Interplay Between KLF4, STAT, IRF, and NF-κB in VSMC and Macrophage Plasticity During Vascular Inflammation and Atherosclerosis
Abstract
1. Introduction
2. VSMC and MØ Plasticity in Atherosclerosis
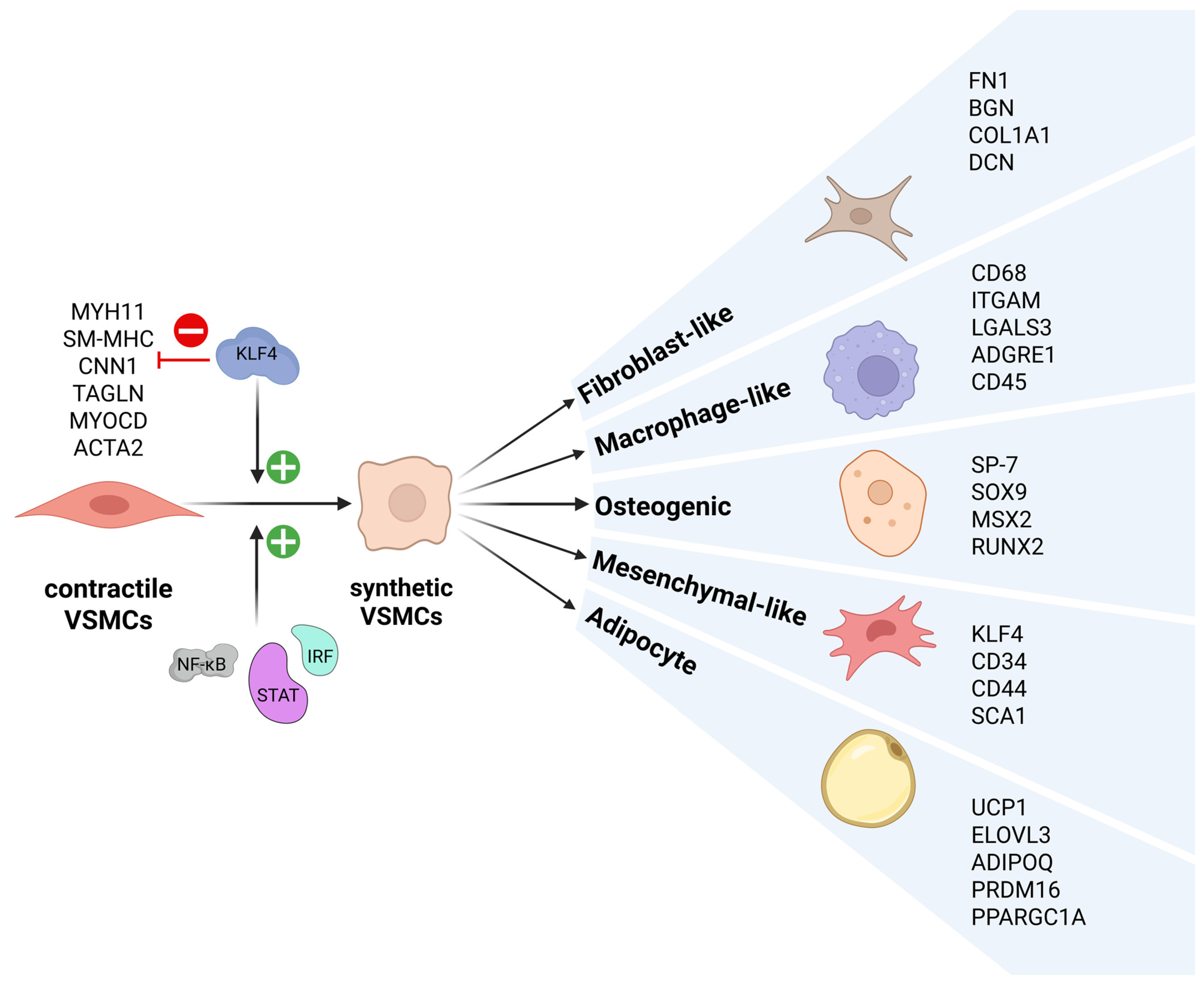
2.1. VSMC Phenotypic Switching
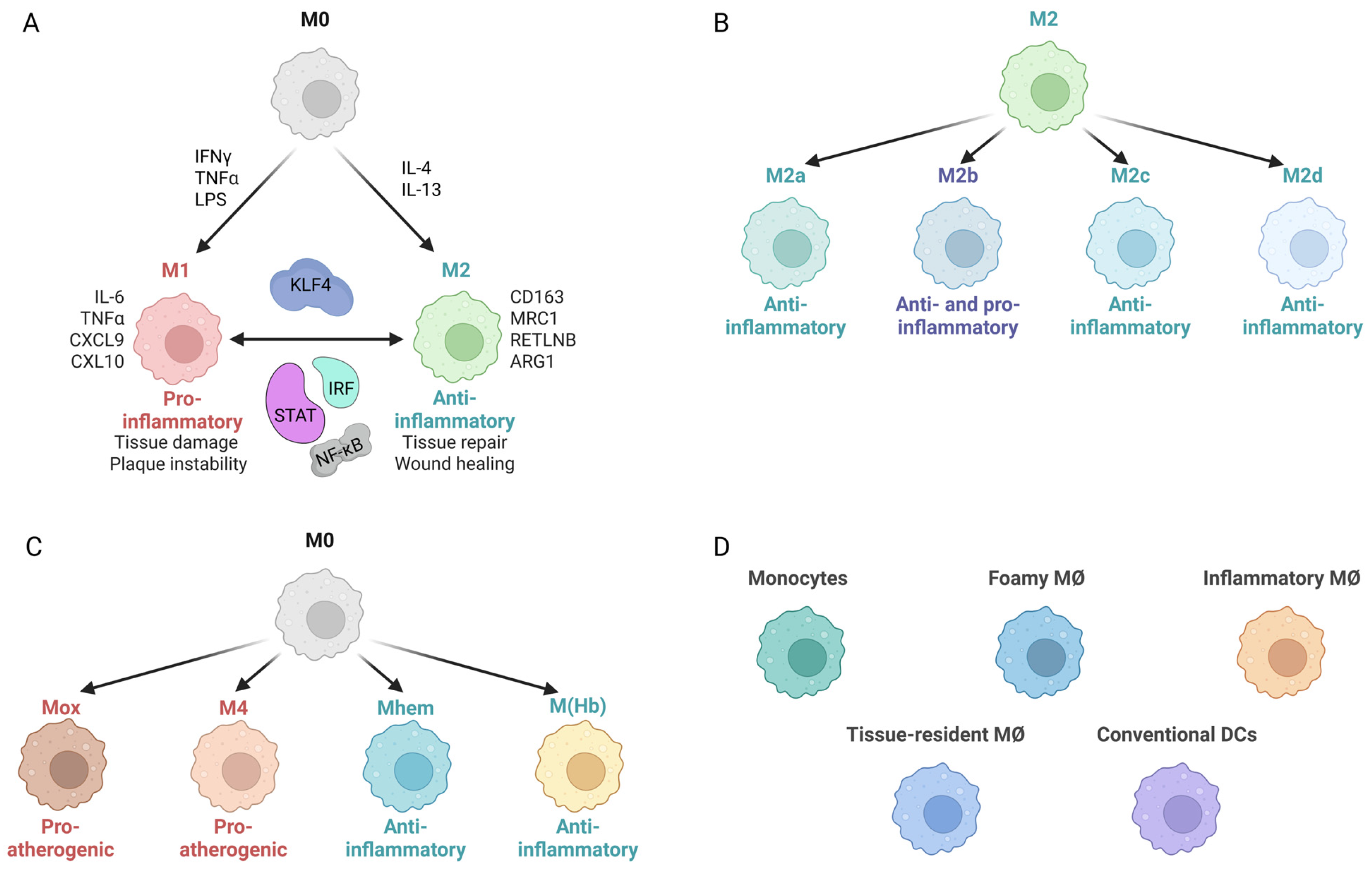
2.2. MØ Polarization
3. TLRs and IFNs in Atherosclerosis
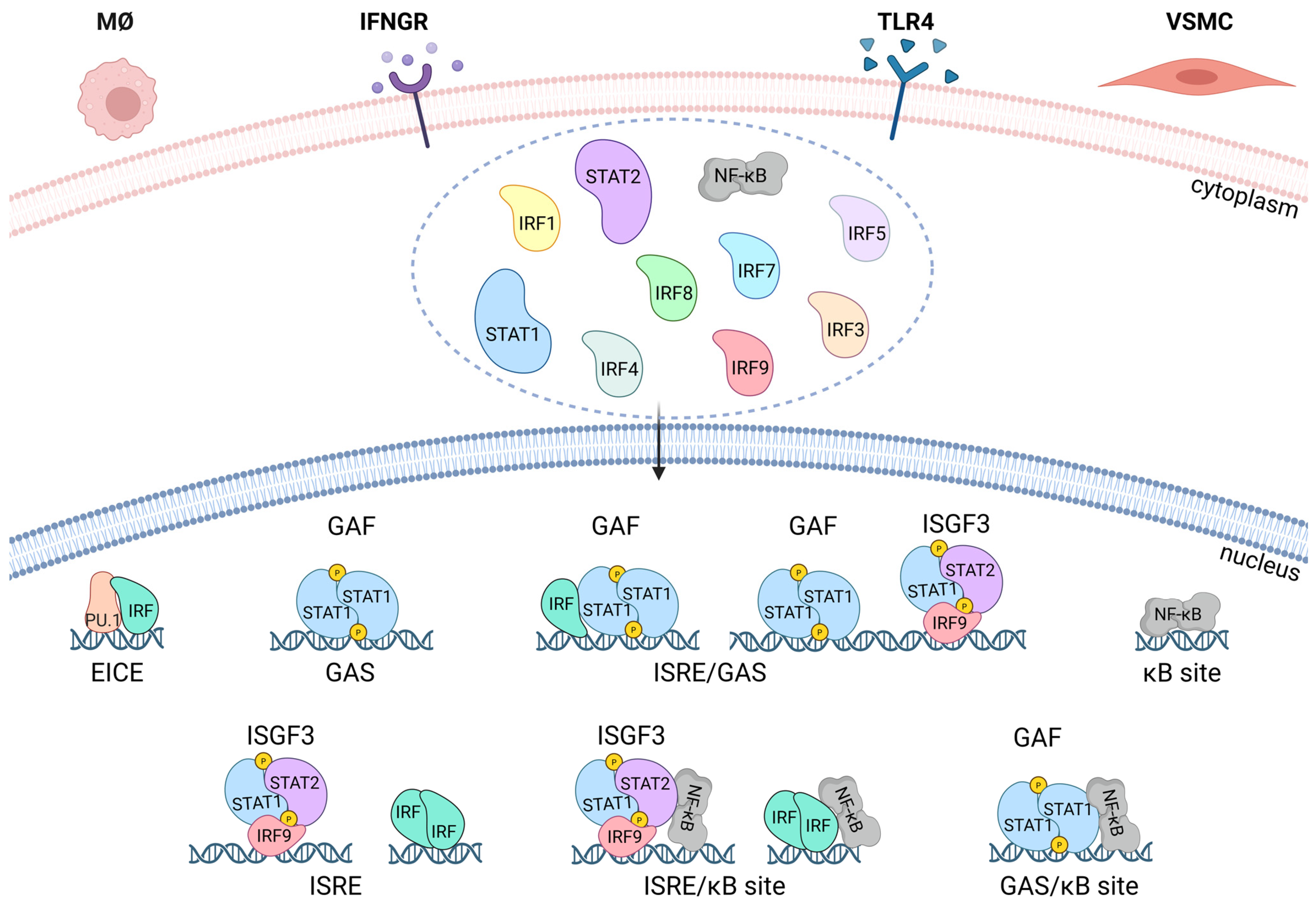
4. STATs, IRFs, and NF-κB in IFNγ and TLR4 Signaling
5. STATs, IRFs and NF-κB in Inflammation and Atherosclerosis
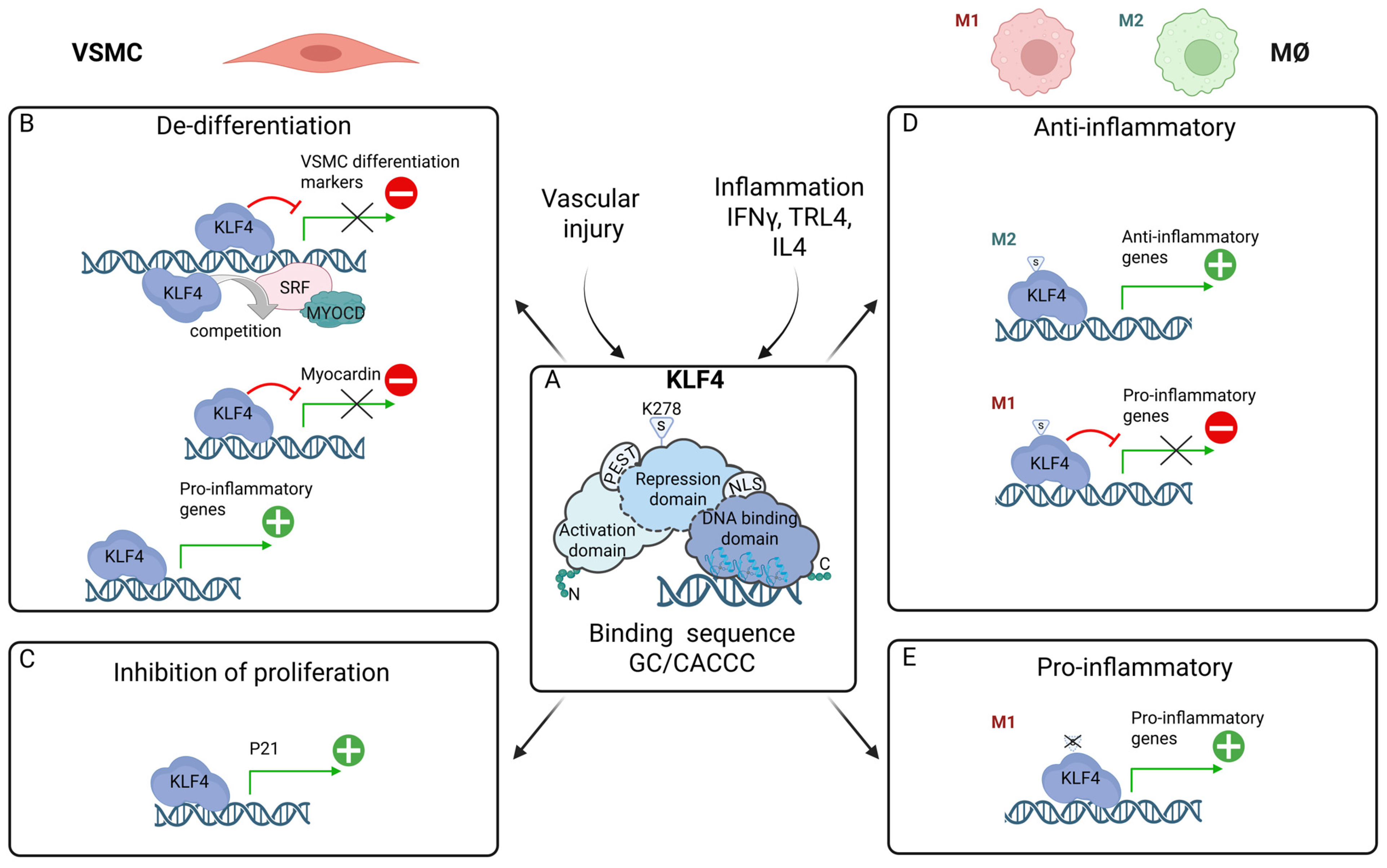
6. KLF4 in VSMC Phenotypic Switching: Promotion of a Pro-Atherogenic Phenotype, Inhibition of Neointima Formation
7. KLF4 in MØ Polarization: Multi-Functional Regulator of Pro- and Anti-Inflammatory Phenotypes
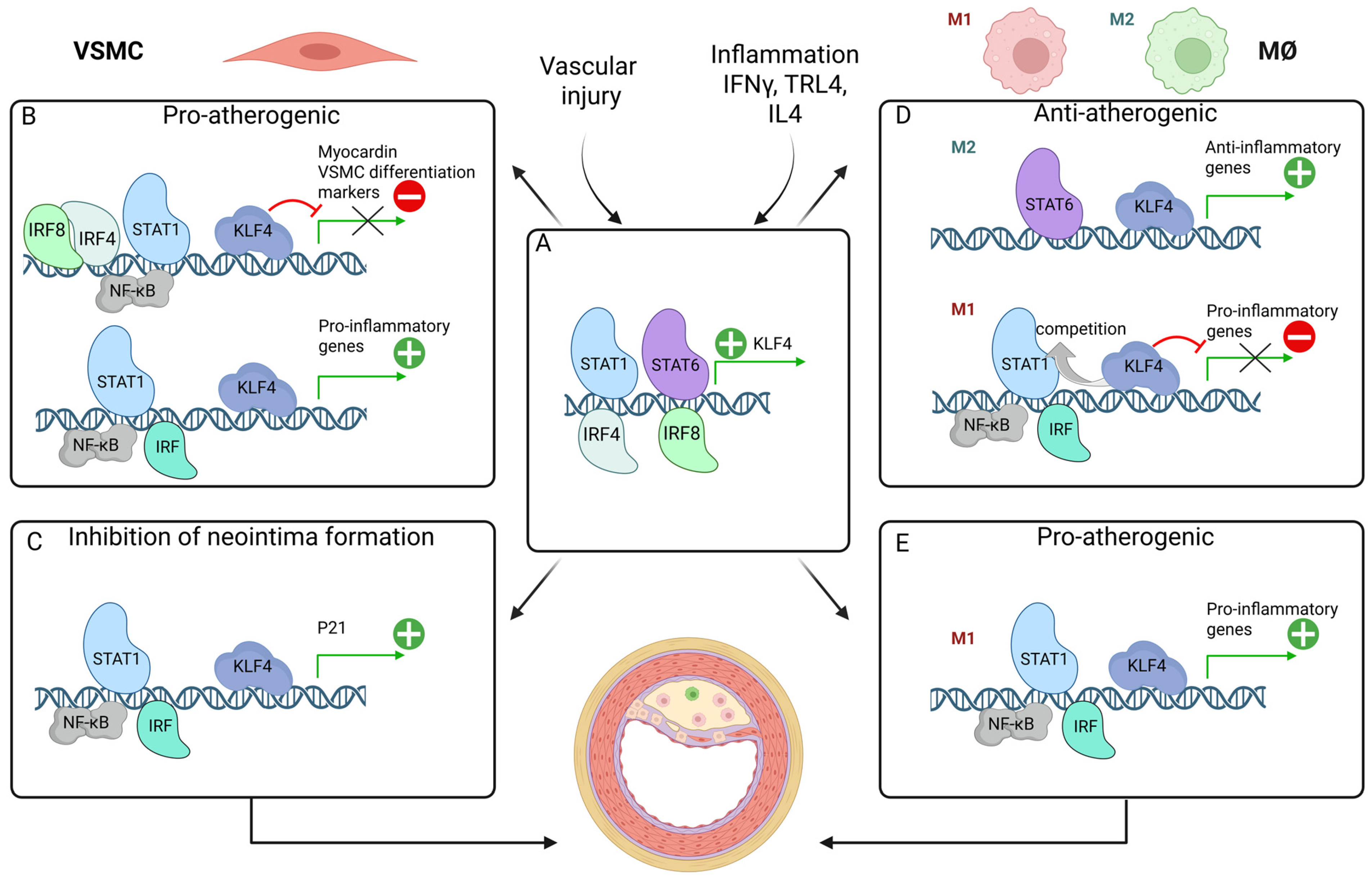
8. Interplay Between KLF4, STATs, IRFs, and NF-κB in Inflammation and Atherosclerosis
8.1. Potentiating VSMC Phenotypic Switching: Pro-Atherogenic
8.2. Inhibition of Neointima Formation
8.3. Modulation of Macrophage Differentiation: Anti-Atherogenic vs. Pro-Atherogenic
9. Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EC | endothelial cells |
| TLR | Toll-like receptors |
| MØ | macrophages |
| Th1 | T helper 1 lymphocytes |
| IFN | interferon |
| DC | dendritic cells |
| VSMC | vascular smooth muscle cells |
| KLF4 | Krüppel-like factor 4 |
| STAT | Signal Transducer and Activator of Transcription |
| IRF | Interferon Regulatory Factor |
| NF-κB | Nuclear factor-κB |
| SM-MHC/MYH11 | smooth muscle myosin heavy chain |
| CNN1 | calponin |
| SM22α | transgelin |
| MYOCD | myocardin |
| TNF-a | tumor necrosis factor alpha |
| PDGF-BB | platelet-derived growth factor BB |
| ROS | reactive oxygen species |
| LPS | lipopolysaccharide |
| NO | nitric oxide |
| ApoEKO | Apolipoprotein E Knockout |
| LDL | low-density lipoprotein |
| LDLr | low-density lipoprotein receptor |
| WT | wild-type |
| HFD | high fat diet |
| GAF | γ-activated factor |
| GAS | IFNγ-activated sequence |
| ISGF3 | interferon-stimulated gene factor 3 |
| ISRE | IFN-stimulated response element |
| CVD | cardiovascular disease |
| Dnmt3a | DNA methyltransferase 3a |
| DBD | DNA-binding domain |
| TAD | Transcriptional activation domain |
| iNOS | Inducible Nitric Oxide Synthase |
| MSC | mesenchymal stem cells |
| SUMO | small ubiquitin-like modifier |
| SENP1 | small ubiquitin-like modifier specific peptidase 1 |
| TG | transgenic |
| CKD | chronic kidney disease |
| scRNAseq | single-cell RNA sequencing |
References
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 56. [Google Scholar] [CrossRef]
- Hansson, G.K.; Hermansson, A. The Immune System in Atherosclerosis. Nat. Immunol. 2011, 12, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Ramji, D.P.; Davies, T.S. Cytokines in Atherosclerosis: Key Players in All Stages of Disease and Promising Therapeutic Targets. Cytokine Growth Factor Rev. 2015, 26, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Szelag, M.; Piaszyk-Borychowska, A.; Plens-Galaska, M.; Wesoly, J.; Bluyssen, H.A.R. Targeted Inhibition of STATs and IRFs as a Potential Treatment Strategy in Cardiovascular Disease. Oncotarget 2016, 7, 48788–48812. [Google Scholar] [CrossRef] [PubMed]
- Björkegren, J.L.M.; Lusis, A.J. Atherosclerosis: Recent Developments. Cell 2022, 185, 1630–1645. [Google Scholar] [CrossRef]
- De Winther, M.P.J.; Bäck, M.; Evans, P.; Gomez, D.; Goncalves, I.; Jørgensen, H.F.; Koenen, R.R.; Lutgens, E.; Norata, G.D.; Osto, E.; et al. Translational Opportunities of Single-Cell Biology in Atherosclerosis. Eur. Heart J. 2023, 44, 1216–1230. [Google Scholar]
- Gimbrone, M.A.; García-Cardeña, G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef]
- Wu, J.; He, S.; Song, Z.; Chen, S.; Lin, X.; Sun, H.; Zhou, P.; Peng, Q.; Du, S.; Zheng, S.; et al. Macrophage Polarization States in Atherosclerosis. Front. Immunol. 2023, 14, 1185587. [Google Scholar] [CrossRef]
- Sreejit, G.; Fleetwood, A.J.; Murphy, A.J.; Nagareddy, P.R. Origins and Diversity of Macrophages in Health and Disease. Clin. Transl. Immunol. 2020, 9, e1222. [Google Scholar] [CrossRef]
- Saigusa, R.; Winkels, H.; Ley, K. T Cell Subsets and Functions in Atherosclerosis. Nat. Rev. Cardiol. 2020, 17, 387–401. [Google Scholar] [CrossRef]
- Britsch, S.; Langer, H.; Duerschmied, D.; Becher, T. The Evolving Role of Dendritic Cells in Atherosclerosis. Int. J. Mol. Sci. 2024, 25, 2450. [Google Scholar] [CrossRef]
- Grootaert, M.O.J.; Bennett, M.R. Vascular Smooth Muscle Cells in Atherosclerosis: Time for a Re-Assessment. Cardiovasc. Res. 2021, 117, 2326–2339. [Google Scholar] [CrossRef] [PubMed]
- Yap, C.; Mieremet, A.; De Vries, C.J.M.; Micha, D.; De Waard, V. Six Shades of Vascular Smooth Muscle Cells Illuminated by KLF4 (Krüppel-Like Factor 4). Arterioscler. Thromb. Vasc. Biol. 2021, 41, 2693–2707. [Google Scholar] [CrossRef] [PubMed]
- Herta, T.; Bhattacharyya, A.; Hippenstiel, S.; Zahlten, J. The Role of KLF4 in Phagocyte Activation during Infectious Diseases. Front. Immunol. 2025, 16, 1584873. [Google Scholar] [CrossRef]
- Sikorski, K.; Wesoly, J.; Bluyssen, H.A.R. Data Mining of Atherosclerotic Plaque Transcriptomes Predicts STAT1-Dependent Inflammatory Signal Integration in Vascular Disease. Int. J. Mol. Sci. 2014, 15, 14313–14331. [Google Scholar] [CrossRef]
