Disrupting the Gut–Brain Axis: How Artificial Sweeteners Rewire Microbiota and Reward Pathways
Abstract
1. Introduction
1.1. Artificial Sweeteners and Public Health
1.2. From Safety to Complexity
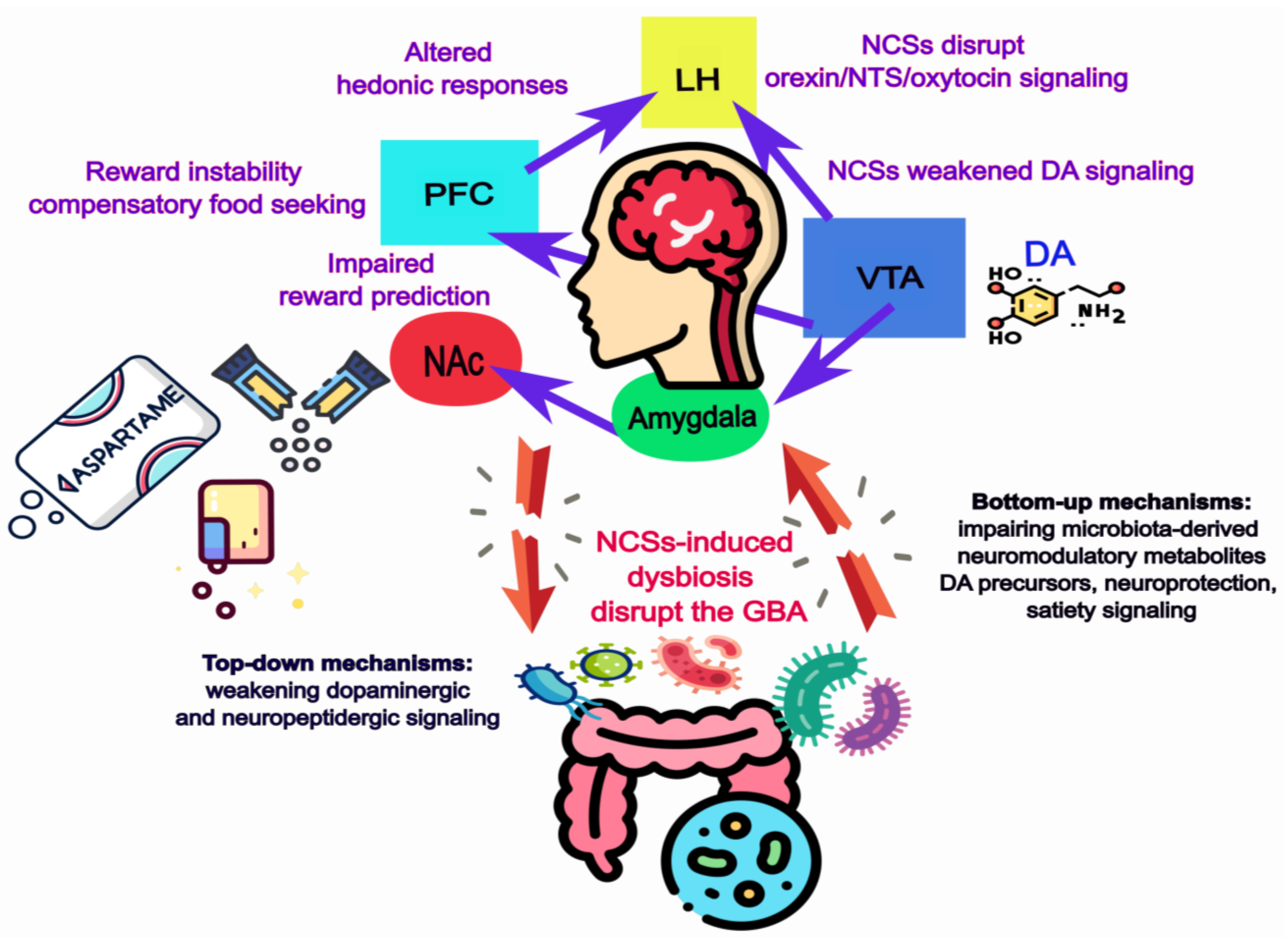
1.3. The Gut–Brain Axis as Mediator
1.4. Aim of the Review
2. NCSs, Gut Microbiota and Homeostatic Feeding
2.1. Not All Sweeteners Are “Born” Equal
2.2. Translational Evidence in Humans
2.3. Microbial Metabolites and Neuroactive Compounds
2.4. Gut Barrier Function and Inflammation: Diet as a Moderator
2.5. Direct Influence of NCSs on Hypothalamic Circuits
2.6. Emerging Insights
2.7. Limitations, Inconsistencies, and Potential Confounders
2.8. Summary
3. Non-Caloric Sweeteners and Neural Reward Processing
3.1. Evolutionary Foundations of Sweet Taste and Nutritional Coupling
3.2. NCSs and Dopaminergic Reward Circuits
3.3. Beyond Dopamine: Neuropeptidergic Modulation of Sweetness and Reward
3.4. Gut Microbial Mediators of NCS-Induced Alterations in Hedonic Feeding
3.5. Integrative Perspective
4. Conclusions and Outlook
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Ace-K | Acesulfame potassium |
| AgRP | Agouti-related peptide |
| ARC | Arcuate nucleus |
| BBB | Blood–brain barrier |
| BNST | Bed nucleus of the stria terminalis |
| BOLD | Blood-oxygen-level-dependent |
| CCK | Cholecystokinin |
| CNS | Central nervous system |
| DA | Dopamine/dopaminergic |
| DAT | Dopamine transporter |
| DS | Dorsal striatum |
| ENS | Enteric nervous system |
| FFAR2/GPR41 | Free fatty acid receptor 2 |
| FFAR3/GPR43 | Free fatty acid receptor 3 |
| fMRI | Functional magnetic resonance imaging |
| FOS | Fructo-oligosaccharides |
| GABA | Gamma-aminobutyric acid |
| GBA | Gut–brain axis |
| GF | Germ-free |
| GLP-1 | Glucagon-like peptide-1 |
| GM | Gut microbiota |
| GRAS | Generally Recognized as Safe |
| HFD | High-fat diet |
| LH | Lateral hypothalamus |
| LPS | Lipopolysaccharide |
| L-DOPA | L-3,4-dihydroxyphenylalanine |
| MCH | Melanin-concentrating hormone |
| NAc | Nucleus accumbens |
| NCSs | Non-caloric sweeteners/Non-nutritive sweeteners |
| NPY | Neuropeptide Y |
| NTS | Neurotensin |
| OFC | Orbitofrontal cortex |
| PFC | Prefrontal cortex |
| POMC | Pro-opiomelanocortin |
| PULs | Polysaccharide utilization loci |
| PYY | Peptide YY |
| SCFAs | Short-chain fatty acids |
| SERT | Serotonin transporter |
| TH | Tyrosine hydroxylase |
| VTA | Ventral tegmental area |
| VS | Ventral striatum |
| VMH | Ventromedial hypothalamus |
| WD | Westernized diet |
References
- Sylvetsky, A.C.; Rother, K.I. Trends in the Consumption of Low-Calorie Sweeteners. Physiol. Behav. 2016, 164, 446–450. [Google Scholar] [CrossRef]
- Chatelan, A.; Raeisi-Dehkordi, H.; Salehi-Abargouei, A. Substituting Low-Calorie Sweetened Beverages for Sugar-Sweetened Beverages to Prevent Obesity and Cardiometabolic Diseases: Still a Good Idea? Curr. Dev. Nutr. 2024, 8, 102105. [Google Scholar] [CrossRef] [PubMed]
- Fowler, S.P.; Williams, K.; Resendez, R.G.; Hunt, K.J.; Hazuda, H.P.; Stern, M.P. Fueling the Obesity Epidemic? Artificially Sweetened Beverage Use and Long-Term Weight Gain. Obesity 2008, 16, 1894–1900. [Google Scholar] [CrossRef]
- Martyn, D.; Darch, M.; Roberts, A.; Lee, H.Y.; Tian, T.Y.; Kaburagi, N.; Belmar, P. Low-/No-Calorie Sweeteners: A Review of Global Intakes. Nutrients 2018, 10, 357. [Google Scholar] [CrossRef]
- Malek, A.M.; Hunt, K.J.; DellaValle, D.M.; Greenberg, D.; Peter, J.V.S.; Marriott, B.P. Reported Consumption of Low-Calorie Sweetener in Foods, Beverages, and Food and Beverage Additions by US Adults: NHANES 2007-2012. Curr. Dev. Nutr. 2018, 2, nzy054. [Google Scholar] [CrossRef]
- Roberts, A. The Safety and Regulatory Process for Low Calorie Sweeteners in the United States. Physiol. Behav. 2016, 164, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Herzog, H.; Zhang, L.; Fontana, L.; Neely, G.G. Impact of Non-Sugar Sweeteners on Metabolism beyond Sweet Taste Perception. Trends Endocrinol. Metab. 2025, 36, 563–576. [Google Scholar] [CrossRef]
- Sylvetsky, A.C.; Rother, K.I. Nonnutritive Sweeteners in Weight Management and Chronic Disease: A Review. Obesity 2018, 26, 635–640. [Google Scholar] [CrossRef]
- Mathur, K.; Agrawal, R.; Nagpure, S.; Deshpande, D. Effect of Artificial Sweeteners on Insulin Resistance among Type-2 Diabetes Mellitus Patients. J. Fam. Med. Prim. Care 2020, 9, 69. [Google Scholar] [CrossRef] [PubMed]
- Gershon, M.D.; Margolis, K.G. The Gut, Its Microbiome, and the Brain: Connections and Communications. J. Clin. Investig. 2021, 131, e143768. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between Microbiota and Immunity in Health and Disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- van de Wouw, M.; Schellekens, H.; Dinan, T.G.; Cryan, J.F. Microbiota-Gut-Brain Axis: Modulator of Host Metabolism and Appetite. J. Nutr. 2017, 147, 727–745. [Google Scholar] [CrossRef] [PubMed]
- Suez, J.; Korem, T.; Zeevi, D.; Zilberman-Schapira, G.; Thaiss, C.A.; Maza, O.; Israeli, D.; Zmora, N.; Gilad, S.; Weinberger, A.; et al. Artificial Sweeteners Induce Glucose Intolerance by Altering the Gut Microbiota. Nature 2014, 514, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Schebendach, J.; Klein, D.A.; Mayer, L.E.S.; Attia, E.; Devlin, M.J.; Foltin, R.W.; Walsh, B.T. Assessment of the Motivation to Use Artificial Sweetener Among Individuals with an Eating Disorder. Appetite 2016, 109, 131. [Google Scholar] [CrossRef] [PubMed]
- Green, E.; Murphy, C. Altered Processing of Sweet Taste in the Brain of Diet Soda Drinkers. Physiol. Behav. 2012, 107, 560–567. [Google Scholar] [CrossRef]
- Dai, X.; Guo, Z.; Chen, D.; Li, L.; Song, X.; Liu, T.; Jin, G.; Li, Y.; Liu, Y.; Ajiguli, A.; et al. Maternal Sucralose Intake Alters Gut Microbiota of Offspring and Exacerbates Hepatic Steatosis in Adulthood. Gut Microbes 2020, 11, 1043–1063. [Google Scholar] [CrossRef]
