Early Changes in Cardiac Macrophage Subsets in Heart Failure with Preserved Ejection Fraction
Abstract
1. Introduction
2. Results
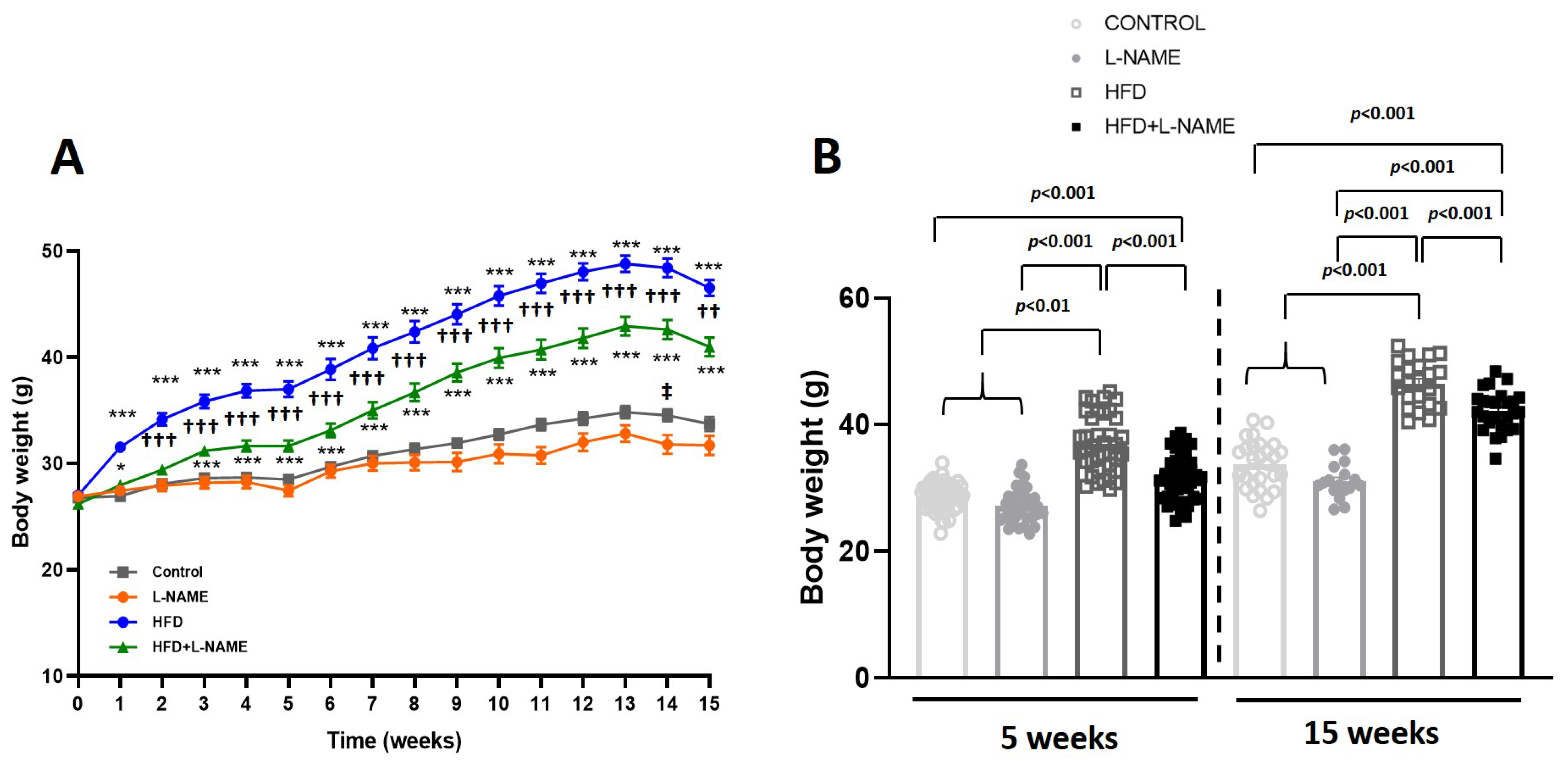
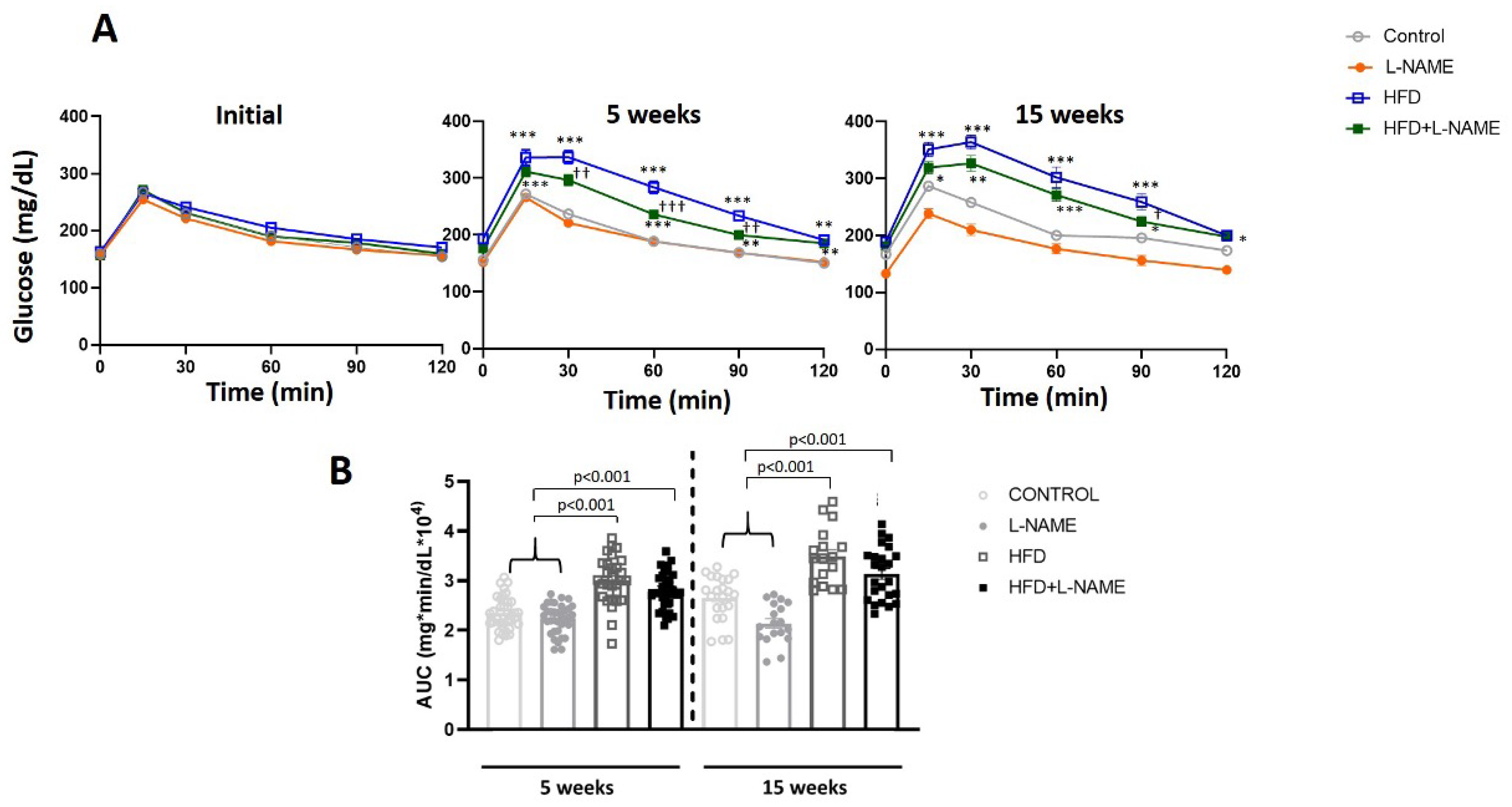
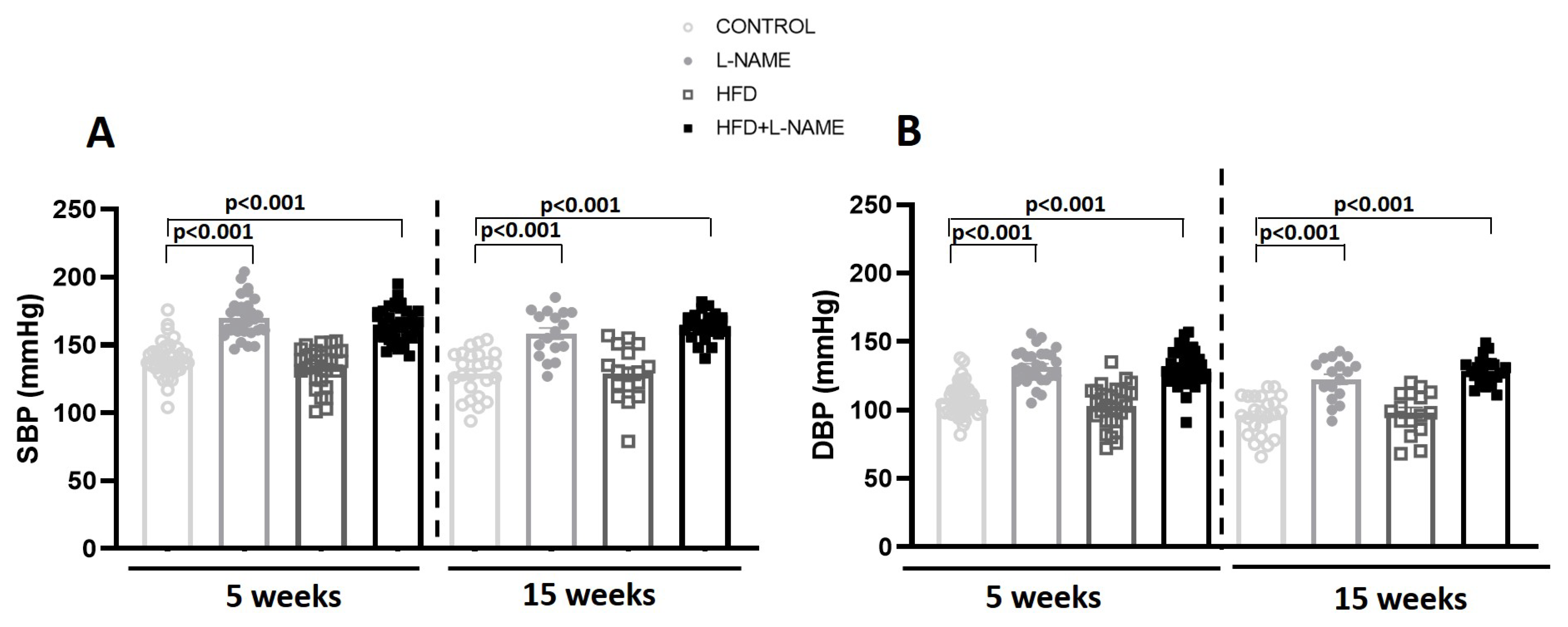
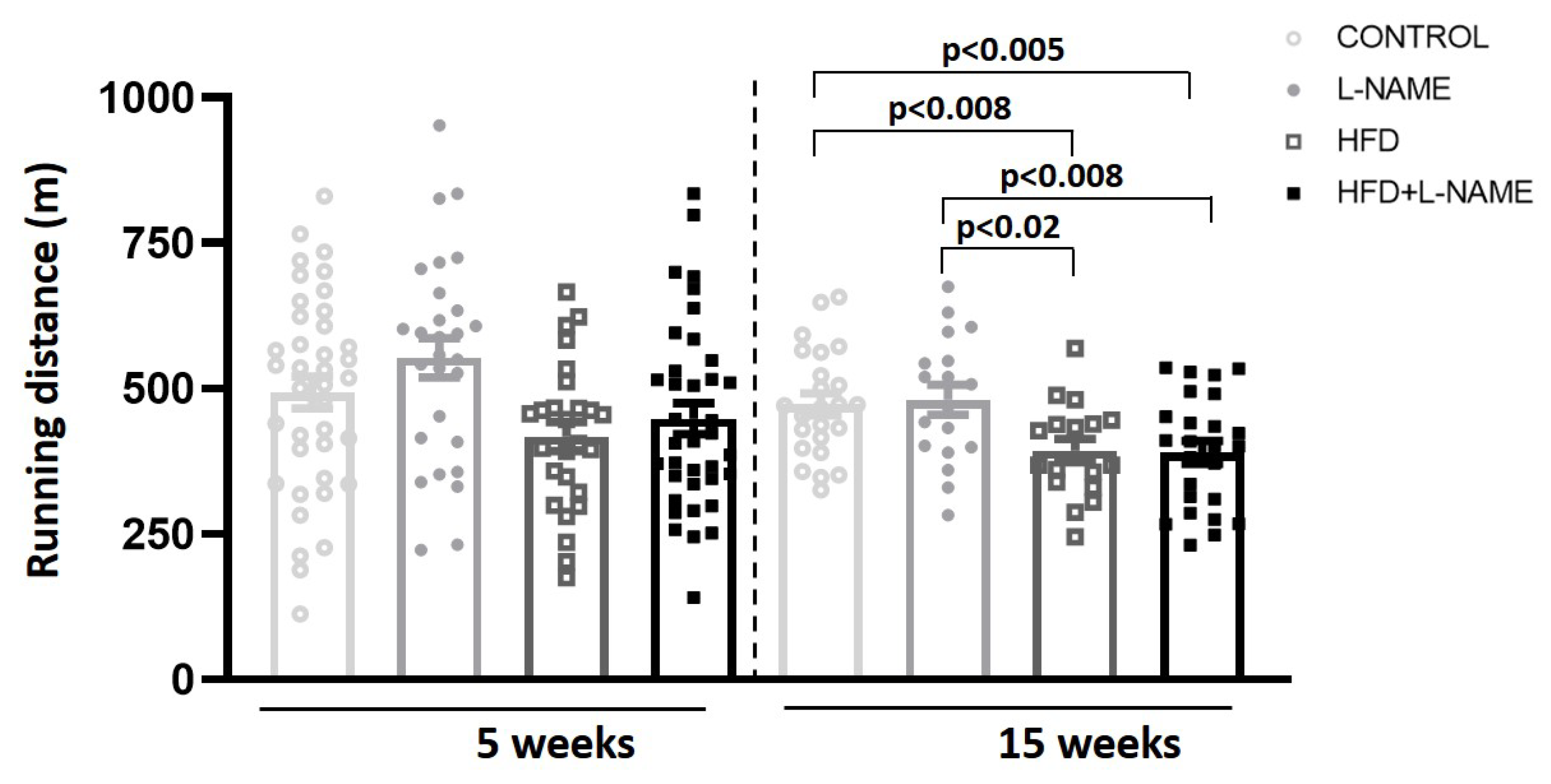
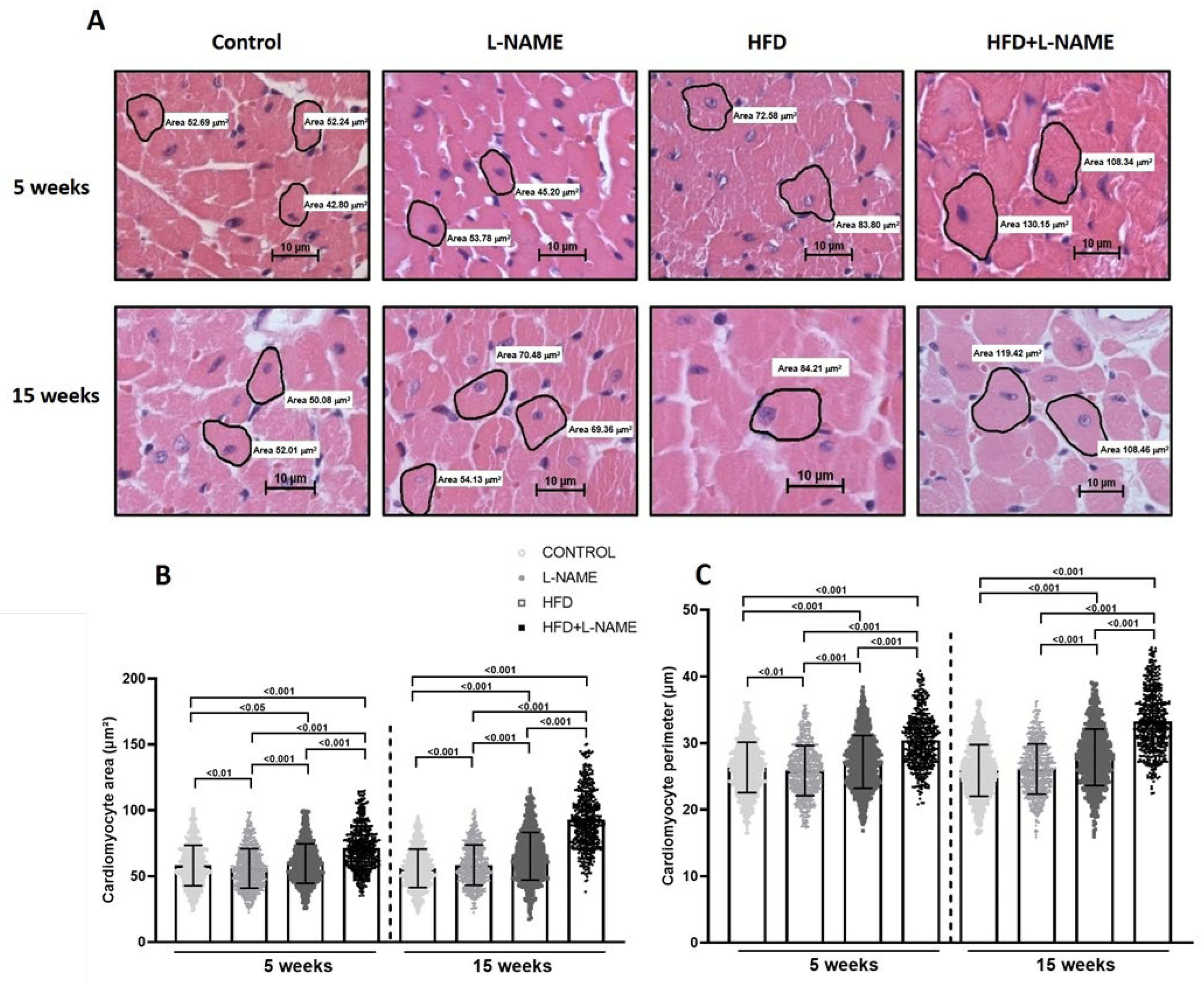
2.1. HFD+L-NAME Mouse Model Reproduces Major Clinical Features of HFpEF
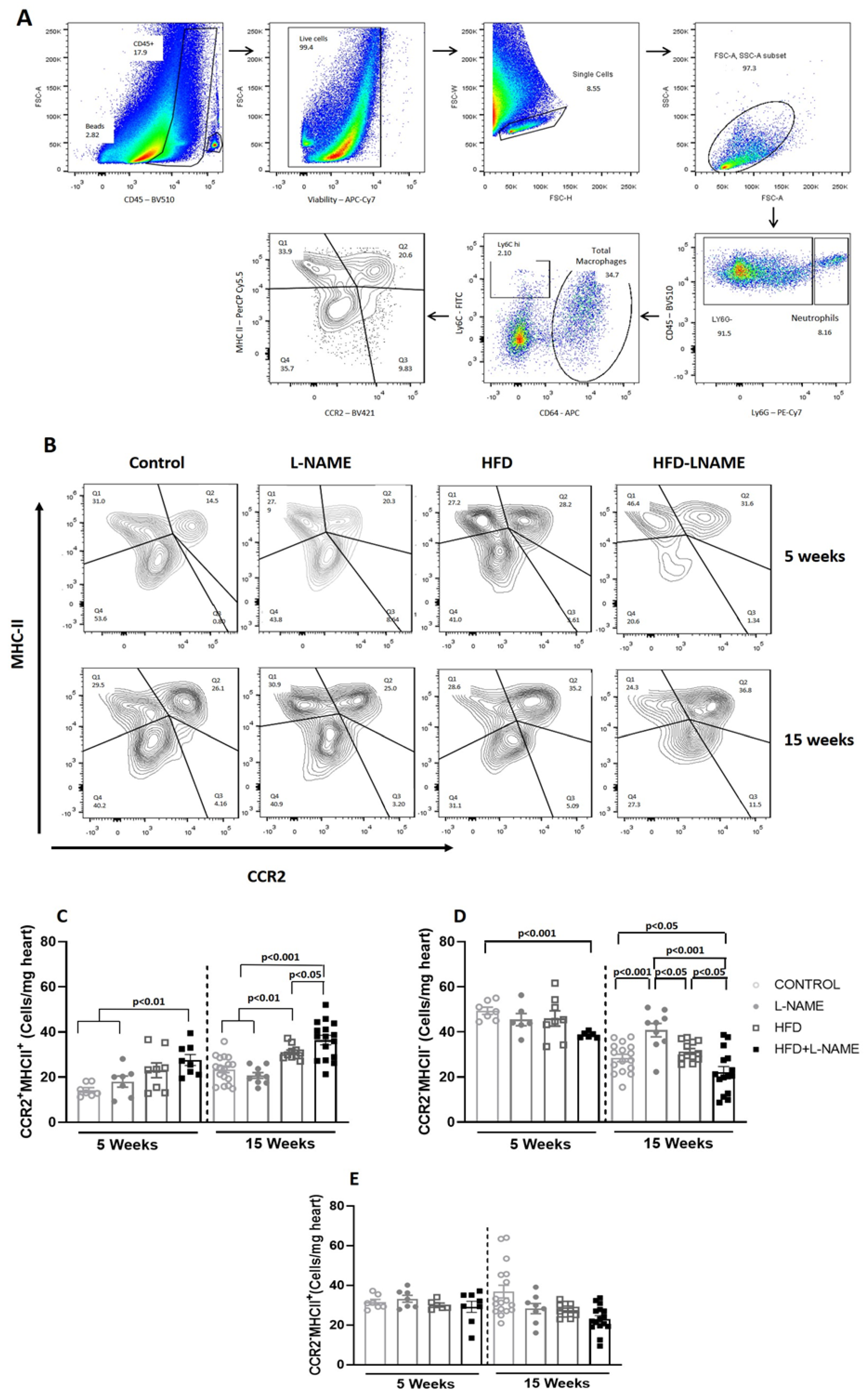
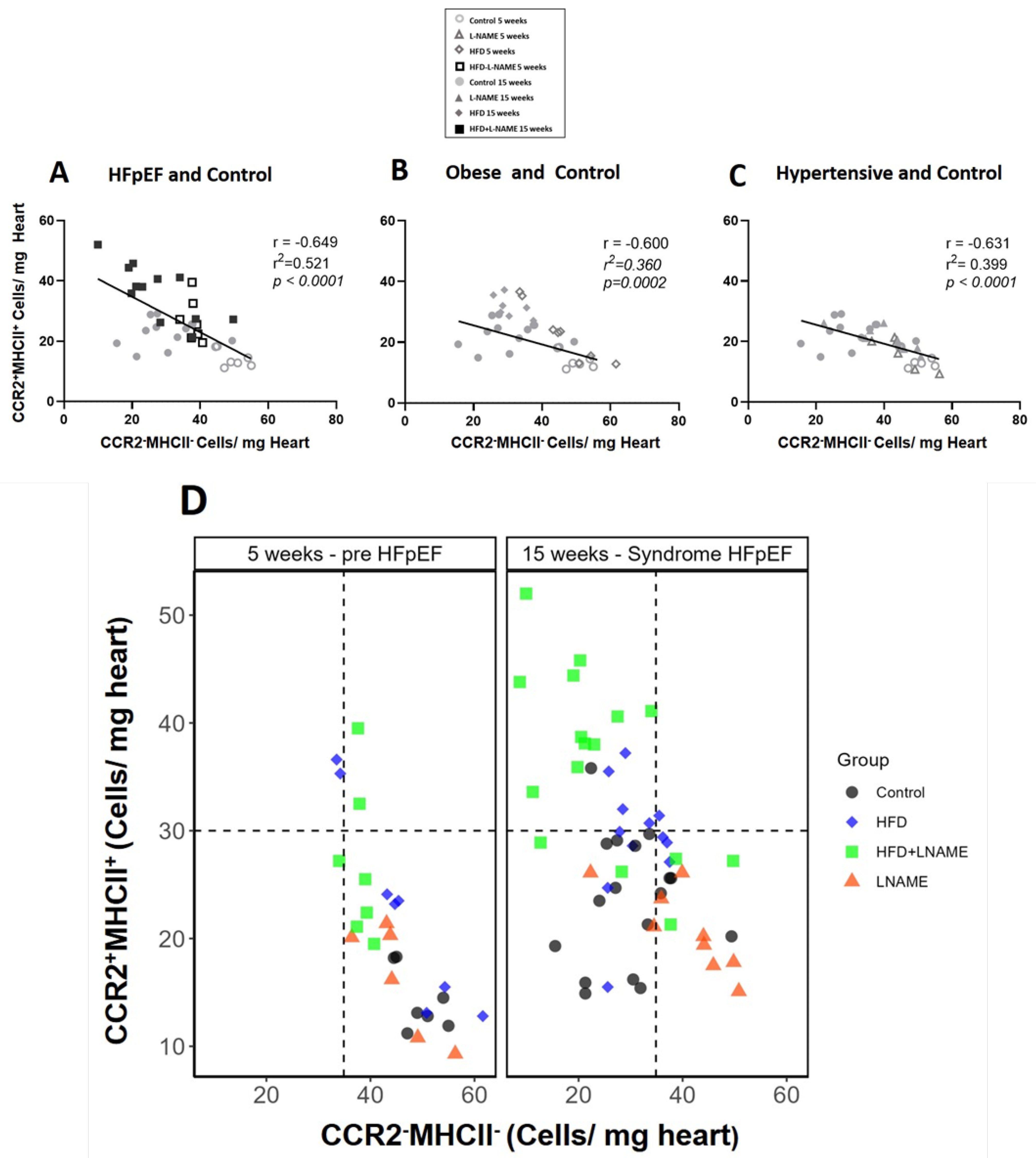
2.2. Progression to Heart Failure with Preserved Ejection Fraction (HFpEF) Is Marked by Early Decrease in Resident Protective CCR2−MHCII− Macrophages and Increase in Proinflammatory CCR2+MHCII+ Macrophages
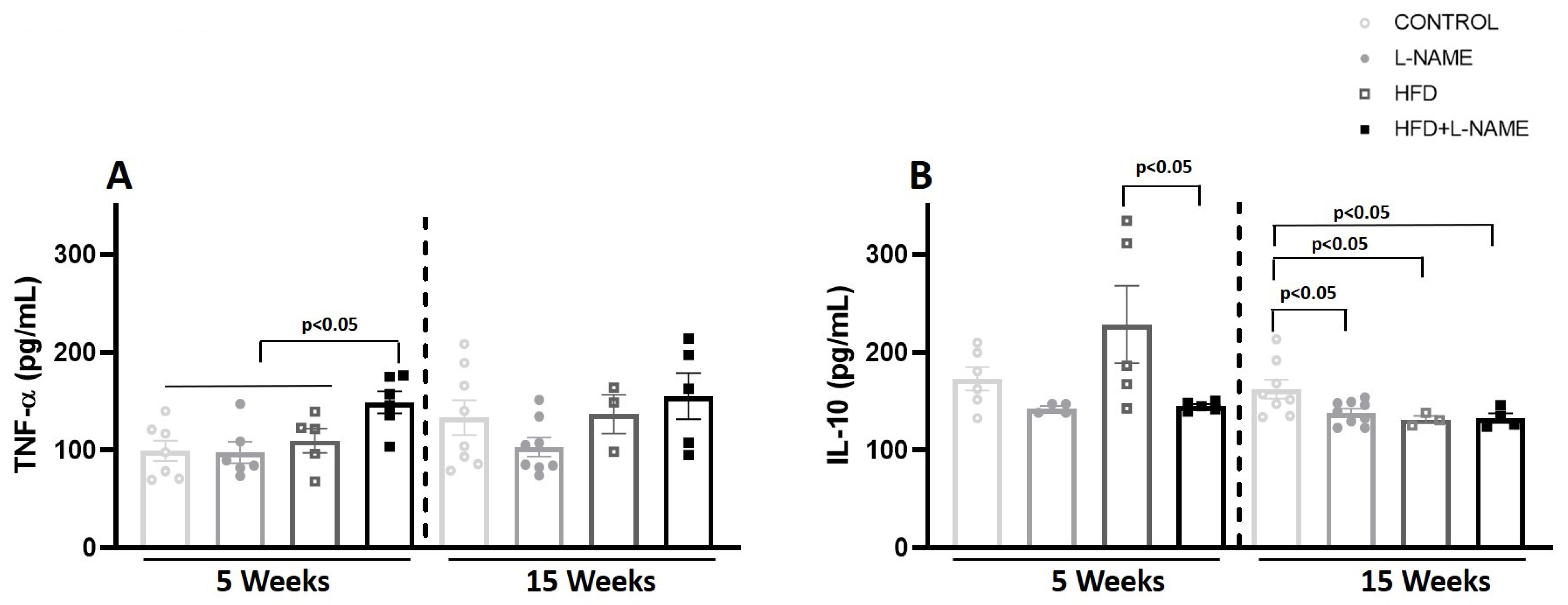
2.3. Circulating TNF-α and IL-10 During HFpEF Progression
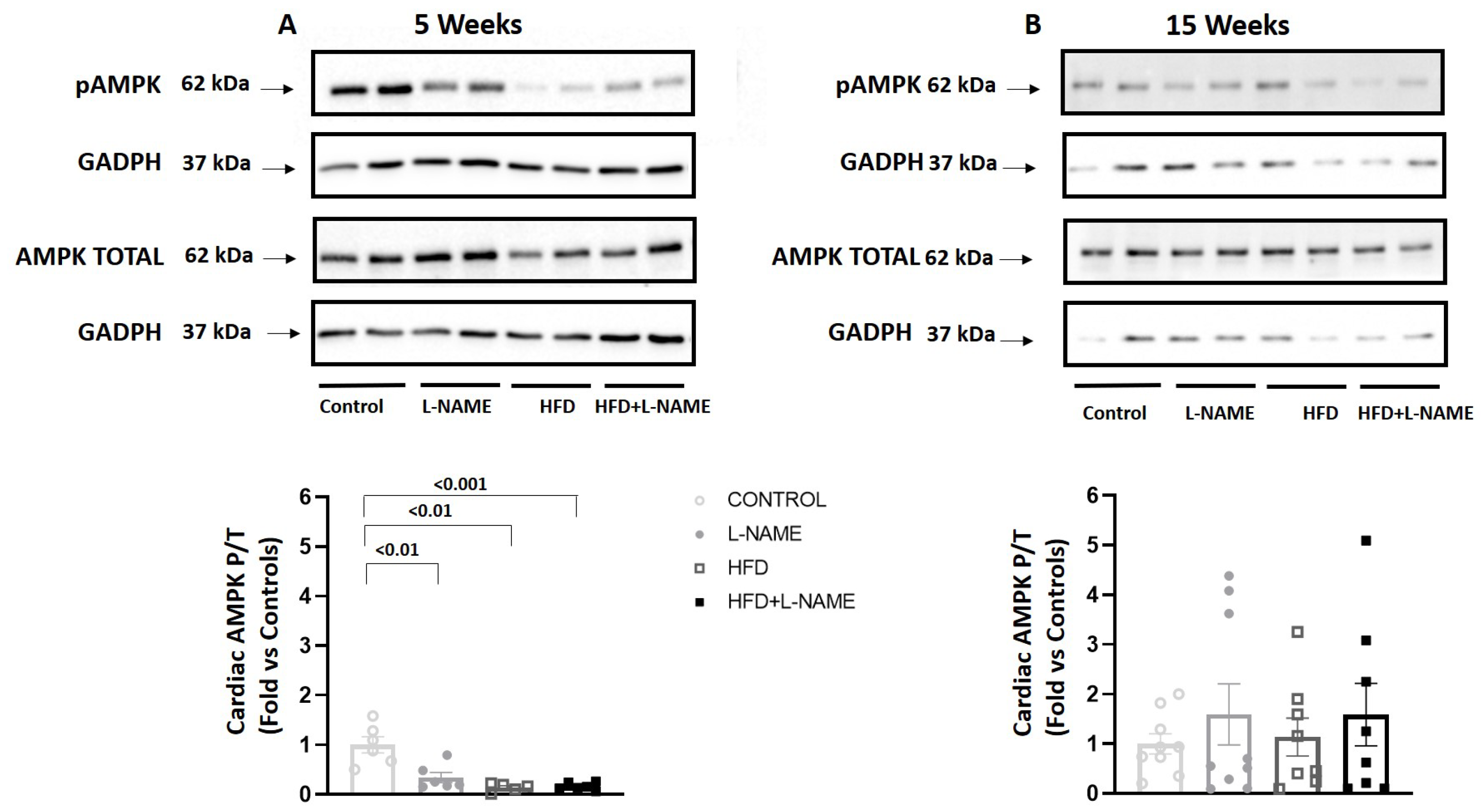
2.4. Cardiac AMP-Activated Protein Kinase (AMPK) Activation During HFpEF Progression
3. Discussion
4. Materials and Methods
4.1. Experimental Model
4.2. Tail-Cuff Blood Pressure
4.3. Exercise Exhaustion Test
4.4. Intraperitoneal Glucose Tolerance Test
4.5. Blood Lipid
4.6. Conventional Echocardiography and Doppler Imaging
4.7. Left Ventricular Hypertrophy Measurement
4.8. Cardiac Fibrosis
4.9. Flow Cytometry
4.10. Plasma Insulin, IL-10 and TNF-α
4.11. Western Blot Analysis to Determine Cardiac Adenosine Monophosphate (AMP)–Activated Protein Kinase (AMPK) Activity
4.12. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lam, C.S.P.; Voors, A.A.; de Boer, R.A.; Solomon, S.D.; van Veldhuisen, D.J. Heart Failure with Preserved Ejection Fraction: From Mechanisms to Therapies. Eur. Heart J. 2018, 39, 2780–2792. [Google Scholar] [CrossRef]
- van der Meer, P.; Gaggin, H.K.; Dec, G.W. ACC/AHA Versus ESC Guidelines on Heart Failure: JACC Guideline Comparison. J. Am. Coll. Cardiol. 2019, 73, 2756–2768. [Google Scholar] [CrossRef]
