Atractylodes macrocephala Koidz. Polysaccharide Alleviates Chemotherapy-Induced Depression-Like Behaviors Through the Gut–Brain Axis
Abstract
1. Introduction
2. Results
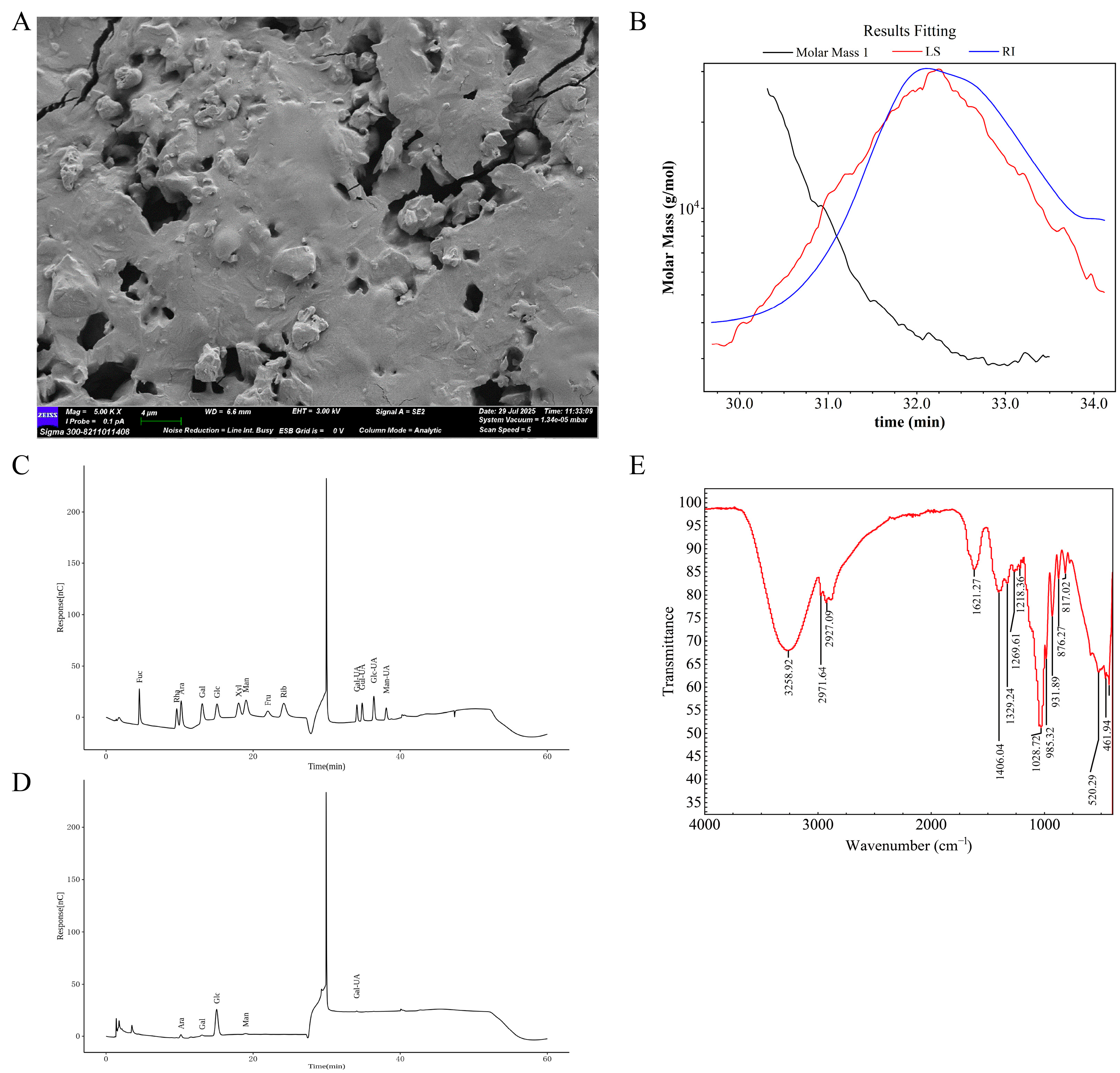
2.1. Structural Analysis of AP
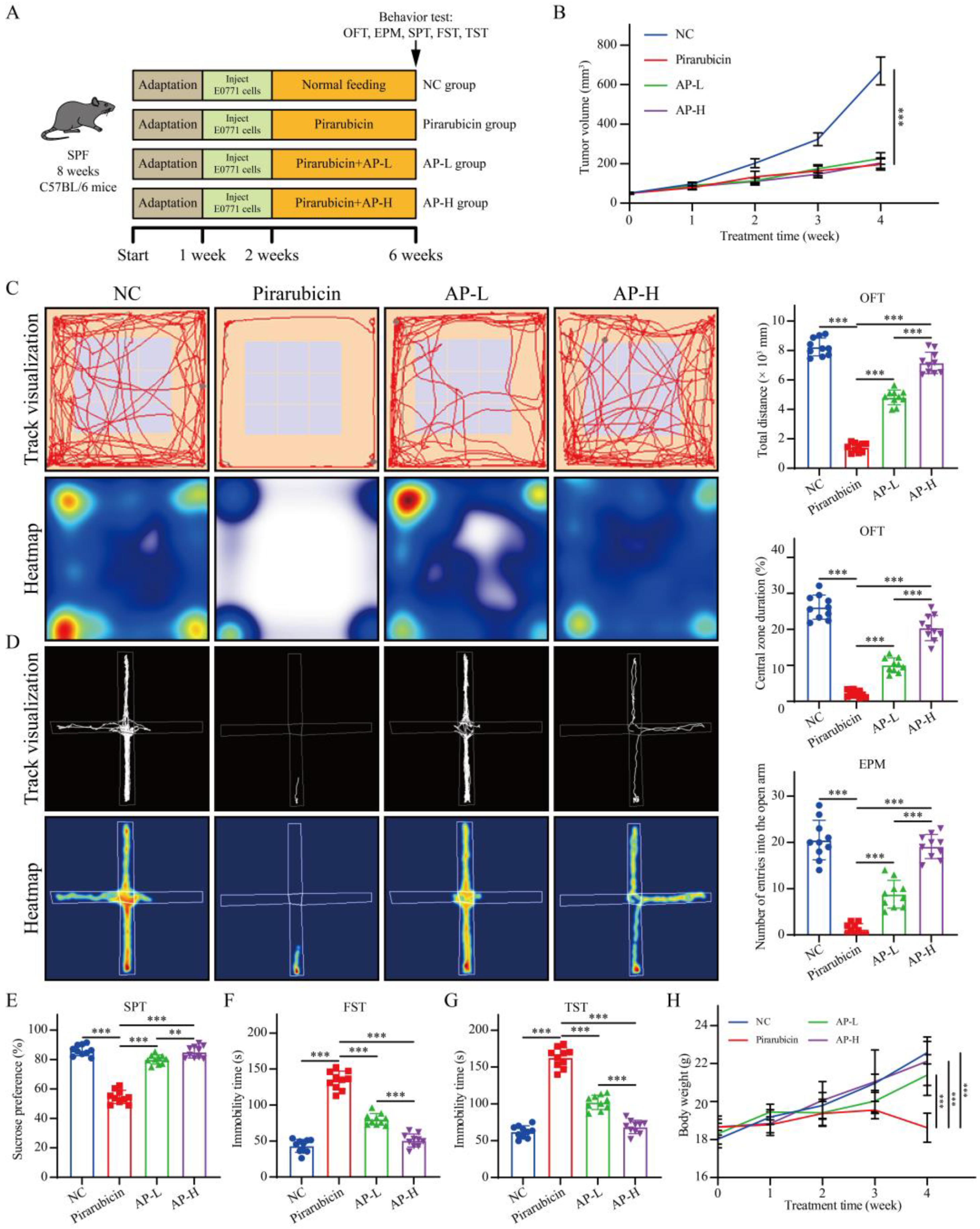
2.2. AP Alleviates Depression-like Behaviors Caused by Chemotherapy
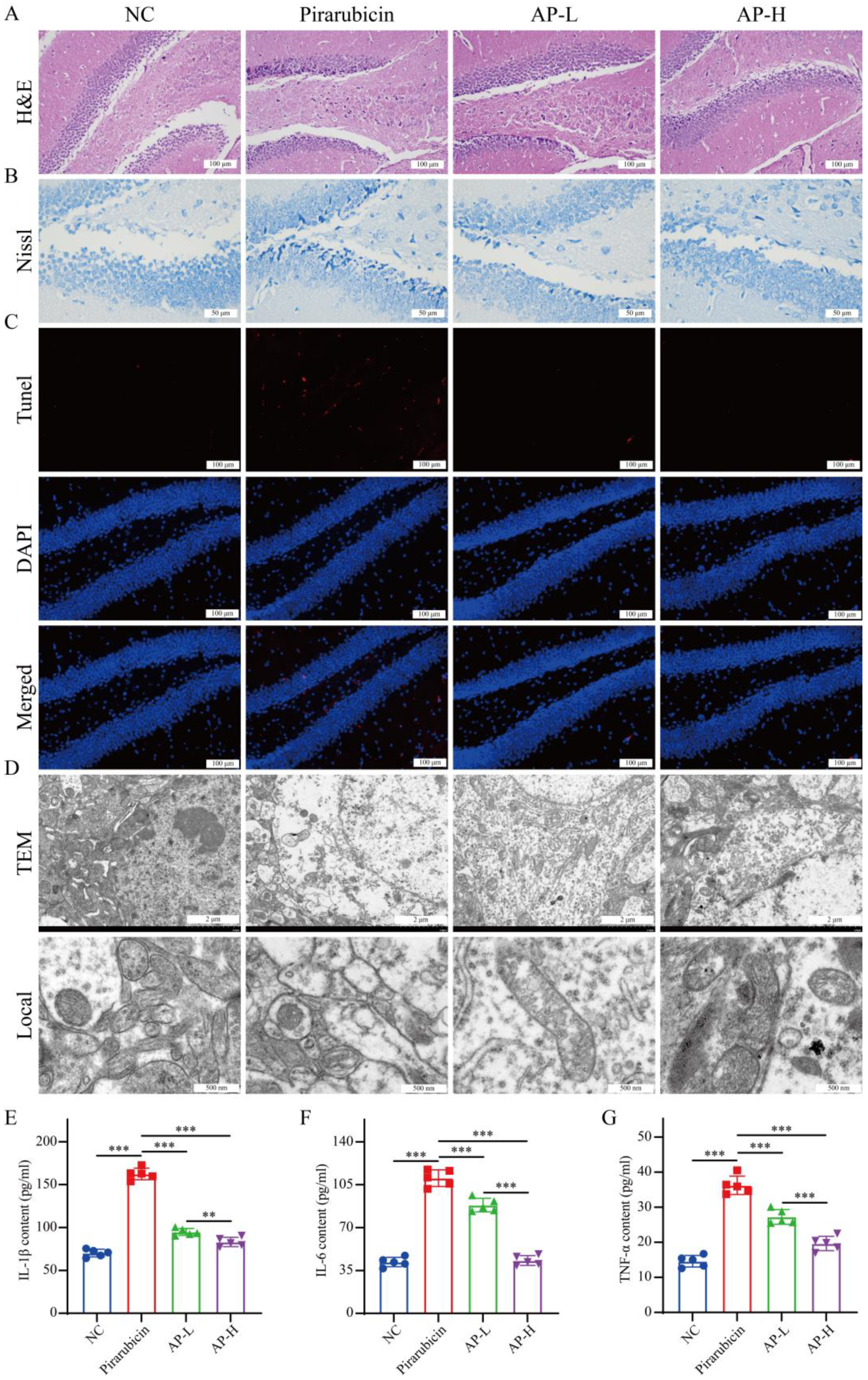
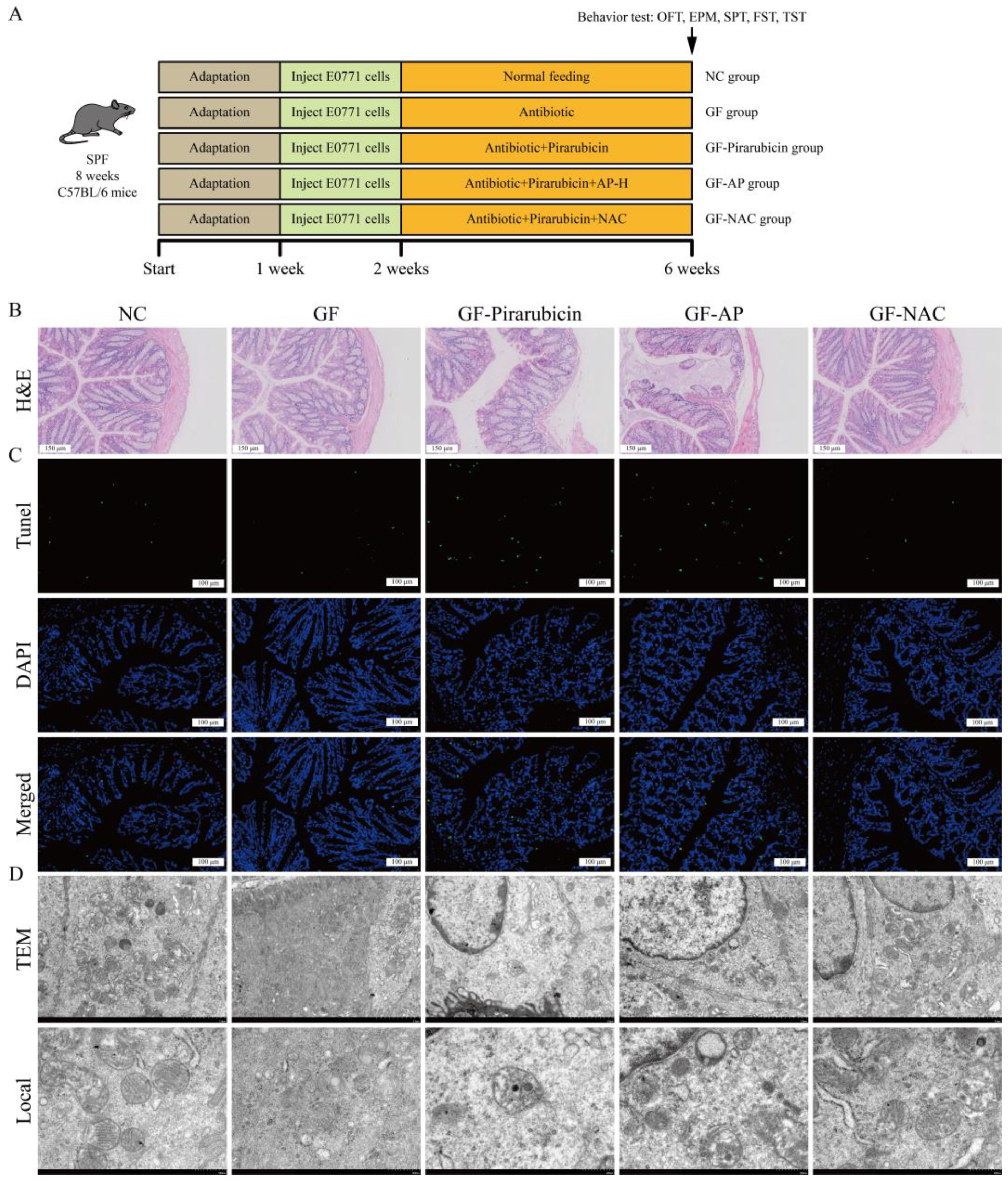
2.3. AP Prevents Hippocampal Damage Caused by Chemotherapy
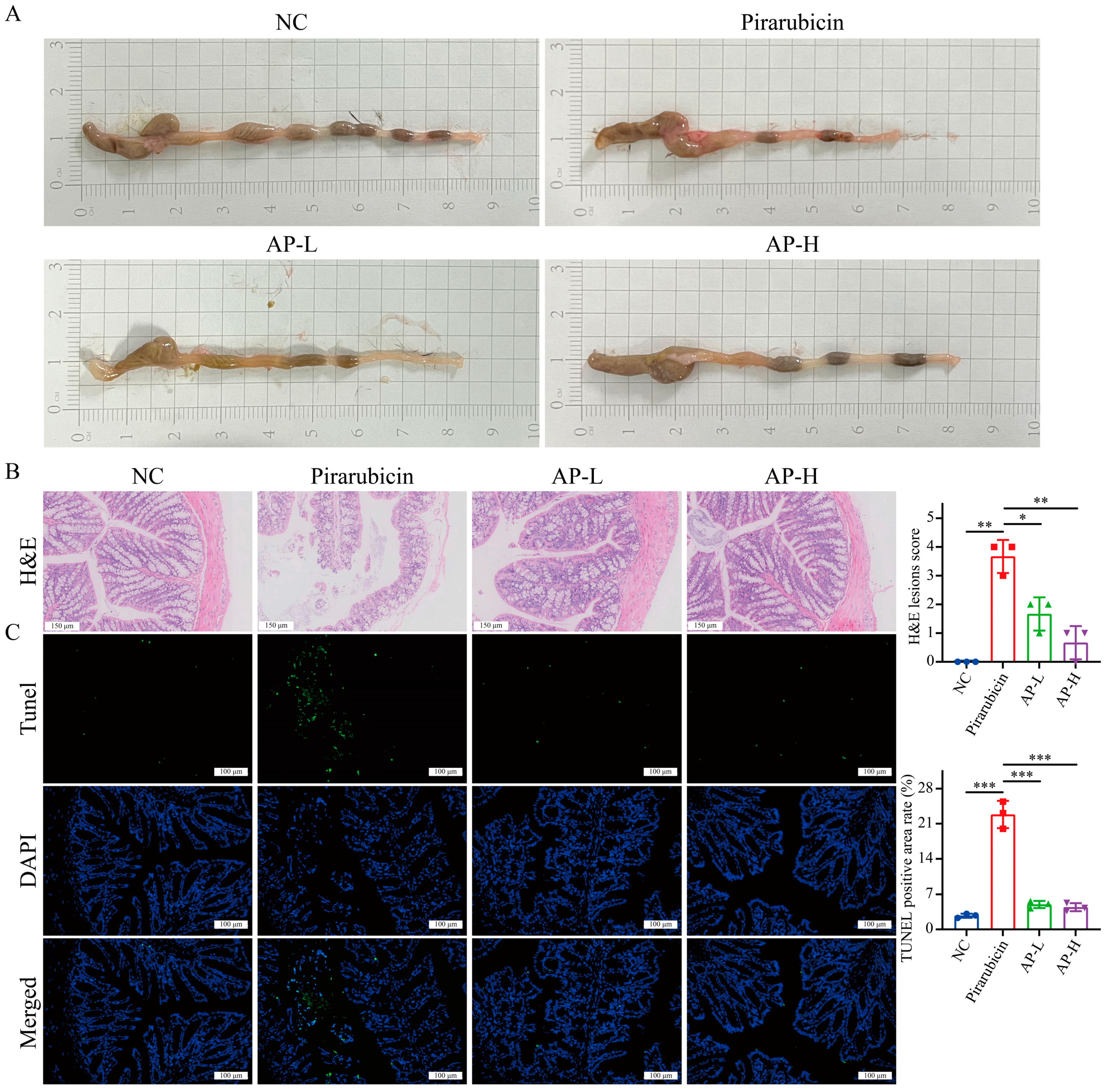
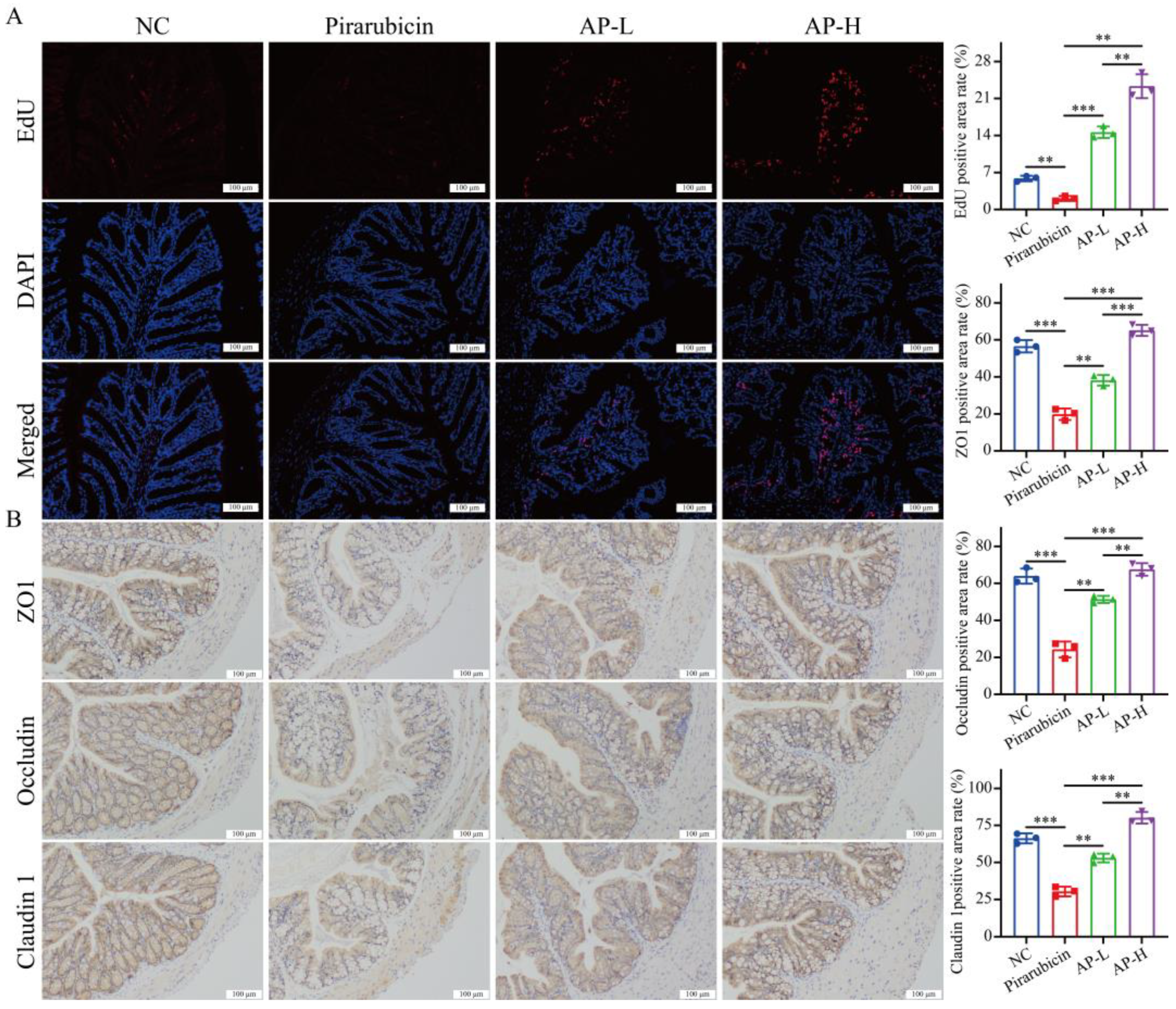
2.4. AP Strengthens Intestinal Barrier
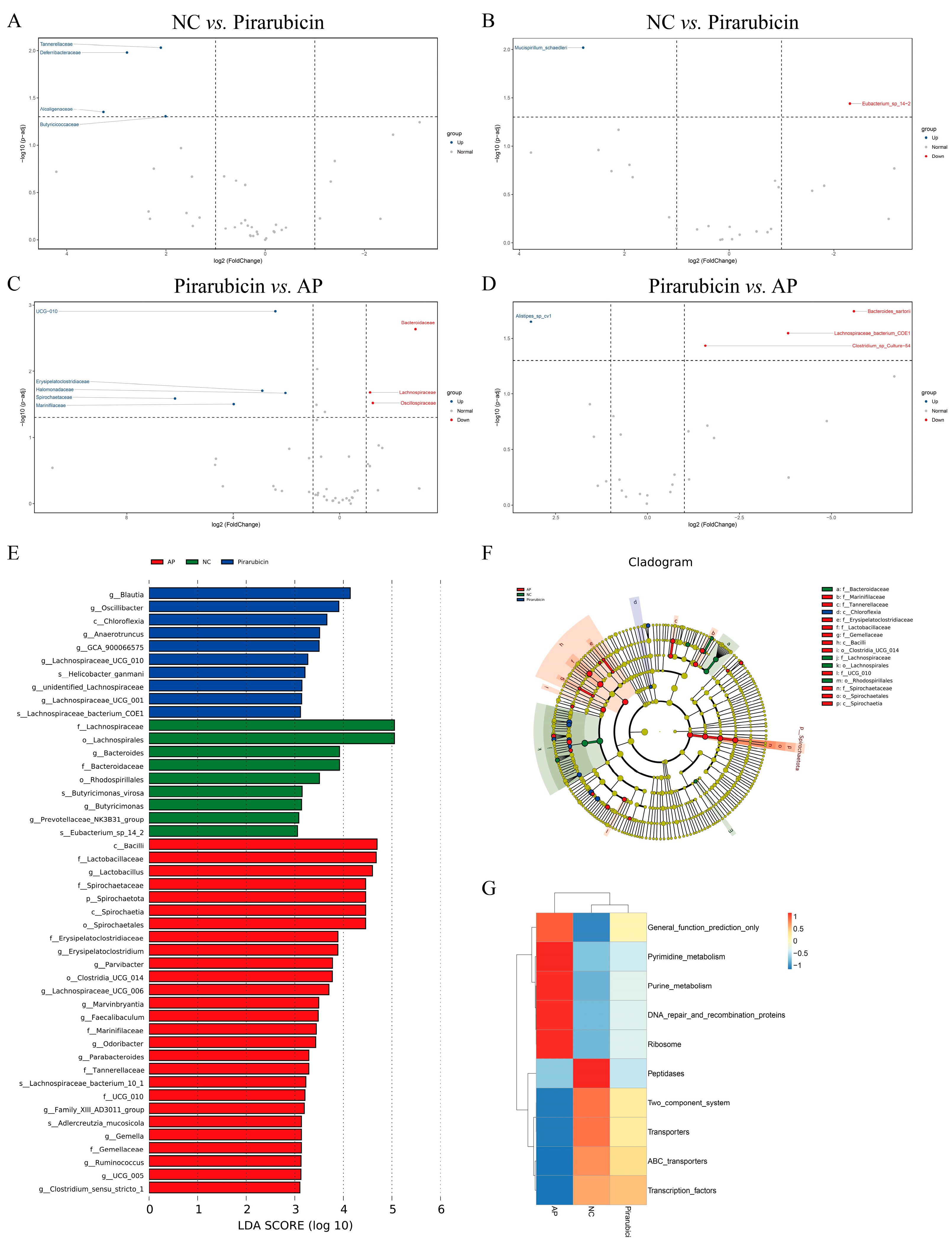
2.5. AP Restores Community Structure of Intestinal Flora
2.5.1. Diversity Analysis
2.5.2. Species Analysis, LefSe Analysis, and Functional Predictive Analysis
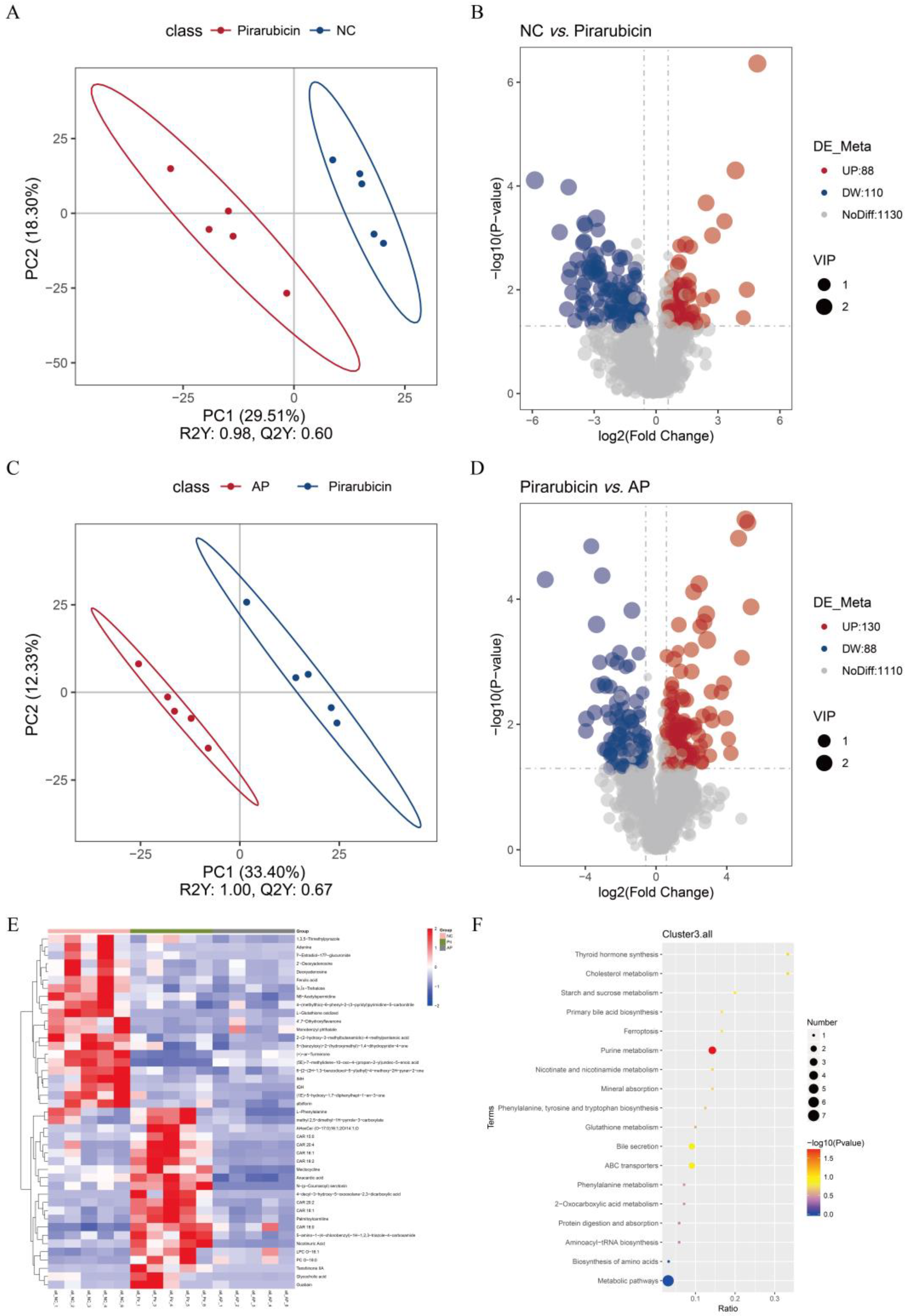
2.6. AP Improves Microbial Metabolites
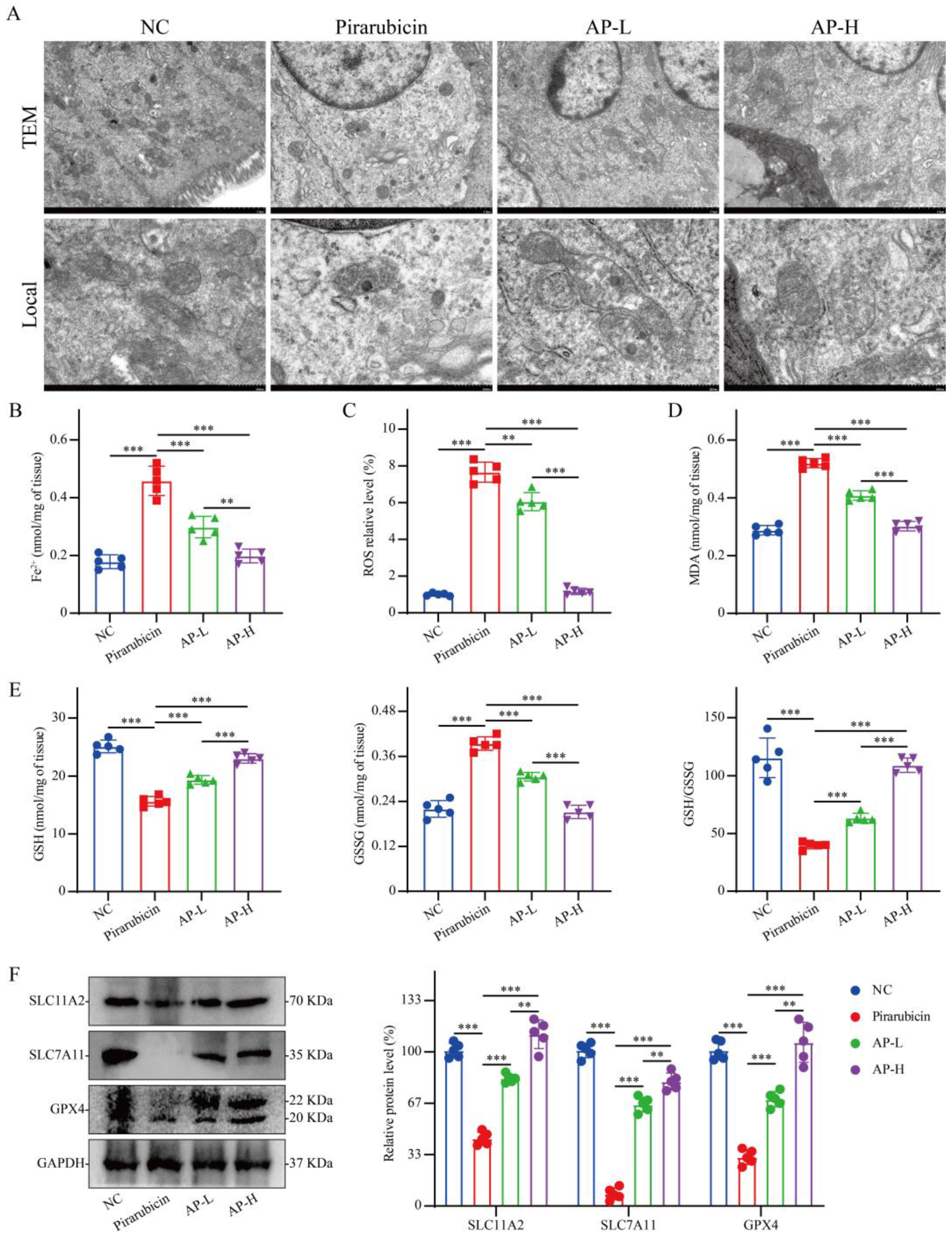
2.7. AP Reduces Ferroptosis in Intestinal Tissue
2.8. Intestinal Flora Mediates the Anti-Depressant Effect of AP
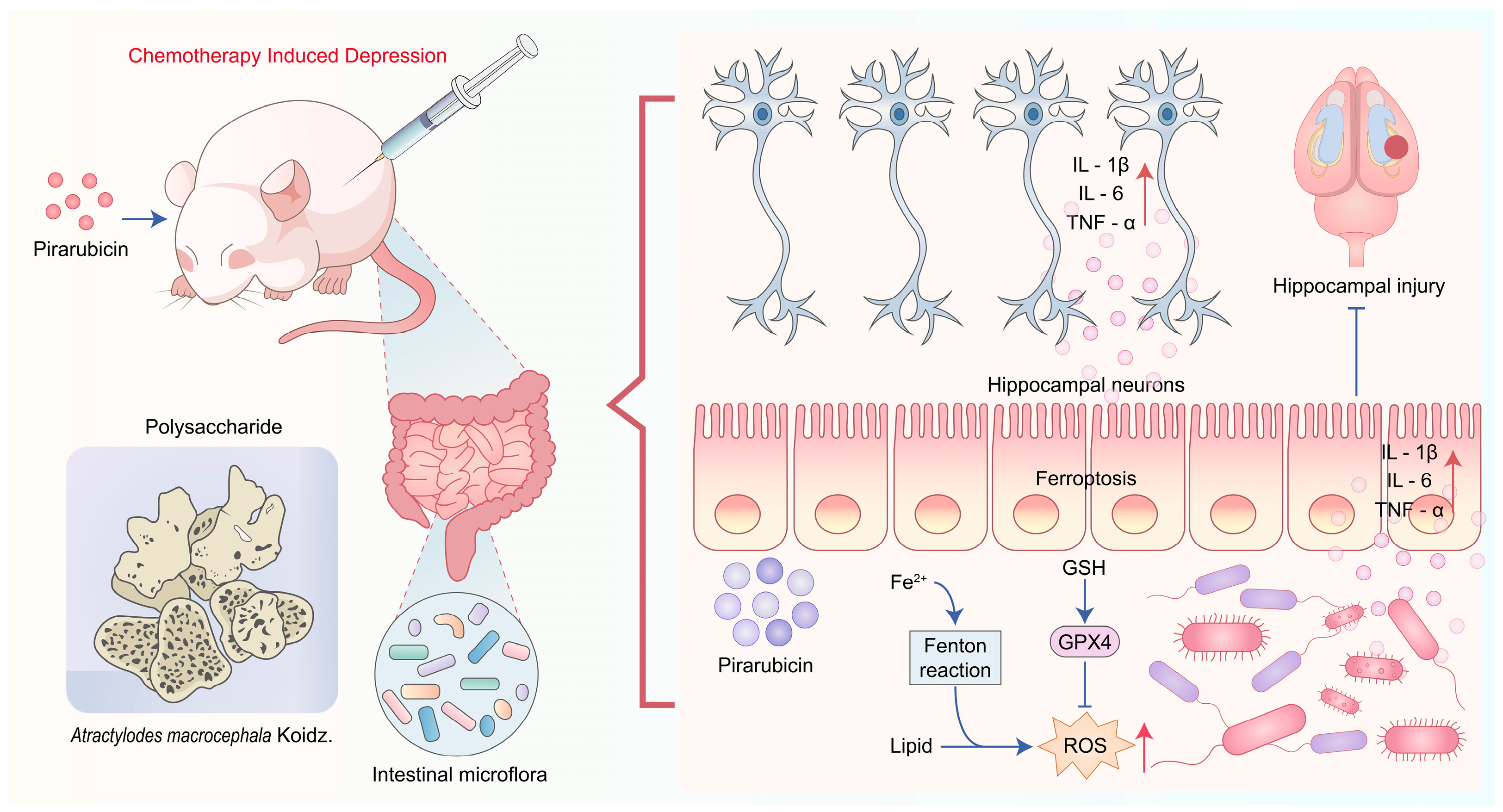
3. Discussion
4. Materials and Methods
4.1. AP Preparation
4.2. Other Chemicals and Reagents
4.3. Cell Culture
4.4. Establishment, Grouping, and Administration of Animal Models
4.5. Behavioral Analysis
4.6. Euthanasia and Sample Collection
4.7. Pathological Analysis
4.8. Enzyme-Linked Immunosorbent Assay (ELISA)
4.9. 16S rDNA Sequencing
4.10. Metabolomics Analysis
4.11. Ferroptosis-Related Index Detection
4.12. Western Blot (WB)
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AP | Atractylodes macrocephala Koidz. polysaccharide |
| CID | Chemotherapy-induced depression |
| BBB | blood–brain barrier |
| FT-IR | Fourier-transform infrared spectroscopy |
| SEM | scanning electron microscopy |
| DMEM | Dulbecco’s modified Eagle’s medium |
| FBS | fetal bovine serum |
| PBS | phosphate-buffered saline |
| TUNEL | Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling |
| IL-1β | Interleukin-1β |
| ELISA | Enzyme-linked immunosorbent assay |
| IL-6 | Interleukin-6 |
| TNF-α | Tumor necrosis factor-alpha |
| MDA | Malondialdehyde |
| GSH | Glutathione |
| GSSG | Glutathione disulfide |
| SPF | specific pathogen-free |
| OFT | open field test |
| EPM | elevated plus maze |
| SPT | sucrose preference test |
| H&E | hematoxylin and eosin |
| IHC | immunohistochemistry |
| TEM | transmission electron microscope |
