Integrating Experimental and Computational Approaches to Cardioprotection: Vascular Reactivity, Molecular Docking, and ADMET Modeling of Melicoccus bijugatus (Guinep)
Abstract
1. Introduction
2. Results
2.1. Effect of M. bijugatus on Contractile Response
2.2. Redocking Validation
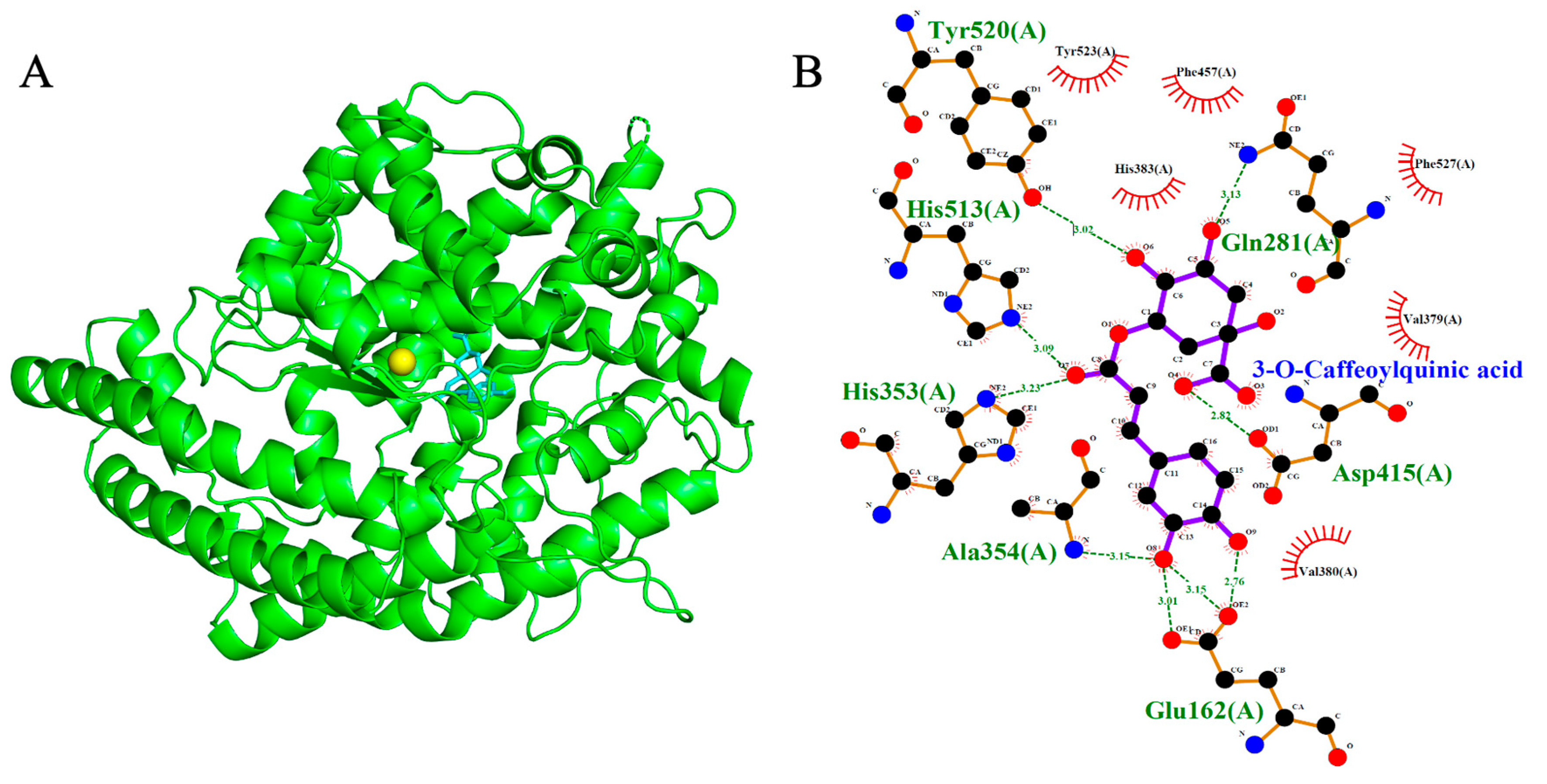
2.3. Binding Affinity and Docking Pose Analysis
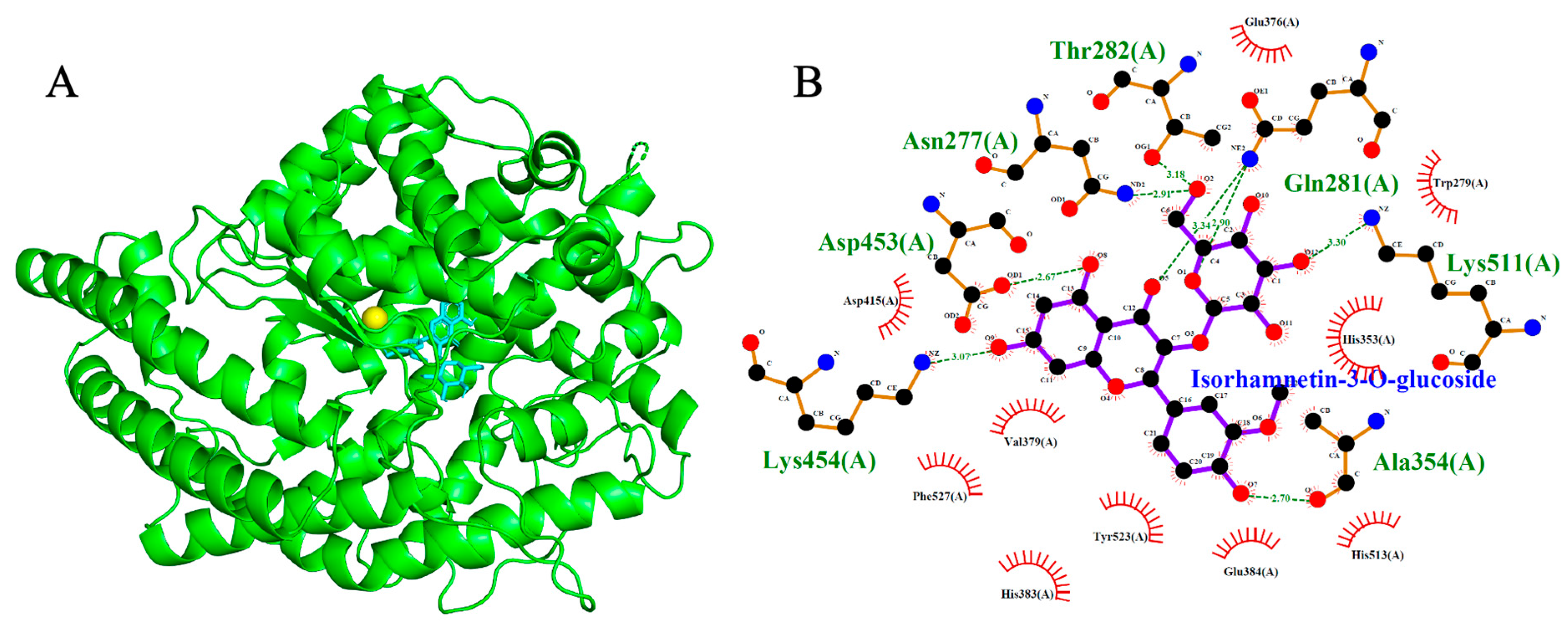
2.4. Angiotensin-Converting Enzyme (ACE, PDB: 1O86)
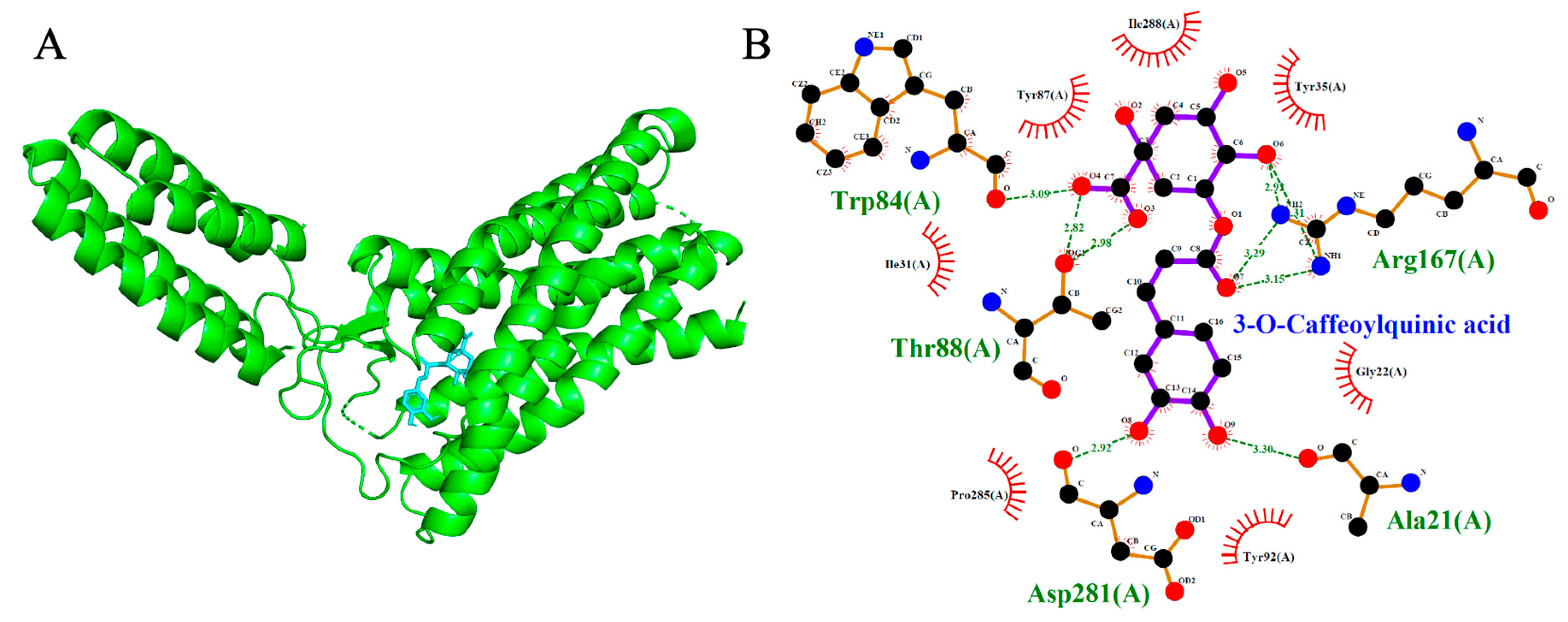
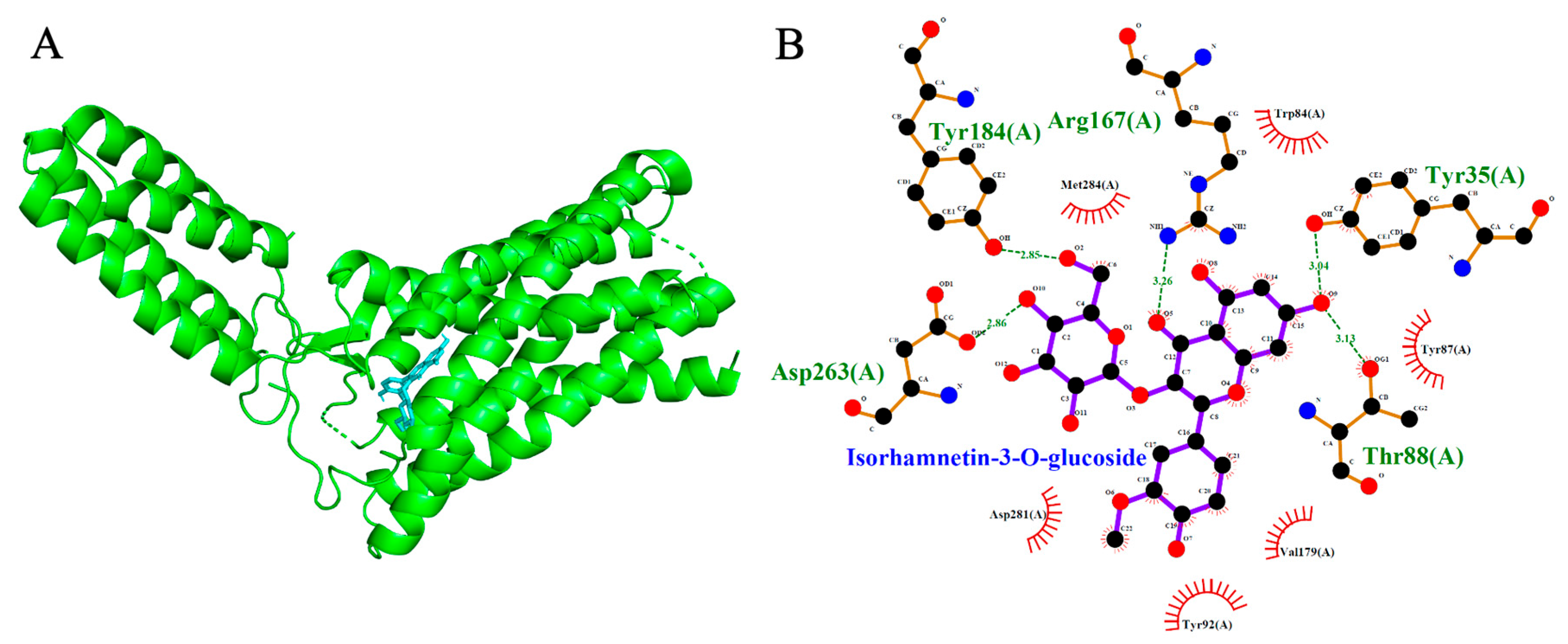
2.5. Angiotensin II Type 1 Receptor (AT1R, PDB: 4ZUD)
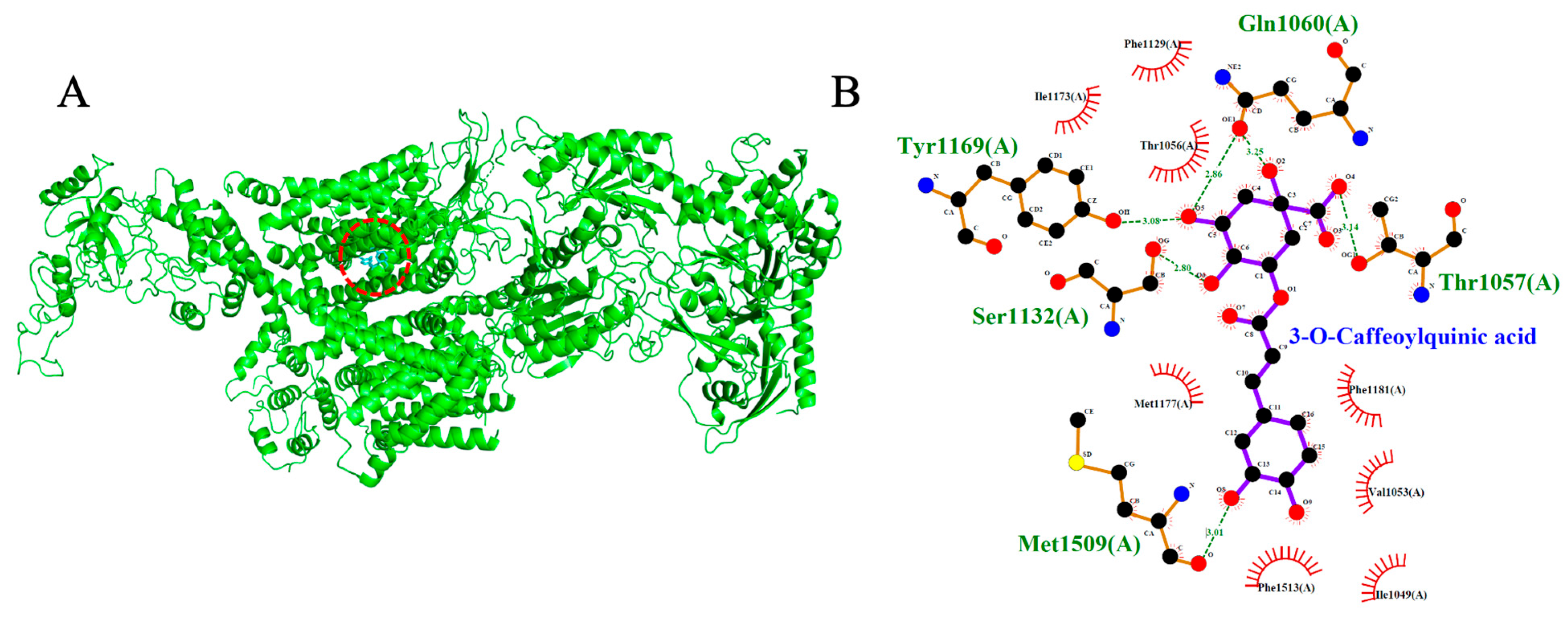
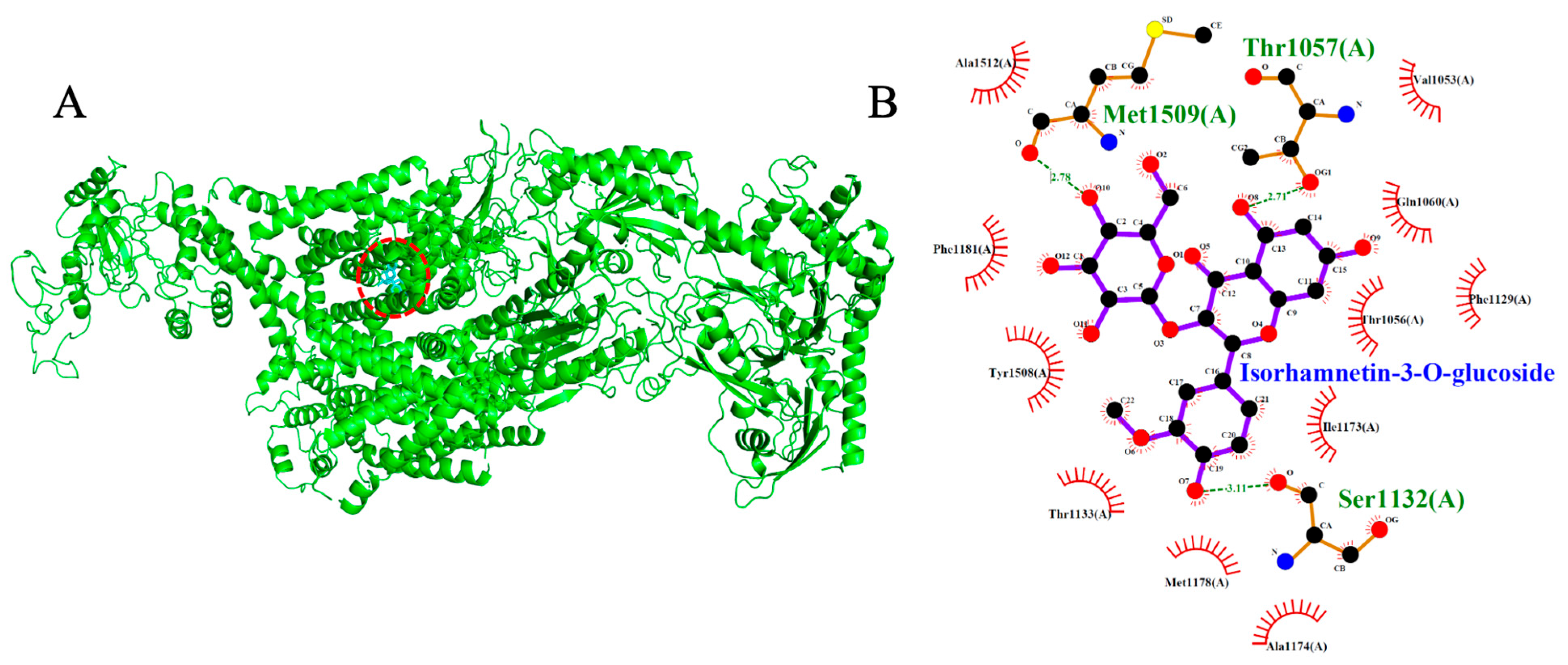
2.6. Voltage-Gated Calcium Channel (VGCC, PDB: 8WE8)
2.7. ADMET Pharmacokinetic Properties
2.8. Biological Activity Based on Spectra
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Plant Material and Extraction
4.3. Animals
4.4. Vascular Reactivity Experiments
4.5. Protein and Ligand Preparation
4.6. Molecular Docking
4.7. Docking Validation
4.8. Docking Protocol
4.9. Prediction of ADMET Pharmacokinetic Properties
4.10. Prediction of Biological Activity Based on Spectra
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bystrom, L.M. The Potential Health Effects of Melicoccus bijugatus Jacq. Fruits: Phytochemical, Chemotaxonomic and Ethnobotanical Investigations. Fitoterapia 2012, 83, 266–271. [Google Scholar] [CrossRef]
- Nwokocha, C.R.; Gordon, A.; Palacios, J.; Paredes, A.; Cifuentes, F.; Francis, S.; Watson, J.A.; Delgoda, R.; Nwokocha, M.; Alexander-Lindo, R.; et al. Hypotensive and Antihypertensive Effects of an Aqueous Extract from Guinep Fruit (Melicoccus bijugatus Jacq) in Rats. Sci. Rep. 2020, 10, 18623. [Google Scholar] [CrossRef]
- Nwokocha, C.R.; Warren, I.; Palacios, J.; Simirgiotis, M.; Nwokocha, M.; Harrison, S.; Thompson, R.; Paredes, A.; Bórquez, J.; Lavado, A.; et al. Modulatory Effect of Guinep (Melicoccus bijugatus Jacq) Fruit Pulp Extract on Isoproterenol-Induced Myocardial Damage in Rats. Identification of Major Metabolites Using High Resolution UHPLC Q-Orbitrap Mass Spectrometry. Molecules 2019, 24, 235. [Google Scholar] [CrossRef]