- Frantz, S.; Falcao-Pires, I.; Balligand, J.-L.; Bauersachs, J.; Brutsaert, D.; Ciccarelli, M.; Dawson, D.; de Windt, L.J.; Giacca, M.; Hamdani, N.; et al. The innate immune system in chronic cardiomyopathy: A European Society of Cardiology (ESC) scientific statement from the Working Group on Myocardial Function of the ESC. Eur. J. Heart Fail. 2018, 20, 445–459. [Google Scholar] [CrossRef]
- Bayes-Genis, A.; Bisbal, F.; Núñez, J.; Santas, E.; Lupón, J.; Rossignol, P.; Paulus, W. Transitioning from Preclinical to Clinical Heart Failure with Preserved Ejection Fraction: A Mechanistic Approach. J. Clin. Med. 2020, 9, 1110. [Google Scholar] [CrossRef] [PubMed]
- Paulus, W.J.; Tschope, C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J. Am. Coll. Cardiol. 2013, 62, 263–271. [Google Scholar] [CrossRef]
- Zaman, R.; Hamidzada, H.; Epelman, S. Exploring cardiac macrophage heterogeneity in the healthy and diseased myocardium. Curr. Opin. Immunol. 2021, 68, 54–63. [Google Scholar] [CrossRef]
- Epelman, S.; Lavine, K.J.; Beaudin, A.E.; Sojka, D.K.; Carrero, J.A.; Calderon, B.; Brija, T.; Gautier, E.L.; Ivanov, S.; Satpathy, A.T.; et al. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity 2014, 40, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Lavine, K.J.; Epelman, S.; Uchida, K.; Weber, K.J.; Nichols, C.G.; Schilling, J.D.; Ornitz, D.M.; Randolph, G.J.; Mann, D.L. Distinct macrophage lineages contribute to disparate patterns of cardiac recovery and remodeling in the neonatal and adult heart. Proc. Natl. Acad. Sci. USA 2014, 111, 16029–16034, Erratum in Proc. Natl. Acad. Sci. USA 2016, 113, E1414. [Google Scholar] [CrossRef]
- Molawi, K.; Wolf, Y.; Kandalla, P.K.; Favret, J.; Hagemeyer, N.; Frenzel, K.; Pinto, A.R.; Klapproth, K.; Henri, S.; Malissen, B.; et al. Progressive replacement of embryo-derived cardiac macrophages with age. J. Exp. Med. 2014, 211, 2151–2158. [Google Scholar] [CrossRef]
