Human Small Airway Epithelia Reveal Dichloroacetate as a Broad-Spectrum Antiviral Against Respiratory Viruses
Abstract
1. Introduction
2. Results
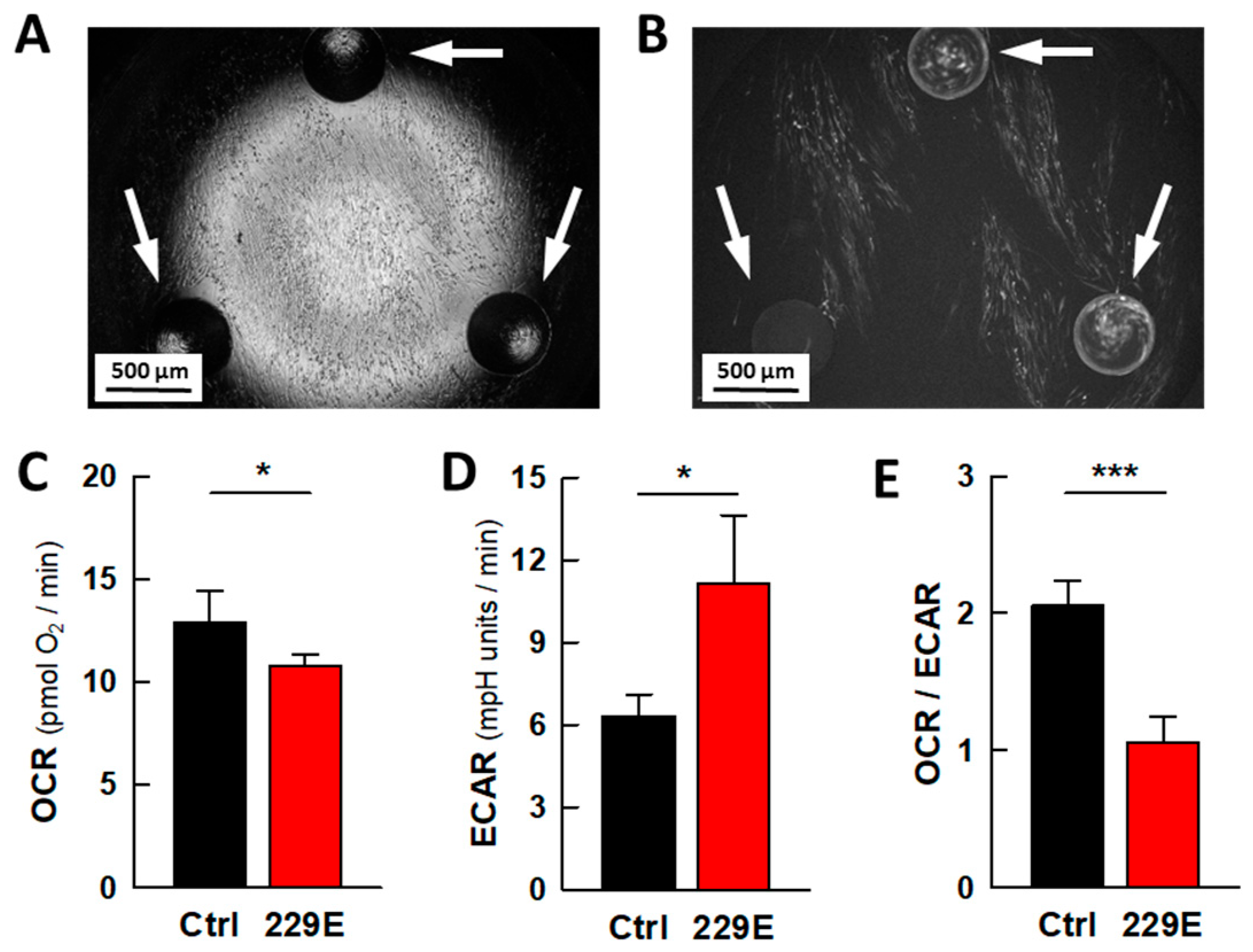
2.1. Viral Infection Leads to Metabolic Rewiring in MRC5 Fibroblasts
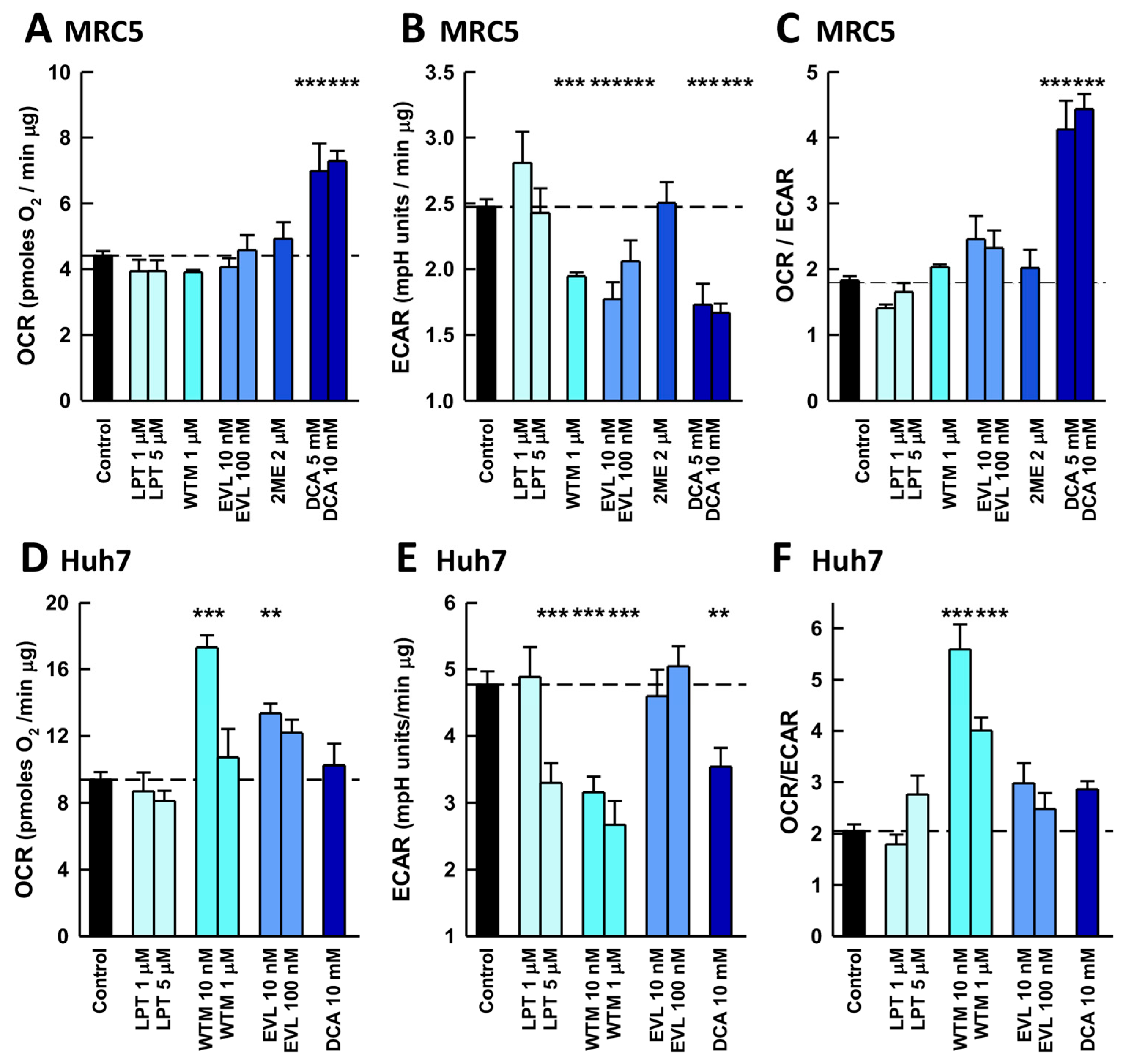
2.2. Antiviral Effects of Inhibitors of Pathways Involved in the Warburg Effect
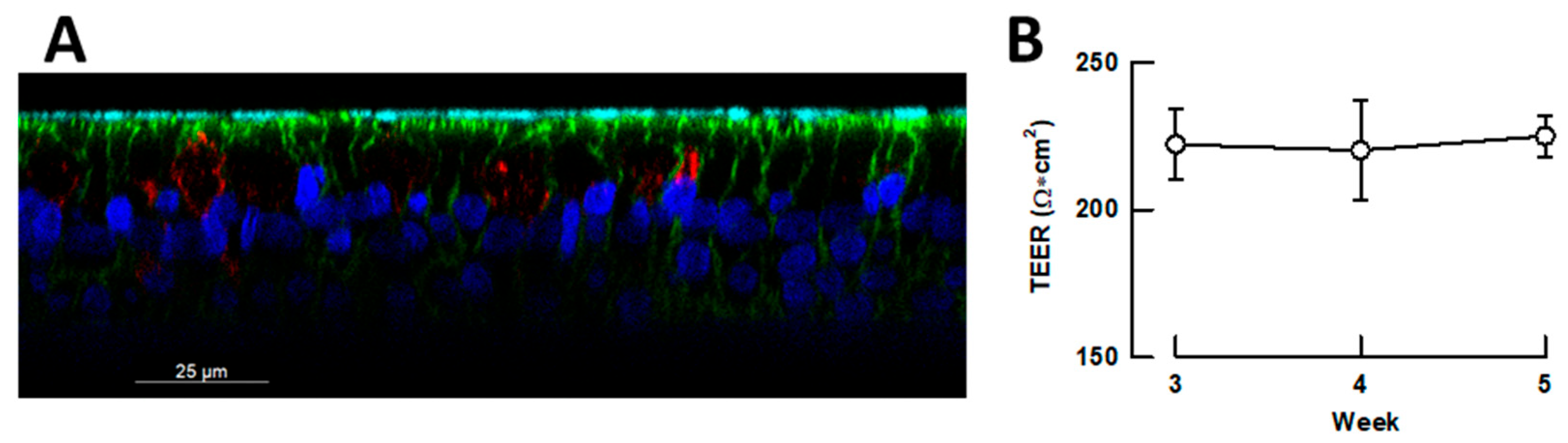
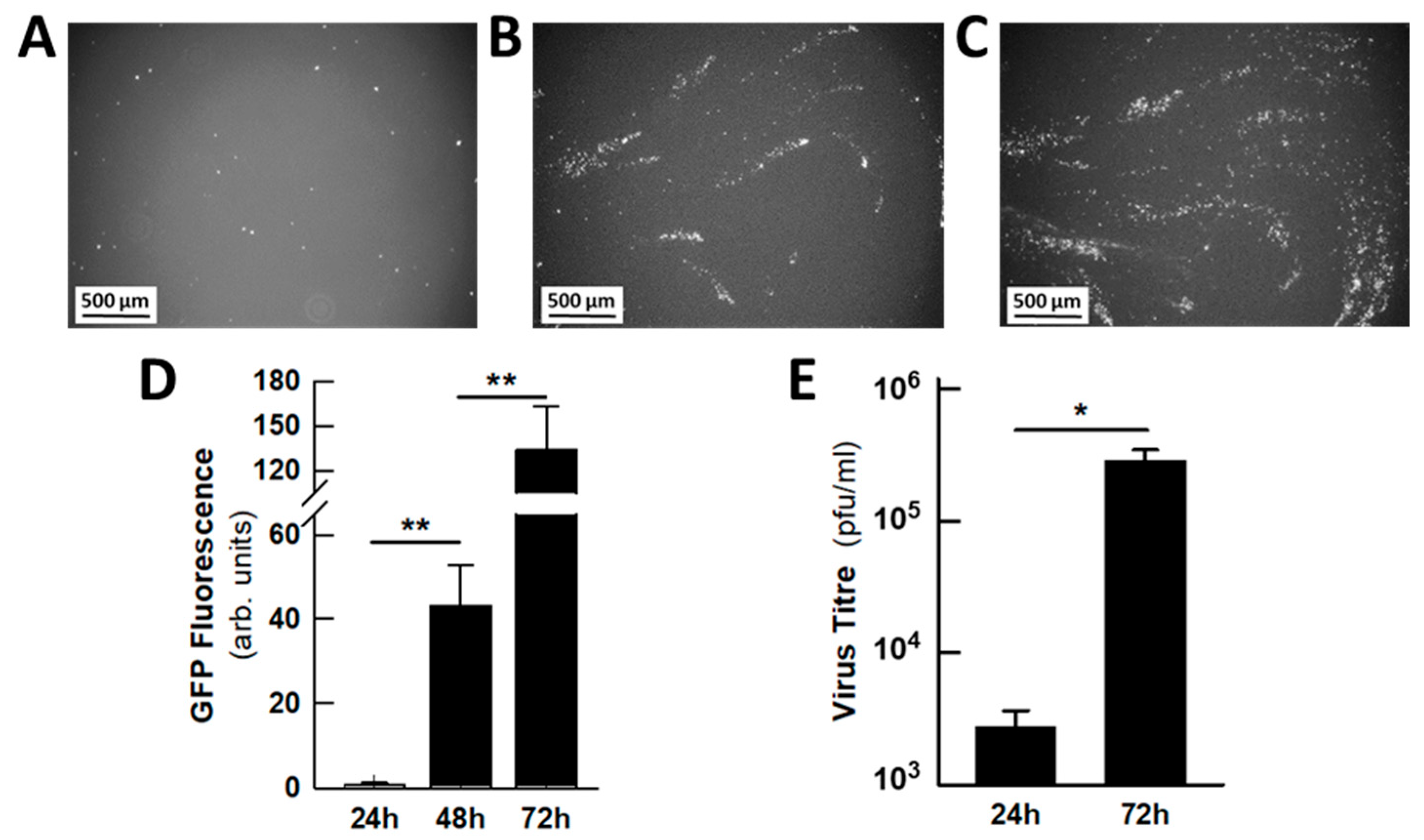
2.3. Antiviral Activity of DCA in Small Airway Epithelia
3. Discussion
4. Materials and Methods
4.1. Cell Lines
4.2. Viruses
4.3. Differentiation of Small Airway Epithelia in Air-Liquid Interface Cultures
4.4. Viral Infections
4.5. Quantification of Viral Infection
4.6. Energy Metabolism
4.7. Confocal Microscopy
4.8. Reagents and Media
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALI | Air-liquid interface |
| DAPI | 4′,6-diamidino-2-phenylindole |
| DCA | Dichloroacetate |
| DMEM | Dulbecco’s modified Eagle’s medium |
| D-PBS | Dulbecco’s phosphate-buffered saline |
| ECAR | Extracellular acidification rate |
| EGFR | Epidermal growth factor receptor |
| EVL | Everolimus |
| ffu | Fluorescent focus units |
| HER2 | Human epidermal growth factor receptor 2 |
| HIF-1α | Hypoxia inducible factor 1α |
| HIFBS | Heat-inactivated fetal bovine serum |
| hpi | Hours post-infection |
| hSAEC | Primary human small airway epithelial cells |
| LDH-A | Lactate dehydrogenase A |
| LPT | Lapatinib |
| mTOR | Mammalian target of rapamycin |
| OCR | Oxygen consumption rate |
| MEM | Minimal essential medium |
| MOI | Multiplicity of infection |
| PBS | Phosphate-buffered saline |
| PDC | Pyruvate dehydrogenase complex |
| PDH | Pyruvate dehydrogenase |
| PDK | Pyruvate dehydrogenase kinase |
| PFA | Paraformaldehyde |
| PI3K | Growth factor-regulated phosphoinositide 3-kinase |
| pfu | Plaque forming units |
| RSV | Respiratory syncytial virus |
| RT | Room temperature |
| TEER | Transepithelial electrical resistance |
| WTM | Wortmaninn |
| 2DOG | 2-deoxyglucose |
| 2ME | 2-methoxyestradiol |
References
- He, Y.; Liu, W.J.; Jia, N.; Richardson, S.; Huang, C. Viral Respiratory Infections in a Rapidly Changing Climate: The Need to Prepare for the next Pandemic. eBioMedicine 2023, 93, 104593. [Google Scholar] [CrossRef]
- Jordan, R.; Ford-Scheimer, S.L.; Alarcon, R.M.; Atala, A.; Borenstein, J.T.; Brimacombe, K.R.; Cherry, S.; Clevers, H.; Davis, M.I.; Funnell, S.G.P.; et al. Report of the Assay Guidance Workshop on 3-Dimensional Tissue Models for Antiviral Drug Development. J. Infect. Dis. 2023, 228, S337–S354. [Google Scholar] [CrossRef] [PubMed]
- Touret, F.; Driouich, J.-S.; Cochin, M.; Petit, P.R.; Gilles, M.; Barthélémy, K.; Moureau, G.; Mahon, F.-X.; Malvy, D.; Solas, C.; et al. Preclinical Evaluation of Imatinib Does Not Support Its Use as an Antiviral Drug against SARS-CoV-2. Antivir. Res. 2021, 193, 105137. [Google Scholar] [CrossRef]
