Are pNF-H, IL-6, BDNF, and NSP Reliable Biomarkers of Cognitive Function in Prostate Cancer Patients?
Abstract
1. Introduction
1.1. Inflammation
1.2. Coagulation and Fibrinolysis
1.3. Brain Tissue Damage
1.4. Other Biomarkers of Cognitive Decline
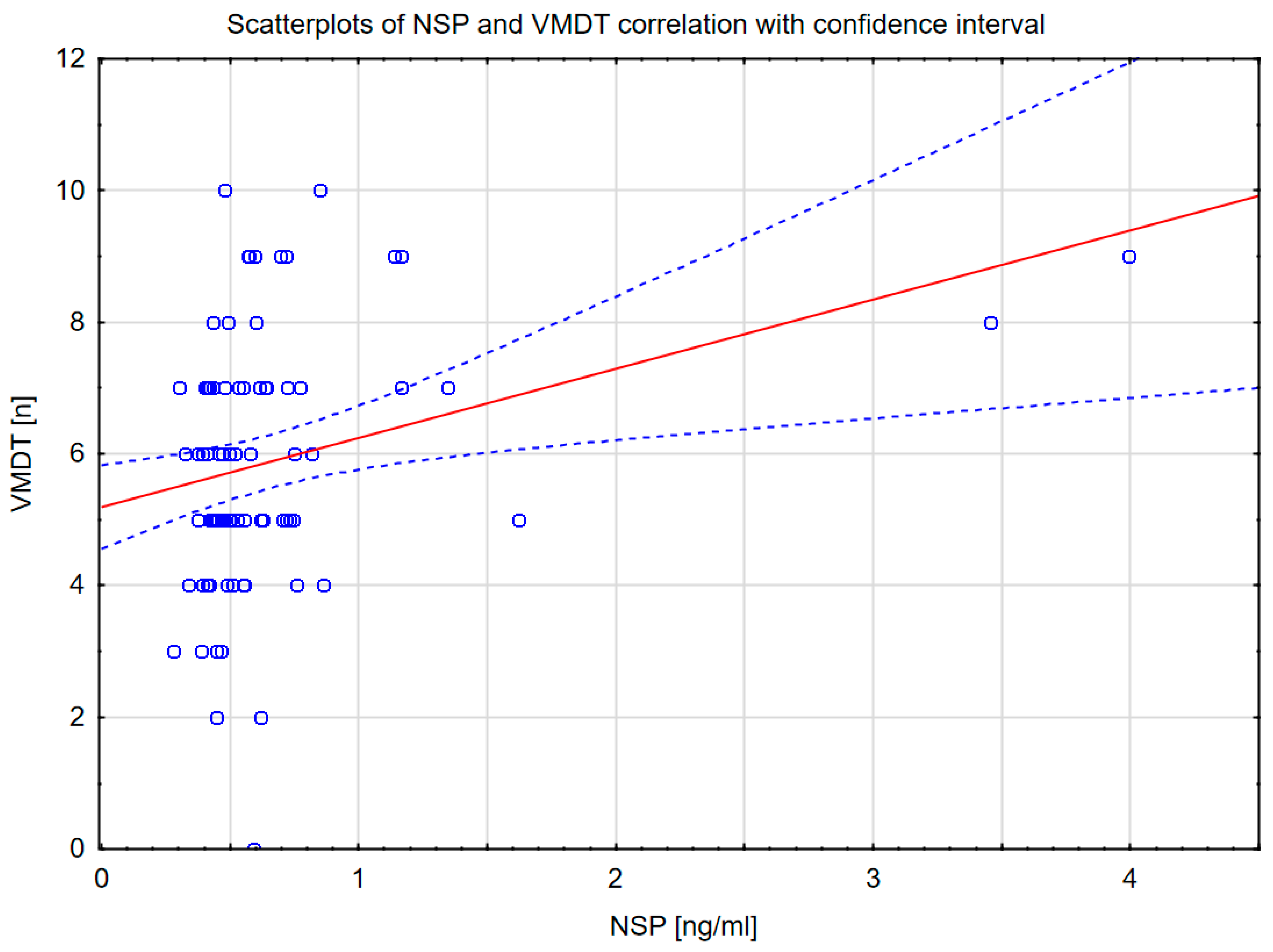
2. Results
3. Discussion
3.1. Il-6
3.2. NSP
3.3. pNf-H
3.4. BDNF
4. Materials and Methods
4.1. Simple Reaction Time (SRT)
4.2. Verbal Memory Test (VMT)
4.3. Verbal Memory Deferred Test (VMDT)
4.4. GoNoGo Test
4.5. Visual Working Memory Test (VWMT)
4.6. Statistical Analysis
5. Conclusions
Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Aβ | beta-amyloid |
| AD | Alzheimer’s disease |
| BDNF | brain-derived neurotrophic factor |
| BMI | body mass index |
| CNS | central nervous system |
| CRP | C-reactive protein |
| GG | the Grade Group according to the International Society of Urological Pathology classification |
| GNG | GoNoGo Test |
| GS | the Gleason Score |
| ICIQ-UI SF | The International Consultation on Incontinence Questionnaire Short Form |
| IIEF-5 | the International Index of Erectile Function, 5-item version |
| Il-6 | interleukin 6 |
| NSP | neuroserpin |
| PC | prostate cancer |
| pNF-H | the high-molecular-weight neurofilament heavy subunit |
| PSA | prostate-specific antigen |
| SRT | Simple reaction time |
| TNM | the Tumor, Node, Metastasis staging system |
| VMDT | Verbal memory deferred test |
| VMT | Verbal memory test |
| VWMT | Visual working memory test |
References
- Popiołek, A.K.; Chyrek-Tomaszewska, A.; Stachowicz-Karpińska, A.; Bieliński, M.K.; Borkowska, A. Biochemical Parameters in Cognitive Functions. Neuropsychiatr. Dis. Treat. 2020, 16, 2479–2489. [Google Scholar] [CrossRef] [PubMed]
- Dove, A.; Shang, Y.; Xu, W.; Grande, G.; Laukka, E.J.; Fratiglioni, L.; Marseglia, A. The Impact of Diabetes on Cognitive Impairment and Its Progression to Dementia. Alzheimer’s Dement. 2021, 17, 1769–1778. [Google Scholar] [CrossRef]
- Ouk, T.; Amr, G.; Azzaoui, R.; Delassus, L.; Fossaert, E.; Tailleux, A.; Bordet, R.; Modine, T. Lipid-Lowering Drugs Prevent Neurovascular and Cognitive Consequences of Cardiopulmonary Bypass. Vascul. Pharmacol. 2016, 80, 59–66. [Google Scholar] [CrossRef]
- Magnuson, A.; Ahles, T.; Chen, B.T.; Mandelblatt, J.; Janelsins, M.C. Cognitive Function in Older Adults with Cancer: Assessment, Management, and Research Opportunities. J. Clin. Oncol. 2021, 39, 2138–2149. [Google Scholar] [CrossRef]
- Jarzemski, P.; Brzoszczyk, B.; Popiołek, A.; Stachowicz-Karpińska, A.; Gołota, S.; Bieliński, M.; Borkowska, A. Cognitive Function, Depression, and Anxiety in Patients Undergoing Radical Prostatectomy with and without Adjuvant Treatment. Neuropsychiatr. Dis. Treat. 2019, 15, 819–829. [Google Scholar] [CrossRef]
- Tewari, A.K.; Stockert, J.A.; Yadav, S.S.; Yadav, K.K.; Khan, I. Inflammation and Prostate Cancer. Adv. Exp. Med. Biol. 2018, 1095, 41–65. [Google Scholar] [CrossRef] [PubMed]
- De Marzo, A.M.; Platz, E.A.; Sutcliffe, S.; Xu, J.; Grönberg, H.; Drake, C.G.; Nakai, Y.; Isaacs, W.B.; Nelson, W.G. Inflammation in Prostate Carcinogenesis. Nat. Rev. Cancer 2007, 7, 256–269. [Google Scholar] [CrossRef] [PubMed]
- Archer, M.; Dogra, N.; Kyprianou, N. Inflammation as a Driver of Prostate Cancer Metastasis and Therapeutic Resistance. Cancers 2020, 12, 2984. [Google Scholar] [CrossRef]
- Rani, A.; Dasgupta, P.; Murphy, J.J. Prostate Cancer. Am. J. Pathol. 2019, 189, 2119–2137. [Google Scholar] [CrossRef]
- Országhová, Z.; Mego, M.; Chovanec, M. Long-Term Cognitive Dysfunction in Cancer Survivors. Front. Mol. Biosci. 2021, 8, 770413. [Google Scholar] [CrossRef]
