Mannose Derivatives as Anti-Infective Agents
Abstract
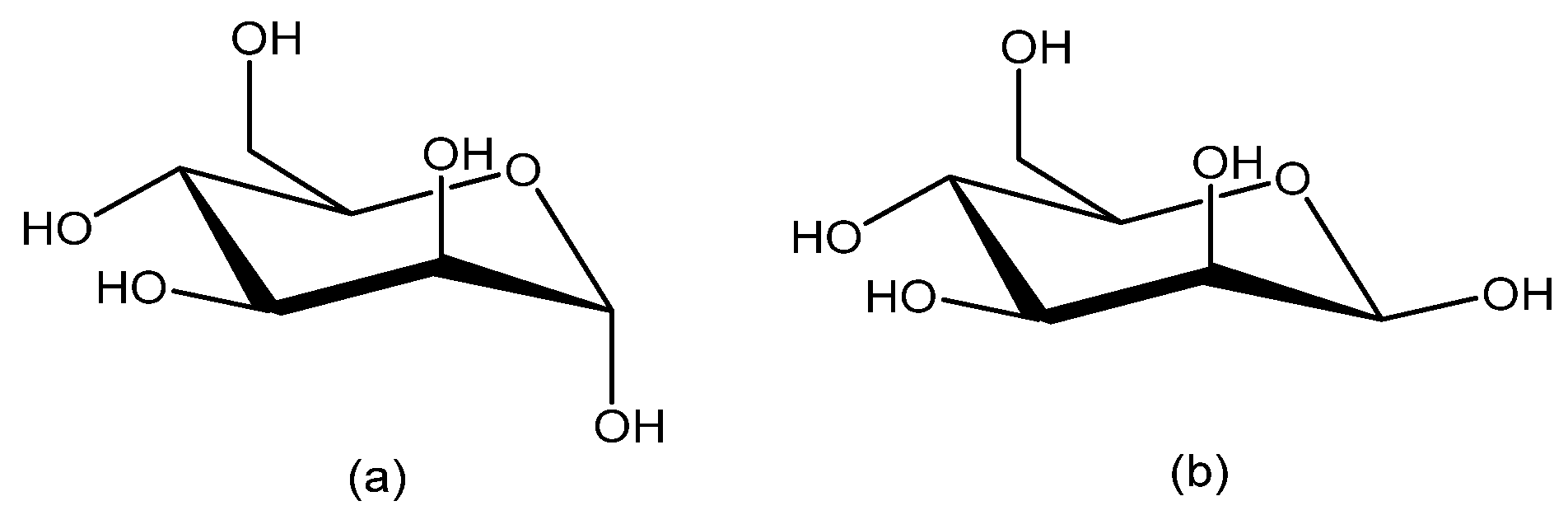
1. Introduction
2. Antibacterial Activities
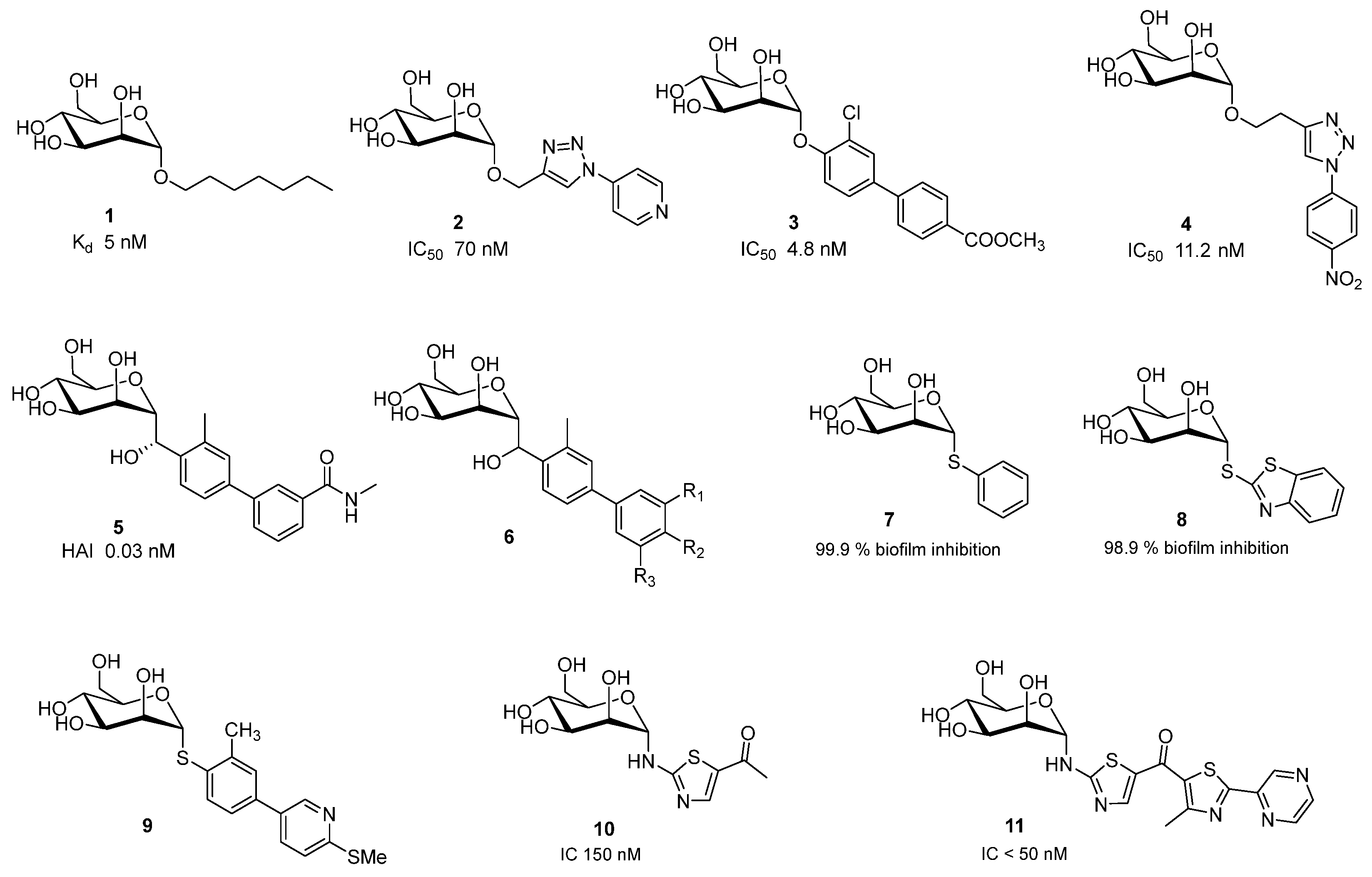
2.1. Escherichia coli
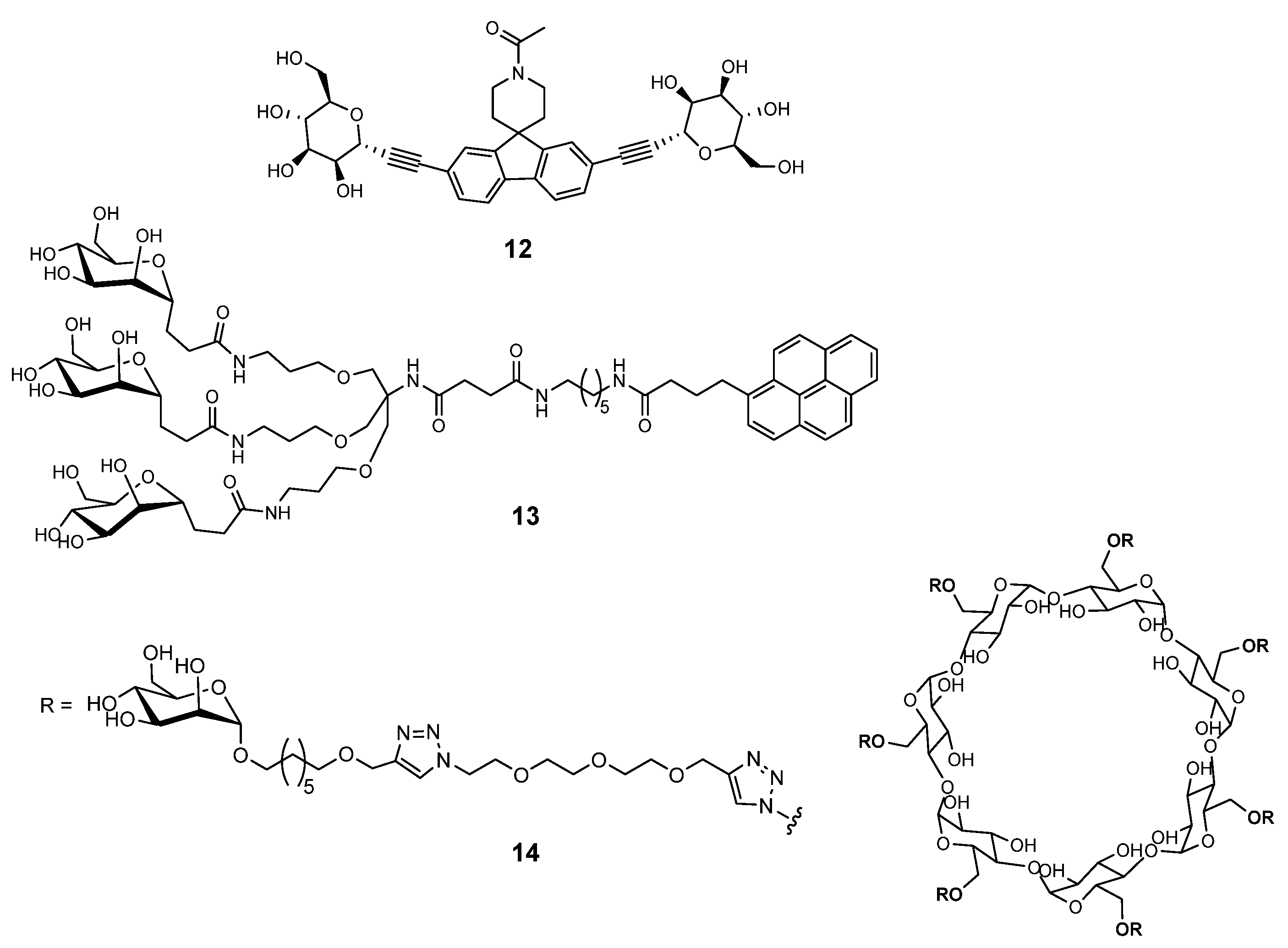
2.2. Pseudomonas aeruginosa and Burkholderia cepacia Complex
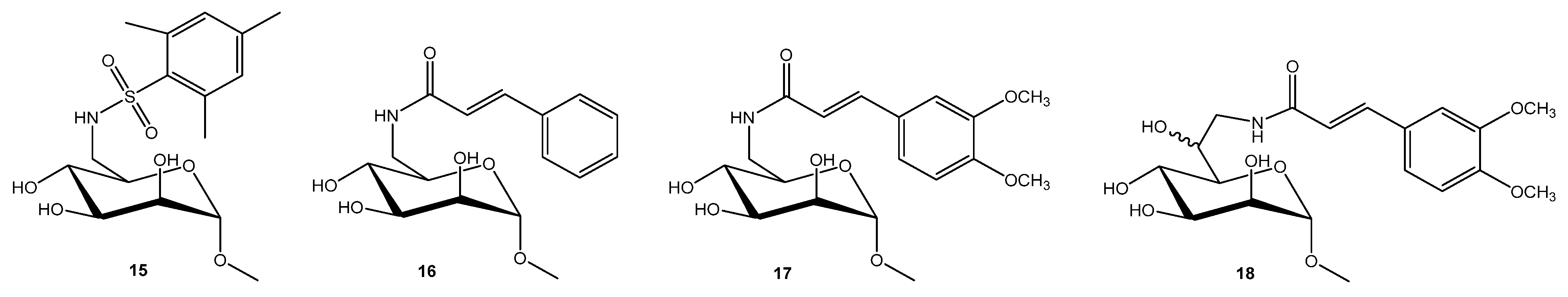
2.2.1. Pseudomonas aeruginosa
2.2.2. Burkholderia cepacia Complex
2.3. Mycobacterium tuberculosis
2.4. Methicillin-Resistant Staphylococcus aureus (MRSA)
2.5. Other Bacteria
2.6. Mannose-Containing Antibiotics
3. Antiviral Activities
3.1. Human Immunodeficiency Virus (HIV)
3.2. Ebola Virus
3.3. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)
3.4. Human Papillomavirus and Herpes Simplex
4. Antiparasitic Activities
5. Antifungal Activities
6. Conclusions
Funding
Conflicts of Interest
Abbreviations
| AZTMP | Azt 5′-Monophosphate |
| BH30 | Boltornh30 |
| BSA | Bovine Serum Albumin |
| CBA | Carbohydrate-Binding Agents |
| DC | Dendritic Cells |
| DC-SIGN | Dendritic Cell-Specific Intercellular Adhesion Molecule-3-Grabbing Non-Integrin |
| GPI | Glycosylphosphatidylinositol |
| HIV | Human Immunodeficiency Virus |
| HSA | Human Serum Albumin |
| IC50 | half-maximal inhibitory concentration |
| Man | Mannose |
| αMeMan | methyl α-D-mannopyranoside |
| MIC | minimum inhibitory concentration |
| Mtb | Mycobacterium Tuberculosis |
| PEG | Polyethylene Glycol |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Coronavirus 2 |
| SLN | Solid Lipid Nanoparticles |
| Tb | Tuberculosis |
| TT | Tetanus Toxoid |
| UPEC | Uropathogenic Escherichia Coli |
| UTI | Urinary Tract Infection |
References
- Tian, M.; Li, X.; Yu, L.; Qian, J.; Bai, X.; Yang, J.; Deng, R.; Lu, C.; Zhao, H.; Liu, Y. Glycosylation as an Intricate Post-Translational Modification Process Takes Part in Glycoproteins Related Immunity. Cell Commun. Signal. 2025, 23, 214. [Google Scholar] [CrossRef]
- Leusmann, S.; Ménová, P.; Shanin, E.; Titz, A.; Rademacher, C. Glycomimetics for the Inhibition and Modulation of Lectins. Chem. Soc. Rev. 2023, 52, 3663–3740. [Google Scholar] [CrossRef]
- Francesconi, O.; Roelens, S. Biomimetic Carbohydrate-Binding Agents (CBAs): Binding Affinities and Biological Activities. Chembiochem 2019, 20, 1329–1346. [Google Scholar] [CrossRef]
- Chen, S.; Wang, K.; Wang, Q. Mannose: A Promising Player in Clinical and Biomedical Applications. Curr. Drug Deliv. 2024, 21, 1435–1444. [Google Scholar] [CrossRef] [PubMed]
- Dhanalakshmi, M.; Sruthi, D.; Jinuraj, K.R.; Das, K.; Dave, S.; Andal, N.M.; Das, J. Mannose: A Potential Saccharide Candidate in Disease Management. Med. Chem. Res. 2023, 32, 391–408. [Google Scholar] [CrossRef] [PubMed]
- Imberty, A.; Chabre, Y.M.; Roy, R. Glycomimetics and Glycodendrimers as High Affinity Microbial Anti-Adhesins. Chem.—A Eur. J. 2008, 14, 7490–7499. [Google Scholar] [CrossRef]
- Hoyos, P.; Perona, A.; Juanes, O.; Rumbero, Á.; Hernáiz, M.J. Synthesis of Glycodendrimers with Antiviral and Antibacterial Activity. Chem.—A Eur. J. 2021, 27, 7593–7624. [Google Scholar] [CrossRef]
- Ribić, R.; Petrović Peroković, V.; Meštrović, T.; Neuberg, M.; Bradić, N. Cranberry-Derived Phenolic Compounds Contribute to the Inhibition of FimH-Mediated Escherichia coli Hemagglutination. Antibiotics 2025, 14, 418. [Google Scholar] [CrossRef]
