Comparative Bone-Protective Effects of Tocotrienol Isomers from Palm and Annatto in Dexamethasone-Induced Osteoporotic Male Rats
Abstract
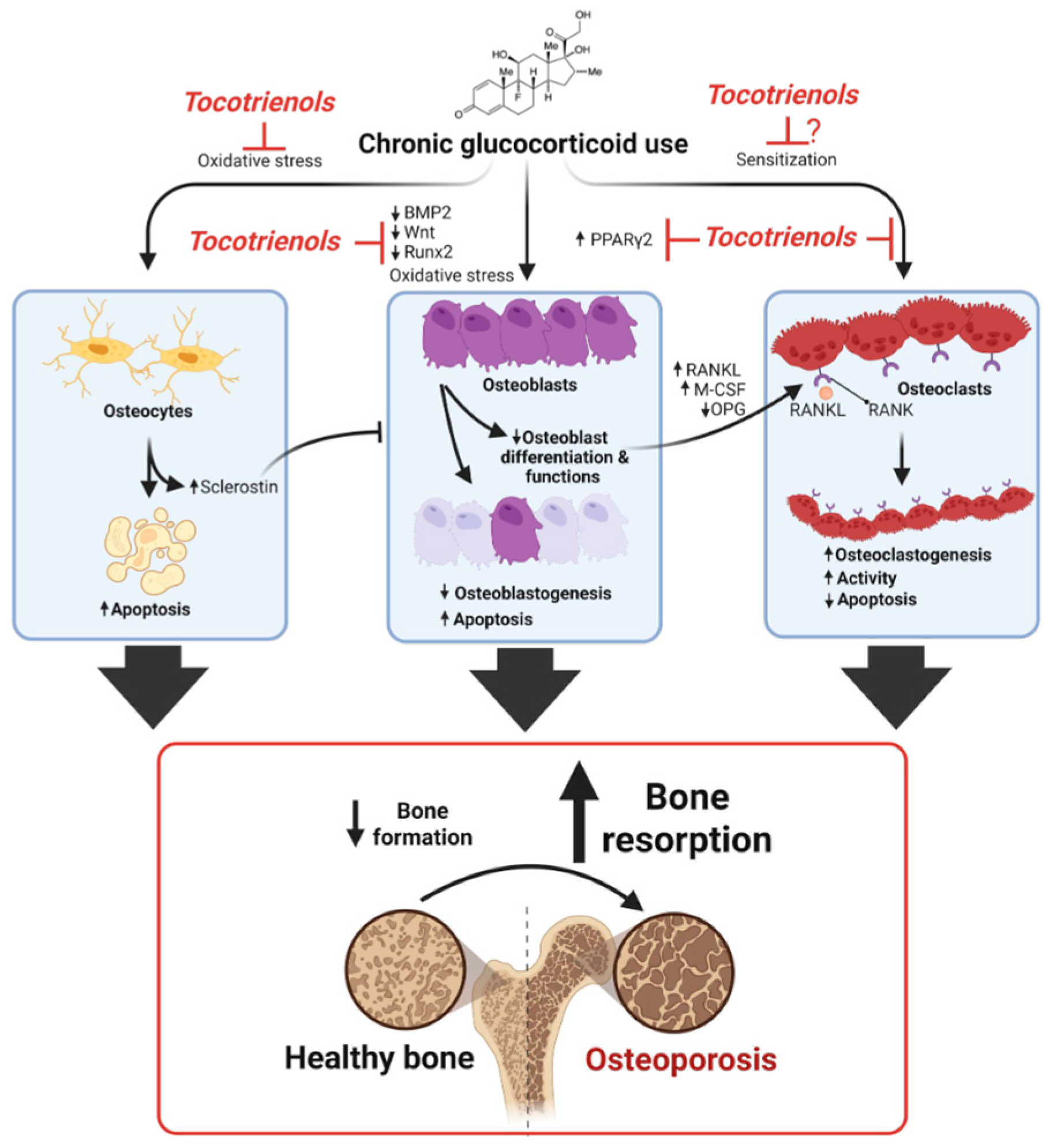
1. Introduction
2. Results
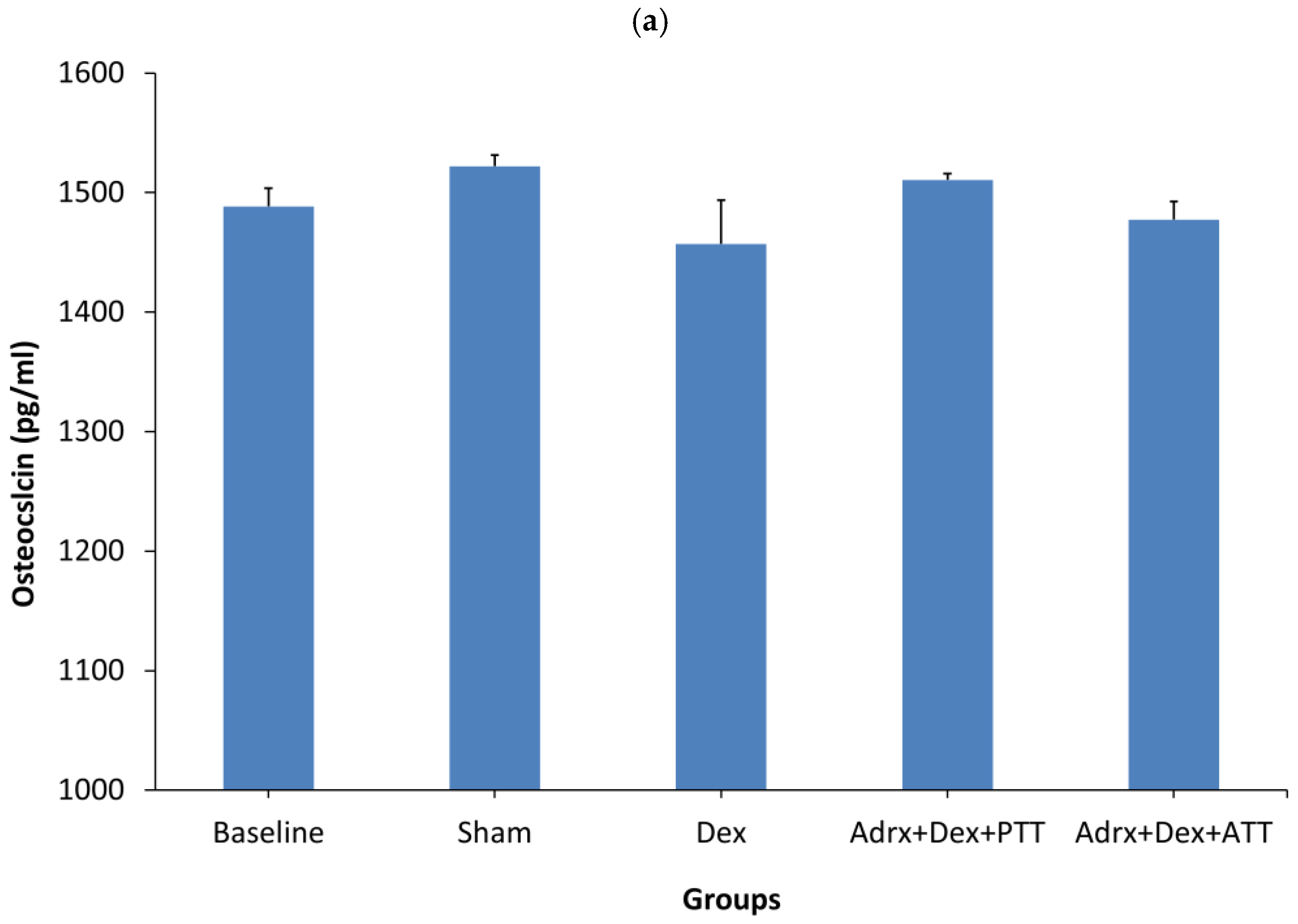
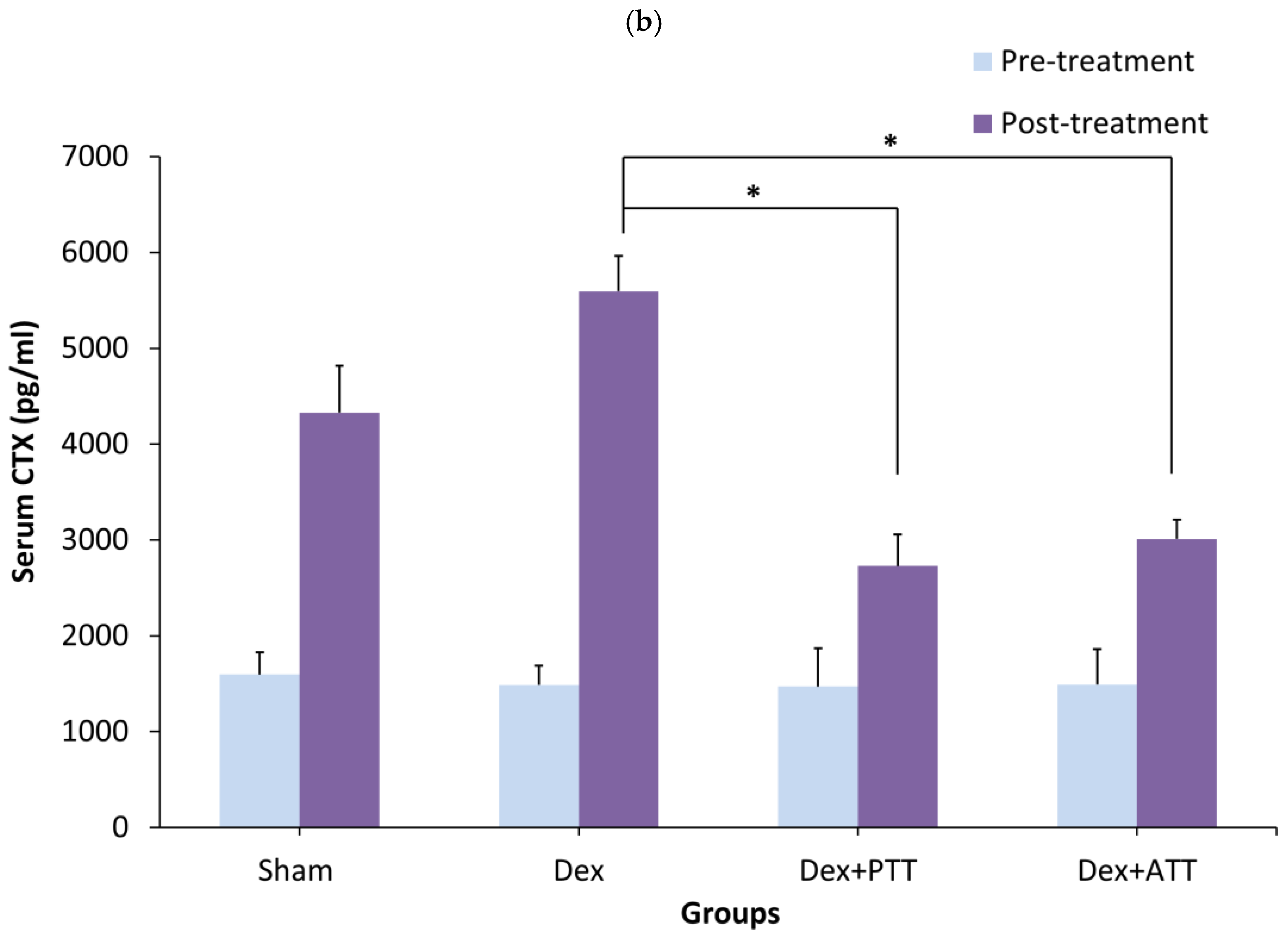
2.1. Bone Biochemical Markers
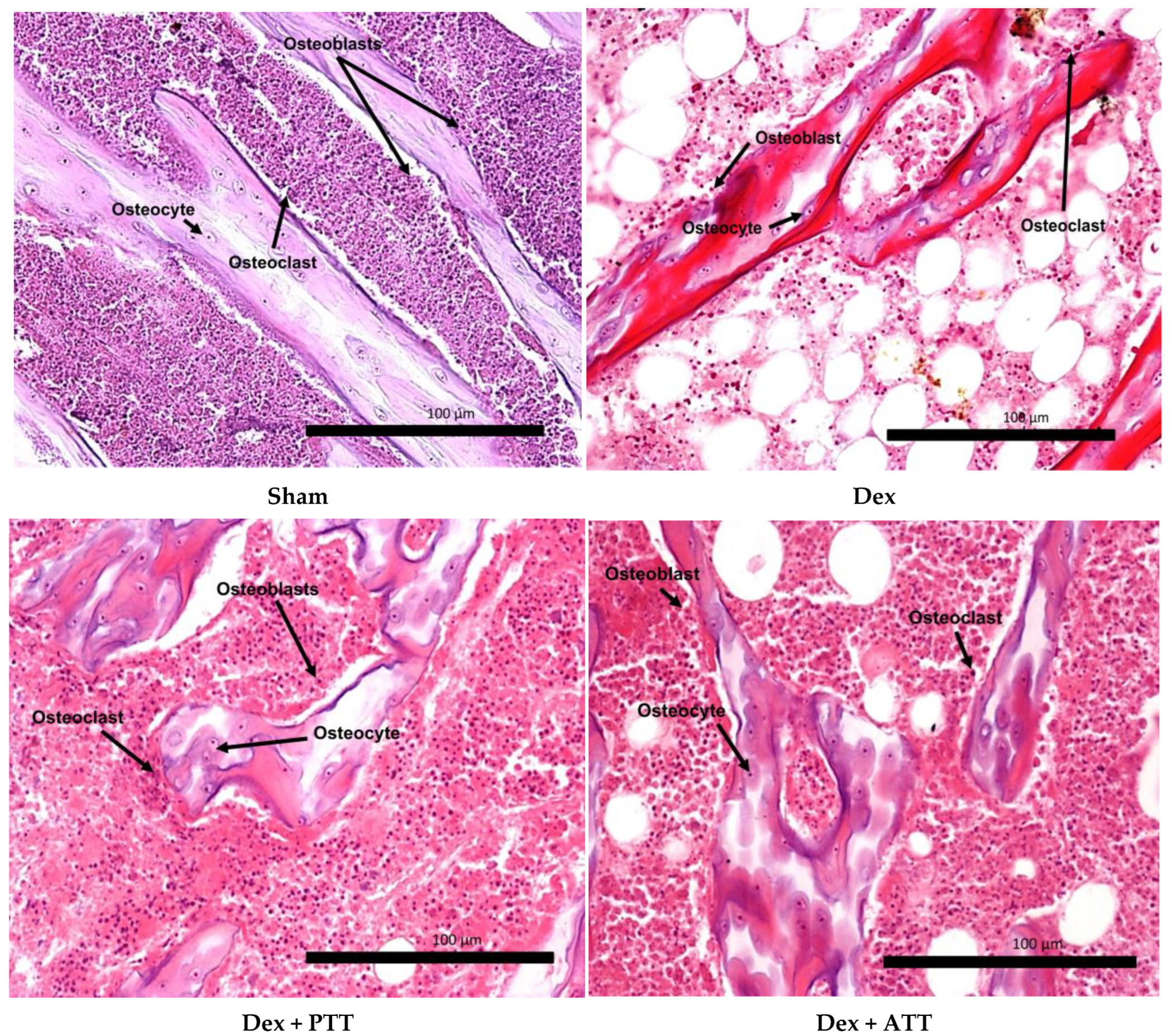
