 | bicuculline | 54.28 | 0.3 |
| 36 | MOL009149 | cheilanthifoline | 69.67 | 0.88 |
| 37 | MOL000519 | coniferin | 46.51 | 0.72 |
| Target | Betweenness | Closeness | Degree |
|---|---|---|---|
| GAPDH | 3135.52 | 0.0034 | 143 |
| AKT1 | 2177.38 | 0.0033 | 141 |
| TNF | 2308.29 | 0.0033 | 135 |
| SRC | 3516.03 | 0.0031 | 119 |
| EGFR | 1237.43 | 0.0031 | 117 |
| PPARγ | 1571.36 | 0.0031 | 114 |
| Time | A% | B% |
|---|---|---|
| 0 | 95 | 5 |
| 2 | 95 | 5 |
| 4 | 70 | 30 |
| 8 | 50 | 50 |
| 10 | 20 | 80 |
| 14 | 0 | 100 |
| 15 | 0 | 100 |
| 15.1 | 95 | 5 |
| 16 | 95 | 5 |
| Parameters | Positive Ion | Negative Ion |
|---|---|---|
| Spray Voltage (V) | 3800 | −3000 |
| Capillary Temperature (°C) | 320 | 320 |
| Aux gas heater temperature (°C) | 350 | 350 |
| Sheath Gas Flow Rate (Arb) | 35 | 35 |
| Aux gas flow rate (Arb) | 8 | 8 |
| S-lens RF level | 50 | 50 |
| Mass range (m/z) | 100–1200 | 100–1200 |
| Full ms resolution | 70,000 | 70,000 |
| MS/MS resolution | 17,500 | 17,500 |
| NCE/stepped NCE | 10, 20, 40 | 10, 20, 40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Zeng, M.; Shen, X.; Zhang, B. Exploring the Mechanisms of n-Butanol Extract from Tibetan Medicine Biebersteinia heterostemon in Improving Type 2 Diabetes Based on Network Pharmacology and Cellular Experiments. Int. J. Mol. Sci. 2025, 26, 9866. https://doi.org/10.3390/ijms26209866
Chen S, Zeng M, Shen X, Zhang B. Exploring the Mechanisms of n-Butanol Extract from Tibetan Medicine Biebersteinia heterostemon in Improving Type 2 Diabetes Based on Network Pharmacology and Cellular Experiments. International Journal of Molecular Sciences. 2025; 26(20):9866. https://doi.org/10.3390/ijms26209866
Chicago/Turabian StyleChen, Shengwen, Mengting Zeng, Xiuxiu Shen, and Benyin Zhang. 2025. "Exploring the Mechanisms of n-Butanol Extract from Tibetan Medicine Biebersteinia heterostemon in Improving Type 2 Diabetes Based on Network Pharmacology and Cellular Experiments" International Journal of Molecular Sciences 26, no. 20: 9866. https://doi.org/10.3390/ijms26209866
APA StyleChen, S., Zeng, M., Shen, X., & Zhang, B. (2025). Exploring the Mechanisms of n-Butanol Extract from Tibetan Medicine Biebersteinia heterostemon in Improving Type 2 Diabetes Based on Network Pharmacology and Cellular Experiments. International Journal of Molecular Sciences, 26(20), 9866. https://doi.org/10.3390/ijms26209866





