Bottom Sediments as Dynamic Arenas for Anthropogenic Pollutants: Profiling Sources, Unraveling Fate Mechanisms, and Assessing Ecological Consequences
Abstract
1. Introduction
2. Review Methodology
3. Pollutant Profile and Pathways of Input in Bottom Sediments
3.1. Nutrients: Nitrogen and Phosphorus
3.2. Mineral Compounds
3.3. Microplastics
3.4. Organic Compounds
3.4.1. Pharmaceutical Active Compounds
3.4.2. Agricultural Pesticides
3.4.3. Polycyclic Aromatic Hydrocarbons
4. Fate and Transformation Mechanisms of Pollutants in Bottom Sediments
4.1. Physical Transport Mechanisms: Sedimentation
4.2. Redox-Driven Speciation and Transformation
4.3. Sorption Dynamics and Environmental Factors
4.4. Microbial Biodegradation and Biotransformation
4.5. Synergistic and Antagonistic Interactions Among Pollutants
4.5.1. Microplastics as Vectors and Modulators of Pollutants
4.5.2. Nutrient-Driven Modulation of Pollutant Fate
4.5.3. Heavy Metal–Organic Contaminant Coupling Mechanisms
5. Environmental Impacts of Pollutants in Sediments
5.1. Toxicological Effects on Benthic Fauna
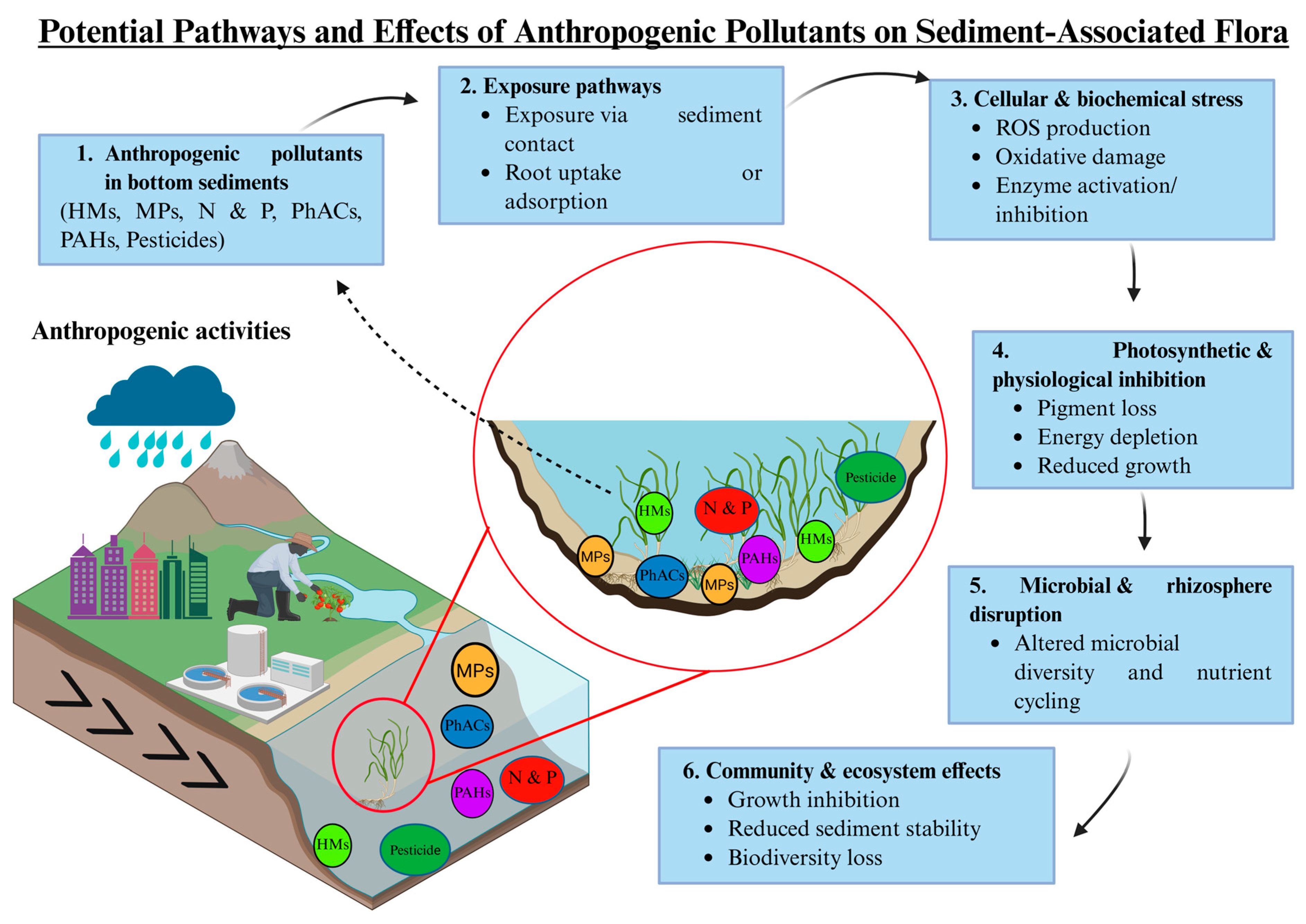
5.2. Effects on Sediment-Associated Flora
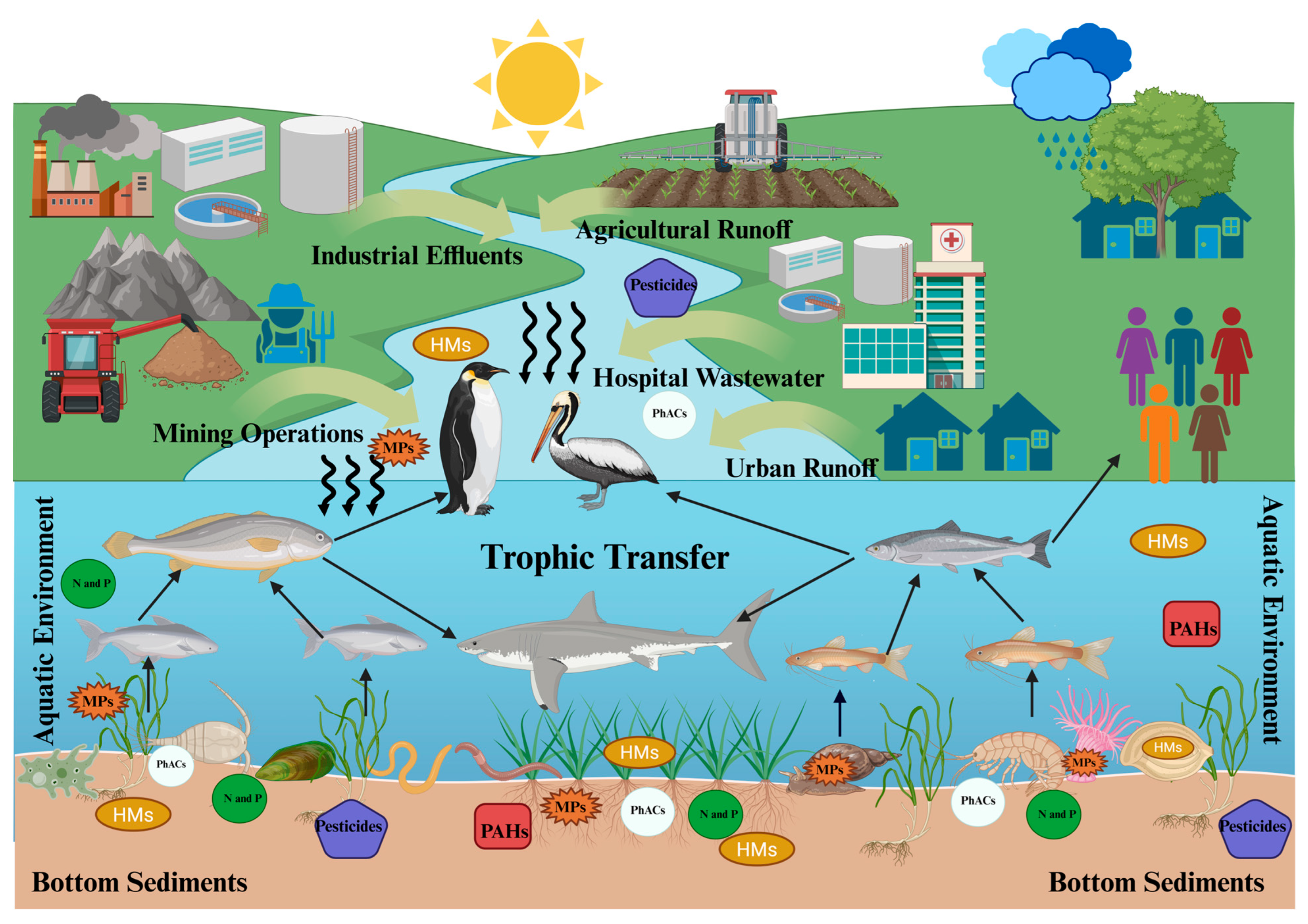
5.3. Bioaccumulation and Biomagnification in Aquatic Food Webs
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, S.; Gray, A.B.; Murphy-Hagan, C.; Hapich, H.; Cowger, W.; Perna, J.; Le, T.; Nogi, H.; Badwal, B.; McLaughlin, K.; et al. Microplastic Pollution in the Water Column and Benthic Sediment of the San Pedro Bay, California, USA. Environ. Res. 2025, 269, 120866. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, M. The Effects of Land Use on Concentrations of Nutrients and Selected Metals in Bottom Sediments and the Risk Assessment for Rivers of the Warta River Catchment, Poland. Land 2021, 10, 589. [Google Scholar] [CrossRef]
- Kucharski, D.; Giebułtowicz, J.; Drobniewska, A.; Nałęcz-Jawecki, G.; Skowronek, A.; Strzelecka, A.; Mianowicz, K.; Drzewicz, P. The Study on Contamination of Bottom Sediments from the Odra River Estuary (SW Baltic Sea) by Tributyltin Using Environmetric Methods. Chemosphere 2022, 308, 136133. [Google Scholar] [CrossRef] [PubMed]
- Pizzini, S.; Giubilato, E.; Morabito, E.; Barbaro, E.; Bonetto, A.; Calgaro, L.; Feltracco, M.; Semenzin, E.; Vecchiato, M.; Zangrando, R.; et al. Contaminants of Emerging Concern in Water and Sediment of the Venice Lagoon, Italy. Environ. Res. 2024, 249, 118401. [Google Scholar] [CrossRef]
- Peris, A.; Barbieri, M.V.; Postigo, C.; Rambla-Alegre, M.; López de Alda, M.; Eljarrat, E. Pesticides in Sediments of the Ebro River Delta Cultivated Area (NE Spain): Occurrence and Risk Assessment for Aquatic Organisms. Environ. Pollut. 2022, 305, 119239. [Google Scholar] [CrossRef]
- Reznikov, S.A.; Yakunina, O.V.; Adzhiev, R.A.; Makarova, I.V. Concentration of Polycyclic Aromatic Hydrocarbons in Lake Baikal Bottom Sediments in the Areas of Strong Anthropogenic Impact. Russ. Meteorol. Hydrol. 2022, 47, 700–707. [Google Scholar] [CrossRef]
- Li, X.; Li, G.; Wang, J.; Li, X.; Yang, Y.; Song, D. Elucidating Polyethylene Microplastic Degradation Mechanisms and Metabolic Pathways via Iron-Enhanced Microbiota Dynamics in Marine Sediments. J. Hazard. Mater. 2024, 466, 133655. [Google Scholar] [CrossRef]
- Jolaosho, T.L.; Rasaq, M.F.; Omotoye, E.V.; Araomo, O.V.; Adekoya, O.S.; Abolaji, O.Y.; Hungbo, J.J. Microplastics in Freshwater and Marine Ecosystems: Occurrence, Characterization, Sources, Distribution Dynamics, Fate, Transport Processes, Potential Mitigation Strategies, and Policy Interventions. Ecotoxicol. Environ. Saf. 2025, 294, 118036. [Google Scholar] [CrossRef]
- Han, H.; Lu, X.; David, F.B.; Joshi, U.M.; Zhang, L. Nitrogen Dynamics at the Sediment-Water Interface in a Tropical Reservoir. Ecol. Eng. 2014, 73, 146–153. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Sidhu, G.P.S.; Handa, N.; Kohli, S.K.; Yadav, P.; Bali, A.S.; Parihar, R.D.; et al. Worldwide Pesticide Usage and Its Impacts on Ecosystem. SN Appl. Sci. 2019, 1, 1446. [Google Scholar] [CrossRef]
- Bavumiragira, J.P.; Ge, J.; Yin, H. Fate and Transport of Pharmaceuticals in Water Systems: A Processes Review. Sci. Total Environ. 2022, 823, 153635. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.; He, J.; Lü, C.; Dong, S.; Wang, J.; Xie, Z.; Zhang, F. Spatial Variations and Distributions of Phosphorus and Nitrogen in Bottom Sediments from a Typical North-Temperate Lake, China. Environ. Earth Sci. 2014, 71, 3063–3079. [Google Scholar] [CrossRef]
- Sultana, S.; Sultana, N.; Moniruzzaman, M.; Dastagir, M.R.; Hossain, M.K. Unmasking Heavy Metal Contamination: Tracing, Risk Estimating and Source Fingerprinting from Coastal Sediments of the Payra River in Bangladesh. Mar. Pollut. Bull. 2025, 211, 117455. [Google Scholar] [CrossRef]
- McIlwraith, H.K.; Dias, M.; Orihel, D.M.; Rennie, M.D.; Harrison, A.L.; Hoffman, M.J.; Provencher, J.F.; Rochman, C.M. A Multicompartment Assessment of Microplastic Contamination in Semi-Remote Boreal Lakes. Environ. Toxicol. Chem. 2024, 43, 999–1011. [Google Scholar] [CrossRef]
- Yang, C.; Yang, P.; Geng, J.; Yin, H.; Chen, K. Sediment Internal Nutrient Loading in the Most Polluted Area of a Shallow Eutrophic Lake (Lake Chaohu, China) and Its Contribution to Lake Eutrophication. Environ. Pollut. 2020, 262, 114292. [Google Scholar] [CrossRef]
- Sun, C.; Wang, S.; Wang, H.; Hu, X.; Yang, F.; Tang, M.; Zhang, M.; Zhong, J. Internal Nitrogen and Phosphorus Loading in a Seasonally Stratified Reservoir: Implications for Eutrophication Management of Deep-Water Ecosystems. J. Environ. Manag. 2022, 319, 115681. [Google Scholar] [CrossRef]
- Waqas, W.; Yuan, Y.; Ali, S.; Zhang, M.; Shafiq, M.; Ali, W.; Chen, Y.; Xiang, Z.; Chen, R.; Ikhwanuddin, M.; et al. Toxic Effects of Heavy Metals on Crustaceans and Associated Health Risks in Humans: A Review. Environ. Chem. Lett. 2024, 22, 1391–1411. [Google Scholar] [CrossRef]
- Demina, L.L.; Galkin, S.V.; Solomatina, A.S. Trace Elements and Organic Carbon in Benthic Organisms and Bottom Sediments of the East-Siberian Sea. Geochem. Int. 2024, 62, 1077–1095. [Google Scholar] [CrossRef]