- Fisher, C.R.; Mba Medie, F.; Luu, R.J.; Gaibler, R.B.; Mulhern, T.J.; Miller, C.R.; Zhang, C.J.; Rubio, L.D.; Marr, E.E.; Vijayakumar, V.; et al. A High-Throughput, High-Containment Human Primary Epithelial Airway Organ-on-Chip Platform for SARS-CoV-2 Therapeutic Screening. Cells 2023, 12, 2639. [Google Scholar] [CrossRef] [PubMed]
- Teo, A.; Chua, C.L.L.; Chan, L.L.Y. Airway Models in a Pandemic: Suitability of Models in Modeling SARS-CoV-2. PLoS Pathog. 2022, 18, e1010432. [Google Scholar] [CrossRef]
- Plebani, R.; Bai, H.; Si, L.; Li, J.; Zhang, C.; Romano, M. 3D Lung Tissue Models for Studies on SARS-CoV-2 Pathophysiology and Therapeutics. Int. J. Mol. Sci. 2022, 23, 10071. [Google Scholar] [CrossRef] [PubMed]
- Pezzulo, A.A.; Starner, T.D.; Scheetz, T.E.; Traver, G.L.; Tilley, A.E.; Harvey, B.-G.; Crystal, R.G.; McCray, P.B.; Zabner, J. The Air-Liquid Interface and Use of Primary Cell Cultures Are Important to Recapitulate the Transcriptional Profile of in Vivo Airway Epithelia. Am. J. Physiol. Lung Cell Mol. Physiol. 2011, 300, L25–L31. [Google Scholar] [CrossRef]
- Kumar, S.; Sarma, P.; Kaur, H.; Prajapat, M.; Bhattacharyya, A.; Avti, P.; Sehkhar, N.; Kaur, H.; Bansal, S.; Mahendiratta, S.; et al. Clinically Relevant Cell Culture Models and Their Significance in Isolation, Pathogenesis, Vaccine Development, Repurposing and Screening of New Drugs for SARS-CoV-2: A Systematic Review. Tissue Cell 2021, 70, 101497. [Google Scholar] [CrossRef]
- Dittmar, M.; Lee, J.S.; Whig, K.; Segrist, E.; Li, M.; Kamalia, B.; Castellana, L.; Ayyanathan, K.; Cardenas-Diaz, F.L.; Morrisey, E.E.; et al. Drug Repurposing Screens Reveal Cell-Type-Specific Entry Pathways and FDA-Approved Drugs Active against SARS-Cov-2. Cell Rep. 2021, 35, 108959. [Google Scholar] [CrossRef]
- Nepali, K.; Sharma, R.; Sharma, S.; Thakur, A.; Liou, J.-P. Beyond the Vaccines: A Glance at the Small Molecule and Peptide-Based Anti-COVID19 Arsenal. J. Biomed. Sci. 2022, 29, 65. [Google Scholar] [CrossRef]
- Kleinehr, J.; Wilden, J.J.; Boergeling, Y.; Ludwig, S.; Hrincius, E.R. Metabolic Modifications by Common Respiratory Viruses and Their Potential as New Antiviral Targets. Viruses 2021, 13, 2068. [Google Scholar] [CrossRef]
- Goodwin, C.M.; Xu, S.; Munger, J. Stealing the Keys to the Kitchen: Viral Manipulation of the Host Cell Metabolic Network. Trends Microbiol. 2015, 23, 789–798. [Google Scholar] [CrossRef]
- Sanchez, E.L.; Lagunoff, M. Viral Activation of Cellular Metabolism. Virology 2015, 479–480, 609–618. [Google Scholar] [CrossRef]
- Allen, C.N.S.; Arjona, S.P.; Santerre, M.; Sawaya, B.E. Hallmarks of Metabolic Reprogramming and Their Role in Viral Pathogenesis. Viruses 2022, 14, 602. [Google Scholar] [CrossRef] [PubMed]
