Thiotaurine Attenuates TNF-α-Induced Inflammation in Human Chondrocytes via NF-κB Pathway Suppression and Thiol-Dependent Persulfidation
Abstract
1. Introduction
2. Results
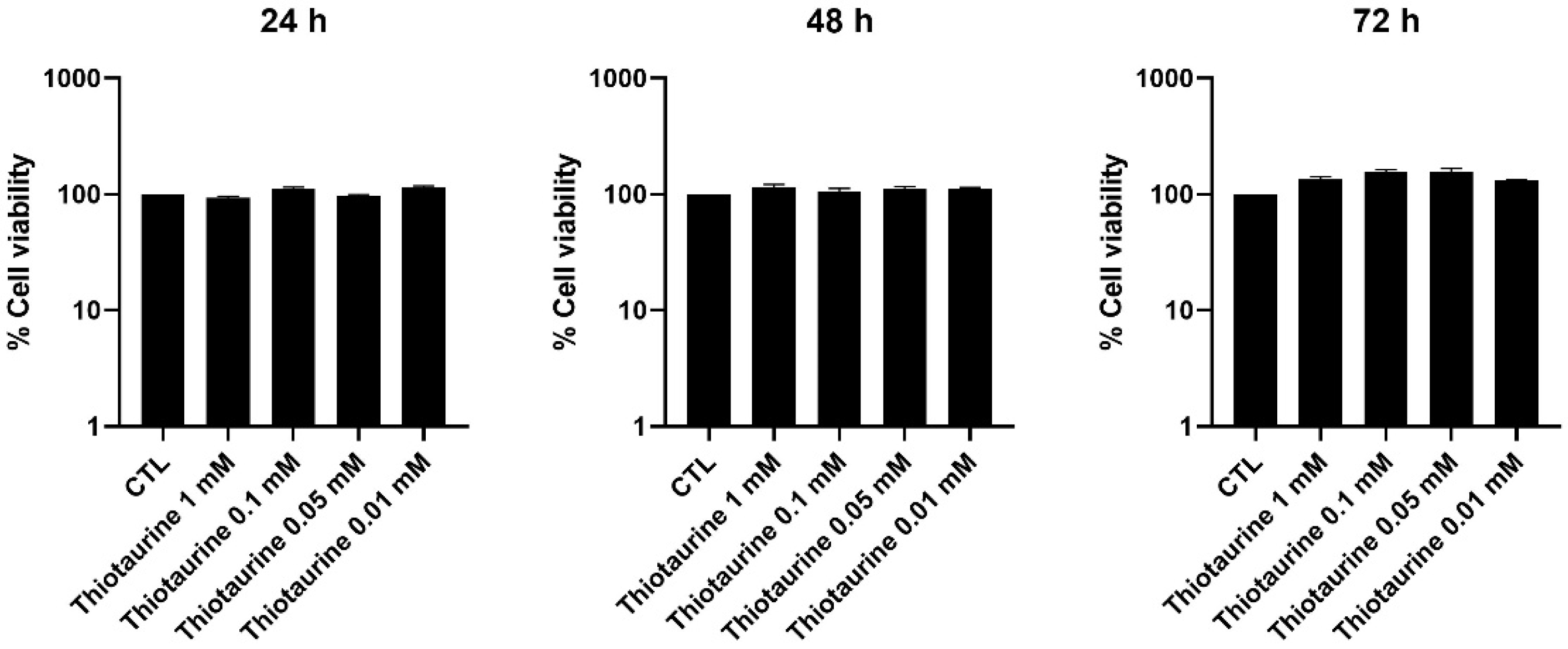
2.1. Cellular Viability After Thiotaurine Treatment
2.2. Effect of Thiotaurine on Inflammatory Mediators
2.2.1. Interleukin Modulation
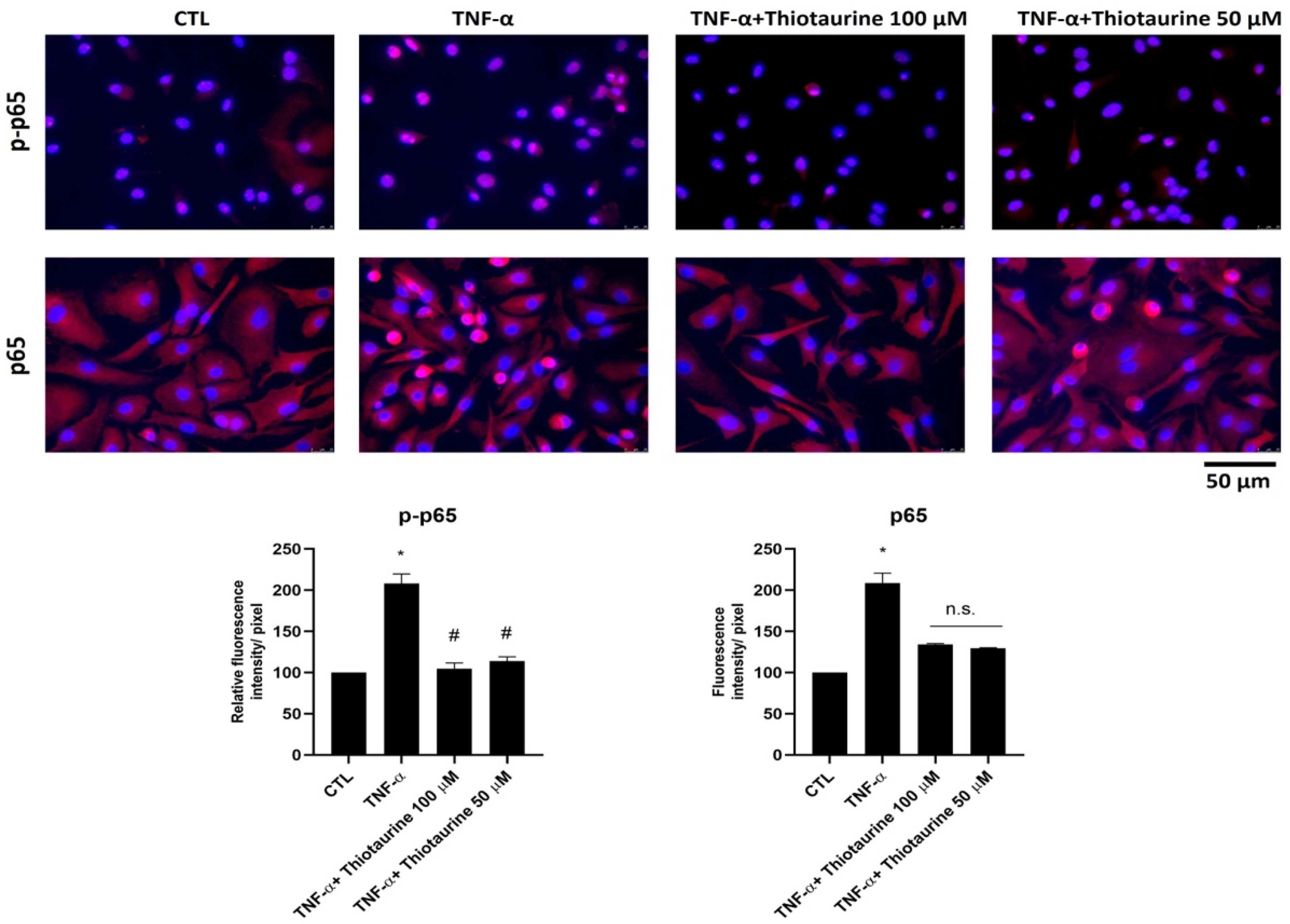
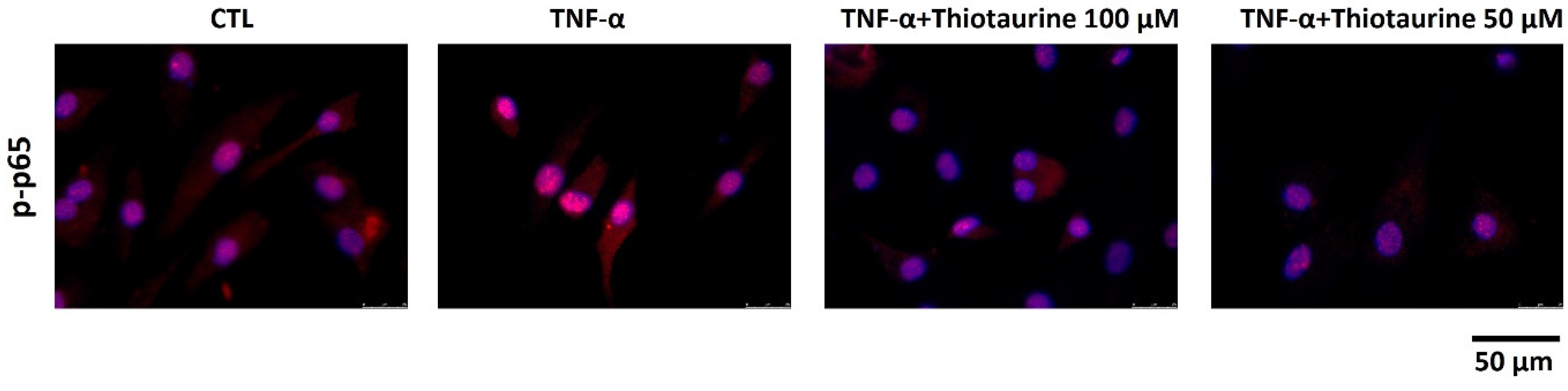
2.2.2. Effect on p65 Phosphorylation (p-p65) and Expression
2.3. In Vitro Effect of Thiotaurine on Persulfide Formation
3. Discussion
4. Materials and Methods
4.1. Thiotaurine Synthesis
4.2. Human Primary Cell Isolation and Culture
4.3. Cell Treatment
4.4. MTS Assay
4.5. RNA Extraction and Reverse Transcription
4.6. Quantitative Real-Time PCR
4.7. Immunofluorescence Analysis
4.8. ELISA
4.9. Persulphide Determination Assay
4.10. Densitometric Analysis
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kolluru, G.K.; Shen, X.; Bir, S.C.; Kevil, C.G. Hydrogen sulfide chemical biology: Pathophysiological roles and detection. Nitric Oxide-Biol. Chem. 2013, 35, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Szabõ, C. Hydrogen sulphide and its therapeutic potential. Nat. Rev. Drug Discov. 2007, 6, 917–935. [Google Scholar] [CrossRef] [PubMed]
- Francioso, A.; Baseggio Conrado, A.; Mosca, L.; Fontana, M. Chemistry and Biochemistry of Sulfur Natural Compounds: Key Intermediates of Metabolism and Redox Biology. Oxid. Med. Cell. Longev. 2020, 2020, 8294158. [Google Scholar] [CrossRef]
- Gadalla, M.M.; Snyder, S.H. Hydrogen sulfide as a gasotransmitter. J. Neurochem. 2010, 113, 14–26. [Google Scholar] [CrossRef]
- Chan, S.J.; Wong, P.T.H. Hydrogen sulfide in stroke: Protective or deleterious? Neurochem. Int. 2017, 105, 78–87. [Google Scholar] [CrossRef]
- Burguera, E.F.; Meijide-Failde, R.; Blanco, F.J. Hydrogen Sulfide and Inflammatory Joint Diseases. Curr. Drug Targets 2017, 18, 1641–1652. [Google Scholar] [CrossRef]