- Schillaci, O.; Scimeca, M.; Trivigno, D.; Chiaravalloti, A.; Facchetti, S.; Anemona, L.; Bonfiglio, R.; Santeusanio, G.; Tancredi, V.; Bonanno, E.; et al. Prostate Cancer and Inflammation: A New Molecular Imaging Challenge in the Era of Personalized Medicine. Nucl. Med. Biol. 2019, 68–69, 66–79. [Google Scholar] [CrossRef]
- Sfanos, K.S.; De Marzo, A.M. Prostate Cancer and Inflammation: The Evidence. Histopathology 2012, 60, 199–215. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.P.; Li, J.; Tewari, A.K. Inflammation and Prostate Cancer: The Role of Interleukin 6 (IL-6). BJU Int. 2014, 113, 986–992. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Basel, D.; Chow, S.-O.; Fong-Yee, C.; Kim, S.; Buttgereit, F.; Dunstan, C.R.; Zhou, H.; Seibel, M.J. Targeting IL-6 and RANKL Signaling Inhibits Prostate Cancer Growth in Bone. Clin. Exp. Metastasis 2014, 31, 921–933. [Google Scholar] [CrossRef] [PubMed]
- Lwaleed, B.A.; Lam, L.; Lasebai, M.; Cooper, A.J. Expression of Tissue Factor and Tissue Factor Pathway Inhibitor in Microparticles and Subcellular Fractions of Normal and Malignant Prostate Cell Lines. Blood Coagul. Fibrinolysis 2013, 24, 339–343. [Google Scholar] [CrossRef]
- Winther-Larsen, A.; Sandfeld-Paulsen, B.; Hvas, A.-M. Hyperfibrinolysis in Patients with Solid Malignant Neoplasms: A Systematic Review. Semin. Thromb. Hemost. 2021, 47, 581–588. [Google Scholar] [CrossRef]
- Quinn, T.J.; Gallacher, J.; Deary, I.J.; Lowe, G.D.O.; Fenton, C.; Stott, D.J. Association between Circulating Hemostatic Measures and Dementia or Cognitive Impairment: Systematic Review and Meta-Analyzes. J. Thromb. Haemost. 2011, 9, 1475–1482. [Google Scholar] [CrossRef]
- Reumann, R.; Vierk, R.; Zhou, L.; Gries, F.; Kraus, V.; Mienert, J.; Romswinkel, E.; Morellini, F.; Ferrer, I.; Nicolini, C.; et al. The Serine Protease Inhibitor Neuroserpin Is Required for Normal Synaptic Plasticity and Regulates Learning and Social Behavior. Learn. Mem. 2017, 24, 650–659. [Google Scholar] [CrossRef]
- D’Acunto, E.; Fra, A.; Visentin, C.; Manno, M.; Ricagno, S.; Galliciotti, G.; Miranda, E. Neuroserpin: Structure, Function, Physiology and Pathology. Cell. Mol. Life Sci. 2021, 78, 6409–6430. [Google Scholar] [CrossRef]
- Hermann, M.; Reumann, R.; Schostak, K.; Kement, D.; Gelderblom, M.; Bernreuther, C.; Frischknecht, R.; Schipanski, A.; Marik, S.; Krasemann, S.; et al. Deficits in Developmental Neurogenesis and Dendritic Spine Maturation in Mice Lacking the Serine Protease Inhibitor Neuroserpin. Mol. Cell. Neurosci. 2020, 102, 103420. [Google Scholar] [CrossRef]
- Borges, V.M.; Lee, T.W.; Christie, D.L.; Birch, N.P. Neuroserpin Regulates the Density of Dendritic Protrusions and Dendritic Spine Shape in Cultured Hippocampal Neurons. J. Neurosci. Res. 2010, 88, 2610–2617. [Google Scholar] [CrossRef]
- Chuang, H.-N.; van Rossum, D.; Sieger, D.; Siam, L.; Klemm, F.; Bleckmann, A.; Bayerlová, M.; Farhat, K.; Scheffel, J.; Schulz, M.; et al. Carcinoma Cells Misuse the Host Tissue Damage Response to Invade the Brain. Glia 2013, 61, 1331–1346. [Google Scholar] [CrossRef]
- Petr, J.; Hogeboom, L.; Nikulin, P.; Wiegers, E.; Schroyen, G.; Kallehauge, J.; Chmelík, M.; Clement, P.; Nechifor, R.E.; Fodor, L.-A.; et al. A Systematic Review on the Use of Quantitative Imaging to Detect Cancer Therapy Adverse Effects in Normal-Appearing Brain Tissue. Magn. Reson. Mater. Phys. Biol. Med. 2022, 35, 163–186. [Google Scholar] [CrossRef] [PubMed]
- Shaw, G.; Yang, C.; Ellis, R.; Anderson, K.; Parker Mickle, J.; Scheff, S.; Pike, B.; Anderson, D.K.; Howland, D.R. Hyperphosphorylated Neurofilament NF-H Is a Serum Biomarker of Axonal Injury. Biochem. Biophys. Res. Commun. 2005, 336, 1268–1277. [Google Scholar] [CrossRef]