- Li, P.G.; Xu, J.W.; Ikeda, K.; Kobayakawa, A.; Kayano, Y.; Mitani, T.; Ikami, T.; Yamori, Y. Caffeic Acid Inhibits Vascular Smooth Muscle Cell Proliferation Induced by Angiotensin II in Stroke-Prone Spontaneously Hypertensive Rats. Hypertens. Res. 2005, 28, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Yasuko, K.; Tomohiro, N.; Sei-Itsu, M.; Ai-Na, L.; Yasuo, F.; Takashi, T. Caffeic Acid Is a Selective Inhibitor for Leukotriene Biosynthesis. Biochim. Biophys. Acta (BBA)/Lipids Lipid Metab. 1984, 792, 92–97. [Google Scholar] [CrossRef]
- Manna, S.K.; Mukhopadhyay, A.; Aggarwal, B.B. Resveratrol Suppresses TNF-Induced Activation of Nuclear Transcription Factors NF-ΚB, Activator Protein-1, and Apoptosis: Potential Role of Reactive Oxygen Intermediates and Lipid Peroxidation. J. Immunol. 2000, 164, 6509–6519. [Google Scholar] [CrossRef] [PubMed]
- Bystrom, L.M.; Lewis, B.A.; Brown, D.L.; Rodriguez, E.; Obendorf, R.L. Characterisation of Phenolics by LC-UV/Vis, LC-MS/MS and Sugars by GC in Melicoccus bijugatus Jacq. “Montgomery” Fruits. Food Chem. 2008, 111, 1017–1024. [Google Scholar] [CrossRef]
- Sibi, G.; Rabina, S. Inhibition of Pro-Inflammatory Mediators and Cytokines by Chlorella Vulgaris Extracts. Pharmacogn. Res. 2016, 8, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Schuier, M.; Sies, H.; Illek, B.; Fischer, H. Cocoa-Related Flavonoids Inhibit CFTR-Mediated Chloride Transport across T84 Human Colon Epithelia. J. Nutr. 2005, 135, 2320–2325. [Google Scholar] [CrossRef]
- Carmona-Hernandez, J.C.; Gonzalez-Correa, C.H.; Fausto-Gonzalez, C.J. Antioxidant Potential of Polyphenols from Colombian Melicoccus bijugatus Fruit. Int. J. Innov. Res. Sci. Stud. 2025, 8, 769–776. [Google Scholar] [CrossRef]
- Feng, H.; Chen, G.; Zhang, Y.; Guo, M. Exploring Multifunctional Bioactive Components from Podophyllum Sinense Using Multi-Target Ultrafiltration. Front. Pharmacol. 2021, 12, 749189. [Google Scholar] [CrossRef]
- Meng, X.-Y.; Zhang, H.-X.; Mezei, M.; Cui, M. Molecular Docking: A Powerful Approach for Structure-Based Drug Discovery. Curr. Comput. Aided-Drug Des. 2012, 7, 146–157. [Google Scholar] [CrossRef]