- Munteanu, C.; Turnea, M.A.; Rotariu, M. Hydrogen Sulfide: An Emerging Regulator of Oxidative Stress and Cellular Homeostasis—A Comprehensive One-Year Review. Antioxidants 2023, 12, 1737. [Google Scholar] [CrossRef]
- Kabil, O.; Banerjee, R. Enzymology of H2S biogenesis, decay and signaling. Antioxid. Redox Signal. 2014, 20, 770–782. [Google Scholar] [CrossRef]
- Singh, S.; Padovani, D.; Leslie, R.A.; Chiku, T.; Banerjee, R. Relative contributions of cystathionine β-synthase and γ-cystathionase to H2S biogenesis via alternative trans-sulfuration reactions. J. Biol. Chem. 2009, 284, 22457–22466. [Google Scholar] [CrossRef] [PubMed]
- Chiku, T.; Padovani, D.; Zhu, W.; Singh, S.; Vitvitsky, V.; Banerjee, R. H2S Biogenesis by Human Cystathionine γ-Lyase Leads to the Novel Sulfur Metabolites Lanthionine and Homolanthionine and Is Responsive to the Grade of Hyperhomocysteinemia. J. Biol. Chem. 2009, 284, 11601–11612. [Google Scholar] [CrossRef]
- Goldring, M.B.; Otero, M. Inflammation in osteoarthritis. Curr. Opin. Rheumatol. 2011, 23, 471–478. [Google Scholar] [CrossRef]
- Daheshia, M.; Yao, J.Q. The Interleukin 1β Pathway in the Pathogenesis of Osteoarthritis. J. Rheumatol. 2008, 35, 2306–2312. [Google Scholar] [CrossRef]
- Kapoor, M.; Martel-Pelletier, J.; Lajeunesse, D.; Pelletier, J.P.; Fahmi, H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 33–42. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.-C.; Jo, J.; Park, J.; Kang, H.K.; Park, Y. NF-kB Signaling Pathways in Osteoarthritic Cartilage Destruction. Cells 2019, 8, 734. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wu, S.; Zhang, R.; Zhong, Q.; Zhang, X.; Sun, X. Therapeutic potential of hydrogen sulfide in osteoarthritis development. Front. Pharmacol. 2024, 15, 1336693. [Google Scholar] [CrossRef] [PubMed]