- Schiattarella, G.G.; Altamirano, F.; Tong, D.; French, K.M.; Villalobos, E.; Kim, S.Y.; Luo, X.; Jiang, N.; May, H.I.; Wang, Z.V.; et al. Nitrosative stress drives heart failure with preserved ejection fraction. Nature 2019, 568, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Smart, C.D.; Madhur, M.S. The immunology of heart failure with preserved ejection fraction. Clin. Sci. 2023, 137, 1225–1247. [Google Scholar] [CrossRef]
- Berger, J.H.; Shi, Y.; Matsuura, T.R.; Batmanov, K.; Chen, X.; Tam, K.; Marshall, M.; Kue, R.; Patel, J.; Taing, R.; et al. Two-hit mouse model of heart failure with preserved ejection fraction combining diet-induced obesity and renin-mediated hypertension. Sci. Rep. 2025, 15, 422. [Google Scholar] [CrossRef]
- Sanhueza-Olivares, F.; Valenzuela-Arce, F.; Calle-Chalco, X.; Silva, D.; Muñoz-Córdova, F.; Mella-Torres, A.; Ortega-Muñoz, A.; Troncoso, M.F.; Muñoz-Rodriguez, C.; Pino de la Fuente, F.; et al. Vascular remodelling in a mouse model of heart failure with preserved ejection fraction. J. Physiol. 2025, early view. [Google Scholar] [CrossRef]
- Gao, S.; Liu, X.P.; Li, T.T.; Chen, L.; Feng, Y.P.; Wang, Y.K.; Yin, Y.J.; Little, P.J.; Wu, X.Q.; Xu, S.W.; et al. Animal models of heart failure with preserved ejection fraction (HFpEF): From metabolic pathobiology to drug discovery. Acta Pharmacol. Sin. 2024, 45, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Wang, Z.; Liu, X.; He, Y. Confronting the global obesity epidemic: Investigating the role and underlying mechanisms of vitamin D in metabolic syndrome management. Front. Nutr. 2024, 11, 1416344. [Google Scholar] [CrossRef] [PubMed]
- Noubiap, J.J.; Nansseu, J.R.; Lontchi-Yimagou, E.; Nkeck, J.R.; Nyaga, U.F.; Ngouo, A.T.; Tounouga, D.N.; Tianyi, F.L.; Foka, A.J.; Ndoadoumgue, A.L.; et al. Global, regional, and country estimates of metabolic syndrome burden in children and adolescents in 2020: A systematic review and modelling analysis. Lancet Child Adolesc. Health 2022, 6, 158–170. [Google Scholar] [CrossRef] [PubMed]
