Iron in Vascular Calcification: Pro-Calcific Agent or Protective Modulator?
Abstract
1. Introduction
2. Cardiovascular Calcification
2.1. Types of Cardiovascular Calcification
2.2. Calcification Is an Actively Regulated Process
2.2.1. Osteochondrogenic Transcription Factors Regulate Cardiovascular Calcification
2.2.2. Calcification Inducers
2.2.3. Calcification Inhibitors
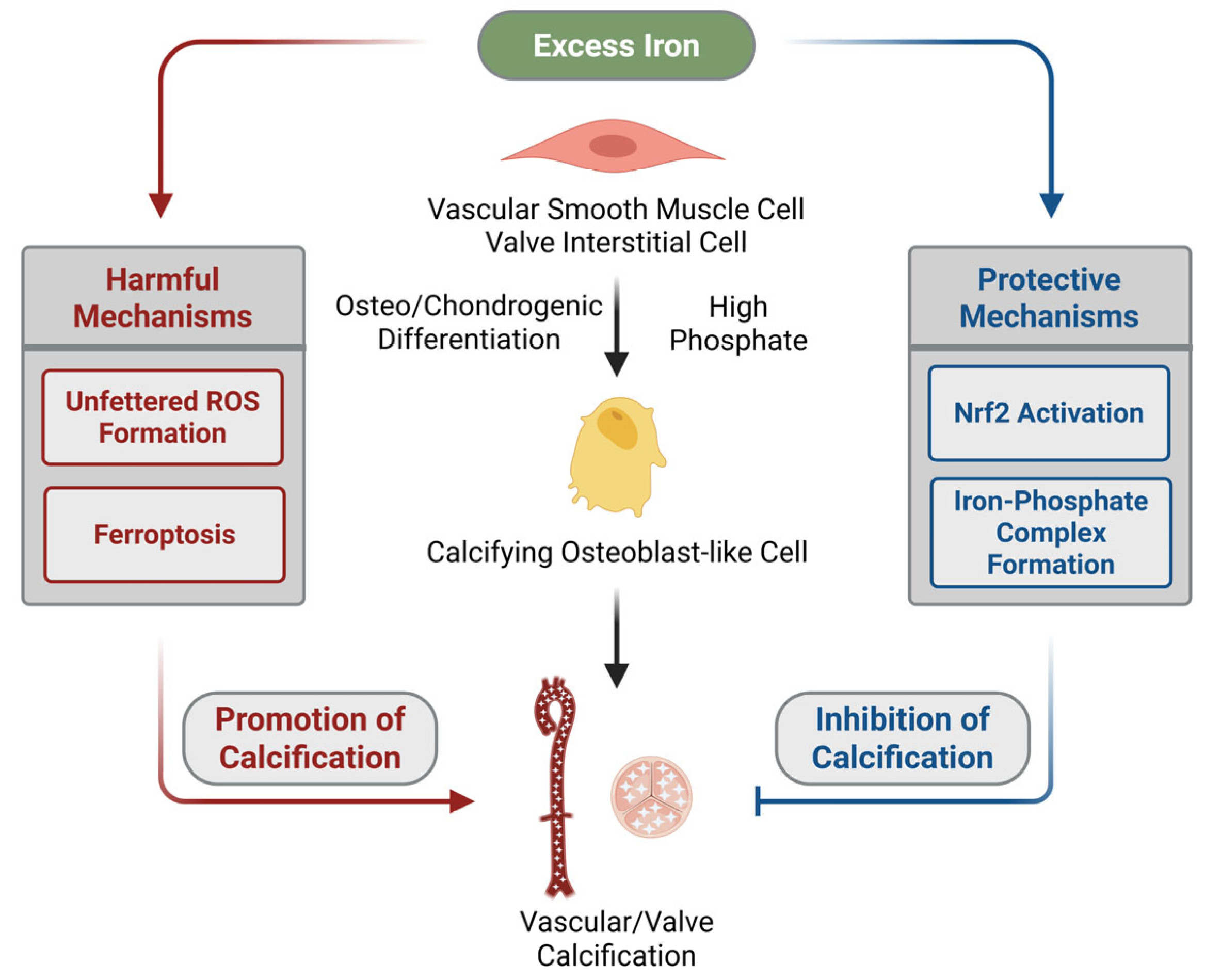
3. Iron Is a Janus-Faced Modulator of Cardiovascular Calcification
3.1. In Vitro, Ex Vivo, and In Vivo Evidence That Excess Iron Promotes Vascular Calcification
3.2. In Vitro, Ex Vivo, and In Vivo Evidence That Excess Iron Inhibits Vascular Calcification
3.3. The Potential Role of Iron in Promoting Valve Calcification
3.4. The Potential Role of Iron in Inhibiting Valve Calcification
3.5. The Role of Iron-Loaded Macrophages in Intimal Calcification
4. Key Cellular Mechanisms Underlying the Modulatory Effect of Iron on Cardiovascular Calcification
4.1. ROS Production and Activation of the Nuclear Factor Erythroid 2-Related Factor 2 (Nrf2) Antioxidant Pathway
4.2. Ferroptosis
4.3. Iron–Phosphate Complex Formation
5. Iron Dysregulation in CKD and Its Association with Vascular Calcification
6. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AGEs | Advanced Glycation End-products |
| ALP | Alkaline Phosphatase |
| BMP-2 | Bone Morphogenetic Protein 2 |
| CACS | Coronary Artery Calcification Score |
| CAVD | Calcific Aortic Valve Disease |
| CKD | Chronic Kidney Disease |
| DMF | Dimethyl Fumarate |
| FtH | Ferritin Heavy Chain |
| FtL | Ferritin Light Chain |
| GPX4 | Glutathione Peroxidase 4 |
| GSH | Glutathione |
| HAoSMCs | Human Aortic Smooth Muscle Cells |
| HIF | Hypoxia-Inducible Factor |
| HO-1 | Heme Oxygenase-1 |
| Keap1 | Kelch-like ECH-associated Protein 1 |
| MAPK | Mitogen-Activated Protein Kinase |
| MGP | Matrix Gla Protein |
| Msx2 | Msh Homeobox 2 |
| NQO1 | NAD(P)H Quinone Dehydrogenase 1 |
| Nrf2 | Nuclear Factor Erythroid 2-Related Factor 2 |
| OCN | Osteocalcin |
| OM | Osteogenic Medium |
| OPG | Osteoprotegerin |
| PA21 | Sucroferric Oxyhydroxide (iron-based phosphate binder) |
| PiT-1 | Sodium-dependent Phosphate Transporter 1 |
| RBCs | Red Blood Cells |
| ROS | Reactive Oxygen Species |
| Runx2 | Runt-related Transcription Factor 2 |
| Sirt1 | Sirtuin 1 |
| SLC7A11 | Cystine/Glutamate Antiporter (system Xc– subunit) |
| SOX9 | SRY-Box Transcription Factor 9 |
| TNF-α | Tumor Necrosis Factor Alpha |
| TSAT | Transferrin Saturation |
| VICs | Valvular Interstitial Cells |
| VSMCs | Vascular Smooth Muscle Cells |
| α-SMA | Alpha-Smooth Muscle Actin |
References
- Neven, E.; De Schutter, T.M.; Behets, G.J.; Gupta, A.; D’Haese, P.C. Iron and Vascular Calcification. Is There a Link? Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transplant. Assoc. -Eur. Ren. Assoc. 2011, 26, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Ciceri, P.; Cozzolino, M. The Emerging Role of Iron in Heart Failure and Vascular Calcification in CKD. Clin. Kidney J. 2021, 14, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Jie, W.; Huang, H. Vascular Calcification: Molecular Mechanisms and Therapeutic Interventions. MedComm 2023, 4, e200. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, O.M. Treatment of Iron Deficiency Anemia in CKD and End-Stage Kidney Disease. Kidney Int. Rep. 2021, 6, 2261–2269. [Google Scholar] [CrossRef]
- Mizuiri, S.; Nishizawa, Y.; Doi, T.; Yamashita, K.; Shigemoto, K.; Usui, K.; Arita, M.; Naito, T.; Doi, S.; Masaki, T. Iron, Coronary Artery Calcification, and Mortality in Patients Undergoing Hemodialysis. Ren. Fail. 2021, 43, 371–380. [Google Scholar] [CrossRef]
- Giachelli, C.M. Vascular Calcification Mechanisms. J. Am. Soc. Nephrol. JASN 2004, 15, 2959–2964. [Google Scholar] [CrossRef]
- Hutcheson, J.D.; Goettsch, C.; Rogers, M.A.; Aikawa, E. Revisiting Cardiovascular Calcification: A Multifaceted Disease Requiring a Multidisciplinary Approach. Semin. Cell Dev. Biol. 2015, 46, 68–77. [Google Scholar] [CrossRef]
- Rogers, M.A.; Aikawa, E. Cardiovascular Calcification: Artificial Intelligence and Big Data Accelerate Mechanistic Discovery. Nat. Rev. Cardiol. 2019, 16, 261–274. [Google Scholar] [CrossRef]
- Libby, P.; Theroux, P. Pathophysiology of Coronary Artery Disease. Circulation 2005, 111, 3481–3488. [Google Scholar] [CrossRef]
- Wilson, P.W.; D’Agostino, R.B.; Levy, D.; Belanger, A.M.; Silbershatz, H.; Kannel, W.B. Prediction of Coronary Heart Disease Using Risk Factor Categories. Circulation 1998, 97, 1837–1847. [Google Scholar] [CrossRef]
- Jinnouchi, H.; Sato, Y.; Sakamoto, A.; Cornelissen, A.; Mori, M.; Kawakami, R.; Gadhoke, N.V.; Kolodgie, F.D.; Virmani, R.; Finn, A.V. Calcium Deposition within Coronary Atherosclerotic Lesion: Implications for Plaque Stability. Atherosclerosis 2020, 306, 85–95. [Google Scholar] [CrossRef]
