Markers of Antiviral Response in SLE Patients After Vaccination Against SARS-CoV-2
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
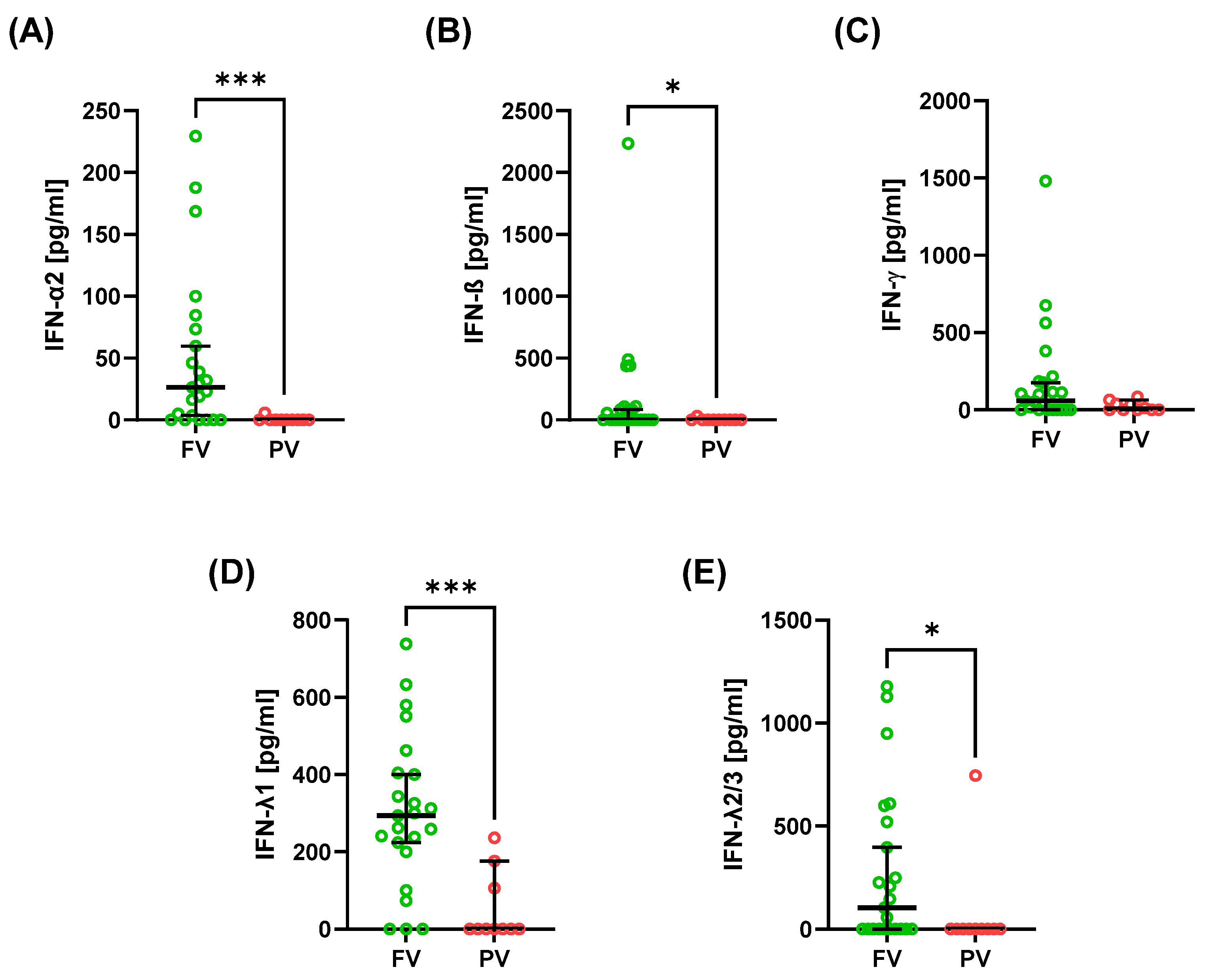
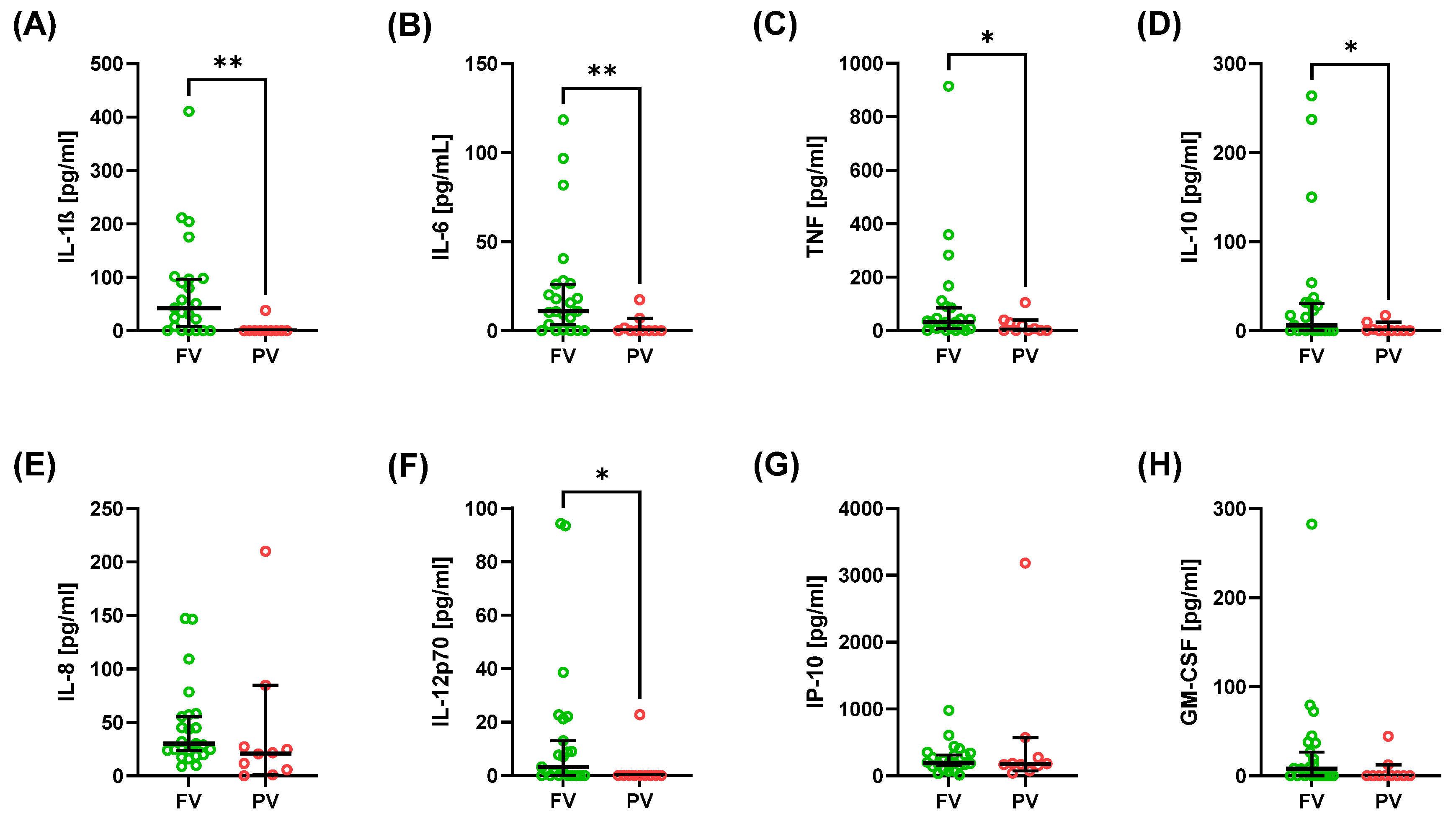
2.2. Cytokine and Interferon Profiles
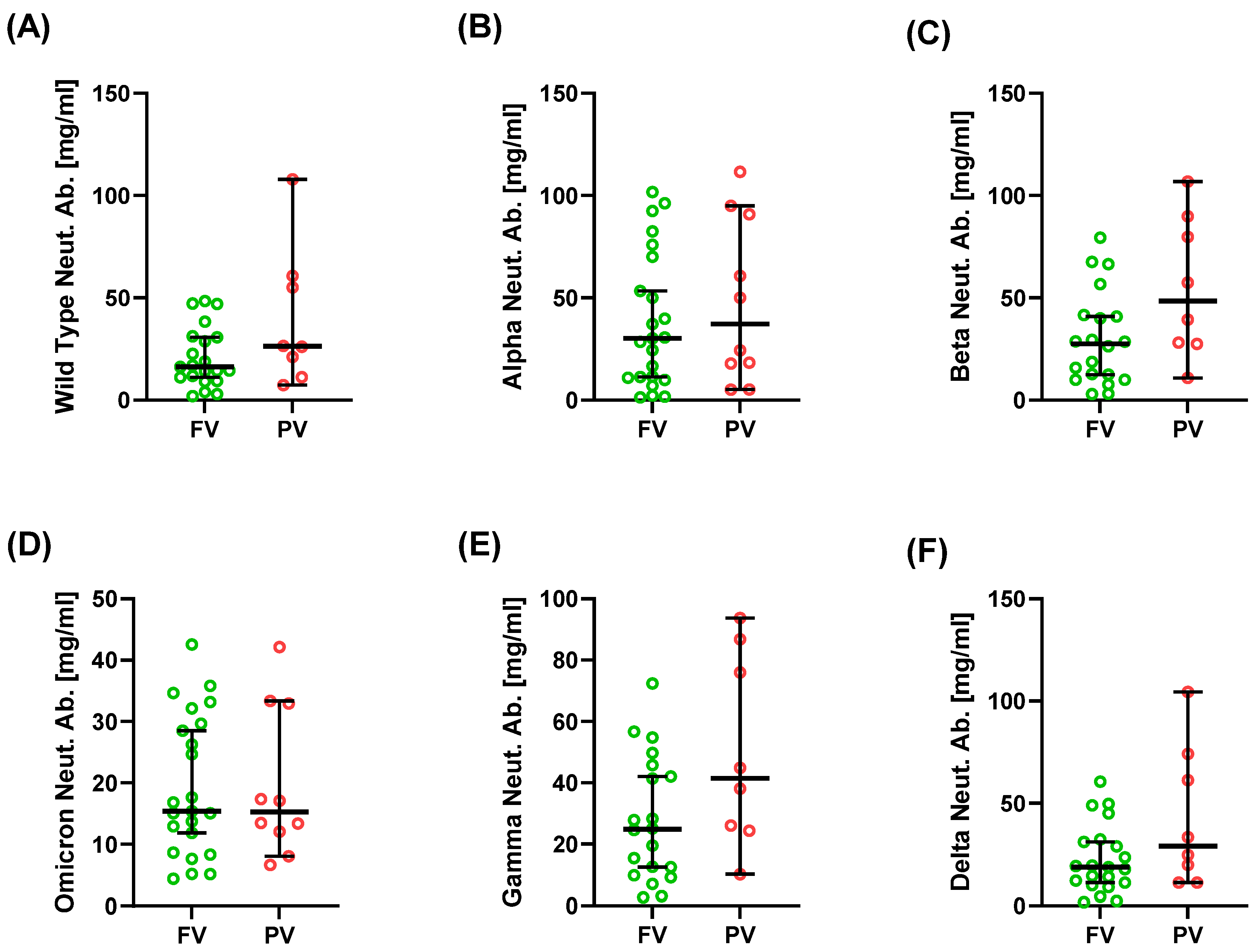
2.3. Neutralizing Antibodies Against SARS-CoV-2 Variants
2.4. T- and B-Cell Subpopulations
2.5. Gene Expression Signatures
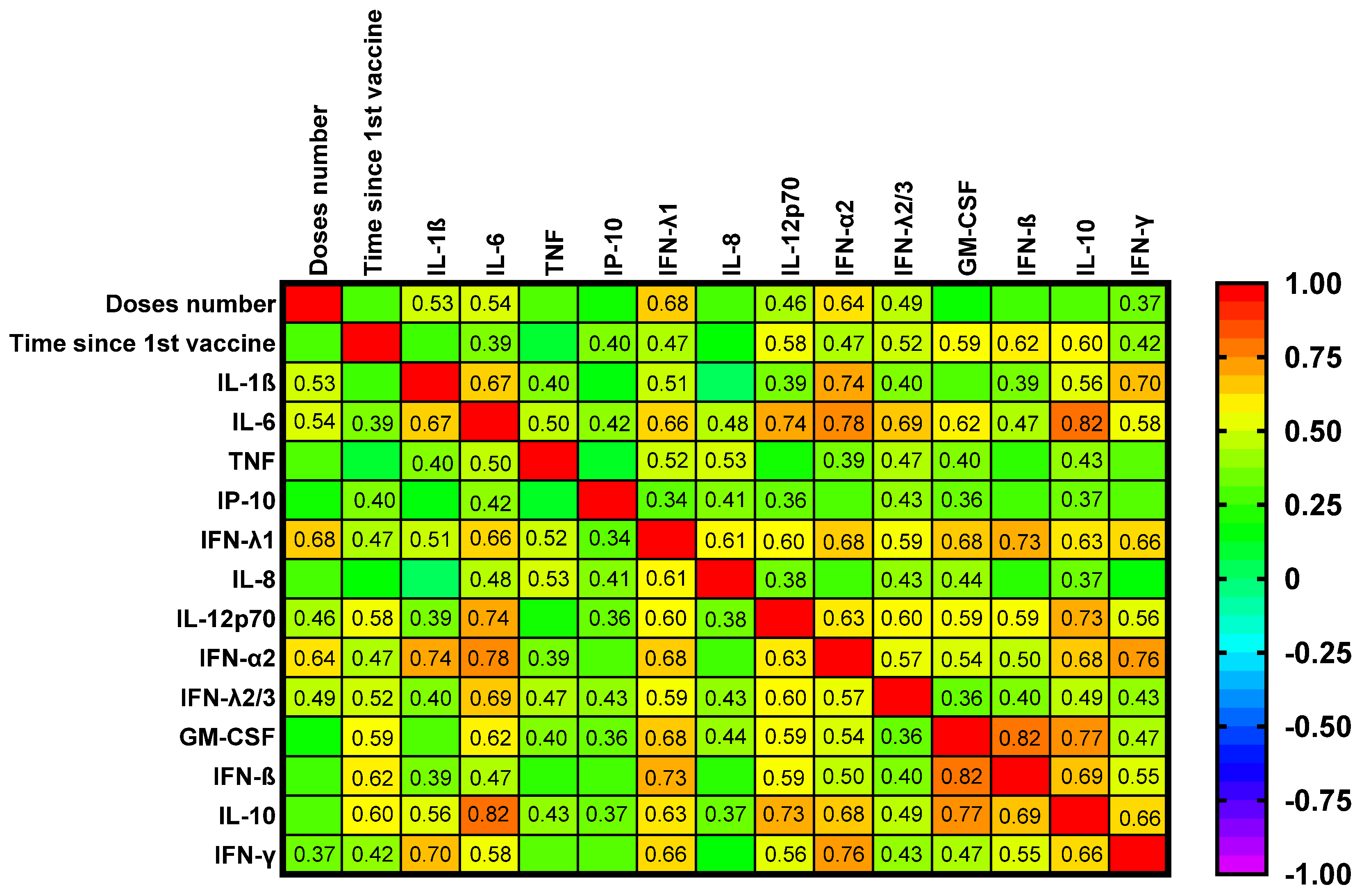
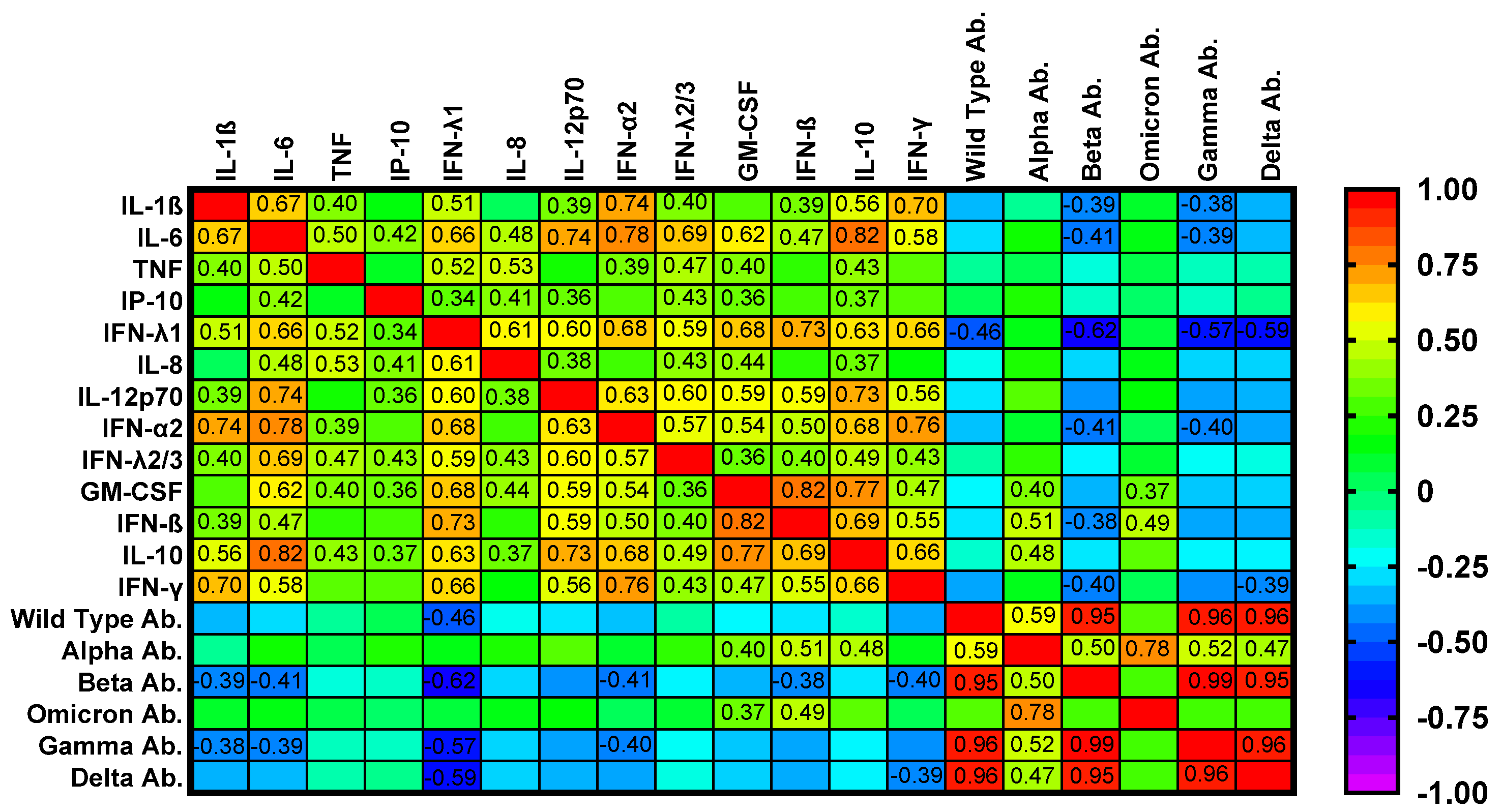
2.6. Correlation Analyses
2.7. Summary of Results
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Study Design and Patient Population
5.2. Vaccination Status
5.3. Clinical and Laboratory Assessment
5.4. Measurement of Cytokines, Chemokines, and Neutralizing Antibodies
5.5. Phenotypic Analysis of T- and B-Cell Subpopulations Ex Vivo
5.6. Molecular Analysis of T- and B-Cell Gene Expression
5.7. Phenotypic Analysis of T- and B-Cell Subpopulations by Flow Cytometry
5.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACR | American College of Rheumatology |
| ACE2 | angiotensin-converting enzyme 2 |
| APC | allophycocyanin (fluorochrome) |
| APOBEC3G | apolipoprotein B mRNA editing enzyme catalytic subunit 3G |
| BAFF | B-cell activating factor (TNFSF13B) |
| BMI | body mass index |
| cDNA | complementary DNA |
| cGAS | cyclic GMP–AMP synthase |
| CCR7 | C–C chemokine receptor type 7 (CD197) |
| CD | cluster of differentiation |
| CI | confidence interval |
| CKD-EPI | Chronic Kidney Disease Epidemiology Collaboration (equation) |
| COVID-19 | coronavirus disease 2019 |
| CRP | C-reactive protein |
| CXCL10 | C-X-C motif chemokine ligand 10 (see IP-10) |
| ΔCt | delta threshold cycle (comparative Ct) |
| DNM | double-negative memory (B cells) |
| eGFR | estimated glomerular filtration rate |
| EDTA | ethylenediaminetetraacetic acid |
| ELISpot | enzyme-linked immunospot |
| ESR | erythrocyte sedimentation rate |
| EULAR | European Alliance of Associations for Rheumatology |
| FACS | fluorescence-activated cell sorting |
| FITC | fluorescein isothiocyanate |
| FOXP3 | forkhead box P3 |
| FV | fully vaccinated |
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase |
| GATA3 | GATA-binding protein 3 |
| GM-CSF | granulocyte–macrophage colony-stimulating factor |
| HLA-DR | human leukocyte antigen–DR isotype |
| IDSA | Infectious Diseases Society of America |
| IFN | interferon |
| IgA | immunoglobulin A |
| IgG | immunoglobulin G |
| IgM | immunoglobulin M |
| IGRA | interferon-γ release assay |
| IL | interleukin |
| IP-10 | interferon-γ–induced protein 10 (CXCL10) |
| IRF8 | interferon regulatory factor 8 |
| ISG | interferon-stimulated gene |
| LN | lupus nephritis |
| mRNA | messenger RNA |
| MWU | Mann–Whitney U (test) |
| NETs | neutrophil extracellular traps |
| Neut. Ab. | neutralizing antibodies |
| NSM | non-switched memory (B cells) |
| PB | plasmablasts |
| PBMCs | peripheral blood mononuclear cells |
| PBS | phosphate-buffered saline |
| PE | phycoerythrin |
| PV | partially vaccinated |
| qPCR | quantitative polymerase chain reaction |
| RBC | red blood cell |
| RORC | RAR-related orphan receptor C (RORγt) |
| RT | reverse transcription |
| RT-qPCR | reverse-transcription quantitative PCR |
| S1 | SARS-CoV-2 spike protein subunit 1 |
| SARS-CoV-2 | severe acute respiratory syndrome coronavirus 2 |
| SDI | SLICC/ACR Damage Index |
| SLE | systemic lupus erythematosus |
| SLEDAI | Systemic Lupus Erythematosus Disease Activity Index |
| SLAM-R | Systemic Lupus Activity Measure–Revised |
| sCr | serum creatinine |
| SLICC | Systemic Lupus International Collaborating Clinics |
| SM | switched memory (B cells) |
| STING | stimulator of interferon genes |
| TB | transitional B cells |
| TBX21 | T-box transcription factor TBX21 (T-bet) |
| Tcm | central memory T cells |
| Tem | effector memory T cells |
| Temra | effector memory T cells re-expressing CD45RA |
| TGFB | transforming growth factor beta (gene) |
| TLR | toll-like receptor |
| TNF | tumor necrosis factor (gene) |
| Tregs | regulatory T cells |
| TRIM21 | tripartite motif-containing 21 (Ro52) |
| UDG | uracil-DNA glycosylase |
| WT | wild type |
| pDCs | plasmacytoid dendritic cells |
References