- Hakkou, Z.; Maciuk, A.; Leblais, V.; Bouanani, N.E.; Mekhfi, H.; Bnouham, M.; Aziz, M.; Ziyyat, A.; Rauf, A.; Hadda, T.B.; et al. Antihypertensive and Vasodilator Effects of Methanolic Extract of Inula Viscosa: Biological Evaluation and POM Analysis of Cynarin, Chlorogenic Acid as Potential Hypertensive. Biomed. Pharmacother. 2017, 93, 62–69. [Google Scholar] [CrossRef]
- Ibarra, M.; Moreno, L.; Vera, R.; Cogolludo, A.; Duarte, J.; Tamargo, J.; Perez-Vizcaino, F. Effects of the Flavonoid Quercetin and Its Methylated Metabolite Isorhamnetin in Isolated Arteries from Spontaneously Hypertensive Rats. Planta Med. 2003, 69, 995–1000. [Google Scholar] [CrossRef]
- Guerrero, L.; Castillo, J.; Quiñones, M.; Garcia-Vallvé, S.; Arola, L.; Pujadas, G.; Muguerza, B. Inhibition of Angiotensin-Converting Enzyme Activity by Flavonoids: Structure-Activity Relationship Studies. PLoS ONE 2012, 7, e49493. [Google Scholar] [CrossRef]
- Francis, S.; Laurieri, N.; Nwokocha, C.; Delgoda, R. Treatment of Rats with Apocynin Has Considerable Inhibitory Effects on Arylamine N-Acetyltransferase Activity in the Liver. Sci. Rep. 2016, 6, 26906. [Google Scholar] [CrossRef]
- Palacios, J.; Villarroel, C.; Asunción-Alvarez, D.; Cifuentes, F.; Paredes, A.; Nwokocha, C.R.; Castro-Álvarez, A.; Parra, C. Metabolites Isolated from Senecio Nutans Sch. Bip and Their Synthesized Oximes Inhibit Angiotensin I-Converting Enzyme Activity in Vascular Smooth Muscle. Int. J. Mol. Sci. 2025, 26, 3786. [Google Scholar] [CrossRef]
- Pires, D.E.V.; Blundell, T.L.; Ascher, D.B. PkCSM: Predicting Small-Molecule Pharmacokinetic and Toxicity Properties Using Graph-Based Signatures. J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef]
- ACS. Way2Drug: Updated Data and New Features for Efficient Prediction of Biological Activities. 2023. Available online: https://way2drug.com/PassOnline/ (accessed on 23 July 2025).
- Guimaraes, P.S.; Santiago, N.M.; Xavier, C.H.; Velloso, E.P.; Fontes, M.A.; Santos, R.A.; Campagnole-Santos, M.J. Chronic Infusion of Angiotensin-(1-7) into the Lateral Ventricle of the Brain Attenuates Hypertension in DOCA-Salt Rats. Am. J. Physiol. Heart Circ. Physiol. 2012, 303, H393–H400. [Google Scholar] [CrossRef]
