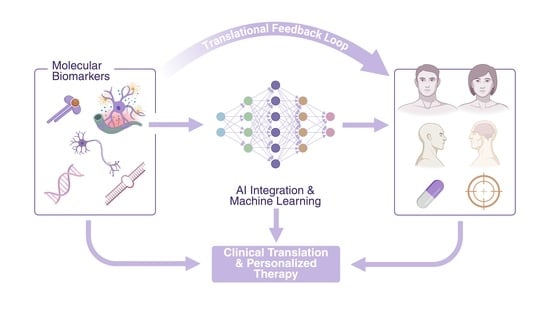
Special Issue “Translating Molecular Psychiatry: From Biomarkers to Personalized Therapies”
1. Introduction: Psychiatry at the Molecular Crossroads
2. Mapping the Terrain: Current Insights from the Special Issue
2.1. Immune-Related Insights in Mood Disorders
2.2. Schizophrenia (SCZ): Treatment Optimization and BBB Dysfunction
2.3. Precision Biomarkers and AI in Psychiatric Diagnosis
2.4. Novel Therapies in Alzheimer’s and Developmental Disorders
3. Present Challenges and Gaps
4. Building the Bridge: Advances Highlighted in This Special Issue
5. The Road Ahead
5.1. Near-Term (2–4 Years)
5.2. Long-Term (5–10 Years)
6. Conclusions: From Discovery to Translation
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| AI | artificial intelligence |
| BBB | blood–brain barrier |
| BD | bipolar disorder |
| CP | cerebral palsy |
| GBS | group B streptococcus |
| hs-CRP | high-sensitivity C-reactive protein |
| IL-1 | interleukin-1 |
| IL-1Ra | interleukin-1 receptor antagonist |
| IL-6 | interleukin-6 |
| LAI | long-acting injectable |
| OIS | one-injection start |
| PNEI | psychoneuroendocrineimmunology |
| SCZ | schizophrenia |
| TIS | two-injection start |
References
- Harrison, P.J.; Geddes, J.R.; Tunbridge, E.M. The Emerging Neurobiology of Bipolar Disorder. Trends Neurosci. 2018, 41, 18–30. [Google Scholar] [CrossRef]
- Abi-Dargham, A.; Moeller, S.J.; Ali, F.; DeLorenzo, C.; Domschke, K.; Horga, G.; Jutla, A.; Kotov, R.; Paulus, M.P.; Rubio, J.M.; et al. Candidate biomarkers in psychiatric disorders: State of the field. World Psychiatry 2023, 22, 236–262. [Google Scholar] [CrossRef]
- Vieta, E.; Berk, M.; Schulze, T.G.; Carvalho, A.F.; Suppes, T.; Calabrese, J.R.; Gao, K.; Miskowiak, K.W.; Grande, I. Bipolar disorders. Nat. Rev. Dis. Primers 2018, 4, 18008. [Google Scholar] [CrossRef]
- Waszkiewicz, N. Mentally Sick or Not-(Bio)Markers of Psychiatric Disorders Needed. J. Clin. Med. 2020, 9, 2375. [Google Scholar] [CrossRef]
- Dacquino, C.; De Rossi, P.; Spalletta, G. Schizophrenia and bipolar disorder: The road from similarities and clinical heterogeneity to neurobiological types. Clin. Chim. Acta 2015, 449, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M. Parkinson’s Disease: Bridging Gaps, Building Biomarkers, and Reimagining Clinical Translation. Cells 2025, 14, 1161. [Google Scholar] [CrossRef]
- Gruzdev, S.K.; Yakovlev, A.A.; Druzhkova, T.A.; Guekht, A.B.; Gulyaeva, N.V. The Missing Link: How Exosomes and miRNAs can Help in Bridging Psychiatry and Molecular Biology in the Context of Depression, Bipolar Disorder and Schizophrenia. Cell. Mol. Neurobiol. 2019, 39, 729–750. [Google Scholar] [CrossRef] [PubMed]
- Comai, S.; Manchia, M.; Bosia, M.; Miola, A.; Poletti, S.; Benedetti, F.; Nasini, S.; Ferri, R.; Rujescu, D.; Leboyer, M.; et al. Moving toward precision and personalized treatment strategies in psychiatry. Int. J. Neuropsychopharmacol. 2025, 28, pyaf025. [Google Scholar] [CrossRef]
- McIntyre, R.S.; Berk, M.; Brietzke, E.; Goldstein, B.I.; López-Jaramillo, C.; Kessing, L.V.; Malhi, G.S.; Nierenberg, A.A.; Rosenblat, J.D.; Majeed, A.; et al. Bipolar disorders. Lancet 2020, 396, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Goes, F.S. Diagnosis and management of bipolar disorders. BMJ 2023, 381, e073591. [Google Scholar] [CrossRef]
- Wolfers, T.; Doan, N.T.; Kaufmann, T.; Alnæs, D.; Moberget, T.; Agartz, I.; Buitelaar, J.K.; Ueland, T.; Melle, I.; Franke, B.; et al. Mapping the Heterogeneous Phenotype of Schizophrenia and Bipolar Disorder Using Normative Models. JAMA Psychiatry 2018, 75, 1146–1155. [Google Scholar] [CrossRef] [PubMed]
- Cheon, E.J.; Bearden, C.E.; Sun, D.; Ching, C.R.K.; Andreassen, O.A.; Schmaal, L.; Veltman, D.J.; Thomopoulos, S.I.; Kochunov, P.; Jahanshad, N.; et al. Cross disorder comparisons of brain structure in schizophrenia, bipolar disorder, major depressive disorder, and 22q11.2 deletion syndrome: A review of ENIGMA findings. Psychiatry Clin. Neurosci. 2022, 76, 140–161. [Google Scholar] [CrossRef] [PubMed]
- Mandal, P.K.; Gaur, S.; Roy, R.G.; Samkaria, A.; Ingole, R.; Goel, A. Schizophrenia, Bipolar and Major Depressive Disorders: Overview of Clinical Features, Neurotransmitter Alterations, Pharmacological Interventions, and Impact of Oxidative Stress in the Disease Process. ACS Chem. Neurosci. 2022, 13, 2784–2802. [Google Scholar] [CrossRef]