- Shattock, A.J.; Johnson, H.C.; Sim, S.Y.; Carter, A.; Lambach, P.; Hutubessy, R.C.W.; Thompson, K.M.; Badizadegan, K.; Lambert, B.; Ferrari, M.J.; et al. Contribution of vaccination to improved survival and health: Modelling 50 years of the Expanded Programme on Immunization. Lancet 2024, 403, 2307–2316. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, B. The contribution of vaccination to global health: Past, present and future. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130433. [Google Scholar] [CrossRef] [PubMed]
- Riedel, S. Edward Jenner and the History of Smallpox and Vaccination. In Baylor University Medical Center Proceedings; Taylor & Francis: Abingdon-on-Thames, UK, 2005; Volume 18, pp. 21–25. [Google Scholar]
- Smith, K.A. Louis Pasteur, the Father of Immunology? Front. Immunol. 2012, 3, 68. [Google Scholar] [CrossRef] [PubMed]
- Pollard, A.J.; Bijker, E.M. A guide to vaccinology: From basic principles to new developments. Nat. Rev. Immunol. 2020, 21, 83–100, Correction in Nat. Rev. Immunol. 2021, 21, 129. https://doi.org/10.1038/s41577-020-00497-5. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef]
- Zimmermann, P.; Curtis, N. Factors That Influence the Immune Response to Vaccination. Clin. Microbiol. Rev. 2019, 32, e00084-18. [Google Scholar] [CrossRef]
- Plotkin, S.A. Correlates of Protection Induced by Vaccination. Clin. Vaccine Immunol. 2010, 17, 1055–1065. [Google Scholar] [CrossRef]
- van Assen, S.; Agmon-Levin, N.; Elkayam, O.; Cervera, R.; Doran, M.F.; Dougados, M.; Emery, P.; Geborek, P.; Ioannidis, J.P.A.; Jayne, D.R.W.; et al. EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann. Rheum. Dis. 2011, 70, 414–422. [Google Scholar] [CrossRef]
- Rubin, L.G.; Levin, M.J.; Ljungman, P.; Davies, E.G.; Avery, R.; Tomblyn, M.; Bousvaros, A.; Dhanireddy, S.; Sung, L.; Keyserling, H.; et al. Executive Summary: 2013 IDSA Clinical Practice Guideline for Vaccination of the Immunocompromised Host. Clin. Infect. Dis. 2014, 58, 309–318. [Google Scholar] [CrossRef]
- Landewé, R.B.M.; Kroon, F.P.B.; Alunno, A.; Najm, A.; Bijlsma, J.W.; Burmester, G.-R.R.; Caporali, R.; Combe, B.; Conway, R.; Curtis, J.R.; et al. EULAR recommendations for the management and vaccination of people with rheumatic and musculoskeletal diseases in the context of SARS-CoV-2: The November 2021 update. Ann. Rheum. Dis. 2022, 81, 1628–1639. [Google Scholar] [CrossRef]