- Eisenreich, W.; Leberfing, J.; Rudel, T.; Heesemann, J.; Goebel, W. Interactions of SARS-CoV-2 with Human Target Cells—A Metabolic View. Int. J. Mol. Sci. 2024, 25, 9977. [Google Scholar] [CrossRef]
- Eisenreich, W.; Rudel, T.; Heesemann, J.; Goebel, W. How Viral and Intracellular Bacterial Pathogens Reprogram the Metabolism of Host Cells to Allow Their Intracellular Replication. Front. Cell Infect. Microbiol. 2019, 9, 42. [Google Scholar] [CrossRef]
- Claus, C.; Liebert, U.G. A Renewed Focus on the Interplay between Viruses and Mitochondrial Metabolism. Arch. Virol. 2014, 159, 1267–1277. [Google Scholar] [CrossRef] [PubMed]
- Krakauer, D.C.; Payne, R.J. The Evolution of Virus-Induced Apoptosis. Proc. Biol. Sci. 1997, 264, 1757–1762. [Google Scholar] [CrossRef]
- Dai, X.; Hakizimana, O.; Zhang, X.; Kaushik, A.C.; Zhang, J. Orchestrated Efforts on Host Network Hijacking: Processes Governing Virus Replication. Virulence 2020, 11, 183–198. [Google Scholar] [CrossRef] [PubMed]
- Lunt, S.Y.; Vander Heiden, M.G. Aerobic Glycolysis: Meeting the Metabolic Requirements of Cell Proliferation. Annu. Rev. Cell Dev. Biol. 2011, 27, 441–464. [Google Scholar] [CrossRef]
- Girdhar, K.; Powis, A.; Raisingani, A.; Chrudinová, M.; Huang, R.; Tran, T.; Sevgi, K.; Dogus Dogru, Y.; Altindis, E. Viruses and Metabolism: The Effects of Viral Infections and Viral Insulins on Host Metabolism. Annu. Rev. Virol. 2021, 8, 373–391. [Google Scholar] [CrossRef]
- Pant, A.; Dsouza, L.; Yang, Z. Alteration in Cellular Signaling and Metabolic Reprogramming during Viral Infection. mBio 2021, 12, e0063521. [Google Scholar] [CrossRef]
- Cairns, R.A.; Harris, I.S.; Mak, T.W. Regulation of Cancer Cell Metabolism. Nat. Rev. Cancer 2011, 11, 85–95. [Google Scholar] [CrossRef]
- Basile, M.S.; Cavalli, E.; McCubrey, J.; Hernández-Bello, J.; Muñoz-Valle, J.F.; Fagone, P.; Nicoletti, F. The PI3K/Akt/mTOR Pathway: A Potential Pharmacological Target in COVID-19. Drug Discov. Today 2022, 27, 848–856. [Google Scholar] [CrossRef]
- Sharma, D.; Singh, M.; Gupta, R.; Garg, M.; Altieri, A.; Kurkin, A.; Kumar, V.; Rani, R. Targeting Virus-Induced Reprogrammed Cell Metabolism via Glycolytic Inhibitors: An Effective Therapeutic Approach Against SARS-CoV-2. Mini Rev. Med. Chem. 2023, 23, 120–130. [Google Scholar] [CrossRef]
- Ayres, J.S. A Metabolic Handbook for the COVID-19 Pandemic. Nat. Metab. 2020, 2, 572–585. [Google Scholar] [CrossRef] [PubMed]
- Guarnieri, J.W.; Lie, T.; Albrecht, Y.E.S.; Hewin, P.; Jurado, K.A.; Widjaja, G.A.; Zhu, Y.; McManus, M.J.; Kilbaugh, T.J.; Keith, K.; et al. Mitochondrial Antioxidants Abate SARS-CoV-2 Pathology in Mice. Proc. Natl. Acad. Sci. USA 2024, 121, e2321972121. [Google Scholar] [CrossRef] [PubMed]
- Flores-Torres, A.S.; Rezinciuc, S.; Bezavada, L.; Shulkin, B.L.; Cormier, S.A.; Smallwood, H.S. Respiratory Syncytial Virus Elicits Glycolytic Metabolism in Pediatric Upper and Lower Airways. Viruses 2025, 17, 703. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Kepp, O.; Vander Heiden, M.G.; Kroemer, G. Metabolic Targets for Cancer Therapy. Nat. Rev. Drug Discov. 2013, 12, 829–846. [Google Scholar] [CrossRef]