- Otsuka, F.; Sakakura, K.; Yahagi, K.; Joner, M.; Virmani, R. Has Our Understanding of Calcification in Human Coronary Atherosclerosis Progressed? Arterioscler. Thromb. Vasc. Biol. 2014, 34, 724–736, Correction in Arterioscler. Thromb. Vasc. Biol. 2014, 34, e17. [Google Scholar] [CrossRef]
- Mackey, R.H.; Venkitachalam, L.; Sutton-Tyrrell, K. Calcifications, Arterial Stiffness and Atherosclerosis. Adv. Cardiol. 2007, 44, 234–244. [Google Scholar] [CrossRef]
- Madhavan, M.V.; Tarigopula, M.; Mintz, G.S.; Maehara, A.; Stone, G.W.; Généreux, P. Coronary Artery Calcification: Pathogenesis and Prognostic Implications. J. Am. Coll. Cardiol. 2014, 63, 1703–1714. [Google Scholar] [CrossRef]
- Onnis, C.; Virmani, R.; Kawai, K.; Nardi, V.; Lerman, A.; Cademartiri, F.; Scicolone, R.; Boi, A.; Congiu, T.; Faa, G.; et al. Coronary Artery Calcification: Current Concepts and Clinical Implications. Circulation 2024, 149, 251–266. [Google Scholar] [CrossRef] [PubMed]
- Lanzer, P.; Boehm, M.; Sorribas, V.; Thiriet, M.; Janzen, J.; Zeller, T.; St Hilaire, C.; Shanahan, C. Medial Vascular Calcification Revisited: Review and Perspectives. Eur. Heart J. 2014, 35, 1515–1525. [Google Scholar] [CrossRef]
- Johnson, R.C.; Leopold, J.A.; Loscalzo, J. Vascular Calcification: Pathobiological Mechanisms and Clinical Implications. Circ. Res. 2006, 99, 1044–1059, Correction in Circ. Res. 2009, 105, e8. [Google Scholar] [CrossRef] [PubMed]
- Lanzer, P.; Hannan, F.M.; Lanzer, J.D.; Janzen, J.; Raggi, P.; Furniss, D.; Schuchardt, M.; Thakker, R.; Fok, P.-W.; Saez-Rodriguez, J.; et al. Medial Arterial Calcification: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 78, 1145–1165. [Google Scholar] [CrossRef]
- Shanahan, C.M.; Cary, N.R.B.; Salisbury, J.R.; Proudfoot, D.; Weissberg, P.L.; Edmonds, M.E. Medial Localization of Mineralization-Regulating Proteins in Association With Mönckeberg’s Sclerosis. Circulation 1999, 100, 2168–2176. [Google Scholar] [CrossRef]
- Dao, H.H.; Essalihi, R.; Bouvet, C.; Moreau, P. Evolution and Modulation of Age-Related Medial Elastocalcinosis: Impact on Large Artery Stiffness and Isolated Systolic Hypertension. Cardiovasc. Res. 2005, 66, 307–317. [Google Scholar] [CrossRef]
- Moncla, L.-H.M.; Briend, M.; Bossé, Y.; Mathieu, P. Calcific Aortic Valve Disease: Mechanisms, Prevention and Treatment. Nat. Rev. Cardiol. 2023, 20, 546–559. [Google Scholar] [CrossRef]
- Miller, J.D.; Weiss, R.M.; Heistad, D.D. Calcific Aortic Valve Stenosis: Methods, Models, and Mechanisms. Circ. Res. 2011, 108, 1392–1412. [Google Scholar] [CrossRef]
- Komori, T.; Yagi, H.; Nomura, S.; Yamaguchi, A.; Sasaki, K.; Deguchi, K.; Shimizu, Y.; Bronson, R.T.; Gao, Y.H.; Inada, M.; et al. Targeted Disruption of Cbfa1 Results in a Complete Lack of Bone Formation Owing to Maturational Arrest of Osteoblasts. Cell 1997, 89, 755–764. [Google Scholar] [CrossRef]
- Nakashima, K.; Zhou, X.; Kunkel, G.; Zhang, Z.; Deng, J.M.; Behringer, R.R.; de Crombrugghe, B. The Novel Zinc Finger-Containing Transcription Factor Osterix Is Required for Osteoblast Differentiation and Bone Formation. Cell 2002, 108, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Bi, W.; Deng, J.M.; Zhang, Z.; Behringer, R.R.; de Crombrugghe, B. Sox9 Is Required for Cartilage Formation. Nat. Genet. 1999, 22, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Wilkie, A.O.; Tang, Z.; Elanko, N.; Walsh, S.; Twigg, S.R.; Hurst, J.A.; Wall, S.A.; Chrzanowska, K.H.; Maxson, R.E. Functional Haploinsufficiency of the Human Homeobox Gene MSX2 Causes Defects in Skull Ossification. Nat. Genet. 2000, 24, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Tyson, K.L.; Reynolds, J.L.; McNair, R.; Zhang, Q.; Weissberg, P.L.; Shanahan, C.M. Osteo/Chondrocytic Transcription Factors and Their Target Genes Exhibit Distinct Patterns of Expression in Human Arterial Calcification. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 489–494. [Google Scholar] [CrossRef]
- Speer, M.Y.; Li, X.; Hiremath, P.G.; Giachelli, C.M. Runx2/Cbfa1, but Not Loss of Myocardin, Is Required for Smooth Muscle Cell Lineage Reprogramming toward Osteochondrogenesis. J. Cell. Biochem. 2010, 110, 935–947. [Google Scholar] [CrossRef]
- Jono, S.; McKee, M.D.; Murry, C.E.; Shioi, A.; Nishizawa, Y.; Mori, K.; Morii, H.; Giachelli, C.M. Phosphate Regulation of Vascular Smooth Muscle Cell Calcification. Circ. Res. 2000, 87, E10–E17. [Google Scholar] [CrossRef]
- Yang, H.; Curinga, G.; Giachelli, C.M. Elevated Extracellular Calcium Levels Induce Smooth Muscle Cell Matrix Mineralization in Vitro1. Kidney Int. 2004, 66, 2293–2299. [Google Scholar] [CrossRef]
- Muteliefu, G.; Enomoto, A.; Jiang, P.; Takahashi, M.; Niwa, T. Indoxyl Sulphate Induces Oxidative Stress and the Expression of Osteoblast-Specific Proteins in Vascular Smooth Muscle Cells. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transplant. Assoc. -Eur. Ren. Assoc. 2009, 24, 2051–2058. [Google Scholar] [CrossRef]
- Stabley, J.N.; Towler, D.A. Arterial Calcification in Diabetes Mellitus: Preclinical Models and Translational Implications. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 205–217. [Google Scholar] [CrossRef]