2.2. Effects of Annatto Tocotrienol on Bone Structural Histomorphometry in Dexamethasone-Induced Osteoporotic Bone
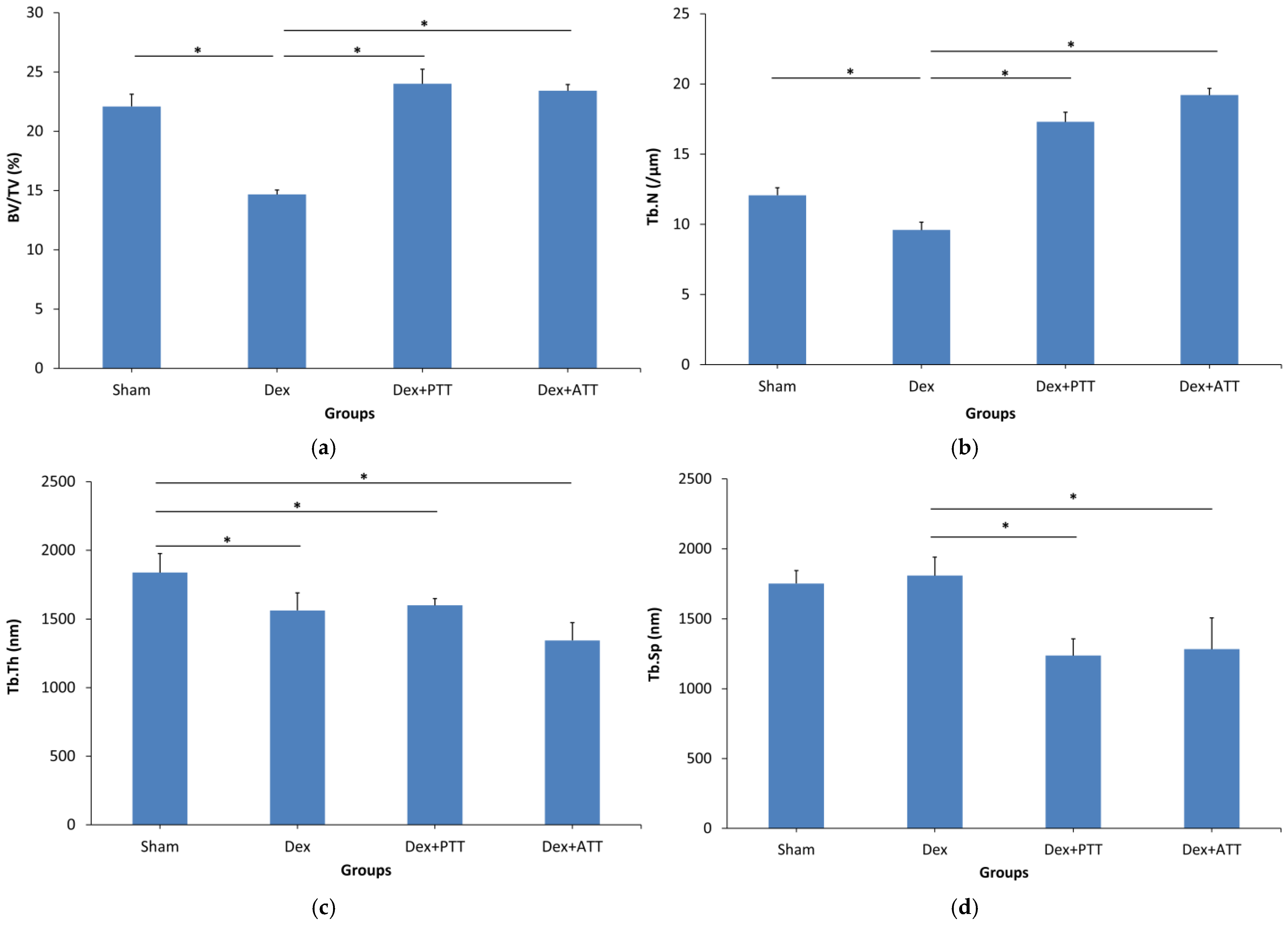
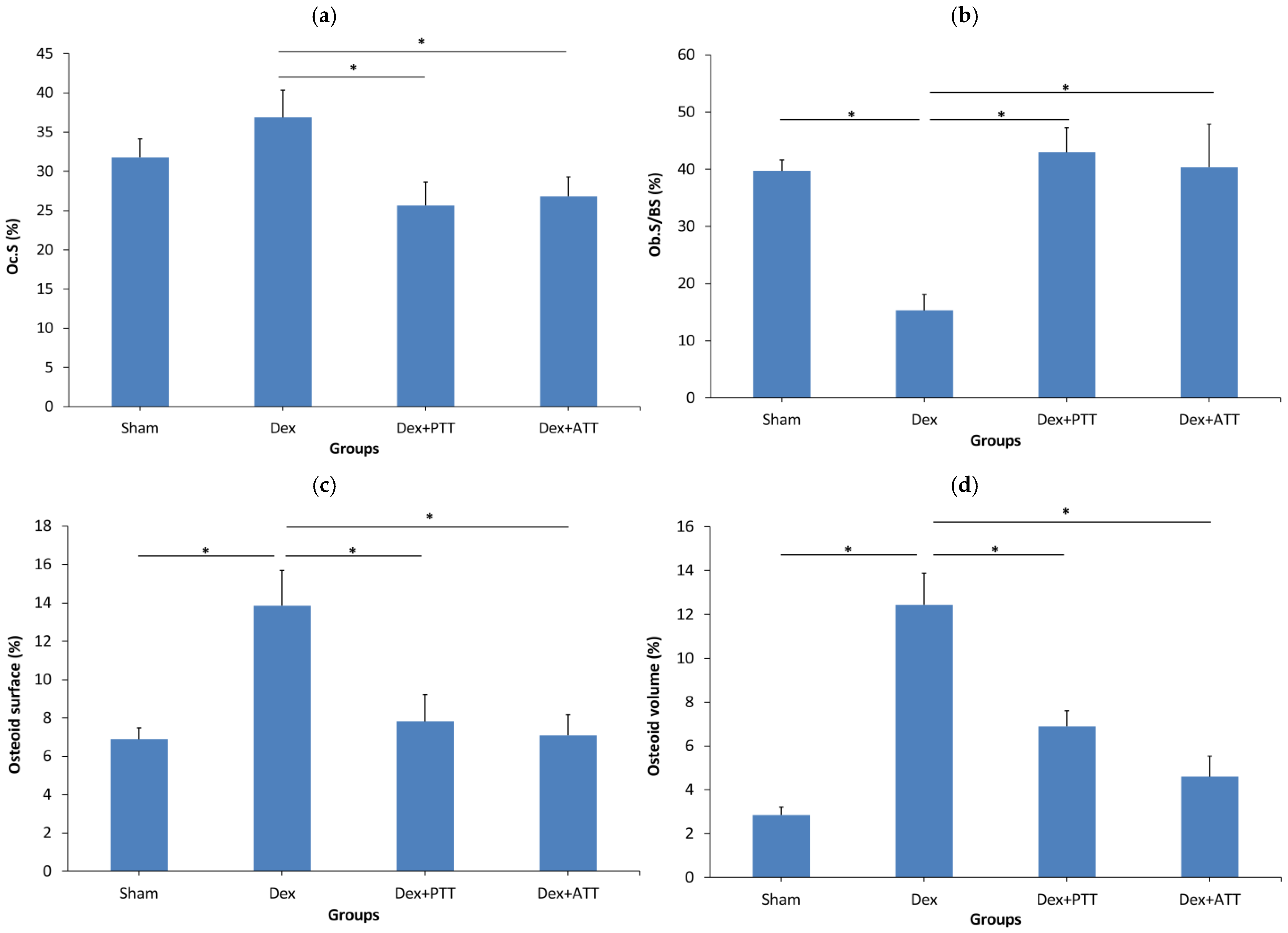
2.3. Static Parameters
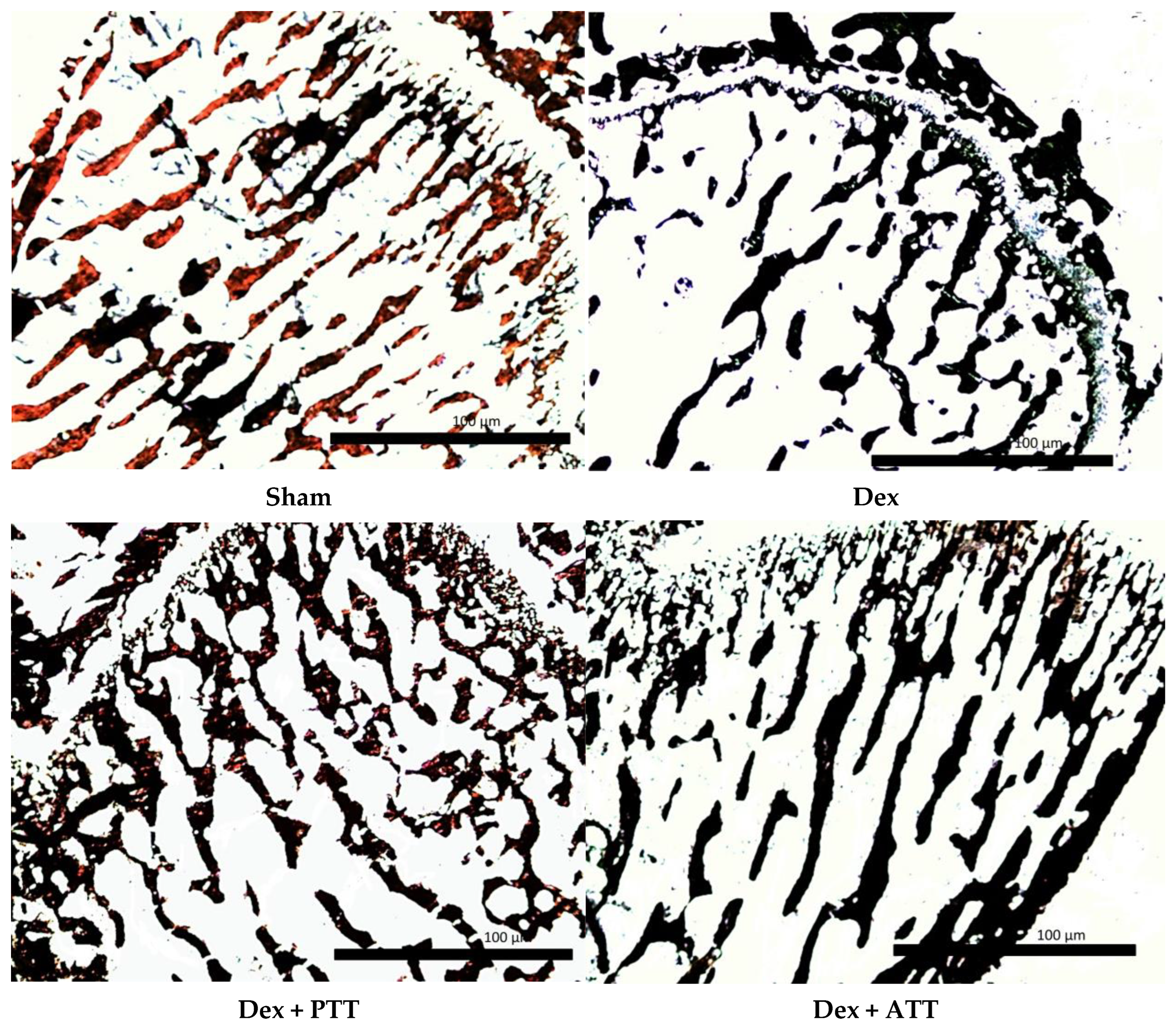
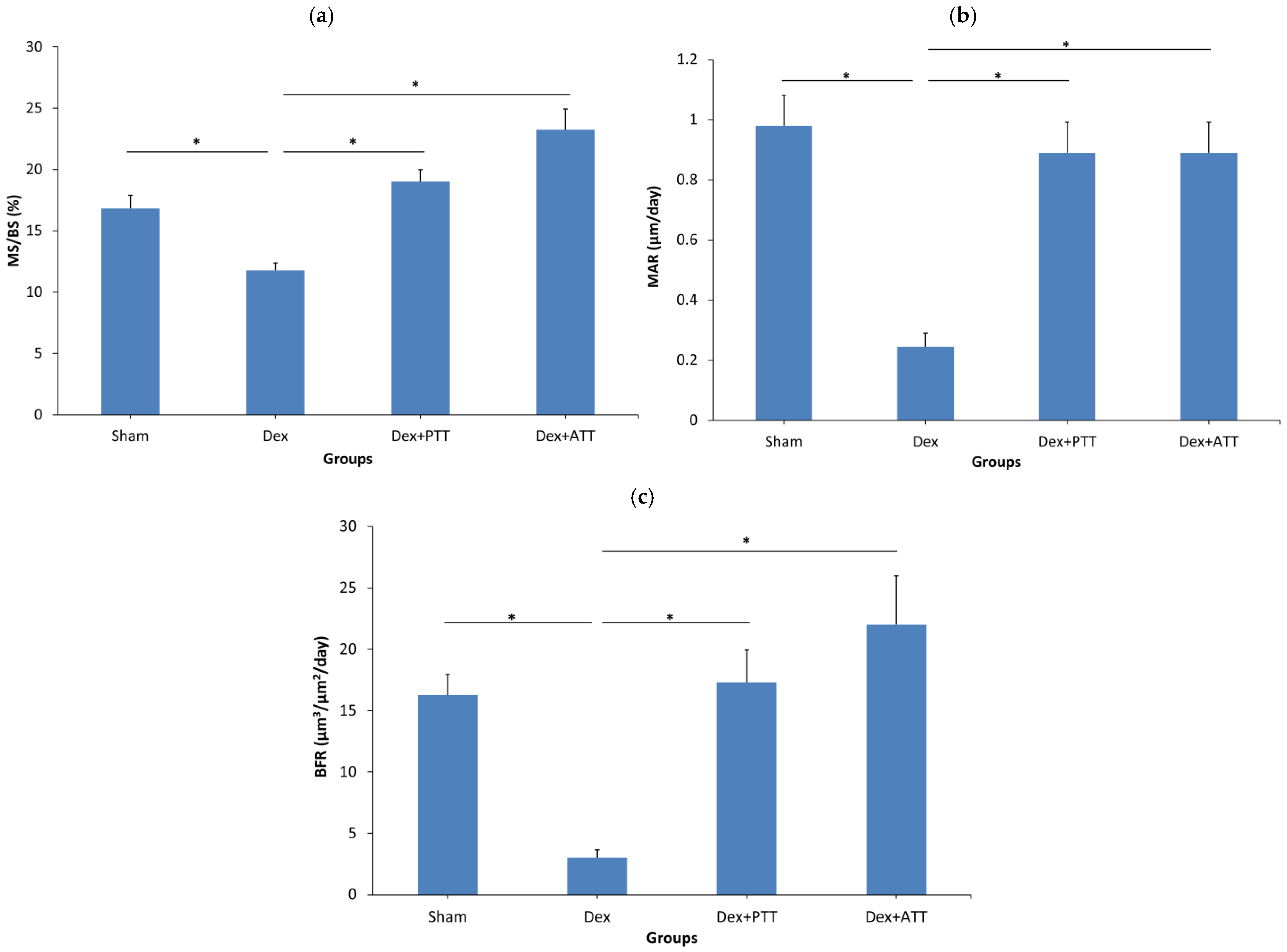
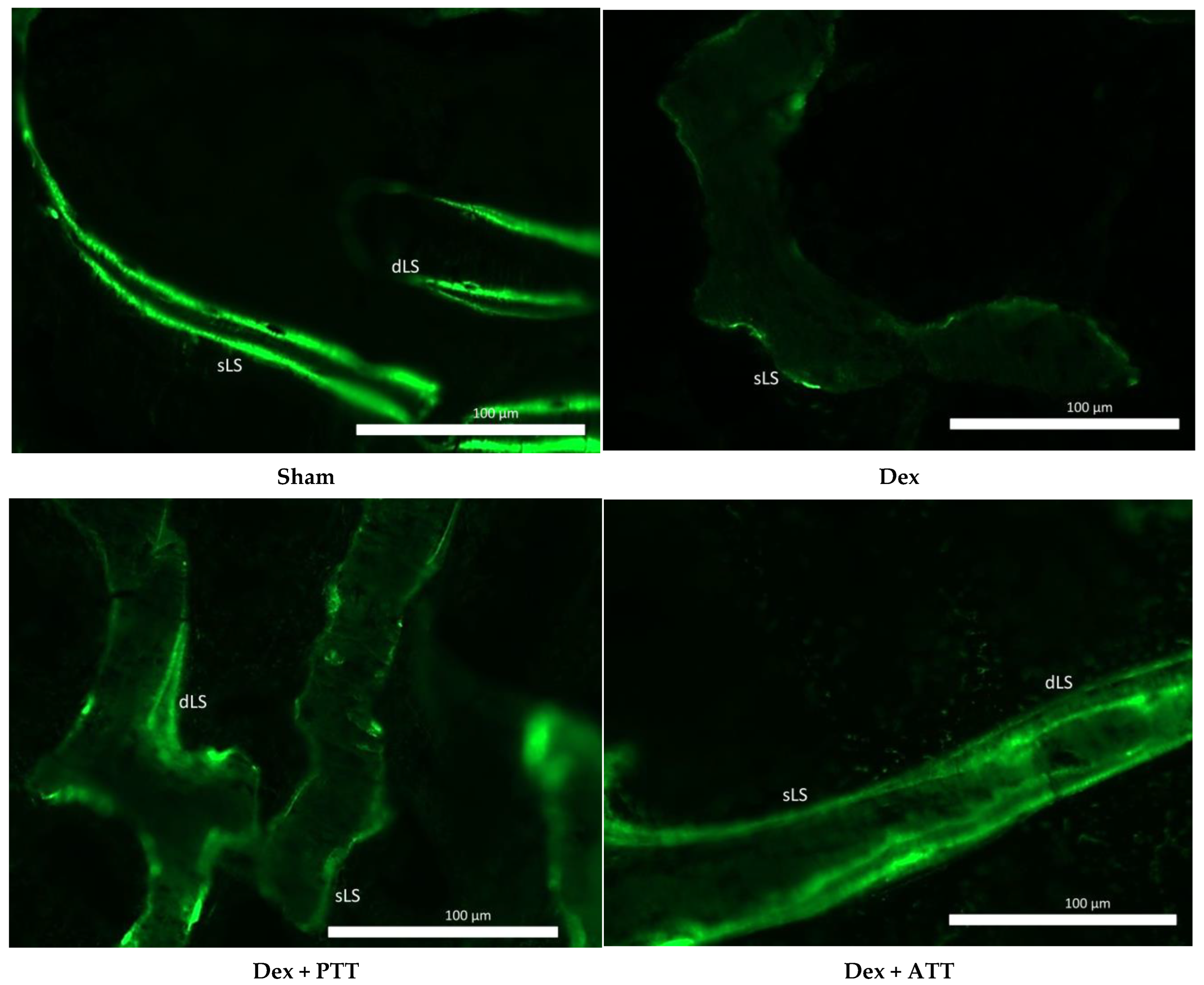