- Rykova, E.Y.; Klimontov, V.V.; Shmakova, E.; Korbut, A.I.; Merkulova, T.I.; Kzhyshkowska, J. Anti-Inflammatory Effects of SGLT2 Inhibitors: Focus on Macrophages. Int. J. Mol. Sci. 2025, 26, 1670. [Google Scholar] [CrossRef]
- Cui, Y.; Chen, J.; Zhang, Z.; Shi, H.; Sun, W.; Yi, Q. The role of AMPK in macrophage metabolism, function and polarisation. J. Transl. Med. 2023, 21, 892. [Google Scholar] [CrossRef]
- Dzik, J. Metabolic evolutionary roots of the macrophage immune response in amoeba-bacteria interactions: The conserved role of hypoxia-induced factor and AMP kinase. Acta Biochim. Pol. 2021, 68, 457–476. [Google Scholar] [CrossRef]
- Peixoto, C.A.; Oliveira, W.H.; Araújo, S.M.D.R.; Nunes, A.K.S. AMPK activation: Role in the signaling pathways of neuroinflammation and neurodegeneration. Exp. Neurol. 2017, 298, 31–41. [Google Scholar] [CrossRef]
- Yang, Z.; Lin, S.; Feng, W.; Liu, Y.; Song, Z.; Pan, G.; Zhang, Y.; Dai, X.; Ding, X.; Chen, L.; et al. A potential therapeutic target in traditional Chinese medicine for ulcerative colitis: Macrophage polarization. Front. Pharmacol. 2022, 13, 999179. [Google Scholar] [CrossRef]
- Sag, D.; Carling, D.; Stout, R.D.; Suttles, J. Adenosine 5’-monophosphate-activated protein kinase promotes macrophage polarization to an anti-inflammatory functional phenotype. J. Immunol. 2008, 181, 8633–8641. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Huang, L.; Shi, X.; Yang, L.; Hua, F.; Ma, J.; Zhu, W.; Liu, X.; Xuan, R.; Shen, Y.; et al. Metformin protects against myocardial ischemia-reperfusion injury and cell pyroptosis via AMPK/NLRP3 inflammasome pathway. Aging 2020, 12, 24270–24287. [Google Scholar] [CrossRef] [PubMed]
- Gélinas, R.; Mailleux, F.; Dontaine, J.; Bultot, L.; Demeulder, B.; Ginion, A.; Daskalopoulos, E.P.; Esfahani, H.; Dubois-Deruy, E.; Lauzier, B.; et al. AMPK activation counteracts cardiac hypertrophy by reducing O-GlcNAcylation. Nat. Commun. 2018, 9, 374. [Google Scholar] [CrossRef] [PubMed]