- Bhuyan, M.S. Effects of Microplastics on Fish and in Human Health. Front. Environ. Sci. 2022, 10, 827289. [Google Scholar] [CrossRef]
- Meynard, A.; Espinoza-González, C.; Núñez, A.; Castañeda, F.; Contreras-Porcia, L. Synergistic, Antagonistic, and Additive Effects of Heavy Metals (Copper and Cadmium) and Polycyclic Aromatic Hydrocarbons (PAHs) Under Binary and Tertiary Combinations in Key Habitat-Forming Kelp Species of Chile. Environ. Sci. Pollut. Res. 2021, 28, 18300–18307. [Google Scholar] [CrossRef]
- Sugiyama, I.; Halevy, I. Long-Term Interaction of Metals and Phosphate with Ferrihydrite in Artificial Seawater and Implications for Past and Present Environments. Chem. Geol. 2024, 670, 122448. [Google Scholar] [CrossRef]
- Wang, M.; Yu, Y.; Ren, Y.; Wang, J.; Chen, H. Effect of Antibiotic and/or Heavy Metal on Nitrogen Cycle of Sediment-Water Interface in Aquaculture System: Implications from Sea Cucumber Culture. Environ. Pollut. 2023, 325, 121453. [Google Scholar] [CrossRef] [PubMed]
- Hermans, M.; Lenstra, W.K.; van Helmond, N.A.G.M.; Behrends, T.; Egger, M.; Séguret, M.J.M.; Gustafsson, E.; Gustafsson, B.G.; Slomp, C.P. Impact of Natural Re-Oxygenation on the Sediment Dynamics of Manganese, Iron and Phosphorus in a Euxinic Baltic Sea Basin. Geochim. Cosmochim. Acta 2019, 246, 174–196. [Google Scholar] [CrossRef]
- Han, Y.-S.; Kim, S.-H.; Chon, C.-M.; Kwon, S.; Kim, J.G.; Choi, H.W.; Ahn, J.S. Effect of FeS on Mercury Behavior in Mercury-Contaminated Stream Sediment: A Case Study of Pohang Gumu Creek in South Korea. J. Hazard. Mater. 2020, 393, 122373. [Google Scholar] [CrossRef]
- Debnath, A.; Singh, P.K.; Chandra Sharma, Y. Metallic Contamination of Global River Sediments and Latest Developments for Their Remediation. J. Environ. Manag. 2021, 298, 113378. [Google Scholar] [CrossRef]
- Acquavita, A.; Floreani, F.; Covelli, S. Occurrence and Speciation of Arsenic and Mercury in Alluvial and Coastal Sediments. Curr. Opin. Environ. Sci. Health 2021, 22, 100272. [Google Scholar] [CrossRef]
- Sharma, N.; Wang, Z.; Catalano, J.G.; Giammar, D.E. Dynamic Responses of Trace Metal Bioaccessibility to Fluctuating Redox Conditions in Wetland Soils and Stream Sediments. ACS Earth Space Chem. 2022, 6, 1331–1344. [Google Scholar] [CrossRef]
- Droz, B.; Drouin, G.; Maurer, L.; Villette, C.; Payraudeau, S.; Imfeld, G. Phase Transfer and Biodegradation of Pesticides in Water–Sediment Systems Explored by Compound-Specific Isotope Analysis and Conceptual Modeling. Environ. Sci. Technol. 2021, 55, 4720–4728. [Google Scholar] [CrossRef]
- Elyamine, A.M.; Kan, J.; Meng, S.; Tao, P.; Wang, H.; Hu, Z. Aerobic and Anaerobic Bacterial and Fungal Degradation of Pyrene: Mechanism Pathway Including Biochemical Reaction and Catabolic Genes. Int. J. Mol. Sci. 2021, 22, 8202. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, Z.; Sun, H.; Xing, R.; Zhou, S. Degradation of Microplastics by Hydroxyl Radicals Generated during Microbially Driven Humus Redox Transformation. Water Res. 2022, 221, 118731. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, L. Microplastic Migration and Transformation Pathways and Exposure Health Risks. Environ. Pollut. 2025, 368, 125700. [Google Scholar] [CrossRef]
- Cao, X.; Li, X.; Wang, H.; Zhang, S.; Zhang, H.; Sakamaki, T.; Li, X. The Promotion of the Polycyclic Aromatic Hydrocarbons Degradation Mechanism by Humic Acid as Electron Mediator in a Sediment Microbial Electrochemical System. Bioresour. Technol. 2024, 404, 130909. [Google Scholar] [CrossRef]
- Jia, H.; Zhao, J.; Li, L.; Li, X.; Wang, C. Transformation of Polycyclic Aromatic Hydrocarbons (PAHs) on Fe (III)-Modified Clay Minerals: Role of Molecular Chemistry and Clay Surface Properties. Appl. Catal. B Environ. 2014, 154, 238–245. [Google Scholar] [CrossRef]
- Yin, H.; Yin, P.; Yang, Z. Seasonal Sediment Phosphorus Release across Sediment-Water Interface and Its Potential Role in Supporting Algal Blooms in a Large Shallow Eutrophic Lake (Lake Taihu, China). Sci. Total Environ. 2023, 896, 165252. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.; Liu, P.; Hu, X.; Zhu, S. Harmful Algal Blooms in Eutrophic Marine Environments: Causes, Monitoring, and Treatment. Water 2024, 16, 2525. [Google Scholar] [CrossRef]
- Antunes, M.; Antunes, M.T.; Fernandes, A.N.; da Silva Crespo, J.; Giovanela, M. Nutrient Contents in Bottom Sediment Samples from a Southern Brazilian Microbasin. Environ. Earth Sci. 2013, 69, 959–968. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, F.; He, Z.; Guo, J.; Qu, X.; Xie, F.; Giesy, J.P.; Liao, H.; Guo, F. Characterization of Organic Phosphorus in Lake Sediments by Sequential Fractionation and Enzymatic Hydrolysis. Environ. Sci. Technol. 2013, 47, 7679–7687. [Google Scholar] [CrossRef]
- Qiu, Z.; Liu, Q.; Zhang, R.; Zhan, C.; Liu, S.; Zhang, J.; Liu, H.; Xiao, W.; Liu, X. Distribution Characteristics and Pollution Assessment of Phosphorus Forms, TOC, and TN in the Sediments of Daye Lake, Central China. J. Soils Sediments 2023, 23, 1023–1036. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, L.; Zhang, H.; Wu, X.; Mei, D. Phosphorus Adsorption Characteristics at the Sediment-Water Interface and Relationship with Sediment Properties in FUSHI Reservoir, China. Environ. Earth Sci. 2012, 67, 15–22. [Google Scholar] [CrossRef]
- Danyang, W.; Xianqiang, T.; Rui, L.; Wenjun, Y. Spatial Distribution Patterns of Nitrogen and Phosphorus in Water and Bed Sediment of the Three Gorges Reservoir. J. Clean. Prod. 2021, 322, 129026. [Google Scholar] [CrossRef]
- Ipeaiyeda, A.R.; Sonibare, K.B.; Alabi, A.H. Speciation of Phosphorus and Nitrogen in Sediments of Ogun River in Abeokuta, Southwestern Nigeria. Afr. J. Environ. Sci. Technol. 2024, 18, 145–157. [Google Scholar] [CrossRef]
- Wang, Y.-P.; Xu, W.-W.; Han, C.; Hu, W.-P. Distribution of Nitrogen and Phosphorus in Lake Chaohu Sediments and Pollution Evaluation. Huan Jing Ke Xue = Huanjing Kexue 2021, 42, 699–711. [Google Scholar]
