Navigating the Effects of Anti-Atherosclerotic Supplements and Acknowledging Associated Bleeding Risks
Abstract
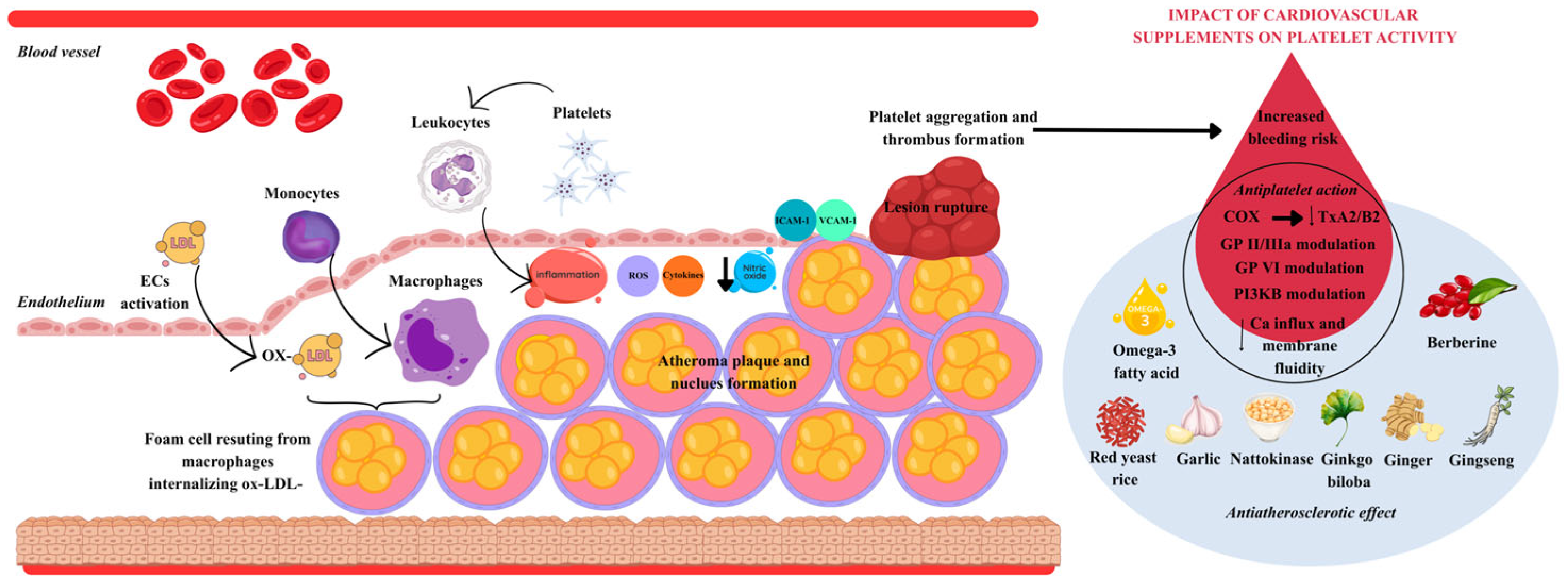
1. Introduction
2. Nutraceuticals and Cardiovascular Health: Anti-Atherosclerotic Potential and Safety Concerns
2.1. Anti-Atherosclerotic Supplements Acting as Lipid Lowering Agents
2.1.1. Omega-3 Fatty Acids
2.1.2. Berberine
2.1.3. Red Yeast Rice
2.1.4. Garlic
2.1.5. Nattokinase
2.2. Nutraceutical and Botanical Bioactives That Inhibit LDL Oxidation
2.2.1. Astaxanthin
2.2.2. Resveratrol
2.2.3. Coenzyme Q10 (CoQ10)
2.2.4. Vitamins C and E
2.3. Supplements with Anti-Atherosclerotic Effects That Reduce Vascular Inflammation
2.3.1. Ginkgo Biloba
2.3.2. Ginger
2.3.3. Ginseng
2.3.4. Curcumin
3. Hemostatic Safety of Nutraceutical and Botanical Bioactives: Bleeding Risks and Drug–Drug Interactions
3.1. Pharmacodynamic (Antiplatelet/Fibrinolytic) Effects
3.2. Micronutrient-Related Coagulation Effects
3.3. Pharmacokinetic Interactions with Antithrombotics
3.4. Agents with Limited or No Consistent Clinical Bleeding Signal
3.5. Extracellular Vesicles at the Thrombosis–Bleeding Interface
4. Future Research Directions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| cAMP | Cyclic Adenosine Monophosphate |
| COX1 | Cyclooxygenase1 |
| CRP | C-Reactive Protein |
| CVD | Cardiovascular Disease |
| CYP3A | Cytochrome P450 3A |
| DHA | Docosahexaenoic Acid |
| DSHEA | Dietary Supplement Health and Education Act |
| EGFR | Epidermal Growth Factor Receptor |
| EPA | Eicosapentaenoic Acid |
| ERK | Extracellular Signal-Regulated Kinase |
| FDA | Food and Drug Administration |
| FMD | Flow-Mediated Dilation |
| GPVI | Glycoprotein VI |
| HDLC | High-Density Lipoprotein Cholesterol |
| HO1 | Heme Oxygenase1 |
| hsCRP | High-Sensitivity C-Reactive Protein |
| ICAM1 | Intercellular Adhesion Molecule1 |
| IL6 | Interleukin6 |
| INR | International Normalized Ratio |
| IP3 | Inositol1,4,5trisphosphate |
| JAKSTAT | Janus Kinase Signal Transducer and Activator of Transcription |
| LDL | Low-Density Lipoprotein |
| LDLC | Low-Density Lipoprotein Cholesterol |
| LDLR | Low-Density Lipoprotein Receptor |
| LOX1 | Lectinlike Oxidized LDL Receptor1 |
| MAPK | Mitogen-Activated Protein Kinase |
| MCP1 | Monocyte Chemoattractant Protein1 |
| MI | Myocardial Infarction |
| NFκB | Nuclear Factor kappa light chain enhancer of Activated B cells |
| NK | Nattokinase |
| NO | Nitric Oxide |
| NOX4 | NADPH Oxidase 4 |
| Nrf2 | Nuclear Factor Erythroid 2related Factor 2 |
| NRYR | Natto Red Yeast Rice |
| oxLDL | Oxidized Low-Density Lipoprotein |
| PAF | Platelet-Activating Factor |
| PAI1 | Plasminogen Activator Inhibitor1 |
| PCSK9 | Proprotein Convertase Subtilisin/Kexin Type 9 |
| Pgp | P-glycoprotein |
| PI3K | Phosphoinositide 3Kinase |
| PI3Kβ | Phosphoinositide 3Kinase Beta |
| PLC | Phospholipase C |
| PNLIP | Pancreatic lipase |
| PXDN | Peroxidasin |
| PXR | Pregnane X Receptor |
| ROS | Reactive Oxygen Species |
| RYR | Red Yeast Rice |
| SIRT1 | Sirtuin 1 |
| tPA | Tissue Plasminogen Activator |
| TNFα | Tumor Necrosis Factor alpha |
| TLR4 | Toll-like Receptor 4 |
| TRAF6 | TNF Receptor Associated Factor 6 |
| TXA2 | Thromboxane A2 |
| VCAM1 | Vascular Cell Adhesion Molecule1 |
| VASP | Vasodilator-Stimulated Phosphoprotein |
| VLDL | Very-Low-Density Lipoprotein |
References
- Björkegren, J.L.; Lusis, A.J. Atherosclerosis: Recent developments. Cell 2022, 185, 1630–1645. [Google Scholar] [CrossRef] [PubMed]
- Siegel-Axel, D.I.; Gawaz, M. Platelets and endothelial cells. Semin. Thromb. Hemost. 2007, 33, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Watanabe, T. Atherosclerosis: Known and unknown. Pathol. Int. 2022, 72, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Jebari-Benslaiman, S.; Galicia-García, U.; Larrea-Sebal, A.; Olaetxea, J.R.; Alloza, I.; Vandenbroeck, K.; Benito-Vicente, A.; Martín, C. Pathophysiology of atherosclerosis. Int. J. Mol. Sci. 2022, 23, 3346. [Google Scholar] [CrossRef]
