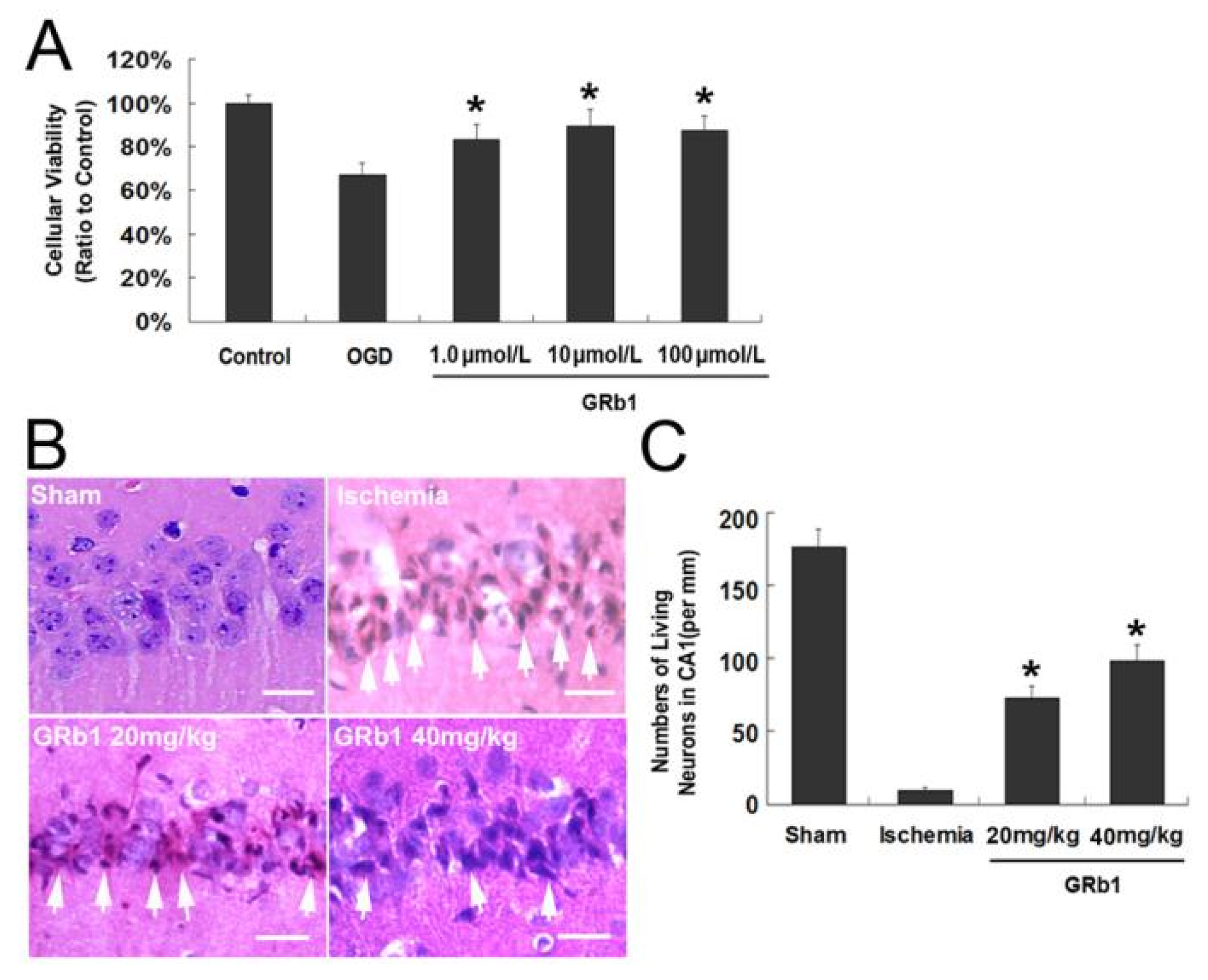
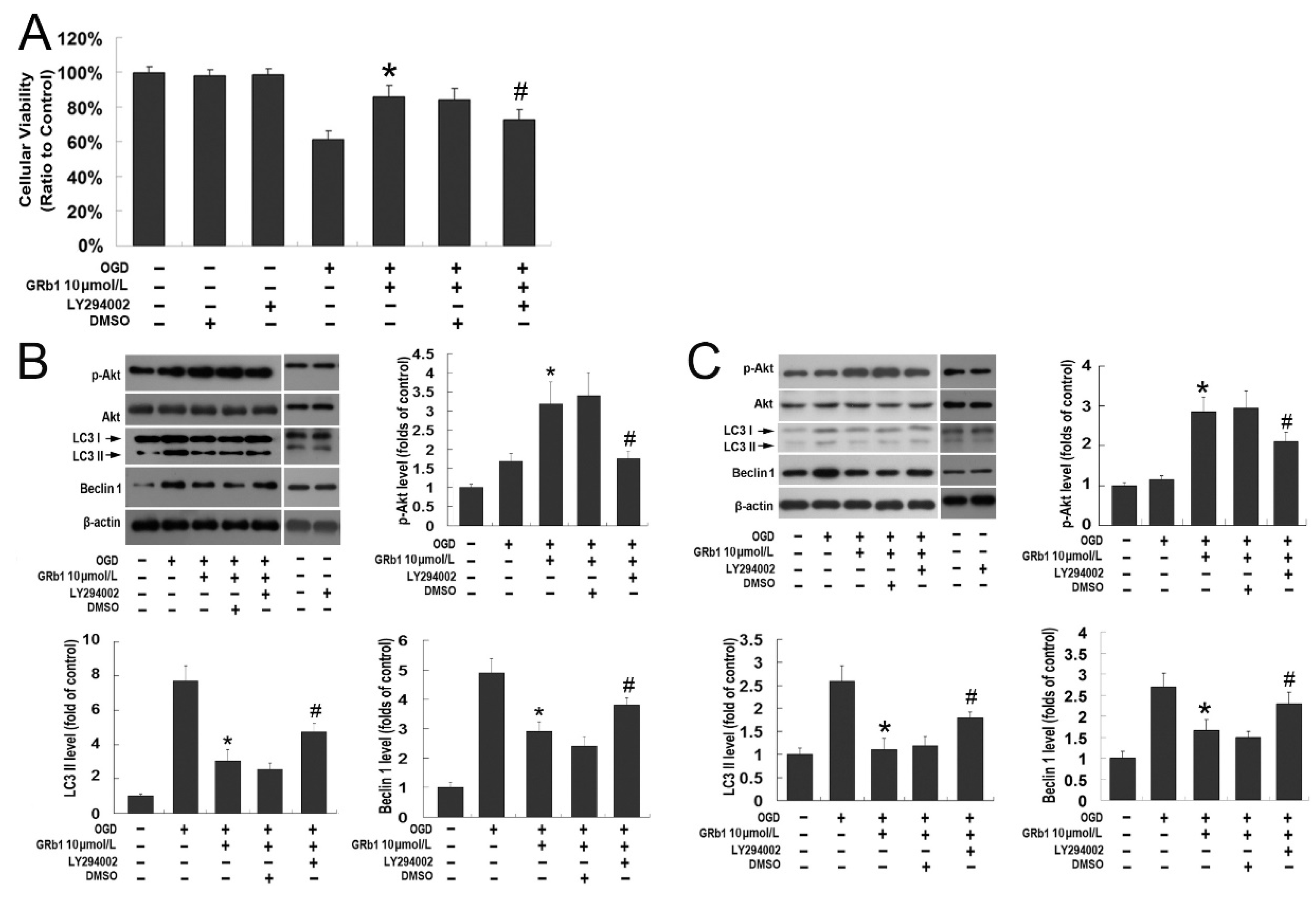
Correction: Luo et al. Inhibition of Autophagy via Activation of PI3K/Akt Pathway Contributes to the Protection of Ginsenoside Rb1 against Neuronal Death Caused by Ischemic Insults. Int. J. Mol. Sci. 2014, 15, 15426–15442
References
- Luo, T.; Liu, G.; Ma, H.; Lu, B.; Xu, H.; Wang, Y.; Wu, J.; Ge, P.; Liang, J. Inhibition of Autophagy via Activation of PI3K/Akt Pathway Contributes to the Protection of Ginsenoside Rb1 against Neuronal Death Caused by Ischemic Insults. Int. J. Mol. Sci. 2014, 15, 15426–15442. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Luo, T.; Qi, L.; Wang, B.; Luo, Y.; Ge, P. Ischemic Postconditioning Alleviates Neuronal Injury Caused by Relief of Carotid Stenosis in a Rat Model of Cerebral Hypoperfusion. Int. J. Mol. Sci. 2012, 13, 13338–13351. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, T.; Liu, G.; Ma, H.; Lu, B.; Xu, H.; Wang, Y.; Wu, J.; Ge, P.; Liang, J. Correction: Luo et al. Inhibition of Autophagy via Activation of PI3K/Akt Pathway Contributes to the Protection of Ginsenoside Rb1 against Neuronal Death Caused by Ischemic Insults. Int. J. Mol. Sci. 2014, 15, 15426–15442. Int. J. Mol. Sci. 2025, 26, 9839. https://doi.org/10.3390/ijms26209839
