The Caenorhabditis elegans sdhb-1(R244H) Model Shows Characteristics of Human PPGL Tumor Cells
Abstract
1. Introduction
2. Results
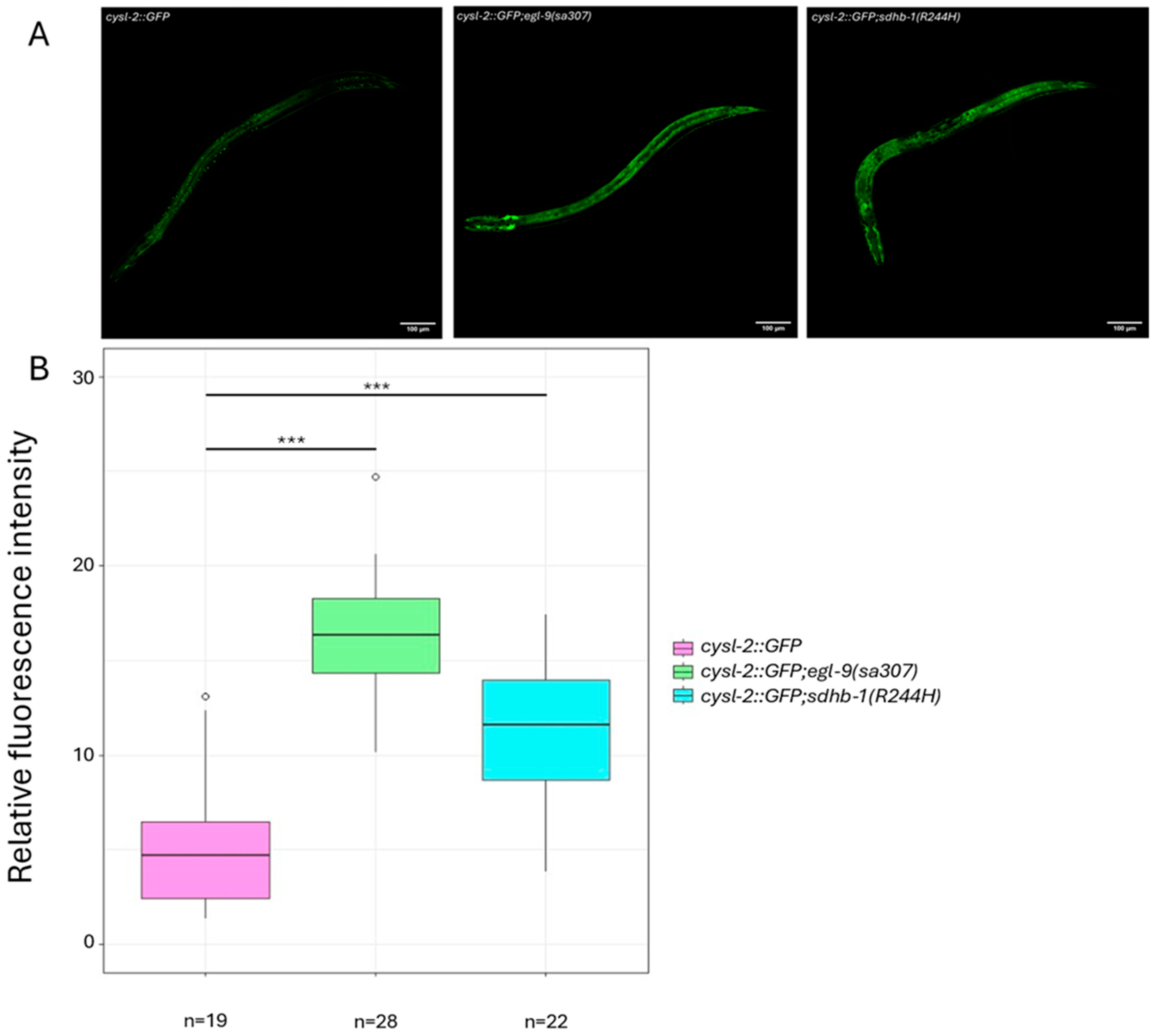
2.1. Pseudohypoxia Is Activated Under Normoxic Conditions in sdhb-1(R244H) Mutants
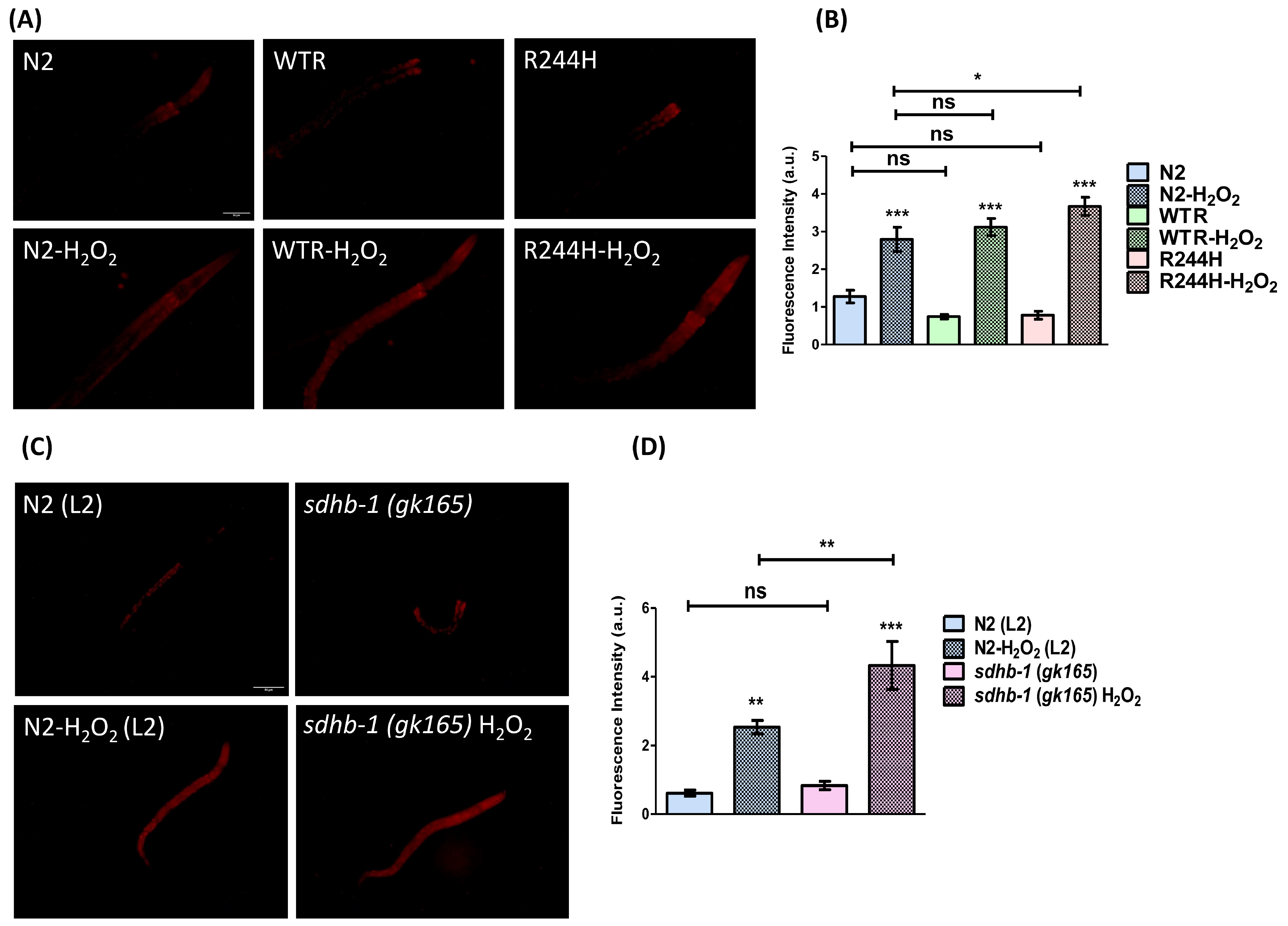
2.2. Impact of sdhb-1 Null Mutation and R244H Point Mutation on Overall Oxidative Status
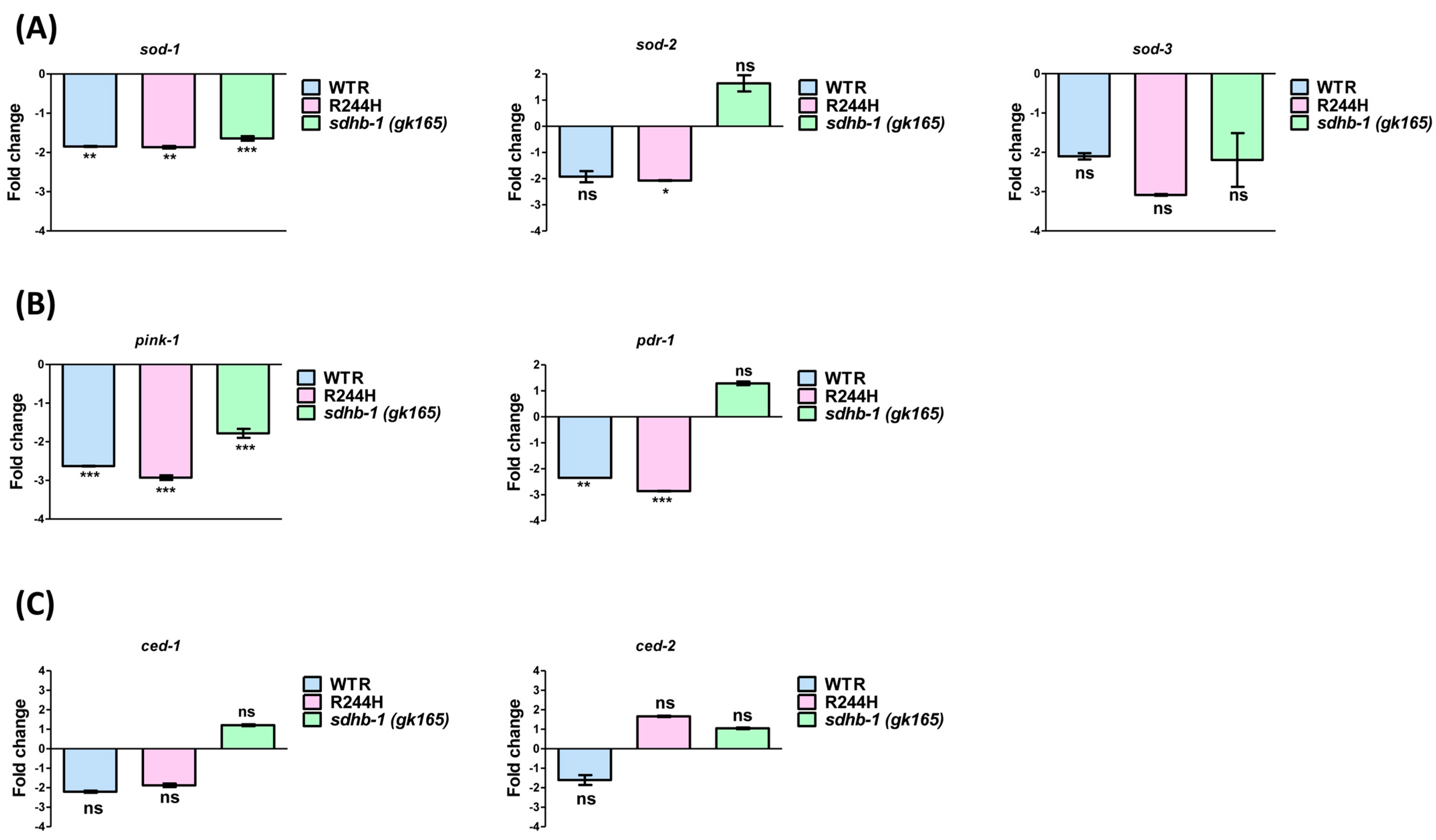
2.3. Impact of sdhb-1 Null Mutation and R244H Point Mutation on Levels of Antioxidant Enzymes, Mitophagy and Apoptosis Markers
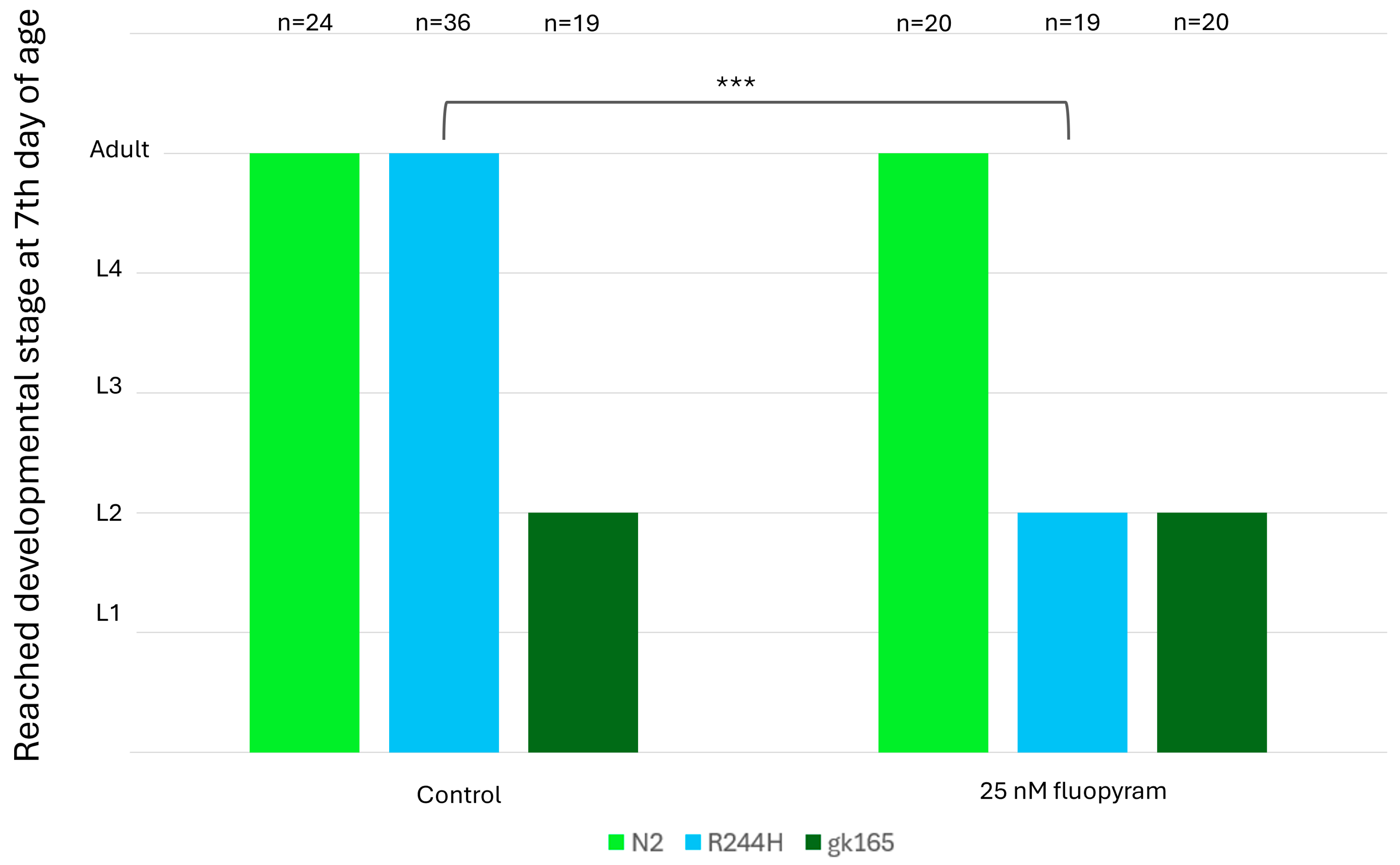
2.4. Fluopyram Treatment Selectively Affects the Development of sdhb-1(R244H) Mutants
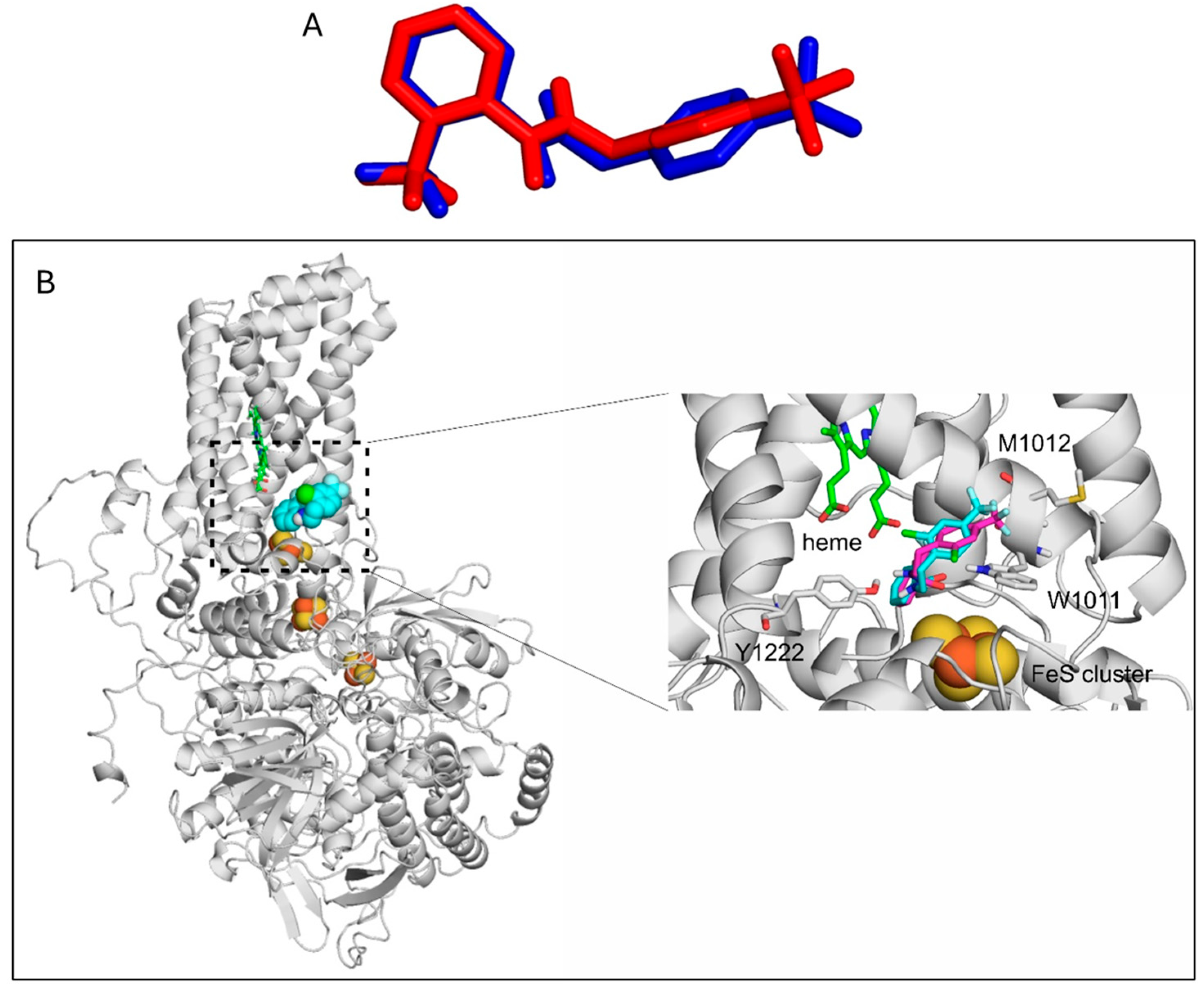
2.5. Binding Mode of Fluopyram to the Wild-Type and Mutant SDH Complex
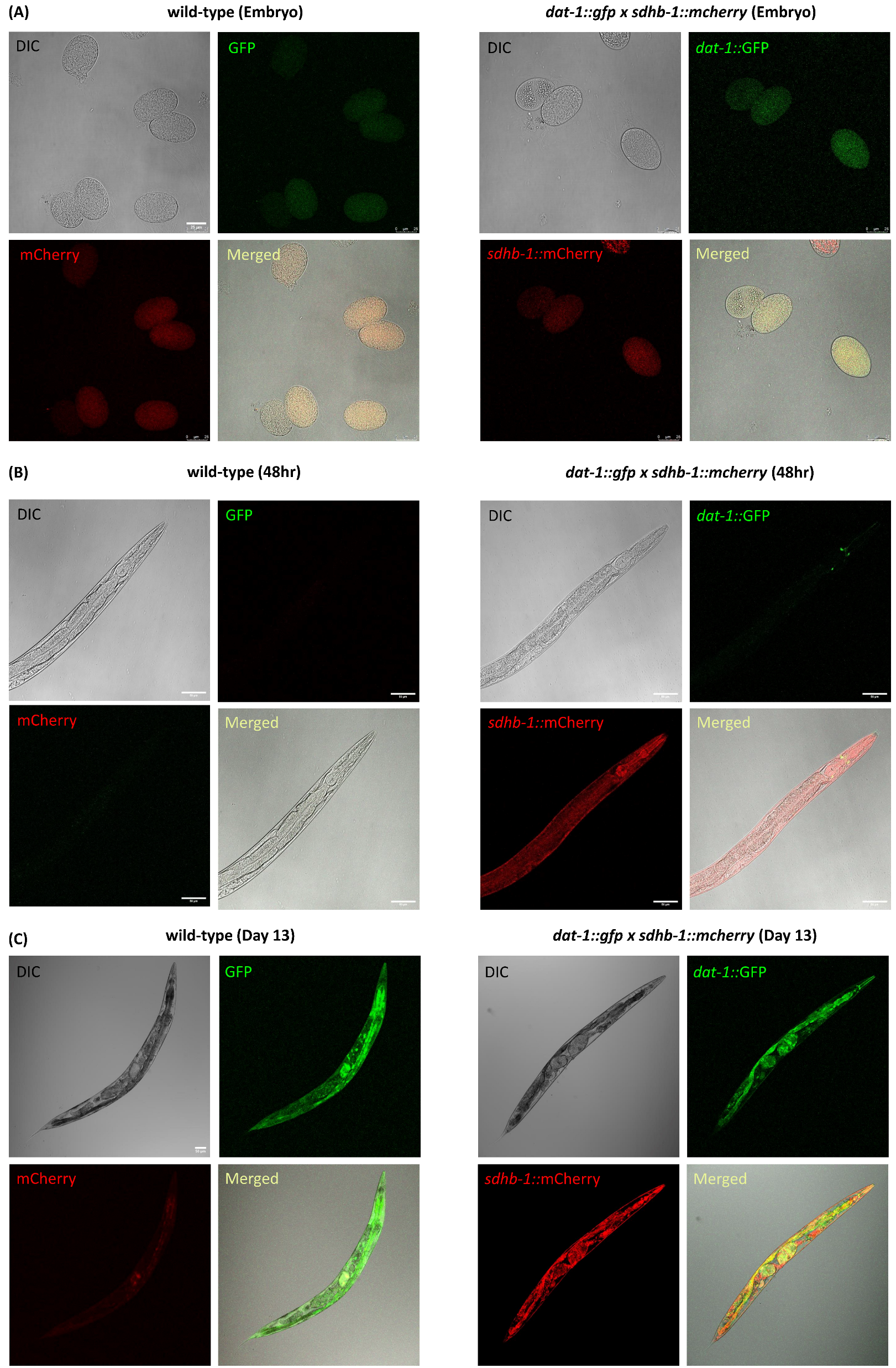
2.6. Expression Pattern of SDHB-1 and DAT-1 Throughout Different Stages of Worm Development
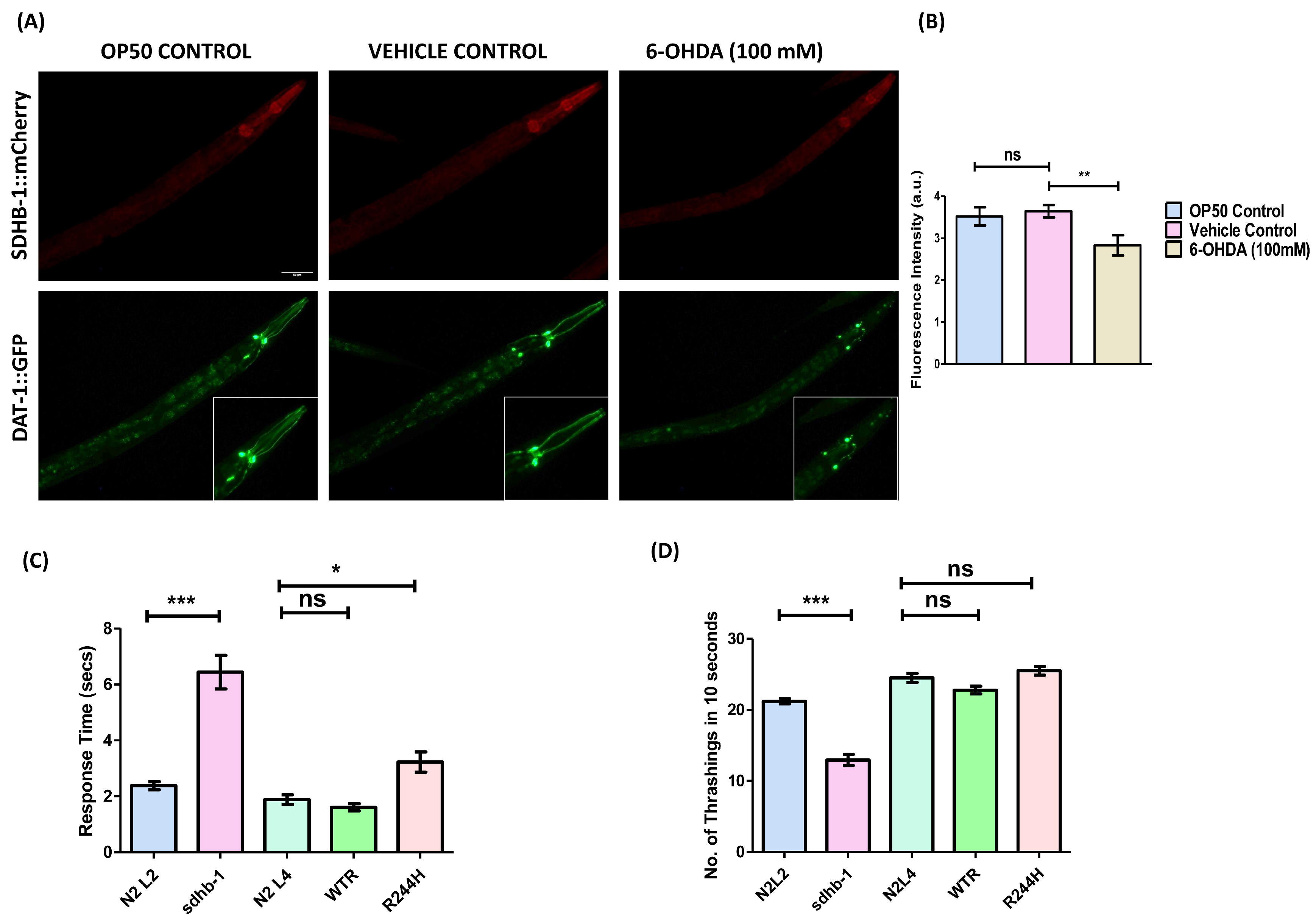
2.7. Correlating Mitochondrial Dysfunction and Dopaminergic Neurodegeneration in the Context of B Subunit of Succinate Dehydrogenase (SDHB)
3. Discussion
4. Materials and Methods
4.1. Caenorhabditis elegans Strains
4.2. Oxidative Stress Measurement
4.3. Image Acquisition
4.4. Thrashing Assay
4.5. 1-Nonanol Aversion Assay
4.6. qRT-PCR and Semi-qPCR Experiments
4.7. Single Worm qRT-PCR Experiments
4.8. 6-OHDA Treatment
4.9. Residual SDH Activity
4.10. Modeling Studies
4.10.1. Ligand Preparation
4.10.2. Target Preparation
4.10.3. Docking
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DAT-1 | dopamine transporter |
| DHE | dihydroethidium |
| ETC | electron transport chain |
| HIF | hypoxia-inducible factor |
| LDH | lactate dehydrogenase |
| NDRG1 | N-myc downstream regulated 1 |
| 6-OHDA | 6-hydroxydopamine |
| PHEO | pheochromocytoma |
| PGL | paraganglioma |
| PINK | PTEN-induced putative kinase |
| PPGL | pheochromocytoma/paraganglioma |
| ROS | reactive oxygen species |
| SDHB | succinate dehydrogenase B subunit |