- Murdaca, G.; Orsi, A.; Spanò, F.; Puppo, F.; Durando, P.; Icardi, G.; Ansaldi, F. Influenza and pneumococcal vaccinations of patients with systemic lupus erythematosus: Current views upon safety and immunogenicity. Autoimmun. Rev. 2014, 13, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Garg, M.; Mufti, N.; Palmore, T.N.; Hasni, S.A. Recommendations and barriers to vaccination in systemic lupus erythematosus. Autoimmun. Rev. 2018, 17, 990–1001. [Google Scholar] [CrossRef] [PubMed]
- Pugès, M.; Biscay, P.; Barnetche, T.; Truchetet, M.; Richez, C.; Seneschal, J.; Gensous, N.; Lazaro, E.; Duffau, P. Immunogenicity and impact on disease activity of influenza and pneumococcal vaccines in systemic lupus erythematosus: A systematic literature review and meta-analysis. Rheumatology 2016, 55, 1664–1672. [Google Scholar] [CrossRef] [PubMed]
- Yuki, E.F.N.; Borba, E.F.; Pasoto, S.G.; Seguro, L.P.; Lopes, M.; Saad, C.G.S.; Medeiros-Ribeiro, A.C.; Silva, C.A.; de Andrade, D.C.O.; Kupa, L.d.V.K.; et al. Impact of Distinct Therapies on Antibody Response to SARS-CoV-2 Vaccine in Systemic Lupus Erythematosus. Arthritis Care Res. 2022, 74, 562–571. [Google Scholar] [CrossRef]
- Furer, V.; Eviatar, T.; Zisman, D.; Peleg, H.; Paran, D.; Levartovsky, D.; Zisapel, M.; Elalouf, O.; Kaufman, I.; Meidan, R.; et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: A multicentre study. Ann. Rheum. Dis. 2021, 80, 1330–1338, Correction in Ann. Rheum. Dis. 2022, 81, 133. https://doi.org/10.1136/annrheumdis-2021-220647corr1. [Google Scholar] [CrossRef]
- Petri, M.; Joyce, D.; Haag, K.; Fava, A.; Goldman, D.W.; Zhong, D.; Xiao, S.; Milstone, A.; Magder, L.S. Effect of Systemic Lupus Erythematosus and Immunosuppressive Agents on COVID-19 Vaccination Antibody Response. Arthritis Care Res. 2023, 75, 1878–1885. [Google Scholar] [CrossRef]
- Adawi, M.; Bragazzi, N.L.; McGonagle, D.; Watad, S.; Mahroum, N.; Damiani, G.; Conic, R.; Bridgewood, C.; Mahagna, H.; Giacomelli, L.; et al. Immunogenicity, safety and tolerability of anti-pneumococcal vaccination in systemic lupus erythematosus patients: An evidence-informed and PRISMA compliant systematic review and meta-analysis. Autoimmun. Rev. 2019, 18, 73–92. [Google Scholar] [CrossRef]