- Zicha, J.; Dobesová, Z.; Kunes, J. Antihypertensive Mechanisms of Chronic Captopril or N-Acetylcysteine Treatment in L-NAME Hypertensive Rats. Hypertens. Res. 2006, 29, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, X.; Zhuang, Y.; Li, Y.; Tian, H.; Shi, P.; Li, G. Isolation of Novel ACE-Inhibitory and Antioxidant Peptides from Quinoa Bran Albumin Assisted with an in Silico Approach: Characterization, in Vivo Antihypertension, and Molecular Docking. Molecules 2019, 24, 4562. [Google Scholar] [CrossRef]
- Mahmoud, M.R.; Shahien, M.M.; Ibrahim, S.; S Alenazi, F.; Hussein, W.; Abdallah, M.H.; Aljadani, A.; Alreshidi, F.; E El-Horany, H.; M Osman Elhussein, G.E.; et al. Novel Insights in the Hypertension Treatment & Type 2 Diabetics Induced by Angiotensin Receptor Blockers: MD Simulation Studies & Molecular Docking of Some Promising Natural Therapies. ACS Omega 2024, 9, 21234–21244. [Google Scholar] [CrossRef]
- Saddala, M.S.; Lennikov, A.; Mukwaya, A.; Yang, Y.; Hill, M.A.; Lagali, N.; Huang, H. Discovery of Novel L-Type Voltage-Gated Calcium Channel Blockers and Application for the Prevention of Inflammation and Angiogenesis. J. Neuroinflammation 2020, 17, 132. [Google Scholar] [CrossRef] [PubMed]
- Hollman, P.C.H.; Katan, M.B. Dietary Flavonoids: Intake, Health Effects and Bioavailability. Food Chem. Toxicol. 1999, 37, 937–942. [Google Scholar] [CrossRef]
- Zhu, F.; Huang, B.; Hu, C.Y.; Jiang, Q.Y.; Lu, Z.G.; Lu, M.; Wang, M.H.; Gong, M.; Qiao, C.P.; Chen, W.; et al. Effects of Total Flavonoids of Hippophae rhamnoides L. on Intracellular Free Calcium in Cultured Vascular Smooth Muscle Cells of Spontaneously Hypertensive Rats and Wistar-Kyoto Rats. Chin. J. Integr. Med. 2005, 11, 287–292. [Google Scholar] [CrossRef]
- Cifuentes, F.; Palacios, J.; Kuzmicic, J.; Carvajal, L.; Muñoz, F.; Quispe, C.; Nwokocha, C.R.; Morales, G.; Norambuena-Soto, I.; Chiong, M.; et al. Vasodilator and Hypotensive Effects of Pure Compounds and Hydroalcoholic Extract of Xenophyllum poposum (Phil) V.A Funk (Compositae) on Rats. Phytomedicine 2018, 50, 99–108. [Google Scholar] [CrossRef]