- Karesioglu, E.; Cikriklar, H.I.; Durak, V.A.; Aydin, B.; Ardic, A.; Armagan, E. Serum pNF-H Levels in the First Six Hours after Experimental Mild Traumatic Brain Injury in Rats. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 6928–6934. [Google Scholar] [CrossRef] [PubMed]
- Ahadi, R.; Khodagholi, F.; Daneshi, A.; Vafaei, A.; Mafi, A.A.; Jorjani, M. Diagnostic Value of Serum Levels of GFAP, pNF-H, and NSE Compared With Clinical Findings in Severity Assessment of Human Traumatic Spinal Cord Injury. Spine 2015, 40, E823–E830. [Google Scholar] [CrossRef]
- Gatson, J.W.; Liu, M.-M.; Rivera-Chavez, F.A.; Minei, J.P.; Wolf, S.E. Serum Levels of Neurofilament-H Are Elevated in Patients Suffering From Severe Burns. J. Burn. Care Res. 2015, 36, 545–550. [Google Scholar] [CrossRef]
- Takahashi, H.; Aoki, Y.; Nakajima, A.; Sonobe, M.; Terajima, F.; Saito, M.; Taniguchi, S.; Yamada, M.; Watanabe, F.; Furuya, T.; et al. Phosphorylated Neurofilament Subunit NF-H Becomes Elevated in the Cerebrospinal Fluid of Patients with Acutely Worsening Symptoms of Compression Myelopathy. J. Clin. Neurosci. 2014, 21, 2175–2178. [Google Scholar] [CrossRef]
- Holmström, U.; Tsitsopoulos, P.P.; Holtz, A.; Salci, K.; Shaw, G.; Mondello, S.; Marklund, N. Cerebrospinal Fluid Levels of GFAP and pNF-H Are Elevated in Patients with Chronic Spinal Cord Injury and Neurological Deterioration. Acta Neurochir. 2020, 162, 2075–2086. [Google Scholar] [CrossRef]
- Anderson, K.J.; Scheff, S.W.; Miller, K.M.; Roberts, K.N.; Gilmer, L.K.; Yang, C.; Shaw, G. The Phosphorylated Axonal form of the Neurofilament Subunit NF-H (pNF-H) as a Blood Biomarker of Traumatic Brain Injury. J. Neurotrauma 2008, 25, 1079–1085. [Google Scholar] [CrossRef] [PubMed]
- Benady, A.; Freidin, D.; Pick, C.G.; Rubovitch, V. GM1 Ganglioside Prevents Axonal Regeneration Inhibition and Cognitive Deficits in a Mouse Model of Traumatic Brain Injury. Sci. Rep. 2018, 8, 13340. [Google Scholar] [CrossRef]
- Heiskanen, M.; Banuelos, I.; Manninen, E.; Andrade, P.; Hämäläinen, E.; Puhakka, N.; Pitkänen, A. Plasma Neurofilament Heavy Chain Is a Prognostic Biomarker for the Development of Severe Epilepsy after Experimental Traumatic Brain Injury. Epilepsia 2024, 65, 3703–3716. [Google Scholar] [CrossRef] [PubMed]
- Shibahashi, K.; Doi, T.; Tanaka, S.; Hoda, H.; Chikuda, H.; Sawada, Y.; Takasu, Y.; Chiba, K.; Nozaki, T.; Hamabe, Y.; et al. The Serum Phosphorylated Neurofilament Heavy Subunit as a Predictive Marker for Outcome in Adult Patients after Traumatic Brain Injury. J. Neurotrauma 2016, 33, 1826–1833. [Google Scholar] [CrossRef]
- Zurek, J.; Bartlová, L.; Fedora, M. Hyperphosphorylated Neurofilament NF-H as a Predictor of Mortality after Brain Injury in Children. Brain Inj. 2011, 25, 221–226. [Google Scholar] [CrossRef]
- Trifilio, E.; Bottari, S.; McQuillan, L.E.; Barton, D.J.; Lamb, D.G.; Robertson, C.; Rubenstein, R.; Wang, K.K.; Wagner, A.K.; Williamson, J.B. Temporal Profile of Serum Neurofilament Light (NF-L) and Heavy (pNF-H) Level Associations with 6-Month Cognitive Performance in Patients with Moderate-Severe Traumatic Brain Injury. J. Head Trauma Rehabil. 2024, 39, E470–E480. [Google Scholar] [CrossRef]
- Mietani, K.; Sumitani, M.; Ogata, T.; Shimojo, N.; Inoue, R.; Abe, H.; Kawamura, G.; Yamada, Y. Dysfunction of the Blood-Brain Barrier in Postoperative Delirium Patients, Referring to the Axonal Damage Biomarker Phosphorylated Neurofilament Heavy Subunit. PLoS ONE 2019, 14, e0222721. [Google Scholar] [CrossRef]