| SOD | superoxide dismutase |
| VEGFA | vascular endothelial growth factor A |
References
- Raphael, M.J.; Chan, D.L.; Law, C.; Singh, S. Principles of Diagnosis and Management of Neuroendocrine Tumours. CMAJ 2017, 189, E398–E404. [Google Scholar] [CrossRef] [PubMed]
- Juhlin, C.C. The Road Ahead: A Brief Guide to Navigating the 2022 WHO Classification of Endocrine and Neuroendocrine Tumours. J. Clin. Pathol. 2024, 78, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.Q.; Bezerra-Neto, J.E.; Mendonça, B.B.; Latronico, A.C.; Fragoso, M.C.B. V Primary Malignant Tumors of the Adrenal Glands. Clinics 2018, 73, e756s. [Google Scholar] [CrossRef]
- Dahia, P.L.M. Pheochromocytoma and Paraganglioma Pathogenesis: Learning from Genetic Heterogeneity. Nat. Rev. Cancer 2014, 14, 108–119. [Google Scholar] [CrossRef]
- Amar, L.; Bertherat, J.; Baudin, E.; Ajzenberg, C.; Bressac-de Paillerets, B.; Chabre, O.; Chamontin, B.; Delemer, B.; Giraud, S.; Murat, A.; et al. Genetic Testing in Pheochromocytoma or Functional Paraganglioma. J. Clin. Oncol. 2005, 23, 8812–8818. [Google Scholar] [CrossRef]
- Timmers, H.J.L.M.; Kozupa, A.; Eisenhofer, G.; Raygada, M.; Adams, K.T.; Solis, D.; Lenders, J.W.M.; Pacak, K. Clinical Presentations, Biochemical Phenotypes, and Genotype-Phenotype Correlations in Patients with Succinate Dehydrogenase Subunit B-Associated Pheochromocytomas and Paragangliomas. J. Clin. Endocrinol. Metab. 2007, 92, 779–786. [Google Scholar] [CrossRef]
- Takács-Vellai, K.; Farkas, Z.; Ősz, F.; Stewart, G.W. Model Systems in SDHx-Related Pheochromocytoma/Paraganglioma. Cancer Metastasis Rev. 2021, 40, 1177–1201. [Google Scholar] [CrossRef]
- Saskői, É.; Hujber, Z.; Nyírő, G.; Likó, I.; Mátyási, B.; Petővári, G.; Mészáros, K.; Kovács, A.L.; Patthy, L.; Supekar, S.; et al. The SDHB Arg230His Mutation Causing Familial Paraganglioma Alters Glycolysis in a New Caenorhabditis Elegans Model. Dis. Model. Mech. 2020, 13, dmm044925. [Google Scholar] [CrossRef] [PubMed]