- GBD 2021 Antimicrobial Resistance Collaborators. Global Burden of Bacterial Antimicrobial Resistance 1990–2021: A Systematic Analysis with Forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef]
- Sarshar, M.; Behzadi, P.; Ambrosi, C.; Zagaglia, C.; Palamara, A.T.; Scribano, D. FimH and Anti-Adhesive Therapeutics: A Disarming Strategy Against Uropathogens. Antibiotics 2020, 9, 397. [Google Scholar] [CrossRef]
- Ribić, R.; Meštrović, T.; Neuberg, M.; Kozina, G. Effective Anti-Adhesives of Uropathogenic Escherichia coli. Acta Pharm. 2018, 68, 1–18. [Google Scholar] [CrossRef]
- Lenger, S.M.; Bradley, M.S.; Thomas, D.A.; Bertolet, M.H.; Lowder, J.L.; Sutcliffe, S. D-Mannose vs Other Agents for Recurrent Urinary Tract Infection Prevention in Adult Women: A Systematic Review and Meta-Analysis. Am. J. Obs. Gynecol. 2020, 223, 265.e1–265.e13. [Google Scholar] [CrossRef]
- Cooper, T.E.; Teng, C.; Howell, M.; Teixeira-Pinto, A.; Jaure, A.; Wong, G. D-Mannose for Preventing and Treating Urinary Tract Infections. Cochrane Database Syst. Rev. 2022, 8, CD013608. [Google Scholar] [CrossRef]
- Bachmann, P.; Afanasyev, P.; Boehringer, D.; Glockshuber, R. Structures of the Escherichia coli Type 1 Pilus during Pilus Rod Assembly and after Assembly Termination. Nat. Commun. 2025, 16, 4988. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, S.; Fiege, B.; Eris, D.; Silbermann, M.; Jakob, R.P.; Navarra, G.; Maier, T.; Ernst, B. Conformational Switch of the Bacterial Adhesin FimH in the Absence of the Regulatory Domain: Engineering a Minimalistic Allosteric System. J. Biol. Chem. 2018, 293, 1835–1849. [Google Scholar] [CrossRef] [PubMed]
- Aprikian, P.; Tchesnokova, V.; Kidd, B.; Yakovenko, O.; Yarov-Yarovoy, V.; Trinchina, E.; Vogel, V.; Thomas, W.; Sokurenko, E. Interdomain Interaction in the FimH Adhesin of Escherichia coli Regulates the Affinity to Mannose. J. Biol. Chem. 2007, 282, 23437–23446. [Google Scholar] [CrossRef] [PubMed]
- de Monerri, N.C.S.; Che, Y.; Lees, J.A.; Jasti, J.; Wu, H.; Griffor, M.C.; Kodali, S.; Hawkins, J.C.; Lypowy, J.; Ponce, C.; et al. Structure-Based Design of an Immunogenic, Conformationally Stabilized FimH Antigen for a Urinary Tract Infection Vaccine. PLoS Pathog. 2025, 21, e1012325. [Google Scholar] [CrossRef]
- Bertuccini, L.; Costanzo, M.; Iosi, F.; Tinari, A.; Terruzzi, F.; Stronati, L.; Aloi, M.; Cucchiara, S.; Superti, F. Lactoferrin Prevents Invasion and Inflammatory Response Following E. coli Strain LF82 Infection in Experimental Model of Crohn’s Disease. Dig. Liver Dis. 2014, 46, 496–504. [Google Scholar] [CrossRef]
- Ofek, I.; Mirelman, D.; Sharon, N. Adherence of Escherichia coli to Human Mucosal Cells Mediated by Mannose Receptors. Nature 1977, 265, 623–625. [Google Scholar] [CrossRef]
- Mydock-McGrane, L.K.; Cusumano, Z.T.; Janetka, J.W. Mannose-Derived FimH Antagonists: A Promising Anti-Virulence Therapeutic Strategy for Urinary Tract Infections and Crohn’s Disease. Expert. Opin. Ther. Pat. 2016, 26, 175–197. [Google Scholar] [CrossRef]
- Mousavifar, L.; Roy, R. Recent Development in the Design of Small “drug-like” and Nanoscale Glycomimetics against Escherichia coli Infections. Drug Discov. Today 2021, 26, 2124–2137. [Google Scholar] [CrossRef] [PubMed]
- Bouckaert, J.; Berglund, J.; Schembri, M.; De Genst, E.; Cools, L.; Wuhrer, M.; Hung, C.-S.; Pinkner, J.; Slättegård, R.; Zavialov, A.; et al. Receptor Binding Studies Disclose a Novel Class of High-Affinity Inhibitors of the Escherichia coli FimH Adhesin. Mol. Microbiol. 2005, 55, 441–455. [Google Scholar] [CrossRef] [PubMed]