- Gusev, E.; Sarapultsev, A. Atherosclerosis and inflammation: Insights from the theory of general pathological processes. Int. J. Mol. Sci. 2023, 24, 7910. [Google Scholar] [CrossRef]
- Mussbacher, M.; Schossleitner, K.; Kral-Pointner, J.B.; Salzmann, M.; Schrammel, A.; Schmid, J.A. More than just a monolayer: The multifaceted role of endothelial cells in the pathophysiology of atherosclerosis. Curr. Atheroscler. Rep. 2022, 24, 483–492. [Google Scholar] [CrossRef]
- Wu, X.-M.; Zhang, N.; Li, J.-S.; Yang, Z.-H.; Huang, X.-L.; Yang, X.-F. Purinergic receptors mediate endothelial dysfunction and participate in atherosclerosis. Purinergic Signal. 2023, 19, 265–272. [Google Scholar] [CrossRef]
- Jiang, H.; Zhou, Y.; Nabavi, S.M.; Sahebkar, A.; Little, P.J.; Xu, S.; Weng, J.; Ge, J. Mechanisms of oxidized LDL-mediated enothelial dysfunction and its consequences for the development of atherosclerosis. Front. Cardiovasc. Med. 2022, 9, 925923. [Google Scholar] [CrossRef]
- Batty, M.; Bennett, M.R.; Yu, E. The role of oxidative stress in atherosclerosis. Cells 2022, 11, 3843. [Google Scholar] [CrossRef]
- Momi, S.; Falcinelli, E.; Petito, E.; Ciarrocca Taranta, G.; Ossoli, A.; Gresele, P. Matrix metalloproteinase-2 on activated platelets triggers endothelial PAR-1 initiating atherosclerosis. Eur. Heart J. 2022, 43, 504–514. [Google Scholar] [CrossRef]
- Gardin, C.; Ferroni, L.; Leo, S.; Tremoli, E.; Zavan, B. Platelet-derived exosomes in atherosclerosis. Int. J. Mol. Sci. 2022, 23, 12546. [Google Scholar] [CrossRef] [PubMed]
- Theofilis, P.; Sagris, M.; Antonopoulos, A.S.; Oikonomou, E.; Tsioufis, C.; Tousoulis, D. Inflammatory mediators of platelet activation: Focus on atherosclerosis and COVID-19. Int. J. Mol. Sci. 2021, 22, 11170. [Google Scholar] [CrossRef] [PubMed]
- Bravo, G.M.; Annarapu, G.; Carmona, E.; Nawarskas, J.; Clark, R.; Novelli, E.; Alvidrez, R.I.M. Platelets in thrombosis and atherosclerosis: A double-edged sword. Am. J. Pathol. 2024, 194, 1608–1621. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tang, C. Targeting platelet in atherosclerosis plaque formation: Current knowledge and future perspectives. Int. J. Mol. Sci. 2020, 21, 9760. [Google Scholar] [CrossRef]
- Serebruany, V.L.; Malinin, A.I.; Eisert, R.M.; Sane, D.C. Risk of bleeding complications with antiplatelet agents: Meta-analysis of 338,191 patients enrolled in 50 randomized controlled trials. Am. J. Hematol. 2004, 75, 40–47. [Google Scholar] [CrossRef]
- Nording, H.; Baron, L.; Langer, H.F. Platelets as therapeutic targets to prevent atherosclerosis. Atherosclerosis 2020, 307, 97–108. [Google Scholar] [CrossRef]
- Parma, L.; Baganha, F.; Quax, P.H.; de Vries, M.R. Plaque angiogenesis and intraplaque hemorrhage in atherosclerosis. Eur. J. Pharmacol. 2017, 816, 107–115. [Google Scholar] [CrossRef]
- Levy, A.; Moreno, P. Intraplaque hemorrhage. Curr. Mol. Med. 2006, 6, 479–488. [Google Scholar] [CrossRef]
- Kolodgie, F.D.; Gold, H.K.; Burke, A.P.; Fowler, D.R.; Kruth, H.S.; Weber, D.K.; Farb, A.; Guerrero, L.; Hayase, M.; Kutys, R. Intraplaque hemorrhage and progression of coronary atheroma. N. Engl. J. Med. 2003, 349, 2316–2325. [Google Scholar] [CrossRef]
- AlAli, M.; Alqubaisy, M.; Aljaafari, M.N.; AlAli, A.O.; Baqais, L.; Molouki, A.; Abushelaibi, A.; Lai, K.-S.; Lim, S.-H.E. Nutraceuticals: Transformation of Conventional Foods into Health Promoters/Disease Preventers and Safety Considerations. Molecules 2021, 26, 2540. [Google Scholar] [CrossRef]