- Stine, Z.E.; Schug, Z.T.; Salvino, J.M.; Dang, C.V. Targeting Cancer Metabolism in the Era of Precision Oncology. Nat. Rev. Drug Discov. 2022, 21, 141–162. [Google Scholar] [CrossRef]
- Holness, M.J.; Sugden, M.C. Regulation of Pyruvate Dehydrogenase Complex Activity by Reversible Phosphorylation. Biochem. Soc. Trans. 2003, 31, 1143–1151. [Google Scholar] [CrossRef]
- Vicenti, I.; Dragoni, F.; Giannini, A.; Giammarino, F.; Spinicci, M.; Saladini, F.; Boccuto, A.; Zazzi, M. Development of a Cell-Based Immunodetection Assay for Simultaneous Screening of Antiviral Compounds Inhibiting Zika and Dengue Virus Replication. SLAS Discov. 2020, 25, 506–514. [Google Scholar] [CrossRef]
- Pandamooz, S.; Jurek, B.; Meinung, C.-P.; Baharvand, Z.; Sahebi Shahem-Abadi, A.; Haerteis, S.; Miyan, J.A.; Downing, J.; Dianatpour, M.; Borhani-Haghighi, A.; et al. Experimental Models of SARS-CoV-2 Infection: Possible Platforms to Study COVID-19 Pathogenesis and Potential Treatments. Annu. Rev. Pharmacol. Toxicol. 2022, 62, 25–53. [Google Scholar] [CrossRef]
- Baldassi, D.; Gabold, B.; Merkel, O. Air-Liquid Interface Cultures of the Healthy and Diseased Human Respiratory Tract: Promises, Challenges and Future Directions. Adv. Nanobiomed Res. 2021, 1, 2000111. [Google Scholar] [CrossRef] [PubMed]
- Hasan, S.; Sebo, P.; Osicka, R. A Guide to Polarized Airway Epithelial Models for Studies of Host-Pathogen Interactions. FEBS J. 2018, 285, 4343–4358. [Google Scholar] [CrossRef] [PubMed]
- Mavin, E.; Verdon, B.; Carrie, S.; Saint-Criq, V.; Powell, J.; Kuttruff, C.A.; Ward, C.; Garnett, J.P.; Miwa, S. Real-Time Measurement of Cellular Bioenergetics in Fully Differentiated Human Nasal Epithelial Cells Grown at Air-Liquid-Interface. Am. J. Physiol. Lung Cell Mol. Physiol. 2020, 318, L1158–L1164. [Google Scholar] [CrossRef] [PubMed]
- Zarkoob, H.; Allué-Guardia, A.; Chen, Y.-C.; Garcia-Vilanova, A.; Jung, O.; Coon, S.; Song, M.J.; Park, J.-G.; Oladunni, F.; Miller, J.; et al. Modeling SARS-CoV-2 and Influenza Infections and Antiviral Treatments in Human Lung Epithelial Tissue Equivalents. Commun. Biol. 2022, 5, 810. [Google Scholar] [CrossRef]
- Dichtl, S.; Posch, W.; Wilflingseder, D. The Breathtaking World of Human Respiratory in Vitro Models: Investigating Lung Diseases and Infections in 3D Models, Organoids, and Lung-on-Chip. Eur. J. Immunol. 2024, 54, e2250356. [Google Scholar] [CrossRef]
- Raymonda, M.H.; Ciesla, J.H.; Monaghan, M.; Leach, J.; Asantewaa, G.; Smorodintsev-Schiller, L.A.; Lutz, M.M.; Schafer, X.L.; Takimoto, T.; Dewhurst, S.; et al. Pharmacologic Profiling Reveals Lapatinib as a Novel Antiviral against SARS-CoV-2 in Vitro. Virology 2022, 566, 60–68. [Google Scholar] [CrossRef]