- Suez, J.; Cohen, Y.; Valdés-Mas, R.; Mor, U.; Dori-Bachash, M.; Federici, S.; Zmora, N.; Leshem, A.; Heinemann, M.; Linevsky, R.; et al. Personalized Microbiome-Driven Effects of Non-Nutritive Sweeteners on Human Glucose Tolerance. Cell 2022, 185, 3307–3328.e19. [Google Scholar] [CrossRef]
- Palmnäs, M.S.A.; Cowan, T.E.; Bomhof, M.R.; Su, J.; Reimer, R.A.; Vogel, H.J.; Hittel, D.S.; Shearer, J. Low-Dose Aspartame Consumption Differentially Affects Gut Microbiota-Host Metabolic Interactions in the Diet-Induced Obese Rat. PLoS ONE 2014, 9, e109841. [Google Scholar] [CrossRef]
- Davidson, T.L.; Martin, A.A.; Clark, K.; Swithers, S.E. Intake of High-Intensity Sweeteners Alters the Ability of Sweet Taste to Signal Caloric Consequences: Implications for the Learned Control of Energy and Body Weight Regulation. Q. J. Exp. Psychol. 2011, 64, 1430. [Google Scholar] [CrossRef]
- Smeets, P.A.M.; Weijzen, P.; de Graaf, C.; Viergever, M.A. Consumption of Caloric and Non-Caloric Versions of a Soft Drink Differentially Affects Brain Activation during Tasting. Neuroimage 2011, 54, 1367–1374. [Google Scholar] [CrossRef]
- Chakravartti, S.P.; Jann, K.; Veit, R.; Liu, H.; Yunker, A.G.; Angelo, B.; Monterosso, J.R.; Xiang, A.H.; Kullmann, S.; Page, K.A. Non-Caloric Sweetener Effects on Brain Appetite Regulation in Individuals across Varying Body Weights. Nat. Metab. 2025, 7, 574–585. [Google Scholar] [CrossRef]
- Liu, C.M.; Kanoski, S.E. Homeostatic and Non-Homeostatic Controls of Feeding Behavior: Distinct vs. Common Neural Systems. Physiol. Behav. 2018, 193, 223. [Google Scholar] [CrossRef]
- Berthoud, H.R.; Münzberg, H.; Morrison, C.D. Blaming the Brain for Obesity: Integration of Hedonic and Homeostatic Mechanisms. Gastroenterology 2017, 152, 1728–1738. [Google Scholar] [CrossRef]
- Rudenga, K.J.; Small, D.M. Amygdala Response to Sucrose Consumption Is Inversely Related to Artificial Sweetener Use. Appetite 2012, 58, 504–507. [Google Scholar] [CrossRef]
- Frank, G.K.W.; Oberndorfer, T.A.; Simmons, A.N.; Paulus, M.P.; Fudge, J.L.; Yang, T.T.; Kaye, W.H. Sucrose Activates Human Taste Pathways Differently from Artificial Sweetener. Neuroimage 2008, 39, 1559–1569. [Google Scholar] [CrossRef]
- Bartolotto, C. Does Consuming Sugar and Artificial Sweeteners Change Taste Preferences? Perm. J. 2015, 19, 81. [Google Scholar] [CrossRef]
- Uebanso, T.; Ohnishi, A.; Kitayama, R.; Yoshimoto, A.; Nakahashi, M.; Shimohata, T.; Mawatari, K.; Takahashi, A. Effects of Low-Dose Non-Caloric Sweetener Consumption on Gut Microbiota in Mice. Nutrients 2017, 9, 560. [Google Scholar] [CrossRef]
- Abou-Donia, M.B.; El-Masry, E.M.; Abdel-Rahman, A.A.; McLendon, R.E.; Schiffman, S.S. Splenda Alters Gut Microflora and Increases Intestinal P-Glycoprotein and Cytochrome p-450 in Male Rats. J. Toxicol. Environ. Health. A 2008, 71, 1415–1429. [Google Scholar] [CrossRef]
- Wang, Q.P.; Browman, D.; Herzog, H.; Gregory Neely, G. Non-Nutritive Sweeteners Possess a Bacteriostatic Effect and Alter Gut Microbiota in Mice. PLoS ONE 2018, 13, e0199080. [Google Scholar] [CrossRef]
- Cutuli, D.; Decandia, D.; Giacovazzo, G.; Coccurello, R. Physical Exercise as Disease-Modifying Alternative against Alzheimer’s Disease: A Gut-Muscle-Brain Partnership. Int. J. Mol. Sci. 2023, 24, 14686. [Google Scholar] [CrossRef]
- Cutuli, D.; Giacovazzo, G.; Decandia, D.; Coccurello, R. Alzheimer’s Disease and Depression in the Elderly: A Trajectory Linking Gut Microbiota and Serotonin Signaling. Front. Psychiatry 2022, 13, 1010169. [Google Scholar] [CrossRef]
- Gauthier, E.; Milagro, F.I.; Navas-Carretero, S. Effect of Low-and Non-Calorie Sweeteners on the Gut Microbiota: A Review of Clinical Trials and Cross-Sectional Studies. Nutrition 2024, 117, 112237. [Google Scholar] [CrossRef]
- Conz, A.; Salmona, M.; Diomede, L. Effect of Non-Nutritive Sweeteners on the Gut Microbiota. Nutrients 2023, 15, 1869. [Google Scholar] [CrossRef]
- Ruiz-Ojeda, F.J.; Plaza-Díaz, J.; Sáez-Lara, M.J.; Gil, A. Effects of Sweeteners on the Gut Microbiota: A Review of Experimental Studies and Clinical Trials. Adv. Nutr. 2019, 10, S31–S48, Erratum in Adv. Nutr. 2020, 11, 468. [Google Scholar] [CrossRef]
- Debras, C.; Chazelas, E.; Sellem, L.; Porcher, R.; Druesne-Pecollo, N.; Esseddik, Y.; De Edelenyi, F.S.; Agaësse, C.; De Sa, A.; Lutchia, R.; et al. Artificial Sweeteners and Risk of Cardiovascular Diseases: Results from the Prospective NutriNet-Santé Cohort. BMJ 2022, 378, e071204. [Google Scholar] [CrossRef]
- Debras, C.; Deschasaux-Tanguy, M.; Chazelas, E.; Sellem, L.; Druesne-Pecollo, N.; Esseddik, Y.; de Edelenyi, F.S.; Agaësse, C.; de Sa, A.; Lutchia, R.; et al. Artificial Sweeteners and Risk of Type 2 Diabetes in the Prospective NutriNet-Santé Cohort. Diabetes Care 2023, 46, 1681–1690. [Google Scholar] [CrossRef]
- Witkowski, M.; Nemet, I.; Alamri, H.; Wilcox, J.; Gupta, N.; Nimer, N.; Haghikia, A.; Li, X.S.; Wu, Y.; Saha, P.P.; et al. The Artificial Sweetener Erythritol and Cardiovascular Event Risk. Nat. Med. 2023, 29, 710–718. [Google Scholar] [CrossRef]
- Gerasimidis, K.; Bryden, K.; Chen, X.; Papachristou, E.; Verney, A.; Roig, M.; Hansen, R.; Nichols, B.; Papadopoulou, R.; Parrett, A. The Impact of Food Additives, Artificial Sweeteners and Domestic Hygiene Products on the Human Gut Microbiome and Its Fibre Fermentation Capacity. Eur. J. Nutr. 2020, 59, 3213–3230. [Google Scholar] [CrossRef]
- Shil, A.; Olusanya, O.; Ghufoor, Z.; Forson, B.; Marks, J.; Chichger, H. Artificial Sweeteners Disrupt Tight Junctions and Barrier Function in the Intestinal Epithelium through Activation of the Sweet Taste Receptor, T1R3. Nutrients 2020, 12, 1862. [Google Scholar] [CrossRef]
- Santos, P.S.; Caria, C.R.P.; Gotardo, E.M.F.; Ribeiro, M.L.; Pedrazzoli, J.; Gambero, A. Artificial Sweetener Saccharin Disrupts Intestinal Epithelial Cells’ Barrier Function in Vitro. Food Funct. 2018, 9, 3815–3822. [Google Scholar] [CrossRef]