- Mirzai, S.; Laffin, L.J. Supplements for Lipid Lowering: What Does the Evidence Show? Curr. Cardiol. Rep. 2023, 25, 795–805. [Google Scholar] [CrossRef]
- Vogel, J.H.K.; Bolling, S.F.; Costello, R.B.; Guarneri, E.M.; Krucoff, M.W.; Longhurst, J.C.; Olshansky, B.; Pelletier, K.R.; Tracy, C.M.; Vogel, R.A.; et al. Integrating complementary medicine into cardiovascular medicine: A report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. J. Am. Coll. Cardiol. 2005, 46, 123–136. [Google Scholar] [CrossRef]
- Starr, R.R. Too little, too late: Ineffective regulation of dietary supplements in the United States. Am. J. Public Health 2015, 105, 478–485. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Collins, R.; Reith, C.; Emberson, J.; Armitage, J.; Baigent, C.; Blackwell, L.; Blumenthal, R.; Danesh, J.; Davey Smith, G.; DeMets, D.; et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet 2016, 388, 2532–2561, Correction in Lancet 2017, 389, 602. [Google Scholar] [CrossRef] [PubMed]
- Grant, J.K.; Dangl, M.; Ndumele, C.E.; Michos, E.D.; Martin, S.S. A historical, evidence-based, and narrative review on commonly used dietary supplements in lipid-lowering. J. Lipid Res. 2024, 65, 100493. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Skulas-Ray, A.C.; Wilson, P.W.F.; Harris, W.S.; Brinton, E.A.; Kris-Etherton, P.M.; Richter, C.K. Omega-3 fatty acids for the management of hypertriglyceridemia: A science advisory from the American Heart Association. Circulation 2019, 140, e673–e691. [Google Scholar] [CrossRef]
- Backes, J.; Anzalone, D.; Hilleman, D.; Catini, J. The clinical relevance of omega-3 fatty acids in the management of hypertriglyceridemia. Lipids Health Dis. 2016, 15, 118. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Green, C.J.; Pramfalk, C.; Charlton, C.A.; Gunn, P.J.; Cornfield, T.; Pavlides, M.; Karpe, F.; Hodson, L. Hepatic de novo lipogenesis is suppressed and fat oxidation is increased by omega-3 fatty acids at the expense of glucose metabolism. BMJ Open Diabetes Res. Care 2020, 8, e000871. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Adili, R.; Hawley, M.; Holinstat, M. Regulation of platelet function and thrombosis by omega-3 and omega-6 polyunsaturated fatty acids. Prostaglandins Other Lipid Mediat. 2018, 139, 10–18. [Google Scholar] [CrossRef]
- DeFilippis, A.; Rai, S.N.; Cambon, A.; Miles, R.; Jaffe, A.S.; Moser, A.B.; O Jones, R.; Bolli, R.; Schulman, S.P. Fatty acids and TxA2 generation, in the absence of platelet-COX-1 activity. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 428–433. [Google Scholar] [CrossRef]
- Bäck, M. Omega-3 fatty acids in atherosclerosis and coronary artery disease. Futur. Sci. OA 2017, 3, FSO236. [Google Scholar] [CrossRef]
- Fredman, G.; Van Dyke, T.E.; Serhan, C.N. Resolvin E1 Regulates Adenosine Diphosphate Activation of Human Platelets. Arter. Thromb. Vasc. Biol. 2010, 30, 2005–2013. [Google Scholar] [CrossRef] [PubMed]
- Kacik, M.; Olivan-Viguera, A.; Köhler, R. Modulation of KCa3.1 Channels by Eicosanoids, Omega-3 Fatty Acids, and Molecular Determinants. PLoS ONE 2014, 9, e112081. [Google Scholar] [CrossRef] [PubMed]
- Larson, M.K.; Tormoen, G.W.; Weaver, L.J.; Luepke, K.J.; Patel, I.A.; Hjelmen, C.E.; Ensz, N.M.; McComas, L.S.; Mccarty, O.J.T. Exogenous modification of platelet membranes with the omega-3 fatty acids EPA and DHA reduces platelet procoagulant activity and thrombus formation. Am. J. Physiol. Physiol. 2013, 304, C273–C279. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, A.; Stanger, L.; Freedman, C.J.; Standley, M.; Hoang, T.; Adili, R.; Tsai, W.; Van Hoorebeke, C.; Holman, T.R.; Holinstat, M. DHA 12-LOX-derived oxylipins regulate platelet activation and thrombus formation through a PKA-dependent signaling pathway. J. Thromb. Haemost. 2020, 19, 839–851. [Google Scholar] [CrossRef]