- Mack, M.; Cihak, J.; Simonis, C.; Luckow, B.; Proudfoot, A.E.I.; Plachý, J.; Bruhl, H.; Frink, M.; Anders, H.J.; Vielhauer, V.; et al. Expression and characterization of the chemokine receptors CCR2 and CCR5 in mice. J. Immunol. 2001, 166, 4697–4704. [Google Scholar] [CrossRef]
- Hahn, V.S.; Yanek, L.R.; Vaishnav, J.; Ying, W.; Vaidya, D.; Lee, Y.Z.J.; Riley, S.J.; Subramanya, V.; Brown, E.E.; Hopkins, C.D.; et al. Endomyocardial Biopsy Characterization of Heart Failure with Preserved Ejection Fraction and Prevalence of Cardiac Amyloidosis. JACC Heart Fail. 2020, 8, 712–724. [Google Scholar] [CrossRef]
- Hulsmans, M.; Sager, H.B.; Roh, J.D.; Valero-Muñoz, M.; Houstis, N.E.; Iwamoto, Y.; Sun, Y.; Wilson, R.M.; Wojtkiewicz, G.; Tricot, B.; et al. Cardiac macrophages promote diastolic dysfunction. J. Exp. Med. 2018, 215, 423–440. [Google Scholar] [CrossRef]
- Glezeva, N.; Voon, V.; Watson, C.; Horgan, S.; McDonald, K.; Ledwidge, M.; Baugh, J. Exaggerated inflammation and monocytosis associate with diastolic dysfunction in heart failure with preserved ejection fraction: Evidence of M2 macrophage activation in disease pathogenesis. J. Card. Fail. 2015, 21, 167–177. [Google Scholar] [CrossRef]
- Hiraiwa, H.; Yura, Y.; Okumura, T.; Murohara, T. Interplay of the heart, spleen, and bone marrow in heart failure: The role of splenic extramedullary hematopoiesis. Heart Fail. Rev. 2024, 29, 1049–1063. [Google Scholar] [CrossRef]
- Gonzalez, L.; Novoa, U.; Moya, J.; Gabrielli, L.; Jalil, J.E.; García, L.; Chiong, M.; Lavandero, S.; Ocaranza, M.P. Angiotensin-(1-9) reduces cardiovascular and renal inflammation in experimental renin-independent hypertension. Biochem. Pharmacol. 2018, 156, 357–370. [Google Scholar] [CrossRef]
- Zhang, T.Y.; Zhao, B.J.; Wang, T.; Wang, J. Effect of aging and sex on cardiovascular structure and function in wildtype mice assessed with echocardiography. Sci. Rep. 2021, 11, 22800. [Google Scholar] [CrossRef] [PubMed]