| ANOVA | one-way analysis of variance |
| GPC-RI-MALS | Gel permeation chromatography–refractive index–multi-angle light scattering |
| HPLC | High-performance liquid chromatography |
| PCA | Principal component analysis |
| PCoA | Principal coordinate analysis |
| NMDS | Non-metric multidimensional scaling |
| LDA | Linear discriminant analysis |
| LEfSe | LDA effect size |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| ROS | Reactive oxygen species |
| NAC | N-acetylcysteine |
| FMT | Fecal microbiota transplantation |
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Gradishar, W.J.; Moran, M.S.; Abraham, J.; Abramson, V.; Aft, R.; Agnese, D.; Allison, K.H.; Anderson, B.; Bailey, J.; Burstein, H.J.; et al. Breast Cancer, Version 3.2024, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2024, 22, 331–357. [Google Scholar] [CrossRef]
- Hendy, A.; Ibrahim, R.K.; Darwish, A.; Al, S.S.; Shalby, A.; Khubrani, R.; Alawad, A.E.; Abdelaliem, S.; Wahba, N. Sleep disturbance, cancer-related fatigue, and depression as determinants of quality of life among breast cancer patients undergoing chemotherapy: A cross-sectional study. BMC Cancer 2025, 25, 1122. [Google Scholar] [CrossRef]
- Lyu, W.; Ouyang, M.; Ma, X.; Han, T.; Pi, D.; Qiu, S. Kai-Xin-San Attenuates Doxorubicin-Induced Cognitive Impairment by Reducing Inflammation, Oxidative Stress, and Neural Degeneration in 4T1 Breast Cancer Mice. Evid. Based Complement. Altern. Med. 2021, 2021, 5521739. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, H.; Liang, Z.; Cui, J.; Liu, S.; Chen, Y.; Pi, D.; Ouyang, M. Elucidating the mechanism of traditional Chinese medicine formula (Yifei-sanjie Pill) in alleviating the chemobrain based on network pharmacology and experimental verification. J. Tradit. Complement. Med. 2024, in press. [Google Scholar] [CrossRef]
- Zuberi, A.; Rehman, U.; Gupta, G.; Ghazwani, M.; Hani, U.; Kesharwani, P. Smart liposomal systems for brain cancer: Technological innovations in drug delivery. Colloid Surf. B Biointerfaces 2025, 255, 114904. [Google Scholar] [CrossRef]
- Tzikos, G.; Chamalidou, E.; Christopoulou, D.; Apostolopoulou, A.; Gkarmiri, S.; Pertsikapa, M.; Menni, A.; Theodorou, I.M.; Stavrou, G.; Doutsini, N.; et al. Psychobiotics Ameliorate Depression and Anxiety Status in Surgical Oncology Patients: Results from the ProDeCa Study. Nutrients 2025, 17, 857. [Google Scholar] [CrossRef]