- Inoue, R.; Sumitani, M.; Ogata, T.; Chikuda, H.; Matsubara, T.; Kato, S.; Shimojo, N.; Uchida, K.; Yamada, Y. Direct Evidence of Central Nervous System Axonal Damage in Patients with Postoperative Delirium: A Preliminary Study of pNF-H as a Promising Serum Biomarker. Neurosci. Lett. 2017, 653, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Natori, A.; Ogata, T.; Sumitani, M.; Kogure, T.; Yamauchi, T.; Yamauchi, H. Potential Role of pNF-H, a Biomarker of Axonal Damage in the Central Nervous System, as a Predictive Marker of Chemotherapy-Induced Cognitive Impairment. Clin. Cancer Res. 2015, 21, 1348–1352. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Zhao, L.; Tang, Y. The Function of BDNF and Its Receptor in the Male Genitourinary System and Its Potential Clinical Application. Curr. Issues Mol. Biol. 2022, 45, 110–121. [Google Scholar] [CrossRef]
- March, B.; Lockhart, K.R.; Faulkner, S.; Smolny, M.; Rush, R.; Hondermarck, H. ELISA-Based Quantification of Neurotrophic Growth Factors in Urine from Prostate Cancer Patients. FASEB BioAdvances 2021, 3, 888–896. [Google Scholar] [CrossRef]
- Li, T.; Yu, Y.; Song, Y.; Li, X.; Lan, D.; Zhang, P.; Xiao, Y.; Xing, Y. Activation of BDNF/TrkB Pathway Promotes Prostate Cancer Progression via Induction of Epithelial-Mesenchymal Transition and Anoikis Resistance. FASEB J. 2020, 34, 9087–9101. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.-G.; Sereika, S.M.; Cummings, M.H.; Erickson, K.I.; Bender, C.M.; Conley, Y.P. DNA Methylation of BDNF and RASA2 Genes Is Associated With Cognitive Function in Postmenopausal Women With Breast Cancer. Oncol. Nurs. Forum 2024, 51, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Lange, M.; Joly, F.; Vardy, J.; Ahles, T.; Dubois, M.; Tron, L.; Winocur, G.; De Ruiter, M.B.; Castel, H. Cancer-Related Cognitive Impairment: An Update on State of the Art, Detection, and Management Strategies in Cancer Survivors. Ann. Oncol. 2019, 30, 1925–1940. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Saligan, L.N.; Li, X.; Xiang, L.; Kwiat, C.; Nguyen, C.; Crouch, A.; Von Ah, D. Associations of Brain-Derived Neurotrophic Factor Rs6265 Polymorphism and Cognitive Function in Breast Cancer Survivors from a Cross-Sectional Study. Cancer Med. 2024, 13, e6975. [Google Scholar] [CrossRef]
- Wrann, C.D.; White, J.P.; Salogiannnis, J.; Laznik-Bogoslavski, D.; Wu, J.; Ma, D.; Lin, J.D.; Greenberg, M.E.; Spiegelman, B.M. Exercise Induces Hippocampal BDNF through a PGC-1α/FNDC5 Pathway. Cell Metab. 2013, 18, 649–659. [Google Scholar] [CrossRef]
- Popiołek, A.; Brzoszczyk, B.; Jarzemski, P.; Piskunowicz, M.; Jarzemski, M.; Borkowska, A.; Bieliński, M. Quality of Life of Prostate Cancer Patients Undergoing Prostatectomy and Affective Temperament. Cancer Manag. Res. 2022, 14, 1743–1755. [Google Scholar] [CrossRef]
- Malekan, M.; Nezamabadi, S.S.; Samami, E.; Mohebalizadeh, M.; Saghazadeh, A.; Rezaei, N. BDNF and Its Signaling in Cancer. J. Cancer Res. Clin. Oncol. 2023, 149, 2621–2636. [Google Scholar] [CrossRef]
- Sartori, A.C.; Vance, D.E.; Slater, L.Z.; Crowe, M. The Impact of Inflammation on Cognitive Function in Older Adults: Implications for Healthcare Practice and Research. J. Neurosci. Nurs. 2012, 44, 206–217. [Google Scholar] [CrossRef]
- Gruol, D.L. IL-6 Regulation of Synaptic Function in the CNS. Neuropharmacology 2015, 96 Pt A, 42–54. [Google Scholar] [CrossRef]
- Mena-Vázquez, N.; Ortiz-Márquez, F.; Ramírez-García, T.; Cabezudo-García, P.; García-Studer, A.; Mucientes-Ruiz, A.; Lisbona-Montañez, J.M.; Borregón-Garrido, P.; Ruiz-Limón, P.; Redondo-Rodríguez, R.; et al. Impact of Inflammation on Cognitive Function in Patients with Highly Inflammatory Rheumatoid Arthritis. RMD Open 2024, 10, e004422. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, Q.; Zhang, X.; Song, Y.; Zheng, J.; An, Y.; Lu, Y. Correlation between Inflammatory Biomarkers, Cognitive Function and Glycemic and Lipid Profiles in Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Clin. Biochem. 2023, 121–122, 110683. [Google Scholar] [CrossRef]