- Tanaka, M. Beyond the boundaries: Transitioning from categorical to dimensional paradigms in mental health diagnostics. Adv. Clin. Exp. Med. 2024, 33, 1295–1301. [Google Scholar] [CrossRef]
- Capatina, T.F.; Oatu, A.; Babasan, C.; Trifu, S. Translating Molecular Psychiatry: From Biomarkers to Personalized Therapies—A Narrative Review. Int. J. Mol. Sci. 2025, 26, 4285. [Google Scholar] [CrossRef]
- García-Gutiérrez, M.S.; Navarrete, F.; Sala, F.; Gasparyan, A.; Austrich-Olivares, A.; Manzanares, J. Biomarkers in Psychiatry: Concept, Definition, Types and Relevance to the Clinical Reality. Front. Psychiatry 2020, 11, 432. [Google Scholar] [CrossRef]
- Venkatasubramanian, G.; Keshavan, M.S. Biomarkers in Psychiatry—A Critique. Ann. Neurosci. 2016, 23, 3–5. [Google Scholar] [CrossRef]
- Do, K.Q. Bridging the gaps towards precision psychiatry: Mechanistic biomarkers for early detection and intervention. Psychiatry Res. 2023, 321, 115064. [Google Scholar] [CrossRef]
- Kraguljac, N.V.; McDonald, W.M.; Widge, A.S.; Rodriguez, C.I.; Tohen, M.; Nemeroff, C.B. Neuroimaging Biomarkers in Schizophrenia. Am. J. Psychiatry 2021, 178, 509–521. [Google Scholar] [CrossRef]
- Roy, B.; Yoshino, Y.; Allen, L.; Prall, K.; Schell, G.; Dwivedi, Y. Exploiting Circulating MicroRNAs as Biomarkers in Psychiatric Disorders. Mol. Diagn. Ther. 2020, 24, 279–298. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Vécsei, L. From Microbial Switches to Metabolic Sensors: Rewiring the Gut-Brain Kynurenine Circuit. Biomedicines 2025, 13, 2020. [Google Scholar] [CrossRef]
- Prompiengchai, S.; Dunlop, K. Breakthroughs and challenges for generating brain network-based biomarkers of treatment response in depression. Neuropsychopharmacology 2024, 50, 230–245. [Google Scholar] [CrossRef]
- Abi-Dargham, A.; Horga, G. The search for imaging biomarkers in psychiatric disorders. Nat. Med. 2016, 22, 1248–1255. [Google Scholar] [CrossRef] [PubMed]
- Szabó, Á.; Galla, Z.; Spekker, E.; Szűcs, M.; Martos, D.; Takeda, K.; Ozaki, K.; Inoue, H.; Yamamoto, S.; Toldi, J.; et al. Oxidative and Excitatory Neurotoxic Stresses in CRISPR/Cas9-Induced Kynurenine Aminotransferase Knockout Mice: A Novel Model for Despair-Based Depression and Post-Traumatic Stress Disorder. Front. Biosci. 2025, 30, 25706. [Google Scholar] [CrossRef]
- Lozupone, M.; Seripa, D.; Stella, E.; La Montagna, M.; Solfrizzi, V.; Quaranta, N.; Veneziani, F.; Cester, A.; Sardone, R.; Bonfiglio, C.; et al. Innovative biomarkers in psychiatric disorders: A major clinical challenge in psychiatry. Expert. Rev. Proteomics 2017, 14, 809–824. [Google Scholar] [CrossRef]
- Kraus, B.; Zinbarg, R.; Braga, R.M.; Nusslock, R.; Mittal, V.A.; Gratton, C. Insights from personalized models of brain and behavior for identifying biomarkers in psychiatry. Neurosci. Biobehav. Rev. 2023, 152, 105259. [Google Scholar] [CrossRef]
- Berdeville, C.; Silva-Amaral, D.; Dalgalarrondo, P.; Banzato, C.E.M.; Martins-de-Souza, D. A scoping review of protein biomarkers for schizophrenia: State of progress, underlying biology, and methodological considerations. Neurosci. Biobehav. Rev. 2025, 168, 105949. [Google Scholar] [CrossRef] [PubMed]
- Ríos, U.; Pérez, S.; Martínez, C.; Moya, P.R.; Arancibia, M. Inflammation and Cognition in Bipolar Disorder: Diverging Paths of Interleukin-6 and Outcomes. Int. J. Mol. Sci. 2025, 26, 6372. [Google Scholar] [CrossRef] [PubMed]
- Bottaccioli, A.G.; Bologna, M.; Bottaccioli, F. Rethinking Depression-Beyond Neurotransmitters: An Integrated Psychoneuroendocrineimmunology Framework for Depression’s Pathophysiology and Tailored Treatment. Int. J. Mol. Sci. 2025, 26, 2759. [Google Scholar] [CrossRef]
- Trovini, G.; Lombardozzi, G.; Kotzalidis, G.D.; Lionetto, L.; Russo, F.; Sabatino, A.; Serra, E.; Castorina, S.; Civita, G.; Frezza, S.; et al. Optimising Aripiprazole Long-Acting Injectable: A Comparative Study of One- and Two-Injection Start Regimens in Schizophrenia with and Without Substance Use Disorders and Relationship to Early Serum Levels. Int. J. Mol. Sci. 2025, 26, 1394. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, J.; Wang, X.; Han, M.; Fei, Y.; Wang, J. Blood-Brain Barrier Disruption in Schizophrenia: Insights, Mechanisms, and Future Directions. Int. J. Mol. Sci. 2025, 26, 873. [Google Scholar] [CrossRef]