- Cavallini, D.; De Marco, C.; Mondovì, B. Chromatographic Evidence on the Occurrence of Thiotaurine in the Urine of Rats Fed with Cystine. J. Biol. Chem. 1959, 234, 854–857. [Google Scholar] [CrossRef]
- Baseggio Conrado, A.; Capuozzo, E.; Mosca, L.; Francioso, A.; Fontana, M. Thiotaurine: From Chemical and Biological Properties to Role in H2S Signaling. In Taurine 11; Advances in Experimental Medicine and Biology; Springer: Singapore, 2019; Volume 1155, pp. 755–771. [Google Scholar]
- Capuozzo, E.; Conrado, A.B.; Fontana, M. Thiotaurine modulates human neutrophil activation. In Taurine 9; Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2015; Volume 803. [Google Scholar] [CrossRef]
- Karpowicz, S.J.; Anderson, L. Enzymatic and non-enzymatic conversion of cystamine to thiotaurine and taurine. Biochim. Biophys. Acta-Gen. Subj. 2022, 1866, 130225. [Google Scholar] [CrossRef]
- Capuozzo, E.; Giorgi, A.; Canterini, S.; Baseggio Conrado, A.; Giarrusso, P.; Schininà, M.E.; Fontana, M. A Proteomic Approach to Study the Effect of Thiotaurine on Human Neutrophil Activation. In Taurine 10; Advances in Experimental Medicine and Biology; Springer: Dordrecht, The Netherlands, 2017; Volume 975, pp. 563–571. [Google Scholar]
- Dragotto, J.; Capuozzo, E.; Fontana, M.; Curci, A.; Fiorenza, M.T.; Canterini, S. Thiotaurine protects mouse cerebellar granule neurons from potassium deprivation-induced apoptosis by inhibiting the activation of Caspase-3. In Taurine 9; Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2015; Volume 803. [Google Scholar] [CrossRef]
- Israel, A. The IKK Complex, a Central Regulator of NF-kB Activation. Cold Spring Harb. Perspect. Biol. 2010, 2, a000158. [Google Scholar] [CrossRef]
- Burrage, P.S. Matrix Metalloproteinases: Role In Arthritis. Front. Biosci. 2006, 11, 529. [Google Scholar] [CrossRef]