- van Berkel, A.; Rao, J.U.; Kusters, B.; Demir, T.; Visser, E.; Mensenkamp, A.R.; van der Laak, J.A.W.M.; Oosterwijk, E.; Lenders, J.W.M.; Sweep, F.C.G.J.; et al. Correlation Between In Vivo 18F-FDG PET and Immunohistochemical Markers of Glucose Uptake and Metabolism in Pheochromocytoma and Paraganglioma. J. Nucl. Med. 2014, 55, 1253–1259. [Google Scholar] [CrossRef]
- Fliedner, S.M.J.; Kaludercic, N.; Jiang, X.-S.; Hansikova, H.; Hajkova, Z.; Sladkova, J.; Limpuangthip, A.; Backlund, P.S.; Wesley, R.; Martiniova, L.; et al. Warburg Effect’s Manifestation in Aggressive Pheochromocytomas and Paragangliomas: Insights from a Mouse Cell Model Applied to Human Tumor Tissue. PLoS ONE 2012, 7, e40949. [Google Scholar] [CrossRef]
- Selak, M.A.; Armour, S.M.; MacKenzie, E.D.; Boulahbel, H.; Watson, D.G.; Mansfield, K.D.; Pan, Y.; Simon, M.C.; Thompson, C.B.; Gottlieb, E. Succinate Links TCA Cycle Dysfunction to Oncogenesis by Inhibiting HIF-Alpha Prolyl Hydroxylase. Cancer Cell 2005, 7, 77–85. [Google Scholar] [CrossRef]
- Bayley, J.-P.; Devilee, P. Hypothesis: Why Different Types of SDH Gene Variants Cause Divergent Tumor Phenotypes. Genes 2022, 13, 1025. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Rath, E.M.; Tsang, V.H.M.; Duff, A.P.; Robinson, B.G.; Church, W.B.; Benn, D.E.; Dwight, T.; Clifton-Bligh, R.J. Structural and Functional Consequences of Succinate Dehydrogenase Subunit B Mutations. Endocr. Relat. Cancer 2015, 22, 387–397. [Google Scholar] [CrossRef]
- Shen, C.; Nettleton, D.; Jiang, M.; Kim, S.K.; Powell-Coffman, J.A. Roles of the HIF-1 Hypoxia-Inducible Factor During Hypoxia Response in Caenorhabditis Elegans. J. Biol. Chem. 2005, 280, 20580–20588. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Guo, R.; Powell-Coffman, J.A. The Caenorhabditis Elegans Hif-1 Gene Encodes a BHLH-PAS Protein That Is Required for Adaptation to Hypoxia. Proc. Natl. Acad. Sci. USA 2001, 98, 7916–7921. [Google Scholar] [CrossRef]