- Piaszyk-Borychowska, A.; Széles, L.; Csermely, A.; Chiang, H.C.; Wesoły, J.; Lee, C.K.; Nagy, L.; Bluyssen, H.A.R. Signal Integration of IFN-I and IFN-II with TLR4 Involves Sequential Recruitment of STAT1-Complexes and NFκB to Enhance pro-Inflammatory Transcription. Front. Immunol. 2019, 10, 1253. [Google Scholar] [CrossRef]
- Zhang, F.; Guo, X.; Xia, Y.; Mao, L. An Update on the Phenotypic Switching of Vascular Smooth Muscle Cells in the Pathogenesis of Atherosclerosis. Cell. Mol. Life Sci. 2022, 79, 6. [Google Scholar] [CrossRef]
- Cao, G.; Xuan, X.; Hu, J.; Zhang, R.; Jin, H.; Dong, H. How Vascular Smooth Muscle Cell Phenotype Switching Contributes to Vascular Disease. Cell Commun. Signal. 2022, 20, 180. [Google Scholar] [CrossRef]
- Chen, R.; McVey, D.G.; Shen, D.; Huang, X.; Ye, S. Phenotypic Switching of Vascular Smooth Muscle Cells in Atherosclerosis. J. Am. Heart Assoc. 2023, 12, e031121. [Google Scholar] [CrossRef]
- Mosquera, J.V.; Auguste, G.; Wong, D.; Turner, A.W.; Hodonsky, C.J.; Alvarez-Yela, A.C.; Song, Y.; Cheng, Q.; Lino Cardenas, C.L.; Theofilatos, K.; et al. Integrative Single-Cell Meta-Analysis Reveals Disease-Relevant Vascular Cell States and Markers in Human Atherosclerosis. Cell Rep. 2023, 42, 113380. [Google Scholar] [CrossRef]
- Alencar, G.F.; Owsiany, K.M.; Karnewar, S.; Sukhavasi, K.; Mocci, G.; Nguyen, A.T.; Williams, C.M.; Shamsuzzaman, S.; Mokry, M.; Henderson, C.A.; et al. Stem Cell Pluripotency Genes Klf4 and Oct4 Regulate Complex SMC Phenotypic Changes Critical in Late-Stage Atherosclerotic Lesion Pathogenesis. Circulation 2020, 142, 2045–2059. [Google Scholar] [CrossRef] [PubMed]
- Piccolo, V.; Curina, A.; Genua, M.; Ghisletti, S.; Simonatto, M.; Sabò, A.; Amati, B.; Ostuni, R.; Natoli, G. Opposing Macrophage Polarization Programs Show Extensive Epigenomic and Transcriptional Cross-Talk. Nat. Immunol. 2017, 18, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Rojas, J.; Salazar, J.; Sofía Martínez, M.; Palmar, J.; Bautista, J.; Chávez-Castillo, M.; Gómez, A.; Bermúdez, V. Macrophage Heterogeneity and Plasticity: Impact of Macrophage Biomarkers on Atherosclerosis. Scientifica 2015, 2015, 851252. [Google Scholar] [CrossRef] [PubMed]
- Barrett, T.J. Macrophages in Atherosclerosis Regression. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 20–33. [Google Scholar] [CrossRef]
- Kuznetsova, T.; Prange, K.H.M.; Glass, C.K.; de Winther, M.P.J. Transcriptional and Epigenetic Regulation of Macrophages in Atherosclerosis. Nat. Rev. Cardiol. 2020, 17, 216–228. [Google Scholar] [CrossRef]
- Shioi, A.; Ikari, Y. Plaque Calcification during Atherosclerosis Progression and Regression. J. Atheroscler. Thromb. 2018, 25, 294–303. [Google Scholar] [CrossRef]
- Jinnouchi, H.; Guo, L.; Sakamoto, A.; Torii, S.; Sato, Y.; Cornelissen, A.; Kuntz, S.; Paek, K.H.; Fernandez, R.; Fuller, D.; et al. Diversity of Macrophage Phenotypes and Responses in Atherosclerosis. Cell. Mol. Life Sci. 2020, 77, 1919–1932. [Google Scholar] [CrossRef]
- Gordon, S. Alternative Activation of Macrophages. Nat. Rev. Immunol. 2003, 3, 23–35. [Google Scholar] [CrossRef]
- Domschke, G.; Gleissner, C.A. CXCL4-Induced Macrophages in Human Atherosclerosis. Cytokine 2019, 122, 154141. [Google Scholar] [CrossRef]
- Murray, P.J.; Wynn, T.A. Protective and Pathogenic Functions of Macrophage Subsets. Nat. Rev. Immunol. 2011, 11, 723–737. [Google Scholar] [CrossRef]
- Chinetti-Gbaguidi, G.; Colin, S.; Staels, B. Macrophage Subsets in Atherosclerosis. Nat. Rev. Cardiol. 2015, 12, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Nagenborg, J.; Goossens, P.; Biessen, E.A.L.; Donners, M.M.P.C. Heterogeneity of Atherosclerotic Plaque Macrophage Origin, Phenotype and Functions: Implications for Treatment. Eur. J. Pharmacol. 2017, 816, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Tanay, A.; Regev, A. Scaling Single-Cell Genomics from Phenomenology to Mechanism. Nature 2017, 541, 331–338. [Google Scholar] [CrossRef]
- Cochain, C.; Vafadarnejad, E.; Arampatzi, P.; Pelisek, J.; Winkels, H.; Ley, K.; Wolf, D.; Saliba, A.-E.; Zernecke, A. Single Cell Rna Seq Reveals the Transcriptional Landscape and Heterogeneity of Aortic Macrophages. Circ. Res. 2018, 122, 1661–1674. [Google Scholar] [CrossRef]
- Willemsen, L.; Pj De Winther, M. Macrophage Subsets in Atherosclerosis as Defined by Single-Cell Technologies. J. Pathol. 2020, 250, 705–714. [Google Scholar] [CrossRef]
- Winkels, H.; Ehinger, E.; Vassallo, M.; Buscher, K.; Dinh, H.Q.; Kobiyama, K.; Hamers, A.A.J.; Cochain, C.; Vafadarnejad, E.; Saliba, A.-E.; et al. Atlas of the Immune Cell Repertoire in Mouse Atherosclerosis Defined by Single-Cell RNA-Sequencing and Mass Cytometry. Circ. Res. 2018, 122, 1675–1688. [Google Scholar] [CrossRef]
- Yang, P.; Rong, X.; Gao, Z.; Wang, J.; Liu, Z. Metabolic and Epigenetic Regulation of Macrophage Polarization in Atherosclerosis: Molecular Mechanisms and Targeted Therapies. Pharmacol. Res. 2025, 212, 107588. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, Q.; Ban, R.; Zhao, X.; Sun, H.; Lin, J.; Guo, T.; Wang, T.; Xia, K.; Xin, Z.; et al. Single-Nucleus RNA Sequencing Reveals That Macrophages and Smooth Muscle Cells Promote Carotid Atherosclerosis Progression through Mitochondrial Autophagy. Medicine 2024, 103, E37171. [Google Scholar] [CrossRef]