- Mehana, E.-S.E.; Khafaga, A.F.; El-Blehi, S.S. The role of matrix metalloproteinases in osteoarthritis pathogenesis: An updated review. Life Sci. 2019, 234, 116786. [Google Scholar] [CrossRef]
- Andrés, C.M.C.; Lobo, F.; de la Lastra, J.M.P.; Munguira, E.B.; Juan, C.A.; Pérez Lebeña, E. Reactive Sulfur Species and Protein Persulfidation: An Emerging Redox Axis in Human Health and Disease. Curr. Issues Mol. Biol. 2025, 47, 765. [Google Scholar] [CrossRef]
- Filipovic, M.R. Persulfidation (S-sulfhydration) and H2S. In Chemistry, Biochemistry and Pharmacology of Hydrogen Sulfide; Handbook of Experimental Pharmacology; Springer: Cham, Switzerland, 2015; Volume 230, pp. 29–59. [Google Scholar]
- Calvert, J.W.; Jha, S.; Gundewar, S.; Elrod, J.W.; Ramachandran, A.; Pattillo, C.B.; Kevil, C.G.; Lefer, D.J. Hydrogen Sulfide Mediates Cardioprotection Through Nrf2 Signaling. Circ. Res. 2009, 105, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Magli, E.; Perissutti, E.; Santagada, V.; Caliendo, G.; Corvino, A.; Esposito, G.; Esposito, G.; Fiorino, F.; Migliaccio, M.; Scognamiglio, A.; et al. H2S Donors and Their Use in Medicinal Chemistry. Biomolecules 2021, 11, 1899. [Google Scholar] [CrossRef] [PubMed]
- Gotor, C.; Aroca, A.; Romero, L.C. Persulfidation is the mechanism underlying sulfide-signaling of autophagy. Autophagy 2022, 18, 695–697. [Google Scholar] [CrossRef]
- Sun, H.J.; Leng, B.; Wu, Z.Y.; Bian, J.S. Polysulfide and hydrogen sulfide ameliorate cisplatin-induced nephrotoxicity and renal inflammation through persulfidating stat3 and ikkβ. Int. J. Mol. Sci. 2020, 21, 7805. [Google Scholar] [CrossRef]
- He, B.; Zhang, Z.; Huang, Z.; Duan, X.; Wang, Y.; Cao, J.; Li, L.; He, K.; Nice, E.C.; He, W.; et al. Protein persulfidation: Rewiring the hydrogen sulfide signaling in cell stress response. Biochem. Pharmacol. 2023, 209, 115444. [Google Scholar] [CrossRef]