2.4. Dynamic Parameters
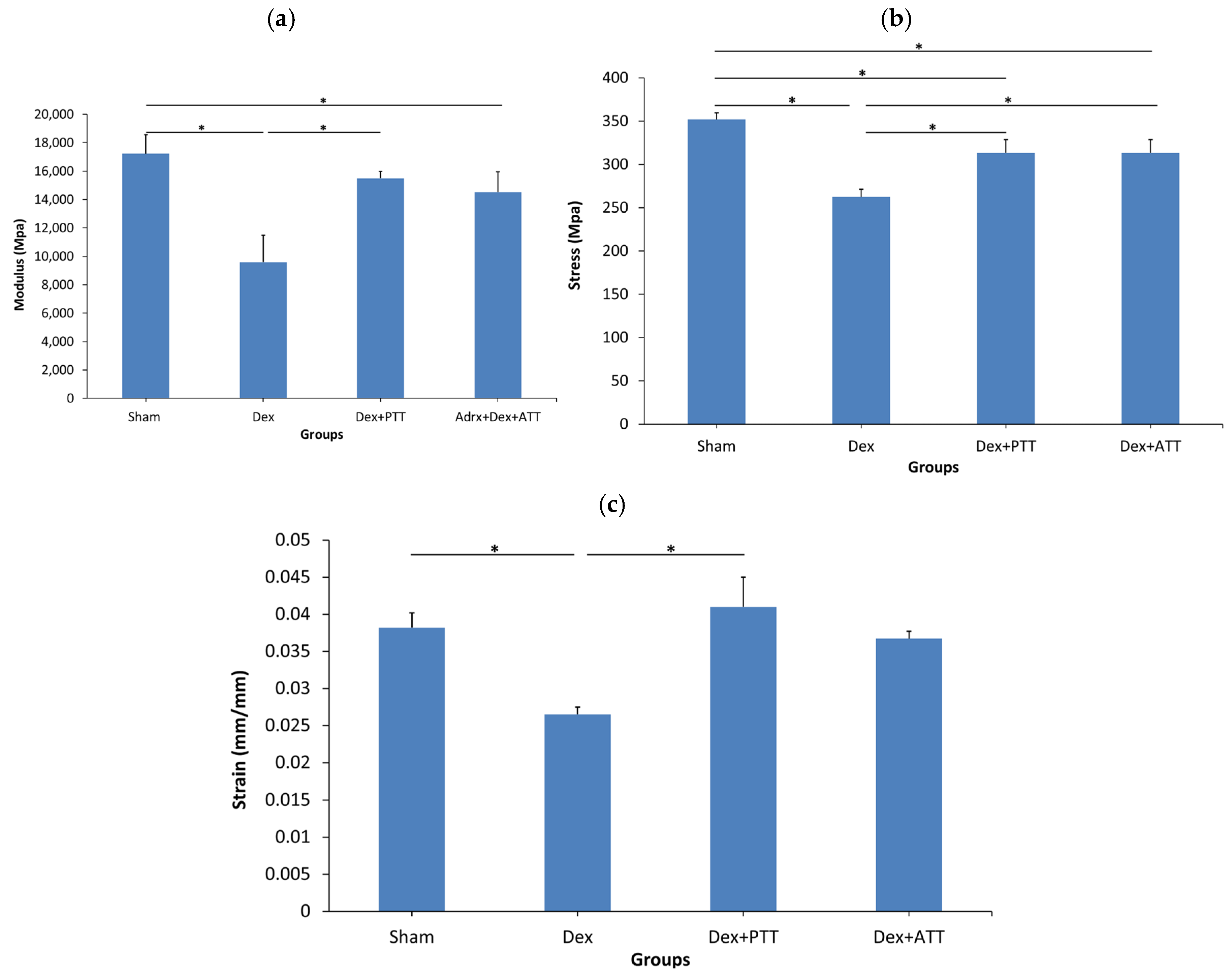
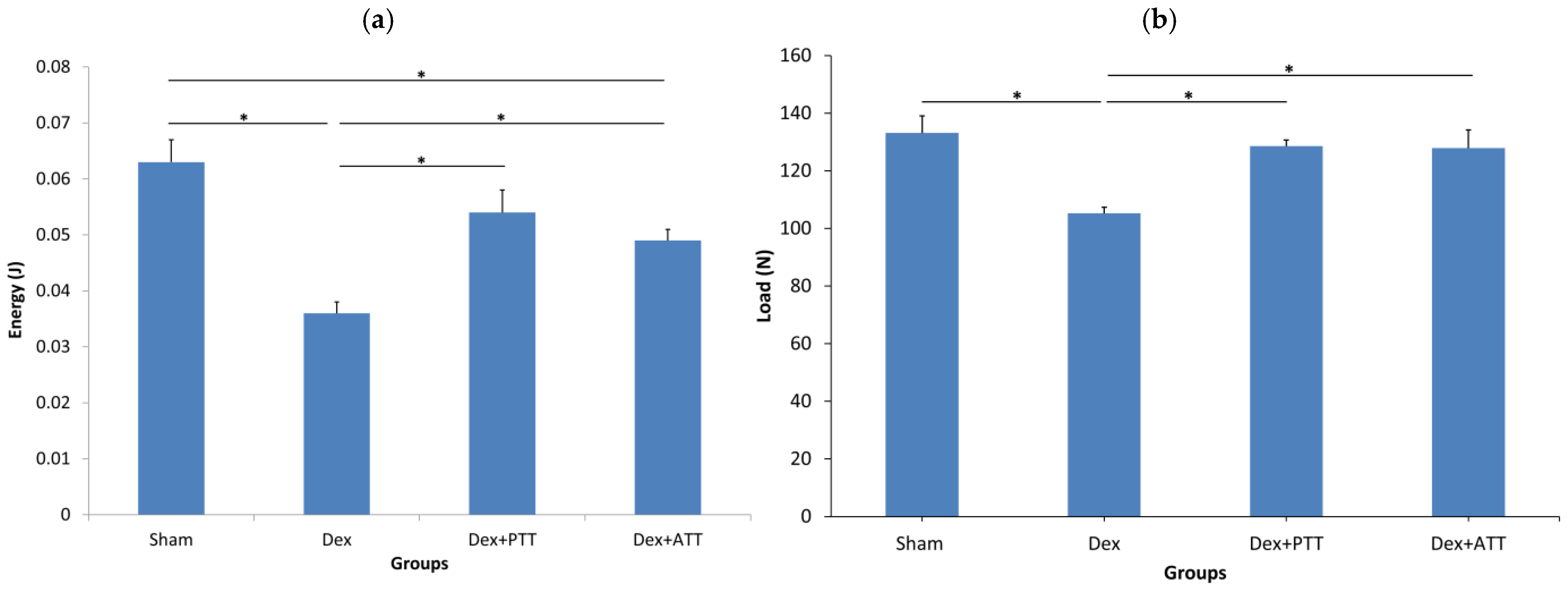
2.5. Effects of Tocotrienols on Bone Biomechanical Strength in Dexamethasone-Induced Osteoporotic Bone
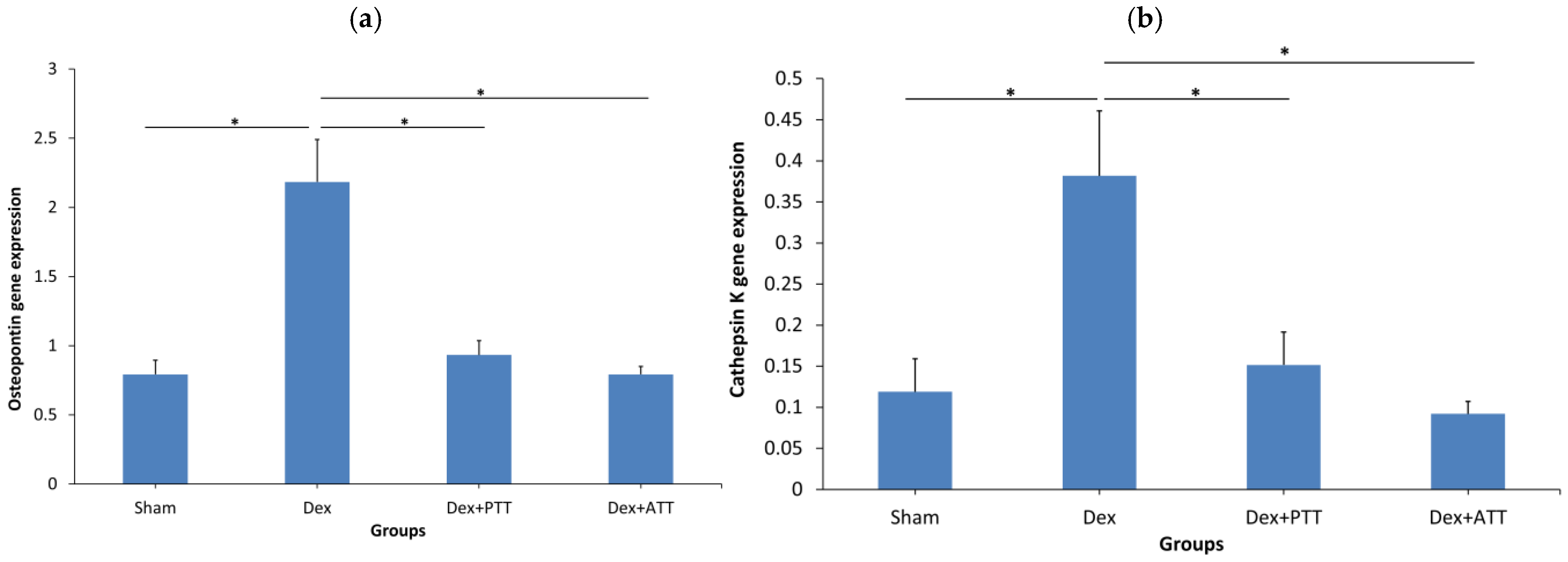
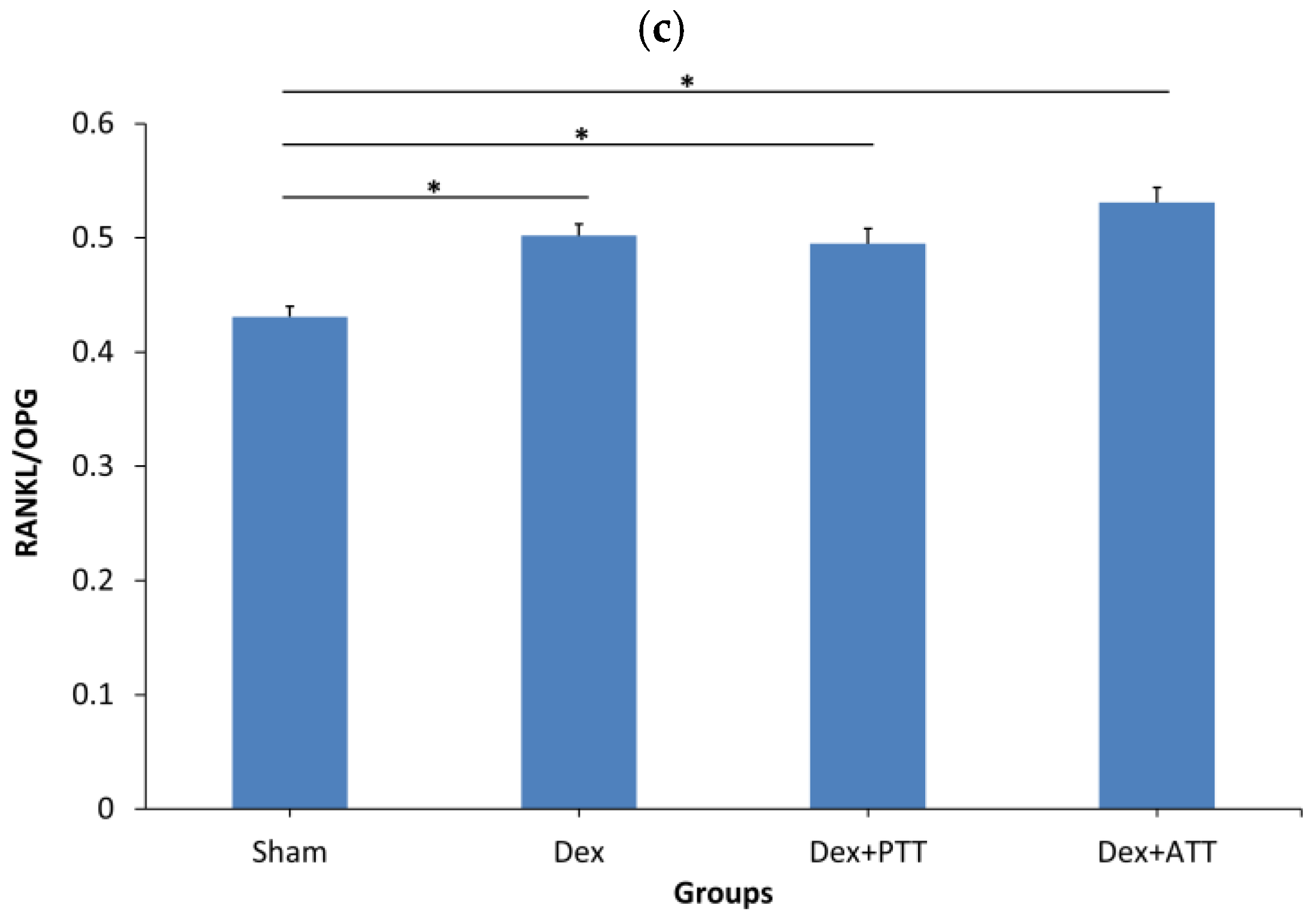
2.6. Effects of Tocotrienols on Bone Formation and Resorption Gene Expressions in Dexamethasone-Induced Osteoporotic Bone
2.7. Effects of Tocotrienols on Lipid Peroxidation and Oxidative Stress Enzymes in Dexamethasone-Induced Osteoporotic Bone
3. Discussion
4. Materials and Methods
4.1. Animals and Treatment
4.2. Sample Collection
4.3. Measurement of Serum Bone Biochemical Markers
4.4. Bone Biomechanical Test
4.5. Bone Histomorphometric Analysis
- Bone Volume/Tissue Volume (BV/TV, %): BV ÷ TV
- Trabecular Thickness (Tb.Th, µm): BV ÷ (½BS)
- Trabecular Number (Tb.N,/mm): (BV/TV) ÷ Tb.Th
- Trabecular Separation (Tb.Sp, µm): (1 − Tb.Th) ÷ Tb.N
- Single-Labeled Surface per Bone Surface (sLS/BS, %): proportion of bone surface with a single fluorescent label.