- Ribeiro-Santos, R.; de Campos-Carli, S.M.; Ferretjans, R.; Teixeira-Carvalho, A.; Martins-Filho, O.A.; Teixeira, A.L.; Salgado, J.V. The Association of Cognitive Performance and IL-6 Levels in Schizophrenia Is Influenced by Age and Antipsychotic Treatment. Nord. J. Psychiatry 2020, 74, 187–193. [Google Scholar] [CrossRef]
- Sutin, A.R.; Stephan, Y.; Luchetti, M.; Terracciano, A. Purpose in Life and Markers of Immunity and Inflammation: Testing Pathways of Episodic Memory. J. Psychosom. Res. 2023, 174, 111487. [Google Scholar] [CrossRef]
- Tegeler, C.; O’Sullivan, J.L.; Bucholtz, N.; Goldeck, D.; Pawelec, G.; Steinhagen-Thiessen, E.; Demuth, I. The Inflammatory Markers CRP, IL-6, and IL-10 Are Associated with Cognitive Function--Data from the Berlin Aging Study II. Neurobiol. Aging 2016, 38, 112–117. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, J.; Wu, Y.; Xie, M.; Tao, S.; Lv, Q.; Wang, Q. Plasma IL-6 Levels and Their Association with Brain Health and Dementia Risk: A Population-Based Cohort Study. Brain. Behav. Immun. 2024, 120, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.H.; Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Alnaaim, S.A.; Alexiou, A.; Papadakis, M.; Saad, H.M.; Batiha, G.E.-S. The Probable Role of Tissue Plasminogen Activator/Neuroserpin Axis in Alzheimer’s Disease: A New Perspective. Acta Neurol. Belg. 2024, 124, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Pentz, R.; Iulita, M.F.; Ducatenzeiler, A.; Bennett, D.A.; Cuello, A.C. The Human Brain NGF Metabolic Pathway Is Impaired in the Pre-Clinical and Clinical Continuum of Alzheime’s Disease. Mol. Psychiatry 2021, 26, 6023–6037. [Google Scholar] [CrossRef]
- Barba, L.; Bellomo, G.; Oeckl, P.; Chiasserini, D.; Gaetani, L.; Torrigiani, E.G.; Paoletti, F.P.; Steinacker, P.; Abu-Rumeileh, S.; Parnetti, L.; et al. CSF Neurosecretory Proteins VGF and Neuroserpin in Patients with Alzheimer’s and Lewy Body Diseases. J. Neurol. Sci. 2024, 462, 123059. [Google Scholar] [CrossRef]
- Zheng, D.; Chen, H.; Davids, J.; Bryant, M.; Lucas, A. Serpins for Diagnosis and Therapy in Cancer. Cardiovasc. Haematol. Disord.-Drug Targets 2013, 13, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Godinez, A.; Rajput, R.; Chitranshi, N.; Gupta, V.; Basavarajappa, D.; Sharma, S.; You, Y.; Pushpitha, K.; Dhiman, K.; Mirzaei, M.; et al. Neuroserpin, a Crucial Regulator for Axogenesis, Synaptic Modelling and Cell-Cell Interactions in the Pathophysiology of Neurological Disease. Cell. Mol. Life Sci. 2022, 79, 172. [Google Scholar] [CrossRef]
- Valiente, M.; Obenauf, A.C.; Jin, X.; Chen, Q.; Zhang, X.H.-F.; Lee, D.J.; Chaft, J.E.; Kris, M.G.; Huse, J.T.; Brogi, E.; et al. Serpins Promote Cancer Cell Survival and Vascular Co-Option in Brain Metastasis. Cell 2014, 156, 1002–1016. [Google Scholar] [CrossRef] [PubMed]
- Fabbro, S.; Seeds, N.W. Plasminogen Activator Activity Is Inhibited While Neuroserpin Is Up-Regulated in the Alzheimer Disease Brain. J. Neurochem. 2009, 109, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Yepes, M. The Plasminogen Activating System in the Pathogenesis of Alzheimer’s Disease. Neural Regen. Res. 2021, 16, 1973–1977. [Google Scholar] [CrossRef] [PubMed]
- Kucuksayan, E.; Kucuksayan, H.; Sozen, M.E.; Sircan-Kucuksayan, A. Elevated Level of Neuroserpin Is an Indication for the Resistance to Gambogic Acid-Induced Apoptosis and Oxidative Stress in Triple-Negative Breast Cancer Cells. Asian Biomed. Res. Rev. News 2024, 18, 69–80. [Google Scholar] [CrossRef]