- Wellens, A.; Lahmann, M.; Touaibia, M.; Vaucher, J.; Oscarson, S.; Roy, R.; Remaut, H.; Bouckaert, J. The Tyrosine Gate as a Potential Entropic Lever in the Receptor-Binding Site of the Bacterial Adhesin FimH. Biochemistry 2012, 51, 4790–4799. [Google Scholar] [CrossRef] [PubMed]
- Gouin, S.G.; Roos, G.; Bouckaert, J. Discovery and Application of FimH Antagonists. In Carbohydrates as Drugs; Seeberger, P.H., Rademacher, C., Eds.; Springer International Publishing: Cham, Switzerland, 2014; pp. 123–168. ISBN 978-3-319-08675-0. [Google Scholar]
- Touaibia, M.; Krammer, E.-M.; Shiao, T.C.; Yamakawa, N.; Wang, Q.; Glinschert, A.; Papadopoulos, A.; Mousavifar, L.; Maes, E.; Oscarson, S.; et al. Sites for Dynamic Protein-Carbohydrate Interactions of O- and C-Linked Mannosides on the E. coli FimH Adhesin. Molecules 2017, 22, 1101. [Google Scholar] [CrossRef]
- Schwardt, O.; Rabbani, S.; Hartmann, M.; Abgottspon, D.; Wittwer, M.; Kleeb, S.; Zalewski, A.; Smieško, M.; Cutting, B.; Ernst, B. Design, Synthesis and Biological Evaluation of Mannosyl Triazoles as FimH Antagonists. Bioorg. Med. Chem. 2011, 19, 6454–6473. [Google Scholar] [CrossRef]
- Klein, T.; Abgottspon, D.; Wittwer, M.; Rabbani, S.; Herold, J.; Jiang, X.; Kleeb, S.; Lüthi, C.; Scharenberg, M.; Bezençon, J.; et al. FimH Antagonists for the Oral Treatment of Urinary Tract Infections: From Design and Synthesis to in Vitro and in Vivo Evaluation. J. Med. Chem. 2010, 53, 8627–8641. [Google Scholar] [CrossRef]
- Mydock-McGrane, L.; Cusumano, Z.; Han, Z.; Binkley, J.; Kostakioti, M.; Hannan, T.; Pinkner, J.S.; Klein, R.; Kalas, V.; Crowley, J.; et al. Antivirulence C-Mannosides as Antibiotic-Sparing, Oral Therapeutics for Urinary Tract Infections. J. Med. Chem. 2016, 59, 9390–9408. [Google Scholar] [CrossRef]
- Zhou, Z.; Tang, R.; Fang, Y.; Lv, T.; Liu, J.; Wang, X. Facile Synthesis of FimH Antagonist and Its Analogues: Simple Entry to Complex C-Mannoside Inhibitors of E. coli Adhesion . ACS Med. Chem. Lett. 2024, 15, 1724–1730. [Google Scholar] [CrossRef]
- Mohammed, A.F.; Othman, S.A.; Abou-Ghadir, O.F.; Kotb, A.A.; Mostafa, Y.A.; El-Mokhtar, M.A.; Abdu-Allah, H.H.M. Design, Synthesis, Biological Evaluation and Docking Study of Some New Aryl and Heteroaryl Thiomannosides as FimH Antagonists. Bioorg. Chem. 2024, 145, 107258. [Google Scholar] [CrossRef]
- Sattigeri, J.A.; Garg, M.; Bhateja, P.; Soni, A.; Rauf, A.R.A.; Gupta, M.; Deshmukh, M.S.; Jain, T.; Alekar, N.; Barman, T.K.; et al. Synthesis and Evaluation of Thiomannosides, Potent and Orally Active FimH Inhibitors. Bioorg. Med. Chem. Lett. 2018, 28, 2993–2997. [Google Scholar] [CrossRef]
- Mydock-McGrane, L.K.; Hannan, T.J.; Janetka, J.W. Rational Design Strategies for FimH Antagonists: New Drugs on the Horizon for Urinary Tract Infection and Crohn’s Disease. Expert. Opin. Drug Discov. 2017, 12, 711–731. [Google Scholar] [CrossRef]
- asif Enterome Highlights Microbiome Publication Describing Sibofimloc’s Novel Mechanism of Action for the Treatment of Crohn’s Disease. Enterome 2021. Available online: https://www.enterome.com/news-events/enterome-highlights-microbiome-publication-describing-sibofimlocs-novel-mechanism-of-action-for-the-treatment-of-crohns-disease/ (accessed on 7 September 2025).