- Chen, N.X.; Duan, D.; O’Neill, K.D.; Moe, S.M. High Glucose Increases the Expression of Cbfa1 and BMP-2 and Enhances the Calcification of Vascular Smooth Muscle Cells. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transplant. Assoc. -Eur. Ren. Assoc. 2006, 21, 3435–3442. [Google Scholar] [CrossRef]
- Tanikawa, T.; Okada, Y.; Tanikawa, R.; Tanaka, Y. Advanced Glycation End Products Induce Calcification of Vascular Smooth Muscle Cells through RAGE/P38 MAPK. J. Vasc. Res. 2009, 46, 572–580. [Google Scholar] [CrossRef]
- Shioi, A.; Katagi, M.; Okuno, Y.; Mori, K.; Jono, S.; Koyama, H.; Nishizawa, Y. Induction of Bone-Type Alkaline Phosphatase in Human Vascular Smooth Muscle Cells. Circ. Res. 2002, 91, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Lencel, P.; Delplace, S.; Pilet, P.; Leterme, D.; Miellot, F.; Sourice, S.; Caudrillier, A.; Hardouin, P.; Guicheux, J.; Magne, D. Cell-Specific Effects of TNF-α and IL-1β on Alkaline Phosphatase: Implication for Syndesmophyte Formation and Vascular Calcification. Lab. Investig. J. Tech. Methods Pathol. 2011, 91, 1434–1442. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Chang, Q.; Xin, M.; Wang, Q.; Li, H.; Qian, J. Endogenous Bone Morphogenetic Protein 2 Plays a Role in Vascular Smooth Muscle Cell Calcification Induced by Interleukin 6 in Vitro. Int. J. Immunopathol. Pharmacol. 2017, 30, 227–237. [Google Scholar] [CrossRef]
- Parhami, F.; Morrow, A.D.; Balucan, J.; Leitinger, N.; Watson, A.D.; Tintut, Y.; Berliner, J.A.; Demer, L.L. Lipid Oxidation Products Have Opposite Effects on Calcifying Vascular Cell and Bone Cell Differentiation. A Possible Explanation for the Paradox of Arterial Calcification in Osteoporotic Patients. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 680–687. [Google Scholar] [CrossRef] [PubMed]
- Bear, M.; Butcher, M.; Shaughnessy, S.G. Oxidized Low-Density Lipoprotein Acts Synergistically with Beta-Glycerophosphate to Induce Osteoblast Differentiation in Primary Cultures of Vascular Smooth Muscle Cells. J. Cell. Biochem. 2008, 105, 185–193. [Google Scholar] [CrossRef]
- Rogers, M.A.; Atkins, S.K.; Zheng, K.H.; Singh, S.A.; Chelvanambi, S.; Pham, T.H.; Kuraoka, S.; Stroes, E.S.G.; Aikawa, M.; Aikawa, E. Lipoprotein(a) Induces Vesicular Cardiovascular Calcification Revealed With Single-Extracellular Vesicle Analysis. Front. Cardiovasc. Med. 2022, 9, 778919. [Google Scholar] [CrossRef]
- Balogh, E.; Tóth, A.; Méhes, G.; Trencsényi, G.; Paragh, G.; Jeney, V. Hypoxia Triggers Osteochondrogenic Differentiation of Vascular Smooth Muscle Cells in an HIF-1 (Hypoxia-Inducible Factor 1)-Dependent and Reactive Oxygen Species-Dependent Manner. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1088–1099. [Google Scholar] [CrossRef]
- Mokas, S.; Larivière, R.; Lamalice, L.; Gobeil, S.; Cornfield, D.N.; Agharazii, M.; Richard, D.E. Hypoxia-Inducible Factor-1 Plays a Role in Phosphate-Induced Vascular Smooth Muscle Cell Calcification. Kidney Int. 2016, 90, 598–609. [Google Scholar] [CrossRef] [PubMed]
- Csiki, D.M.; Ababneh, H.; Tóth, A.; Lente, G.; Szöőr, Á.; Tóth, A.; Fillér, C.; Juhász, T.; Nagy, B.; Balogh, E.; et al. Hypoxia-Inducible Factor Activation Promotes Osteogenic Transition of Valve Interstitial Cells and Accelerates Aortic Valve Calcification in a Mice Model of Chronic Kidney Disease. Front. Cardiovasc. Med. 2023, 10, 1168339. [Google Scholar] [CrossRef] [PubMed]
- Tóth, A.; Csiki, D.M.; Nagy, B.; Balogh, E.; Lente, G.; Ababneh, H.; Szöőr, Á.; Jeney, V. Daprodustat Accelerates High Phosphate-Induced Calcification Through the Activation of HIF-1 Signaling. Front. Pharmacol. 2022, 13, 798053. [Google Scholar] [CrossRef] [PubMed]
- Schinke, T.; Amendt, C.; Trindl, A.; Pöschke, O.; Müller-Esterl, W.; Jahnen-Dechent, W. The Serum Protein Alpha2-HS Glycoprotein/Fetuin Inhibits Apatite Formation in Vitro and in Mineralizing Calvaria Cells. A Possible Role in Mineralization and Calcium Homeostasis. J. Biol. Chem. 1996, 271, 20789–20796. [Google Scholar] [CrossRef]
- Luo, G.; Ducy, P.; McKee, M.D.; Pinero, G.J.; Loyer, E.; Behringer, R.R.; Karsenty, G. Spontaneous Calcification of Arteries and Cartilage in Mice Lacking Matrix GLA Protein. Nature 1997, 386, 78–81. [Google Scholar] [CrossRef]
- Orriss, I.R.; Arnett, T.R.; Russell, R.G.G. Pyrophosphate: A Key Inhibitor of Mineralisation. Curr. Opin. Pharmacol. 2016, 28, 57–68. [Google Scholar] [CrossRef]
- ter Braake, A.D.; Tinnemans, P.T.; Shanahan, C.M.; Hoenderop, J.G.J.; de Baaij, J.H.F. Magnesium Prevents Vascular Calcification in Vitro by Inhibition of Hydroxyapatite Crystal Formation. Sci. Rep. 2018, 8, 2069. [Google Scholar] [CrossRef]
- Zumbrennen-Bullough, K.; Babitt, J.L. The Iron Cycle in Chronic Kidney Disease (CKD): From Genetics and Experimental Models to CKD Patients. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transplant. Assoc. -Eur. Ren. Assoc. 2014, 29, 263–273. [Google Scholar] [CrossRef]