- Moyon, Q.; Sterlin, D.; Miyara, M.; Anna, F.; Mathian, A.; Lhote, R.; Ghillani-Dalbin, P.; Breillat, P.; Mudumba, S.; de Alba, S.; et al. BNT162b2 vaccine-induced humoral and cellular responses against SARS-CoV-2 variants in systemic lupus erythematosus. Ann. Rheum. Dis. 2021, 81, 575–583. [Google Scholar] [CrossRef]
- Izmirly, P.M.; Kim, M.Y.; Samanovic, M.; Fernandez-Ruiz, R.; Ohana, S.; Deonaraine, K.K.; Engel, A.J.; Masson, M.; Xie, X.; Cornelius, A.R.; et al. Evaluation of Immune Response and Disease Status in Systemic Lupus Erythematosus Patients Following SARS–CoV-2 Vaccination. Arthritis Rheumatol. 2021, 74, 284–294. [Google Scholar] [CrossRef]
- Tunitsky-Lifshitz, Y.; Maoz-Segal, R.; Niznik, S.; Shavit, R.; Yahia, S.H.; Langevitz, P.; Agmon-Levin, N. The third dose of BNT162b2 COVID-19 vaccine is efficacious and safe for systemic lupus erythematosus patients receiving belimumab. Lupus 2023, 32, 675–679. [Google Scholar] [CrossRef]
- Larsen, E.S.; Nilsson, A.C.; Möller, S.; Voss, A.B.; Johansen, I.S. Immunogenicity and risk of disease flare after a three-dose regimen with SARS-CoV-2 vaccination in patients with systemic lupus erythematosus: Results from the prospective cohort study COVAC-SLE. Clin. Exp. Rheumatol. 2022, 41, 676–684. [Google Scholar] [CrossRef]
- Tan, S.Y.S.; Yee, A.M.; Sim, J.J.L.; Lim, C.C. COVID-19 vaccination in systemic lupus erythematosus: A systematic review of its effectiveness, immunogenicity, flares and acceptance. Rheumatology 2022, 62, 1757–1772. [Google Scholar] [CrossRef]
- Tang, W.; Askanase, A.D.; Khalili, L.; Merrill, J.T. SARS-CoV-2 vaccines in patients with SLE. Lupus Sci. Med. 2021, 8, e000479, Correction in Lupus Sci. Med. 2021, 8, e000479corr1. https://doi.org/10.1136/lupus-2021-000479corr1. [Google Scholar] [CrossRef]
- Khatri, G.; Shaikh, S.; Rai, A.; Cheema, H.A.; Essar, M.Y. Systematic lupus erythematous patients following COVID-19 vaccination: Its flares up and precautions. Ann. Med. Surg. 2022, 80, 104282. [Google Scholar] [CrossRef] [PubMed]