- Zhang, T.; Pan, Y.; Sawa, T.; Akaike, T.; Matsunaga, T. Supersulfide donors and their therapeutic targets in inflammatory diseases. Front. Immunol. 2025, 16, 1581385. [Google Scholar] [CrossRef] [PubMed]
- Filipovic, M.R.; Zivanovic, J.; Alvarez, B.; Banerjee, R. Chemical Biology of H2S Signaling through Persulfidation. Chem. Rev. 2018, 118, 1253–1337. [Google Scholar] [CrossRef]
- Egbujor, M.C.; Petrosino, M.; Zuhra, K.; Saso, L. The Role of Organosulfur Compounds as Nrf2 Activators and Their Antioxidant Effects. Antioxidants 2022, 11, 1255. [Google Scholar] [CrossRef]
- Ida, T.; Sawa, T.; Ihara, H.; Tsuchiya, Y.; Watanabe, Y.; Kumagai, Y.; Suematsu, M.; Motohashi, H.; Fujii, S.; Matsunaga, T.; et al. Reactive cysteine persulfides and S-polythiolation regulate oxidative stress and redox signaling. Proc. Natl. Acad. Sci. USA 2014, 111, 7606–7611. [Google Scholar] [CrossRef]
- Mishanina, T.V.; Libiad, M.; Banerjee, R. Biogenesis of reactive sulfur species for signaling by hydrogen sulfide oxidation pathways. Nat. Chem. Biol. 2015, 11, 457–464. [Google Scholar] [CrossRef]
- Iciek, M.; Bilska-Wilkosz, A.; Górny, M. Sulfane sulfur—New findings on an old topic. Acta Biochim. Pol. 2019, 66, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Toohey, J.; Cooper, A. Thiosulfoxide (Sulfane) Sulfur: New Chemistry and New Regulatory Roles in Biology. Molecules 2014, 19, 12789–12813. [Google Scholar] [CrossRef] [PubMed]
- Francioso, A.; Dupré, S.; Fontana, M. Chemistry of Outlandish Natural Products Belonging to Sulfur Metabolism: Unrevealed Green Syntheses and Separation Strategies from the Cavallini’s Old School. Separations 2022, 9, 45. [Google Scholar] [CrossRef]
- Stoppoloni, D.; Politi, L.; Leopizzi, M.; Gaetani, S.; Guazzo, R.; Basciani, S.; Moreschini, O.; De Santi, M.; Scandurra, R.; Scotto d’Abusco, A. Effect of glucosamine and its peptidyl-derivative on the production of extracellular matrix components by human primary chondrocytes. Osteoarthr. Cartil. 2015, 23, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]