- Bi, F.; Zhang, J.; Xie, R.; Yu, D.; Wei, H.; Wang, Y.; Hua, Z.; Qi, X.; Huang, B.; Yang, G. Adenosine Triphosphate-Responsive Glyconanorods through Self-Assembly of β-Cyclodextrin-Based Glycoconjugates for Targeted and Effective Bacterial Sensing and Killing. Biomacromolecules 2023, 24, 1003–1013. [Google Scholar] [CrossRef]
- Sehad, C.; Shiao, T.C.; Sallam, L.M.; Azzouz, A.; Roy, R. Effect of Dendrimer Generation and Aglyconic Linkers on the Binding Properties of Mannosylated Dendrimers Prepared by a Combined Convergent and Onion Peel Approach. Molecules 2018, 23, 1890. [Google Scholar] [CrossRef] [PubMed]
- Loris, R.; Tielker, D.; Jaeger, K.-E.; Wyns, L. Structural Basis of Carbohydrate Recognition by the Lectin LecB from Pseudomonas Aeruginosa. J. Mol. Biol. 2003, 331, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Sommer, R.; Exner, T.E.; Titz, A. A Biophysical Study with Carbohydrate Derivatives Explains the Molecular Basis of Monosaccharide Selectivity of the Pseudomonas Aeruginosa Lectin LecB. PLoS ONE 2014, 9, e112822. [Google Scholar] [CrossRef] [PubMed]
- Wojtczak, K.; Byrne, J.P. Structural Considerations for Building Synthetic Glycoconjugates as Inhibitors for Pseudomonas Aeruginosa Lectins. ChemMedChem 2022, 17, e202200081. [Google Scholar] [CrossRef]
- Hauck, D.; Joachim, I.; Frommeyer, B.; Varrot, A.; Philipp, B.; Möller, H.M.; Imberty, A.; Exner, T.E.; Titz, A. Discovery of Two Classes of Potent Glycomimetic Inhibitors of Pseudomonas Aeruginosa LecB with Distinct Binding Modes. ACS Chem. Biol. 2013, 8, 1775–1784. [Google Scholar] [CrossRef]
- Sommer, R.; Hauck, D.; Varrot, A.; Wagner, S.; Audfray, A.; Prestel, A.; Möller, H.M.; Imberty, A.; Titz, A. Cinnamide Derivatives of D-Mannose as Inhibitors of the Bacterial Virulence Factor LecB from Pseudomonas Aeruginosa. ChemistryOpen 2015, 4, 756–767. [Google Scholar] [CrossRef]
- Hofmann, A.; Sommer, R.; Hauck, D.; Stifel, J.; Göttker-Schnetmann, I.; Titz, A. Synthesis of Mannoheptose Derivatives and Their Evaluation as Inhibitors of the Lectin LecB from the Opportunistic Pathogen Pseudomonas Aeruginosa. Carbohydr. Res. 2015, 412, 34–42. [Google Scholar] [CrossRef]
- Gerland, B.; Goudot, A.; Pourceau, G.; Meyer, A.; Dugas, V.; Cecioni, S.; Vidal, S.; Souteyrand, E.; Vasseur, J.-J.; Chevolot, Y.; et al. Synthesis of a Library of Fucosylated Glycoclusters and Determination of Their Binding toward Pseudomonas Aeruginosa Lectin B (PA-IIL) Using a DNA-Based Carbohydrate Microarray. Bioconjug. Chem. 2012, 23, 1534–1547. [Google Scholar] [CrossRef]
- Dupin, L.; Noël, M.; Bonnet, S.; Meyer, A.; Géhin, T.; Bastide, L.; Randriantsoa, M.; Souteyrand, E.; Cottin, C.; Vergoten, G.; et al. Screening of a Library of Oligosaccharides Targeting Lectin LecB of Pseudomonas Aeruginosa and Synthesis of High Affinity Oligoglycoclusters. Molecules 2018, 23, 3073. [Google Scholar] [CrossRef]
- Limqueco, E.; Passos Da Silva, D.; Reichhardt, C.; Su, F.-Y.; Das, D.; Chen, J.; Srinivasan, S.; Convertine, A.; Skerrett, S.J.; Parsek, M.R.; et al. Mannose Conjugated Polymer Targeting P. Aeruginosa Biofilms. ACS Infect. Dis. 2020, 6, 2866–2871. [Google Scholar] [CrossRef]
- Taouai, M.; Chakroun, K.; Sommer, R.; Michaud, G.; Giacalone, D.; Ben Maaouia, M.A.; Vallin-Butruille, A.; Mathiron, D.; Abidi, R.; Darbre, T.; et al. Glycocluster Tetrahydroxamic Acids Exhibiting Unprecedented Inhibition of Pseudomonas Aeruginosa Biofilms. J. Med. Chem. 2019, 62, 7722–7738. [Google Scholar] [CrossRef] [PubMed]
- Lameignere, E.; Malinovská, L.; Sláviková, M.; Duchaud, E.; Mitchell, E.P.; Varrot, A.; Sedo, O.; Imberty, A.; Wimmerová, M. Structural Basis for Mannose Recognition by a Lectin from Opportunistic Bacteria Burkholderia Cenocepacia. Biochem. J. 2008, 411, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Lameignere, E.; Shiao, T.C.; Roy, R.; Wimmerova, M.; Dubreuil, F.; Varrot, A.; Imberty, A. Structural Basis of the Affinity for Oligomannosides and Analogs Displayed by BC2L-A, a Burkholderia Cenocepacia Soluble Lectin. Glycobiology 2010, 20, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, R.; Malinovska, L.; Lameignère, E.; Adamova, L.; de Castro, C.; Cioci, G.; Stanetty, C.; Kosma, P.; Molinaro, A.; Wimmerova, M.; et al. Burkholderia Cenocepacia Lectin A Binding to Heptoses from the Bacterial Lipopolysaccharide. Glycobiology 2012, 22, 1387–1398. [Google Scholar] [CrossRef]
- Csávás, M.; Malinovská, L.; Perret, F.; Gyurkó, M.; Illyés, Z.T.; Wimmerová, M.; Borbás, A. Tri- and Tetravalent Mannoclusters Cross-Link and Aggregate BC2L-A Lectin from Burkholderia Cenocepacia. Carbohydr. Res. 2017, 437, 1–8. [Google Scholar] [CrossRef]
- Geng, X.; Wang, G.; Guo, Z.; Gu, G. Synthesis of the Oligosaccharides of Burkholderia Pseudomallei and B. Mallei Capsular Polysaccharide and Preliminary Immunological Studies of Their Protein Conjugates. J. Org. Chem. 2020, 85, 2369–2384. [Google Scholar] [CrossRef]
- Rieger, J.; Stoffelbach, F.; Cui, D.; Imberty, A.; Lameignere, E.; Putaux, J.-L.; Jérôme, R.; Jérôme, C.; Auzély-Velty, R. Mannosylated Poly(Ethylene Oxide)-b-Poly(Epsilon-Caprolactone) Diblock Copolymers: Synthesis, Characterization, and Interaction with a Bacterial Lectin. Biomacromolecules 2007, 8, 2717–2725. [Google Scholar] [CrossRef]
- Gest, P.; Kaur, D.; Pham, H.T.; van der Woerd, M.; Hansen, E.; Brennan, P.J.; Jackson, M.; Guerin, M.E. Preliminary Crystallographic Analysis of GpgS, a Key Glucosyltransferase Involved in Methylglucose Lipopolysaccharide Biosynthesis in Mycobacterium Tuberculosis. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2008, 64, 1121–1124. [Google Scholar] [CrossRef]
- Urresti, S.; Albesa-Jové, D.; Schaeffer, F.; Pham, H.T.; Kaur, D.; Gest, P.; van der Woerd, M.J.; Carreras-González, A.; López-Fernández, S.; Alzari, P.M.; et al. Mechanistic Insights into the Retaining Glucosyl-3-Phosphoglycerate Synthase from Mycobacteria. J. Biol. Chem. 2012, 287, 24649–24661. [Google Scholar] [CrossRef]