- Van Doorn, H.R.; Yu, H. Viral Respiratory Infections. In Hunter’s Tropical Medicine and Emerging Infectious Diseases; Elsevier: Amsterdam, The Netherlands, 2020; pp. 284–288. ISBN 978-0-323-55512-8. [Google Scholar]
- Michi, A.N.; Proud, D. A Toolbox for Studying Respiratory Viral Infections Using Air-Liquid Interface Cultures of Human Airway Epithelial Cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2021, 321, L263–L280. [Google Scholar] [CrossRef]
- Vastag, L.; Koyuncu, E.; Grady, S.L.; Shenk, T.E.; Rabinowitz, J.D. Divergent Effects of Human Cytomegalovirus and Herpes Simplex Virus-1 on Cellular Metabolism. PLoS Pathog. 2011, 7, e1002124. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Janocha, A.J.; Leahy, R.A.; Klatte, R.; Dudzinski, D.; Mavrakis, L.A.; Comhair, S.A.A.; Lauer, M.E.; Cotton, C.U.; Erzurum, S.C. A Novel Method for Pulmonary Research: Assessment of Bioenergetic Function at the Air-Liquid Interface. Redox Biol. 2014, 2, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Chavda, V.P.; Vora, L.K.; Gajjar, N.; Apostolopoulos, V.; Shah, N.; Chen, Z.-S. 2-Deoxy-D-Glucose and Its Derivatives for the COVID-19 Treatment: An Update. Front. Pharmacol. 2022, 13, 899633. [Google Scholar] [CrossRef] [PubMed]
- Jung, G.-S.; Jeon, J.-H.; Choi, Y.-K.; Jang, S.Y.; Park, S.Y.; Kim, S.-W.; Byun, J.-K.; Kim, M.-K.; Lee, S.; Shin, E.-C.; et al. Pyruvate Dehydrogenase Kinase Regulates Hepatitis C Virus Replication. Sci. Rep. 2016, 6, 30846. [Google Scholar] [CrossRef]
- Lee, S.R.; Roh, J.Y.; Ryu, J.; Shin, H.-J.; Hong, E.-J. Activation of TCA Cycle Restrains Virus-Metabolic Hijacking and Viral Replication in Mouse Hepatitis Virus-Infected Cells. Cell Biosci. 2022, 12, 7. [Google Scholar] [CrossRef]
- Gilbert-Jaramillo, J.; Komarasamy, T.V.; Balasubramaniam, V.R.; Heather, L.C.; James, W.S. Targeting Glucose Metabolism with Dichloroacetate (DCA) Reduces Zika Virus Replication in Brain Cortical Progenitors at Different Stages of Maturation. Antivir. Res. 2024, 228, 105933. [Google Scholar] [CrossRef]
- Mingo-Casas, P.; Blázquez, A.-B.; Gómez de Cedrón, M.; San-Félix, A.; Molina, S.; Escribano-Romero, E.; Calvo-Pinilla, E.; Jiménez de Oya, N.; Ramírez de Molina, A.; Saiz, J.-C.; et al. Glycolytic Shift during West Nile Virus Infection Provides New Therapeutic Opportunities. J. Neuroinflamm. 2023, 20, 217. [Google Scholar] [CrossRef]
- Garreta, E.; Prado, P.; Stanifer, M.L.; Monteil, V.; Marco, A.; Ullate-Agote, A.; Moya-Rull, D.; Vilas-Zornoza, A.; Tarantino, C.; Romero, J.P.; et al. A Diabetic Milieu Increases ACE2 Expression and Cellular Susceptibility to SARS-CoV-2 Infections in Human Kidney Organoids and Patient Cells. Cell Metab. 2022, 34, 857–873.e9. [Google Scholar] [CrossRef]
- Kennedy, B.E.; Sadek, M.; Gujar, S.A. Targeted Metabolic Reprogramming to Improve the Efficacy of Oncolytic Virus Therapy. Mol. Ther. 2020, 28, 1417–1421. [Google Scholar] [CrossRef]