- Sánchez-Tapia, M.; Miller, A.W.; Granados-Portillo, O.; Tovar, A.R.; Torres, N. The Development of Metabolic Endotoxemia Is Dependent on the Type of Sweetener and the Presence of Saturated Fat in the Diet. Gut Microbes 2020, 12, 1801301. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Chi, L.; Gao, B.; Tu, P.; Ru, H.; Lu, K. The Artificial Sweetener Acesulfame Potassium Affects the Gut Microbiome and Body Weight Gain in CD-1 Mice. PLoS ONE 2017, 12, e0178426. [Google Scholar] [CrossRef]
- Basson, A.R.; Rodriguez-Palacios, A.; Cominelli, F. Artificial Sweeteners: History and New Concepts on Inflammation. Front. Nutr. 2021, 8, 746247. [Google Scholar] [CrossRef]
- Li, S.; Liu, M.; Han, Y.; Liu, C.; Cao, S.; Cui, Y.; Zhu, X.; Wang, Z.; Liu, B.; Shi, Y. Gut Microbiota-Derived Gamma-Aminobutyric Acid Improves Host Appetite by Inhibiting Satiety Hormone Secretion. mSystems 2024, 9, e0101524. [Google Scholar] [CrossRef]
- Wang, Q.P.; Lin, Y.Q.; Zhang, L.; Wilson, Y.A.; Oyston, L.J.; Cotterell, J.; Qi, Y.; Khuong, T.M.; Bakhshi, N.; Planchenault, Y.; et al. Sucralose Promotes Food Intake through NPY and a Neuronal Fasting Response. Cell Metab. 2016, 24, 75–90. [Google Scholar] [CrossRef]
- Yunes, R.A.; Poluektova, E.U.; Dyachkova, M.S.; Klimina, K.M.; Kovtun, A.S.; Averina, O.V.; Orlova, V.S.; Danilenko, V.N. GABA Production and Structure of GadB/GadC Genes in Lactobacillus and Bifidobacterium Strains from Human Microbiota. Anaerobe 2016, 42, 197–204. [Google Scholar] [CrossRef]
- Otaru, N.; Ye, K.; Mujezinovic, D.; Berchtold, L.; Constancias, F.; Cornejo, F.A.; Krzystek, A.; de Wouters, T.; Braegger, C.; Lacroix, C.; et al. GABA Production by Human Intestinal Bacteroides spp.: Prevalence, Regulation, and Role in Acid Stress Tolerance. Front. Microbiol. 2021, 12, 656895. [Google Scholar] [CrossRef]
- Rodriguez-Palacios, A.; Harding, A.; Menghini, P.; Himmelman, C.; Retuerto, M.; Nickerson, K.P.; Lam, M.; Croniger, C.M.; McLean, M.H.; Durum, S.K.; et al. The Artificial Sweetener Splenda Promotes Gut Proteobacteria, Dysbiosis, and Myeloperoxidase Reactivity in Crohn’s Disease–Like Ileitis. Inflamm. Bowel Dis. 2018, 24, 1005–1020. [Google Scholar] [CrossRef]
- Baldelli, V.; Scaldaferri, F.; Putignani, L.; Del Chierico, F. The Role of Enterobacteriaceae in Gut Microbiota Dysbiosis in Inflammatory Bowel Diseases. Microorganisms 2021, 9, 697. [Google Scholar] [CrossRef]
- Zeng, M.Y.; Inohara, N.; Nuñez, G. Mechanisms of Inflammation-Driven Bacterial Dysbiosis in the Gut. Mucosal Immunol. 2017, 10, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Tagliamonte, S.; Laiola, M.; Ferracane, R.; Vitale, M.; Gallo, M.A.; Meslier, V.; Pons, N.; Ercolini, D.; Vitaglione, P. Mediterranean Diet Consumption Affects the Endocannabinoid System in Overweight and Obese Subjects: Possible Links with Gut Microbiome, Insulin Resistance and Inflammation. Eur. J. Nutr. 2021, 60, 3703–3716. [Google Scholar] [CrossRef]
- Si, J.; Kang, H.; You, H.J.; Ko, G.P. Revisiting the Role of Akkermansia Muciniphila as a Therapeutic Bacterium. Gut Microbes 2022, 14, 2078619. [Google Scholar] [CrossRef]
- O’Riordan, K.J.; Collins, M.K.; Moloney, G.M.; Knox, E.G.; Aburto, M.R.; Fülling, C.; Morley, S.J.; Clarke, G.; Schellekens, H.; Cryan, J.F. Short Chain Fatty Acids: Microbial Metabolites for Gut-Brain Axis Signalling. Mol. Cell Endocrinol. 2022, 546, 111572. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhou, E.; Berbée, J.F.P.; Van Dijk, K.W.; Rensen, P.; Wang, Y. Oral Butyrate Induces Satiety and Improves Insulin Resistance via Gut Microbiota. Atherosclerosis 2020, 315, e100. [Google Scholar] [CrossRef]
- Singh, A.; Chelikani, P. Peptide YY Mediates the Satiety Effects of the Short Chain Fatty Acid–Butyrate (P08-004-19). Curr. Dev. Nutr. 2019, 3, nzz044.P08-004-19. [Google Scholar] [CrossRef]
- Yang, M.; Bose, S.; Lim, S.; Seo, J.; Shin, J.; Lee, D.; Chung, W.H.; Song, E.J.; Nam, Y.D.; Kim, H. Beneficial Effects of Newly Isolated Akkermansia Muciniphila Strains from the Human Gut on Obesity and Metabolic Dysregulation. Microorganisms 2020, 8, 1413. [Google Scholar] [CrossRef] [PubMed]
- Olivier-Van Stichelen, S.; Rother, K.I.; Hanover, J.A. Maternal Exposure to Non-Nutritive Sweeteners Impacts Progeny’s Metabolism and Microbiome. Front. Microbiol. 2019, 10, 440007. [Google Scholar] [CrossRef]
- Guo, S.; Al-Sadi, R.; Said, H.M.; Ma, T.Y. Lipopolysaccharide Causes an Increase in Intestinal Tight Junction Permeability in Vitro and in Vivo by Inducing Enterocyte Membrane Expression and Localization of TLR-4 and CD14. Am. J. Pathol. 2013, 182, 375. [Google Scholar] [CrossRef]
- de Git, K.C.G.; Adan, R.A.H. Leptin Resistance in Diet-Induced Obesity: The Role of Hypothalamic Inflammation. Obes. Rev. 2015, 16, 207–224. [Google Scholar] [CrossRef] [PubMed]
- de La Serre, C.B.; de Lartigue, G.; Raybould, H.E. Chronic Exposure to Low Dose Bacterial Lipopolysaccharide Inhibits Leptin Signaling in Vagal Afferent Neurons. Physiol. Behav. 2015, 139, 188–194. [Google Scholar] [CrossRef]
- Contreras-Chavez, G.G.; Estrada, J.A.; Contreras, I. Changes in Appetite Regulation-Related Signaling Pathways in the Brain of Mice Supplemented with Non-Nutritive Sweeteners. J. Mol. Neurosci. 2021, 71, 1144–1155. [Google Scholar] [CrossRef]
- Sohn, J.W. Network of Hypothalamic Neurons That Control Appetite. BMB Rep. 2015, 48, 229–233. [Google Scholar] [CrossRef]
- Friedman, J.M. Leptin and the Endocrine Control of Energy Balance. Nat. Metab. 2019, 1, 754–764. [Google Scholar] [CrossRef] [PubMed]
- Rafiei, N.; Mitchell, C.S.; Tedesco, C.R.; Chen, J.; Choi, E.A.; Roughley, S.; Jean-Richard-dit-Bressel, P.; Kumar, N.N.; McNally, G.P.; Herzog, H.; et al. Chemogenetic Activation of Arcuate Nucleus NPY and NPY/AgRP Neurons Increases Feeding Behaviour in Mice. Neuropeptides 2024, 107, 102454. [Google Scholar] [CrossRef]
- Beutler, L.R.; Chen, Y.; Ahn, J.S.; Lin, Y.C.; Essner, R.A.; Knight, Z.A. Dynamics of Gut-Brain Communication Underlying Hunger. Neuron 2017, 96, 461–475.e5. [Google Scholar] [CrossRef]