- Saleem, M.; Jeelani, G.; Pall, I.A.; Ganai, J.; Kumar, S. Water and Sediment Geochemistry of an Urban Lake: Implications to Weathering and Anthropogenic Activity. Int. J. Sediment Res. 2022, 37, 809–822. [Google Scholar] [CrossRef]
- Rzetala, M.A.; Machowski, R.; Solarski, M.; Bakota, D.; Płomiński, A.; Rzetala, M. Toxic Metals, Non-Metals and Metalloids in Bottom Sediments as a Geoecological Indicator of a Water Body’s Suitability for Recreational Use. Int. J. Environ. Res. Public Health 2023, 20, 4334. [Google Scholar] [CrossRef]
- El-Saadani, Z.; Mingqi, W.; He, Z.; Hamukwaya, S.L.; Abdel Wahed, M.S.M.; Khatita, A.A. Environmental Geochemistry and Fractionation of Cadmium Metal in Surficial Bottom Sediments and Water of the Nile River, Egypt. Toxics 2022, 10, 221. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.K.; Islam, F.; Karmaker, K.D.; Akhtar, U.S.; Parvin, A.; Parvin, A.; Moniruzzaman, M.; Saha, B.; Suchi, P.D.; Hossain, M.A.; et al. Source-Specific Geochemical and Health Risk Assessment of Anthropogenically Induced Metals in a Tropical Urban Waterway. Mar. Pollut. Bull. 2024, 203, 116483. [Google Scholar] [CrossRef] [PubMed]
- Farhat, H.I.; Aly, W. Effect of Site on Sedimentological Characteristics and Metal Pollution in Two Semi-Enclosed Embayments of Great Freshwater Reservoir: Lake Nasser, Egypt. J. Afr. Earth Sci. 2018, 141, 194–206. [Google Scholar] [CrossRef]
- Lin, J.; Zhang, S.; Liu, D.; Yu, Z.; Zhang, L.; Cui, J.; Xie, K.; Li, T.; Fu, C. Mobility and Potential Risk of Sediment-Associated Heavy Metal Fractions under Continuous Drought-Rewetting Cycles. Sci. Total Environ. 2018, 625, 79–86. [Google Scholar] [CrossRef]
- Harmesa; Cordova, M.R. A Preliminary Study on Heavy Metal Pollutants Chrome (Cr), Cadmium (Cd), and Lead (Pb) in Sediments and Beach Morning Glory Vegetation (Ipomoea Pes-Caprae) from Dasun Estuary, Rembang, Indonesia. Mar. Pollut. Bull. 2021, 162, 111819. [Google Scholar] [CrossRef]
- Osuna-Martínez, C.C.; Armienta, M.A.; Bergés-Tiznado, M.E.; Páez-Osuna, F. Arsenic in Waters, Soils, Sediments, and Biota from Mexico: An Environmental Review. Sci. Total Environ. 2021, 752, 142062. [Google Scholar] [CrossRef]
- Paul, V.; Sankar, M.S.; Vattikuti, S.; Dash, P.; Arslan, Z. Pollution Assessment and Land Use Land Cover Influence on Trace Metal Distribution in Sediments from Five Aquatic Systems in Southern USA. Chemosphere 2021, 263, 128243. [Google Scholar] [CrossRef]
- Kluska, M.; Jabłońska, J. Pollution Assessment and Spatial Distribution of Heavy Metals in Surface Waters and Bottom Sediments of the Krzna River (Poland). Water 2024, 16, 1008. [Google Scholar] [CrossRef]
- Chaudhary, D.K.; Seo, D.G.; Han, S.; Hong, Y. Distribution of Mercury in Modern Bottom Sediments of the Beaufort Sea in Relation to the Processes of Early Diagenesis: Microbiological Aspect. Mar. Pollut. Bull. 2024, 202, 116300. [Google Scholar] [CrossRef]
- Belesov, A.V.; Rezviy, T.V.; Pokryshkin, S.A.; Chukhchin, D.G.; Kozhevnikov, A.Y. New Insights into the Role of Sediments in Microplastic Inputs from the Northern Dvina River (Russia) to the White and Barents Seas. Mar. Pollut. Bull. 2024, 202, 116310. [Google Scholar] [CrossRef]
- Mor, S.; Ravindra, K. Municipal Solid Waste Landfills in Lower- and Middle-Income Countries: Environmental Impacts, Challenges and Sustainable Management Practices. Process Saf. Environ. Prot. 2023, 174, 510–530. [Google Scholar] [CrossRef]
- Gholizadeh, M.; Shadi, A.; Abadi, A.; Nemati, M.; Senapathi, V.; Karthikeyan, S.; Kulandaisamy, P. Exploring the Microplastic Pollution: Unveiling Origins and Varieties in Coastal Sediments and Waters of the Bushehr Province, Persian Gulf, Iran. Mar. Pollut. Bull. 2024, 198, 115939. [Google Scholar] [CrossRef]
- Uddin, S.; Fowler, S.W.; Uddin, M.F.; Behbehani, M.; Naji, A. A Review of Microplastic Distribution in Sediment Profiles. Mar. Pollut. Bull. 2021, 163, 111973. [Google Scholar] [CrossRef] [PubMed]
- Chubarenko, I.; Esiukova, E.; Zobkov, M.; Isachenko, I. Microplastics Distribution in Bottom Sediments of the Baltic Sea Proper. Mar. Pollut. Bull. 2022, 179, 113743. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Hossain, K.B.; Wei, H.; Chen, J.; Jia, R.; Gao, X.; Jin, H.; Cai, M. Fate and Mass Budget of Microplastic in the Beibu Gulf, the Northern South China Sea. Mar. Environ. Res. 2024, 202, 106797. [Google Scholar] [CrossRef] [PubMed]
- Uddin, S.; Fowler, S.W.; Behbehani, M. An Assessment of Microplastic Inputs into the Aquatic Environment from Wastewater Streams. Mar. Pollut. Bull. 2020, 160, 111538. [Google Scholar] [CrossRef]
- Sambandam, M.; Dhineka, K.; Sivadas, S.K.; Kaviarasan, T.; Begum, M.; Hoehn, D.; Sivyer, D.; Mishra, P.; Murthy, M.V.R. Occurrence, Characterization, and Source Delineation of Microplastics in the Coastal Waters and Shelf Sediments of the Central East Coast of India, Bay of Bengal. Chemosphere 2022, 303, 135135. [Google Scholar] [CrossRef]
- Duis, K.; Coors, A. Microplastics in the Aquatic and Terrestrial Environment: Sources (with a Specific Focus on Personal Care Products), Fate and Effects. Environ. Sci. Eur. 2016, 28, 2. [Google Scholar] [CrossRef]
- Chaudhry, A.K.; Sachdeva, P. Microplastics’ Origin, Distribution, and Rising Hazard to Aquatic Organisms and Human Health: Socio-Economic Insinuations and Management Solutions. Reg. Stud. Mar. Sci. 2021, 48, 102018. [Google Scholar] [CrossRef]
- Soriano, Y.; Gimeno-García, E.; Campo, J.; Hernández-Crespo, C.; Andreu, V.; Picó, Y. Exploring Organic and Inorganic Contaminant Histories in Sediment Cores across the Anthropocene: Accounting for Site/Area Dependent Factors. J. Hazard. Mater. 2024, 470, 134168. [Google Scholar] [CrossRef] [PubMed]
- Nantaba, F.; Wasswa, J.; Kylin, H.; Bouwman, H.; Palm, W.U.; Kümmerer, K. Spatial Trends and Ecotoxic Risk Assessment of Selected Pharmaceuticals in Sediments from Lake Victoria, Uganda, East Africa. Sci. Total Environ. 2024, 906, 167348. [Google Scholar] [CrossRef]
- Pires, P.; Pereira, A.M.P.T.; Pena, A.; Silva, L.J.G. Non-Steroidal Anti-Inflammatory Drugs in the Aquatic Environment and Bivalves: The State of the Art. Toxics 2024, 12, 415. [Google Scholar] [CrossRef]