- Ali, S.S.; Asif, M.; Khan, N.A. Anti-Atherosclerosis and Anti-Hypertensive Effects of Flavonoid Isorhamnetin Isolated from the Bark of Cordia dichotoma L. J. Pharm. Res. Int. 2021, 33, 316–338. [Google Scholar] [CrossRef]
- Suzuki, A.; Yamamoto, N.; Jokura, H.; Yamamoto, M.; Fujii, A.; Tokimitsu, I.; Saito, I. Chlorogenic Acid Attenuates Hypertension and Improves Endothelial Function in Spontaneously Hypertensive Rats. J. Hypertens. 2006, 24, 1065–1073. [Google Scholar] [CrossRef]
- de Angelis, I.; Turco, L. Caco-2 Cells as a Model for Intestinal Absorption. Curr. Protoc. Toxicol. 2011, 47, 20.6.1–20.6.15. [Google Scholar] [CrossRef] [PubMed]
- Gong, G.; Guan, Y.Y.; Zhang, Z.L.; Rahman, K.; Wang, S.J.; Zhou, S.; Luan, X.; Zhang, H. Isorhamnetin: A Review of Pharmacological Effects. Biomed. Pharmacother. 2020, 128, 110301. [Google Scholar] [CrossRef]
- Nulton-Persson, A.C.; Szweda, L.I.; Sadek, H.A. Inhibition of Cardiac Mitochondrial Respiration by Salicylic Acid and Acetylsalicylate. J. Cardiovasc. Pharmacol. 2004, 44, 591–595. [Google Scholar] [CrossRef]
- Ye, L.; Hu, P.; Feng, L.P.; Huang, L.L.; Wang, Y.; Yan, X.; Xiong, J.; Xia, H.L. Protective Effects of Ferulic Acid on Metabolic Syndrome: A Comprehensive Review. Molecules 2023, 28, 281. [Google Scholar] [CrossRef]
- Vinet, R.; Araos, P.; Gentina, J.C.; Knox, M.; Guzmán, L. P-Coumaric Acid Reduces High Glucose-Mediated Impairment of Endothelium-Dependent Relaxation in Rat Aorta. Bol. Latinoam. Caribe Plantas Med. Aromat. 2014, 13, 232–237. [Google Scholar]
- Palacios, J.; Paredes, A.; Catalán, M.A.; Nwokocha, C.R.; Cifuentes, F. Novel Oxime Synthesized from a Natural Product of Senecio nutans SCh. Bip. (Asteraceae) Enhances Vascular Relaxation in Rats by an Endothelium-Independent Mechanism. Molecules 2022, 27, 3333. [Google Scholar] [CrossRef] [PubMed]
- Logan, K.; Nwokocha, C.; Asemota, H.; Gray, W. Characterization of ACE Inhibitory Activity in Dioscorea Alata Cv and Its Implication as a Natural Antihypertensive Extract. J. Ethnopharmacol. 2024, 319, 117221. [Google Scholar] [CrossRef] [PubMed]