- Checa-Robles, F.J.; Salvetat, N.; Cayzac, C.; Menhem, M.; Favier, M.; Vetter, D.; Ouna, I.; Nani, J.V.; Hayashi, M.A.F.; Brietzke, E.; et al. RNA Editing Signatures Powered by Artificial Intelligence: A New Frontier in Differentiating Schizophrenia, Bipolar, and Schizoaffective Disorders. Int. J. Mol. Sci. 2024, 25, 12981. [Google Scholar] [CrossRef]
- Șerban, M.; Toader, C.; Covache-Busuioc, R.A. Brain Tumors, AI and Psychiatry: Predicting Tumor-Associated Psychiatric Syndromes with Machine Learning and Biomarkers. Int. J. Mol. Sci. 2025, 26, 8114. [Google Scholar] [CrossRef]
- Barbalho, S.M.; Laurindo, L.F.; de Oliveira Zanuso, B.; da Silva, R.M.S.; Gallerani Caglioni, L.; Nunes Junqueira de Moraes, V.B.F.; Fornari Laurindo, L.; Dogani Rodrigues, V.; da Silva Camarinha Oliveira, J.; Beluce, M.E.; et al. AdipoRon’s Impact on Alzheimer’s Disease-A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2025, 26, 484. [Google Scholar] [CrossRef]
- Ayash, T.A.; Allard, M.J.; Chevin, M.; Sébire, G. IL-1 Blockade Mitigates Autism and Cerebral Palsy Traits in Offspring In-Utero Exposed to Group B Streptococcus Chorioamnionitis. Int. J. Mol. Sci. 2024, 25, 11393. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Battaglia, S.; Liloia, D. Navigating Neurodegeneration: Integrating Biomarkers, Neuroinflammation, and Imaging in Parkinson’s, Alzheimer’s, and Motor Neuron Disorders. Biomedicines 2025, 13, 1045. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.F.; Solmi, M.; Sanches, M.; Machado, M.O.; Stubbs, B.; Ajnakina, O.; Sherman, C.; Sun, Y.R.; Liu, C.S.; Brunoni, A.R.; et al. Evidence-based umbrella review of 162 peripheral biomarkers for major mental disorders. Transl. Psychiatry 2020, 10, 152. [Google Scholar] [CrossRef]
- Rowland, T.; Perry, B.I.; Upthegrove, R.; Barnes, N.; Chatterjee, J.; Gallacher, D.; Marwaha, S. Neurotrophins, cytokines, oxidative stress mediators and mood state in bipolar disorder: Systematic review and meta-analyses. Br. J. Psychiatry 2018, 213, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, F.P.; Borges, M.C.; Horta, B.L.; Bowden, J.; Davey Smith, G. Inflammatory Biomarkers and Risk of Schizophrenia: A 2-Sample Mendelian Randomization Study. JAMA Psychiatry 2017, 74, 1226–1233. [Google Scholar] [CrossRef]
- Kageyama, Y.; Kasahara, T.; Kato, M.; Sakai, S.; Deguchi, Y.; Tani, M.; Kuroda, K.; Hattori, K.; Yoshida, S.; Goto, Y.; et al. The relationship between circulating mitochondrial DNA and inflammatory cytokines in patients with major depression. J. Affect. Disord. 2018, 233, 15–20. [Google Scholar] [CrossRef]
- Salvetat, N.; Checa-Robles, F.J.; Delacrétaz, A.; Cayzac, C.; Dubuc, B.; Vetter, D.; Dainat, J.; Lang, J.P.; Gamma, F.; Weissmann, D. AI algorithm combined with RNA editing-based blood biomarkers to discriminate bipolar from major depressive disorders in an external validation multicentric cohort. J. Affect. Disord. 2024, 356, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.A.F.; Salvetat, N.; Cayzac, C.; Checa-Robles, F.J.; Dubuc, B.; Mereuze, S.; Nani, J.V.; Molina, F.; Brietzke, E.; Weissmann, D. Euthymic and depressed bipolar patients are characterized by different RNA editing patterns in blood. Psychiatry Res. 2023, 328, 115422. [Google Scholar] [CrossRef]
- Pinto, J.V.; Moulin, T.C.; Amaral, O.B. On the transdiagnostic nature of peripheral biomarkers in major psychiatric disorders: A systematic review. Neurosci. Biobehav. Rev. 2017, 83, 97–108. [Google Scholar] [CrossRef]
- Teixeira, A.L.; Colpo, G.D.; Fries, G.R.; Bauer, I.E.; Selvaraj, S. Biomarkers for bipolar disorder: Current status and challenges ahead. Expert. Rev. Neurother. 2019, 19, 67–81. [Google Scholar] [CrossRef]
- Fernandes, B.S.; Dai, Y.; Jia, P.; Zhao, Z. Charting the proteome landscape in major psychiatric disorders: From biomarkers to biological pathways towards drug discovery. Eur. Neuropsychopharmacol. 2022, 61, 43–59. [Google Scholar] [CrossRef]
- Gandal, M.J.; Zhang, P.; Hadjimichael, E.; Walker, R.L.; Chen, C.; Liu, S.; Won, H.; van Bakel, H.; Varghese, M.; Wang, Y.; et al. Transcriptome-wide isoform-level dysregulation in ASD, schizophrenia, and bipolar disorder. Science 2018, 362, eaat8127. [Google Scholar] [CrossRef]