- Salahinejad, A.; Meuthen, D.; Attaran, A.; Chivers, D.P.; Ferrari, M.C.O. Effects of Common Antiepileptic Drugs on Teleost Fishes. Sci. Total Environ. 2023, 866, 161324. [Google Scholar] [CrossRef]
- Kucharski, D.; Nałęcz-Jawecki, G.; Drzewicz, P.; Skowronek, A.; Mianowicz, K.; Strzelecka, A.; Giebułtowicz, J. The Assessment of Environmental Risk Related to the Occurrence of Pharmaceuticals in Bottom Sediments of the Odra River Estuary (SW Baltic Sea). Sci. Total Environ. 2022, 828, 154446. [Google Scholar] [CrossRef]
- Kondor, A.C.; Molnár, É.; Jakab, G.; Vancsik, A.; Filep, T.; Szeberényi, J.; Szabó, L.; Maász, G.; Pirger, Z.; Weiperth, A.; et al. Pharmaceuticals in Water and Sediment of Small Streams under the Pressure of Urbanization: Concentrations, Interactions, and Risks. Sci. Total Environ. 2022, 808, 152160. [Google Scholar] [CrossRef]
- Mac Loughlin, T.M.; Peluso, M.L.; Marino, D.J.G. Multiple Pesticides Occurrence, Fate, and Environmental Risk Assessment in a Small Horticultural Stream of Argentina. Sci. Total Environ. 2022, 802, 149893. [Google Scholar] [CrossRef]
- San Juan, M.R.F.; Lavarías, S.M.L.; Aparicio, V.; Larsen, K.E.; Lerner, J.E.C.; Cortelezzi, A. Ecological Risk Assessment of Pesticides in Sediments of Pampean Streams, Argentina. Chemosphere 2023, 313, 137598. [Google Scholar] [CrossRef]
- Bensadi, L.; Azzoug, M.; Benslimane, A.; Benlaribi, R.; Bouledouar, S.; Merzeg, F.A. Distribution, Levels, Sources and Risk Assessment of Polycyclic Aromatic Hydrocarbons in the Bottom Sediments of a Mediterranean River under Multiple Anthropopressures (Soummam River), Algeria. Mar. Pollut. Bull. 2024, 202, 116416. [Google Scholar] [CrossRef] [PubMed]
- Koltovskaya, E.V.; Nemirovskaya, I.A. Concentration and Composition of Polycyclic Aromatic Hydrocarbons in Bottom Sediments of the Barents and Norwegian Seas. Oceanology 2023, 63, S143–S154. [Google Scholar] [CrossRef]
- Skic, K.; Boguta, P.; Klimkowicz-Pawlas, A.; Ukalska-Jaruga, A.; Baran, A. Effect of Sorption Properties on the Content, Ecotoxicity, and Bioaccumulation of Polycyclic Aromatic Hydrocarbons (PAHs) in Bottom Sediments. J. Hazard. Mater. 2023, 442, 130073. [Google Scholar] [CrossRef]
- Baran, A.; Klimkowicz-Pawlas, A.; Ukalska-Jaruga, A.; Mierzwa-Hersztek, M.; Gondek, K.; Szara-Bąk, M.; Tarnawski, M.; Spałek, I. Distribution of Polycyclic Aromatic Hydrocarbons (PAHs) in the Bottom Sediments of a Dam Reservoir, Their Interaction with Organic Matter and Risk to Benthic Fauna. J. Soils Sediments 2021, 21, 2418–2431. [Google Scholar] [CrossRef]
- Semenov, M.Y.; Marinaite, I.I.; Silaev, A.V.; Begunova, L.A. Composition, Concentration and Origin of Polycyclic Aromatic Hydrocarbons in Waters and Bottom Sediments of Lake Baikal and Its Tributaries. Water 2023, 15, 2324. [Google Scholar] [CrossRef]
- Qiu, X.; Pu, M.; Zhang, H.; Xu, B.; Wang, J.; Xuan, R. Occurrence, Distribution, and Correlation of Antibiotics in the Aquatic Ecosystem of Poyang Lake Basin, China. J. Hazard. Mater. 2024, 479, 135656. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Z.; Zhan, X.; Zhang, T. Summary of Experiments and Influencing Factors of Sediment Settling Velocity in Still Water. Water 2024, 16, 938. [Google Scholar] [CrossRef]
- Deng, Z.; Huang, D.; He, Q.; Chassagne, C. Review of the Action of Organic Matter on Mineral Sediment Flocculation. Front. Earth Sci. 2022, 10, 965919. [Google Scholar] [CrossRef]
- Saha, R.; Dutta, S.M. Pesticides’ Mode of Action on Aquatic Life. Toxicol. Rep. 2024, 13, 101780. [Google Scholar] [CrossRef]
- Wang, P.; Shen, X.; Qiu, S.; Zhang, L.; Ma, Y.; Liang, J. Clay-Based Materials for Heavy Metals Adsorption: Mechanisms, Advancements, and Future Prospects in Environmental Remediation. Crystals 2024, 14, 1046. [Google Scholar] [CrossRef]
- Rodrigues, P.; Oliva-Teles, L.; Guimarães, L.; Carvalho, A.P. Occurrence of Pharmaceutical and Pesticide Transformation Products in Freshwater: Update on Environmental Levels, Toxicological Information and Future Challenges. Rev. Environ. Contam. Toxicol. 2022, 260, 14. [Google Scholar] [CrossRef]
- Li, Q.; Bu, Q.; Bai, Z.; Wu, X.; Yu, G.; Cao, H.; Yang, L.; Tang, J. The Microbial Oxidation of Pharmaceuticals in an Anaerobic Aqueous Environment: Effect of Dissolved Organic Matter Fractions from Different Sources. Sci. Total Environ. 2023, 899, 165682. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Yu, P.; Zhang, J.; Guo, Z.; Li, Y.; Wang, S.; Hu, Z. Biotic/Abiotic Transformation Mechanisms of Phenanthrene in Iron-Rich Constructed Wetland under Redox Fluctuation. Water Res. 2024, 261, 122033. [Google Scholar] [CrossRef]
- Barbieri, M.V.; Chiron, S. Relevance of Photocatalytic Redox Transformations of Selected Pharmaceuticals in a Copper-and Iron-Rich Mediterranean Intermittent River. Chemosphere 2023, 339, 139762. [Google Scholar] [CrossRef]
- Jia, H.; Zhao, S.; Shi, Y.; Zhu, L.; Wang, C.; Sharma, V.K. Transformation of Polycyclic Aromatic Hydrocarbons and Formation of Environmentally Persistent Free Radicals on Modified Montmorillonite: The Role of Surface Metal Ions and Polycyclic Aromatic Hydrocarbon Molecular Properties. Environ. Sci. Technol. 2018, 52, 5725–5733. [Google Scholar] [CrossRef]
- Wang, A.; Dang, Z.; Wang, Y.; Fan, H.; Miao, S. Efficient Inorganic Stabilization Materials for Chromium and Arsenic Pollution in Water and Soil. Appl. Sci. 2025, 15, 7069. [Google Scholar] [CrossRef]
- Senze, M.; Kowalska-Góralska, M.; Czyż, K.; Wondołowska-Grabowska, A. Release of Selected Metals (Al, Cd, Cu, Mn, Ni, Fe, Zn) from River Bottom Sediments: An Experimental Study. Limnol. Rev. 2023, 23, 50–69. [Google Scholar] [CrossRef]
- Chen, L.; Wang, D.; Sun, T.; Fan, T.; Wu, S.; Fang, G.; Yang, M.; Zhou, D. Quantification of the Redox Properties of Microplastics and Their Effect on Arsenite Oxidation. Fundam. Res. 2023, 3, 777–785. [Google Scholar] [CrossRef]