- Double-Labeled Surface per Bone Surface (dLS/BS, %): proportion of bone surface with two fluorescent labels.
- Mineralized Surface per Bone Surface (MS/BS, %): calculated as:
4.6. Gene Expression Measurements
4.7. Measurement of Lipid Peroxidation and Oxidative Stress Enzymes
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silverman, S.; Curtis, J.; Saag, K.; Flahive, J.; Adachi, J.; Anderson, F.; Chapurlat, R.; Cooper, C.; Diez-Perez, A.; Greenspan, S.; et al. International management of bone health in glucocorticoid-exposed individuals in the observational GLOW study. Osteoporos. Int. 2014, 26, 419–420. [Google Scholar] [CrossRef]
- Weinstein, R.S. Glucocorticoid-Induced Osteoporosis. Rev. Endocr. Metab. Disord. 2001, 2, 65–73. [Google Scholar] [CrossRef]
- Carbonare, L.D.; Bertoldo, F.; Valenti, M.; Zenari, S.; Zanatta, M.; Sella, S.; Giannini, S.; Cascio, V.L. Histomorphometric analysis of glucocorticoid-induced osteoporosis. Micron 2005, 36, 645–652. [Google Scholar] [CrossRef]
- Wang, L.T.; Chen, L.R.; Chen, K.H. Hormone-Related and Drug-Induced Osteoporosis: A Cellular and Molecular Overview. Int. J. Mol. Sci. 2023, 24, 5814. [Google Scholar] [CrossRef]
- Weinstein, R.S.; Chen, J.-R.; Powers, C.C.; Stewart, S.A.; Landes, R.D.; Bellido, T.; Jilka, R.L.; Parfitt, A.M.; Manolagas, S.C. Promotion of osteoclast survival and antagonism of bisphosphonate-induced osteoclast apoptosis by glucocorticoids. J. Clin. Investig. 2002, 109, 1041–1048. [Google Scholar] [CrossRef]
- Deng, S.; Dai, G.; Chen, S.; Nie, Z.; Zhou, J.; Fang, H.; Peng, H. Dexamethasone induces osteoblast apoptosis through ROS-PI3K/AKT/GSK3β signaling pathway. Biomed Pharmacother 2019, 110, 602–608. [Google Scholar] [CrossRef]
- Hodsman, A.B.; Fraher, L.J.; Ostbye, T.; Adachi, J.D.; Steer, B.M. An evaluation of several biochemical markers for bone formation and resorption in a protocol utilizing cyclical parathyroid hormone and calcitonin therapy for osteoporosis. J. Clin. Investig. 1993, 91, 1138–1148. [Google Scholar] [CrossRef]
- Kaji, H.; Sugimoto, T.; Kanatani, M.; Nishiyama, K.; Chihara, K. Dexamethasone Stimulates Osteoclast-like Cell Formation by Directly Acting on Hemopoietic Blast Cells and Enhances Osteoclast-like Cell Formation Stimulated by Parathyroid Hormone and Prostaglandin E2. J. Bone Miner. Res. 1997, 12, 734–741. [Google Scholar] [CrossRef]
- Piemontese, M.; Xiong, J.; Fujiwara, Y.; Thostenson, J.D.; O’Brien, C.A. Cortical bone loss caused by glucocorticoid excess requires RANKL production by osteocytes and is associated with reduced OPG expression in mice. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E587–E593. [Google Scholar] [CrossRef]
- Chen, M.; Fu, W.; Xu, H.; Liu, C.J. Pathogenic mechanisms of glucocorticoid-induced osteoporosis. Cytokine Growth Factor Rev 2023, 70, 54–66. [Google Scholar] [CrossRef]
- Negre-Salvayre, A.; Auge, N.; Ayala, V.; Basaga, H.; Boada, J.; Brenke, R.; Chapple, S.; Cohen, G.; Feher, J.; Grune, T.; et al. Pathological aspects of lipid peroxidation. Free Radic. Res. 2010, 44, 1125–1171. [Google Scholar] [CrossRef]
- Garrett, I.R.; Boyce, B.F.; Oreffo, R.O.; Bonewald, L.; Poser, J.; Mundy, G.R. Oxygen-derived free radicals stimulate osteoclastic bone resorption in rodent bone in vitro and in vivo. J. Clin. Investig. 1990, 85, 632–639. [Google Scholar] [CrossRef]
- Parhami, F.; Garfinkel, A.; Demer, L.L. Role of Lipids in Osteoporosis. Arter. Thromb. Vasc. Biol. 2000, 20, 2346–2348. [Google Scholar] [CrossRef]
- Guo, T.; Zhang, L.; Konermann, A.; Zhou, H.; Jin, F.; Liu, W. Manganese superoxide dismutase is required to maintain osteoclast differentiation and function under static force. Sci. Rep. 2015, 5, 8016. [Google Scholar] [CrossRef]
- Meister, M.L.; Mo, H.; Ji, X.; Shen, C. Tocotrienols in Bone Protection: Evidence from Preclinical Studies. eFood 2020, 1, 217–225. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Sundaram, C.; Prasad, S.; Kannappan, R. Tocotrienols, the vitamin E of the 21st century: Its potential against cancer and other chronic diseases. Biochem. Pharmacol. 2010, 80, 1613–1631. [Google Scholar] [CrossRef]
- Ahmad, N.; Khalid, B.; Luke, D.; Nirwana, S.I. Tocotrienol offers better protection than tocopherol from free radical-induced damage of rat bone. Clin. Exp. Pharmacol. Physiol. 2005, 32, 761–770. [Google Scholar] [CrossRef]
- Xu, H.; Watkins, B.A.; Seifert, M.F. Vitamin E stimulates trabecular bone formation and alters epiphyseal cartilage morphometry. Calcif. Tissue Int. 1995, 57, 293–300. [Google Scholar] [CrossRef]
- Chin, K.-Y.; Ima-Nirwana, S. The biological effects of tocotrienol on bone: A review on evidence from rodent models. Drug Des. Dev. Ther. 2015, 9, 2049–2060. [Google Scholar] [CrossRef]
- Shen, C.-L.; Mo, H.; Yang, S.; Wang, S.; Felton, C.K.; Tomison, M.D.; Soelaiman, I.N. Safety and efficacy of tocotrienol supplementation for bone health in postmenopausal women: Protocol for a dose-response double-blinded placebo-controlled randomised trial. BMJ Open 2016, 6, e012572. [Google Scholar] [CrossRef]
- Ahsan, H.; Ahad, A.; Iqbal, J.; Siddiqui, W.A. Pharmacological potential of tocotrienols: A review. Nutr. Metab. 2014, 11, 52. [Google Scholar] [CrossRef]
- Abdul-Majeed, S.; Mohamed, N.; Soelaiman, I.-N. Effects of Tocotrienol and Lovastatin Combination on Osteoblast and Osteoclast Activity in Estrogen-Deficient Osteoporosis. Evidence-Based Complement. Altern. Med. 2012, 2012, 960742. [Google Scholar] [CrossRef]
- Chin, K.Y. Updates in the skeletal and joint protective effects of tocotrienol: A mini review. Front. Endocrinol. 2024, 15, 1417191. [Google Scholar] [CrossRef]
- Watts, N.B.; Cooper, C.; Lindsay, R.; Eastell, R.; Manhart, M.D.; Barton, I.P.; van Staa, T.-P.; Adachi, J.D. Relationship between changes in bone mineral density and vertebral fracture risk associated with risedronate: Greater increases in bone mineral density do not relate to greater decreases in fracture risk. J. Clin. Densitom. 2004, 7, 255–261. [Google Scholar] [CrossRef]
- Wang, L.; Banu, J.; Mcmahan, C.; Kalu, D. Male rodent model of age-related bone loss in men. Bone 2001, 29, 141–148. [Google Scholar] [CrossRef]
- Suhana, M.R.E.; Farihah, H.S.; Faizah, O.; Nazrun, A.S.; Norazlina, M.; Norliza, M.; Ima-Nirwana, S. Effect of 11β-HSD1 dehydrogenase activity on bone histomorphometry of glucocorticoid-induced osteoporotic male Sprague-Dawley rats. Singap. Med. J. 2011, 52, 786–793. [Google Scholar]
- Ima-Nirwana, S.; Suhaniza, S. Effects of Tocopherols and Tocotrienols on Body Composition and Bone Calcium Content in Adrenalectomized Rats Replaced with Dexamethasone. J. Med. Food 2004, 7, 45–51. [Google Scholar] [CrossRef]
- Ibáñez, L.; Ferrándiz, M.L.; Brines, R.; Guede, D.; Cuadrado, A.; Alcaraz, M.J. Effects of Nrf2 Deficiency on Bone Microarchitecture in an Experimental Model of Osteoporosis. Oxidative Med. Cell. Longev. 2014, 2014, 726590. [Google Scholar] [CrossRef]