- Ibraheem, K.; Smith, A.; Collett, A.; Georgopoulos, N.T. Prevention of chemotherapy drug-mediated human hair follicle damage: Combined use of cooling with antioxidant suppresses oxidative stress and prevents matrix keratinocyte cytotoxicity. Front. Pharmacol. 2025, 16, 1558593. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhang, X.; Li, M.; Wu, W.; Sun, X.; Zhang, L.; Gong, T. Novel lipid hybrid albumin nanoparticle greatly lowered toxicity of pirarubicin. Mol. Pharm. 2013, 10, 3832–3841. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Lian, X.; Dong, J.; Wan, Z.; Xia, C.; Song, X.; Fu, Y.; Gong, T.; Zhang, Z. Co-delivery of Pirarubicin and Paclitaxel by Human Serum Albumin Nanoparticles to Enhance Antitumor Effect and Reduce Systemic Toxicity in Breast Cancers. Mol. Pharm. 2015, 12, 4085–4098. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Yuan, L.; Liu, Z.; Li, R.; Cai, Y.; Liu, X. Resveratrol ameliorates cognitive impairment with improvement of gut microbiota, inflammation, neurogenesis, and synaptic proteins in a mouse model of Gulf War Illness. Eur. J. Pharmacol. 2025, 1002, 177768. [Google Scholar] [CrossRef]
- Huang, S.; He, H.; Li, H.; Li, C.; Wang, F.; Hu, Y.; Liu, Y.; Chen, L.; Chen, H. Modulating the intestinal flora involves the effect of Atractylodis macrocephalae Rhizoma polysaccharide on spleen deficiency diarrhea. Int. J. Biol. Macromol. 2025, 321, 146562. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lu, Y.; Wang, Y.; Ding, N.; Huang, Z.; Hu, J.; Tong, J.; Zhang, Y.; Deng, L.; Luo, X.; et al. Xiaoyaosan improves depression-like behaviours in mice with post-stroke depression by modulating gut microbiota and microbial metabolism and regulating P2X7R/NLRP3 inflammasome. Phytomedicine 2025, 145, 157078. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Hamo, M.; Atchley, T.; Nabors, B.; Markert, J.; Estevez-Ordonez, D. The effect of antidepressants on glioblastoma survival: A systematic review and meta-analysis. Neurooncol. Adv. 2025, 7, vdaf75. [Google Scholar] [CrossRef] [PubMed]
- Baxter, S.M.; McShane, C.M.; McIntosh, S.A.; Bennett, D.; Lohfeld, L.; Middleton, D.R.S.; Savage, G.; Fitzpatrick, D.; Kane, J.; McBrien, A.; et al. Stage at diagnosis and breast cancer-specific mortality in breast cancer patients treated with antidepressants, anxiolytics, and antipsychotics: A population-based cohort study from Northern Ireland. Breast Cancer Res. Treat. 2025, 213, 137–150. [Google Scholar] [CrossRef]