- Shi, X.; Chen, Z.; Liu, X.; Wei, W.; Ni, B.-J. The Photochemical Behaviors of Microplastics through the Lens of Reactive Oxygen Species: Photolysis Mechanisms and Enhancing Photo-Transformation of Pollutants. Sci. Total Environ. 2022, 846, 157498. [Google Scholar] [CrossRef]
- Zhu, K.; Jia, H.; Sun, Y.; Dai, Y.; Zhang, C.; Guo, X.; Wang, T.; Zhu, L. Long-Term Phototransformation of Microplastics under Simulated Sunlight Irradiation in Aquatic Environments: Roles of Reactive Oxygen Species. Water Res. 2020, 173, 115564. [Google Scholar] [CrossRef]
- Wang, S.; Jin, X.; Bu, Q.; Jiao, L.; Wu, F. Effects of Dissolved Oxygen Supply Level on Phosphorus Release from Lake Sediments. Colloids Surfaces A Physicochem. Eng. Asp. 2008, 316, 245–252. [Google Scholar] [CrossRef]
- Bao, Y.; Bolan, N.S.; Lai, J.; Wang, Y.; Jin, X.; Kirkham, M.B.; Wu, X.; Fang, Z.; Zhang, Y.; Wang, H. Interactions between Organic Matter and Fe (Hydr) Oxides and Their Influences on Immobilization and Remobilization of Metal (Loid) s: A Review. Crit. Rev. Environ. Sci. Technol. 2022, 52, 4016–4037. [Google Scholar] [CrossRef]
- Jin, X.; He, Y.; Kirumba, G.; Hassan, Y.; Li, J. Phosphorus Fractions and Phosphate Sorption-Release Characteristics of the Sediment in the Yangtze River Estuary Reservoir. Ecol. Eng. 2013, 55, 62–66. [Google Scholar] [CrossRef]
- Murphy, A.E.; Bulseco, A.N.; Ackerman, R.; Vineis, J.H.; Bowen, J.L. Sulphide Addition Favours Respiratory Ammonification (DNRA) over Complete Denitrification and Alters the Active Microbial Community in Salt Marsh Sediments. Environ. Microbiol. 2020, 22, 2124–2139. [Google Scholar] [CrossRef]
- Suter, E.A.; Pachiadaki, M.G.; Montes, E.; Edgcomb, V.P.; Scranton, M.I.; Taylor, C.D.; Taylor, G.T. Diverse Nitrogen Cycling Pathways across a Marine Oxygen Gradient Indicate Nitrogen Loss Coupled to Chemoautotrophic Activity. Environ. Microbiol. 2021, 23, 2747–2764. [Google Scholar] [CrossRef]
- Zhou, L.; Zhao, B.; Zhuang, W.-Q. Double-Edged Sword Effects of Dissimilatory Nitrate Reduction to Ammonium (DNRA) Bacteria on Anammox Bacteria Performance in an MBR Reactor. Water Res. 2023, 233, 119754. [Google Scholar] [CrossRef]
- Zhang, H.-B.; Wang, H.-F.; Liu, J.-B.; Bi, Z.; Jin, R.-F.; Tian, T. Anaerobic Ammonium Oxidation Coupled to Iron (III) Reduction Catalyzed by a Lithoautotrophic Nitrate-Reducing Iron (II) Oxidizing Enrichment Culture. ISME J. 2024, 18, wrae149. [Google Scholar] [CrossRef]
- Devi, U.; Bhattacharyya, K.G. Mobility and Bioavailability of Cd, Co, Cr, Cu, Mn and Zn in Surface Runoff Sediments in the Urban Catchment Area of Guwahati, India. Appl. Water Sci. 2018, 8, 18. [Google Scholar] [CrossRef]
- Tu, C.; Jin, Z.; Che, F.; Cao, X.; Song, X.; Lu, C.; Huang, W. Characterization of Phosphorus Sorption and Microbial Community in Lake Sediments during Overwinter and Recruitment Periods of Cyanobacteria. Chemosphere 2022, 307, 135777. [Google Scholar] [CrossRef]
- Yu, Y.; Mo, W.Y.; Luukkonen, T. Adsorption Behaviour and Interaction of Organic Micropollutants with Nano and Microplastics–a Review. Sci. Total Environ. 2021, 797, 149140. [Google Scholar] [CrossRef] [PubMed]
- Alimi, O.S.; Farner Budarz, J.; Hernandez, L.M.; Tufenkji, N. Microplastics and Nanoplastics in Aquatic Environments: Aggregation, Deposition, and Enhanced Contaminant Transport. Environ. Sci. Technol. 2018, 52, 1704–1724. [Google Scholar] [CrossRef]
- Kah, M.; Oliver, D.; Kookana, R. Sequestration and Potential Release of PFAS from Spent Engineered Sorbents. Sci. Total Environ. 2021, 765, 142770. [Google Scholar] [CrossRef]
- Zhao, N.; Ju, F.; Pan, H.; Tang, Z.; Ling, H. Molecular Dynamics Simulation of the Interaction of Water and Humic Acid in the Adsorption of Polycyclic Aromatic Hydrocarbons. Environ. Sci. Pollut. Res. 2020, 27, 25754–25765. [Google Scholar] [CrossRef]
- Ukalska-Jaruga, A.; Smreczak, B. The Impact of Organic Matter on Polycyclic Aromatic Hydrocarbon (PAH) Availability and Persistence in Soils. Molecules 2020, 25, 2470. [Google Scholar] [CrossRef]
- Li, W.; Xu, S.; Chen, X.; Han, D.; Mu, B. Influencing Factors and Nutrient Release from Sediments in the Water Level Fluctuation Zone of Biliuhe Reservoir, a Drinking Water Reservoir. Water 2023, 15, 3659. [Google Scholar] [CrossRef]
- Bento, B.; Hintelmann, H. Assessment of Mercury Methylation and Methylmercury Demethylation Potentials in Water and Sediments along the Wabigoon River System. Sci. Total Environ. 2024, 951, 175658. [Google Scholar] [CrossRef]
- Bacha, A.-U.-R.; Nabi, I.; Zhang, L. Mechanisms and the Engineering Approaches for the Degradation of Microplastics. Acs Es&T Eng. 2021, 1, 1481–1501. [Google Scholar]
- Xie, L.; Zhu, K.; Chen, N.; Deng, Y.; Jiang, W.; Jia, H. A Critical Review of an Environmental Risk Substance Induced by Aging Microplastics: Insights into Environmentally Persistent Free Radicals. Environ. Sci. Technol. 2024, 58, 22502–22518. [Google Scholar] [CrossRef]
- Di, X.; Sun, T.; Hu, M.; Wang, D.; Zhang, H. Significant Microplastic Accumulation and Burial in the Intertidal Sedimentary Environments of the Yellow River Delta. J. Hazard. Mater. 2025, 487, 137134. [Google Scholar] [CrossRef]
- Hong, Y.; Liao, D.; Chen, J.; Khan, S.; Su, J.; Li, H. A Comprehensive Study of the Impact of Polycyclic Aromatic Hydrocarbons (PAHs) Contamination on Salt Marsh Plants Spartina Alterniflora: Implication for Plant-Microbe Interactions in Phytoremediation. Environ. Sci. Pollut. Res. 2015, 22, 7071–7081. [Google Scholar] [CrossRef]
- Zhu, L.; Xu, G.; Zhang, Q.; Wang, G.; Wang, W.; Wang, Q. Semirational Design Strategy to Enhance the Thermostability and Catalytic Activity of Cytochrome P450 105D7 for the Degradation of the Pharmaceutically Active Compounds: Diclofenac. Environ. Sci. Technol. 2024, 58, 15681–15690. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Reible, D.D. PAH Degradation and Redox Control in an Electrode Enhanced Sediment Cap. J. Chem. Technol. Biotechnol. 2012, 87, 1222–1228. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, P.; Bhatt, K.; Huang, Y.; Lin, Z.; Chen, S. Esterase Is a Powerful Tool for the Biodegradation of Pyrethroid Insecticides. Chemosphere 2020, 244, 125507. [Google Scholar] [CrossRef] [PubMed]