- Powell-Coffman, J.A. Hypoxia Signaling and Resistance in C. Elegans. Trends Endocrinol. Metab. 2010, 21, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Cserepes, M.; Türk, D.; Tóth, S.; Pape, V.F.S.; Gaál, A.; Gera, M.; Szabó, J.E.; Kucsma, N.; Várady, G.; Vértessy, B.G.; et al. Unshielding Multidrug Resistant Cancer Through Selective Iron Depletion of P-Glycoprotein-Expressing Cells. Cancer Res. 2020, 80, 663–674. [Google Scholar] [CrossRef]
- Ma, D.K.; Vozdek, R.; Bhatla, N.; Horvitz, H.R. CYSL-1 Interacts with the O2-Sensing Hydroxylase EGL-9 to Promote H2S-Modulated Hypoxia-Induced Behavioral Plasticity in C. elegans. Neuron 2012, 73, 925–940. [Google Scholar] [CrossRef]
- Saikolappan, S.; Kumar, B.; Shishodia, G.; Koul, S.; Koul, H.K. Reactive Oxygen Species and Cancer: A Complex Interaction. Cancer Lett. 2019, 452, 132–143. [Google Scholar] [CrossRef]
- Ilanchezhian, M.; Jha, A.; Pacak, K.; Del Rivero, J. Emerging Treatments for Advanced/Metastatic Pheochromocytoma and Paraganglioma. Curr. Treat. Options Oncol. 2020, 21, 85. [Google Scholar] [CrossRef]
- Saito, Y.; Ishii, K.-A.; Aita, Y.; Ikeda, T.; Kawakami, Y.; Shimano, H.; Hara, H.; Takekoshi, K. Loss of SDHB Elevates Catecholamine Synthesis and Secretion Depending on ROS Production and HIF Stabilization. Neurochem. Res. 2016, 41, 696–706. [Google Scholar] [CrossRef]
- Ishii, T.; Yasuda, K.; Akatsuka, A.; Hino, O.; Hartman, P.S.; Ishii, N. A Mutation in the SDHC Gene of Complex II Increases Oxidative Stress, Resulting in Apoptosis and Tumorigenesis. Cancer Res. 2005, 65, 203–209. [Google Scholar] [CrossRef]
- Goffrini, P.; Ercolino, T.; Panizza, E.; Giachè, V.; Cavone, L.; Chiarugi, A.; Dima, V.; Ferrero, I.; Mannelli, M. Functional Study in a Yeast Model of a Novel Succinate Dehydrogenase Subunit B Gene Germline Missense Mutation (C191Y) Diagnosed in a Patient Affected by a Glomus Tumor. Hum. Mol. Genet. 2009, 18, 1860–1868. [Google Scholar] [CrossRef]
- Smith, E.H.; Janknecht, R.; Maher, L.J. Succinate Inhibition of Alpha-Ketoglutarate-Dependent Enzymes in a Yeast Model of Paraganglioma. Hum. Mol. Genet. 2007, 16, 3136–3148. [Google Scholar] [CrossRef] [PubMed]