- Pereira, P.J.B.; Empadinhas, N.; Albuquerque, L.; Sá-Moura, B.; da Costa, M.S.; Macedo-Ribeiro, S. Mycobacterium Tuberculosis Glucosyl-3-Phosphoglycerate Synthase: Structure of a Key Enzyme in Methylglucose Lipopolysaccharide Biosynthesis. PLoS ONE 2008, 3, e3748. [Google Scholar] [CrossRef] [PubMed]
- Gopalaswamy, R.; Dusthackeer, V.N.A.; Kannayan, S.; Subbian, S. Extrapulmonary Tuberculosis—An Update on the Diagnosis, Treatment and Drug Resistance. J. Respir. 2021, 1, 141–164. [Google Scholar] [CrossRef]
- Kumar, M.; Virmani, T.; Kumar, G.; Deshmukh, R.; Sharma, A.; Duarte, S.; Brandão, P.; Fonte, P. Nanocarriers in Tuberculosis Treatment: Challenges and Delivery Strategies. Pharmaceuticals 2023, 16, 1360. [Google Scholar] [CrossRef] [PubMed]
- Gairola, A.; Benjamin, A.; Weatherston, J.D.; Cirillo, J.D.; Wu, H.-J. Recent Developments in Drug Delivery for Treatment of Tuberculosis by Targeting Macrophages. Adv. Ther. 2022, 5, 2100193. [Google Scholar] [CrossRef]
- Prabhu, P.; Fernandes, T.; Damani, M.; Chaubey, P.; Narayanan, S.; Sawarkar, S. 2 Receptor Specific Ligand Conjugated Nanocarriers: An Effective Strategy for Targeted Therapy of Tuberculosis. Curr. Drug Deliv. 2022, 19, 830–845. [Google Scholar] [CrossRef]
- Prabhu, P.; Fernandes, T.; Chaubey, P.; Kaur, P.; Narayanan, S.; Vk, R.; Sawarkar, S.P. Mannose-Conjugated Chitosan Nanoparticles for Delivery of Rifampicin to Osteoarticular Tuberculosis. Drug Deliv. Transl. Res. 2021, 11, 1509–1519. [Google Scholar] [CrossRef]
- Mistry, N.; Bandyopadhyaya, R.; Mehra, S. Enhancement of Antimycobacterial Activity of Rifampicin Using Mannose-Anchored Lipid Nanoparticles against Intramacrophage Mycobacteria. ACS Appl. Bio Mater. 2022, 5, 5779–5789. [Google Scholar] [CrossRef]
- Turner, N.A.; Sharma-Kuinkel, B.K.; Maskarinec, S.A.; Eichenberger, E.M.; Shah, P.P.; Carugati, M.; Holland, T.L.; Fowler, V.G. Methicillin-Resistant Staphylococcus Aureus: An Overview of Basic and Clinical Research. Nat. Rev. Microbiol. 2019, 17, 203–218. [Google Scholar] [CrossRef]
- Lv, H.; Yang, M.; Yang, Y.; Tang, Z.; Guo, Y.; Zhou, J.; Gui, Y.; Huang, R.; Cai, J.; Yu, B.; et al. Metal Ion and Antibiotic Co-Loaded Nanoparticles for Combating Methicillin-Rresistant Staphylococcus Aureus-Induced Osteomyelitis. ACS Nano 2025, 19, 5253–5268. [Google Scholar] [CrossRef]
- Yang, X.; Fang, R.; Li, X.; Kong, W.; Jin, Y.; Jiao, R.; Liu, Z.; Zhang, M.; Peng, Q.; Zhang, Y.; et al. Engineered Nanovesicles for the Precise and Noninvasive Treatment of Deep Osteomyelitis Caused by MRSA Infection with Enhanced Immune Response. ACS Appl. Mater. Interfaces 2025, 17, 11795–11810. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Xie, B.; Peng, H.; Shi, G.; Sreenivas, B.; Guo, J.; Wang, C.; He, Y. Eradicating Intracellular MRSA via Targeted Delivery of Lysostaphin and Vancomycin with Mannose-Modified Exosomes. J. Control. Release 2021, 329, 454–467. [Google Scholar] [CrossRef] [PubMed]
- Baurhoo, B.; Ferket, P.; Ashwell, C.M.; de Oliviera, J.; Zhao, X. Cell Walls of Saccharomyces Cerevisiae Differentially Modulated Innate Immunity and Glucose Metabolism during Late Systemic Inflammation. PLoS ONE 2012, 7, e30323. [Google Scholar] [CrossRef]
- Piotrowski, M.; Wultańska, D.; Obuch-Woszczatyński, P.; Pituch, H. Fructooligosaccharides and Mannose Affect Clostridium Difficile Adhesion and Biofilm Formation in a Concentration-Dependent Manner. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1975–1984. [Google Scholar] [CrossRef]
- Arif, M.; Ahmad, R.; Sharaf, M.; Muhammad, J.; Abdalla, M.; Eltayb, W.A.; Liu, C.-G. Antibacterial and Antibiofilm Activity of Mannose-Modified Chitosan/PMLA Nanoparticles against Multidrug-Resistant Helicobacter pylori. Int. J. Biol. Macromol. 2022, 223, 418–432. [Google Scholar] [CrossRef]
- Shea, K.W.; Cunha, B.A. Teicoplanin. Med. Clin. N. Am. 1995, 79, 833–844. [Google Scholar] [CrossRef]
- Molina, K.C.; Miller, M.A.; Mueller, S.W.; Van Matre, E.T.; Krsak, M.; Kiser, T.H. Clinical Pharmacokinetics and Pharmacodynamics of Dalbavancin. Clin. Pharmacokinet. 2022, 61, 363–374. [Google Scholar] [CrossRef]
- Mattox, J.; Belliveau, P.; Durand, C. Oritavancin: A Novel Lipoglycopeptide. Consult. Pharm. 2016, 31, 86–95. [Google Scholar] [CrossRef]
- Debono, M.; Merkel, K.E.; Molloy, R.M.; Barnhart, M.; Presti, E.; Hunt, A.H.; Hamill, R.L. Actaplanin, New Glycopeptide Antibiotics Produced by Actinoplanes Missouriensis. The Isolation and Preliminary Chemical Characterization of Actaplanin. J. Antibiot. 1984, 37, 85–95. [Google Scholar] [CrossRef]
- O’Hare, M.D.; Ghosh, G.; Felmingham, D.; Grüneberg, R.N. In-Vitro Studies with Ramoplanin (MDL 62,198): A Novel Lipoglycopeptide Antimicrobial. J. Antimicrob. Chemother. 1990, 25, 217–220. [Google Scholar] [CrossRef]
- Singh, M.P.; Petersen, P.J.; Weiss, W.J.; Janso, J.E.; Luckman, S.W.; Lenoy, E.B.; Bradford, P.A.; Testa, R.T.; Greenstein, M. Mannopeptimycins, New Cyclic Glycopeptide Antibiotics Produced by Streptomyces Hygroscopicus LL-AC98: Antibacterial and Mechanistic Activities. Antimicrob. Agents Chemother. 2003, 47, 62–69. [Google Scholar] [CrossRef]
- Lozach, P.-Y.; Burleigh, L.; Staropoli, I.; Navarro-Sanchez, E.; Harriague, J.; Virelizier, J.-L.; Rey, F.A.; Desprès, P.; Arenzana-Seisdedos, F.; Amara, A. Dendritic Cell-Specific Intercellular Adhesion Molecule 3-Grabbing Non-Integrin (DC-SIGN)-Mediated Enhancement of Dengue Virus Infection Is Independent of DC-SIGN Internalization Signals. J. Biol. Chem. 2005, 280, 23698–23708. [Google Scholar] [CrossRef]
- Andreini, M.; Doknic, D.; Sutkeviciute, I.; Reina, J.J.; Duan, J.; Chabrol, E.; Thepaut, M.; Moroni, E.; Doro, F.; Belvisi, L.; et al. Second Generation of Fucose-Based DC-SIGN Ligands: Affinity Improvement and Specificity versus Langerin. Org. Biomol. Chem. 2011, 9, 5778–5786. [Google Scholar] [CrossRef] [PubMed]
- Švajger, U.; Anderluh, M.; Jeras, M.; Obermajer, N. C-Type Lectin DC-SIGN: An Adhesion, Signalling and Antigen-Uptake Molecule That Guides Dendritic Cells in Immunity. Cell. Signal. 2010, 22, 1397–1405. [Google Scholar] [CrossRef] [PubMed]
- Global HIV & AIDS Statistics—Fact Sheet | UNAIDS. Available online: https://www.unaids.org/en/resources/fact-sheet (accessed on 7 September 2025).