- Mahmoudi, E.; Green, M.J.; Cairns, M.J. Dysregulation of circRNA expression in the peripheral blood of individuals with schizophrenia and bipolar disorder. J. Mol. Med. 2021, 99, 981–991. [Google Scholar] [CrossRef]
- Tasic, L.; Larcerda, A.L.T.; Pontes, J.G.M.; da Costa, T.; Nani, J.V.; Martins, L.G.; Santos, L.A.; Nunes, M.F.Q.; Adelino, M.P.M.; Pedrini, M.; et al. Peripheral biomarkers allow differential diagnosis between schizophrenia and bipolar disorder. J. Psychiatr. Res. 2019, 119, 67–75. [Google Scholar] [CrossRef]
- Bharadwaj, R.; Nath, P.; Phukan, J.K.; Deb, K.; Gogoi, V.; Bhattacharyya, D.K.; Barah, P. Integrative ceRNA network analysis identifies unique and shared molecular signatures in Bipolar Disorder and Schizophrenia. J. Psychiatr. Res. 2024, 176, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Matsumoto, M.; Iijima, K.; Sumiyoshi, T. Specificity and Continuity of Schizophrenia and Bipolar Disorder: Relation to Biomarkers. Curr. Pharm. Des. 2020, 26, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Khatun, M.T.; Rana, H.K.; Hossain, M.A.; Lakshmanna, K.; Rahman, M.M.; Parvin, A.; Rahman, M.H. Bioinformatics and systems biology approaches to identify molecular targets and pathways shared between schizophrenia and bipolar disorder. Inform. Med. Unlocked 2024, 49, 101556. [Google Scholar] [CrossRef]
- Aryal, S.; Bonanno, K.; Song, B.; Mani, D.R.; Keshishian, H.; Carr, S.A.; Sheng, M.; Dejanovic, B. Deep proteomics identifies shared molecular pathway alterations in synapses of patients with schizophrenia and bipolar disorder and mouse model. Cell Rep. 2023, 42, 112497. [Google Scholar] [CrossRef]
- Smeland, O.B.; Bahrami, S.; Frei, O.; Shadrin, A.; O’Connell, K.; Savage, J.; Watanabe, K.; Krull, F.; Bettella, F.; Steen, N.E.; et al. Genome-wide analysis reveals extensive genetic overlap between schizophrenia, bipolar disorder, and intelligence. Mol. Psychiatry 2020, 25, 844–853. [Google Scholar] [CrossRef]
- Karthik, S.; Sudha, M. Predicting bipolar disorder and schizophrenia based on non-overlapping genetic phenotypes using deep neural network. Evol. Intell. 2021, 14, 619–634. [Google Scholar] [CrossRef]
- Ruderfer, D.M.; Ripke, S.; McQuillin, A.; Boocock, J.; Stahl, E.A.; Pavlides, J.M.W.; Mullins, N.; Charney, A.W.; Ori, A.P.; Loohuis, L.M.O. Genomic dissection of bipolar disorder and schizophrenia, including 28 subphenotypes. Cell 2018, 173, 1705–1715.e16. [Google Scholar] [CrossRef]
- Jang, Y.; Park, S.; Won, H.-H.; Myung, W. Identifying the Unique Genetic Architecture of Schizophrenia Distinguished from Bipolar Disorder Using Genomic Structural Equation Modeling. Int. J. Neuropsychopharmacol. 2025, 28, i362. [Google Scholar] [CrossRef]
- Namkung, H.; Yukitake, H.; Fukudome, D.; Lee, B.J.; Tian, M.; Ursini, G.; Saito, A.; Lam, S.; Kannan, S.; Srivastava, R. The miR-124-AMPAR pathway connects polygenic risks with behavioral changes shared between schizophrenia and bipolar disorder. Neuron 2023, 111, 220–235.e229. [Google Scholar] [CrossRef] [PubMed]
- Martos, D.; Lőrinczi, B.; Szatmári, I.; Vécsei, L.; Tanaka, M. Decoupling Behavioral Domains via Kynurenic Acid Analog Optimization: Implications for Schizophrenia and Parkinson’s Disease Therapeutics. Cells 2025, 14, 973. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Szatmári, I.; Vécsei, L. Quinoline Quest: Kynurenic Acid Strategies for Next-Generation Therapeutics via Rational Drug Design. Pharmaceuticals 2025, 18, 607. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Li, Y.; Zhou, Y.; Zhao, J.; Lu, Y.; Xu, Z. AdipoRon attenuates depression-like behavior in T2DM mice via inhibiting inflammation and regulating autophagy. Brain Res. Bull. 2025, 224, 111308. [Google Scholar] [CrossRef]
- Brien, M.E.; Gaudreault, V.; Hughes, K.; Hayes, D.J.L.; Heazell, A.E.P.; Girard, S. A Systematic Review of the Safety of Blocking the IL-1 System in Human Pregnancy. J. Clin. Med. 2021, 11, 225. [Google Scholar] [CrossRef]
- Buckley, L.F.; Abbate, A. Interleukin-1 blockade in cardiovascular diseases: A clinical update. Eur. Heart J. 2018, 39, 2063–2069. [Google Scholar] [CrossRef] [PubMed]
- Gergues, M.M.; Lalani, L.K.; Kheirbek, M.A. Identifying dysfunctional cell types and circuits in animal models for psychiatric disorders with calcium imaging. Neuropsychopharmacology 2024, 50, 274–284. [Google Scholar] [CrossRef]