- Vinchi, F.; Muckenthaler, M.U.; Da Silva, M.C.; Balla, G.; Balla, J.; Jeney, V. Atherogenesis and Iron: From Epidemiology to Cellular Level. Front. Pharmacol. 2014, 5, 94. [Google Scholar] [CrossRef]
- Aierken, Y.; He, H.; Li, R.; Lin, Z.; Xu, T.; Zhang, L.; Wu, Y.; Liu, Y. Inhibition of Slc39a14/Slc39a8 Reduce Vascular Calcification via Alleviating Iron Overload Induced Ferroptosis in Vascular Smooth Muscle Cells. Cardiovasc. Diabetol. 2024, 23, 186. [Google Scholar] [CrossRef]
- Kawada, S.; Nagasawa, Y.; Kawabe, M.; Ohyama, H.; Kida, A.; Kato-Kogoe, N.; Nanami, M.; Hasuike, Y.; Kuragano, T.; Kishimoto, H.; et al. Iron-Induced Calcification in Human Aortic Vascular Smooth Muscle Cells through Interleukin-24 (IL-24), with/without TNF-Alpha. Sci. Rep. 2018, 8, 658. [Google Scholar] [CrossRef]
- Ye, Y.; Chen, A.; Li, L.; Liang, Q.; Wang, S.; Dong, Q.; Fu, M.; Lan, Z.; Li, Y.; Liu, X.; et al. Repression of the Antiporter SLC7A11/Glutathione/Glutathione Peroxidase 4 Axis Drives Ferroptosis of Vascular Smooth Muscle Cells to Facilitate Vascular Calcification. Kidney Int. 2022, 102, 1259–1275. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Yang, N.; Si, H.; Liu, T.; Wang, H.; Geng, H.; Qin, Q.; Guo, Z. Iron Overload Impairs Renal Function and Is Associated with Vascular Calcification in Rat Aorta. Biometals Int. J. Role Met. Ions Biol. Biochem. Med. 2022, 35, 1325–1339. [Google Scholar] [CrossRef]
- Van den Branden, A.; Opdebeeck, B.; Adriaensen, S.; Evenepoel, P.; Vanden Berghe, T.; Verhulst, A. Intravenous Iron Treatment Fuels Chronic Kidney Disease-Induced Arterial Media Calcification in Rats. J. Pathol. 2025, 265, 172–183. [Google Scholar] [CrossRef]
- Zarjou, A.; Jeney, V.; Arosio, P.; Poli, M.; Antal-Szalmás, P.; Agarwal, A.; Balla, G.; Balla, J. Ferritin Prevents Calcification and Osteoblastic Differentiation of Vascular Smooth Muscle Cells. J. Am. Soc. Nephrol. JASN 2009, 20, 1254–1263. [Google Scholar] [CrossRef] [PubMed]
- Becs, G.; Zarjou, A.; Agarwal, A.; Kovács, K.É.; Becs, Á.; Nyitrai, M.; Balogh, E.; Bányai, E.; Eaton, J.W.; Arosio, P.; et al. Pharmacological Induction of Ferritin Prevents Osteoblastic Transformation of Smooth Muscle Cells. J. Cell. Mol. Med. 2016, 20, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Ciceri, P.; Elli, F.; Braidotti, P.; Falleni, M.; Tosi, D.; Bulfamante, G.; Block, G.A.; Cozzolino, M. Iron Citrate Reduces High Phosphate-Induced Vascular Calcification by Inhibiting Apoptosis. Atherosclerosis 2016, 254, 93–101. [Google Scholar] [CrossRef]
- Ciceri, P.; Falleni, M.; Tosi, D.; Martinelli, C.; Bulfamante, G.; Block, G.A.; Messa, P.; Cozzolino, M. High-Phosphate Induced Vascular Calcification Is Reduced by Iron Citrate through Inhibition of Extracellular Matrix Osteo-Chondrogenic Shift in VSMCs. Int. J. Cardiol. 2019, 297, 94–103. [Google Scholar] [CrossRef]
- Ciceri, P.; Falleni, M.; Tosi, D.; Martinelli, C.; Cannizzo, S.; Marchetti, G.; D’Arminio Monforte, A.; Bulfamante, G.; Block, G.A.; Messa, P.; et al. Therapeutic Effect of Iron Citrate in Blocking Calcium Deposition in High Pi-Calcified VSMC: Role of Autophagy and Apoptosis. Int. J. Mol. Sci. 2019, 20, 5925. [Google Scholar] [CrossRef]
- Wang, P.; Guo, C.; Pan, H.; Chen, W.; Peng, D. Iron Sucrose: A Double-Edged Sword in High Phosphate Media-Induced Vascular Calcification. Calcif. Tissue Int. 2021, 108, 798–807. [Google Scholar] [CrossRef]
- Rajendran, R.; Minqin, R.; Ronald, J.A.; Rutt, B.K.; Halliwell, B.; Watt, F. Does Iron Inhibit Calcification during Atherosclerosis? Free Radic. Biol. Med. 2012, 53, 1675–1679. [Google Scholar] [CrossRef]
- Seto, T.; Hamada, C.; Tomino, Y. Suppressive Effects of Iron Overloading on Vascular Calcification in Uremic Rats. J. Nephrol. 2014, 27, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Phan, O.; Maillard, M.; Peregaux, C.; Mordasini, D.; Stehle, J.-C.; Funk, F.; Burnier, M. PA21, a New Iron-Based Noncalcium Phosphate Binder, Prevents Vascular Calcification in Chronic Renal Failure Rats. J. Pharmacol. Exp. Ther. 2013, 346, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Neven, E.; Corremans, R.; Vervaet, B.A.; Funk, F.; Walpen, S.; Behets, G.J.; D’Haese, P.C.; Verhulst, A. Renoprotective Effects of Sucroferric Oxyhydroxide in a Rat Model of Chronic Renal Failure. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transplant. Assoc. -Eur. Ren. Assoc. 2020, 35, 1689–1699. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, M.; Goto, A.; Iwasaki, T.; Nakanishi, T.; Kuma, A.; Nanami, M.; Kuragano, T. Effect of Iron Administration on the Aortic Iron Content and Vascular Calcification in Phosphorus-Loaded Chronic Kidney Disease Rats. BMC Nephrol. 2023, 24, 373. [Google Scholar] [CrossRef]
- Laguna-Fernandez, A.; Carracedo, M.; Jeanson, G.; Nagy, E.; Eriksson, P.; Caligiuri, G.; Franco-Cereceda, A.; Bäck, M. Iron Alters Valvular Interstitial Cell Function and Is Associated with Calcification in Aortic Stenosis. Eur. Heart J. 2016, 37, 3532–3535. [Google Scholar] [CrossRef]