- Boroujeni, M.E.; Lopacinska, N.; Antonczyk, A.; Kluzek, K.; Wesoly, J.; Bluyssen, H.A. Integrative Multi-Omics Analysis of IFNγ-Induced Macrophages and Atherosclerotic Plaques Reveals Macrophage-Dependent STAT1-Driven Transcription in Atherosclerosis. Front. Immunol. 2024, 16, 1590953. [Google Scholar] [CrossRef]
- Pourcet, B.; Staels, B. Alternative Macrophages in Atherosclerosis: Not Always Protective! J. Clin. Investig. 2018, 128, 910–912. [Google Scholar] [CrossRef]
- Antonczyk, A.; Krist, B.; Sajek, M.; Michalska, A.; Piaszyk-Borychowska, A.; Plens-Galaska, M.; Wesoly, J.; Bluyssen, H.A.R. Direct Inhibition of IRF-Dependent Transcriptional Regulatory Mechanisms Associated with Disease. Front. Immunol. 2019, 10, 1176. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, Y.; Li, H.; Wang, F.; Yao, S. Toll-like Receptor 4: A Potential Therapeutic Target for Multiple Human Diseases. Biomed. Pharmacother. 2023, 166, 115338. [Google Scholar] [CrossRef]
- Bagheri, B.; Khatibiyan Feyzabadi, Z.; Nouri, A.; Azadfallah, A.; Mahdizade Ari, M.; Hemmati, M.; Darban, M.; Alavi Toosi, P.; Banihashemian, S.Z. Atherosclerosis and Toll-Like Receptor4 (TLR4), Lectin-Like Oxidized Low-Density Lipoprotein-1 (LOX-1), and Proprotein Convertase Subtilisin/Kexin Type9 (PCSK9). Mediat. Inflamm. 2024, 2024, 5830491. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.E.; Georgiou, E.; Monaco, C. The Expression and Functions of Toll-Like Receptors in Atherosclerosis. Mediat. Inflamm. 2010, 2010, 393946. [Google Scholar] [CrossRef] [PubMed]
- Helen Xu, X.; Shah, P.K.; Faure, E.; Equils, O.; Thomas, L.; Fishbein, M.C.; Luthringer, D.; Xu, X.-P.; Rajavashisth, T.B.; Yano, J.; et al. Toll-Like Receptor-4 Is Expressed by Macrophages in Murine and Human Lipid-Rich Atherosclerotic Plaques and Upregulated by Oxidized LDL. Circulation 2001, 104, 3103–3108. [Google Scholar] [CrossRef]
- Methe, H.; Kim, J.-O.; Kofler, S.; Weis, M.; Nabauer, M.; Koglin, J. Expansion of Circulating Toll-Like Receptor 4-Positive Monocytes in Patients With Acute Coronary Syndrome. Circulation 2005, 111, 2654–2661. [Google Scholar] [CrossRef]
- Michelsen, K.S.; Wong, M.H.; Shah, P.K.; Zhang, W.; Yano, J.; Doherty, T.M.; Akira, S.; Rajavashisth, T.B.; Arditi, M. Lack of Toll-like Receptor 4 or Myeloid Differentiation Factor 88 Reduces Atherosclerosis and Alters Plaque Phenotype in Mice Deficient in Apolipoprotein E. Proc. Natl. Acad. Sci. USA 2004, 101, 10679–10684. [Google Scholar] [CrossRef]
- Lu, Z.; Zhang, X.; Li, Y.; Jin, J.; Huang, Y. TLR4 Antagonist Reduces Early-Stage Atherosclerosis in Diabetic Apolipoprotein E-Deficient Mice. J. Endocrinol. 2013, 216, 61–71. [Google Scholar] [CrossRef]
- Laugerette, F.; Vors, C.; Peretti, N.; Michalski, M.C. Complex Links between Dietary Lipids, Endogenous Endotoxins and Metabolic Inflammation. Biochimie 2011, 93, 39–45. [Google Scholar] [CrossRef]
- Singh, R.K.; Haka, A.S.; Asmal, A.; Barbosa-Lorenzi, V.C.; Grosheva, I.; Chin, H.F.; Xiong, Y.; Hla, T.; Maxfield, F.R. TLR4 (Toll-Like Receptor 4)-Dependent Signaling Drives Extracellular Catabolism of LDL (Low-Density Lipoprotein) Aggregates. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 86–102. [Google Scholar] [CrossRef]
- Yin, Q.; Jiang, D.; Li, L.; Yang, Y.; Wu, P.; Luo, Y.; Yang, R.; Li, D. LPS Promotes Vascular Smooth Muscle Cells Proliferation Through the TLR4/Rac1/Akt Signalling Pathway. Cell. Physiol. Biochem. 2018, 44, 2189–2200. [Google Scholar] [CrossRef]
- Yang, X.; Coriolan, D.; Murthy, V.; Schultz, K.; Golenbock, D.T.; Beasley, D. Proinflammatory Phenotype of Vascular Smooth Muscle Cells: Role of Efficient Toll-like Receptor 4 Signaling Proinflammatory Pheno-Type of Vascular Smooth Muscle Cells: Role of Efficient Toll-like Receptor 4 Signaling. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, 1069–1076. [Google Scholar] [CrossRef]
- Guo, L.; Chen, C.H.; Zhang, L.L.; Cao, X.J.; Ma, Q.L.; Deng, P.; Zhu, G.; Gao, C.Y.; Li, B.H.; Pi, Y.; et al. IRAK1 Mediates TLR4-Induced ABCA1 Downregulation and Lipid Accumulation in VSMCs. Cell Death Dis. 2015, 6, e1949. [Google Scholar] [CrossRef] [PubMed]
- Strela, F.B.; Brun, B.F.; Berger, R.C.M.; Melo, S.; de Oliveira, E.M.; Barauna, V.G.; Vassallo, P.F. Lipopolysaccharide Exposure Modulates the Contractile and Migratory Phenotypes of Vascular Smooth Muscle Cells. Life Sci. 2020, 241, 117098. [Google Scholar] [CrossRef] [PubMed]
- Michalska, A.; Blaszczyk, K.; Wesoly, J.; Bluyssen, H.A.R. A Positive Feedback Amplifier Circuit That Regulates Interferon (IFN)-Stimulated Gene Expression and Controls Type I and Type II IFN Responses. Front. Immunol. 2018, 9, 1135. [Google Scholar] [CrossRef] [PubMed]
- Eskandarian Boroujeni, M.; Sekrecka, A.; Antonczyk, A.; Hassani, S.; Sekrecki, M.; Nowicka, H.; Lopacinska, N.; Olya, A.; Kluzek, K.; Wesoly, J.; et al. Dysregulated Interferon Response and Immune Hyperactivation in Severe COVID-19: Targeting STATs as a Novel Therapeutic Strategy. Front. Immunol. 2022, 13, 888897. [Google Scholar] [CrossRef]