- Whiteley, J.T.; Fernandes, S.; Sharma, A.; Mendes, A.P.D.; Racha, V.; Benassi, S.K.; Marchetto, M.C. Reaching into the toolbox: Stem cell models to study neuropsychiatric disorders. Stem Cell Rep. 2022, 17, 187–210. [Google Scholar] [CrossRef]
- Price, R.B.; Duman, R. Neuroplasticity in cognitive and psychological mechanisms of depression: An integrative model. Mol. Psychiatry 2020, 25, 530–543. [Google Scholar] [CrossRef]
- Bortolato, B.; Hyphantis, T.N.; Valpione, S.; Perini, G.; Maes, M.; Morris, G.; Kubera, M.; Köhler, C.A.; Fernandes, B.S.; Stubbs, B.; et al. Depression in cancer: The many biobehavioral pathways driving tumor progression. Cancer Treat. Rev. 2017, 52, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M. From Serendipity to Precision: Integrating AI, Multi-Omics, and Human-Specific Models for Personalized Neuropsychiatric Care. Biomedicines 2025, 13, 167. [Google Scholar] [CrossRef]
- Rees, E.; Owen, M.J. Translating insights from neuropsychiatric genetics and genomics for precision psychiatry. Genome Med. 2020, 12, 43. [Google Scholar] [CrossRef] [PubMed]
- Arns, M.; van Dijk, H.; Luykx, J.J.; van Wingen, G.; Olbrich, S. Stratified psychiatry: Tomorrow’s precision psychiatry? Eur. Neuropsychopharmacol. 2022, 55, 14–19. [Google Scholar] [CrossRef]
- Kas, M.J.H.; Hyman, S.; Williams, L.M.; Hidalgo-Mazzei, D.; Huys, Q.J.M.; Hotopf, M.; Cuthbert, B.; Lewis, C.M.; De Picker, L.J.; Lalousis, P.A.; et al. Towards a consensus roadmap for a new diagnostic framework for mental disorders. Eur. Neuropsychopharmacol. 2025, 90, 16–27. [Google Scholar] [CrossRef]
- Dhieb, D.; Bastaki, K. Pharmaco-Multiomics: A New Frontier in Precision Psychiatry. Int. J. Mol. Sci. 2025, 26, 1082. [Google Scholar] [CrossRef]
- Foley, É.M.; Slaney, C.; Donnelly, N.A.; Kaser, M.; Ziegler, L.; Khandaker, G.M. A novel biomarker of interleukin 6 activity and clinical and cognitive outcomes in depression. Psychoneuroendocrinology 2024, 164, 107008. [Google Scholar] [CrossRef]
- Solmi, M.; Suresh Sharma, M.; Osimo, E.F.; Fornaro, M.; Bortolato, B.; Croatto, G.; Miola, A.; Vieta, E.; Pariante, C.M.; Smith, L.; et al. Peripheral levels of C-reactive protein, tumor necrosis factor-α, interleukin-6, and interleukin-1β across the mood spectrum in bipolar disorder: A meta-analysis of mean differences and variability. Brain Behav. Immun. 2021, 97, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Mario, A.; Ivana, L.; Anita, M.; Silvio, M.; Claudia, A.; Mariaclaudia, M.; Antonello, B. Inflammatory Biomarkers, Cognitive Functioning, and Brain Imaging Abnormalities in Bipolar Disorder: A Systematic Review. Clin. Neuropsychiatry 2024, 21, 32–62. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Sastre, P.; Gómez-Sánchez-Lafuente, C.; Martín-Martín, J.; Herrera-Imbroda, J.; Mayoral-Cleries, F.; Santos-Amaya, I.; Rodríguez de Fonseca, F.; Guzmán-Parra, J.; Rivera, P.; Suárez, J. Pharmacotherapeutic value of inflammatory and neurotrophic biomarkers in bipolar disorder: A systematic review. Prog. Neuropsychopharmacol. Biol. Psychiatry 2024, 134, 111056. [Google Scholar] [CrossRef] [PubMed]
- Rosenblat, J.D.; Brietzke, E.; Mansur, R.B.; Maruschak, N.A.; Lee, Y.; McIntyre, R.S. Inflammation as a neurobiological substrate of cognitive impairment in bipolar disorder: Evidence, pathophysiology and treatment implications. J. Affect. Disord. 2015, 188, 149–159. [Google Scholar] [CrossRef]
- Bavaresco, D.V.; da Rosa, M.I.; Uggioni, M.L.R.; Ferraz, S.D.; Pacheco, T.R.; Toé, H.; da Silveira, A.P.; Quadros, L.F.A.; de Souza, T.D.; Varela, R.B.; et al. Increased inflammatory biomarkers and changes in biological rhythms in bipolar disorder: A case-control study. J. Affect. Disord. 2020, 271, 115–122. [Google Scholar] [CrossRef]
- Tanaka, M.; He, Z.; Han, S.; Battaglia, S. Editorial: Noninvasive brain stimulation: A promising approach to study and improve emotion regulation. Front. Behav. Neurosci. 2025, 19, 1633936. [Google Scholar] [CrossRef]
- Figueiredo Godoy, A.C.; Frota, F.F.; Araújo, L.P.; Valenti, V.E.; Pereira, E.; Detregiachi, C.R.P.; Galhardi, C.M.; Caracio, F.C.; Haber, R.S.A.; Fornari Laurindo, L.; et al. Neuroinflammation and Natural Antidepressants: Balancing Fire with Flora. Biomedicines 2025, 13, 1129. [Google Scholar] [CrossRef]