- Natesh, R.; Schwager, S.L.U.; Sturrock, E.D.; Acharya, K.R. Crystal Structure of the Human Angiotensin-Converting Enzyme-Lisinopril Complex. Nature 2003, 421, 551–554. [Google Scholar] [CrossRef]
- Zhang, H.; Unal, H.; Desnoyer, R.; Han, G.W.; Patel, N.; Katritch, V.; Karnik, S.S.; Cherezov, V.; Stevens, R.C. Structural Basis for Ligand Recognition and Functional Selectivity at Angiotensin Receptor. J. Biol. Chem. 2015, 290, 29127–29139. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Yao, X.; Chen, J.; Huang, G.; Fan, X.; Xue, L.; Li, Z.; Wu, T.; Zheng, Y.; Huang, J.; et al. Structural Basis for Human Cav1.2 Inhibition by Multiple Drugs and the Neurotoxin Calciseptine. Cell 2023, 186, 5363–5374.e16. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Alves, V.M.; Muratov, E.; Fourches, D.; Strickland, J.; Kleinstreuer, N.; Andrade, C.H.; Tropsha, A. Predicting Chemically-Induced Skin Reactions. Part II: QSAR Models of Skin Permeability and the Relationships between Skin Permeability and Skin Sensitization. Toxicol. Appl. Pharmacol. 2015, 284, 273–280. [Google Scholar] [CrossRef]
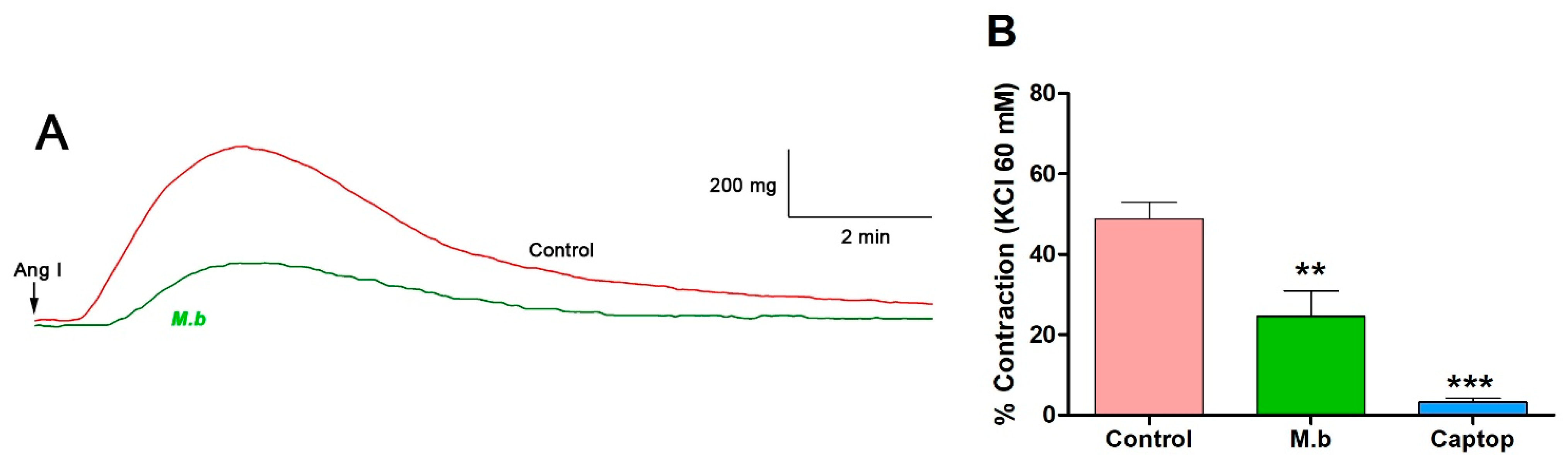
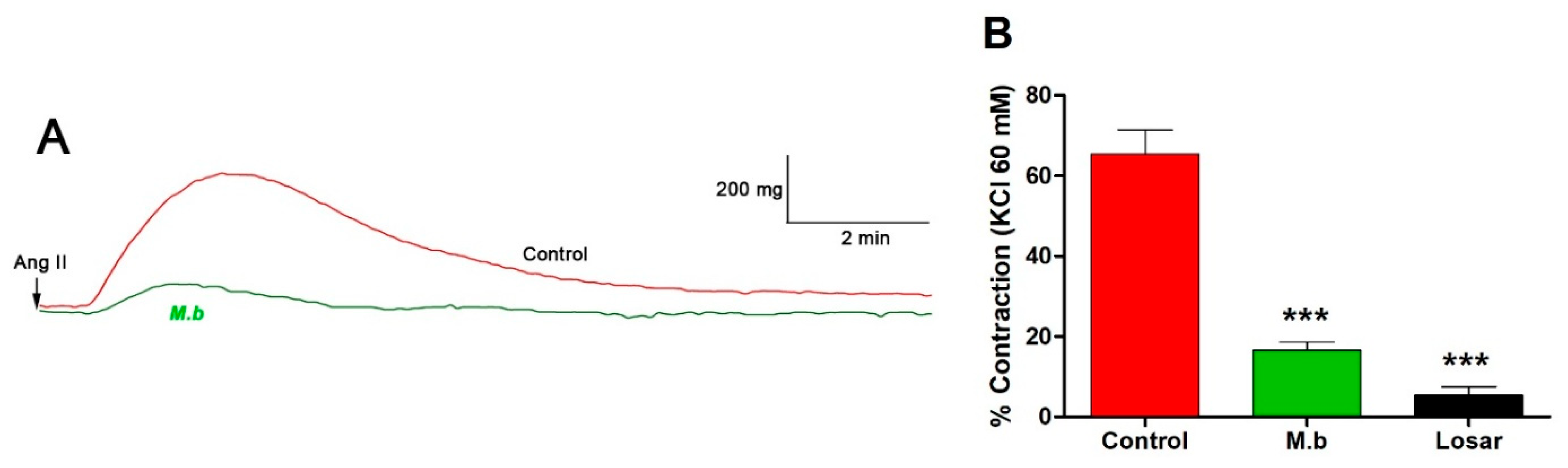
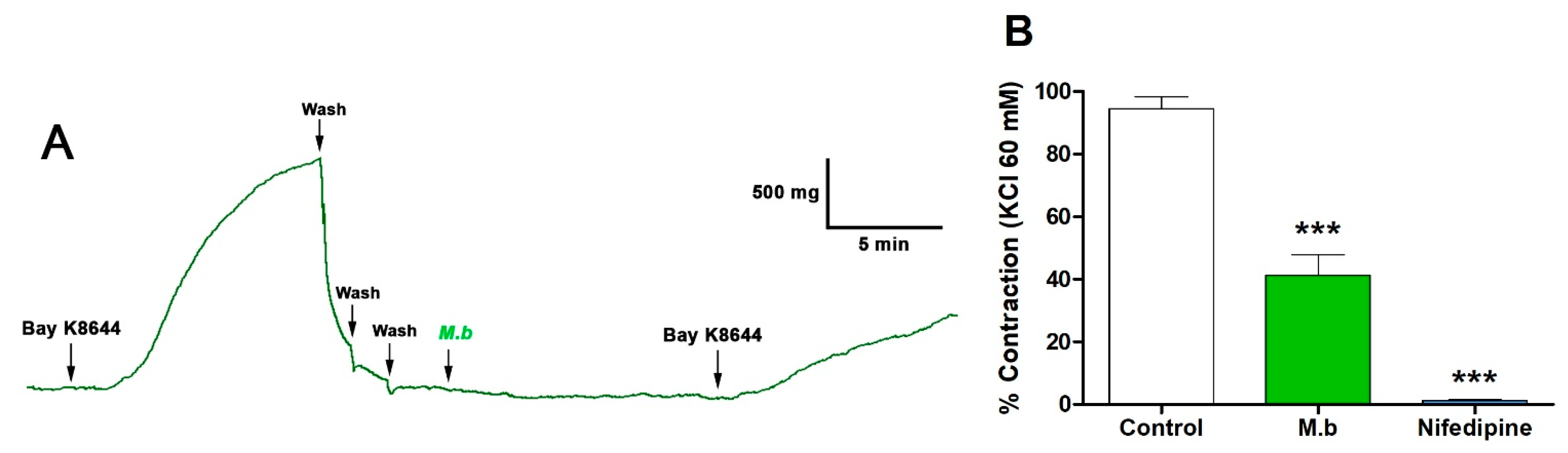

| Ligand | Affinity (kcal/mol) | Number of H Bonds | Amino Acid Residues |
|---|---|---|---|
| * Lisinopril | −8.2 | 4 | Tyr523, Ala354, Glu384, His513 |
| * Captopril | −5.6 | 3 | Gln281, Lys511, Tyr520 |
| 3-O-Caffeoylquinic acid | −8.4 | 7 | Asp415, Tyr520, His513, His353, Ala354, Glu162, Gln281 |
| Isorhamnetin-3-O-glucoside | −9.2 | 7 | Asp453, Lys454, Ala354, Gln281, Lys511, Asn277, Thr282 |
| Ligand | Affinity (kcal/mol) | Number of H Bonds | Amino Acid Residues |
|---|---|---|---|
| * Olmesartan | −8.8 | 1 | Thr88 |
| * Losartan | −8.8 | 3 | Cys180, Tyr87, Thr88 |
| 3-O-Caffeoylquinic acid | −7.5 | 5 | Trp84, Tyr88, Asp281, Ala21, Arg167 |
| Isorhamnetin-3-O-glucoside | −8.2 | 5 | Tyr184, Asp263, Thr88, Tyr35, Arg 167 |
| Ligand | Affinity (kcal/mol) | Number of H Bonds | Amino Acid Residues |
|---|---|---|---|
| * Amlodipine | −6.4 | - | - |
| * Nifedipine | −6.9 | - | - |
| * Verapamil | −7.5 | 2 | Thr1056, Ser1132 |
| * Diltiazem | −8.1 | - | - |