- Stam, O.C.G.; Daemen, M.J.A.P.; van Rijswijk, J.W.; de Mol, B.A.J.M.; van der Wal, A.C. Intraleaflet Hemorrhages Are a Common Finding in Symptomatic Aortic and Mitral Valves. Cardiovasc. Pathol. Off. J. Soc. Cardiovasc. Pathol. 2017, 30, 12–18. [Google Scholar] [CrossRef]
- Morvan, M.; Arangalage, D.; Franck, G.; Perez, F.; Cattan-Levy, L.; Codogno, I.; Jacob-Lenet, M.-P.; Deschildre, C.; Choqueux, C.; Even, G.; et al. Relationship of Iron Deposition to Calcium Deposition in Human Aortic Valve Leaflets. J. Am. Coll. Cardiol. 2019, 73, 1043–1054. [Google Scholar] [CrossRef]
- Xu, R.; Huang, Y.; Zhu, D.; Guo, J. Iron Promotes Slc7a11-Deficient Valvular Interstitial Cell Osteogenic Differentiation: A Possible Mechanism by Which Ferroptosis Participates in Intraleaflet Hemorrhage-Induced Calcification. Free. Radic. Biol. Med. 2022, 184, 158–169, Erratum in Free. Radic. Biol. Med. 2022, 186, 31. [Google Scholar] [CrossRef]
- Qin, Z.; Bäck, M.; Franco-Cereceda, A.; Pawelzik, S.-C. Increased Calcification by Erythrophagocytosis in Aortic Valvular Interstitial Cells. ESC Heart Fail. 2025, 12, 1469–1473. [Google Scholar] [CrossRef]
- Sikura, K.É.; Potor, L.; Szerafin, T.; Zarjou, A.; Agarwal, A.; Arosio, P.; Poli, M.; Hendrik, Z.; Méhes, G.; Oros, M.; et al. Potential Role of H-Ferritin in Mitigating Valvular Mineralization. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 413–431. [Google Scholar] [CrossRef]
- Balogh, E.; Chowdhury, A.; Ababneh, H.; Csiki, D.M.; Tóth, A.; Jeney, V. Heme-Mediated Activation of the Nrf2/HO-1 Axis Attenuates Calcification of Valve Interstitial Cells. Biomedicines 2021, 9, 427. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, S.M.; Carpentier, A.F.; Chen, L.; Shen, M.; Quintero, L.J.; Witzel, T.H. Calcium Mitigation in Bioprosthetic Tissues by Iron Pretreatment: The Challenge of Iron Leaching. Ann. Thorac. Surg. 1995, 60, S332–S338. [Google Scholar] [CrossRef] [PubMed]
- Manji, R.A.; Menkis, A.H.; Ekser, B.; Cooper, D.K.C. The Future of Bioprosthetic Heart Valves. Indian J. Med. Res. 2012, 135, 150–151. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Fan, C.; Li, X.; Zhao, L. The Role of Macrophage Polarization in Vascular Calcification. Biochem. Biophys. Res. Commun. 2024, 710, 149863. [Google Scholar] [CrossRef]
- Murray, P.J.; Wynn, T.A. Protective and Pathogenic Functions of Macrophage Subsets. Nat. Rev. Immunol. 2011, 11, 723–737. [Google Scholar] [CrossRef]
- Italiani, P.; Boraschi, D. From Monocytes to M1/M2 Macrophages: Phenotypical vs. Functional Differentiation. Front. Immunol. 2014, 5, 514. [Google Scholar] [CrossRef]
- Moore, K.J.; Sheedy, F.J.; Fisher, E.A. Macrophages in Atherosclerosis: A Dynamic Balance. Nat. Rev. Immunol. 2013, 13, 709–721. [Google Scholar] [CrossRef]
- Chinetti-Gbaguidi, G.; Colin, S.; Staels, B. Macrophage Subsets in Atherosclerosis. Nat. Rev. Cardiol. 2015, 12, 10–17. [Google Scholar] [CrossRef]
- Kadl, A.; Meher, A.K.; Sharma, P.R.; Lee, M.Y.; Doran, A.C.; Johnstone, S.R.; Elliott, M.R.; Gruber, F.; Han, J.; Chen, W.; et al. Identification of a Novel Macrophage Phenotype That Develops in Response to Atherogenic Phospholipids via Nrf2. Circ. Res. 2010, 107, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Jinnouchi, H.; Guo, L.; Sakamoto, A.; Torii, S.; Sato, Y.; Cornelissen, A.; Kuntz, S.; Paek, K.H.; Fernandez, R.; Fuller, D.; et al. Diversity of Macrophage Phenotypes and Responses in Atherosclerosis. Cell. Mol. Life Sci. CMLS 2019, 77, 1919–1932. [Google Scholar] [CrossRef] [PubMed]
- Boyle, J.J.; Harrington, H.A.; Piper, E.; Elderfield, K.; Stark, J.; Landis, R.C.; Haskard, D.O. Coronary Intraplaque Hemorrhage Evokes a Novel Atheroprotective Macrophage Phenotype. Am. J. Pathol. 2009, 174, 1097–1108. [Google Scholar] [CrossRef] [PubMed]
- Boyle, J.J.; Johns, M.; Kampfer, T.; Nguyen, A.T.; Game, L.; Schaer, D.J.; Mason, J.C.; Haskard, D.O. Activating Transcription Factor 1 Directs Mhem Atheroprotective Macrophages through Coordinated Iron Handling and Foam Cell Protection. Circ. Res. 2012, 110, 20–33. [Google Scholar] [CrossRef]
- Finn, A.V.; Nakano, M.; Narula, J.; Kolodgie, F.D.; Virmani, R. Concept of Vulnerable/Unstable Plaque. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1282–1292. [Google Scholar] [CrossRef]
- Finn, A.V.; Nakano, M.; Polavarapu, R.; Karmali, V.; Saeed, O.; Zhao, X.; Yazdani, S.; Otsuka, F.; Davis, T.; Habib, A.; et al. Hemoglobin Directs Macrophage Differentiation and Prevents Foam Cell Formation in Human Atherosclerotic Plaques. J. Am. Coll. Cardiol. 2012, 59, 166–177. [Google Scholar] [CrossRef]
- Soares, M.P.; Hamza, I. Macrophages and Iron Metabolism. Immunity 2016, 44, 492–504. [Google Scholar] [CrossRef]
- Sakamoto, A.; Kawakami, R.; Mori, M.; Guo, L.; Paek, K.H.; Mosquera, J.V.; Cornelissen, A.; Ghosh, S.K.B.; Kawai, K.; Konishi, T.; et al. CD163+ Macrophages Restrain Vascular Calcification, Promoting the Development of High-Risk Plaque. JCI Insight 2023, 8, e154922. [Google Scholar] [CrossRef]
- Greenwald, R.A. Handbook Methods for Oxygen Radical Research; CRC Press: Boca Raton, FL, USA, 2018; ISBN 978-1-351-08137-5. [Google Scholar]