- Su, Z.; Alhadeff, A.L.; Betley, J.N. Nutritive, Post-Ingestive Signals Are the Primary Regulators of AgRP Neuron Activity. Cell Rep. 2017, 21, 2724–2736. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yan, J.; Chen, K.; Song, L.; Sun, B.; Wei, X. Effects of Saccharin Supplementation on Body Weight, Sweet Receptor MRNA Expression and Appetite Signals Regulation in Post-Weanling Rats. Peptides 2018, 107, 32–38. [Google Scholar] [CrossRef]
- Movahedian, M.; Golzan, S.A.; Asbaghi, O.; Prabahar, K.; Hekmatdoost, A. Assessing the Impact of Non-Nutritive Sweeteners on Anthropometric Indices and Leptin Levels in Adults: A GRADE-Assessed Systematic Review, Meta-Analysis, and Meta-Regression of Randomized Clinical Trials. Crit. Rev. Food Sci. Nutr. 2024, 64, 11161–11178. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, D.M.; Abdelgawad, M.A.; Ghoneim, M.M.; Alhossan, A.; Al-Serwi, R.H.; Farouk, A. Impact of Some Natural and Artificial Sweeteners Consumption on Different Hormonal Levels and Inflammatory Cytokines in Male Rats: In Vivo and In Silico Studies. ACS Omega 2024, 9, 30364–30380. [Google Scholar] [CrossRef]
- Alard, J.; Cudennec, B.; Boutillier, D.; Peucelle, V.; Descat, A.; Decoin, R.; Kuylle, S.; Jablaoui, A.; Rhimi, M.; Wolowczuk, I.; et al. Multiple Selection Criteria for Probiotic Strains with High Potential for Obesity Management. Nutrients 2021, 13, 713. [Google Scholar] [CrossRef]
- Yadav, H.; Lee, J.H.; Lloyd, J.; Walter, P.; Rane, S.G. Beneficial Metabolic Effects of a Probiotic via Butyrate-Induced GLP-1 Hormone Secretion. J. Biol. Chem. 2013, 288, 25088–25097. [Google Scholar] [CrossRef]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous Bacteria from the Gut Microbiota Regulate Host Serotonin Biosynthesis. Cell 2015, 161, 264–276, Erratum in Cell 2015, 163, 258. https://doi.org/10.1016/j.cell.2015.09.017. [Google Scholar] [CrossRef]
- Engevik, M.A.; Luck, B.; Visuthranukul, C.; Ihekweazu, F.D.; Engevik, A.C.; Shi, Z.; Danhof, H.A.; Chang-Graham, A.L.; Hall, A.; Endres, B.T.; et al. Human-Derived Bifidobacterium Dentium Modulates the Mammalian Serotonergic System and Gut-Brain Axis. Cell Mol. Gastroenterol. Hepatol. 2021, 11, 221–248. [Google Scholar] [CrossRef]
- van Galen, K.A.; ter Horst, K.W.; Serlie, M.J. Serotonin, Food Intake, and Obesity. Obes. Rev. 2021, 22, e13210. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.N.; Feng, L.J.; Wang, B.M.; Jiang, K.; Li, S.; Xu, X.; Wang, W.Q.; Zhao, J.W.; Wang, Y.M. Lactobacillus Acidophilus and Bifidobacterium Longum Supernatants Upregulate the Serotonin Transporter Expression in Intestinal Epithelial Cells. Saudi J. Gastroenterol. 2018, 24, 59–66. [Google Scholar] [CrossRef]
- Desbonnet, L.; Garrett, L.; Clarke, G.; Bienenstock, J.; Dinan, T.G. The Probiotic Bifidobacteria Infantis: An Assessment of Potential Antidepressant Properties in the Rat. J. Psychiatr. Res. 2008, 43, 164–174. [Google Scholar] [CrossRef]
- Ruddick, J.P.; Evans, A.K.; Nutt, D.J.; Lightman, S.L.; Rook, G.A.W.; Lowry, C.A. Tryptophan Metabolism in the Central Nervous System: Medical Implications. Expert Rev. Mol. Med. 2006, 8, 1–27. [Google Scholar] [CrossRef]
- Li, C.H.; Wang, C.T.; Lin, Y.J.; Kuo, H.Y.; Wu, J.S.; Hong, T.C.; Chang, C.J.; Wu, H.T. Long-Term Consumption of the Sugar Substitute Sorbitol Alters Gut Microbiome and Induces Glucose Intolerance in Mice. Life Sci. 2022, 305, 120770. [Google Scholar] [CrossRef]
- Hesse, S.; van de Giessen, E.; Zientek, F.; Petroff, D.; Winter, K.; Dickson, J.C.; Tossici-Bolt, L.; Sera, T.; Asenbaum, S.; Darcourt, J.; et al. Association of Central Serotonin Transporter Availability and Body Mass Index in Healthy Europeans. Eur. Neuropsychopharmacol. 2014, 24, 1240–1247. [Google Scholar] [CrossRef]
- Fletcher, P.J.; Burton, M.J. Microstructural Analysis of the Anorectic Action of Peripherally Administered 5-HT. Pharmacol. Biochem. Behav. 1986, 24, 1133–1136. [Google Scholar] [CrossRef]
- Tolhurst, G.; Heffron, H.; Lam, Y.S.; Parker, H.E.; Habib, A.M.; Diakogiannaki, E.; Cameron, J.; Grosse, J.; Reimann, F.; Gribble, F.M. Short-Chain Fatty Acids Stimulate Glucagon-like Peptide-1 Secretion via the G-Protein-Coupled Receptor FFAR2. Diabetes 2012, 61, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Psichas, A.; Sleeth, M.L.; Murphy, K.G.; Brooks, L.; Bewick, G.A.; Hanyaloglu, A.C.; Ghatei, M.A.; Bloom, S.R.; Frost, G. The Short Chain Fatty Acid Propionate Stimulates GLP-1 and PYY Secretion via Free Fatty Acid Receptor 2 in Rodents. Int. J. Obes. 2014, 39, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Legget, K.T.; Cornier, M.A.; Bessesen, D.H.; Mohl, B.; Thomas, E.A.; Tregellas, J.R. Greater Reward-Related Neuronal Response to Hedonic Foods in Women Compared with Men. Obesity 2018, 26, 362–367. [Google Scholar] [CrossRef]
- Beauchamp, G.K. Why Do We like Sweet Taste: A Bitter Tale? Physiol. Behav. 2016, 164, 432–437. [Google Scholar] [CrossRef]
- Ramirez, I. Why Do Sugars Taste Good? Neurosci. Biobehav. Rev. 1990, 14, 125–134. [Google Scholar] [CrossRef]
- Treesukosol, Y.; Smith, K.R.; Spector, A.C. The Functional Role of the T1R Family of Receptors in Sweet Taste and Feeding. Physiol. Behav. 2011, 105, 14. [Google Scholar] [CrossRef] [PubMed]
- Mennella, J.A.; Bobowski, N.K.; Reed, D.R. The Development of Sweet Taste: From Biology to Hedonics. Rev. Endocr. Metab. Disord. 2016, 17, 171. [Google Scholar] [CrossRef]
- Venable, E.M.; Carmody, R.N. Decoupled Nutrient Status: A Framework to Disentangle Host from Microbial Responses to Diets That Vary in Digestibility. Front. Food Sci. Technol. 2024, 4, 1469470. [Google Scholar] [CrossRef]
- Swithers, S.E. Artificial Sweeteners Produce the Counterintuitive Effect of Inducing Metabolic Derangements. Trends Endocrinol. Metab. 2013, 24, 431. [Google Scholar] [CrossRef]
- Coccurello, R.; Maccarrone, M. Hedonic Eating and the “Delicious Circle”: From Lipid-Derived Mediators to Brain Dopamine and Back. Front. Neurosci. 2018, 12, 271. [Google Scholar] [CrossRef]
- Hernandez, L.; Hoebel, B.G. Food Reward and Cocaine Increase Extracellular Dopamine in the Nucleus Accumbens as Measured by Microdialysis. Life Sci. 1988, 42, 1705–1712. [Google Scholar] [CrossRef]