- Sever, B.; Otsuka, M.; Fujita, M.; Ciftci, H. A Review of FDA-Approved Anti-HIV-1 Drugs, Anti-Gag Compounds, and Potential Strategies for HIV-1 Eradication. Int. J. Mol. Sci. 2024, 25, 3659. [Google Scholar] [CrossRef]
- Wilen, C.B.; Tilton, J.C.; Doms, R.W. HIV: Cell Binding and Entry. Cold Spring Harb. Perspect. Med. 2012, 2, a006866. [Google Scholar] [CrossRef]
- Gamboa Marin, O.J.; Ng, K.; Verma, N.; Flavien Yapi, A.G.; Pantophlet, R.; Gauthier, C. Lewis-X-Containing Triterpenoid Saponins Inhibit DC-SIGN- and L-SIGN-Mediated Transfer of HIV-1 Infection. Chemistry 2025, 31, e202500993. [Google Scholar] [CrossRef]
- Sattin, S.; Daghetti, A.; Thépaut, M.; Berzi, A.; Sánchez-Navarro, M.; Tabarani, G.; Rojo, J.; Fieschi, F.; Clerici, M.; Bernardi, A. Inhibition of DC-SIGN-Mediated HIV Infection by a Linear Trimannoside Mimic in a Tetravalent Presentation. ACS Chem. Biol. 2010, 5, 301–312. [Google Scholar] [CrossRef]
- Berzi, A.; Reina, J.J.; Ottria, R.; Sutkeviciute, I.; Antonazzo, P.; Sanchez-Navarro, M.; Chabrol, E.; Biasin, M.; Trabattoni, D.; Cetin, I.; et al. A Glycomimetic Compound Inhibits DC-SIGN-Mediated HIV Infection in Cellular and Cervical Explant Models. AIDS 2012, 26, 127–137. [Google Scholar] [CrossRef]
- Wells, L.; Vierra, C.; Hardman, J.; Han, Y.; Dimas, D.; Gwarada-Phillips, L.N.; Blackeye, R.; Eggers, D.K.; LaBranche, C.C.; Král, P.; et al. Sulfoglycodendrimer Therapeutics for HIV-1 and SARS-CoV-2. Adv. Ther. 2021, 4, 2000210. [Google Scholar] [CrossRef]
- Kensinger, R.D.; Catalone, B.J.; Krebs, F.C.; Wigdahl, B.; Schengrund, C.-L. Novel Polysulfated Galactose-Derivatized Dendrimers as Binding Antagonists of Human Immunodeficiency Virus Type 1 Infection. Antimicrob. Agents Chemother. 2004, 48, 1614–1623. [Google Scholar] [CrossRef]
- Martínez, C.; Merchán, A.; Perona, A.; Ramírez-López, P.; Suárez, J.R.; Hernáiz, M.J. Design and Sustainable Synthesis of Small Mannose-Based Glycodendrons as Ligands for HIV-1 Envelope Protein Gp120: Toward an Explanation for Their Binding. Catal. Today 2024, 429, 114493. [Google Scholar] [CrossRef]
- Parris, G.E. 2-Deoxy-D-Glucose as a Potential Drug against Fusogenic Viruses Including HIV. Med. Hypotheses 2008, 70, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, E.; Dehuyser, L.; Sigwalt, D.; Flacher, V.; Bernacchi, S.; Chaloin, O.; Remy, J.-S.; Mueller, C.G.; Baati, R.; Wagner, A. Dynamic Micelles of Mannoside Glycolipids Are More Efficient than Polymers for Inhibiting HIV-1 Trans-Infection. Bioconjug. Chem. 2013, 24, 1813–1823. [Google Scholar] [CrossRef] [PubMed]
- Molema, G.; Jansen, R.W.; Pauwels, R.; de Clercq, E.; Meijer, D.K. Targeting of Antiviral Drugs to T4-Lymphocytes. Anti-HIV Activity of Neoglycoprotein-AZTMP Conjugates in Vitro. Biochem. Pharmacol. 1990, 40, 2603–2610. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.; Brigido, L.; Müller, W.E.; Hansen, J.E.; Ezekowitz, R.A.; Mills, J. Screening for Inhibitors of HIV Gp120-CD4 Binding Using an Enzyme-Linked Immunoabsorbent Assay. J. Virol. Methods 1993, 42, 1–12. [Google Scholar] [CrossRef]
- Hoorelbeke, B.; Xue, J.; LiWang, P.J.; Balzarini, J. Role of the Carbohydrate-Binding Sites of Griffithsin in the Prevention of DC-SIGN-Mediated Capture and Transmission of HIV-1. PLoS ONE 2013, 8, e64132. [Google Scholar] [CrossRef]
- Nabatov, A.A.; de Jong, M.A.W.P.; de Witte, L.; Bulgheresi, S.; Geijtenbeek, T.B.H. C-Type Lectin Mermaid Inhibits Dendritic Cell Mediated HIV-1 Transmission to CD4+ T Cells. Virology 2008, 378, 323–328. [Google Scholar] [CrossRef]