- Miglani, R.; Parveen, N.; Kumar, A.; Ansari, M.A.; Khanna, S.; Rawat, G.; Panda, A.K.; Bisht, S.S.; Upadhyay, J.; Ansari, M.N. Degradation of Xenobiotic Pollutants: An Environmentally Sustainable Approach. Metabolites 2022, 12, 818. [Google Scholar] [CrossRef]
- Agarwal, V.; Miles, Z.D.; Winter, J.M.; Eustáquio, A.S.; El Gamal, A.A.; Moore, B.S. Enzymatic Halogenation and Dehalogenation Reactions: Pervasive and Mechanistically Diverse. Chem. Rev. 2017, 117, 5619–5674. [Google Scholar] [CrossRef]
- Gieg, L.M.; Fowler, S.J.; Berdugo-Clavijo, C. Syntrophic Biodegradation of Hydrocarbon Contaminants. Curr. Opin. Biotechnol. 2014, 27, 21–29. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Y.; He, X.; Lu, Q. Degradation or Humification: Rethinking Strategies to Attenuate Organic Pollutants. Trends Biotechnol. 2022, 40, 1061–1072. [Google Scholar] [CrossRef]
- Zhang, T.; Jiang, B.; Xing, Y.; Ya, H.; Lv, M.; Wang, X. Current Status of Microplastics Pollution in the Aquatic Environment, Interaction with Other Pollutants, and Effects on Aquatic Organisms. Environ. Sci. Pollut. Res. 2022, 29, 16830–16859. [Google Scholar] [CrossRef]
- Atugoda, T.; Vithanage, M.; Wijesekara, H.; Bolan, N.; Sarmah, A.K.; Bank, M.S.; You, S.; Ok, Y.S. Interactions between Microplastics, Pharmaceuticals and Personal Care Products: Implications for Vector Transport. Environ. Int. 2021, 149, 106367. [Google Scholar] [CrossRef]
- Goveas, L.C. Interactions between Polyaromatic Hydrocarbons and Microplastics: Environmental Mechanisms and Ecotoxicological Impacts. Environ. Geochem. Health 2025, 47, 320. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Q.; Sheng, Y. Nutrients in Overlying Water Affect the Environmental Behavior of Heavy Metals in Coastal Sediments. Environ. Res. 2023, 238, 117135. [Google Scholar] [CrossRef]
- Ye, F.; Duan, L.; Sun, Y.; Yang, F.; Liu, R.; Gao, F.; Wang, Y.; Xu, Y. Nitrogen Removal in Freshwater Sediments of Riparian Zone: N-Loss Pathways and Environmental Controls. Front. Microbiol. 2023, 14, 1239055. [Google Scholar] [CrossRef]
- Zhou, C.; Gao, Y.; Gaulier, C.; Luo, M.; Zhang, X.; Bratkic, A.; Davison, W.; Baeyens, W. Advances in Understanding Mobilization Processes of Trace Metals in Marine Sediments. Environ. Sci. Technol. 2020, 54, 15151–15161. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yang, Y.; Wu, N.; Gao, M.; Tan, Y. Combined Toxicity of Pyrethroid Insecticides and Heavy Metals: A Review. Environ. Chem. Lett. 2019, 17, 1693–1706. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J. Multivalent Metal Catalysts in Fenton/Fenton-like Oxidation System: A Critical Review. Chem. Eng. J. 2023, 466, 143147. [Google Scholar] [CrossRef]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef]
- Hu, X.; Tlili, A.; Schirmer, K.; Bao, M.; Bürgmann, H. Metal Concentration in Freshwater Sediments Is Linked to Microbial Biodiversity and Community Composition. Environ. Int. 2025, 199, 109465. [Google Scholar] [CrossRef]
- Cheng, W.; Kalahroodi, E.L.; Marsac, R.; Hanna, K. Adsorption of Quinolone Antibiotics to Goethite under Seawater Conditions: Application of a Surface Complexation Model. Environ. Sci. Technol. 2018, 53, 1130–1138. [Google Scholar] [CrossRef]
- Primost, M.A.; Chierichetti, M.A.; Castaños, C.; Bigatti, G.; Miglioranza, K.S.B. Persistent Organic Pollutants (POPs), Current Use Pesticides (CUPs) and Polycyclic Aromatic Hydrocarbons (PAHs) in Edible Marine Invertebrates from a Patagonian Harbor. Mar. Pollut. Bull. 2024, 207, 116940. [Google Scholar] [CrossRef]
- Impellitteri, F.; Multisanti, C.R.; Rusanova, P.; Piccione, G.; Falco, F.; Faggio, C. Exploring the Impact of Contaminants of Emerging Concern on Fish and Invertebrates Physiology in the Mediterranean Sea. Biology 2023, 12, 767. [Google Scholar] [CrossRef]
- Lushchak, V.I. Environmentally Induced Oxidative Stress in Fish. In Oxidative Stress in Aquatic Ecosystems; John Wiley & Sons: Hoboken, NJ, USA, 2011; Volume 101, pp. 295–307. [Google Scholar] [CrossRef]
- Franco, R.; Sánchez-Olea, R.; Reyes-Reyes, E.M.; Panayiotidis, M.I. Environmental Toxicity, Oxidative Stress and Apoptosis: Menage a Trois. Mutat. Res. Toxicol. Environ. Mutagen. 2009, 674, 3–22. [Google Scholar] [CrossRef]
- Mdaini, Z.; Telahigue, K.; Hajji, T.; Rabeh, I.; Pharand, P.; El Cafsi, M.; Tremblay, R.; Gagné, J.P. Bioaccumulation of Polycyclic Aromatic Hydrocarbons (PAH) in Polychaeta Marphysa Sanguinea in the Anthropogenically Impacted Tunis Lagoon: DNA Damage and Immune Biomarkers. Mar. Pollut. Bull. 2022, 184, 114104. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.; Costa, P.M.; Ferreira, A.M.; Costa, M.H. Comparative DNA Damage and Oxidative Effects of Carcinogenic and Non-Carcinogenic Sediment-Bound PAHs in the Gills of a Bivalve. Aquat. Toxicol. 2013, 142–143, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Trestrail, C.; Walpitagama, M.; Miranda, A.; Nugegoda, D.; Shimeta, J. Microplastics Alter Digestive Enzyme Activities in the Marine Bivalve, Mytilus galloprovincialis. Sci. Total Environ. 2021, 779, 146418. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yu, P.; Cai, M.; Wu, D.; Zhang, M.; Chen, M.; Zhao, Y. Effects of Microplastics on the Innate Immunity and Intestinal Microflora of Juvenile Eriocheir Sinensis. Sci. Total Environ. 2019, 685, 836–846. [Google Scholar] [CrossRef]
- Gedik, K.; Eryaşar, A.R. Microplastic Pollution Profile of Mediterranean Mussels (Mytilus galloprovincialis) Collected along the Turkish Coasts. Chemosphere 2020, 260, 127570. [Google Scholar] [CrossRef]
- Sharma, S.; Bhardwaj, A.; Thakur, M.; Saini, A. Understanding Microplastic Pollution of Marine Ecosystem: A Review. Environ. Sci. Pollut. Res. 2024, 31, 41402–41445. [Google Scholar] [CrossRef]
- Guo, J.; Ren, J.; Chang, C.; Duan, Q.; Li, J.; Kanerva, M.; Yang, F.; Mo, J. Freshwater Crustacean Exposed to Active Pharmaceutical Ingredients: Ecotoxicological Effects and Mechanisms. Environ. Sci. Pollut. Res. 2023, 30, 48868–48902. [Google Scholar] [CrossRef]