- Moss, J.W. Interferon-γ: Promising Therapeutic Target in Atherosclerosis. World J. Exp. Med. 2015, 5, 154. [Google Scholar] [CrossRef]
- Boshuizen, M.C.S.; De Winther, M.P.J. Interferons as Essential Modulators of Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1579–1588. [Google Scholar] [CrossRef]
- Voloshyna, I.; Littlefield, M.J.; Reiss, A.B. Atherosclerosis and Interferon-γ: New Insights and Therapeutic Targets. Trends Cardiovasc. Med. 2014, 24, 45–51. [Google Scholar] [CrossRef]
- Elyasi, A.; Voloshyna, I.; Ahmed, S.; Kasselman, L.J.; Behbodikhah, J.; De Leon, J.; Reiss, A.B. The Role of Interferon-γ in Cardiovascular Disease: An Update. Inflamm. Res. 2020, 69, 975–988. [Google Scholar] [CrossRef]
- Yu, X.H.; Zhang, J.; Zheng, X.L.; Yang, Y.H.; Tang, C.K. Interferon-γ in Foam Cell Formation and Progression of Atherosclerosis. Clin. Chim. Acta 2015, 441, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bai, Y.; Qin, L.; Zhang, P.; Yi, T.; Teesdale, S.A.; Zhao, L.; Pober, J.S.; Tellides, G. Interferon-γ Induces Human Vascular Smooth Muscle Cell Proliferation and Intimal Expansion by Phosphatidylinositol 3-Kinase-Dependent Mammalian Target of Rapamycin Raptor Complex 1 Activation. Circ. Res. 2007, 101, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Buono, C.; Come, C.E.; Stavrakis, G.; Maguire, G.F.; Connelly, P.W.; Lichtman, A.H. Influence of Interferon-on the Extent and Phenotype of Diet-Induced Atherosclerosis in the LDLR-Deficient Mouse. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Pablo, A.M.; Jiang, X.-C.; Wang, N.; Tall, A.R.; Schindler, C. IFN-g Potentiates Atherosclerosis in ApoE Knock-out Mice. J. Clin. Investig. 1997, 99, 2752–2761. [Google Scholar] [CrossRef]
- Tellides, G.; Tereb, D.A.; Kirkiles-Smith, N.C.; Kim, R.W.; Wilson, J.H.; Schechner, J.S.; Lorber, M.I.; Pober, J.S. Interferon-G Elicits arteriosclerosis in the absence of leukocytes. Nature 2000, 403, 207–211. [Google Scholar] [CrossRef]
- Whitman, S.C.; Ravisankar, P.; Elam, H.; Daugherty, A. Exogenous Interferon-γ Enhances Atherosclerosis in Apolipoprotein E-/-Mice. Am. J. Pathol. 2000, 157, 1819–1824. [Google Scholar] [CrossRef]
- Platanitis, E.; Decker, T. Regulatory Networks Involving STATs, IRFs, and NFκB in Inflammation. Front. Immunol. 2018, 9, 2542. [Google Scholar] [CrossRef]
- Sekrecka, A.; Kluzek, K.; Sekrecki, M.; Boroujeni, M.E.; Hassani, S.; Yamauchi, S.; Sada, K.; Wesoly, J.; Bluyssen, H.A.R. Time-Dependent Recruitment of GAF, ISGF3 and IRF1 Complexes Shapes IFNα and IFNγ-Activated Transcriptional Responses and Explains Mechanistic and Functional Overlap. Cell. Mol. Life Sci. 2023, 80, 187. [Google Scholar] [CrossRef]
- Matsumoto, M.; Tanaka, N.; Harada, H.; Kimura, T.; Yokochi, T.; Kitagawa, M.; Schindler, C.; Taniguchi, T. Activation of the Transcription Factor ISGF3 by Interferon-Gamma. Biol. Chem. 1999, 380, 699–703. [Google Scholar] [CrossRef]
- Kimura, T.; Kadokawa, Y.; Harada, H.; Matsumoto, M.; Sato, M.; Kashiwazaki, Y.; Tarutani, M.; Tan, R.S.P.; Takasugi, T.; Matsuyama, T.; et al. Essential and Non-Redundant Roles of P48 (ISGF3γ) and IRF-1 in Both Type I and Type II Interferon Responses, as Revealed by Gene Targeting Studies. Genes Cells 1996, 1, 115–124. [Google Scholar] [CrossRef]
- Zimmermann, A.; Trilling, M.; Wagner, M.; Wilborn, M.; Bubic, I.; Jonjic, S.; Koszinowski, U.; Hengel, H. A Cytomegaloviral Protein Reveals a Dual Role for STAT2 in IFN-γ Signaling and Antiviral Responses. J. Exp. Med. 2005, 201, 1543–1553. [Google Scholar] [CrossRef]
- Platanitis, E.; Demiroz, D.; Schneller, A.; Fischer, K.; Capelle, C.; Hartl, M.; Gossenreiter, T.; Müller, M.; Novatchkova, M.; Decker, T. A Molecular Switch from STAT2-IRF9 to ISGF3 Underlies Interferon-Induced Gene Transcription. Nat. Commun. 2019, 10, 2921. [Google Scholar] [CrossRef]
- Langlais, D.; Barreiro, L.B.; Gros, P. The Macrophage IRF8/IRF1 Regulome Is Required for Protection against Infections and Is Associated with Chronic Inflammation. J. Exp. Med. 2016, 213, 585–603. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Vinkemeier, U.; Zhao, Y.; Jeruzalmi, D.; Darnell, J.E.; Kuriyan, J. Crystal Structure of a Tyrosine Phosphorylated STAT-1 Dimer Bound to DNA. Cell 1998, 93, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. Toll-like Receptors and Their Crosstalk with Other Innate Receptors in Infection and Immunity. Immunity 2011, 34, 637–650. [Google Scholar] [CrossRef] [PubMed]
- Hertzog, P.J.; O’Neill, L.A.; Hamilton, J.A. The Interferon in TLR Signaling: More than Just Antiviral. Trends Immunol. 2003, 24, 534–539. [Google Scholar] [CrossRef]
- Farlik, M.; Reutterer, B.; Schindler, C.; Greten, F.; Vogl, C.; Müller, M.; Decker, T. Nonconventional Initiation Complex Assembly by STAT and NF-ΚB Transcription Factors Regulates Nitric Oxide Synthase Expression. Immunity 2010, 33, 25–34. [Google Scholar] [CrossRef]
- Tamassia, N.; Calzetti, F.; Ear, T.; Cloutier, A.; Gasperini, S.; Bazzoni, F.; Mcdonald, P.P.; Cassatella, M.A. Molecular Mechanisms Underlying the Synergistic Induction of CXCL10 by LPS and IFN-c in Human Neutrophils. Eur. J. Immunol. 2007, 37, 2627–2634. [Google Scholar] [CrossRef]