- Chiba, H.; Inokoshi, J.; Nakashima, H.; Omura, S.; Tanaka, H. Actinohivin, a Novel Anti-Human Immunodeficiency Virus Protein from an Actinomycete, Inhibits Viral Entry to Cells by Binding High-Mannose Type Sugar Chains of Gp120. Biochem. Biophys. Res. Commun. 2004, 316, 203–210. [Google Scholar] [CrossRef]
- Malvy, D.; McElroy, A.K.; de Clerck, H.; Günther, S.; van Griensven, J. Ebola Virus Disease. Lancet 2019, 393, 936–948. [Google Scholar] [CrossRef]
- Barrientos, L.G.; O’Keefe, B.R.; Bray, M.; Sanchez, A.; Gronenborn, A.M.; Boyd, M.R. Cyanovirin-N Binds to the Viral Surface Glycoprotein, GP1,2 and Inhibits Infectivity of Ebola Virus. Antivir. Res. 2003, 58, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Rojo, J.; Delgado, R. Glycodendritic Structures: Promising New Antiviral Drugs. J. Antimicrob. Chemother. 2004, 54, 579–581. [Google Scholar] [CrossRef] [PubMed]
- Lasala, F.; Arce, E.; Otero, J.R.; Rojo, J.; Delgado, R. Mannosyl Glycodendritic Structure Inhibits DC-SIGN-Mediated Ebola Virus Infection in Cis and in Trans. Antimicrob. Agents Chemother. 2003, 47, 3970–3972. [Google Scholar] [CrossRef] [PubMed]
- Luczkowiak, J.; Sattin, S.; Sutkevičiūtė, I.; Reina, J.J.; Sánchez-Navarro, M.; Thépaut, M.; Martínez-Prats, L.; Daghetti, A.; Fieschi, F.; Delgado, R.; et al. Pseudosaccharide Functionalized Dendrimers as Potent Inhibitors of DC-SIGN Dependent Ebola Pseudotyped Viral Infection. Bioconjug. Chem. 2011, 22, 1354–1365. [Google Scholar] [CrossRef]
- Muñoz, A.; Sigwalt, D.; Illescas, B.M.; Luczkowiak, J.; Rodríguez-Pérez, L.; Nierengarten, I.; Holler, M.; Remy, J.-S.; Buffet, K.; Vincent, S.P.; et al. Synthesis of Giant Globular Multivalent Glycofullerenes as Potent Inhibitors in a Model of Ebola Virus Infection. Nat. Chem. 2016, 8, 50–57. [Google Scholar] [CrossRef]
- Ning, X.; Budhadev, D.; Pollastri, S.; Nehlmeier, I.; Kempf, A.; Manfield, I.; Turnbull, W.B.; Pöhlmann, S.; Bernardi, A.; Li, X.; et al. Polyvalent Glycomimetic-Gold Nanoparticles Revealing Critical Roles of Glycan Display on Multivalent Lectin-Glycan Interaction Biophysics and Antiviral Properties. JACS Au 2024, 4, 3295–3309. [Google Scholar] [CrossRef]
- Casalino, L.; Gaieb, Z.; Goldsmith, J.A.; Hjorth, C.K.; Dommer, A.C.; Harbison, A.M.; Fogarty, C.A.; Barros, E.P.; Taylor, B.C.; McLellan, J.S.; et al. Beyond Shielding: The Roles of Glycans in the SARS-CoV-2 Spike Protein. ACS Cent. Sci. 2020, 6, 1722–1734. [Google Scholar] [CrossRef]
- Thépaut, M.; Luczkowiak, J.; Vivès, C.; Labiod, N.; Bally, I.; Lasala, F.; Grimoire, Y.; Fenel, D.; Sattin, S.; Thielens, N.; et al. DC/L-SIGN Recognition of Spike Glycoprotein Promotes SARS-CoV-2 Trans-Infection and Can Be Inhibited by a Glycomimetic Antagonist. PLoS Pathog. 2021, 17, e1009576. [Google Scholar] [CrossRef]
- Cramer, J.; Lakkaichi, A.; Aliu, B.; Jakob, R.P.; Klein, S.; Cattaneo, I.; Jiang, X.; Rabbani, S.; Schwardt, O.; Zimmer, G.; et al. Sweet Drugs for Bad Bugs: A Glycomimetic Strategy against the DC-SIGN-Mediated Dissemination of SARS-CoV-2. J. Am. Chem. Soc. 2021, 143, 17465–17478. [Google Scholar] [CrossRef]
- Delaunay, C.; Pollastri, S.; Thépaut, M.; Cavazzoli, G.; Belvisi, L.; Bouchikri, C.; Labiod, N.; Lasala, F.; Gimeno, A.; Franconetti, A.; et al. Unprecedented Selectivity for Homologous Lectin Targets: Differential Targeting of the Viral Receptors L-SIGN and DC-SIGN. Chem. Sci. 2024, 15, 15352–15366. [Google Scholar] [CrossRef]