- Tóth, A.; Balogh, E.; Jeney, V. Regulation of Vascular Calcification by Reactive Oxygen Species. Antioxid. Basel Switz. 2020, 9, 963. [Google Scholar] [CrossRef]
- Münzel, T.; Camici, G.G.; Maack, C.; Bonetti, N.R.; Fuster, V.; Kovacic, J.C. Impact of Oxidative Stress on the Heart and Vasculature Part 2 of a 3-Part Series. J. Am. Coll. Cardiol. 2017, 70, 212–229. [Google Scholar] [CrossRef]
- Agharazii, M.; St-Louis, R.; Gautier-Bastien, A.; Ung, R.-V.; Mokas, S.; Larivière, R.; Richard, D.E. Inflammatory Cytokines and Reactive Oxygen Species as Mediators of Chronic Kidney Disease-Related Vascular Calcification. Am. J. Hypertens. 2015, 28, 746–755. [Google Scholar] [CrossRef]
- Liberman, M.; Bassi, E.; Martinatti, M.K.; Lario, F.C.; Wosniak, J.; Pomerantzeff, P.M.A.; Laurindo, F.R.M. Oxidant Generation Predominates around Calcifying Foci and Enhances Progression of Aortic Valve Calcification. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 463–470. [Google Scholar] [CrossRef]
- Mody, N.; Parhami, F.; Sarafian, T.A.; Demer, L.L. Oxidative Stress Modulates Osteoblastic Differentiation of Vascular and Bone Cells. Free Radic. Biol. Med. 2001, 31, 509–519. [Google Scholar] [CrossRef]
- Byon, C.H.; Javed, A.; Dai, Q.; Kappes, J.C.; Clemens, T.L.; Darley-Usmar, V.M.; McDonald, J.M.; Chen, Y. Oxidative Stress Induces Vascular Calcification through Modulation of the Osteogenic Transcription Factor Runx2 by AKT Signaling. J. Biol. Chem. 2008, 283, 15319–15327. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q. Role of Nrf2 in Oxidative Stress and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Wakabayashi, N.; Katoh, Y.; Ishii, T.; Igarashi, K.; Engel, J.D.; Yamamoto, M. Keap1 Represses Nuclear Activation of Antioxidant Responsive Elements by Nrf2 through Binding to the Amino-Terminal Neh2 Domain. Genes Dev. 1999, 13, 76–86. [Google Scholar] [CrossRef]
- Kerins, M.J.; Ooi, A. The Roles of NRF2 in Modulating Cellular Iron Homeostasis. Antioxid. Redox Signal. 2018, 29, 1756–1773. [Google Scholar] [CrossRef] [PubMed]
- Ha, C.-M.; Park, S.; Choi, Y.-K.; Jeong, J.-Y.; Oh, C.J.; Bae, K.-H.; Lee, S.J.; Kim, J.-H.; Park, K.-G.; Jun, D.Y.; et al. Activation of Nrf2 by Dimethyl Fumarate Improves Vascular Calcification. Vascul. Pharmacol. 2014, 63, 29–36. [Google Scholar] [CrossRef]
- Zhang, P.; Li, Y.; Du, Y.; Li, G.; Wang, L.; Zhou, F. Resveratrol Ameliorated Vascular Calcification by Regulating Sirt-1 and Nrf2. Transplant. Proc. 2016, 48, 3378–3386. [Google Scholar] [CrossRef]
- Aghagolzadeh, P.; Radpour, R.; Bachtler, M.; van Goor, H.; Smith, E.R.; Lister, A.; Odermatt, A.; Feelisch, M.; Pasch, A. Hydrogen Sulfide Attenuates Calcification of Vascular Smooth Muscle Cells via KEAP1/NRF2/NQO1 Activation. Atherosclerosis 2017, 265, 78–86. [Google Scholar] [CrossRef]
- Ji, R.; Sun, H.; Peng, J.; Ma, X.; Bao, L.; Fu, Y.; Zhang, X.; Luo, C.; Gao, C.; Jin, Y.; et al. Rosmarinic Acid Exerts an Antagonistic Effect on Vascular Calcification by Regulating the Nrf2 Signalling Pathway. Free Radic. Res. 2019, 53, 187–197. [Google Scholar] [CrossRef]
- Wei, R.; Enaka, M.; Muragaki, Y. Activation of KEAP1/NRF2/P62 Signaling Alleviates High Phosphate-Induced Calcification of Vascular Smooth Muscle Cells by Suppressing Reactive Oxygen Species Production. Sci. Rep. 2019, 9, 10366. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Zhou, Q.; Zheng, X.; Sun, B.; Zhao, S. Mitoquinone Attenuates Vascular Calcification by Suppressing Oxidative Stress and Reducing Apoptosis of Vascular Smooth Muscle Cells via the Keap1/Nrf2 Pathway. Free Radic. Biol. Med. 2020, 161, 23–31. [Google Scholar] [CrossRef]
- Dixon, S.J.; Stockwell, B.R. The Role of Iron and Reactive Oxygen Species in Cell Death. Nat. Chem. Biol. 2014, 10, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Hou, W.; Song, X.; Yu, Y.; Huang, J.; Sun, X.; Kang, R.; Tang, D. Ferroptosis: Process and Function. Cell Death Differ. 2016, 23, 369–379. [Google Scholar] [CrossRef] [PubMed]
- An, Q.; Sun, H.; Yang, B.; Cheng, M.; Jin, J.; Zhang, D.; Chang, L.; Zhang, S.; Bai, Y.; Xu, J. Histone Methyltransferase G9a Drives Ferroptosis to Aggravate Vascular Calcification by Inhibiting SLC7A11 Transcription. Cell. Signal. 2025, 135, 112010. [Google Scholar] [CrossRef]
- Xiong, L.; Xiao, Q.; Chen, R.; Huang, L.; Gao, J.; Wang, L.; Li, G.; Li, Y. Histone Deacetylase 9 Promotes Osteogenic Trans-Differentiation of Vascular Smooth Muscle Cells via Ferroptosis in Chronic Kidney Disease Vascular Calcification. Ren. Fail. 2024, 46, 2422435. [Google Scholar] [CrossRef]