Luo T, Liu G, Ma H, Lu B, Xu H, Wang Y, Wu J, Ge P, Liang J. Correction: Luo et al. Inhibition of Autophagy via Activation of PI3K/Akt Pathway Contributes to the Protection of Ginsenoside Rb1 against Neuronal Death Caused by Ischemic Insults. Int. J. Mol. Sci. 2014, 15, 15426–15442. International Journal of Molecular Sciences. 2025; 26(20):9839. https://doi.org/10.3390/ijms26209839
Chicago/Turabian StyleLuo, Tianfei, Guiying Liu, Hongxi Ma, Bin Lu, Haiyang Xu, Yujing Wang, Jiang Wu, Pengfei Ge, and Jianmin Liang. 2025. "Correction: Luo et al. Inhibition of Autophagy via Activation of PI3K/Akt Pathway Contributes to the Protection of Ginsenoside Rb1 against Neuronal Death Caused by Ischemic Insults. Int. J. Mol. Sci. 2014, 15, 15426–15442" International Journal of Molecular Sciences 26, no. 20: 9839. https://doi.org/10.3390/ijms26209839
APA StyleLuo, T., Liu, G., Ma, H., Lu, B., Xu, H., Wang, Y., Wu, J., Ge, P., & Liang, J. (2025). Correction: Luo et al. Inhibition of Autophagy via Activation of PI3K/Akt Pathway Contributes to the Protection of Ginsenoside Rb1 against Neuronal Death Caused by Ischemic Insults. Int. J. Mol. Sci. 2014, 15, 15426–15442. International Journal of Molecular Sciences, 26(20), 9839. https://doi.org/10.3390/ijms26209839