- Colombo, M. Deep and Beautiful. The Reward Prediction Error Hypothesis of Dopamine. Stud. Hist. Philos. Biol. Biomed. Sci. 2014, 45, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Lak, A.; Stauffer, W.R.; Schultz, W. Dopamine Neurons Learn Relative Chosen Value from Probabilistic Rewards. Elife 2016, 5, e18044. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Gong, R.; Rodriguez, V.; Quach, K.T.; Chen, X.; Sternson, S.M. Hedonic Eating Is Controlled by Dopamine Neurons That Oppose GLP-1R Satiety. Science 2025, 387, eadt0773. [Google Scholar] [CrossRef] [PubMed]
- Tellez, L.A.; Han, W.; Zhang, X.; Ferreira, T.L.; Perez, I.O.; Shammah-Lagnado, S.J.; Van Den Pol, A.N.; De Araujo, I.E. Separate Circuitries Encode the Hedonic and Nutritional Values of Sugar. Nat. Neurosci. 2016, 19, 465–470. [Google Scholar] [CrossRef]
- Aoyama, K.; Nagano, A. Effects of Saccharin Consumption on Operant Responding for Sugar Reward and Incubation of Sugar Craving in Rats. Foods 2020, 9, 1823. [Google Scholar] [CrossRef]
- Meye, F.J.; Adan, R.A.H. Feelings about Food: The Ventral Tegmental Area in Food Reward and Emotional Eating. Trends Pharmacol. Sci. 2014, 35, 31–40. [Google Scholar] [CrossRef]
- van Opstal, A.M.; Kaal, I.; van den Berg-Huysmans, A.A.; Hoeksma, M.; Blonk, C.; Pijl, H.; Rombouts, S.A.R.B.; van der Grond, J. Dietary Sugars and Non-Caloric Sweeteners Elicit Different Homeostatic and Hedonic Responses in the Brain. Nutrition 2019, 60, 80–86. [Google Scholar] [CrossRef]
- Van Opstal, A.M.; Hafkemeijer, A.; van den Berg-Huysmans, A.A.; Hoeksma, M.; Mulder, T.P.J.; Pijl, H.; Rombouts, S.A.R.B.; van der Grond, J. Brain Activity and Connectivity Changes in Response to Nutritive Natural Sugars, Non-Nutritive Natural Sugar Replacements and Artificial Sweeteners. Nutr. Neurosci. 2021, 24, 395–405. [Google Scholar] [CrossRef]
- Seeley, W.W.; Menon, V.; Schatzberg, A.F.; Keller, J.; Glover, G.H.; Kenna, H.; Reiss, A.L.; Greicius, M.D. Dissociable Intrinsic Connectivity Networks for Salience Processing and Executive Control. J. Neurosci. 2007, 27, 2349. [Google Scholar] [CrossRef]
- Hamelin, H.D.S.; Poizat, G.; Florian, C.D.S.; Kursa, M.B.; Pittaras, E.; Callebert, J.; Rampon, C.; Taouis, M.; Hamed, A.; Granon, S. Prolonged Consumption of Sweetened Beverages Lastingly Deteriorates Cognitive Functions and Reward Processing in Mice. Cereb. Cortex 2022, 32, 1365–1378. [Google Scholar] [CrossRef]
- Mathes, C.M.; Terrill, S.J.; Taborda-Bejarano, J.P.; Chometton, S.; Witt, M.J.; Mendiratta, G.; Gilman, E.G.; Hartswick, D.R.; Anderson, B.M.; Schier, L.A. Neurobehavioral Plasticity in the Rodent Gustatory System Induced by Regular Consumption of a Low-Calorie Sweetener during Adolescence. Sci. Rep. 2025, 15, 2359. [Google Scholar] [CrossRef] [PubMed]
- Bonnavion, P.; Mickelsen, L.E.; Fujita, A.; de Lecea, L.; Jackson, A.C. Hubs and Spokes of the Lateral Hypothalamus: Cell Types, Circuits and Behaviour. J. Physiol. 2016, 594, 6443–6462. [Google Scholar] [CrossRef] [PubMed]
- Allen, G.V.; Cechetto, D.F. Neurotensin in the Lateral Hypothalamic Area: Origin and Function. Neuroscience 1995, 69, 533–544. [Google Scholar] [CrossRef]
- Broberger, C.; De Lecea, L.; Sutcliffe, J.G.; Hökfelt, T. Hypocretin/Orexin- and Melanin-Concentrating Hormone-Expressing Cells Form Distinct Populations in the Rodent Lateral Hypothalamus: Relationship to the Neuropeptide y and Agouti Gene-Related Protein Systems. J. Comp. Neurol. 1998, 402, 460–474. [Google Scholar] [CrossRef]
- Sharpe, M.J.; Marchant, N.J.; Whitaker, L.R.; Richie, C.T.; Zhang, Y.J.; Campbell, E.J.; Koivula, P.P.; Necarsulmer, J.C.; Mejias-Aponte, C.; Morales, M.; et al. Lateral Hypothalamic GABAergic Neurons Encode Reward Predictions That Are Relayed to the Ventral Tegmental Area to Regulate Learning. Curr. Biol. 2017, 27, 2089. [Google Scholar] [CrossRef]
- Sheng, H.; Lei, C.; Yuan, Y.; Fu, Y.; Cui, D.; Yang, L.; Shao, D.; Cao, Z.; Yang, H.; Guo, X.; et al. Nucleus Accumbens Circuit Disinhibits Lateral Hypothalamus Glutamatergic Neurons Contributing to Morphine Withdrawal Memory in Male Mice. Nat. Commun. 2023, 14, 71. [Google Scholar] [CrossRef]
- Mcintyre, C.; Li, X.F.; De Burgh, R.; Ivanova, D.; Lass, G.; O’byrne, K.T. GABA Signaling in the Posterodorsal Medial Amygdala Mediates Stress-Induced Suppression of LH Pulsatility in Female Mice. Endocrinology 2022, 164, 164. [Google Scholar] [CrossRef]
- Weera, M.M.; Shackett, R.S.; Kramer, H.M.; Middleton, J.W.; Gilpin, N.W. Central Amygdala Projections to Lateral Hypothalamus Mediate Avoidance Behavior in Rats. J. Neurosci. 2021, 41, 61–72. [Google Scholar] [CrossRef]
- Barbano, M.F.; Zhang, S.; Chen, E.; Espinoza, O.; Mohammad, U.; Alvarez-Bagnarol, Y.; Liu, B.; Hahn, S.; Morales, M. Lateral Hypothalamic Glutamatergic Inputs to VTA Glutamatergic Neurons Mediate Prioritization of Innate Defensive Behavior over Feeding. Nat. Commun. 2024, 15, 403. [Google Scholar] [CrossRef] [PubMed]
- Geisler, S.; Derst, C.; Veh, R.W.; Zahm, D.S. Glutamatergic Afferents of the Ventral Tegmental Area in the Rat. J. Neurosci. 2007, 27, 5730–5743. [Google Scholar] [CrossRef]
- Fadel, J.; Deutch, A.Y. Anatomical Substrates of Orexin-Dopamine Interactions: Lateral Hypothalamic Projections to the Ventral Tegmental Area. Neuroscience 2002, 111, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, N.; Borgland, S.L. Diversity in the Lateral Hypothalamic Input to the Ventral Tegmental Area. Neuropharmacology 2019, 154, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Marinescu, A.M.; Labouesse, M.A. The Nucleus Accumbens Shell: A Neural Hub at the Interface of Homeostatic and Hedonic Feeding. Front. Neurosci. 2024, 18, 1437210, Erratum in Front. Neurosci. 2024, 18, 1531676. [Google Scholar] [CrossRef]
- Liu, D.; Zheng, X.; Hui, Y.; Xu, Y.; Du, J.; Du, Z.; Che, Y.; Wu, F.; Yu, G.; Zhang, J.; et al. Lateral Hypothalamus Orexinergic Projection to the Medial Prefrontal Cortex Modulates Chronic Stress-Induced Anhedonia but Not Anxiety and Despair. Transl. Psychiatry 2024, 14, 149. [Google Scholar] [CrossRef]