- Li, C.; Meng, G.; Su, L.; Chen, A.; Xia, M.; Xu, C.; Yu, D.; Jiang, A.; Wei, J. Dichloroacetate Blocks Aerobic Glycolytic Adaptation to Attenuated Measles Virus and Promotes Viral Replication Leading to Enhanced Oncolysis in Glioblastoma. Oncotarget 2015, 6, 1544–1555. [Google Scholar] [CrossRef]
- Stacpoole, P.W.; Moore, G.W.; Kornhauser, D.M. Metabolic Effects of Dichloroacetate in Patients with Diabetes Mellitus and Hyperlipoproteinemia. N. Engl. J. Med. 1978, 298, 526–530. [Google Scholar] [CrossRef] [PubMed]
- Roche, T.E.; Hiromasa, Y. Pyruvate Dehydrogenase Kinase Regulatory Mechanisms and Inhibition in Treating Diabetes, Heart Ischemia, and Cancer. Cell Mol. Life Sci. 2007, 64, 830–849. [Google Scholar] [CrossRef]
- Abdelmalak, M.; Lew, A.; Ramezani, R.; Shroads, A.L.; Coats, B.S.; Langaee, T.; Shankar, M.N.; Neiberger, R.E.; Subramony, S.H.; Stacpoole, P.W. Long-Term Safety of Dichloroacetate in Congenital Lactic Acidosis. Mol. Genet. Metab. 2013, 109, 139–143. [Google Scholar] [CrossRef]
- Kankotia, S.; Stacpoole, P.W. Dichloroacetate and Cancer: New Home for an Orphan Drug? Biochim. Biophys. Acta 2014, 1846, 617–629. [Google Scholar] [CrossRef]
- Michelakis, E.D.; Gurtu, V.; Webster, L.; Barnes, G.; Watson, G.; Howard, L.; Cupitt, J.; Paterson, I.; Thompson, R.B.; Chow, K.; et al. Inhibition of Pyruvate Dehydrogenase Kinase Improves Pulmonary Arterial Hypertension in Genetically Susceptible Patients. Sci. Transl. Med. 2017, 9, eaao4583. [Google Scholar] [CrossRef]
- Whitehouse, S.; Cooper, R.H.; Randle, P.J. Mechanism of Activation of Pyruvate Dehydrogenase by Dichloroacetate and Other Halogenated Carboxylic Acids. Biochem. J. 1974, 141, 761–774. [Google Scholar] [CrossRef]
- Stacpoole, P.W.; McCall, C.E. The Pyruvate Dehydrogenase Complex: Life’s Essential, Vulnerable and Druggable Energy Homeostat. Mitochondrion 2023, 70, 59–102. [Google Scholar] [CrossRef] [PubMed]
- Michelakis, E.D.; Sutendra, G.; Dromparis, P.; Webster, L.; Haromy, A.; Niven, E.; Maguire, C.; Gammer, T.-L.; Mackey, J.R.; Fulton, D.; et al. Metabolic Modulation of Glioblastoma with Dichloroacetate. Sci. Transl. Med. 2010, 2, 31ra34. [Google Scholar] [CrossRef] [PubMed]
- Prins, K.W.; Thenappan, T.; Weir, E.K.; Kalra, R.; Pritzker, M.; Archer, S.L. Repurposing Medications for Treatment of Pulmonary Arterial Hypertension: What’s Old Is New Again. J. Am. Heart Assoc. 2019, 8, e011343. [Google Scholar] [CrossRef]
- Leow, H.W.; Koscielniak, M.; Williams, L.; Saunders, P.T.K.; Daniels, J.; Doust, A.M.; Jones, M.-C.; Ferguson, G.D.; Bagger, Y.; Horne, A.W.; et al. Dichloroacetate as a Possible Treatment for Endometriosis-Associated Pain: A Single-Arm Open-Label Exploratory Clinical Trial (EPiC). Pilot. Feasibility Stud. 2021, 7, 67. [Google Scholar] [CrossRef]