- Slane, B.G.; Aykin-Burns, N.; Smith, B.J.; Kalen, A.L.; Goswami, P.C.; Domann, F.E.; Spitz, D.R. Mutation of Succinate Dehydrogenase Subunit C Results in Increased O2·−, Oxidative Stress, and Genomic Instability. Cancer Res. 2006, 66, 7615–7620. [Google Scholar] [CrossRef]
- Ha, N.M.; Tran, S.H.; Shim, Y.-H.; Kang, K. Caenorhabditis Elegans as a Powerful Tool in Natural Product Bioactivity Research. Appl. Biol. Chem. 2022, 65, 18. [Google Scholar] [CrossRef]
- Zhao, H.; Kalivendi, S.; Zhang, H.; Joseph, J.; Nithipatikom, K.; Vásquez-Vivar, J.; Kalyanaraman, B. Superoxide Reacts with Hydroethidine but Forms a Fluorescent Product That Is Distinctly Different from Ethidium: Potential Implications in Intracellular Fluorescence Detection of Superoxide. Free Radic. Biol. Med. 2003, 34, 1359–1368. [Google Scholar] [CrossRef]
- Min, H.; Youn, E.; Kim, J.; Son, S.Y.; Lee, C.H.; Shim, Y.-H. Effects of Phosphoethanolamine Supplementation on Mitochondrial Activity and Lipogenesis in a Caffeine Ingestion Caenorhabditis Elegans Model. Nutrients 2020, 12, 3348. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, T.; Imai, H. Hydrogen Peroxide Produced by Superoxide Dismutase SOD-2 Activates Sperm in Caenorhabditis Elegans. J. Biol. Chem. 2017, 292, 14804–14813. [Google Scholar] [CrossRef] [PubMed]
- Ahier, A.; Dai, C.-Y.; Kirmes, I.; Cummins, N.; Hung, G.C.C.; Götz, J.; Zuryn, S. PINK1 and Parkin Shape the Organism-Wide Distribution of a Deleterious Mitochondrial Genome. Cell Rep. 2021, 35, 109203. [Google Scholar] [CrossRef]
- Schleker, A.S.S.; Rist, M.; Matera, C.; Damijonaitis, A.; Collienne, U.; Matsuoka, K.; Habash, S.S.; Twelker, K.; Gutbrod, O.; Saalwächter, C.; et al. Mode of Action of Fluopyram in Plant-Parasitic Nematodes. Sci. Rep. 2022, 12, 11954. [Google Scholar] [CrossRef]
- Vidal-Gadea, A.G.; Pierce-Shimomura, J.T. Conserved Role of Dopamine in the Modulation of Behavior. Commun. Integr. Biol. 2012, 5, 440–447. [Google Scholar] [CrossRef]