- Harris, G.C.; Wimmer, M.; Aston-Jones, G. A Role for Lateral Hypothalamic Orexin Neurons in Reward Seeking. Nature 2005, 437, 556–559. [Google Scholar] [CrossRef]
- Aston-Jones, G.; Smith, R.J.; Sartor, G.C.; Moorman, D.E.; Massi, L.; Tahsili-Fahadan, P.; Richardson, K.A. Lateral Hypothalamic Orexin/Hypocretin Neurons: A Role in Reward-Seeking and Addiction. Brain Res. 2010, 1314, 74–90. [Google Scholar] [CrossRef]
- Cason, A.M.; Aston-Jones, G. Attenuation of Saccharin-Seeking in Rats by Orexin/Hypocretin Receptor 1 Antagonist. Psychopharmacology 2013, 228, 499. [Google Scholar] [CrossRef]
- Shiuchi, T.; Haque, M.S.; Okamoto, S.; Inoue, T.; Kageyama, H.; Lee, S.; Toda, C.; Suzuki, A.; Bachman, E.S.; Kim, Y.B.; et al. Hypothalamic Orexin Stimulates Feeding-Associated Glucose Utilization in Skeletal Muscle via Sympathetic Nervous System. Cell Metab. 2009, 10, 466–480. [Google Scholar] [CrossRef]
- Kempadoo, K.A.; Tourino, C.; Cho, S.L.; Magnani, F.; Leinninger, G.M.; Stuber, G.D.; Zhang, F.; Myers, M.G.; Deisseroth, K.; de Lecea, L.; et al. Hypothalamic Neurotensin Projections Promote Reward by Enhancing Glutamate Transmission in the VTA. J. Neurosci. 2013, 33, 7618–7626. [Google Scholar] [CrossRef] [PubMed]
- Leinninger, G.M.; Opland, D.M.; Jo, Y.H.; Faouzi, M.; Christensen, L.; Cappellucci, L.A.; Rhodes, C.J.; Gnegy, M.E.; Becker, J.B.; Pothos, E.N.; et al. Leptin Action via Neurotensin Neurons Controls Orexin, the Mesolimbic Dopamine System and Energy Balance. Cell Metab. 2011, 14, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Gazit Shimoni, N.; Tose, A.J.; Seng, C.; Jin, Y.; Lukacsovich, T.; Yang, H.; Verharen, J.P.H.; Liu, C.; Tanios, M.; Hu, E.; et al. Changes in Neurotensin Signalling Drive Hedonic Devaluation in Obesity. Nature 2025, 641, 1238–1247. [Google Scholar] [CrossRef]
- Salaya-Velazquez, N.F.; López-Muciño, L.A.; Mejía-Chávez, S.; Sánchez-Aparicio, P.; Domínguez-Guadarrama, A.A.; Venebra-Muñoz, A. Anandamide and Sucralose Change ΔfosB Expression in the Reward System. Neuroreport 2020, 31, 240–244. [Google Scholar] [CrossRef]
- Howard, J.D.; Gottfried, J.A.; Tobler, P.N.; Kahnt, T. Identity-Specific Coding of Future Rewards in the Human Orbitofrontal Cortex. Proc. Natl. Acad. Sci. USA 2015, 112, 5195–5200. [Google Scholar] [CrossRef]
- Takahashi, Y.K.; Roesch, M.R.; Stalnaker, T.A.; Haney, R.Z.; Calu, D.J.; Taylor, A.R.; Burke, K.A.; Schoenbaum, G. The Orbitofrontal Cortex and Ventral Tegmental Area Are Necessary for Learning from Unexpected Outcomes. Neuron 2009, 62, 269. [Google Scholar] [CrossRef]
- Liu, C.M.; Hsu, T.M.; Suarez, A.N.; Subramanian, K.S.; Fatemi, R.A.; Cortella, A.M.; Noble, E.E.; Roitman, M.F.; Kanoski, S.E. Central Oxytocin Signaling Inhibits Food Reward-Motivated Behaviors and VTA Dopamine Responses to Food-Predictive Cues in Male Rats. Horm. Behav. 2020, 126, 104855. [Google Scholar] [CrossRef]
- Patel, D.; Sundar, M.; Lorenz, E.; Leong, K.C. Oxytocin Attenuates Expression, but Not Acquisition, of Sucrose Conditioned Place Preference in Rats. Front. Behav. Neurosci. 2020, 14, 603232. [Google Scholar] [CrossRef]
- Billings, L.B.; Spero, J.A.; Vollmer, R.R.; Amico, J.A. Oxytocin Null Mice Ingest Enhanced Amounts of Sweet Solutions during Light and Dark Cycles and during Repeated Shaker Stress. Behav. Brain Res. 2006, 171, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Heijtz, R.D.; Wang, S.; Anuar, F.; Qian, Y.; Björkholm, B.; Samuelsson, A.; Hibberd, M.L.; Forssberg, H.; Pettersson, S. Normal Gut Microbiota Modulates Brain Development and Behavior. Proc. Natl. Acad. Sci. USA 2011, 108, 3047–3052. [Google Scholar] [CrossRef]
- Ousey, J.; Boktor, J.C.; Mazmanian, S.K. Gut Microbiota Suppress Feeding Induced by Palatable Foods. Curr. Biol. 2023, 33, 147–157.e7. [Google Scholar] [CrossRef]
- de Wouters d’Oplinter, A.; Rastelli, M.; Van Hul, M.; Delzenne, N.M.; Cani, P.D.; Everard, A. Gut Microbes Participate in Food Preference Alterations during Obesity. Gut Microbes 2021, 13, 1959242. [Google Scholar] [CrossRef] [PubMed]
- Salahpour, A.; Ramsey, A.J.; Medvedev, I.O.; Kile, B.; Sotnikova, T.D.; Holmstrand, E.; Ghisi, V.; Nicholls, P.J.; Wong, L.; Murphy, K.; et al. Increased Amphetamine-Induced Hyperactivity and Reward in Mice Overexpressing the Dopamine Transporter. Proc. Natl. Acad. Sci. USA 2008, 105, 4405–4410. [Google Scholar] [CrossRef]
- Drew, M.R.; Simpson, E.H.; Kellendonk, C.; Herzberg, W.G.; Lipatova, O.; Fairhurst, S.; Kandel, E.R.; Malapani, C.; Balsam, P.D. Transient Overexpression of Striatal D2 Receptors Impairs Operant Motivation and Interval Timing. J. Neurosci. 2007, 27, 7731. [Google Scholar] [CrossRef] [PubMed]
- Rekdal, V.M.; Bess, E.N.; Bisanz, J.E.; Turnbaugh, P.J.; Balskus, E.P. Discovery and Inhibition of an Interspecies Gut Bacterial Pathway for Levodopa Metabolism. Science 2019, 364, 1055. [Google Scholar] [CrossRef]
- Coccurello, R.; Marrone, M.C.; Maccarrone, M. The Endocannabinoids-Microbiota Partnership in Gut-Brain Axis Homeostasis: Implications for Autism Spectrum Disorders. Front. Pharmacol. 2022, 13, 1668. [Google Scholar] [CrossRef]
- Gu, F.; Wu, Y.; Liu, Y.; Dou, M.; Jiang, Y.; Liang, H. Lactobacillus Casei Improves Depression-like Behavior in Chronic Unpredictable Mild Stress-Induced Rats by the BDNF-TrkB Signal Pathway and the Intestinal Microbiota. Food Funct. 2020, 11, 6148–6157. [Google Scholar] [CrossRef]
- Wang, Y.; Tong, Q.; Ma, S.R.; Zhao, Z.X.; Pan, L.B.; Cong, L.; Han, P.; Peng, R.; Yu, H.; Lin, Y.; et al. Oral Berberine Improves Brain Dopa/Dopamine Levels to Ameliorate Parkinson’s Disease by Regulating Gut Microbiota. Signal Transduct. Target. Ther. 2021, 6, 77. [Google Scholar] [CrossRef] [PubMed]
- Méndez-García, L.A.; Bueno-Hernández, N.; Cid-Soto, M.A.; De León, K.L.; Mendoza-Martínez, V.M.; Espinosa-Flores, A.J.; Carrero-Aguirre, M.; Esquivel-Velázquez, M.; León-Hernández, M.; Viurcos-Sanabria, R.; et al. Ten-Week Sucralose Consumption Induces Gut Dysbiosis and Altered Glucose and Insulin Levels in Healthy Young Adults. Microorganisms 2022, 10, 434. [Google Scholar] [CrossRef]