- Deidda, I.; Russo, R.; Bonaventura, R.; Costa, C.; Zito, F.; Lampiasi, N. Neurotoxicity in Marine Invertebrates: An Update. Biology 2021, 10, 161. [Google Scholar] [CrossRef]
- Banerjee, P.; Garai, P.; Saha, N.C.; Saha, S.; Sharma, P.; Maiti, A.K. A Critical Review on the Effect of Nitrate Pollution in Aquatic Invertebrates and Fish. Water. Air Soil Pollut. 2023, 234, 333. [Google Scholar] [CrossRef]
- Bian, D.D.; Shi, Y.X.; Zhang, X.; Liu, X.; Jiang, J.J.; Zhu, X.R.; Zhang, D.Z.; Liu, Q.N.; Zhu, B.J.; Tang, B.P. Nitrite Toxicity in Shrimp Aquaculture: Mechanisms, Health Impacts, and Sustainable Mitigation Strategies. Rev. Aquac. 2025, 17, e70062. [Google Scholar] [CrossRef]
- Xiao, H.; Peng, S.; Liu, X.; Jia, J.; Wang, H. Phytoremediation of Nutrients and Organic Carbon from Contaminated Water by Aquatic Macrophytes and the Physiological Response. Environ. Technol. Innov. 2021, 21, 101295. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, K.; Lv, J.; Peng, X.; Tang, Y.; Zhao, L.; Cheng, Y.; Liu, Q. Effects of Nitrogen and Phosphorus in Sediment on the Occurrence of Cladophora sp. (Cladophoraceae) in Aquaculture Ponds. Biology 2024, 13, 739. [Google Scholar] [CrossRef]
- Chang, W.; Zhu, X.; Sun, J.; Pang, Y.; Zhang, S. Effects of Lead Pollution on Bacterial Communities in Biofilm Attached to Submerged Plants. Water Sci. Technol. 2022, 86, 1358–1372. [Google Scholar] [CrossRef]
- Suzhen, H.; Xuhui, H.; Hongkuan, C.; Qixuan, S.; Xingzhang, L.; Zheng, Z. Role of Phosphorus in Vallisneria Natans and Biofilm Exposure to Pb2+ and Cd2+ Stress. Sci. Total Environ. 2022, 835, 155235. [Google Scholar] [CrossRef]
- Lin, L.; Xu, E.G.; Liu, M.; Yang, Y.; Zhou, A.; Suyamud, B.; Pan, X.; Yuan, W. Microbiological Processes of Submicrometer Plastics Affecting Submerged Plant Growth in a Chronic Exposure Microcosm. Environ. Sci. Technol. Lett. 2023, 10, 33–39. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, D.; Deng, H.; Zhang, J. Responses of Submerged Plant Vallisneria Natans Growth and Leaf Biofilms to Water Contaminated with Microplastics. Sci. Total Environ. 2022, 818, 151750. [Google Scholar] [CrossRef]
- Rathnayake, R.; Amarathunga, A.A.D.; De Silva, D.S.M.; McGoran, A.R.; Bakir, A.; Sivyer, D.B.; Reeve, C.; Narangoda, S. Comprehensive Analysis of Microplastic Abundance in Macrophytes, Macrophyte-Associated Sediments, and Water in Tropical Coastal Lagoons in Sri Lanka. Water 2025, 17, 157. [Google Scholar] [CrossRef]
- Sfriso, A.A.; Tomio, Y.; Juhmani, A.-S.; Sfriso, A.; Munari, C.; Mistri, M. Macrophytes: A Temporary Sink for Microplastics in Transitional Water Systems. Water 2021, 13, 3032. [Google Scholar] [CrossRef]
- Wang, B.; Shen, Q.; Han, C.; Zheng, Y.; Wang, Z.; Liu, C.; Zhang, L.; Ren, J. New Insights into the Growth Response of the Macrophyte Vallisneria Natans Exposed to Phosphite. Sci. Total Environ. 2022, 851, 158189. [Google Scholar] [CrossRef]
- Fan, P.; Liu, C.; Ke, Z.; Zhou, W.; Wu, Z. Growth and Physiological Responses in a Submerged Clonal Aquatic Plant and Multiple-Endpoint Assessment under Prolonged Exposure to Ciprofloxacin. Ecotoxicol. Environ. Saf. 2022, 239, 113690. [Google Scholar] [CrossRef]
- Lee, J.W.; Choi, H.; Hwang, U.K.; Kang, J.C.; Kang, Y.J.; Kim, K.I.; Kim, J.H. Toxic Effects of Lead Exposure on Bioaccumulation, Oxidative Stress, Neurotoxicity, and Immune Responses in Fish: A Review. Environ. Toxicol. Pharmacol. 2019, 68, 101–108. [Google Scholar] [CrossRef]
- Aslam, H.; Mansour, M.; Honey, S.; Ashraf, M.A.; Ullah, A.; Umar, A.; Nusrat, N.; Khan, M.U.; Sohail, J.; Hashim, M.M.; et al. Facts of the Main Rigorous Heavy Metals Affecting Waterfowls Health, Genetics, and Migration Habits. J. Toxicol. Stud. 2024, 3, 1990. [Google Scholar] [CrossRef]
- Morcillo, P.; Cordero, H.; Meseguer, J.; Esteban, M.Á.; Cuesta, A. In Vitro Immunotoxicological Effects of Heavy Metals on European Sea Bass (Dicentrarchus labrax L.) Head-Kidney Leucocytes. Fish Shellfish Immunol. 2015, 47, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Khan, B.N.; Ullah, H.; Ashfaq, Y.; Hussain, N.; Atique, U.; Aziz, T.; Alharbi, M.; Albekairi, T.H.; Alasmari, A.F. Elucidating the Effects of Heavy Metals Contamination on Vital Organ of Fish and Migratory Birds Found at Fresh Water Ecosystem. Heliyon 2023, 9, e20968. [Google Scholar] [CrossRef]
- Castaño-Ortiz, J.M.; Gil-Solsona, R.; Ospina-Álvarez, N.; Alcaraz-Hernández, J.D.; Farré, M.; León, V.M.; Barceló, D.; Santos, L.H.M.L.M.; Rodríguez-Mozaz, S. Fate of Pharmaceuticals in the Ebro River Delta Region: The Combined Evaluation of Water, Sediment, Plastic Litter, and Biomonitoring. Sci. Total Environ. 2024, 906, 167467. [Google Scholar] [CrossRef]
- Yan, S.; Chen, R.; Wang, M.; Zha, J. Carbamazepine at Environmentally Relevant Concentrations Caused DNA Damage and Apoptosis in the Liver of Chinese Rare Minnows (Gobiocypris rarus) by the Ras/Raf/ERK/P53 Signaling Pathway. Environ. Pollut. 2021, 270, 116245. [Google Scholar] [CrossRef]
- Okuthe, G.E.; Dube, E.; Mafunda, P.S. Effects of Pharmaceuticals and Endocrine-Disrupting Chemicals on Reproductive Biology of Aquatic Fauna: Penguins as Sentinel Species. J. Xenobiotics 2025, 15, 110. [Google Scholar] [CrossRef]
- Ocampos, A.I.; Guinn, M.A.; Elliott, J.; Wittmaack, C.; Sinclair, C.; Abdulla, H.; Orbach, D.N. Pharmaceuticals in the Blubber of Live Free-Swimming Common Bottlenose Dolphins (Tursiops truncatus). iScience 2024, 27, 111507. [Google Scholar] [CrossRef]
- Alava, J.J.; Calle, P.; Tirapé, A.; Biedenbach, G.; Cadena, O.A.; Maruya, K.; Lao, W.; Aguirre, W.; Jiménez, P.J.; Domínguez, G.A. Persistent Organic Pollutants and Mercury in Genetically Identified Inner Estuary Bottlenose Dolphin (Tursiops truncatus) Residents of the Guayaquil Gulf, Ecuador: Ecotoxicological Science in Support of Pollutant Management and Cetacean Conservation. Front. Mar. Sci. 2020, 7, 122. [Google Scholar] [CrossRef]
- Ahmad, M.B.; Islam, A.U.; Hassan, S.; Tarfeen, N.; Nisa, K.U.; Nisar, K.; Hassan, T.; Ganiee, S.A.; Bhat, A.R.; Ganai, B.A. Unraveling the Toxic Link between Pesticides and Brain Cancer: A Review on Molecular Mechanisms, Signaling Pathways and Future Research Trends. Nucleus 2025. [Google Scholar] [CrossRef]