- Schroder, K.; Hertzog, P.J.; Ravasi, T.; Hume, D.A. Interferon-γ: An Overview of Signals, Mechanisms and Functions. J. Leukoc. Biol. 2004, 75, 163–189. [Google Scholar] [CrossRef]
- Hu, X.; Ivashkiv, L.B. Cross-Regulation of Signaling and Immune Responses by IFN-γ and STAT1. Immunity 2009, 31, 539–550. [Google Scholar] [CrossRef]
- Chmielewski, S.; Olejnik, A.; Sikorski, K.; Pelisek, J.; Błaszczyk, K.; Aoqui, C.; Nowicka, H.; Zernecke, A.; Heemann, U.; Wesoly, J.; et al. STAT1-Dependent Signal Integration between IFNγ and TLR4 in Vascular Cells Reflect pro-Atherogenic Responses in Human Atherosclerosis. PLoS ONE 2014, 9, e113318. [Google Scholar] [CrossRef]
- Kleinert, H.; Schwarz, P.M.; Forstermann, U. Regulation of the expression of inducible nitric oxide synthase. Biol. Chem. 2003, 384, 1343–1364. [Google Scholar] [CrossRef]
- Proost, P.; Verpoest, S.; Van De Borne, K.; Schutyser, E.; Struyf, S.; Put, W.; Ronsse, I.; Grillet, B.; Opdenakker, G.; Van Damme, J. Synergistic Induction of CXCL9 and CXCL11 by Toll-like Receptor Ligands and Interferon-γ in Fibroblasts Correlates with Elevated Levels of CXCR3 Ligands in Septic Arthritis Synovial Fluids. J. Leukoc. Biol. 2004, 75, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Febbraio, M.; Podrez, E.; Cathcart, M.K.; Stark, G.R.; Chisolm, G.M. Signal Transducer and Activator of Transcription 1 Is Required for Optimal Foam Cell Formation and Atherosclerotic Lesion Development. Circulation 2007, 115, 2939–2947. [Google Scholar] [CrossRef]
- Lim, W.-S.; Timmins, J.M.; Seimon, T.A.; Sadler, A.; Kolodgie, F.D.; Virmani, R.; Tabas, I. Signal Transducer and Activator of Transcription-1 Is Critical for Apoptosis in Macrophages Subjected to Endoplasmic Reticulum Stress In Vitro and in Advanced Atherosclerotic Lesions In Vivo. Circulation 2008, 117, 940–951, Erratum in Circulation 2008, 117, e303. [Google Scholar] [CrossRef] [PubMed]
- Kirchmer, M.N.; Franco, A.; Albasanz-Puig, A.; Murray, J.; Yagi, M.; Gao, L.; Dong, Z.M.; Wijelath, E.S. Modulation of Vascular Smooth Muscle Cell Phenotype by STAT-1 and STAT-3. Atherosclerosis 2014, 234, 169–175. [Google Scholar] [CrossRef]
- Torella, D.; Curcio, A.; Gasparri, C.; Galuppo, V.; De Serio, D.; Surace, F.C.; Cavaliere, A.L.; Leone, A.; Coppola, C.; Ellison, G.M.; et al. Fludarabine Prevents Smooth Muscle Proliferation in Vitro and Neointimal Hyperplasia in Vivo through Specific Inhibition of STAT-1 Activation. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, 2935–2943. [Google Scholar] [CrossRef]
- Antonczyk, A.; Kluzek, K.; Herbich, N.; Boroujeni, M.E.; Krist, B.; Wronka, D.; Karlik, A.; Przybyl, L.; Plewinski, A.; Wesoly, J.; et al. Identification of ALEKSIN as a Novel Multi-IRF Inhibitor of IRF- and STAT-Mediated Transcription in Vascular Inflammation and Atherosclerosis. Front. Pharmacol. 2024, 15, 1471182. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, T.; Zheng, X.; Huangfu, N. The Role of Interferon Regulatory Factors in Atherosclerosis. Front. Cardiovasc. Med. 2025, 12, 1606034. [Google Scholar] [CrossRef]
- Du, M.; Wang, X.; Mao, X.; Yang, L.; Huang, K.; Zhang, F.; Wang, Y.; Luo, X.; Wang, C.; Peng, J.; et al. Absence of Interferon Regulatory Factor 1 Protects against Atherosclerosis in Apolipoprotein E-Deficient Mice. Theranostics 2019, 9, 4688–4703. [Google Scholar] [CrossRef]
- Shen, Y.; Sun, Z.; Mao, S.; Zhang, Y.; Jiang, W.; Wang, H. IRF-1 Contributes to the Pathological Phenotype of VSMCs during Atherogenesis by Increasing CCL19 Transcription. Aging 2021, 13, 933–943. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, W.L.; Jiang, X.; Wang, P.X.; Fang, C.; Zhu, X.Y.; Huang, Z.; She, Z.G.; Li, H. Ablation of Interferon Regulatory Factor 3 Protects against Atherosclerosis in Apolipoprotein E-Deficient Mice. Hypertension 2017, 69, 510–520. [Google Scholar] [CrossRef]
- Leipner, J.; Dederichs, T.S.; von Ehr, A.; Rauterberg, S.; Ehlert, C.; Merz, J.; Dufner, B.; Hoppe, N.; Krebs, K.; Heidt, T.; et al. Myeloid Cell-Specific Irf5 Deficiency Stabilizes Atherosclerotic Plaques in Apoe–/–Mice. Mol. Metab. 2021, 53, 101250. [Google Scholar] [CrossRef]
- Seneviratne, A.N.; Edsfeldt, A.; Cole, J.E.; Kassiteridi, C.; Swart, M.; Park, I.; Green, P.; Khoyratty, T.; Saliba, D.; Goddard, M.E.; et al. Interferon Regulatory Factor 5 Controls Necrotic Core Formation in Atherosclerotic Lesions by Impairing Efferocytosis. Circulation 2017, 136, 1140–1154. [Google Scholar] [CrossRef]
- Edsfeldt, A.; Swart, M.; Singh, P.; Dib, L.; Sun, J.; Cole, J.E.; Park, I.; Al-Sharify, D.; Persson, A.; Nitulescu, M.; et al. Interferon Regulatory Factor-5-Dependent CD11c+ Macrophages Contribute to the Formation of Rupture–Prone Atherosclerotic Plaques. Eur. Heart J. 2022, 43, 1864–1877. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.L.; She, Z.G.; Qin, J.J.; Guo, J.H.; Gong, F.H.; Zhang, P.; Fang, C.; Tian, S.; Zhu, X.Y.; Gong, J.; et al. Interferon Regulatory Factor 4 Inhibits Neointima Formation by Engaging Krüppel-Like Factor 4 Signaling. Circulation 2017, 136, 1412–1433. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhang, S.M.; Zhang, P.; Zhang, X.J.; Zhu, L.H.; Chen, K.; Gao, L.; Zhang, Y.; Kong, X.J.; Tian, S.; et al. Interferon Regulatory Factor 7 Protects against Vascular Smooth Muscle Cell Proliferation and Neointima Formation. J. Am. Heart Assoc. 2014, 3, e001309. [Google Scholar] [CrossRef] [PubMed]