- Jadiya, P.; Chatterjee, M.; Sammi, S.R.; Kaur, S.; Palit, G.; Nazir, A. Sir-2.1 Modulates “calorie-Restriction-Mediated” Prevention of Neurodegeneration in Caenorhabditis Elegans: Implications for Parkinson’s Disease. Biochem. Biophys. Res. Commun. 2011, 413, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Latchoumycandane, C.; Anantharam, V.; Jin, H.; Kanthasamy, A.; Kanthasamy, A. Dopaminergic Neurotoxicant 6-OHDA Induces Oxidative Damage Through Proteolytic Activation of PKCδ in Cell Culture and Animal Models of Parkinson’s Disease. Toxicol. Appl. Pharmacol. 2011, 256, 314–323. [Google Scholar] [CrossRef]
- Hameed, R.; Naseer, A.; Saxena, A.; Akbar, M.; Toppo, P.; Sarkar, A.; Shukla, S.K.; Nazir, A. Functional Implications of NHR-210 Enrichment in C. Elegans Cephalic Sheath Glia: Insights into Metabolic and Mitochondrial Disruptions in Parkinson’s Disease Models. Cell. Mol. Life Sci. 2024, 81, 202. [Google Scholar] [CrossRef]
- Vora, M.; Pyonteck, S.M.; Popovitchenko, T.; Matlack, T.L.; Prashar, A.; Kane, N.S.; Favate, J.; Shah, P.; Rongo, C. The Hypoxia Response Pathway Promotes PEP Carboxykinase and Gluconeogenesis in C. elegans. Nat. Commun. 2022, 13, 6168. [Google Scholar] [CrossRef] [PubMed]
- Cowman, S.J.; Koh, M.Y. Revisiting the HIF Switch in the Tumor and Its Immune Microenvironment. Trends Cancer 2022, 8, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Duarte Hospital, C.; Tête, A.; Debizet, K.; Imler, J.; Tomkiewicz-Raulet, C.; Blanc, E.B.; Barouki, R.; Coumoul, X.; Bortoli, S. SDHi Fungicides: An Example of Mitotoxic Pesticides Targeting the Succinate Dehydrogenase Complex. Environ. Int. 2023, 180, 108219. [Google Scholar] [CrossRef]
- Matsumura-Matsuda, E.; Sekiya, M.; Omoto-Inuzuka, M.; Santo, K.; Shikama, A.; Kuba, M.; Sugano, Y.; Iwasaki, H.; Yatoh, S.; Sato, T.; et al. A Rare Coexistence of Pheochromocytoma and Parkinson’s Disease with Diagnostic Challenges. Intern. Med. 2018, 57, 979–985. [Google Scholar] [CrossRef]