- Hasumi, H.; Ishiguro, H.; Nakamura, M.; Sugiura, S.; Osada, Y.; Miyoshi, Y.; Fujinami, K.; Yao, M.; Hamada, K.; Yamada-Okabe, H.; et al. Neuroserpin (PI-12) Is Upregulated in High-Grade Prostate Cancer and Is Associated with Survival. Int. J. Cancer 2005, 115, 911–916. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.K.W.; Barton, D.J.; McQuillan, L.E.; Kobeissy, F.; Cai, G.; Xu, H.; Yang, Z.; Trifilio, E.; Williamson, J.B.; Rubenstein, R.; et al. Parallel Cerebrospinal Fluid and Serum Temporal Profile Assessment of Axonal Injury Biomarkers Neurofilament-Light Chain and Phosphorylated Neurofilament-Heavy Chain: Associations with Patient Outcome in Moderate-Severe Traumatic Brain Injury. J. Neurotrauma 2024, 41, 1609–1627. [Google Scholar] [CrossRef]
- Jehn, C.F.; Becker, B.; Flath, B.; Nogai, H.; Vuong, L.; Schmid, P.; Lüftner, D. Neurocognitive Function, Brain-Derived Neurotrophic Factor (BDNF) and IL-6 Levels in Cancer Patients with Depression. J. Neuroimmunol. 2015, 287, 88–92. [Google Scholar] [CrossRef]
- Ng, D.Q.; Cheng, I.; Wang, C.; Tan, C.J.; Toh, Y.L.; Koh, Y.Q.; Ke, Y.; Foo, K.M.; Chan, R.J.; Ho, H.K.; et al. Brain-Derived Neurotrophic Factor as a Biomarker in Cancer-Related Cognitive Impairment among Adolescent and Young Adult Cancer Patients. Sci. Rep. 2023, 13, 16298. [Google Scholar] [CrossRef]
- Wolff, B.S.; Raheem, S.A.; Alshawi, S.A.; Regan, J.M.; Feng, L.R.; Saligan, L.N. Induction of Fatigue-like Behavior by Pelvic Irradiation of Male Mice Alters Cognitive Behaviors and BDNF Expression. PLoS ONE 2020, 15, e0235566. [Google Scholar] [CrossRef]
- Mietła, B.; Budzyński, J.; Bieliński, M.; Mieczkowski, A.; Pulkowska-Ulfig, J.; Borkowska, A. Links between Parameters of Long-Term Latent Memory and Progression from Paroxysmal to Permanent Atrial Fibrillation during a Five-Year Observation Period. A Preliminary Study. Kardiol. Pol. 2016, 74, 754–761. [Google Scholar] [CrossRef]
- Gasquoine, P.G. Effects of Physical Activity on Delayed Memory Measures in Randomized Controlled Trials with Nonclinical Older, Mild Cognitive Impairment, and Dementia Participants. J. Clin. Exp. Neuropsychol. 2018, 40, 874–886. [Google Scholar] [CrossRef] [PubMed]
- Weissberger, G.H.; Strong, J.V.; Stefanidis, K.B.; Summers, M.J.; Bondi, M.W.; Stricker, N.H. Diagnostic Accuracy of Memory Measures in Alzheimer’s Dementia and Mild Cognitive Impairment: A Systematic Review and Meta-Analysis. Neuropsychol. Rev. 2017, 27, 354–388. [Google Scholar] [CrossRef] [PubMed]
- Shafiee, A.; Beiky, M.; Mohammadi, I.; Rajai, S.; Jafarabady, K.; Moradi, S.; Beikmohamadi, M.; Teixeira, A.L. Effect of Smoking on Brain-Derived Neurotrophic Factor (BDNF) Blood Levels: A Systematic Review and Meta-Analysis. J. Affect. Disord. 2024, 349, 525–533. [Google Scholar] [CrossRef]
- Dalal, R.; Djakiew, D. Molecular characterization of neurotrophin expression and the corresponding tropomyosin receptor kinases (trks) in epithelial and stromal cells of the human prostate. Mol. Cell Endocrinol. 1997, 134, 15–22. [Google Scholar] [CrossRef]
- March, B.; Faulkner, S.; Jobling, P.; Steigler, A.; Blatt, A.; Denham, J.; Hondermarck, H. Tumour innervation and neurosignalling in prostate cancer. Nat. Rev. Urol. 2020, 17, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Buskbjerg, C.R.; Amidi, A.; Buus, S.; Gravholt, C.H.; Hadi Hosseini, S.M.; Zachariae, R. Androgen deprivation therapy and cognitive decline-associations with brain connectomes, endocrine status, and risk genotypes. Prostate Cancer Prostatic Dis. 2022, 25, 208–218. [Google Scholar] [CrossRef]