- He, W.; Meng, Y.; Liu, W.; Geng, J. Protein Kinase D Knockdown Alleviates Vascular Calcification Through P53-SLC7A11 Axis-Mediated Ferroptosis. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2025, 39, e70706. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, J.; Tang, Q.; Zhu, H.; Chen, Y.; Xiong, H.; Jiang, H. The Significance of Serum SLC7A11 Levels in the Occurrence of Vascular Calcification in Maintenance Peritoneal Dialysis Patients. Nephrol. Carlton Vic. 2024, 29, 663–670. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Gao, M.; Chen, Z.; Lu, J.; Li, Y.; Di, Y.; Zhao, Y.; Liu, B.; Tang, R. Lipocalin-2 Promotes CKD Vascular Calcification by Aggravating VSMCs Ferroptosis through NCOA4/FTH1-Mediated Ferritinophagy. Cell Death Dis. 2024, 15, 865. [Google Scholar] [CrossRef]
- Vasudev, S.C.; Chandy, T.; Umasankar, M.M.; Sharma, C.P. Inhibition of Bioprosthesis Calcification Due to Synergistic Effect of Fe/Mg Ions to Polyethylene Glycol Grafted Bovine Pericardium. J. Biomater. Appl. 2001, 16, 93–107. [Google Scholar] [CrossRef]
- Hilton, R.J.; Seare, M.C.; Andros, N.D.; Kenealey, Z.; Orozco, C.M.; Webb, M.; Watt, R.K. Phosphate Inhibits in Vitro Fe3+ Loading into Transferrin by Forming a Soluble Fe(III)–Phosphate Complex: A Potential Non-Transferrin Bound Iron Species. J. Inorg. Biochem. 2012, 110, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M. Role of Free Radicals and Catalytic Metal Ions in Human Disease: An Overview. Methods Enzymol. 1990, 186, 1–85. [Google Scholar] [CrossRef]
- Stauffer, M.E.; Fan, T. Prevalence of Anemia in Chronic Kidney Disease in the United States. PLoS ONE 2014, 9, e84943. [Google Scholar] [CrossRef]
- Nakanishi, T.; Nanami, M.; Kuragano, T. The Pathogenesis of CKD Complications; Attack of Dysregulated Iron and Phosphate Metabolism. Free. Radic. Biol. Med. 2020, 157, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Iron Deficiency Anemia in Chronic Kidney Disease: Review. Chronic Kidney Disease Anemia. Available online: https://www.scribd.com/document/500236367/Anemia-in-CKD (accessed on 27 August 2025).
- Dmitrieva, O.; de Lusignan, S.; Macdougall, I.C.; Gallagher, H.; Tomson, C.; Harris, K.; Desombre, T.; Goldsmith, D. Association of Anaemia in Primary Care Patients with Chronic Kidney Disease: Cross Sectional Study of Quality Improvement in Chronic Kidney Disease (QICKD) Trial Data. BMC Nephrol. 2013, 14, 24. [Google Scholar] [CrossRef] [PubMed]
- Mizuiri, S.; Nishizawa, Y.; Yamashita, K.; Doi, T.; Okubo, A.; Morii, K.; Usui, K.; Arita, M.; Naito, T.; Shigemoto, K.; et al. Absolute Iron Deficiency, Coronary Artery Calcification and Cardiovascular Mortality in Maintenance Haemodialysis Patients. Nephrol. Carlton Vic. 2024, 29, 415–421. [Google Scholar] [CrossRef]
- Chen, S.; Tang, Y.; Pu, Y.; Xia, X.; Li, Y.; Zou, Y. Predictive Value of Ferritin Heavy Chains in the Development of Coronary Artery Calcification in Patients on Maintenance Hemodialysis: A Prospective Cohort Study. Front. Endocrinol. 2025, 16, 1503940. [Google Scholar] [CrossRef]
| A. Iron Promotes Vascular Calcification | |||
| Experimental Model | Major Finding | Reference | |
| In vitro/ex vivo studies | Cell type: HAoSMCs Calcification induction: high Pi+TNF-alpha Treatment: iron overload: holoferritin | Excess iron accelerates Pi-, and Pi+TNF-α-induced calcification of HAoSMCs through IL-24 upregulation. | Kawada et al., 2018 [52] |
| Cell type: primary rat VSMCs/rat aortic rings Calcification induction: high Ca+high Pi Treatment: ferroptosis inhibition: Ferrostatin-1 | Inhibition of ferroptosis with Ferrostatin-1 ameliorates rat VSMC and aortic ring calcification. | Ye et al., 2022 [53] | |
| Cell type: primary mouse VSMCs Calcification induction: β-GP+high Ca Treatment: iron overload: Iron(II) ammonium citrate; iron deficiency: Desferrioxamine | FAC promotes, and DFO inhibits VSMC osteogenic differentiation and calcification. | Aierken et al., 2024 [51] | |
| In vivo studies | Model: vitamin D3-overloaded mice Treatment: ferroptosis inhibition: Ferrostatin-1 | Inhibition of ferroptosis with Ferrostatin-1 ameliorates aortic calcification. | Ye et al., 2022 [53] |
| Model: iron overload rats Treatment: iron-dextrane, i.p. | Iron treatment triggers iron accumulation and increases arterial calcification. | Song et al., 2022 [54] | |
| Model: adenine-induced CKD in rats Treatment: iron-sucrose, i.v. | Iron treatment triggers iron accumulation and lipid peroxidation, and increases CKD-induced arterial calcification. | Van den Branden et al., 2025 [55] | |
| B. Iron Inhibits Vascular Calcification | |||
| Experimental Model | Major Finding | Reference | |
| In vitro/ex vivo studies | Cell/tissue type: HAoSMCs Calcification induction: high Pi Treatment: iron overload: heme/iron chloride/ferritin; ferroxidases: H ferritin, ceruloplasmin | Excess iron inhibits Pi-induced calcification of HAoSMCs through the upregulation of ferritin. Ferroxidase activity provides the calcification-inhibitory effect. | Zarjou et al., 2009 [56] |
| Cell/tissue type: HAoSMCs Calcification induction: β-GP+vitamin D3 Treatment: iron overload: ferritin; ferritin H induction: 3H-1,2-Dithiole-3-thione | Induction of ferritin prevents osteoblastic transformation and calcification of HAoSMCs. | Becs et al., 2016 [57] | |