| 3-O-Caffeoylquinic acid | −7.7 | 5 | Tyr1169, Ser1132, Met1509, Thr1057, Gln1060 |
| Isorhamnetin-3-O-glucoside | −8.7 | 3 | Thr1057, Ser1132, Met1509 |
| Compound | Absorption | Distribution | Metabolism | Excretion | Toxicity | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Caco2 | AGI | VDss | BBB | CNS | CYP2D6/CYP3A4 Inhibitor | CT | Tox. Oral Acute in Rats (LD50) | Tox. Oral Chronicle in Rats (LOAEL) | Hepatox. | |
| Isorhamnetin-3-O-glucoside | −0.55 | 49.86 | −1.51 | −1.69 | −4.09 | No/Yes | 0.59 | 1.77 | 2.14 | No |
| 3-O-Caffeoylquinic acid | −0.51 | 31.03 | −1.32 | −1.38 | −4.01 | No/Yes | 0.32 | 1.51 | 1.80 | No |
| Salicylic acid glucoside | −0.43 | 29.19 | −1.21 | −1.49 | −4.72 | No/No | 0.32 | 2.31 | 3.67 | No |
| Aflavarin | 0.44 | 92.22 | −1.27 | −1.06 | −2.93 | No/Yes | 0.92 | 2.45 | 1.91 | No |
| Coumaric acid glucoside | −0.37 | 35.02 | −1.17 | −1.16 | −3.96 | No/No | 0.30 | 1.46 | 1.85 | No |
| Feruloyl glucoside | −0.40 | 50.32 | −1.00 | −1.30 | −4.07 | No/Yes | 0.18 | 1.49 | 1.87 | No |
| Captopril | 1.16 | 81.05 | −0.97 | −0.26 | −3.09 | No/No | 0.30 | 2.38 | 1.21 | No |
| Lisinopril | −0.19 | 21.22 | 0.17 | −1.10 | −3.73 | No/Yes | 0.33 | 1.78 | 1.90 | Yes |
| Compounds | Biological Activity | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antihypertensive | Cardioprotective | Vasoprotectective | Coronary Vasodilator | Peripheral Vasodilator | Calcium Regulator | Calcium Channel Blocker | Angiotensin Antagonist | |||||||||
| Pa | Pi | Pa | Pi | Pa | Pi | Pa | Pi | Pa | Pi | Pa | Pi | Pa | Pi | Pa | Pi | |
| Isorhamnetin-3-O-glucoside | - | - | 0.988 | 0.001 | 0.942 | 0.002 | 0.666 | 0.009 | 0.435 | 0.063 | 0.354 | 0.064 | - | - | - | - |
| 3-O-Caffeoylquinic acid | - | - | 0.238 | 0.139 | 0.442 | 0.059 | 0.281 | 0.139 | 0.32 | 0.129 | 0.58 | 0.006 | - | - | - | - |
| Salicylic acid glucoside | - | - | 0.698 | 0.004 | 0.849 | 0.004 | 0.464 | 0.033 | 0.57 | 0.028 | 0.375 | 0.053 | - | - | - | - |
| Aflavarin | - | - | 0.32 | 0.069 | - | - | 0.357 | 0.079 | - | - | 0.317 | 0.093 | - | - | - | - |
| Coumaric acid glucoside | - | - | 0.633 | 0.005 | 0.934 | 0.002 | 0.497 | 0.026 | 0.696 | 0.01 | 0.405 | 0.04 | - | - | - | - |