- Ma, Y.; He, J.; Li, C.; Liu, F.; Wang, Y.; Song, F. Effect of Antidepressants Use on Cancer Morbidity and Mortality: A Propensity Score-Matched Longitudinal Cohort Study. J. Affect. Disord. 2025, 387, 119554. [Google Scholar] [CrossRef]
- Zhou, J.; Guo, X.; Yu, W.; Bu, H.; Du, Y.; Li, S.; Kuang, H.; Wu, L. Pharmacological action, mechanism and structure-activity relationship of traditional Chinese medicine polysaccharide in the treatment of Alzheimer’s disease: A review. Int. J. Biol. Macromol. 2025, 330, 148017. [Google Scholar] [CrossRef]
- Wu, Y.; Liang, Y.; Cui, J.; Chen, J.; Huang, J.; Yang, C.; Zuo, Q.; Chen, Q. Xiaoyao San reverses the pro-breast cancer effect of depression by inhibiting glutamate levels in intestinal flora. J. Ethnopharmacol. 2025, 355, 120636. [Google Scholar] [CrossRef]
- Li, L.; Wang, Z.; Zhang, W.; Chen, L.; Yang, Y.; Yang, W.; Li, M.; Yuan, C.; Zhang, L.; Wang, L. Isolation, Structures, and Bioactivities of Polysaccharides from Achyranthes bidentata: A Review. Molecules 2025, 30, 2523. [Google Scholar] [CrossRef]
- Fan, X.; Li, K.; Qin, X.; Li, Z.; Du, Y. Advances in the Preparation and Bioactivity of Polysaccharides From Medicinal Plants With Different Molecular Weights: A Review. Chem. Biodivers. 2025, 22, e03031. [Google Scholar] [CrossRef]
- Ye, Y.; Ze, L.; Duan, M.; Tan, Z.; Wang, Y.; Zhang, H.; Shang, P. Protective effects of plant polysaccharides on intestinal health via targeted regulation of gut microbiota. J. Sci. Food. Agric. 2025, in press. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ou, W.; Lin, G.; Wang, Y.; Chen, D.; Zeng, Z.; Chen, Z.; Lu, X.; Wu, A.; Lin, C.; et al. PAMK Ameliorates Non-Alcoholic Steatohepatitis and Associated Anxiety/Depression-like Behaviors Through Restoring Gut Microbiota and Metabolites in Mice. Nutrients 2024, 16, 3837. [Google Scholar] [CrossRef]
- Cao, D.; Chen, J.; Zhang, Y.; Rui, H.; Guang, K.; Zhang, L.; Wu, R.; Nian, S.; Song, X. Application of Metabolomics and Microbiome Analysis for Revealing the Endogenous Mechanism of Baizhu Xiaozhong San in Postpartum Rats with Spleen-qi Deficiency. J. Med. Food 2025, 28, 584–594. [Google Scholar] [CrossRef]
- Hu, P.; Yan, X.; Zeng, Y.; Jiang, Z.; Liu, J.; Feng, W. An UPLC-MS/MS method for targeted analysis of microbial and host tryptophan metabolism after administration of polysaccharides from Atractylodes macrocephala Koidz. in ulcerative colitis mice. J. Pharm. Biomed. Anal. 2023, 235, 115585. [Google Scholar] [CrossRef]