- Larson, M.K.; Shearer, G.C.; Ashmore, J.H.; Anderson-Daniels, J.M.; Graslie, E.L.; Tholen, J.T.; Vogelaar, J.L.; Korth, A.J.; Nareddy, V.; Sprehe, M.; et al. Omega-3 fatty acids modulate collagen signaling in human platelets. Prostaglandins Leukot. Essent. Fat. Acids. 2011, 84, 93–98. [Google Scholar] [CrossRef]
- Golanski, J.; Szymanska, P.; Rozalski, M. Effects of Omega-3 Polyunsaturated Fatty Acids and Their Metabolites on Haemostasis-Current Perspectives in Cardiovascular Disease. Int. J. Mol. Sci. 2021, 22, 2394. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rausch, J.; Gillespie, S.; Orchard, T.; Tan, A.; McDaniel, J.C. Systematic review of marine-derived omega-3 fatty acid supplementation effects on leptin, adi-ponectin, and the leptin-to-adiponectin ratio. Nutr. Res. 2021, 85, 135–152. [Google Scholar] [CrossRef]
- Bowman, L.; Mafham, M.; Wallendszus, K.; Stevens, W.; Buck, G.; Barton, J.; Murphy, K.; Aung, T.; Haynes, R.; Cox, J.; et al. Effects of Aspirin for Primary Prevention in Persons with Diabetes Mellitus. N. Engl. J. Med. 2018, 18, 379, 1529–1539. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Ketchum, S.B.; Doyle, R.T., Jr.; Juliano, R.A.; Jiao, L.; Granowitz, C.; et al. REDUCE-IT Investigators. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N. Engl. J. Med. 2019, 380, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Zivkovic, S.; Maric, G.; Cvetinovic, N.; Lepojevic-Stefanovic, D.; Bozic Cvijan, B. Anti-Inflammatory Effects of Lipid-Lowering Drugs and Supplements-A Narrative Review. Nutrients 2023, 15, 1517. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Lincoff, A.M.; Garcia, M.; Bash, D.; Ballantyne, C.M.; Barter, P.J.; Davidson, M.H.; Kastelein, J.J.P.; Koenig, W.; McGuire, D.K.; et al. Effect of high-dose omega-3 fatty acids vs corn oil on major adverse cardiovascular events in patients at high cardiovascular risk: The STRENGTH randomized clinical trial. JAMA 2020, 324, 2268–2280. [Google Scholar] [CrossRef]
- Manson, J.E.; Cook, N.R.; Lee, I.M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Albert, C.M.; Gordon, D.; Copeland, T.; et al. Marine n−3 fatty acids and prevention of cardiovascular disease and cancer. N. Engl. J. Med. 2019, 380, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Hao, J.; Fan, D. Biological properties and clinical applications of berberine. Front. Med. 2020, 14, 564–582. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Kim, W.S.; Kim, K.H.; Yoon, M.J.; Cho, H.J.; Shen, Y.; Ye, J.M.; Lee, C.H.; Oh, W.K.; Kim, C.T.; et al. Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes 2006, 55, 2256–2264. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Wei, J.; Abidi, P.; Lin, M.; Inaba, S.; Li, C.; Wang, Y.; Wang, Z.; Si, S.; Pan, H.; et al. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat. Med. 2004, 10, 1344–1351. [Google Scholar] [CrossRef] [PubMed]
- Ai, X.; Yu, P.; Peng, L.; Luo, L.; Liu, J.; Li, S.; Lai, X.; Luan, F.; Meng, X. Berberine: A Review of its Pharmacokinetics Properties and Therapeutic Potentials in Diverse Vascular Diseases. Front. Pharmacol. 2021, 12, 762654. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, C.; Cheng, Y.; Zhang, Y.; Jin, H.; Zuo, Z.; Wang, A.; Huang, J.; Jiang, J.; Kong, W. Berberine and Its Main Metabolite Berberrubine Inhibit Platelet Activation Through Suppressing the Class I PI3Kβ/Rasa3/Rap1 Pathway. Front. Pharmacol. 2021, 12, 734603. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xie, X.; Ma, X.; Zeng, S.; Tang, W.; Xiao, L.; Zhu, C.; Yu, R. Mechanisms of Berberine for the Treatment of Atherosclerosis Based on Network Pharmacology. Evid.-Based Complement. Altern. Med. 2020, 2020, 3568756. [Google Scholar] [CrossRef]