- Clément, M.; Haddad, Y.; Raffort, J.; Lareyre, F.; Newland, S.A.; Master, L.; Harrison, J.; Ozsvar-Kozma, M.; Bruneval, P.; Binder, C.J.; et al. Deletion of IRF8 (Interferon Regulatory Factor 8)-Dependent Dendritic Cells Abrogates Proatherogenic Adaptive Immunity. Circ. Res. 2018, 122, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Leonard, D.; Svenungsson, E.; Sandling, J.K.; Berggren, O.; Jönsen, A.; Bengtsson, C.; Wang, C.; Jensen-Urstad, K.; Granstam, S.O.; Bengtsson, A.A.; et al. Coronary Heart Disease in Systemic Lupus Erythematosus Is Associated with Interferon Regulatory Factor-8 Gene Variants. Circ. Cardiovasc. Genet. 2013, 6, 255–263. [Google Scholar] [CrossRef]
- Döring, Y. Hematopoietic Interferon Regulatory Factor 8-Deficiency Accelerates Atherosclerosis in Mice. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1613–1623. [Google Scholar] [CrossRef]
- Zhang, S.-M.; Gao, L.; Zhang, X.-F.; Zhang, R.; Zhu, L.-H.; Wang, P.-X.; Tian, S.; Yang, D.; Chen, K.; Huang, L.; et al. Interferon Regulatory Factor 8 Modulates Phenotypic Switching of Smooth Muscle Cells by Regulating the Activity of Myocardin. Mol. Cell. Biol. 2014, 34, 400–414. [Google Scholar] [CrossRef][Green Version]
- Zhang, S.M.; Zhu, L.H.; Chen, H.Z.; Zhang, R.; Zhang, P.; Jiang, D.S.; Gao, L.; Tian, S.; Wang, L.; Zhang, Y.; et al. Interferon Regulatory Factor 9 Is Critical for Neointima Formation Following Vascular Injury. Nat. Commun. 2014, 5, 5160, Erratum in Nat. Commun. 2015, 6, 6882. [Google Scholar] [CrossRef]
- Günthner, R.; Anders, H.J. Interferon-Regulatory Factors Determine Macrophage Phenotype Polarization. Mediat. Inflamm. 2013, 2013, 731023. [Google Scholar] [CrossRef] [PubMed]
- Mancino, A.; Termanini, A.; Barozzi, I.; Ghisletti, S.; Ostuni, R.; Prosperini, E.; Ozato, K.; Natoli, G. A Dual Cis-Regulatory Code Links IRF8 to Constitutive and Inducible Gene Expression in Macrophages. Genes Dev. 2015, 29, 394–408. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-ΚB Signaling in Inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- De Winther, M.P.J.; Kanters, E.; Kraal, G.; Hofker, M.H. Nuclear Factor ΚB Signaling in Atherogenesis. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 904–914. [Google Scholar] [CrossRef]
- Pamukcu, B.; Lip, G.Y.H.; Shantsila, E. The Nuclear Factor—Kappa B Pathway in Atherosclerosis: A Potential Therapeutic Target for Atherothrombotic Vascular Disease. Thromb. Res. 2011, 128, 117–123. [Google Scholar] [CrossRef]
- Zuckerbraun, B.S.; McCloskey, C.A.; Mahidhara, R.S.; Kim, P.K.M.; Taylor, B.S.; Tzeng, E. Overexpression of Mutated IκBα Inhibits Vascular Smooth Muscle Cell Proliferation and Intimal Hyperplasia Formation. J. Vasc. Surg. 2003, 38, 812–819. [Google Scholar] [CrossRef]
- Gareus, R.; Kotsaki, E.; Xanthoulea, S.; van der Made, I.; Gijbels, M.J.J.; Kardakaris, R.; Polykratis, A.; Kollias, G.; de Winther, M.P.J.; Pasparakis, M. Endothelial Cell-Specific NF-ΚB Inhibition Protects Mice from Atherosclerosis. Cell Metab. 2008, 8, 372–383. [Google Scholar] [CrossRef]
- Kanters, E.; Gijbels, M.J.; van der Made, I.; Vergouwe, M.N.; Heeringa, P.; Kraal, G.; Hofker, M.H.; de Winther, M.P.J. Hematopoietic NF-B1 Deficiency Results in Small Atherosclerotic Lesions with an Inflammatory Phenotype. Blood 2004, 103, 934–940. [Google Scholar] [CrossRef]
- Goossens, P.; Vergouwe, M.N.; Gijbels, M.J.J.; Curfs, D.M.J.; van Woezik, J.H.G.; Hoeksema, M.A.; Xanthoulea, S.; Leenen, P.J.M.; Rupec, R.A.; Hofker, M.H.; et al. Myeloid IkBa Deficiency Promotes Atherogenesis by Enhancing Leukocyte Recruitment to the Plaques. PLoS ONE 2011, 6, 22327. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Yamashita, M.; Horimai, C.; Hayashi, M. Smooth Muscle-Selective Inhibition of Nuclear Factor-KB Attenuates Smooth Muscle Phenotypic Switching and Neointima Formation Following Vascular Injury. J. Am. Heart Assoc. 2013, 2, e000230. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Ghaleb, A.M.; Yang, V.W. Krüppel-like Factor 4 (KLF4): What We Currently Know. Gene 2017, 611, 27–37. [Google Scholar] [CrossRef]
- Yang, C.; Xiao, X.; Huang, L.; Zhou, F.; Chen, L.H.; Zhao, Y.Y.; Qu, S.L.; Zhang, C. Role of Kruppel-like Factor 4 in Atherosclerosis. Clin. Chim. Acta 2021, 512, 135–141. [Google Scholar] [CrossRef]
- Frazzi, R. KLF4 Is an Epigenetically Modulated, Context-Dependent Tumor Suppressor. Front. Cell Dev. Biol. 2024, 12, 1392391. [Google Scholar] [CrossRef]
- Moonen, J.R.; Chappell, J.; Shi, M.; Shinohara, T.; Li, D.; Mumbach, M.R.; Zhang, F.; Nair, R.V.; Nasser, J.; Mai, D.H.; et al. KLF4 Recruits SWI/SNF to Increase Chromatin Accessibility and Reprogram the Endothelial Enhancer Landscape under Laminar Shear Stress. Nat. Commun. 2022, 13, 4941. [Google Scholar] [CrossRef]
- Zheng, B.; Han, M.; Wen, J.K. Role of Krüppel-like Factor 4 in Phenotypic Switching and Proliferation of Vascular Smooth Muscle Cells. IUBMB Life 2010, 62, 132–139. [Google Scholar] [CrossRef]
- Owens, G.K.; Kumar, M.S.; Wamhoff, B.R. Molecular Regulation of Vascular Smooth Muscle Cell Differentiation in Development and Disease. Physiol. Rev. 2004, 84, 767–801. [Google Scholar] [CrossRef]