- He, H.; Huang, S.; Yu, C.; Wang, Q.; Liu, Y.; Chen, L.; Hu, Y.; Wang, F.; Chen, H. In vitro digestion of Atractylodes macrocephala polysaccharide and fermentation of human intestinal flora as affected by bran frying. Int. J. Biol. Macromol. 2025, 320, 145913. [Google Scholar] [CrossRef]
- Dinler, E.; Kocamaz, D.; Ozpineci, M.; Polat Olca, S.; Icel, S.; Yildirim, M. Cognitive dysfunction and depression in chemotherapy patients: A cross-sectional study from Turkey. BMC Cancer 2025, 25, 1247. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Wang, W.; Yi, Z.; Tian, H.; Wang, H.; Jiang, C.; Chen, Y.; Pi, D.; Chen, Q.; Wu, Y. Peach Gum Polysaccharide Prevents Chemotherapy-Induced Intestinal Injury and Degenerative Behavior. Integr. Cancer Ther. 2025, 24, 1583683546. [Google Scholar] [CrossRef]
- Wu, Y.; Pi, D.; Chen, Y.; Zuo, Q.; Lin, L.; Ouyang, M. Yifei sanjie Pills Alleviate Chemotherapy-Related Fatigue by Reducing Skeletal Muscle Injury and Inhibiting Tumor Growth in Lung Cancer Mice. Evid. Based Complement. Altern. Med. 2022, 2022, 2357616. [Google Scholar] [CrossRef]
- Li, J.; Yi, C.; Zhu, M.; Yuan, Y.; Chen, G.; Qiu, N.; Shen, L.; Song, L.; Liu, W.; Zhang, X. Immunomodulatory actions of tonifying polysaccharides: Pharmacological effects, mechanisms and therapeutic applications. Front. Immunol. 2025, 16, 1640679. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Jia, B.; Gong, S.; Li, Y.; Wang, J.; Huang, Y.; Guo, J. Protective effects of Atractylodes macrocephala polysaccharides on acetaminophen-induced liver injury. Front. Pharmacol. 2025, 16, 1583334. [Google Scholar] [CrossRef]
- Ren, H.; Dai, L.; Ma, C.; Zhou, L.; Wang, L. Hydrolysates exhibited differential modulatory effects on macrophage compared to the raw polysaccharide (xyloglucomannan) isolated from Atractylodes macrocephala Koidz. Prep. Biochem. Biotechnol. 2025, 55, 620–633. [Google Scholar] [CrossRef]
- Rodriguez-Gonzalez, A.; Orio, L. Microbiota and Alcohol Use Disorder: Are Psychobiotics a Novel Therapeutic Strategy? Curr. Pharm. Des. 2020, 26, 2426–2437. [Google Scholar] [CrossRef]
- Capuco, A.; Urits, I.; Hasoon, J.; Chun, R.; Gerald, B.; Wang, J.K.; Kassem, H.; Ngo, A.L.; Abd-Elsayed, A.; Simopoulos, T.; et al. Current Perspectives on Gut Microbiome Dysbiosis and Depression. Adv. Ther. 2020, 37, 1328–1346. [Google Scholar] [CrossRef]