- Flores, Y.; Zapata-Torres, G.; Nuñez, A.; Matthies, D.J.; Alemán, L.; Hernández-Fuentes, C.; Sánchez, G.; Araya, E.; Guzman, F.; Pedrozo, Z.; et al. Angiotensin-(1-9) Retro-Enantiomer Peptide with Cardioprotective Activity. Circulation 2024, 150, 816–820. [Google Scholar] [CrossRef] [PubMed]
- Ocaranza, M.P.; Moya, J.; Barrientos, V.; Alzamora, R.; Hevia, D.; Morales, C.; Pinto, M.; Escudero, N.; García, L.; Novoa, U.; et al. Angiotensin-(1-9) reverses experimental hypertension and cardiovascular damage by inhibition of the angiotensin converting enzyme/Ang II axis. J. Hypertens. 2014, 32, 771–783, Erratum in J. Hypertens. 2014, 32, 1354. [Google Scholar] [CrossRef]
- Teteris, S.A.; Hochheiser, K.; Kurts, C. Isolation of functional dendritic cells from murine kidneys for immunological characterization. Nephrology 2012, 17, 364–371. [Google Scholar] [CrossRef]
- Bajpai, G.; Lavine, K.J. Isolation of Macrophage Subsets and Stromal Cells from Human and Mouse Myocardial Specimens. J. Vis. Exp. 2019, 154, e60015. [Google Scholar] [CrossRef]
- Sander, H.; Wallace, S.; Plouse, R.; Tiwari, S.; Gomes, A.V. Ponceau S waste: Ponceau S staining for total protein normalization. Anal. Biochem. 2019, 575, 44–53. [Google Scholar] [CrossRef] [PubMed]


| 5 Weeks | 15 Weeks | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | CONTROL | L-NAME | HFD | HFD+ L-NAME | F Value | p-Value | CONTROL | L-NAME | HFD | HFD+ L-NAME | F Value | p-Value |
| n | 8 | 8 | 12 | 12 | 8 | 8 | 12 | 12 | ||||
| TG, mmol/L | 2.81 ± 0.31 | 2.52 ± 0.27 | 3.54 ± 0.18 *** | 3.50 ± 0.21 *** | 10.9 | 0.0001 | 2.92 ± 0.29 | 2.44 ± 0.17 | 4.02 ± 0.23 *** | 3.87 ± 0.20 *** | 10.13 | 0.0001 |
| LDL, mmol/L | 1.42 ± 0.14 | 1.52 ± 0.19 | 3.39 ± 0.23 *** | 3.19 ± 0.18 *** | 12.93 | 0.0001 | 1.52 ± 0.18 | 1.23 ± 0.15 | 3.87 ± 0.11 *** | 3.69 ± 0.20 *** | 13.67 | 0.0001 |