- Barbalho, S.M.; Leme Boaro, B.; da Silva Camarinha Oliveira, J.; Patočka, J.; Barbalho Lamas, C.; Tanaka, M.; Laurindo, L.F. Molecular Mechanisms Underlying Neuroinflammation Intervention with Medicinal Plants: A Critical and Narrative Review of the Current Literature. Pharmaceuticals 2025, 18, 133. [Google Scholar] [CrossRef]
- Lv, S.; Luo, C. Blood-brain barrier dysfunction in schizophrenia: Mechanisms and implications (Review). Int. J. Mol. Med. 2025, 56, 153. [Google Scholar] [CrossRef]
- Najjar, S.; Pahlajani, S.; De Sanctis, V.; Stern, J.N.H.; Najjar, A.; Chong, D. Neurovascular Unit Dysfunction and Blood-Brain Barrier Hyperpermeability Contribute to Schizophrenia Neurobiology: A Theoretical Integration of Clinical and Experimental Evidence. Front. Psychiatry 2017, 8, 83. [Google Scholar] [CrossRef] [PubMed]
- Pong, S.; Karmacharya, R.; Sofman, M.; Bishop, J.R.; Lizano, P. The Role of Brain Microvascular Endothelial Cell and Blood-Brain Barrier Dysfunction in Schizophrenia. Complex Psychiatry 2020, 6, 30–46. [Google Scholar] [CrossRef] [PubMed]
- Arango, C.; Fagiolini, A.; Gorwood, P.; Kane, J.M.; Diaz-Mendoza, S.; Sahota, N.; Correll, C.U. Delphi panel to obtain clinical consensus about using long-acting injectable antipsychotics to treat first-episode and early-phase schizophrenia: Treatment goals and approaches to functional recovery. BMC Psychiatry 2023, 23, 453. [Google Scholar] [CrossRef] [PubMed]
- Verebi, C.; Nectoux, J.; Gorwood, P.; Le Strat, Y.; Duriez, P.; Ramoz, N.; Bienvenu, T. A systematic literature review and meta-analysis of circulating nucleic acids as biomarkers in psychiatry. Prog. Neuropsychopharmacol. Biol. Psychiatry 2023, 125, 110770. [Google Scholar] [CrossRef]
- Salvetat, N.; Checa-Robles, F.J.; Patel, V.; Cayzac, C.; Dubuc, B.; Chimienti, F.; Abraham, J.D.; Dupré, P.; Vetter, D.; Méreuze, S.; et al. A game changer for bipolar disorder diagnosis using RNA editing-based biomarkers. Transl. Psychiatry 2022, 12, 182. [Google Scholar] [CrossRef]
- Hayashi, M.; Salvetat, N.; Cayzac, C.; Checa-Robles, F.; Lozano, C.; Dubuc, B.; Mereuze, S.; Nani, J.; Molina, F.; Brietske, E. The RNA editing patterns are different in blood of euthymic and depressed bipolar patients. Eur. Psychiatry 2023, 66, S573. [Google Scholar] [CrossRef]
- Sathyanarayanan, A.; Mueller, T.T.; Ali Moni, M.; Schueler, K.; Baune, B.T.; Lio, P.; Mehta, D.; Baune, B.T.; Dierssen, M.; Ebert, B.; et al. Multi-omics data integration methods and their applications in psychiatric disorders. Eur. Neuropsychopharmacol. 2023, 69, 26–46. [Google Scholar] [CrossRef]
- Smith, B.J.; Silva-Costa, L.C.; Martins-de-Souza, D. Human disease biomarker panels through systems biology. Biophys. Rev. 2021, 13, 1179–1190. [Google Scholar] [CrossRef]
- Mokhtari, A.; Porte, B.; Belzeaux, R.; Etain, B.; Ibrahim, E.C.; Marie-Claire, C.; Lutz, P.E.; Delahaye-Duriez, A. The molecular pathophysiology of mood disorders: From the analysis of single molecular layers to multi-omic integration. Prog. Neuropsychopharmacol. Biol. Psychiatry 2022, 116, 110520. [Google Scholar] [CrossRef]
- Liu, B.; Liu, J.; Wang, J.G.; Liu, C.L.; Yan, H.J. AdipoRon improves cognitive dysfunction of Alzheimer’s disease and rescues impaired neural stem cell proliferation through AdipoR1/AMPK pathway. Exp. Neurol. 2020, 327, 113249. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chang, Y.; Zhu, J.; Wu, Y.; Jiang, X.; Zheng, S.; Li, G.; Ma, R. AdipoRon mitigates tau pathology and restores mitochondrial dynamics via AMPK-related pathway in a mouse model of Alzheimer’s disease. Exp. Neurol. 2023, 363, 114355. [Google Scholar] [CrossRef] [PubMed]
- Youngstein, T.; Hoffmann, P.; Gül, A.; Lane, T.; Williams, R.; Rowczenio, D.M.; Ozdogan, H.; Ugurlu, S.; Ryan, J.; Harty, L.; et al. International multi-centre study of pregnancy outcomes with interleukin-1 inhibitors. Rheumatology 2017, 56, 2102–2108. [Google Scholar] [CrossRef] [PubMed]
- Carnovale, C.; Tombetti, E.; Battini, V.; Mazhar, F.; Radice, S.; Nivuori, M.; Negro, E.; Tamanini, S.; Brucato, A. Inflammasome Targeted Therapy in Pregnancy: New Insights from an Analysis of Real-World Data from the FAERS Database and a Systematic Review. Front. Pharmacol. 2020, 11, 612259. [Google Scholar] [CrossRef]
- Chen, Z.S.; Kulkarni, P.P.; Galatzer-Levy, I.R.; Bigio, B.; Nasca, C.; Zhang, Y. Modern views of machine learning for precision psychiatry. Patterns 2022, 3, 100602. [Google Scholar] [CrossRef]