- Mehta, S.H.; Prakash, R.; Prisant, L.M.; Isales, C.M.; Morgan, J.C.; Williams, H.; Sethi, K.D. Diagnosis of Pheochromocytoma in the Setting of Parkinson Disease. Nat. Rev. Neurol. 2009, 5, 343–347. [Google Scholar] [CrossRef]
- Correia, S.C.; Moreira, P.I. Hypoxia-Inducible Factor 1: A New Hope to Counteract Neurodegeneration? J. Neurochem. 2010, 112, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Jodeiri Farshbaf, M.; Kiani-Esfahani, A. Succinate Dehydrogenase: Prospect for Neurodegenerative Diseases. Mitochondrion 2018, 42, 77–83. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, L.P.; Luke, C.J.; Perlmutter, D.H.; Silverman, G.A.; Pak, S.C. C. elegans in High-Throughput Drug Discovery. Adv. Drug Deliv. Rev. 2014, 69–70, 247–253. [Google Scholar] [CrossRef]
- Brenner, S. The Genetics of Caenorhabditis Elegans. Genetics 1974, 77, 71–94. [Google Scholar] [CrossRef] [PubMed]
- Gusarov, I.; Shamovsky, I.; Pani, B.; Gautier, L.; Eremina, S.; Katkova-Zhukotskaya, O.; Mironov, A.; Makarov, A.A.; Nudler, E. Dietary Thiols Accelerate Aging of C. elegans. Nat. Commun. 2021, 12, 4336. [Google Scholar] [CrossRef]
- Min, H.; Lee, M.; Cho, K.S.; Lim, H.J.; Shim, Y.-H. Nicotinamide Supplementation Improves Oocyte Quality and Offspring Development by Modulating Mitochondrial Function in an Aged Caenorhabditis Elegans Model. Antioxidants 2021, 10, 519. [Google Scholar] [CrossRef]
- Hoogewijs, D.; Houthoofd, K.; Matthijssens, F.; Vandesompele, J.; Vanfleteren, J.R. Selection and Validation of a Set of Reliable Reference Genes for Quantitative Sod Gene Expression Analysis in C. Elegans. BMC Mol. Biol. 2008, 9, 9. [Google Scholar] [CrossRef]
- Ly, K.; Reid, S.J.; Snell, R.G. Rapid RNA Analysis of Individual Caenorhabditis Elegans. MethodsX 2015, 2, 59–63. [Google Scholar] [CrossRef]
- Schrödinger Schrödinger Release 2024-1: Maestro 2024. Available online: https://www.schrodinger.com/life-science/download/release-notes/release-2024-1/ (accessed on 8 January 2024).