- Kim, J.; Yang, J.; Park, O.-J.; Kang, S.-S.; Kim, W.-S.; Kurokawa, K.; Yun, C.-H.; Kim, H.-H.; Lee, B.L.; Han, S.H. Lipoproteins are an important bacterial component responsible for bone destruction through the induction of osteoclast differentiation and activation. J. Bone Miner. Res. 2013, 28, 2381–2391. [Google Scholar] [CrossRef]
- Baek, K.H.; Oh, K.W.; Lee, W.Y.; Lee, S.S.; Kim, M.K.; Kwon, H.S.; Rhee, E.J.; Han, J.H.; Song, K.H.; Cha, B.Y.; et al. Association of oxidative stress with postmenopausal osteoporosis and the effects of hydrogen peroxide on osteoclast formation in human bone marrow cell cultures. Calcif. Tissue Int. 2010, 87, 226–235. [Google Scholar] [CrossRef]
- Parhami, F.; Morrow, A.D.; Balucan, J.; Leitinger, N.; Watson, A.D.; Tintut, Y.; Berliner, J.A.; Demer, L.L. Lipid oxidation products have opposite effects on calcifying vascular cell and bone cell differentiation. A possible explanation for the paradox of arterial calcification in osteoporotic patients. Arter. Thromb. Vasc. Biol. 1997, 17, 680–687. [Google Scholar] [CrossRef]
- Imai, H.; Nakagawa, Y. Biological significance of phospholipid hydroperoxide glutathione peroxidase (PHGPx, GPx4) in mammalian cells. Free Radic. Biol. Med. 2003, 34, 145–169. [Google Scholar] [CrossRef]
- Zhu, L.-J.; Liu, M.-Y.; Li, H.; Liu, X.; Chen, C.; Han, Z.; Wu, H.-Y.; Jing, X.; Zhou, H.-H.; Suh, H.; et al. The different roles of glucocorticoids in the hippocampus and hypothalamus in chronic stress-induced HPA axis hyperactivity. PLoS ONE 2014, 9, e97689. [Google Scholar] [CrossRef]
- Niki, E.; Yoshida, Y.; Saito, Y.; Noguchi, N. Lipid peroxidation: Mechanisms, inhibition, and biological effects. Biochem. Biophys. Res. Commun. 2005, 338, 668–676. [Google Scholar] [CrossRef]
- Chotiyarnwong, P.; McCloskey, E.V. Pathogenesis of glucocorticoid-induced osteoporosis and options for treatment. Nat. Rev. Endocrinol. 2020, 16, 437–447. [Google Scholar] [CrossRef]
- Norazlina, M.; Hermizi, H.; Faizah, O.; Nazrun, A.S.; Norliza, M.; Ima-Nirwana, S. Vitamin E reversed nicotine-induced toxic effects on bone biochemical markers in male rats. Arch. Med. Sci. 2010, 4, 505–512. [Google Scholar] [CrossRef]
- Chin, K.-Y.; Ima-Nirwana, S. Effects of annatto-derived tocotrienol supplementation on osteoporosis induced by testosterone deficiency in rats. Clin. Interv. Aging 2014, 9, 1247–1259. [Google Scholar] [CrossRef]
- Soelaiman, I.N.; Ming, W.; Abu Bakar, R.; Hashnan, N.A.; Ali, H.M.; Mohamed, N.; Muhammad, N.; Shuid, A.N. Palm tocotrienol supplementation enhanced bone formation in oestrogen-deficient rats. Int. J. Endocrinol. 2012, 2012, 532862. [Google Scholar] [CrossRef]
- Holla, A.; Vidyasagar, S.; Nandakrishna, B.; Bairy, L.; Shastry, B.A.; Kamath, A.; Adiga, S. Effect of glucocorticoids exposure on serum osteocalcin levels. Indian Drugs 2019, 56, 54–58. [Google Scholar] [CrossRef]
- Dempster, D.W. The pathophysiology of bone loss. Clin. Geriatr. Med. 2003, 19, 259–270. [Google Scholar] [CrossRef]
- Chavassieux, P.M.; Arlot, M.E.; Roux, J.P.; Portero, N.; Daifotis, A.; Yates, A.J.; Hamdy, N.A.T.; Malice, M.-P.; Freedholm, D.; Meunier, P.J. Effects of alendronate on bone quality and remodeling in glucocorticoid-induced osteoporosis: A histomorphometric analysis of transiliac biopsies. J. Bone Miner. Res. 2000, 15, 754–762. [Google Scholar] [CrossRef]
- Weinstein, R.S.; Jilka, R.L.; Parfitt, A.M.; Manolagas, S.C. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids. Potential mechanisms of their deleterious effects on bone. J. Clin. Investig. 1998, 102, 274–282. [Google Scholar] [CrossRef]
- Jensen, P.R.; Andersen, T.L.; Hauge, E.-M.; Bollerslev, J.; Delaissé, J.-M. A joined role of canopy and reversal cells in bone remodeling—Lessons from glucocorticoid-induced osteoporosis. Bone 2015, 73, 16–23. [Google Scholar] [CrossRef]
- Bruzzaniti, A.; Baron, R. Molecular regulation of osteoclast activity. Rev. Endocr. Metab. Disord. 2006, 7, 123–139. [Google Scholar] [CrossRef]
- Canalis, E.; Bilezikian, J.P.; Angeli, A.; Giustina, A. Perspectives on glucocorticoid-induced osteoporosis. Bone 2004, 34, 593–598. [Google Scholar] [CrossRef]
- Chen, Y.H.; Peng, S.Y.; Cheng, M.T.; Hsu, Y.P.; Huang, Z.X.; Cheng, W.T.; Wu, S.C. Different susceptibilities of osteoclasts and osteoblasts to glucocorticoid-induced oxidative stress and mitochondrial alterations. Chin J Physiol 2019, 62, 70–79. [Google Scholar] [CrossRef]
- Lems, W.F.; Dijkmans, B.A.C. Should we look for osteoporosis in patients with rheumatoid arthritis? Ann. Rheum. Dis. 1998, 57, 325–327. [Google Scholar] [CrossRef]
- Kim, Y.H.; Jun, J.-H.; Woo, K.M.; Ryoo, H.-M.; Kim, G.-S.; Baek, J.-H. Dexamethasone inhibits the formation of multinucleated osteoclastsvia down-regulation of β3 integrin expression. Arch. Pharmacal Res. 2006, 29, 691–698. [Google Scholar] [CrossRef]
- Jia, J.; Yao, W.; Guan, M.; Dai, W.; Shahnazari, M.; Kar, R.; Bonewald, L.; Jiang, J.X.; Lane, N.E. Glucocorticoid dose determines osteocyte cell fate. FASEB J. 2011, 25, 3366–3376. [Google Scholar] [CrossRef]
- Søe, K.; Delaissé, J.-M. Glucocorticoids maintain human osteoclasts in the active mode of their resorption cycle. J. Bone Miner. Res. 2010, 25, 2184–2192. [Google Scholar] [CrossRef]
- Faralli, J.A.; Gagen, D.; Filla, M.S.; Crotti, T.N.; Peters, D.M. Dexamethasone increases αvβ3 integrin expression and affinity through a calcineurin/NFAT pathway. Biochim. Biophys. Acta BBA Mol. Cell Res. 2013, 1833, 3306–3313. [Google Scholar] [CrossRef]
- Reinholt, F.P.; Hultenby, K.; Oldberg, A.; Heinegård, D. Osteopontin—A possible anchor of osteoclasts to bone. Proc. Natl. Acad. Sci. USA 1990, 87, 4473–4475. [Google Scholar] [CrossRef]
- Wei, Q.-S.; Huang, L.; Tan, X.; Chen, Z.-Q.; Chen, S.-M.; Deng, W.-M. Serum osteopontin levels in relation to bone mineral density and bone turnover markers in postmenopausal women. Scand. J. Clin. Lab. Investig. 2015, 76, 33–39. [Google Scholar] [CrossRef]
- Hofbauer, L.C.; Kühne, C.A.; Viereck, V. The OPG/RANKL/RANK system in metabolic bone diseases. J. Musculoskelet. Neuronal Interact. 2004, 4, 268–275. [Google Scholar]
- Sivagurunathan, S.; Muir, M.M.; Brennan, T.C.; Seale, J.P.; Mason, R.S. Influence of Glucocorticoids on Human Osteoclast Generation and Activity. J. Bone Miner. Res. 2005, 20, 390–398. [Google Scholar] [CrossRef]
- Hofbauer, L.C.; Gori, F.; Riggs, B.L.; Lacey, D.L.; Dunstan, C.R.; Spelsberg, T.C.; Khosla, S. Stimulation of osteoprotegerin ligand and inhibition of osteoprotegerin production by glucocorticoids in human osteoblastic lineage cells: Potential paracrine mechanisms of glucocorticoid-induced osteoporosis. Endocrinology 1999, 140, 4382–4389. [Google Scholar] [CrossRef]
- Shiotani, A.; Takami, M.; Itoh, K.; Shibasaki, Y.; Sasaki, T. Regulation of osteoclast differentiation and function by receptor activator of NFkB ligand and osteoprotegerin. Anat. Rec. 2002, 268, 137–146. [Google Scholar] [CrossRef]