- Dempsey, P. Red Yeast Rice: Lipid Lowering Properties and Safety Considerations. Online J. Complement. Altern. Med. 2021, 6, 101752366. [Google Scholar] [CrossRef]
- Peng, D.; Fong, A.; Pelt, A. The effects of red yeast rice supplementation on cholesterol levels in adults. Am. J. Nurs. 2017, 117, 46–54. [Google Scholar] [CrossRef]
- Trogkanis, E.; Karalexi, M.A.; Sergentanis, T.N.; Kornarou, E.; Vassilakou, T. Safety and Efficacy of the Consumption of the Nutraceutical “Red Yeast Rice Extract” for the Reduction of Hypercholesterolemia in Humans: A Systematic Review and Meta-Analysis. Nutrients 2024, 16, 1453. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Fogacci, F.; Stoian, A.P.; Toth, P.P. Red Yeast Rice for the Improvement of Lipid Profiles in Mild-to-Moderate Hypercholesterolemia: A Narrative Review. Nutrients 2023, 15, 2288. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, M.; Xu, Z.; Wang, Z.; Wang, D.; Yang, M.; Li, H.; Zhang, W.; He, R.; Cheng, H.; Guo, P.; et al. Lipid-lowering, antihypertensive, and antithrombotic effects of nattokinase combined with red yeast rice in patients with stable coronary artery disease: A randomized, double-blinded, placebo-controlled trial. Front. Nutr. 2024, 11, 1380727. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Liu, F.; Li, J.; Liang, F.; Li, J.; Cao, J.; Liu, D.; Huang, K.; Li, H.; Lu, X.; et al. Evaluation of NaTto Red Yeast Rice on Regulating Blood Lipid (ENTRY) Study: A Multicenter, Double-Placebo, Double-Blinded, Randomized Controlled Trial in Chinese Adults. Chronic Dis. Transl. Med. 2025, 11, 122–129. [Google Scholar] [CrossRef]
- Tesfaye, A. Revealing the Therapeutic Uses of Garlic (Allium sativum) and Its Potential for Drug Discovery. Sci. World J. 2021, 2021, 8817288. [Google Scholar] [CrossRef]
- Sun, Y.E.; Wang, W.; Qin, J. Anti-Hyperlipidemia of Garlic by Reducing the Level of Total Cholesterol and Low-Density Lipoprotein: A Meta-Analysis. Medicine 2018, 97, e0255. [Google Scholar] [CrossRef]
- Silagy, C.; Neil, A. Garlic as a Lipid Lowering Agent-A Meta-Analysis. J. R. Coll. Physicians Lond. 1994, 28, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Zhou, H.; Zha, W. Garlic Consumption Can Reduce the Risk of Dyslipidemia: A Meta-Analysis of Randomized Controlled Trials. J. Health Popul. Nutr. 2024, 43, 113. [Google Scholar] [CrossRef]
- Rahman, K.; Lowe, G.M.; Smith, S. Aged Garlic Extract Inhibits Human Platelet Aggregation by Altering Intracellular Signaling and Platelet Shape Change. J. Nutr. 2016, 146, 410S–415S. [Google Scholar] [CrossRef]
- Wang, Z.; Ding, L.; Liu, J.; Savarin, P.; Wang, X.; Zhao, K.; Ding, W.; Hou, Y. Allicin Ameliorates Glucose and Lipid Metabolism via Modulation of Gut Microbiota and Bile Acid Profile in Diabetic Rats. J. Funct. Foods 2023, 111, 105899. [Google Scholar] [CrossRef]
- Laka, K.; Makgoo, L.; Mbita, Z. Cholesterol-Lowering Phytochemicals: Targeting the Mevalonate Pathway for Anticancer Interventions. Front. Genet. 2022, 13, 841639. [Google Scholar] [CrossRef]
- Rahman, K. Effects of Garlic on Platelet Biochemistry and Physiology. Mol. Nutr. Food Res. 2007, 51, 1335–1344. [Google Scholar] [CrossRef]
- Wei, C.; Cai, R.; Song, Y.; Liu, X.; Xu, H.L. Research Progress of Nattokinase in Reducing Blood Lipid. Nutrients 2025, 17, 1784. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Muric, M.; Nikolic, M.; Todorovic, A.; Jakovljevic, V.; Vucicevic, K. Comparative Cardioprotective Effectiveness: NOACs vs. Nattokinase-Bridging Basic Research to Clinical Findings. Biomolecules 2024, 14, 956. [Google Scholar] [CrossRef] [PubMed]