- Murdaca, G.; Orsi, A.; Spanò, F.; Faccio, V.; Puppo, F.; Durando, P.; Icardi, G.; Ansaldi, F. Vaccine-preventable infections in Systemic Lupus Erythematosus. Hum. Vaccines Immunother. 2015, 12, 632–643. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, Y.; Isenberg, D.A. Immunisation of patients with systemic lupus erythematosus: The current state of play. Lupus 1999, 8, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Kim, S.T.; Craft, J. The pathogenesis of systemic lupus erythematosus—An update. Curr. Opin. Immunol. 2012, 24, 651–657. [Google Scholar] [CrossRef]
- Postal, M.; Vivaldo, J.F.; Fernandez-Ruiz, R.; Paredes, J.L.; Appenzeller, S.; Niewold, T.B. Type I interferon in the pathogenesis of systemic lupus erythematosus. Curr. Opin. Immunol. 2020, 67, 87–94. [Google Scholar] [CrossRef]
- Kim, J.-M.; Park, S.-H.; Kim, H.-Y.; Kwok, S.-K. A Plasmacytoid Dendritic Cells-Type I Interferon Axis Is Critically Implicated in the Pathogenesis of Systemic Lupus Erythematosus. Int. J. Mol. Sci. 2015, 16, 14158–14170. [Google Scholar] [CrossRef]
- Caielli, S.; Wan, Z.; Pascual, V. Systemic Lupus Erythematosus Pathogenesis: Interferon and Beyond. Annu. Rev. Immunol. 2023, 41, 533–560. [Google Scholar] [CrossRef]
- Mok, C.C.; Chan, K.L.; Tse, S.M. Hesitancy for SARS-CoV-2 vaccines and post-vaccination flares in patients with systemic lupus erythematosus. Vaccine 2022, 40, 5959–5964. [Google Scholar] [CrossRef]
- Gerosa, M.; Schioppo, T.; Argolini, L.M.; Sciascia, S.; Ramirez, G.A.; Moroni, G.; Sinico, R.A.; Bonelli, G.; Alberici, F.; Mescia, F.; et al. The Impact of Anti-SARS-CoV-2 Vaccine in Patients with Systemic Lupus Erythematosus: A Multicentre Cohort Study. Vaccines 2022, 10, 663. [Google Scholar] [CrossRef]
- Louthrenoo, W.; Tangkum, P.; Kasitanon, N.; Gumtorntip, W.; Winichakoon, P.; Konsamun, S.; Wongthanee, A. Flares and Predicting Factors of Flares in Patients with Systemic Lupus Erythematosus Associated with Different Doses and Types of COVID-19 Vaccines. Vaccines 2024, 12, 1399. [Google Scholar] [CrossRef]
- Lisowska, K.A.; Ciesielska-Figlon, K.; Komorniczak, M.; Bułło-Piontecka, B.; Dębska-Ślizień, A.; Wardowska, A. Peripheral Blood Mononuclear Cells and Serum Cytokines in Patients with Lupus Nephritis after COVID-19. Int. J. Mol. Sci. 2024, 25, 8278. [Google Scholar] [CrossRef]