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Inaoka, D.K.; Shiba, T.; Sato, D.; Balogun, E.O.; Sasaki, T.; Nagahama, M.; Oda, M.; Matsuoka, S.; Ohmori, J.; Honma, T.; et al. Structural Insights into the Molecular Design of Flutolanil Derivatives Targeted for Fumarate Respiration of Parasite Mitochondria. Int. J. Mol. Sci. 2015, 16, 15287–15308. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- UniProt Consortium. UniProt: A Worldwide Hub of Protein Knowledge. Nucleic Acids Res. 2019, 47, D506–D515. [Google Scholar] [CrossRef] [PubMed]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Accurate Structure Prediction of Biomolecular Interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Shahrokh, K.; Orendt, A.; Yost, G.S.; Cheatham, T.E. Quantum Mechanically Derived AMBER-Compatible Heme Parameters for Various States of the Cytochrome P450 Catalytic Cycle. J. Comput. Chem. 2012, 33, 119–133. [Google Scholar] [CrossRef]
- Zsidó, B.Z.; Balog, M.; Erős, N.; Poór, M.; Mohos, V.; Fliszár-Nyúl, E.; Hetényi, C.; Nagane, M.; Hideg, K.; Kálai, T.; et al. Synthesis of Spin-Labelled Bergamottin: A Potent CYP3A4 Inhibitor with Antiproliferative Activity. Int. J. Mol. Sci. 2020, 21, 508. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.J.P.; Klamt, A.; Thiel, W.; Danovich, D.; Rocha, G.B.; Gieseking, R.L.; Moussa, J.E.; Kurtz, H.A.; Korambath, P.; Merz, K.M.; et al. MOPAC (22.0.0) 2022; Molecular Sciences Software Institute (MolSSI): Blacksburg, VA, USA, 2022. [Google Scholar]
- Stewart, J.J.P. Optimization of Parameters for Semiempirical Methods VI: More Modifications to the NDDO Approximations and Re-Optimization of Parameters. J. Mol. Model. 2013, 19, 1–32. [Google Scholar] [CrossRef]
- DeLano, W.L. The PyMOL Molecular Graphics System. Version 2.0 2017. Schrödinger, LLC: New York, NY, USA.
- Van Der Spoel, D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.E.; Berendsen, H.J.C. GROMACS: Fast, Flexible, and Free. J. Comput. Chem. 2005, 26, 1701–1718. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of Simple Potential Functions for Simulating Liquid Water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and Testing of a General Amber Force Field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef]
- Gasteiger, J.; Marsili, M. Iterative Partial Equalization of Orbital Electronegativity—A Rapid Access to Atomic Charges. Tetrahedron 1980, 36, 3219–3228. [Google Scholar] [CrossRef]
- Zsidó, B.Z.; Börzsei, R.; Szél, V.; Hetényi, C. Determination of Ligand Binding Modes in Hydrated Viral Ion Channels to Foster Drug Design and Repositioning. J. Chem. Inf. Model. 2021, 61, 4011–4022. [Google Scholar] [CrossRef] [PubMed]
- Tyukodi, L.; Zsidó, B.Z.; Hetényi, C.; Kőszegi, T.; Huber, I.; Rozmer, Z. Serum Albumin Binding Studies on Antiproliferative Cyclic C5-Curcuminoid Derivatives Using Spectroscopic Methods and Molecular Modelling. J. Mol. Struct. 2023, 1287, 135761. [Google Scholar] [CrossRef]
- Lemli, B.; Vilmányi, P.; Fliszár-Nyúl, E.; Zsidó, B.Z.; Hetényi, C.; Szente, L.; Poór, M. Testing Serum Albumins and Cyclodextrins as Potential Binders of the Mycotoxin Metabolites Alternariol-3-Sulfate, Alternariol-9-Monomethylether and Alternariol-9-Monomethylether-3-Sulfate. Int. J. Mol. Sci. 2022, 23, 14353. [Google Scholar] [CrossRef] [PubMed]


| Gene Name | Primer Sequences |
|---|---|
| act-1 | Forward Primer: TTACTCTTTCACCACCACCGCTGA Reverse Primer: TCGTTTCCGACGGTGATGACTTGT |
| od-1 | Forward Primer: CCAGGCAGTTATTGAAGGAGAA Reverse Primer: TGTGGACCGGCAGAAATG |
| sod-2 | Forward Primer: GAGGCGGTCTCCAAAGGAAA Reverse Primer: CCAGAGATCCGAAGTCGCTC |
| sod-3 | Forward Primer: CTCCAAGCACACTCTCCCAG Reverse Primer: TCCCTTTCGAAACAGCCTCG |
| pink-1 | Forward Primer: AGTCGTCTGGACAAAGTGATG Reverse Primer: TTGCTCGAAGTTGTCGTTCT |
| pdr-1 | Forward Primer: CAGACGTCGTACAGCGAATAC Reverse Primer: TCATAGGGCTCCCAGAAGAA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ősz, F.; Akbar, M.; Zsidó, B.Z.; Hetényi, C.; Orbán, T.I.; Fóthi, Á.; Kovács, G.M.; Pintye, A.; Boda, A.; Nazir, A.; et al. The Caenorhabditis elegans sdhb-1(R244H) Model Shows Characteristics of Human PPGL Tumor Cells. Int. J. Mol. Sci. 2025, 26, 10185. https://doi.org/10.3390/ijms262010185
Ősz F, Akbar M, Zsidó BZ, Hetényi C, Orbán TI, Fóthi Á, Kovács GM, Pintye A, Boda A, Nazir A, et al. The Caenorhabditis elegans sdhb-1(R244H) Model Shows Characteristics of Human PPGL Tumor Cells. International Journal of Molecular Sciences. 2025; 26(20):10185. https://doi.org/10.3390/ijms262010185
Chicago/Turabian StyleŐsz, Fanni, Mahmood Akbar, Balázs Zoltán Zsidó, Csaba Hetényi, Tamás I. Orbán, Ábel Fóthi, Gábor M. Kovács, Alexandra Pintye, Attila Boda, Aamir Nazir, and et al. 2025. "The Caenorhabditis elegans sdhb-1(R244H) Model Shows Characteristics of Human PPGL Tumor Cells" International Journal of Molecular Sciences 26, no. 20: 10185. https://doi.org/10.3390/ijms262010185
APA StyleŐsz, F., Akbar, M., Zsidó, B. Z., Hetényi, C., Orbán, T. I., Fóthi, Á., Kovács, G. M., Pintye, A., Boda, A., Nazir, A., Farkas, Z., & Takács-Vellai, K. (2025). The Caenorhabditis elegans sdhb-1(R244H) Model Shows Characteristics of Human PPGL Tumor Cells. International Journal of Molecular Sciences, 26(20), 10185. https://doi.org/10.3390/ijms262010185