| Cell/tissue type: primary rat VSMCs Calcification induction: high Pi Treatment: iron overload: iron(III) citrate | Excess iron inhibits high Pi-induced VSMC calcification by preventing apoptosis, inducing autophagy, and affecting osteoblastic differentiation. | Ciceri et al., 2016 and 2019 [58,59,60] | |
| Cell/tissue type: rat aortic ring Calcification induction: high Pi Treatment: Iron overload: iron(III) sucrose | Excess iron inhibits high Pi-induced aortic ring calcification and osteogenic differentiation of VSMCs. | Wang et al., 2021 [61] | |
| In vivo studies | Model: high cholesterol-induced atherosclerosis in rabbits | Calcium and iron levels show an inverse correlation in atherosclerotic lesions. | Rajendran et al., 2012 [62] |
| Model: adenine-induced CKD in rats Calcification enhancement: Pi-enriched diet Treatment: iron dextran, i.p. | Reduction in aortic calcification and downregulation of osteogenic markers Runx2 and PiT-1 in animals receiving high-dose iron. | Seto et al., 2014 [63] | |
| Model: adenine-induced CKD in rats Calcification enhancement: Pi-enriched diet Treatment: CaCO3 or iron-based phosphate binder (PA21) | Both CaCO3 and PA21 effectively reduced serum phosphate levels, but PA21 was superior in preventing vascular calcification. | Phan et al., 2013 [64] | |
| Model: adenine-induced CKD in rats Calcification enhancement: Pi-enriched diet Treatment: iron-based phosphate binder (PA21) | PA21 treatment improved renal function, reduced vascular calcium deposition, and decreased expression of Runx2. | Neven et al., 2020 [65] | |
| A. Iron Promotes Valve Calcification | |||
| Experimental Model | Major Finding | Reference | |
| In vitro studies | Cell type: human VICs Treatment: senescent RBCs | Senescent RBCs promote VIC differentiation toward an osteoblast-like phenotype. | Morvan et al., 2019 [69] |
| Cell type: Slc7a11-deficient human VICs Calcification induction: osteogenic differentiation medium Treatment: iron overload: Iron(II) sulphate | Excess iron promotes osteogenic differentiation of Slc7a11-deficient VICs. | Xu et al., 2022 [70] | |
| Cell type: primary mouse VSMCs Calcification induction: β-GP+high Ca Treatment: iron overload: Iron(III) ammonium citrate; iron deficiency: Desferrioxamine | Excess iron promotes, and low iron inhibits VSMC osteogenic differentiation and calcification. | Qin et al., 2025 [71] | |
| In vivo observations | Model: human aortic valve leaflets | Iron accumulation is more prevalent in calcified valves than in non-calcified tissues. Iron-containing valve regions show increased expression of genes involved in calcification. | Laguna-Fernandez et al., 2016 [67] |
| Model: human aortic valve leaflets | There is a spatial overlap between iron and calcium deposits in human aortic valve leaflets. | Morvan et al., 2019 [69] | |
| Model: human aortic valve leaflets | Intra-leaflet hemorrhages are often associated with angiogenesis, microvascular leakage, and calcification. | Stam et al., 2017 [68] | |
| B. Iron Inhibits Valve Calcification | |||
| Experimental Model | Major Finding | Reference | |
| In vitro/ex vivo studies | Cell/tissue type: human VICs Calcification induction: high Pi Treatment: iron overload: heme/iron chloride/ferritin; ferroxidases: H ferritin, ceruloplasmin | Iron-mediated FtH upregulation inhibits high Pi-induced VIC calcification through reduced expression of osteogenic markers such as Runx2 and BMP-2, and decreased oxidative stress. | Sikura et al., 2019 [72] |
| Cell/tissue type: human VICs Calcification induction: high Pi Treatment: iron overload: heme/iron(II) chloride/ferritin; Nrf2 and HO-1 inhibition | Heme inhibits high Pi-induced osteogenic phenotype switch and calcification of VICs through the activation of the Nrf2/HO-1 antioxidant pathway. | Balogh et al., 2021 [73] | |
| In vivo studies | Model: glutaraldehyde-pretreated porcine bioprosthetic heart valve tissue implanted in rats subdermally Calcification induction: Warfarin Treatment: iron overload: pretreatment with iron(III) nitrate | Iron pretreatment attenuates calcium accumulation in the implanted bioprosthetic heart valve tissue. | Carpentier et al., 1995 [74] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balogh, E.; Tóth, A.; Jeney, V. Iron in Vascular Calcification: Pro-Calcific Agent or Protective Modulator? Int. J. Mol. Sci. 2025, 26, 10210. https://doi.org/10.3390/ijms262010210
Balogh E, Tóth A, Jeney V. Iron in Vascular Calcification: Pro-Calcific Agent or Protective Modulator? International Journal of Molecular Sciences. 2025; 26(20):10210. https://doi.org/10.3390/ijms262010210
Chicago/Turabian StyleBalogh, Enikő, Andrea Tóth, and Viktória Jeney. 2025. "Iron in Vascular Calcification: Pro-Calcific Agent or Protective Modulator?" International Journal of Molecular Sciences 26, no. 20: 10210. https://doi.org/10.3390/ijms262010210
APA StyleBalogh, E., Tóth, A., & Jeney, V. (2025). Iron in Vascular Calcification: Pro-Calcific Agent or Protective Modulator? International Journal of Molecular Sciences, 26(20), 10210. https://doi.org/10.3390/ijms262010210