- Lin, E.; Lin, C.H.; Lane, H.Y. Precision Psychiatry Applications with Pharmacogenomics: Artificial Intelligence and Machine Learning Approaches. Int. J. Mol. Sci. 2020, 21, 969. [Google Scholar] [CrossRef]
- Wright, S.N.; Anticevic, A. Generative AI for precision neuroimaging biomarker development in psychiatry. Psychiatry Res. 2024, 339, 115955. [Google Scholar] [CrossRef]
- Tanaka, M.; Battaglia, S. Dualistic Dynamics in Neuropsychiatry: From Monoaminergic Modulators to Multiscale Biomarker Maps. Biomedicines 2025, 13, 1456. [Google Scholar] [CrossRef]
- Johnson, K.B.; Wei, W.Q.; Weeraratne, D.; Frisse, M.E.; Misulis, K.; Rhee, K.; Zhao, J.; Snowdon, J.L. Precision Medicine, AI, and the Future of Personalized Health Care. Clin. Transl. Sci. 2021, 14, 86–93. [Google Scholar] [CrossRef]
- Tossici, G.; Zurloni, V.; Nitri, A. Stress and sport performance: A PNEI multidisciplinary approach. Front. Psychol. 2024, 15, 1358771. [Google Scholar] [CrossRef]
- Milani, A.; Saiani, L.; Misurelli, E.; Lacapra, S.; Pravettoni, G.; Magon, G.; Mazzocco, K. The relevance of the contribution of psychoneuroendocrinoimmunology and psychology of reasoning and decision making to nursing science: A discursive paper. J. Adv. Nurs. 2024, 80, 2943–2957. [Google Scholar] [CrossRef]
- Gökyayla, E.; Türel Ermertcan, A.; Bilaç, C. Functional medicine with dermatology insite: A systematic approach for diagnosis and treatment of chronic diseases. Dermatol. Ther. 2020, 33, e13729. [Google Scholar] [CrossRef]
- Ee, C.; Lake, J.; Firth, J.; Hargraves, F.; de Manincor, M.; Meade, T.; Marx, W.; Sarris, J. An integrative collaborative care model for people with mental illness and physical comorbidities. Int. J. Ment. Health Syst. 2020, 14, 83. [Google Scholar] [CrossRef] [PubMed]
- Warren, A. An integrative approach to dementia care. Front. Aging 2023, 4, 1143408. [Google Scholar] [CrossRef]
- Parsons, M.W.; Dietrich, J. Assessment and Management of Cognitive Symptoms in Patients With Brain Tumors. Am. Soc. Clin. Oncol. Educ. Book 2021, 41, e90–e99. [Google Scholar] [CrossRef] [PubMed]
- Schipmann, S.; Suero Molina, E.; Frasch, A.; Stummer, W.; Wiewrodt, D. Initial psycho-oncological counselling in neuro-oncology: Analysis of topics and needs of brain tumour patients. J. Neuro-Oncol. 2018, 136, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Loughan, A.R.; Reid, M.; Willis, K.D.; Davies, A.; Boutté, R.L.; Barrett, S.; Lo, K. The burden of a brain tumor: Guiding patient centric care in neuro-oncology. J. Neuro-Oncol. 2022, 157, 487–498. [Google Scholar] [CrossRef]
- Agiananda, F.; Aninditha, T.; Sofyan, H.R.; Savitri, I.; Karnasih, A.; Puspaseruni, P.A.; Putri, C.K. AB068. Psychiatric disorder in central nervous system tumor patients and its related factors. Chin. Clin. Oncol. 2024, 13, AB068. [Google Scholar] [CrossRef]
- van Kessel, E.; Baumfalk, A.E.; van Zandvoort, M.J.E.; Robe, P.A.; Snijders, T.J. Tumor-related neurocognitive dysfunction in patients with diffuse glioma: A systematic review of neurocognitive functioning prior to anti-tumor treatment. J. Neuro-Oncol. 2017, 134, 9–18. [Google Scholar] [CrossRef]
- Fox, A.; Zarrella, G.V.; Loughan, A.R.; Lanoye, A.; Palesh, O.; Moeller, F.G.; Braun, S.E. QOL-28. Cancer-Related Distress in Neuro-Oncology: Patients Inform Program Development for Enhancing Quality of Life. Neuro-Oncology 2024, 26, viii268–viii269. [Google Scholar] [CrossRef]
- Ali, F.S.; Hussain, M.R.; Gutiérrez, C.; Demireva, P.; Ballester, L.Y.; Zhu, J.J.; Blanco, A.; Esquenazi, Y. Cognitive disability in adult patients with brain tumors. Cancer Treat. Rev. 2018, 65, 33–40. [Google Scholar] [CrossRef]
- Han, V.X.; Patel, S.; Jones, H.F.; Dale, R.C. Maternal immune activation and neuroinflammation in human neurodevelopmental disorders. Nat. Rev. Neurol. 2021, 17, 564–579. [Google Scholar] [CrossRef]
- Gumusoglu, S.B.; Stevens, H.E. Maternal Inflammation and Neurodevelopmental Programming: A Review of Preclinical Outcomes and Implications for Translational Psychiatry. Biol. Psychiatry 2019, 85, 107–121. [Google Scholar] [CrossRef]