- Horne, A.W.; Ahmad, S.F.; Carter, R.; Simitsidellis, I.; Greaves, E.; Hogg, C.; Morton, N.M.; Saunders, P.T.K. Repurposing Dichloroacetate for the Treatment of Women with Endometriosis. Proc. Natl. Acad. Sci. USA 2019, 116, 25389–25391. [Google Scholar] [CrossRef]
- Stacpoole, P.W.; Kurtz, T.L.; Han, Z.; Langaee, T. Role of Dichloroacetate in the Treatment of Genetic Mitochondrial Diseases. Adv. Drug Deliv. Rev. 2008, 60, 1478–1487. [Google Scholar] [CrossRef] [PubMed]
- Mangal, N.; James, M.O.; Stacpoole, P.W.; Schmidt, S. Model Informed Dose Optimization of Dichloroacetate for the Treatment of Congenital Lactic Acidosis in Children. J. Clin. Pharmacol. 2018, 58, 212–220. [Google Scholar] [CrossRef]
- Stacpoole, P.W.; Henderson, G.N.; Yan, Z.; Cornett, R.; James, M.O. Pharmacokinetics, Metabolism and Toxicology of Dichloroacetate. Drug Metab. Rev. 1998, 30, 499–539. [Google Scholar] [CrossRef]
- Tataranni, T.; Piccoli, C. Dichloroacetate (DCA) and Cancer: An Overview towards Clinical Applications. Oxidative Med. Cell. Longev. 2019, 2019, 8201079. [Google Scholar] [CrossRef]
- Schoenmann, N.; Tannenbaum, N.; Hodgeman, R.M.; Raju, R.P. Regulating Mitochondrial Metabolism by Targeting Pyruvate Dehydrogenase with Dichloroacetate, a Metabolic Messenger. Biochim. Biophys. Acta Mol. Basis Dis. 2023, 1869, 166769. [Google Scholar] [CrossRef]
- Cervantes-Barragan, L.; Züst, R.; Maier, R.; Sierro, S.; Janda, J.; Levy, F.; Speiser, D.; Romero, P.; Rohrlich, P.-S.; Ludewig, B.; et al. Dendritic Cell-Specific Antigen Delivery by Coronavirus Vaccine Vectors Induces Long-Lasting Protective Antiviral and Antitumor Immunity. mBio 2010, 1, e00171-10. [Google Scholar] [CrossRef]
- Latorre, V.; Geller, R. Identification of Cytoplasmic Chaperone Networks Relevant for Respiratory Syncytial Virus Replication. Front. Microbiol. 2022, 13, 880394. [Google Scholar] [CrossRef] [PubMed]
- Segalés, J.; Sánchez-Martín, C.; Pujol-Morcillo, A.; Martín-Ruiz, M.; de Los Santos, P.; Lobato-Alonso, D.; Oliver, E.; Rial, E. Role of UCP2 in the Energy Metabolism of the Cancer Cell Line A549. Int. J. Mol. Sci. 2023, 24, 8123. [Google Scholar] [CrossRef]
- Konecny, G.E.; Venkatesan, N.; Yang, G.; Dering, J.; Ginther, C.; Finn, R.; Rahmeh, M.; Fejzo, M.S.; Toft, D.; Jiang, S.W.; et al. Activity of lapatinib a novel HER2 and EGFR dual kinase inhibitor in human endometrial cancer cells. Br. J. Cancer 2008, 98, 1076–1084. [Google Scholar] [CrossRef] [PubMed]
- Elwaie, T.A.; Abbas, S.E.; Aly, E.I.; George, R.F.; Ali, H.; Kraiouchkine, N.; Abdelwahed, K.S.; Fandy, T.E.; El Sayed, K.A.; Abd Elmageed, Z.Y.; et al. HER2 kinase-targeted breast cancer therapy: Design, synthesis, and in vitro and in vivo evaluation of novel lapatinib congeners as selective and potent HER2 inhibitors with favorable metabolic stability. J. Med. Chem. 2020, 63, 15906–15945. [Google Scholar] [CrossRef] [PubMed]