- Adam, P.J.; Regan, C.P.; Hautmann, M.B.; Owens, G.K. Positive- and Negative-Acting Kruppel-like Transcription Factors Bind a Transforming Growth Factor β Control Element Required for Expression of the Smooth Muscle Cell Differentiation Marker SM22α in Vivo. J. Biol. Chem. 2000, 275, 37798–37806. [Google Scholar] [CrossRef]
- Liu, Y.; Sinha, S.; McDonald, O.G.; Shang, Y.; Hoofnagle, M.H.; Owens, G.K. Kruppel-like Factor 4 Abrogates Myocardin-Induced Activation of Smooth Muscle Gene Expression. J. Biol. Chem. 2005, 280, 9719–9727. [Google Scholar] [CrossRef]
- Zhang, W.; Geiman, D.E.; Shields, J.M.; Dang, D.T.; Mahatan, C.S.; Kaestner, K.H.; Biggs, J.R.; Kraft, A.S.; Yang, V.W. The Gut-Enriched Kruppel-like Factor (Kruppel-like Factor 4) Mediates the Transactivating Effect of P53 on the P21(WAF1)/(Cip)1 Promoter. J. Biol. Chem. 2000, 275, 18391–18398. [Google Scholar] [CrossRef]
- Wassmann, S.; Wassmann, K.; Jung, A.; Velten, M.; Knuefermann, P.; Petoumenos, V.; Becher, U.; Werner, C.; Mueller, C.; Nickenig, G. Induction of P53 by GKLF Is Essential for Inhibition of Proliferation of Vascular Smooth Muscle Cells. J. Mol. Cell. Cardiol. 2007, 43, 301–307. [Google Scholar] [CrossRef]
- Yoshida, T.; Kaestner, K.H.; Owens, G.K. Conditional Deletion of Krüppel-like Factor 4 Delays Downregulation of Smooth Muscle Cell Differentiation Markers but Accelerates Neointimal Formation Following Vascular Injury. Circ. Res. 2008, 102, 1548–1557. [Google Scholar] [CrossRef]
- Salmon, M.; Gomez, D.; Greene, E.; Shankman, L.; Owens, G.K. Molecular Medicine Cooperative Binding of KLF4, PELK-1, and HDAC2 to a G/C Repressor Element in the SM22 Promoter Mediates Transcriptional Silencing During SMC Phenotypic Switching In Vivo. Circ. Res. 2012, 111, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Shankman, L.S.; Gomez, D.; Cherepanova, O.A.; Salmon, M.; Alencar, G.F.; Haskins, R.M.; Swiatlowska, P.; Newman, A.A.C.; Greene, E.S.; Straub, A.C.; et al. KLF4-Dependent Phenotypic Modulation of Smooth Muscle Cells Has a Key Role in Atherosclerotic Plaque Pathogenesis. Nat. Med. 2015, 21, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, M.E. Converting Smooth Muscle Cells to Macrophage-like Cells with KLF4 in Atherosclerotic Plaques. Nat. Med. 2015, 21, 549–551. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhao, J.; Dai, T.; Li, X.; Chen, L.; He, Z.; Guo, M.; Zhao, J.; Xu, L. A Review of KLF4 and Inflammatory Disease: Current Status and Future Perspective. Pharmacol. Res. 2024, 207, 107345. [Google Scholar] [CrossRef]
- Jain, M.K.; Sangwung, P.; Hamik, A. Regulation of an Inflammatory Disease: Krüppel-like Factors and Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 499–508. [Google Scholar] [CrossRef]
- Feinberg, M.W.; Wara, A.K.; Cao, Z.; Lebedeva, M.A.; Rosenbauer, F.; Iwasaki, H.; Hirai, H.; Katz, J.P.; Haspel, R.L.; Gray, S.; et al. The Kruppel-like Factor KLF4 Is a Critical Regulator of Monocyte Differentiation. EMBO J. 2007, 26, 4138–4148. [Google Scholar] [CrossRef]
- Liao, X.; Sharma, N.; Kapadia, F.; Zhou, G.; Lu, Y.; Hong, H.; Paruchuri, K.; Mahabeleshwar, G.H.; Dalmas, E.; Venteclef, N.; et al. Krüppel-like Factor 4 Regulates Macrophage Polarization. J. Clin. Investig. 2011, 121, 2736–2749. [Google Scholar] [CrossRef]
- Wang, K.; Zhou, W.; Cai, Q.; Cheng, J.; Cai, R.; Xing, R. SUMOylation of KLF4 Promotes IL-4 Induced Macrophage M2 Polarization. Cell Cycle 2017, 16, 374–381. [Google Scholar] [CrossRef]
- Wang, K.; Xiong, J.; Lu, Y.; Wang, L.; Tian, T. SENP1-KLF4 Signalling Regulates LPS-Induced Macrophage M1 Polarization. FEBS J. 2023, 290, 209–224. [Google Scholar] [CrossRef]
- Sharma, N.; Lu, Y.; Zhou, G.; Liao, X.; Kapil, P.; Anand, P.; Mahabeleshwar, G.H.; Stamler, J.S.; Jain, M.K. Myeloid KLF4 Deficiency Augments Atherogenesis in ApoE-/-Mice. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2836–2838. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, T.; Liu, Y.; Zhang, H.; Wang, K.; Liu, M.; Chen, G.; Xiao, X. Krüppel-like Factor 4 Inhibits the Expression of Interleukin-1 Beta in Lipopolysaccharide-Induced RAW264.7 Macrophages. FEBS Lett. 2012, 586, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, H.; Liu, Y.; Wang, K.; Feng, Y.; Liu, M.; Xiao, X. KLF4 Regulates the Expression of Interleukin-10 in RAW264.7 Macrophages. Biochem. Biophys. Res. Commun. 2007, 362, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, M.W.; Cao, Z.; Wara, A.K.; Lebedeva, M.A.; SenBanerjee, S.; Jain, M.K. Kruppel-like Factor 4 Is a Mediator of Proinflammatory Signaling in Macrophages. J. Biol. Chem. 2005, 280, 38247–38258. [Google Scholar] [CrossRef]
- Ye, Q.; Luo, F.; Yan, T. Transcription factor KLF4 regulated STAT1 to promote M1 polarization of macrophages in rheumatoid arthritis. Aging 2022, 14, 5669–5680. [Google Scholar] [CrossRef]
- Rosenzweig, J.M.; Glenn, J.D.; Calabresi, P.A.; Whartenby, K.A. KLF4 Modulates Expression of IL-6 in Dendritic Cells via Both Promoter Activation and Epigenetic Modification. J. Biol. Chem. 2013, 288, 23868–23874. [Google Scholar] [CrossRef]