- Almeida, C.; Oliveira, R.; Soares, R.; Barata, P. Influence of gut microbiota dysbiosis on brain function: A systematic review. Porto Biomed. J. 2020, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bibolar, A.C.; Nechita, V.I.; Lung, F.C.; Crecan-Suciu, B.D.; Paunescu, R.L. Gut Feelings: Linking Dysbiosis to Depression-A Narrative Literature Review. Med. Lith. 2025, 61, 1360. [Google Scholar] [CrossRef]
- Li, H.; Xiang, Y.; Zhu, Z.; Wang, W.; Jiang, Z.; Zhao, M.; Cheng, S.; Pan, F.; Liu, D.; Ho, R.C.M.; et al. Rifaximin-mediated gut microbiota regulation modulates the function of microglia and protects against CUMS-induced depression-like behaviors in adolescent rat. J. Neuroinflammation 2021, 18, 254. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ou, G.; Wang, S.; Chen, Y.; Xu, L.; Deng, L.; Xu, H.; Chen, X. The predictive, preventive, and personalized medicine of depression: Gut microbiota and inflammation. EPMA J. 2024, 15, 587–598. [Google Scholar] [CrossRef]
- Chi, R.; Li, M.; Zhang, M.; Zhang, N.; Zhang, G.; Cui, L.; Ma, G. Exploring the Association between Anxiety, Depression, and Gut Microbiota during Pregnancy: Findings from a Pregnancy Cohort Study in Shijiazhuang, Hebei Province, China. Nutrients 2024, 16, 1460. [Google Scholar] [CrossRef] [PubMed]
- Thomann, A.K.; Wustenberg, T.; Wirbel, J.; Knoedler, L.; Thomann, P.A.; Zeller, G.; Ebert, M.P.; Lis, S.; Reindl, W. Depression and fatigue in active IBD from a microbiome perspective-a Bayesian approach to faecal metagenomics. BMC Med. 2022, 20, 366. [Google Scholar] [CrossRef]
- Yang, Y.; Mori, M.; Wai, K.M.; Jiang, T.; Sugimura, Y.; Munakata, W.; Mikami, T.; Murashita, K.; Nakaji, S.; Ihara, K. The Association between Gut Microbiota and Depression in the Japanese Population. Microorganisms 2023, 11, 2286. [Google Scholar] [CrossRef]
- Xiong, Y.; Xiong, Y.; Yang, H.; Guo, F.; Yin, J.; Liao, S.; Chen, Z.; Zhang, W.; Zhou, H.; Weng, C.; et al. Age-dependent pharmacokinetics of nifedipine in spontaneous hypertensive rats are associated with changes in gut microbiota and hepatic CYP3A1 expression. Drug. Metab. Dispos. 2025, 53, 100116. [Google Scholar] [CrossRef]
- Wu, Y.; Gou, W.; Yan, Y.; Liu, C.; Yang, Y.; Chen, D.; Xie, K.; Jiang, Z.; Fu, Y.; Zhu, H.; et al. Gut microbiota and acylcarnitine metabolites connect the beneficial association between equol and adiposity in adults: A prospective cohort study. Am. J. Clin. Nutr. 2022, 116, 1831–1841. [Google Scholar] [CrossRef]