- Kim, E.; Huh, J.R.; Choi, G.B. Prenatal and postnatal neuroimmune interactions in neurodevelopmental disorders. Nat. Immunol. 2024, 25, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Corradini, I.; Focchi, E.; Rasile, M.; Morini, R.; Desiato, G.; Tomasoni, R.; Lizier, M.; Ghirardini, E.; Fesce, R.; Morone, D.; et al. Maternal Immune Activation Delays Excitatory-to-Inhibitory Gamma-Aminobutyric Acid Switch in Offspring. Biol. Psychiatry 2018, 83, 680–691. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.B.; Tran, N.T.; Polglase, G.R.; Hunt, R.W.; Nold, M.F.; Nold-Petry, C.A.; Olson, D.M.; Chemtob, S.; Lodygensky, G.A.; Robertson, S.A.; et al. A systematic review of immune-based interventions for perinatal neuroprotection: Closing the gap between animal studies and human trials. J. Neuroinflamm. 2023, 20, 241. [Google Scholar] [CrossRef]
- Tarhini, S.; Crespo-Quiles, C.; Buhler, E.; Pineau, L.; Pallesi-Pocachard, E.; Villain, S.; Saha, S.; Silvagnoli, L.; Stamminger, T.; Luche, H.; et al. Cytomegalovirus infection of the fetal brain: Intake of aspirin during pregnancy blunts neurodevelopmental pathogenesis in the offspring. J. Neuroinflamm. 2024, 21, 298. [Google Scholar] [CrossRef]
- Bauman, M.D.; Van de Water, J. Translational opportunities in the prenatal immune environment: Promises and limitations of the maternal immune activation model. Neurobiol. Dis. 2020, 141, 104864. [Google Scholar] [CrossRef]
- Careaga, M.; Murai, T.; Bauman, M.D. Maternal Immune Activation and Autism Spectrum Disorder: From Rodents to Nonhuman and Human Primates. Biol. Psychiatry 2017, 81, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Jeremic, D.; Jiménez-Díaz, L.; Navarro-López, J.D. Past, present and future of therapeutic strategies against amyloid-β peptides in Alzheimer’s disease: A systematic review. Ageing Res. Rev. 2021, 72, 101496. [Google Scholar] [CrossRef]
- Ramesh, M.; Govindaraju, T. Multipronged diagnostic and therapeutic strategies for Alzheimer’s disease. Chem. Sci. 2022, 13, 13657–13689. [Google Scholar] [CrossRef] [PubMed]
- Mattsson-Carlgren, N.; Salvadó, G.; Ashton, N.J.; Tideman, P.; Stomrud, E.; Zetterberg, H.; Ossenkoppele, R.; Betthauser, T.J.; Cody, K.A.; Jonaitis, E.M.; et al. Prediction of Longitudinal Cognitive Decline in Preclinical Alzheimer Disease Using Plasma Biomarkers. JAMA Neurol. 2023, 80, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Hu, Y.; Cummings, J.; Mattke, S.; Iwatsubo, T.; Nakamura, A.; Vellas, B.; O’Bryant, S.; Shaw, L.M.; Cho, M.; et al. Blood-based biomarkers for Alzheimer’s disease: Current state and future use in a transformed global healthcare landscape. Neuron 2023, 111, 2781–2799. [Google Scholar] [CrossRef]
- Hampel, H.; Au, R.; Mattke, S.; van der Flier, W.M.; Aisen, P.; Apostolova, L.; Chen, C.; Cho, M.; De Santi, S.; Gao, P.; et al. Designing the next-generation clinical care pathway for Alzheimer’s disease. Nat. Aging 2022, 2, 692–703. [Google Scholar] [CrossRef] [PubMed]
| Category | Key Points (≤3) | Ref. |
|---|---|---|
| Immune-Related Insights in Mood Disorders |
| [28] |
| [29] | |
| SCZ: Treatment Optimization and BBB Dysfunction |
| [30] |
| [31] | |
| Precision Biomarkers and AI in Psychiatric Diagnosis |
| [32] |
| [33] | |
| Novel Therapies in Alzheimer’s and Developmental Disorders |
| [34] |
| [35] |
| Category | Key Gaps | Advances from this Special Issue | Future Directions (Near-Term 2–4 yrs/Long-Term 5–10 yrs) |
|---|---|---|---|
| Mood Disorders |
|
|
|
| SCZ |
|
|
|
| Precision Biomarkers & AI |
|
|
|
| Neurodegeneration & Developmental disorders |
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka, M. Special Issue “Translating Molecular Psychiatry: From Biomarkers to Personalized Therapies”. Int. J. Mol. Sci. 2025, 26, 10238. https://doi.org/10.3390/ijms262010238
Tanaka M. Special Issue “Translating Molecular Psychiatry: From Biomarkers to Personalized Therapies”. International Journal of Molecular Sciences. 2025; 26(20):10238. https://doi.org/10.3390/ijms262010238
Chicago/Turabian StyleTanaka, Masaru. 2025. "Special Issue “Translating Molecular Psychiatry: From Biomarkers to Personalized Therapies”" International Journal of Molecular Sciences 26, no. 20: 10238. https://doi.org/10.3390/ijms262010238
APA StyleTanaka, M. (2025). Special Issue “Translating Molecular Psychiatry: From Biomarkers to Personalized Therapies”. International Journal of Molecular Sciences, 26(20), 10238. https://doi.org/10.3390/ijms262010238