| HDL, mmol/L | 2.57 ± 0.23 | 2.82 ± 0.11 | 2.29 ± 0.18 * | 2.20 ± 0.21 * | 4.12 | 0.03 | 2.71 ± 0.22 | 2.78 ± 0.15 | 2.12 ± 0.13 ** | 2.10 ± 0.22 ** | 2.73 | 0.001 |
| CHO, mmol/L | 2.14 ± 0.15 | 2.21 ± 0.22 | 3.85 ± 0.21 *** | 3.65 ± 0.28 *** | 8.92 | 0.0001 | 2.23 ± 0.18 | 2.34 ± 0.25 | 4.31 ± 0.19 *** | 3.78 ± 0.27 *** | 6.63 | 0.0001 |
| 5 Weeks | 15 Weeks | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | CONTROL | L-NAME | HFD | HFD+ L-NAME | F Value | p-Value | CONTROL | L-NAME | HFD | HFD+ L-NAME | F Value | p-Value |
| n | 8 | 8 | 12 | 12 | 8 | 8 | 12 | 12 | ||||
| LVSWT, mm | 0.69 ± 0.02 | 0.66 ± 0.00 | 0.69 ± 0.02 | 0.68 ± 0.01 | 0.90 | NS | 0.68 ± 0.01 | 0.68 ± 0.01 | 0.76 ± 0.01 *** | 0.74 ± 0.01 *** | 12.12 | 0.000 |
| LVPWT, mm | 0.68 ± 0.01 | 0.67 ± 0.02 | 0.66 ± 0.00 | 0.69 ± 0.01 | 0.79 | NS | 0.68 ± 0.01 | 0.67 ± 0.01 | 0.74 ± 0.01 *** | 0.74 ± 0.01 *** | 13.67 | 0.000 |
| HR, bpm | 232 ± 4 | 225 ± 7 | 226 ± 4 | 226 ± 5 | 0.31 | NS | 233 ± 2 | 224 ± 1 | 226 ± 34 | 233 ± 1 | 2.73 | NS |
| LVEDD, mm | 2.86 ± 0.05 | 2.87 ± 0.03 | 2.92 ± 0.03 | 2.86 ± 0.04 | 0.64 | NS | 2.94 ± 0.01 | 2.93 ± 0.02 | 3.06 ± 0.03 *** | 2.97 ± 0.01 | 6.63 | 0.001 |
| LVESD, mm | 1.85 ± 0.06 | 1.83 ± 0.05 | 1.95 ± 0.02 | 1.90 ± 0.03 | 2.01 | NS | 1.95 ± 0.02 | 1.98 ± 0.02 | 2.04 ± 0.04 †† | 1.98 ± 0.06 | 4.41 | 0.04 |
| LVEF, % | 72.75 ± 2.03 | 72.00 ± 1.43 | 69.58 ± 1.14 | 70.00 ± 1.06 | 1.10 | NS | 69.88 ± 0.83 | 68.22 ± 0.1 | 70.47 ± 1.07 | 70.70 ± 1.08 | 1.30 | NS |
| LVFS, % | 35.37 ± 1.75 | 35.50 ± 1.13 | 33.66 ± 0.93 | 34.28 ± 0.72 | 0.64 | NS | 34.61 ± 1.01 | 32.72 ± 0.69 | 34.11 ± 0.76 | 33.35 ± 1.11 | 0.80 | NS |
| LV mass, g | 58.94 ± 1.14 | 53.39 ± 1.33 | 54.74 ± 1.86 | 54.53 ± 1.69 | 0.61 | NS | 57.52 ± 1.23 | 55.55 ± 1.13 | 68.43 ± 1.70 *** | 64.66 ± 1.07 *** | 9.24 | 0.000 |
| LV mass/TL, g/cm | 34.11 ± 1.12 | 31.50 ± 0.86 | 30.47 ± 0.73 | 31.80 ± 1.10 | 1.26 | NS | 32.96 ± 0.82 | 31.95± 0.79 | 38.15 ± 0.78 *** | 37.24 ± 0.66 *** | 10.15 | 0.000 |