- Lamichhane, G.; Acharya, A.; Marahatha, R.; Modi, B.; Paudel, R.; Adhikari, A.; Raut, B.K.; Aryal, S.; Parajuli, N. Microplastics in Environment: Global Concern, Challenges, and Controlling Measures. Int. J. Environ. Sci. Technol. 2023, 20, 4673–4694. [Google Scholar] [CrossRef] [PubMed]
- de Jersey, A.M.; Lavers, J.L.; Bond, A.L.; Wilson, R.; Zosky, G.R.; Rivers-Auty, J. Seabirds in Crisis: Plastic Ingestion Induces Proteomic Signatures of Multiorgan Failure and Neurodegeneration. Sci. Adv. 2025, 11, eads0834. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhan, F.; Yu, R.-Q.; Chen, L.; Wu, Y. Bio-Accumulation of Organic Contaminants in Indo-Pacific Humpback Dolphins: Preliminary Unique Features of the Brain and Testes. Environ. Pollut. 2020, 267, 115511. [Google Scholar] [CrossRef]
- Sombiri, S.; Balhara, N.; Attri, D.; Kharb, I.; Giri, A. An Overview on Occurrence of Polycyclic Aromatic Hydrocarbons in Food Chain with Special Emphasis on Human Health Ailments. Discov. Environ. 2024, 2, 87. [Google Scholar] [CrossRef]
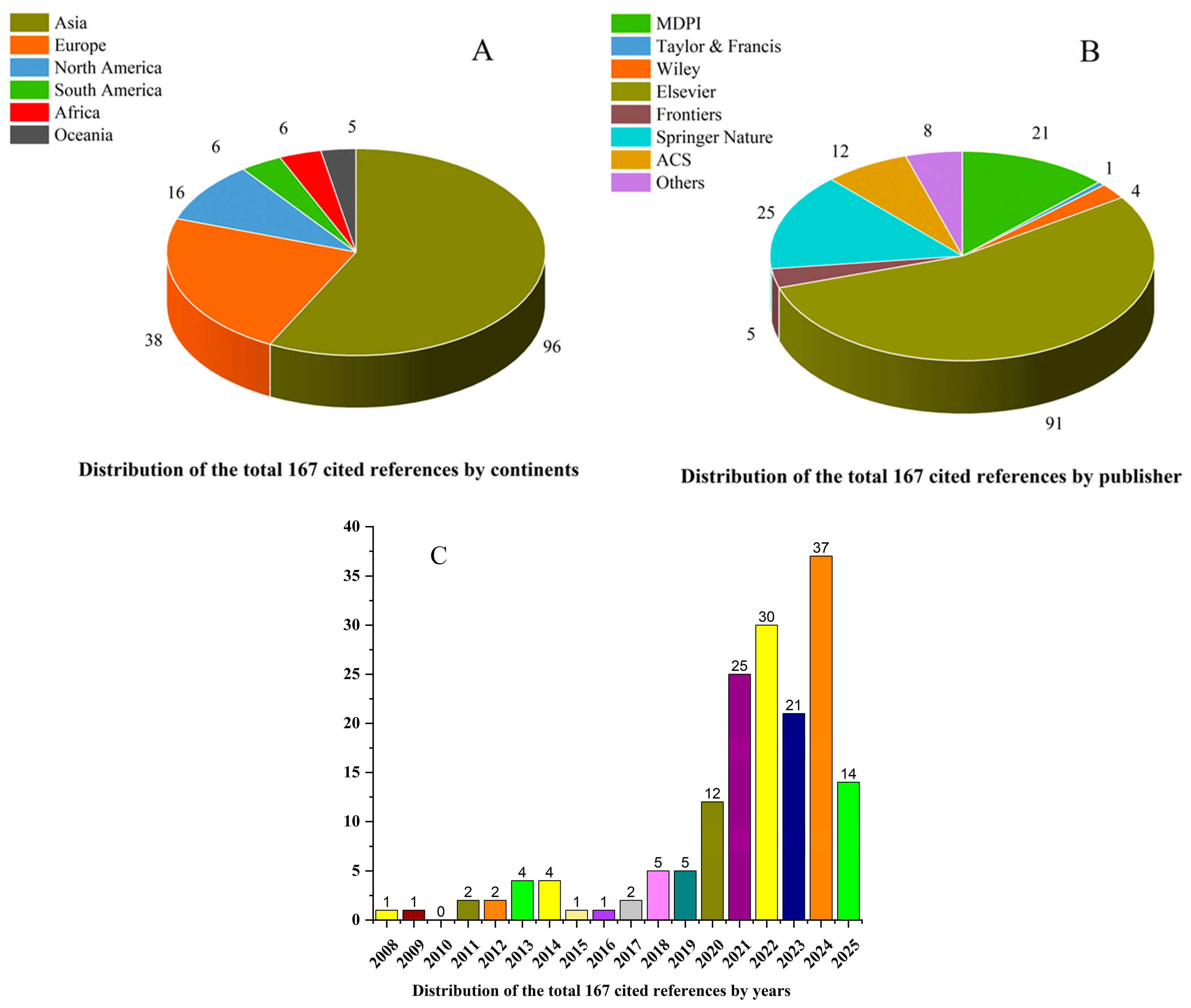

| Site and Location | Nitrogen (mg/kg) | Phosphorus (mg/kg) | Pollution Sources | References |
|---|---|---|---|---|
| Warta River and Tributaries, Poland | 71.5–16,240 | 48.8–8864 | urbanization, land use and agricultural activities | [2] |
| Odra River Estuary (SW Baltic Sea), Poland | 200–18,000 | 80–7940 | industrial effluents, fertilizers and untreated agriculture and municipal waste. | [3] |
| Ogun River, Abeokuta, Nigeria | 840–1960 | 820–2740 | wastewater and runoff from residential areas, farmlands, and slaughterhouse (abattoir) discharges | [41] |
| Marrecas Stream micro basin, Brazil | 2806–5233 | 488–1083 | domestic sewage and poultry farm waste | [36] |
| Lake Chaohu, China | 666 | 509 | Sewage, fertilizer runoff | [42] |
| Three Gorges Reservoir, Yangtze River, China | 622–949 | 916–950 | agricultural activities, urban development | [40] |
| Site and Location | Polymer Types | Concentration (mg/kg) | Abundance (Particles/kg) | Size (µm) | Shape/Morphology | Color | Pollution Sources | References |
|---|---|---|---|---|---|---|---|---|
| San Pedro Bay, CA, USA | PE, PP, PS, CA, Nylon | 416–1102 | 500–3000 | 1.73 | fibers, foam, fragments, film, tire wear particles | black, gray, blue, clear | textiles, personal care products, urban runoff | [1] |
| Bay of Bengal, India | PE, PP, PA, PS, PVC, PET | – | 80–480 | <500 | fibers, fragments, films, pellets | blue, yellow, white, black, red, transparent | riverine runoff, fishing gear, packaging | [61] |
| Bushehr Province (Persian Gulf), Iran | PE, PP, PS, PET, PA | – | 660–2140 | 100–5000 | fibers, fragments, pellets, films | black, white, blue, red, gray, transparent | fishing gear, industrial plastics, tourism | [56] |
| Beibu Gulf (South China Sea), China | CE, PVAL, PE, PP, PS, PET, PU | – | 13.12–155.59 | 330–5000 | fibers, fragments, pellets | white, black, transparent, blue, yellow, gray | aquaculture gear, textiles, industrial plastics | [59] |
| Northern Dvina River, Russia | PE, PP, PET, ABS, PS | 52–334 | 60–650 | <100–>300 | fibers, fragments, films | gray, transparent, black, red, blue | sewage, industrial discharge, shipyards | [54] |
| Ontario Lakes, Canada | PE, PP, PET, PA, PVC, PU | – | 6–320 | 42–>5000 | fibers, fragments, films, foams, spheres | white, transparent, black, gray, blue | atmospheric deposition, urban runoff, industrial inputs | [14] |
| Baltic Sea, Northern Europe | PE, PP, PB, PET/PES, PVC | – | 103–10,179 | 200–5000 | fibers, films, fragments | transparent, blue, brown, black, white, red, green, yellow | wastewater, maritime transport, urban runoff | [58] |
| Pollutant Group | Major Sources | Exposure Pathway (Mechanistic Fate) | Concentration Ranges (mg/kg) | Evidence Strength | Ecological/Human Relevance (Toxicological Endpoints) |
|---|---|---|---|---|---|
| Nutrients (N, P) | agricultural runoff, wastewater discharge, animal waste |
| N (71.5–18,000) and P (48.8–8864) | high |
|
| HMs (Pb, Cd, Hg, As, Cu, Zn) | industrial and mining effluents, urban runoff, agrochemical inputs |
| As (1–46), Cd (0.10–13), Pb (1–272.1), Zn (6–35,300), Hg (0.01–2.40), and Cu (1–298) | high |
|
| PhACs | WWTPs effluents, hospital and livestock waste |
| carbamazepine (0.024–0.3959), diclofenac (0.0089–0.253) and erythromycin (0.038–3.524) | medium |
|
| Pesticides | agricultural runoff, industrial discharge, urban pest control |
| atrazine (0.002–0.183), acetochlor (0.0086–4.315), 2,4-D (0.0391–0.0461) | high |
|
| PAHs | fossil fuel combustion (pyrogenic), oil spills (petrogenic) |
| 0.002–24.3217 | high |
|
| MPs | plastic-waste degradation, wastewater discharge, maritime and fisheries activities |
| 52–1102 | emerging |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maqsood, A.; Łobos-Moysa, E. Bottom Sediments as Dynamic Arenas for Anthropogenic Pollutants: Profiling Sources, Unraveling Fate Mechanisms, and Assessing Ecological Consequences. Int. J. Mol. Sci. 2025, 26, 10219. https://doi.org/10.3390/ijms262010219
Maqsood A, Łobos-Moysa E. Bottom Sediments as Dynamic Arenas for Anthropogenic Pollutants: Profiling Sources, Unraveling Fate Mechanisms, and Assessing Ecological Consequences. International Journal of Molecular Sciences. 2025; 26(20):10219. https://doi.org/10.3390/ijms262010219
Chicago/Turabian StyleMaqsood, Abdullah, and Ewa Łobos-Moysa. 2025. "Bottom Sediments as Dynamic Arenas for Anthropogenic Pollutants: Profiling Sources, Unraveling Fate Mechanisms, and Assessing Ecological Consequences" International Journal of Molecular Sciences 26, no. 20: 10219. https://doi.org/10.3390/ijms262010219
APA StyleMaqsood, A., & Łobos-Moysa, E. (2025). Bottom Sediments as Dynamic Arenas for Anthropogenic Pollutants: Profiling Sources, Unraveling Fate Mechanisms, and Assessing Ecological Consequences. International Journal of Molecular Sciences, 26(20), 10219. https://doi.org/10.3390/ijms262010219