- Srivastav, S.; Neupane, S.; Bhurtel, S.; Katila, N.; Maharjan, S.; Choi, H.; Hong, J.T.; Choi, D.Y. Probiotics Mixture Increases Butyrate, and Subsequently Rescues the Nigral Dopaminergic Neurons from MPTP and Rotenone-Induced Neurotoxicity. J. Nutr. Biochem. 2019, 69, 73–86. [Google Scholar] [CrossRef]
- Wang, L.; Li, S.; Jiang, Y.; Zhao, Z.; Shen, Y.; Zhang, J.; Zhao, L. Neuroprotective Effect of Lactobacillus Plantarum DP189 on MPTP-Induced Parkinson’s Disease Model Mice. J. Funct. Foods 2021, 85, 104635. [Google Scholar] [CrossRef]
- Nápoles-Medina, A.Y.; Aguilar-Uscanga, B.R.; Solís-Pacheco, J.R.; Tejeda-Martínez, A.R.; Ramírez-Jirano, L.J.; Urmeneta-Ortiz, M.F.; Chaparro-Huerta, V.; Flores-Soto, M.E. Oral Administration of Lactobacillus Inhibits the Permeability of Blood-Brain and Gut Barriers in a Parkinsonism Model. Behav. Neurol. 2023, 2023, 6686037. [Google Scholar] [CrossRef]
- Gao, F.; Cheng, C.; Li, R.; Chen, Z.; Tang, K.; Du, G. The Role of Akkermansia Muciniphila in Maintaining Health: A Bibliometric Study. Front. Med. 2025, 12, 1484656. [Google Scholar] [CrossRef] [PubMed]
- Lei, W.; Cheng, Y.; Gao, J.; Liu, X.; Shao, L.; Kong, Q.; Zheng, N.; Ling, Z.; Hu, W. Akkermansia Muciniphila in Neuropsychiatric Disorders: Friend or Foe? Front. Cell Infect. Microbiol. 2023, 13, 1224155. [Google Scholar] [CrossRef]
- Xu, K.; Wang, G.; Gong, J.; Yang, X.; Cheng, Y.; Li, D.; Sheng, S.; Zhang, F. Akkermansia Muciniphila Protects against Dopamine Neurotoxicity by Modulating Butyrate to Inhibit Microglia-Mediated Neuroinflammation. Int. Immunopharmacol. 2025, 152, 114374. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Lei, H.; Chen, G.; Yuan, P.; Cao, Z.; Ser, H.-L.; Zhu, X.; Wu, F.; Liu, C.; Dong, M.; et al. Impaired Intestinal Akkermansia Muciniphila and Aryl Hydrocarbon Receptor Ligands Contribute to Nonalcoholic Fatty Liver Disease in Mice. mSystems 2021, 6, e00985-20. [Google Scholar] [CrossRef] [PubMed]
- Boesmans, L.; Valles-Colomer, M.; Wang, J.; Eeckhaut, V.; Falony, G.; Ducatelle, R.; Van Immerseel, F.; Raes, J.; Verbeke, K. Butyrate Producers as Potential Next-Generation Probiotics: Safety Assessment of the Administration of Butyricicoccus Pullicaecorum to Healthy Volunteers. mSystems 2018, 3, e00094-18. [Google Scholar] [CrossRef]
- Sun, J.; Li, H.; Jin, Y.; Yu, J.; Mao, S.; Su, K.P.; Ling, Z.; Liu, J. Probiotic Clostridium Butyricum Ameliorated Motor Deficits in a Mouse Model of Parkinson’s Disease via Gut Microbiota-GLP-1 Pathway. Brain. Behav. Immun. 2021, 91, 703–715. [Google Scholar] [CrossRef]
- Paiva, I.; Pinho, R.; Pavlou, M.A.; Hennion, M.; Wales, P.; Schütz, A.L.; Rajput, A.; Szego, É.M.; Kerimoglu, C.; Gerhardt, E.; et al. Sodium Butyrate Rescues Dopaminergic Cells from Alpha-Synuclein-Induced Transcriptional Deregulation and DNA Damage. Hum. Mol. Genet. 2017, 26, 2231–2246. [Google Scholar] [CrossRef]
- Byrne, C.S.; Chambers, E.S.; Alhabeeb, H.; Chhina, N.; Morrison, D.J.; Preston, T.; Tedford, C.; Fitzpatrick, J.; Irani, C.; Busza, A.; et al. Increased Colonic Propionate Reduces Anticipatory Reward Responses in the Human Striatum to High-Energy Foods. Am. J. Clin. Nutr. 2016, 104, 5–14. [Google Scholar] [CrossRef]
- Wang, X.; Cai, Z.; Wang, Q.; Wu, C.; Sun, Y.; Wang, Z.; Xu, X.; Xue, W.; Cao, Z.; Zhang, M.; et al. Bacteroides Methylmalonyl-CoA Mutase Produces Propionate That Promotes Intestinal Goblet Cell Differentiation and Homeostasis. Cell Host Microbe 2024, 32, 63–78.e7. [Google Scholar] [CrossRef]
- McKee, L.S.; La Rosa, S.L.; Westereng, B.; Eijsink, V.G.; Pope, P.B.; Larsbrink, J. Polysaccharide Degradation by the Bacteroidetes: Mechanisms and Nomenclature. Environ. Microbiol. Rep. 2021, 13, 559–581. [Google Scholar] [CrossRef] [PubMed]
- Chaudet, M.M.; Rose, D.R. Suggested Alternative Starch Utilization System from the Human Gut Bacterium Bacteroides Thetaiotaomicron. Biochem. Cell Biol. 2016, 94, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Fan, H.; Zhou, J.; Qin, J.; Qin, Z.; Chen, M.; Shen, Y.; Liu, X. In Vitro Inhibitory Effect of Five Natural Sweeteners on α-Glucosidase and α-Amylase. Food Funct. 2024, 15, 2234–2248. [Google Scholar] [CrossRef]
- Nettleton, J.E.; Cho, N.A.; Klancic, T.; Nicolucci, A.C.; Shearer, J.; Borgland, S.L.; Johnston, L.A.; Ramay, H.R.; Noye Tuplin, E.; Chleilat, F.; et al. Maternal Low-Dose Aspartame and Stevia Consumption with an Obesogenic Diet Alters Metabolism, Gut Microbiota and Mesolimbic Reward System in Rat Dams and Their Offspring. Gut 2020, 69, 1807–1817. [Google Scholar] [CrossRef]
- Nettleton, J.E.; Klancic, T.; Schick, A.; Choo, A.C.; Shearer, J.; Borgland, S.L.; Chleilat, F.; Mayengbam, S.; Reimer, R.A. Low-Dose Stevia (Rebaudioside A) Consumption Perturbs Gut Microbiota and the Mesolimbic Dopamine Reward System. Nutrients 2019, 11, 1248. [Google Scholar] [CrossRef]
- Salinas-Velarde, I.D.; Bernal-Morales, B.; Pacheco-Cabrera, P.; Sánchez-Aparicio, P.; Pascual-Mathey, L.I.; Venebra-Muñoz, A. Lower ΔFosB Expression in the Dopaminergic System after Stevia Consumption in Rats Housed under Environmental Enrichment Conditions. Brain Res. Bull. 2021, 177, 172–180. [Google Scholar] [CrossRef]
- Solinas, M.; Thiriet, N.; Rawas, R.E.; Lardeux, V.; Jaber, M. Environmental Enrichment During Early Stages of Life Reduces the Behavioral, Neurochemical, and Molecular Effects of Cocaine. Neuropsychopharmacology 2008, 34, 1102–1111. [Google Scholar] [CrossRef] [PubMed]
- Delbès, A.S.; Castel, J.; Denis, R.G.P.; Morel, C.; Quiñones, M.; Everard, A.; Cani, P.D.; Massiera, F.; Luquet, S.H. Prebiotics Supplementation Impact on the Reinforcing and Motivational Aspect of Feeding. Front. Endocrinol. 2018, 9, 364044. [Google Scholar] [CrossRef] [PubMed]

| Study (Ref) | Model/Design | Sweetener (s) | Exposure Duration/Dose | Key Microbiota Changes | Main Metabolic Findings |
|---|---|---|---|---|---|