| Variable | Fully Vaccinated (n = 23) | Partially Vaccinated (n = 10) | p Value |
|---|---|---|---|
| Age [years] | 43.0 (33.0; 71.0) | 43.0 (39.0; 71.0) | 0.923 |
| Male-to-female ratio | 3:20 | 0:10 | 0.536 |
| SLE duration [years] | 19.0 (2.0; 42.0) | 17.5 (6.0; 37.0) | 0.985 |
| LN duration [years] | 12.0 (1.0; 25.0) | 16.5 (1.0; 32.0) | 0.451 |
| Body mass index (BMI) [kg/m2] | 24.6 (16.7; 34.9) | 25.3 (16.4; 30.5) | 0.862 |
| SLEDAI [points] | 6.0 (0.0; 24.0) | 8.0 (2.0; 26.0) | 0.603 |
| SLAM-R [points] | 4.0 (1.0; 9.0) | 6.5 (2.0; 14.0) | 0.123 |
| SDI [points] | 1.0 (0.0; 5.0) | 0.5 (0.0; 6.0) | 0.603 |
| Complement C3 [g/L] | 1.02 (0.59; 1.89) | 1.07 (0.76; 1.65) | 0.237 |
| Complement C4 [g/L] | 0.16 (0.10; 0.29) | 0.20 (0.07; 0.29) | 0.324 |
| IgG [g/L] | 11.5 (7.7; 16.8) | 11.8 (6.2; 23.0) | 0.819 |
| IgA [g/L] | 2.52 (1.56; 4.83) | 2.62 (0.00; 3.70) | 0.466 |
| IgM [g/L] | 0.88 (0.18; 1.87) | 0.86 (0.00; 1.86) | 0.819 |
| Anti-dsDNA antibodies [IU/mL] | 304.8 (0.0; 800.0) | 176.0 (0.0; 800.0) | 0.564 |
| ESR [mm/h] | 10.0 (2.0; 36.0) | 18.0 (2.0; 42.0) | 0.393 |
| CRP [mg/L] | 1.34 (0.00; 6.09) | 2.06 (0.40; 13.35) | 0.434 |
| sCr [mg/dL] | 0.83 (0.53; 3.80) | 0.87 (0.66; 3.12) | 0.507 |
| eGFR [mL/min/1.73 m2] | 85.2 (13.0; 90.0) | 81.0 (39.0; 90.0) | 0.915 |
| Therapy | |||
| Glucocorticoid use, n (%) | 20 (87%) | 8 (80%) | 0.627 |
| Prednisone equivalent dose [mg/day] | 5 (0; 20) | 5 (0; 10) | 0.499 |
| MMF use, n (%) | 17 (74%) | 1 (10%) | 0.001 |
| CNI use (TAC or CsA), n (%) | 2 (8.7%) | 3 (30%) | 0.149 |
| Antimalarial therapy, n (%) | 15 (65.2%) | 4 (40%) | 0.257 |
| Variable | Fully Vaccinated (n = 23) | Partially Vaccinated (n = 10) | p Value |
|---|---|---|---|
| IL-1β [pg/mL] | 42.83 (0.00; 410.67) | 0.00 (0.00; 38.30) | 0.002 |
| IL-6 [pg/mL] | 11.03 (0.00; 118.47) | 0.00 (0.00; 17.43) | 0.003 |
| TNF-α [pg/mL] | 31.87 (0.00; 914.89) | 3.44 (0.00; 104.41) | 0.046 |
| IP-10 [pg/mL] | 190.20 (11.89; 979.97) | 176.72 (37.09; 3180.58) | 0.681 |
| IFN-λ1 [pg/mL] | 293.78 (0.00; 738.11) | 0.00 (0.00; 236.41) | 0.001 |
| IL-8 [pg/mL] | 30.15 (8.77; 147.14) | 21.03 (0.00; 210.19) | 0.132 |