| Feruloyl glucoside | - | - | 0.652 | 0.005 | 0.905 | 0.003 | 0.661 | 0.01 | 0.724 | 0.008 | 0.385 | 0.048 | - | - | - | - |
| AML | 0.565 | 0.013 | 0.308 | 0.077 | - | - | 0.421 | 0.046 | - | - | 0.423 | 0.034 | 0.663 | 0.002 | - | - |
| NIF | 0.61 | 0.01 | 0.358 | 0.044 | - | - | 0.569 | 0.017 | 0.654 | 0.014 | 0.469 | 0.02 | 0.548 | 0.003 | - | - |
| CAP | 0.559 | 0.014 | - | - | - | - | 0.661 | 0.01 | 0.246 | 0.211 | - | - | 0.592 | 0.002 | ||
| DIL | 0.928 | 0.004 | - | - | - | - | 0.871 | 0.004 | 0.424 | 0.033 | 0.817 | 0.001 | - | - | ||
| LSA | 0.61 | 0.01 | 0.334 | 0.059 | - | - | - | - | - | - | - | - | - | - | 0.995 | 0.001 |
| VE | 0.785 | 0.005 | - | - | - | - | 0.858 | 0.004 | - | - | 0.539 | 0.008 | 0.608 | 0.002 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Logan, K.; Palacios, J.; Lopez, S.; Gray, W.; Nwokocha, C.R. Integrating Experimental and Computational Approaches to Cardioprotection: Vascular Reactivity, Molecular Docking, and ADMET Modeling of Melicoccus bijugatus (Guinep). Int. J. Mol. Sci. 2025, 26, 10228. https://doi.org/10.3390/ijms262010228
Logan K, Palacios J, Lopez S, Gray W, Nwokocha CR. Integrating Experimental and Computational Approaches to Cardioprotection: Vascular Reactivity, Molecular Docking, and ADMET Modeling of Melicoccus bijugatus (Guinep). International Journal of Molecular Sciences. 2025; 26(20):10228. https://doi.org/10.3390/ijms262010228
Chicago/Turabian StyleLogan, Keaton, Javier Palacios, Sussan Lopez, Wesley Gray, and Chukwuemeka R. Nwokocha. 2025. "Integrating Experimental and Computational Approaches to Cardioprotection: Vascular Reactivity, Molecular Docking, and ADMET Modeling of Melicoccus bijugatus (Guinep)" International Journal of Molecular Sciences 26, no. 20: 10228. https://doi.org/10.3390/ijms262010228
APA StyleLogan, K., Palacios, J., Lopez, S., Gray, W., & Nwokocha, C. R. (2025). Integrating Experimental and Computational Approaches to Cardioprotection: Vascular Reactivity, Molecular Docking, and ADMET Modeling of Melicoccus bijugatus (Guinep). International Journal of Molecular Sciences, 26(20), 10228. https://doi.org/10.3390/ijms262010228