| Gene Accession Number | Primer Forward Primer Reverse |
|---|---|
| IL-6 NM_000600 | 5′-GATGGATGCTTCCAATCTG-3′ 5′-CTCTAGGTATACCTCAAACTCC-3′ |
| IL-8 NM_000584 | 5′-GACATCAAAGAAGGACTTG-3′ 5′-GCCACAATTTCAGATCCTG-3′ |
| IL-1β NM_000576 | 5′-ACAGAATCTCCGACCACCACTA-3′ 5′-TCCATGGCCACAACAACTGA-3′ |
| 18S NM_003286 | 5′-CGCCGCTAGAGGTGAAATTC-3′ 5′-CATTCTTGGCAAATGCTTTCG-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mariano, A.; Bigioni, I.; Baseggio Conrado, A.; Francioso, A.; Scotto d’Abusco, A.; Fontana, M. Thiotaurine Attenuates TNF-α-Induced Inflammation in Human Chondrocytes via NF-κB Pathway Suppression and Thiol-Dependent Persulfidation. Int. J. Mol. Sci. 2025, 26, 10208. https://doi.org/10.3390/ijms262010208
Mariano A, Bigioni I, Baseggio Conrado A, Francioso A, Scotto d’Abusco A, Fontana M. Thiotaurine Attenuates TNF-α-Induced Inflammation in Human Chondrocytes via NF-κB Pathway Suppression and Thiol-Dependent Persulfidation. International Journal of Molecular Sciences. 2025; 26(20):10208. https://doi.org/10.3390/ijms262010208
Chicago/Turabian StyleMariano, Alessia, Irene Bigioni, Alessia Baseggio Conrado, Antonio Francioso, Anna Scotto d’Abusco, and Mario Fontana. 2025. "Thiotaurine Attenuates TNF-α-Induced Inflammation in Human Chondrocytes via NF-κB Pathway Suppression and Thiol-Dependent Persulfidation" International Journal of Molecular Sciences 26, no. 20: 10208. https://doi.org/10.3390/ijms262010208
APA StyleMariano, A., Bigioni, I., Baseggio Conrado, A., Francioso, A., Scotto d’Abusco, A., & Fontana, M. (2025). Thiotaurine Attenuates TNF-α-Induced Inflammation in Human Chondrocytes via NF-κB Pathway Suppression and Thiol-Dependent Persulfidation. International Journal of Molecular Sciences, 26(20), 10208. https://doi.org/10.3390/ijms262010208