- Cascio, V.L.; Kanis, J.A.; Beneton, M.N.C.; Bertoldo, F.; Adami, S.; Poggi, G.; Zanolin, M.E. Acute effects of deflazacort and prednisone on rates of mineralization and bone formation. Calcif. Tissue Int. 1995, 56, 109–112. [Google Scholar] [CrossRef]
- Frenkel, B.; White, W.; Tuckermann, J. Glucocorticoid-Induced Osteoporosis. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2015; Volume 872, pp. 179–215. [Google Scholar] [CrossRef]
- Dempster, D.W.; Arlot, M.A.; Meunier, P.J. Mean wall thickness and formation periods of trabecular bone packets in corticosteroid-induced osteoporosis. Calcif. Tissue Int. 1983, 35, 410–417. [Google Scholar] [CrossRef]
- Osterhoff, G.; Morgan, E.F.; Shefelbine, S.J.; Karim, L.; McNamara, L.M.; Augat, P. Bone mechanical properties and changes with osteoporosis. Injury 2016, 47 (Suppl. S2), S11–S20. [Google Scholar] [CrossRef]
- Shuid, A.N.; Azman, K.F.; Muhammad, N.; Mohamed, N.; Soelaiman, I.N. Effects of Palm Tocotrienols on Oxidative Stress and Bone Strength in Ovariectomised Rats. Med. Health 2008, 3, 247–255. [Google Scholar]
- Shuid, A.N.; Mehat, Z.; Mohamed, N.; Muhammad, N.; Soelaiman, I.N. Vitamin E exhibits bone anabolic actions in normal male rats. J. Bone Min. Metab. 2010, 28, 149–156. [Google Scholar] [CrossRef]
- Chin, K.-Y.; Mo, H.; Soelaiman, I.-N. A Review of the Possible Mechanisms of Action of Tocotrienol—A Potential Antiosteoporotic Agent. Curr. Drug Targets 2013, 14, 1533–1541. [Google Scholar] [CrossRef]
- Plotkin, L.I.; Weinstein, R.S.; Parfitt, A.M.; Roberson, P.K.; Manolagas, S.C.; Bellido, T. Prevention of osteocyte and osteoblast apoptosis by bisphosphonates and calcitonin. J. Clin. Investig. 1999, 104, 1363–1374. [Google Scholar] [CrossRef]
- Weinstein, R.S. Glucocorticoids, osteocytes, and skeletal fragility: The role of bone vascularity. Bone 2010, 46, 564–570. [Google Scholar] [CrossRef]
- Nielsen, H.K. Circadian and circatrigintan changes in osteoblastic activity assessed by serum osteocalcin. Physiological and methodological aspects. Dan. Med. Bull. 1994, 41, 216–227. [Google Scholar]
- Chin, K.-Y.; Abdul-Majeed, S.; Fozi, N.F.M.; Ima-Nirwana, S. Annatto Tocotrienol Improves Indices of Bone Static Histomorphometry in Osteoporosis Due to Testosterone Deficiency in Rats. Nutrients 2014, 6, 4974–4983. [Google Scholar] [CrossRef]
- Mohamad, N.-V.; Ima-Nirwana, S.; Chin, K.-Y. Therapeutic potential of annatto tocotrienol with self-emulsifying drug delivery system in a rat model of postmenopausal bone loss. Biomed. Pharmacother. 2021, 137, 111368. [Google Scholar] [CrossRef]
- Ramli, E.S.M.; Ahmad, F.; Salleh, N.I.; Ahmed, A.I.; Hee, C.C.; Kumar, S.N.; Abdullah, M.I.; Yahaya, F.; Soelaiman, I.N. Beneficial Effects of Annatto (Bixa orellana) Tocotrienol on Bone Histomorphometry and Expression of Genes Related to Bone Formation and Resorption in Osteoporosis Induced by Dexamethasone. Int. J. Med. Res. Health Sci. 2018, 7, 85–100. [Google Scholar]
- Maniam, S.; Mohamed, N.; Shuid, A.N.; Soelaiman, I.N. Palm Tocotrienol Exerted Better Antioxidant Activities in Bone than α-Tocopherol. Basic Clin. Pharmacol. Toxicol. 2008, 103, 55–60. [Google Scholar] [CrossRef]
- Fujita, K.; Iwasaki, M.; Ochi, H.; Fukuda, T.; Ma, C.; Miyamoto, T.; Takitani, K.; Negishi-Koga, T.; Sunamura, S.; Kodama, T.; et al. Vitamin E decreases bone mass by stimulating osteoclast fusion. Nat. Med. 2012, 18, 589–594, Erratum in: Nat. Med. 2012, 18, 1445. [Google Scholar] [CrossRef]
- Hermizi, H.; Faizah, O.; Ima-Nirwana, S.; Ahmad Nazrun, S.; Norazlina, M. Beneficial effects of tocotrienol and tocopherol on bone histomorphometric parameters in sprague-dawley male rats after nicotine cessation. Calcif. Tissue Int. 2009, 84, 65–74. [Google Scholar] [CrossRef]
- Packer, L.; Weber, S.U.; Rimbach, G. Molecular Aspects of α-Tocotrienol Antioxidant Action and Cell Signalling. J. Nutr. 2001, 131, 369S–373S. [Google Scholar] [CrossRef]
- Dresner-Pollak, R.; Rosenblatt, M. Blockade of osteoclast-mediated bone resorption through occupancy of the integrin receptor: A potential approach to the therapy of osteoporosis. J. Cell. Biochem. 1994, 56, 323–330. [Google Scholar] [CrossRef]
- Shapses, S.A.; Cifuentes, M.; Spevak, L.; Chowdhury, H.; Brittingham, J.; Boskey, A.L.; Denhardt, D.T. Osteopontin facilitates bone resorption, decreasing bone mineral crystallinity and content during calcium deficiency. Calcif. Tissue Int. 2003, 73, 86–92. [Google Scholar] [CrossRef]
- Koga, T.; Matsui, Y.; Asagiri, M.; Kodama, T.; de Crombrugghe, B.; Nakashima, K.; Takayanagi, H. NFAT and Osterix cooperatively regulate bone formation. Nat. Med. 2005, 11, 880–885. [Google Scholar] [CrossRef]
- Abukhadir, S.S.A.; Mohamed, N.; Makpol, S.; Muhammad, N. Effects of Palm Vitamin E on Bone-Formation-Related Gene Expression in Nicotine-Treated Rats. Evid. Based Complement. Altern. Med. 2012, 2012, 656025. [Google Scholar] [CrossRef]
- Haffa, A.; Krueger, D.; Bruner, J.; Engelke, J.; Gundberg, C.; Akhter, M.; Binkley, N. Diet- or warfarin-induced vitamin K insufficiency elevates circulating undercarboxylated osteocalcin without altering skeletal status in growing female rats. J. Bone Miner. Res. 2000, 15, 872–878. [Google Scholar] [CrossRef]
- Turner, C.H. Determinants of skeletal fragility and bone quality. J. Musculoskelet. Neuronal Interact. 2002, 2, 527–528. [Google Scholar]
- Parfitt, A.M.; Drezner, M.K.; Glorieux, F.H.; Kanis, J.A.; Malluche, H.; Meunier, P.J.; Ott, S.M.; Recker, R.R. Bone histomorphometry: Standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 1987, 2, 595–610. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramli, E.S.M.; Ahmad, F.; Kamaruddin, N.A.; Chin, K.-Y.; Soelaiman, I.N.; Pang, K.-L. Comparative Bone-Protective Effects of Tocotrienol Isomers from Palm and Annatto in Dexamethasone-Induced Osteoporotic Male Rats. Int. J. Mol. Sci. 2025, 26, 10206. https://doi.org/10.3390/ijms262010206
Ramli ESM, Ahmad F, Kamaruddin NA, Chin K-Y, Soelaiman IN, Pang K-L. Comparative Bone-Protective Effects of Tocotrienol Isomers from Palm and Annatto in Dexamethasone-Induced Osteoporotic Male Rats. International Journal of Molecular Sciences. 2025; 26(20):10206. https://doi.org/10.3390/ijms262010206
Chicago/Turabian StyleRamli, Elvy Suhana Mohd, Fairus Ahmad, Nur Aqilah Kamaruddin, Kok-Yong Chin, Ima Nirwana Soelaiman, and Kok-Lun Pang. 2025. "Comparative Bone-Protective Effects of Tocotrienol Isomers from Palm and Annatto in Dexamethasone-Induced Osteoporotic Male Rats" International Journal of Molecular Sciences 26, no. 20: 10206. https://doi.org/10.3390/ijms262010206
APA StyleRamli, E. S. M., Ahmad, F., Kamaruddin, N. A., Chin, K.-Y., Soelaiman, I. N., & Pang, K.-L. (2025). Comparative Bone-Protective Effects of Tocotrienol Isomers from Palm and Annatto in Dexamethasone-Induced Osteoporotic Male Rats. International Journal of Molecular Sciences, 26(20), 10206. https://doi.org/10.3390/ijms262010206