| Suez et al., 2014 [13] | Mouse (C57BL/6J); Fecal microbiota transfer | Saccharin, Sucralose and Aspartame in follow-up human validation | Mice: 11 weeks of saccharin in drinking water (0.1 mg/mL, comparable to ADI); Humans: 7-day exposure to saccharin at FDA-acceptable daily intake levels | In mice: ↑ Bacteroides, ↑ certain Clostridiales species; ↓ Lactobacillus reuteri and Akkermansia muciniphila induce dysbiosis. Fecal microbiota transplantation from saccharin-fed mice transferred glucose intolerance to germ-free recipients. | Glucose intolerance and insulin resistance in both mice and subset of human participants. Demonstrated causality between NCS-induced dysbiosis and host metabolic impairment. |
| Bian et al., 2017 [42] | CD-1 mice | Ace-K | Duration: 4 weeks; Dose: 37.5 mg/kg body weight/day (equivalent to human acceptable daily intake) via drinking water | Sex-dependent alteration of gut microbial diversity and composition: ↓ Lactobacillus and Clostridium (females); ↑ Bacteroides and Sutterella (males); enrichment of genes involved in energy metabolism and xenobiotic degradation. | Sex-dependent effects: males showed ↑ body weight, altered lipid metabolism; females showed no body weight gain. Ace-K modulates metabolic outcomes through microbiota-dependent mechanisms. |
| Uebanso et al., 2017 [27] | C57BL/6J mice; | Ace-K, Sucralose | Duration: 8 weeks; Dose: low-dose sucralose (1.5 mg/kg) or high-dose sucralose (15 mg/kg). Ace-K (15 mg/kg) | Sucralose ↓ Clostridium cluster XIVa. Sucralose specifically reduced total SCFA concentrations (notably butyrate) | No changes in body weight or fasting glucose, changes in SCFA profiles and bacterial taxa suggest early dysbiosis. Even low-dose, chronic NCS exposure can modify gut ecology. |
| Palmnäs et al., 2014 [18] | male Sprague–Dawley rats on high-fat diet (HFD) or standard chow | Aspartame (low dose; 5–7 mg/kg/day via drinking water) | 8–12 weeks (chronic exposure during diet feeding) | ↑ Enterobacteriaceae, ↑ Clostridium leptum, altered overall bacterial diversity; increased fecal propionate (SCFA) levels | No major weight gain difference; ↑ fasting glucose, ↓ insulin sensitivity, altered glucose tolerance; metabolic effects linked to microbiota-derived propionate |
| Olivier-Van Stichelen et al., 2019 [57] | Pregnant and lactating C57BL/6J mice; progeny monitored post-weaning | Sucralose (0.1 mg) and Ace-K (0.25 mg) as upper limit ADI; Sucralose (0.2 mg) and Ace-K (0.5 mg) as twice ADI | Gestation + lactation (maternal exposure only; offspring unexposed after weaning) | Offspring microbiota altered ↓ A. muciniphila; overall increase in Firmicutes | Metabolic deregulation in offspring, in particular glycine metabolism (potential decrease in glutathione synthesis) |
| Abou-Donia et al., 2008 [28] | Sprague-Dawley Male Rat | Splenda® (sucralose-based) by oral gavage at 100, 300, 500, or 1000 mg/kg | 12 weeks | ↓ Bifidobacteria, ↓ Lactobacilli, ↓ Bacteroides, | decrease in beneficial intestinal bacteria, histopathological changes in the colon (e.g., lymphocytic infiltrates into epithelium, increased body weight) |
| Rodriguez-Palacios et al., 2018 [48] | Mice | Splenda® (sucralose-based) “low dose” (1.08 mg/mL); “high dose” (3.5 mg/mL) to drinking water | 6 weeks | ↑ Proteobacteria, ↑ E. coli | Gut dysbiosis, endotoxemia |
| Debras et al., 2022 [35] | Human prospective cohort (NutriNet-Santé) | Mixed NCSs (Aspartame, Ace-K, Sucralose) | Median follow-up 9 years | N/A (epidemiological) | Aspartame ↑ Cerebrovascular disease risk; Ace-K, Sucralose ↑ coronary heart disease risk |
| Debras et al., 2023 [36] | Human cohort (NutriNet-Santé) | Mixed NCSs (Aspartame, Ace-K, Sucralose) | Median follow-up 9.1 years | N/A | ↑ Type 2 diabetes incidence |
| Mechanism | Caloric Sugars (Effects) | NCSs (Effects) | Key References |
|---|---|---|---|
| Dopaminergic reward circuits | Activate sweet receptors + post-ingestive caloric signals → robust DA release in VTA, NAc, PFC; strong reward prediction | Activate receptors without calories → weakened DA signaling, impaired prediction error, compensatory food seeking | [74,75,79,80,81,82] |
| Neuropeptidergic modulation | Balanced orexin, NTS, oxytocin signaling supports hedonic control and decision flexibility | Dysregulated orexin/NTS/oxytocin → rigid reward-based choices, altered hedonic valuation | [85,103,104,105,106,107,108,109,110,111,112,113,114,115] |
| Microbial contributions | Support SCFA producers (A. muciniphila, Clostridium, Lactobacillus); enhance DA precursors, neuroprotection, satiety | Dysbiosis: loss of SCFA-producers, enrichment of pro-inflammatory taxa; impaired DA turnover, barrier integrity | [13,27,28,29,30,31,32,33,34,35,36,37,38,116,117,118,119,120,121,122,123,124,125,126,127,128,131,132,133,134,135,136] |
| Integrated perspective | Coordinated top-down (neural) and bottom-up (microbiota) reinforcement → stable reward + energy homeostasis | Dual disruption: weakened neural reinforcement + impaired microbial metabolite support → reward instability, overeating | [87,138,139,140,141,142,143,144,145] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coccurello, R. Disrupting the Gut–Brain Axis: How Artificial Sweeteners Rewire Microbiota and Reward Pathways. Int. J. Mol. Sci. 2025, 26, 10220. https://doi.org/10.3390/ijms262010220
Coccurello R. Disrupting the Gut–Brain Axis: How Artificial Sweeteners Rewire Microbiota and Reward Pathways. International Journal of Molecular Sciences. 2025; 26(20):10220. https://doi.org/10.3390/ijms262010220
Chicago/Turabian StyleCoccurello, Roberto. 2025. "Disrupting the Gut–Brain Axis: How Artificial Sweeteners Rewire Microbiota and Reward Pathways" International Journal of Molecular Sciences 26, no. 20: 10220. https://doi.org/10.3390/ijms262010220
APA StyleCoccurello, R. (2025). Disrupting the Gut–Brain Axis: How Artificial Sweeteners Rewire Microbiota and Reward Pathways. International Journal of Molecular Sciences, 26(20), 10220. https://doi.org/10.3390/ijms262010220