- O’Sullivan, B.; Brierley, J.; Byrd, D.; Bosman, F.; Kehoe, S.; Kossary, C.; Piñeros, M.; Van Eycken, E.; Weir, H.K.; Gospodarowicz, M. The TNM Classification of Malignant Tumours—Towards Common Understanding and Reasonable Expectations. Lancet Oncol. 2017, 18, 849–851. [Google Scholar] [CrossRef]
- Wu, J.; Alfredsson, L.; Olsson, T.; Hillert, J.A.; Hedström, A.K. Obesity Affects Disease Activity and Progression, Cognitive Functioning, and Quality of Life in People with Multiple Sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2025, 12, e200334. [Google Scholar] [CrossRef]
- van Leenders, G.J.L.H.; van der Kwast, T.H.; Grignon, D.J.; Evans, A.J.; Kristiansen, G.; Kweldam, C.F.; Litjens, G.; McKenney, J.K.; Melamed, J.; Mottet, N.; et al. The 2019 International Society of Urological Pathology (ISUP) Consensus Conference on Grading of Prostatic Carcinoma. Am. J. Surg. Pathol. 2020, 44, e87. [Google Scholar] [CrossRef]
- Rosen, R.C.; Cappelleri, J.C.; Smith, M.D.; Lipsky, J.; Peña, B.M. Development and Evaluation of an Abridged, 5-Item Version of the International Index of Erectile Function (IIEF-5) as a Diagnostic Tool for Erectile Dysfunction. Int. J. Impot. Res. 1999, 11, 319–326. [Google Scholar] [CrossRef]
- Klovning, A.; Avery, K.; Sandvik, H.; Hunskaar, S. Comparison of Two Questionnaires for Assessing the Severity of Urinary Incontinence: The ICIQ-UI SF versus the Incontinence Severity Index. Neurourol. Urodyn. 2009, 28, 411–415. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Median (Q25–Q75) | |
|---|---|---|
| Age [y] | 66.0 (60.0–70.0) | |
| BMI | 27.1 (25.1–29.7) | |
| Years from surgery [y] | 1.5 (1.0–3.2) | |
| GRADE group [n] | 1 | n = 58 |
| 2 | n = 32 | |
| 3 | n = 4 | |
| 4 | n = 3 | |
| 5 | n = 3 | |
| Diabetes (n, %) | 13 (13%) | |
| Hypertension (n, %) | 56 (56%) | |
| MI (n, %) | 9 (9%) | |
| Stroke (n, %) | 7 (7%) | |
| Education | Basic (n, %) | 5 (5%) |
| Vocational (n, %) | 28 (28%) | |
| Secondary (n, %) | 34 (34%) | |
| Higher (n, %) | 33 (33%) | |
| BDNF [ng/mL] | 0.79 (0.27–1.34) | |
| Il-6 [pg/mL] | 3.26 (2.09–9.11) | |
| NSP [ng/mL] | 0.50 (0.43–0.64) | |
| pNf-H [pg/mL] | 50.2 (48.6–52.7) | |
| SRT [n] | 25.0 (25.0–25.0) | |
| SRT RT [ms] | 280.4 (234.3–319.1) | |
| VM_1 [n] | 5.0 (4.0–7.0) | |
| VM_ 2 [n] | 7.0 (6.0–8.0) | |
| VM_3 [n] | 7.0 (7.0–8.0) | |
| VM_4 [n] | 8.0 (7.0–10.0) | |
| VM_5 [n] | 8.0 (7.0–10.0) | |
| VMDT [n] | 6.0 (5.0–7.0) | |
| GoNoGo N correct Go [n] | 74.0 (73.0–75.0) | |
| GoNoGo RT [ms] | 365.8 (323.6–414.3) | |
| GoNoGo N non correct No Go [n] | 5.0 (2.0–8.0) | |
| VWMT [n] | 5.0 (3.0–6.5) | |
| VWMT RT [ms] | 3150.0 (2570.0–4441.0) | |
| Non-Smokers | ACTIVE SMOKERS | p | Obesity | Overweight | Normal Weight | p | No Physical Activity | Physical Activity | p | |
|---|---|---|---|---|---|---|---|---|---|---|
| BDNF [ng/mL] | 0.91 (0.38–1.39) n = 62 | 0.24 (0.10–0.67) n = 10 | 0.08 | 1.05 (0.25–1.33) n = 15 | 0.84 (0.38–1.40) n = 40 | 0.44 (0.16–1.34) n = 20 | 0.47 | 0.56 (0.17–1.33) n = 23 | 0.84 (0.33–1.42) n = 52 | 0.51 |
| IL-6 [pg/mL] | 3.18 (1.95–5.1) n = 66 | 3.34 (2.36–4.91) n = 11 | 0.7 | 5.1 (1.91–16.0) n = 18 | 2.8 (2.1–4.6) n = 42 | 3.42 (2.19–7.58) n = 20 | 0.59 | 2.8 (1.95–10.7) n = 25 | 3.3 (2.16–6.5) n = 55 | 0.81 |
| NSP [ng/mL] | 0.51 (0.43–0.70) n = 67 | 0.49 (0.44–0.60) n = 11 | 0.87 | 0.45 (0.40–0.72) n = 18 | 0.53 (0.44–0.72) n = 43 | 0.5 (0.43–0.59) n = 20 | 0.47 | 0.47 (0.43–0.63) n = 25 | 0.53 (0.42–0.66) n = 56 | 0.75 |
| pNf-H [pg/mL] | 50 (48.6–51.7) n = 62 | 53 (48.1–86.3) n = 10 | 0.3 | 50.8 (48.7–52.1) n = 15 | 49.4 (47.5–53.4) n = 40 | 50.5 (49.5–57.4) n = 20 | 0.44 | 50.2 (48.1–52.7) n = 23 | 50.1 (48.6–52.6) n = 52 | 0.84 |