- Lokhande, K.B.; Apte, G.R.; Shrivastava, A.; Singh, A.; Pal, J.K.; Swamy, K.V.; Gupta, R.K. Sensing the Interactions between Carbohydrate-Binding Agents and N-Linked Glycans of SARS-CoV-2 Spike Glycoprotein Using Molecular Docking and Simulation Studies. J. Biomol. Struct. Dyn. 2022, 40, 3880–3898. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.; Ferruti, P.; Duncan, R. Poly(Amidoamine)s as Potential Endosomolytic Polymers: Evaluation In Vitro and Body Distribution in Normal and Tumour-Bearing Animals. J. Drug Target. 1999, 6, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, R.; Bisazza, A.; Sessa, R.; Primo, L.; Fenili, F.; Manfredi, A.; Ranucci, E.; Ferruti, P. Amphoteric Agmatine Containing Polyamidoamines as Carriers for Plasmid DNA In Vitro and In Vivo Delivery. Biomacromolecules 2010, 11, 2667–2674. [Google Scholar] [CrossRef] [PubMed]
- Mauro, N.; Ferruti, P.; Ranucci, E.; Manfredi, A.; Berzi, A.; Clerici, M.; Cagno, V.; Lembo, D.; Palmioli, A.; Sattin, S. Linear Biocompatible Glyco-Polyamidoamines as Dual Action Mode Virus Infection Inhibitors with Potential as Broad-Spectrum Microbicides for Sexually Transmitted Diseases. Sci. Rep. 2016, 6, 33393. [Google Scholar] [CrossRef]
- Soria-Martinez, L.; Bauer, S.; Giesler, M.; Schelhaas, S.; Materlik, J.; Janus, K.; Pierzyna, P.; Becker, M.; Snyder, N.L.; Hartmann, L.; et al. Prophylactic Antiviral Activity of Sulfated Glycomimetic Oligomers and Polymers. J. Am. Chem. Soc. 2020, 142, 5252–5265. [Google Scholar] [CrossRef]
- Malik, A.; Steinbeis, F.; Carillo, M.A.; Seeberger, P.H.; Lepenies, B.; Varón Silva, D. Immunological Evaluation of Synthetic Glycosylphosphatidylinositol Glycoconjugates as Vaccine Candidates against Malaria. ACS Chem. Biol. 2020, 15, 171–178. [Google Scholar] [CrossRef]
- Carpentieri, A.; Ratner, D.M.; Ghosh, S.K.; Banerjee, S.; Bushkin, G.G.; Cui, J.; Lubrano, M.; Steffen, M.; Costello, C.E.; O’Keefe, B.; et al. The Antiretroviral Lectin Cyanovirin-N Targets Well-Known and Novel Targets on the Surface of Entamoeba Histolytica Trophozoites. Eukaryot. Cell 2010, 9, 1661–1668. [Google Scholar] [CrossRef]
- Paulovičová, E.; Paulovičová, L.; Poláková, M. In Vitro Assessment of Immunobiological Effectivity of Synthetic Non-Ionic Glycolipids. Chem. Biodivers. 2025, 22, e202401368. [Google Scholar] [CrossRef]
- Johnson, M.A.; Bundle, D.R. Designing a New Antifungal Glycoconjugate Vaccine. Chem. Soc. Rev. 2013, 42, 4327–4344. [Google Scholar] [CrossRef]
- Wu, X.; Bundle, D.R. Synthesis of Glycoconjugate Vaccines for Candida Albicans Using Novel Linker Methodology. J. Org. Chem. 2005, 70, 7381–7388. [Google Scholar] [CrossRef]
- Xin, H.; Cartmell, J.; Bailey, J.J.; Dziadek, S.; Bundle, D.R.; Cutler, J.E. Self-Adjuvanting Glycopeptide Conjugate Vaccine against Disseminated Candidiasis. PLoS ONE 2012, 7, e35106. [Google Scholar] [CrossRef]
- Gening, M.L.; Polyanskaya, A.V.; Kuznetsov, A.N.; Titova, A.D.; Yudin, V.I.; Yashunskiy, D.V.; Tsvetkov, Y.E.; Yudina, O.N.; Krylov, V.B.; Nifantiev, N.E. Characterization of Carbohydrate Specificity of Monoclonal Antibodies to Fungal Antigenic Markers Using Biotinylated Oligosaccharides as Coating Antigens. Biochemistry 2024, 89, 2194–2203. [Google Scholar] [CrossRef]


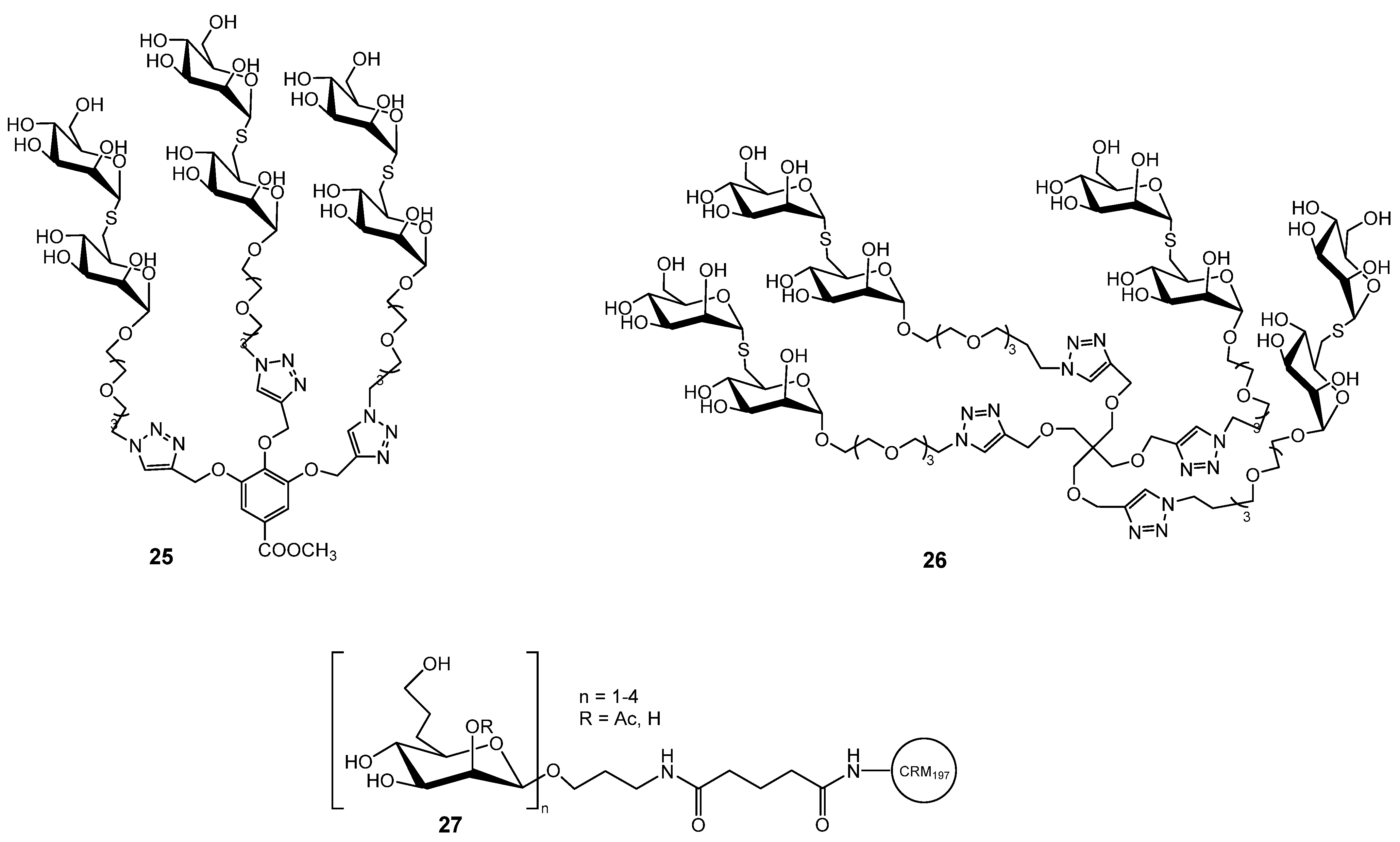
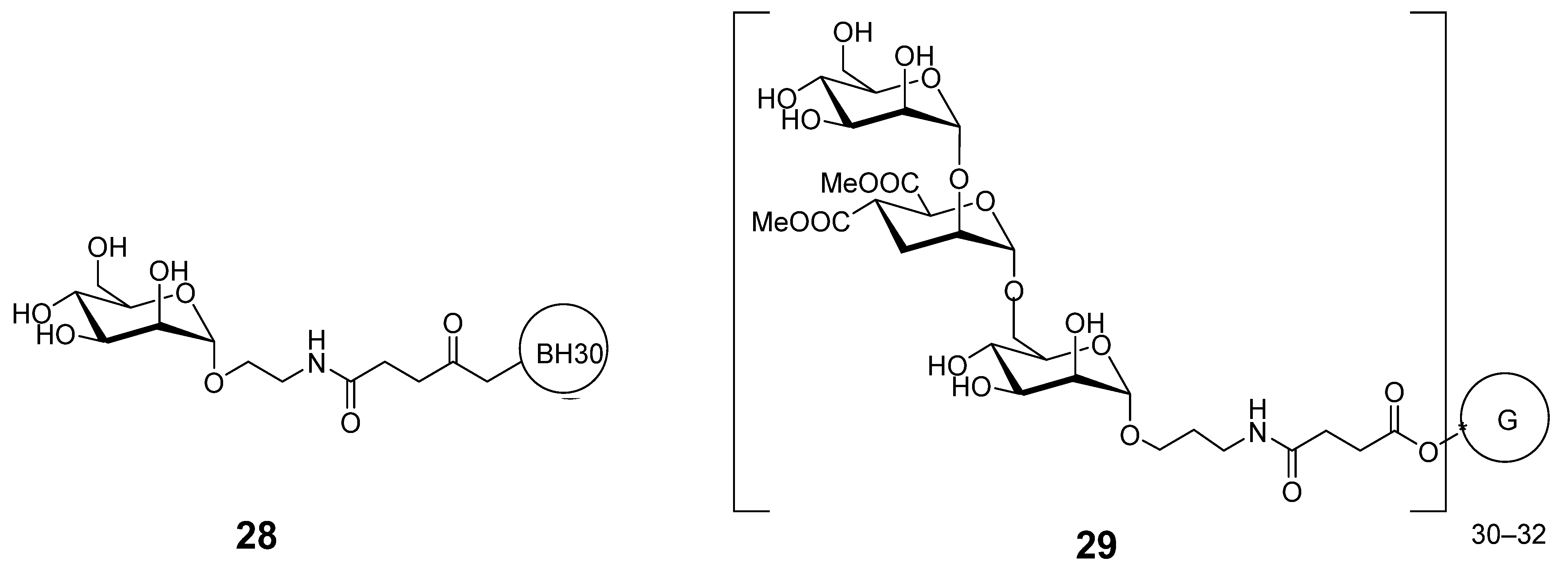




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribić, R. Mannose Derivatives as Anti-Infective Agents. Int. J. Mol. Sci. 2025, 26, 10230. https://doi.org/10.3390/ijms262010230
Ribić R. Mannose Derivatives as Anti-Infective Agents. International Journal of Molecular Sciences. 2025; 26(20):10230. https://doi.org/10.3390/ijms262010230
Chicago/Turabian StyleRibić, Rosana. 2025. "Mannose Derivatives as Anti-Infective Agents" International Journal of Molecular Sciences 26, no. 20: 10230. https://doi.org/10.3390/ijms262010230
APA StyleRibić, R. (2025). Mannose Derivatives as Anti-Infective Agents. International Journal of Molecular Sciences, 26(20), 10230. https://doi.org/10.3390/ijms262010230