| IL-12p70 [pg/mL] | 3.19 (0.00; 94.33) | 0.00 (0.00; 22.77) | 0.014 |
| IFN-α2 [pg/mL] | 26.45 (0.00; 229.38) | 0.00 (0.00; 5.77) | 0.001 |
| IFN-λ2/3 [pg/mL] | 104.21 (0.00; 1177.92) | 0.00 (0.00; 745.10) | 0.031 |
| GM-CSF [pg/mL] | 7.90 (0.00; 282.67) | 0.00 (0.00; 44.38) | 0.076 |
| IFN-β [pg/mL] | 0.00 (0.00; 2237.08) | 0.00 (0.00; 29.89) | 0.045 |
| IL-10 [pg/mL] | 6.36 (0.00; 263.83) | 0.00 (0.00; 17.12) | 0.046 |
| IFN-γ [pg/mL] | 59.70 (0.00; 1480.54) | 6.15 (0.00; 83.45) | 0.086 |
| Alpha Neut. Ab. [mg/mL] | 30.25 (1.30; 101.74) | 37.20 (5.10; 111.51) | 0.570 |
| Beta Neut. Ab. [mg/mL] | 27.45 (2.92; 79.38) | 48.39 (10.85; 106.78) | 0.079 |
| Omicron Neut. Ab. [mg/mL] | 15.38 (4.41; 42.56) | 15.28 (6.65; 42.15) | 0.953 |
| Gamma Neut. Ab. [mg/mL] | 24.91 (2.73; 72.34) | 41.49 (10.17; 93.70) | 0.098 |
| Wild-type Neut. Ab. [mg/mL] | 16.14 (1.91; 48.43) | 26.30 (7.30; 107.88) | 0.164 |
| Delta Neut. Ab. [mg/mL] | 18.75 (1.70; 60.62) | 29.09 (11.15; 104.45) | 0.102 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komorniczak, M.; Lisowska, K.A.; Bułło-Piontecka, B.; Dębska-Ślizień, A.; Wardowska, A. Markers of Antiviral Response in SLE Patients After Vaccination Against SARS-CoV-2. Int. J. Mol. Sci. 2025, 26, 10241. https://doi.org/10.3390/ijms262010241
Komorniczak M, Lisowska KA, Bułło-Piontecka B, Dębska-Ślizień A, Wardowska A. Markers of Antiviral Response in SLE Patients After Vaccination Against SARS-CoV-2. International Journal of Molecular Sciences. 2025; 26(20):10241. https://doi.org/10.3390/ijms262010241
Chicago/Turabian StyleKomorniczak, Michał, Katarzyna Aleksandra Lisowska, Barbara Bułło-Piontecka, Alicja Dębska-Ślizień, and Anna Wardowska. 2025. "Markers of Antiviral Response in SLE Patients After Vaccination Against SARS-CoV-2" International Journal of Molecular Sciences 26, no. 20: 10241. https://doi.org/10.3390/ijms262010241
APA StyleKomorniczak, M., Lisowska, K. A., Bułło-Piontecka, B., Dębska-Ślizień, A., & Wardowska, A. (2025). Markers of Antiviral Response in SLE Patients After Vaccination Against SARS-CoV-2. International Journal of Molecular Sciences, 26(20), 10241. https://doi.org/10.3390/ijms262010241