- Yano, H.; Nakanishi, S.; Kimura, K.; Hanai, N.; Saitoh, Y.; Fukui, Y.; Nonomura, Y.; Matsuda, Y. Inhibition of histamine secretion by wortmannin through the blockade of phosphatidylinositol 3-kinase in RBL-2H3 cells. J. Biol. Chem. 1993, 268, 25846–25856. [Google Scholar] [CrossRef]
- Huang, J.; Chen, L.; Wu, J.; Ai, D.; Zhang, J.Q.; Chen, T.G.; Wang, L. Targeting the PI3K/AKT/mTOR signaling pathway in the treatment of human diseases: Current status, trends, and solutions. J. Med. Chem. 2022, 65, 16033–16061. [Google Scholar] [CrossRef]
- Houghton, P.J. Everolimus. Clin. Cancer Res. 2010, 16, 1368–1372. [Google Scholar] [CrossRef] [PubMed]
- Lui, A.; New, J.; Ogony, J.; Thomas, S.; Lewis-Wambi, J. Everolimus downregulates estrogen receptor and induces autophagy in aromatase inhibitor-resistant breast cancer cells. BMC Cancer 2016, 16, 487. [Google Scholar] [CrossRef]
- Yue, T.L.; Wang, X.; Louden, C.S.; Gupta, S.; Pillarisetti, K.; Gu, J.L.; Hart, T.K.; Lysko, P.G.; Feuerstein, G.Z. 2-Methoxyestradiol, an endogenous estrogen metabolite, induces apoptosis in endothelial cells and inhibits angiogenesis: Possible role for stress-activated protein kinase signaling pathway and Fas expression. Mol. Pharmacol. 1997, 51, 951–962. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez de Iturrate, P.; Hernáez, B.; de los Santos, P.; Sierra-Palomares, Y.; García-Gómez, A.; Sánchez-Cruz, A.; Hernández-Sánchez, C.; Rivas, L.; del Val, M.; Rial, E. Human Small Airway Epithelia Reveal Dichloroacetate as a Broad-Spectrum Antiviral Against Respiratory Viruses. Int. J. Mol. Sci. 2025, 26, 9853. https://doi.org/10.3390/ijms26209853
Martínez de Iturrate P, Hernáez B, de los Santos P, Sierra-Palomares Y, García-Gómez A, Sánchez-Cruz A, Hernández-Sánchez C, Rivas L, del Val M, Rial E. Human Small Airway Epithelia Reveal Dichloroacetate as a Broad-Spectrum Antiviral Against Respiratory Viruses. International Journal of Molecular Sciences. 2025; 26(20):9853. https://doi.org/10.3390/ijms26209853
Chicago/Turabian StyleMartínez de Iturrate, Paula, Bruno Hernáez, Patricia de los Santos, Yolanda Sierra-Palomares, Alba García-Gómez, Alonso Sánchez-Cruz, Catalina Hernández-Sánchez, Luis Rivas, Margarita del Val, and Eduardo Rial. 2025. "Human Small Airway Epithelia Reveal Dichloroacetate as a Broad-Spectrum Antiviral Against Respiratory Viruses" International Journal of Molecular Sciences 26, no. 20: 9853. https://doi.org/10.3390/ijms26209853
APA StyleMartínez de Iturrate, P., Hernáez, B., de los Santos, P., Sierra-Palomares, Y., García-Gómez, A., Sánchez-Cruz, A., Hernández-Sánchez, C., Rivas, L., del Val, M., & Rial, E. (2025). Human Small Airway Epithelia Reveal Dichloroacetate as a Broad-Spectrum Antiviral Against Respiratory Viruses. International Journal of Molecular Sciences, 26(20), 9853. https://doi.org/10.3390/ijms26209853