- Hodis, H.N.; Mack, W.J.; Meiselman, H.J.; Kalra, V.; Liebman, H.; Hwang-Levine, J.; Dustin, L.; Kono, N.; Mert, M.; Wenby, R.B.; et al. Nattokinase atherothrombotic prevention study: A randomized controlled trial. Clin. Hemorheol. Microcirc. 2021, 78, 339–353. [Google Scholar] [CrossRef] [PubMed]
- Urano, T.; Ihara, H.; Umemura, K.; Suzuki, Y.; Oike, M.; Akita, S.; Tsukamoto, Y.; Suzuki, I.; Takada, A. The profibrinolytic enzyme subtilisin NAT purified from Bacillus subtilis cleaves and inactivates plasminogen activator inhibitor type 1. J. Biol. Chem. 2001, 276, 24690–24696. [Google Scholar] [CrossRef]
- Milner, M.; Makise, K. Natto and its active ingredient nattokinase: A potent and safe thrombolytic agent. Altern. Complement. Therap. 2002, 8, 157–164. [Google Scholar] [CrossRef]
- Jang, J.Y.; Kim, T.S.; Cai, J.; Kim, J.; Kim, Y.; Shin, K.; Kim, K.S.; Park, S.K.; Lee, S.P.; Choi, E.K.; et al. Nattokinase improves blood flow by inhibiting platelet aggregation and thrombus formation. Lab. Anim. Res. 2013, 29, 221–225. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hsia, C.H.; Shen, M.C.; Lin, J.S.; Wen, Y.K.; Hwang, K.L.; Cham, T.M.; Yang, N.C. Nattokinase decreases plasma levels of fibrinogen, factor VII, and factor VIII in human subjects. Nutr. Res. 2009, 29, 190–196. [Google Scholar] [CrossRef]
- Liu, X.; Zeng, X.; Mahe, J.; Guo, K.; He, P.; Yang, Q.; Zhang, Z.; Li, Z.; Wang, D.; Zhang, Z.; et al. The Effect of Nattokinase-Monascus Supplements on Dyslipidemia: A Four-Month Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2023, 15, 4239. [Google Scholar] [CrossRef] [PubMed]
- Luquain-Costaz, C.; Delton, I. Oxysterols in vascular cells and role in atherosclerosis. In Implication of Oxysterols and Phy-tosterols in Aging and Human Diseases; Springer International Publishing: Cham, Switzerland, 2023; pp. 213–229. [Google Scholar]
- Samimi, F.; Namiranian, N.; Sharifi-Rigi, A.; Siri, M.; Abazari, O.; Dastghaib, S. Coenzyme Q10: A Key Antioxidant in the Management of Diabetes-Induced Cardiovascular Complications—An Overview of Mechanisms and Clinical Evidence. Int. J. Endocrinol. 2024, 2024, 2247748. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, J.; Feng, J.; Xuan, R. Research progress of Astaxanthin nano-based drug delivery system: Applications, prospects and challenges? Front. Pharmacol. 2023, 14, 1102888. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Gong, N.; Chen, F.; Hu, S.; Zhou, Q.; Gao, X. The Effects of Astaxanthin on Metabolic Syndrome: A Comprehensive Review. Mar. Drugs. 2024, 23, 9. [Google Scholar] [CrossRef]
- Ciaraldi, T.P.; Boeder, S.C.; Mudaliar, S.R.; Giovannetti, E.R.; Henry, R.R.; Pettus, J.H. Astaxanthin, a natural antioxidant, lowers cholesterol and markers of cardiovascular risk in individuals with prediabetes and dyslipidaemia. Diabetes Obes. Metab. 2023, 25, 1985–1994. [Google Scholar] [CrossRef]
- Davinelli, S.; Saso, L.; D’Angeli, F.; Calabrese, V.; Intrieri, M.; Scapagnini, G. Astaxanthin as a modulator of Nrf2, NF-κB, and their crosstalk: Molecular mechanisms and possible clinical applications. Molecules 2022, 27, 502. [Google Scholar] [CrossRef]
- Pereira, C.P.M.; Souza, A.C.R.; Vasconcelos, A.R.; Prado, P.S.; Name, J.J. Antioxidant and anti-inflammatory mechanisms of action of astaxanthin in cardiovascular diseases (Review). Int. J. Mol. Med. 2021, 47, 37–48. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Heidari, M.; Chaboksafar, M.; Alizadeh, M.; Sohrabi, B.; Kheirouri, S. Effects of Astaxanthin supplementation on selected metabolic parameters, anthropometric indices, Sirtuin1 and TNF-α levels in patients with coronary artery disease: A randomized, double-blind, placebo-controlled clinical trial. Front. Nutr. 2023, 10, 1104169. [Google Scholar] [CrossRef]
- Wu, D.; Xu, H.; Chen, J.; Zhang, L. Effects of astaxanthin supplementation on oxidative stress. Int. J. Vitam. Nutr. Res. 2020, 90, 179–194. [Google Scholar] [CrossRef]