- Blau, N.; van Spronsen, F.J.; Levy, H.L. Phenylketonuria. Lancet 2010, 376, 1417–1427. [Google Scholar] [CrossRef]
- He, Y.; Tian, Y.; Xiong, H.; Deng, Z.; Zhang, H.; Guo, F.; Sun, Y. Rice Protein Peptides Ameliorate DSS-Induced Cognitive Impairment and Depressive Behavior in Mice by Modulating Phenylalanine Metabolism and the BDNF/TRKB/CREB Pathway. J. Agric. Food. Chem. 2024, 72, 19812–19825. [Google Scholar] [CrossRef]
- Kciuk, M.; Gielecinska, A.; Mujwar, S.; Kolat, D.; Kaluzinska-Kolat, Z.; Celik, I.; Kontek, R. Doxorubicin-An Agent with Multiple Mechanisms of Anticancer Activity. Cells 2023, 12, 659. [Google Scholar] [CrossRef]
- He, L.; Liu, F.; Li, J. Mitochondrial Sirtuins and Doxorubicin-induced Cardiotoxicity. Cardiovasc. Toxicol. 2021, 21, 179–191. [Google Scholar] [CrossRef]
- Wu, Y.; Pi, D.; Zhou, S.; Wang, W.; Ye, H.; Yi, Z.; Chen, Y.; Ouyang, M. Yiqi Chutan Formula Reverses Cisplatin-Induced Apoptosis and Ferroptosis of Skeletal Muscle by Alleviating Oxidative Stress. Integr. Cancer Ther. 2023, 22, 1563487253. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Kang, H.; Zhang, W.; Wang, J.; Liu, Z.; Jing, J.; Han, L.; Gao, A. Probiotics ameliorate benzene-induced systemic inflammation and hematopoietic toxicity by inhibiting Bacteroidaceae-mediated ferroptosis. Sci. Total Environ. 2023, 899, 165678. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.; Han, S.; Li, K.; Yan, A.; Wang, W.; Lu, W.; Han, J.; Zhang, C. Baicalein ameliorates DSS-induced ulcerative colitis in mice by inhibiting ferroptosis and regulating gut microbiota. Front. Pharmacol. 2025, 16, 1564783. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Hong, B.; Ma, S.; Wu, J.; Yang, Z.; Fan, X.; Shao, L.; Sun, K.; Zhao, J.; Fang, H.; et al. Ecological factors affecting toluene biosynthesis from bacterial communities. Sci. Total Environ. 2025, 959, 178186. [Google Scholar] [CrossRef]
- Li, W.; Wang, X.; Sun, X.; Zhang, Y.; Xie, J. Stir-frying duration modulates structural characterization and functional properties of Atractylodes macrocephala polysaccharides. Int. J. Biol. Macromol. 2025, 306, 141489. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, L.; Pi, D.; Cui, J.; Liang, Y.; Wu, P.; Ouyang, M.; Zuo, Q. Saikosaponin A induces cellular senescence in triple-negative breast cancer by inhibiting the PI3K/Akt signalling pathway. Front. Pharmacol. 2025, 16, 1532579. [Google Scholar] [CrossRef]