- Luo, X.; Chen, J.; Ruan, J.; Chen, Y.; Mo, X.; Xie, J.; Lv, G. Krüppel-like Factor 4 Is a Regulator of Proinflammatory Signaling in Fibroblast-like Synoviocytes through Increased IL-6 Expression. Mediat. Inflamm. 2016, 2016, 1062586. [Google Scholar] [CrossRef]
- Hoekstra, M. Krüppel-like Factor 4-Mediated Smooth Muscle Cell Phenotype Switching to a Galectin-3 Positive Subclass Is a Detrimental Event in the Pathogenesis of Atherosclerotic Cardiovascular Disease. Noncoding RNA Investig. 2020, 4, 8. [Google Scholar] [CrossRef]
- Liao, X.H.; Wang, N.; Zhao, D.W.; Zheng, D.L.; Zheng, L.; Xing, W.J.; Ma, W.J.; Bao, L.Y.; Dong, J.; Zhang, T.C. STAT3 Protein Regulates Vascular Smooth Muscle Cell Phenotypic Switch by Interaction with Myocardin. J. Biol. Chem. 2015, 290, 19641–19652. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.Y.; Shie, J.L.; Tseng, C.C. STAT1 Is Required for IFN-γ-Mediated Gut-Enriched Krüppel-like Factor Expression. Exp. Cell Res. 2002, 281, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Yamashita, M.; Horimai, C.; Hayashi, M. Smooth Muscle-Selective Nuclear Factor-ΚB Inhibition Reduces Phosphate-Induced Arterial Medial Calcification in Mice with Chronic Kidney Disease. J. Am. Heart Assoc. 2017, 6, e007248. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, M.J.; Stang, M.T.; Liu, Y.; Gao, J.; Ren, B.; Zuckerbraun, B.S.; Mahidhara, R.S.; Xing, Q.; Pizzoferrato, E.; Yim, J.H. Interferon Regulatory Factor 1 (IRF-1) Induces P21 WAF1/CIP1 Dependent Cell Cycle Arrest and P21 WAF1/CIP1 Independent Modulation of Survivin in Cancer Cells. Cancer Lett. 2012, 319, 56–65. [Google Scholar] [CrossRef]
- Chin, Y.E.; Kitagawa, M.; Su, W.C.; You, Z.H.; Iwamoto, Y.; Fu, X.Y. Cell growth arrest and induction of cyclin-dependent kinase inhibitor p21 WAF1/CIP1 mediated by STAT1. Science 1996, 272, 719–722. [Google Scholar] [CrossRef]
- Basile, J.R.; Eichten, A.; Zacny, V.; Mü Nger, K. NF-KB-Mediated Induction of P21 Cip1/Waf1 by Tumor Necrosis Factor A Induces Growth Arrest and Cytoprotection in Normal Human Keratinocytes. Mol. Cancer Res. 2003, 1, 262–270. [Google Scholar]
- Luo, W.W.; Lian, H.; Zhong, B.; Shu, H.B.; Li, S. Krüppel-like Factor 4 Negatively Regulates Cellular Antiviral Immune Response. Cell. Mol. Immunol. 2016, 13, 65–72. [Google Scholar] [CrossRef]
- Madonna, S.; Scarponi, C.; Sestito, R.; Pallotta, S.; Cavani, A.; Albanesi, C. The IFN-γ–Dependent Suppressor of Cytokine Signaling 1 Promoter Activity Is Positively Regulated by IFN Regulatory Factor-1 and Sp1 but Repressed by Growth Factor Independence-1b and Krüppel-Like Factor-4, and It Is Dysregulated in Psoriatic Keratinocytes. J. Immunol. 2010, 185, 2467–2481. [Google Scholar] [CrossRef]
- Kurotaki, D.; Osato, N.; Nishiyama, A.; Yamamoto, M.; Ban, T.; Sato, H.; Nakabayashi, J.; Umehara, M.; Miyake, N.; Matsumoto, N.; et al. Essential Role of the IRF8-KLF4 Transcription Factor Cascade in Murine Monocyte Differentiation. Blood 2013, 121, 1839–1849. [Google Scholar] [CrossRef]
- Li, Q.; Wang, M.; Zhang, S.; Jin, M.; Chen, R.; Luo, Y.; Sun, X. Single-Cell RNA Sequencing in Atherosclerosis: Mechanism and Precision Medicine. Front. Pharmacol. 2022, 13, 977490. [Google Scholar] [CrossRef]
- Gastanadui, M.G.; Margaroli, C.; Litovsky, S.; Richter, R.P.; Wang, D.; Xing, D.; Michael Wells, J.; Gaggar, A.; Nanda, V.; Patel, R.P.; et al. Spatial Transcriptomic Approach to Understanding Coronary Atherosclerotic Plaque Stability. Arterioscler. Thromb. Vasc. Biol. 2024, 44, 264–276. [Google Scholar] [CrossRef]
- Bleckwehl, T.; Babler, A.; Tebens, M.; Maryam, S.; Nyberg, M.; Bosteen, M.; Halder, M.; Shaw, I.; Fleig, S.; Pyke, C.; et al. Encompassing View of Spatial and Single-Cell RNA Sequencing Renews the Role of the Microvasculature in Human Atherosclerosis. Nat. Cardiovasc. Res. 2025, 4, 26–44. [Google Scholar] [CrossRef]
- Plens-Galaska, M.; Szelag, M.; Collado, A.; Marques, P.; Vallejo, S.; Ramos-González, M.; Wesoly, J.; Jesus Sanz, M.; Peiró, C.; Bluyssen, H.A.R. Genome-Wide Inhibition of pro-Atherogenic Gene Expression by Multi-STAT Targeting Compounds as a Novel Treatment Strategy of CVDs. Front. Immunol. 2018, 9, 2141. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopacinska, N.; Wesoly, J.; Bluyssen, H.A.R. Interplay Between KLF4, STAT, IRF, and NF-κB in VSMC and Macrophage Plasticity During Vascular Inflammation and Atherosclerosis. Int. J. Mol. Sci. 2025, 26, 10205. https://doi.org/10.3390/ijms262010205
Lopacinska N, Wesoly J, Bluyssen HAR. Interplay Between KLF4, STAT, IRF, and NF-κB in VSMC and Macrophage Plasticity During Vascular Inflammation and Atherosclerosis. International Journal of Molecular Sciences. 2025; 26(20):10205. https://doi.org/10.3390/ijms262010205
Chicago/Turabian StyleLopacinska, Natalia, Joanna Wesoly, and Hans A. R. Bluyssen. 2025. "Interplay Between KLF4, STAT, IRF, and NF-κB in VSMC and Macrophage Plasticity During Vascular Inflammation and Atherosclerosis" International Journal of Molecular Sciences 26, no. 20: 10205. https://doi.org/10.3390/ijms262010205
APA StyleLopacinska, N., Wesoly, J., & Bluyssen, H. A. R. (2025). Interplay Between KLF4, STAT, IRF, and NF-κB in VSMC and Macrophage Plasticity During Vascular Inflammation and Atherosclerosis. International Journal of Molecular Sciences, 26(20), 10205. https://doi.org/10.3390/ijms262010205