- Fassett, R.G.; Coombes, J.S. Astaxanthin in cardiovascular health and disease. Molecules 2012, 17, 2030–2048. [Google Scholar] [CrossRef]
- Xia, N.; Daiber, A.; Förstermann, U.; Li, H. Antioxidant effects of resveratrol in the cardiovascular system. Br. J. Pharmacol. 2017, 174, 1633–1646. [Google Scholar] [CrossRef] [PubMed]
- Obeme-Nmom, J.I.; Abioye, R.O.; Reyes Flores, S.S.; Udenigwe, C.C. Regulation of redox enzymes by nutraceuticals: A review of the roles of antioxidant polyphenols and peptides. Food Funct. 2024, 15, 10956–10980. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Förstermann, U. Resveratrol: A Multifunctional Compound Improving Endothelial Function: Editorial to:“Resveratrol Supplementation Gender Independently Improves Endothelial Reactivity and Suppresses Superoxide Production in Healthy Rats” by S. Soylemez et al. Cardiovasc. Drugs Ther. 2009, 23, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Wang, L.; Qi, L.; Yang, X.; Jin, Y.; Yin, H.; Huang, Y.; Sheng, J.; Wang, X. Resveratrol improved atherosclerosis by increasing LDLR levels via the EGFR-ERK1/2 signaling pathway. Lipids Health Dis. 2025, 24, 167. [Google Scholar] [CrossRef]
- Penumathsa, S.V.; Thirunavukkarasu, M.; Koneru, S.; Juhasz, B.; Zhan, L.; Pant, R.; Menon, V.P.; Otani, H.; Maulik, N. Statin and resveratrol in combination induces cardioprotection against myocardial infarction in hypercholesterolemic rat. J. Mol. Cell. Cardiol. 2007, 42, 508–516. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, X.; Zhu, W.; Liu, Z.; Liu, H.; Zhou, Y.; Cao, Y.; Liu, C.; Xie, Y. Resveratrol enhances autophagic flux and promotes Ox-LDL degradation in HUVECs via upregulation of SIRT1. Oxid. Med. Cell. Longev. 2016, 2016, 7589813. [Google Scholar] [CrossRef]
- Teimouri, M.; Homayouni-Tabrizi, M.; Rajabian, A.; Amiri, H.; Hosseini, H. Anti-inflammatory effects of resveratrol in patients with cardiovascular disease: A systematic review and meta-analysis of randomized controlled trials. Complement. Ther. Med. 2022, 70, 102863. [Google Scholar] [CrossRef]
- Mohammadipoor, N.; Shafiee, F.; Rostami, A.; Kahrizi, M.S.; Soleimanpour, H.; Ghodsi, M.; Ansari, M.J.; Bokov, D.O.; Jannat, B.; Mosharkesh, E.; et al. Resveratrol supplementation efficiently improves endothelial health: A systematic review and meta-analysis of randomized controlled trials. Phytother. Res. 2022, 36, 3529–3539. [Google Scholar] [CrossRef]
- Akbari, M.; Tamtaji, O.R.; Lankarani, K.B.; Tabrizi, R.; Dadgostar, E.; Kolahdooz, F.; Jamilian, M.; Mirzaei, H.; Asemi, Z. The effects of resveratrol supplementation on endothelial function and blood pressures among patients with metabolic syndrome and related disorders: A systematic review and meta-analysis of randomized controlled trials. High Blood Press. Cardiovasc. Prev. 2019, 26, 305–319. [Google Scholar] [CrossRef]
- Godos, J.; Romano, G.L.; Gozzo, L.; Laudani, S.; Paladino, N.; Dominguez Azpíroz, I.; Martínez López, N.M.; Giampieri, F.; Quiles, J.L.; Battino, M.; et al. Resveratrol and vascular health: Evidence from clinical studies and mechanisms of actions related to its metabolites produced by gut microbiota. Front. Pharmacol. 2024, 15, 1368949. [Google Scholar] [CrossRef]
- Cirilli, I.; Damiani, E.; Dludla, P.V.; Hargreaves, I.; Marcheggiani, F.; Millichap, L.E.; Orlando, P.; Silvestri, S.; Tiano, L. Role of Coenzyme Q10 in Health and Disease: An Update on the Last 10 Years (2010–2020). Antioxidants 2021, 10, 1325. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mollazadeh, H.; Tavana, E.; Fanni, G.; Bo, S.; Banach, M.; Pirro, M.; Von Haehling, S.; Jamialahmadi, T.; Sahebkar, A. Effects of statins on mitochondrial pathways. J. Cachexia Sarcopenia Muscle 2021, 12, 237–251. [Google Scholar] [CrossRef]