|
BDNF [ng/mL]
(n = 74) |
IL 6 [pg/mL]
(n = 79) |
NSP [ng/mL]
(n = 80) |
Nfilament H [pg/mL]
(n = 74) | |
|---|---|---|---|---|
| Age [y] | r = −0.065 | r = 0.115 | r = −0.225; p = 0.04 | r = 0.029 |
| BMI | r = 0.040 | r = 0.061 | r = −0.020 | r = −0.002 |
| SRT [n] | r = −0.161 | r = 0.095 | r = 0.172 | r = 0.088 |
| SRT RT [ms] | r = −0.089 | r = −0.106 | r = 0.004 | r = −0.045 |
| VM_1 [n] | r = 0.175 | r = −0.044 | r = 0.028 | r = −0.128 |
| VM_2 [n] | r = 0.176 | r = 0.087 | r = 0.184 | r = −0.204 |
| VM_3 [n] | r = 0.106 | r = 0.210 | r = 0.156 | r = −0.154 |
| VM_4 [n] | r = 0.137 | r = 0.219 | r = 0.203 | r = −0.172 |
| VM_5 [n] | r = 0.129 | r = 0.216 | r = 0.197 | r = −0.179 |
| VMDT [n] | r = 0.231; p = 0.047 | r = 0.101 | r = 0.270; p = 0.015 | r = −0.084 |
| GoNoGo N correct [n] | r = −0.082 | r = 0.088 | r = 0.04 | r = 0.183 |
| GoNoGo RT [ms] | r = −0.064 | r = −0.027 | r = 0.021 | r = 0.089 |
| GoNoGo N non correct No Go [n] | r = 0.205 | r = −0.025 | r = −0.026 | r = −0.181 |
| VWMT [n] | r = 0.032 | r = 0.024 | r = −0.067 | r = 0.057 |
| VWMT RT [ms] | r = −0.016 | r = 0.101 | r = −0.208 | r = 0.073 |
| n = 94 | VMDT_Number Correct | VMDT_Intrusion | VMDT_Perseveration |
|---|---|---|---|
| Age [y] | r = −0.192; | r = −0.055 | r = −0.133 |
| BMI | r = −0.037 | r = 0.030 | r = 0.022 |
| SRT [n] | r = −0.021 | r = 0.047 | r = 0.027 |
| SRT RT [ms] | r = −0.377; p = 0.0001 | r = −0.266; p = 0.008 | r = −0.160 |
| VM_1 [n] | r = 0.307; p = 0.002 | r = −0.042 | r = −175 |
| VM_2 [n] | r = 0.306; p = 0.002 | r = −0.068 | r = −0.135; |
| VM_3 [n] | r = 0.404; p = 0.00004 | r = −0.235; p = 0.021 | r = −0.172 |
| VM_4 [n] | r = 0.573; p < 0.0000001 | r = −0.220; p = 0.031 | r = −0.006 |
| VM_5 [n] | 0.579; p < 0.0000001 | r = −0.255; p = 0.026 | r = −0.002 |
| VM_intrusions [n] | r = 0.027 | r = 0.052 | r = −0.044 |
| VM_perseverations [n] | r = 0.199 | r = 0.196 | r = 0.509; p < 0.0000001 |
| GoNoGo N correct [n] | r = 0.061 | r = 0.133 | r = 0.172 |
| GoNoGo RT [ms] | r = −0.412; p = 0.00003; | r = 0.015 | r = −0.208; p = 0.041 |
| GoNoGo N non correct No Go [n] | 0.137 | r = −0.013 | r = 0.143 |
| VWMT RT [ms] | −0.315; p = 0.001 | r = −0.021 | r = −0.214; p = 0.036 |
| VWMT [n] | 0.199; p = 0.05 | r = 0.102 | r = −0.092 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popiołek, A.; Brzoszczyk, B.; Borkowska, A.; Jarzemski, P.; Kozakiewicz, M.; Szelągowski, A.; Bieliński, M. Are pNF-H, IL-6, BDNF, and NSP Reliable Biomarkers of Cognitive Function in Prostate Cancer Patients? Int. J. Mol. Sci. 2025, 26, 10202. https://doi.org/10.3390/ijms262010202
Popiołek A, Brzoszczyk B, Borkowska A, Jarzemski P, Kozakiewicz M, Szelągowski A, Bieliński M. Are pNF-H, IL-6, BDNF, and NSP Reliable Biomarkers of Cognitive Function in Prostate Cancer Patients? International Journal of Molecular Sciences. 2025; 26(20):10202. https://doi.org/10.3390/ijms262010202
Chicago/Turabian StylePopiołek, Alicja, Bartosz Brzoszczyk, Alina Borkowska, Piotr Jarzemski, Mariusz Kozakiewicz, Adam Szelągowski, and Maciej Bieliński. 2025. "Are pNF-H, IL-6, BDNF, and NSP Reliable Biomarkers of Cognitive Function in Prostate Cancer Patients?" International Journal of Molecular Sciences 26, no. 20: 10202. https://doi.org/10.3390/ijms262010202
APA StylePopiołek, A., Brzoszczyk, B., Borkowska, A., Jarzemski, P., Kozakiewicz, M., Szelągowski, A., & Bieliński, M. (2025). Are pNF-H, IL-6, BDNF, and NSP Reliable Biomarkers of Cognitive Function in Prostate Cancer Patients? International Journal of Molecular Sciences, 26(20), 10202. https://doi.org/10.3390/ijms262010202