| LA, mm | 1.57 ± 0.03 | 1.57 ± 0.03 | 1.53 ± 0.03 | 1.61 ± 0.04 | 1.15 | NS | 1.56 ± 0.03 | 1.62 ± 0.02 | 1.81 ± 0.02 *** | 1.84 ± 0.02 *** | 31.53 | 0.000 |
| E/e’ | 20.37 ± 0.75 | 32.68 ± 1.92 ♢♢♢ | 21.50 ± 0.97 | 39.45 ± 1.72 ‡‡‡ | 19.25 | 0.000 | 24.12 ± 1.91 | 38.56 ± 2.76 ♢♢♢ | 24.89 ± 2.69 | 43.53± 1.97 *** | 30.34 | 0.000 |
| E/A | 1.31 ± 0.54 | 1.82± 0.53 ♢♢♢ | 1.26 ± 0.09 | 1.96 ± 0.32 ‡‡‡ | 15.89 | 0.000 | 1.22 ± 0.65 | 1.94 ± 0.83 ♢♢♢ | 1.37 ± 0.69 | 2.35 ± 1.18 *** | 14.87 | 0.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez, D.; Cordero, K.; Sepúlveda, R.; Venegas, C.; Altamirano, D.; Candia, C.; Ramírez, G.; Araos, P.; Amador, C.A.; Hermoso, M.A.; et al. Early Changes in Cardiac Macrophage Subsets in Heart Failure with Preserved Ejection Fraction. Int. J. Mol. Sci. 2025, 26, 10196. https://doi.org/10.3390/ijms262010196
Gutiérrez D, Cordero K, Sepúlveda R, Venegas C, Altamirano D, Candia C, Ramírez G, Araos P, Amador CA, Hermoso MA, et al. Early Changes in Cardiac Macrophage Subsets in Heart Failure with Preserved Ejection Fraction. International Journal of Molecular Sciences. 2025; 26(20):10196. https://doi.org/10.3390/ijms262010196
Chicago/Turabian StyleGutiérrez, Danae, Karina Cordero, Ruth Sepúlveda, Camilo Venegas, Diego Altamirano, Camila Candia, Gigliola Ramírez, Patricio Araos, Cristian A. Amador, Marcela A. Hermoso, and et al. 2025. "Early Changes in Cardiac Macrophage Subsets in Heart Failure with Preserved Ejection Fraction" International Journal of Molecular Sciences 26, no. 20: 10196. https://doi.org/10.3390/ijms262010196
APA StyleGutiérrez, D., Cordero, K., Sepúlveda, R., Venegas, C., Altamirano, D., Candia, C., Ramírez, G., Araos, P., Amador, C. A., Hermoso, M. A., Gabrielli, L., Jalil, J. E., & Ocaranza, M. P. (2025). Early Changes in Cardiac Macrophage Subsets in Heart Failure with Preserved Ejection Fraction. International Journal of Molecular Sciences, 26(20), 10196. https://doi.org/10.3390/ijms262010196