- Li, F.; Xie, L.; Xiao, Q.; Li, J.; Zhong, H.; Xu, X.; Tu, J.; Luo, Q. Benzo[a]pyrene exposure induces anxiety-like behaviors in the mice through brain metabolic alterations. Sci. Total Environ. 2024, 954, 176215. [Google Scholar] [CrossRef]
- Wu, Y.; Pi, D.; Zhou, S.; Yi, Z.; Dong, Y.; Wang, W.; Ye, H.; Chen, Y.; Zuo, Q.; Ouyang, M. Ginsenoside Rh3 induces pyroptosis and ferroptosis through the Stat3/p53/NRF2 axis in colorectal cancer cells. Acta Biochim. Biophys. Sin. 2023, 55, 587–600. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhou, S.; Pi, D.; Dong, Y.; Wang, W.; Ye, H.; Yi, Z.; Chen, Y.; Lin, L.; Ouyang, M. Deciphering the Molecular Mechanism of Yifei-Sanjie Pill in Cancer-Related Fatigue. J. Oncol. 2023, 2023, 5486017. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Feng, M.; He, F.; Zhang, E.; Li, X.; Cao, Z. Gut microbiota mediates the pro-pyroptosis effect of xierezhuyubuxu decoction in hepatocellular carcinoma. Front. Microbiol. 2024, 15, 1481111. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Z.; Yuan, Y.; Liang, J.C.; Wu, Y.; Cui, J.; Gu, H.; Pi, D.; Yi, Z.; Zhou, S. Atractylodes macrocephala Koidz. Polysaccharide Alleviates Chemotherapy-Induced Depression-Like Behaviors Through the Gut–Brain Axis. Int. J. Mol. Sci. 2025, 26, 10189. https://doi.org/10.3390/ijms262010189
Liang Z, Yuan Y, Liang JC, Wu Y, Cui J, Gu H, Pi D, Yi Z, Zhou S. Atractylodes macrocephala Koidz. Polysaccharide Alleviates Chemotherapy-Induced Depression-Like Behaviors Through the Gut–Brain Axis. International Journal of Molecular Sciences. 2025; 26(20):10189. https://doi.org/10.3390/ijms262010189
Chicago/Turabian StyleLiang, Zheng, Yihan Yuan, July Chen Liang, Yingchao Wu, Jiaqi Cui, Haihong Gu, Dajin Pi, Zhongjia Yi, and Shuyao Zhou. 2025. "Atractylodes macrocephala Koidz. Polysaccharide Alleviates Chemotherapy-Induced Depression-Like Behaviors Through the Gut–Brain Axis" International Journal of Molecular Sciences 26, no. 20: 10189. https://doi.org/10.3390/ijms262010189
APA StyleLiang, Z., Yuan, Y., Liang, J. C., Wu, Y., Cui, J., Gu, H., Pi, D., Yi, Z., & Zhou, S. (2025). Atractylodes macrocephala Koidz. Polysaccharide Alleviates Chemotherapy-Induced Depression-Like Behaviors Through the Gut–Brain Axis. International Journal of Molecular Sciences, 26(20), 10189. https://doi.org/10.3390/ijms262010189