- Daei, S.; Ildarabadi, A.; Goodarzi, S.; Mohamadi-Sartang, M. Effect of coenzyme Q10 supplementation on vascular endothelial function: A systematic review and meta-analysis of randomized controlled trials. High Blood Press. Cardiovasc. Prev. 2024, 31, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Borges, J.Y. The Role of Coenzyme Q10 in Cardiovascular Disease Treatment: An Updated 2024 Systematic Review and Meta-Analysis of Prospective Cohort Studies (1990–2024). medRxiv 2024. [Google Scholar] [CrossRef]
- Dohlmann, T.L.; Kuhlman, A.B.; Morville, T.; Dahl, M.; Asping, M.; Orlando, P.; Silvestri, S.; Tiano, L.; Helge, J.W.; Dela, F.; et al. Coenzyme Q10 supplementation in statin treated patients: A double-blinded randomized placebo-controlled trial. Antioxidants 2022, 11, 1698. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Mao, Q.; Cao, J.; Wang, Y.; Zhou, X.; Fan, L. Effects of coenzyme Q10 on vascular endothelial function in humans: A meta-analysis of randomized controlled trials. Atherosclerosis 2012, 221, 311–316. [Google Scholar] [CrossRef]
- Hamilton, S.J.; Chew, G.T.; Watts, G.F. Coenzyme Q10 improves endothelial dysfunction in statin-treated type 2 diabetic patients. Diabetes Care 2009, 32, 810–812. [Google Scholar] [CrossRef]
- Mortensen, S.A.; Rosenfeldt, F.; Kumar, A.; Dolliner, P.; Filipiak, K.J.; Pella, D.; Alehagen, U.; Steurer, G.; Littarru, G.P. The effect of coenzyme Q10 on morbidity and mortality in chronic heart failure. Eur. J. Heart Fail. 2013, 12, S21. [Google Scholar]
- Alehagen, U.; Johansson, P.; Björnstedt, M.; Rosén, A.; Dahlström, U. Cardiovascular mortality and N-terminal-proBNP reduced after combined selenium and coenzyme Q10 supplementation: A 5-year prospective randomized double-blind placebo-controlled trial among elderly Swedish citizens. Int. J. Cardiol. 2013, 167, 1860–1866. [Google Scholar] [CrossRef]
- Alehagen, U.; Aaseth, J.; Johansson, P. Reduced cardiovascular mortality 10 years after supplementation with selenium and coenzyme Q10 for four years: Follow-up results of a prospective randomized double-blind placebo-controlled trial in elderly citizens. PLoS ONE 2015, 10, e0141641. [Google Scholar] [CrossRef]
- Alehagen, U.; Aaseth, J.; Alexander, J.; Johansson, P. Still reduced cardiovascular mortality 12 years after supplementation with selenium and coenzyme Q10 for four years: A validation of previous 10-year follow-up results of a prospective randomized double-blind placebo-controlled trial in elderly. PLoS ONE 2018, 13, e0193120. [Google Scholar] [CrossRef] [PubMed]
- Vrentzos, E.; Ikonomidis, I.; Pavlidis, G.; Katogiannis, K.; Korakas, E.; Kountouri, A.; Pliouta, L.; Michalopoulou, E.; Pelekanou, E.; Boumpas, D.; et al. Six-month supplementation with high dose coenzyme Q10 improves liver steatosis, endothelial, vascular and myocardial function in patients with metabolic-dysfunction associated steatotic liver disease: A randomized double-blind, placebo-controlled trial. Cardiovasc. Diabetol. 2024, 23, 245. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, İ. Antioxidants: A comprehensive review. Arch. Toxicol. 2025, 99, 1893–1997. [Google Scholar] [CrossRef] [PubMed]
- Trugilho, L.; Alvarenga, L.; Cardozo, L.; Paiva, B.; Brito, J.; Barboza, I.; Almeida, J.; Dos Anjos, J.; Khosla, P.; Ribeiro-Alves, M.; et al. Effects of Tocotrienol on Cardiovascular Risk Markers in Patients with Chronic Kidney Disease: A Randomized Controlled Trial. J. Nutr. Metab. 2025, 2025, 8482883. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Samsam Shariat, S.Z.; Mostafavi, S.A.; Khakpour, F. Antioxidant effects of vitamins C and e on the low-density lipoprotein oxidation mediated by myeloperoxidase. Iran. Biomed. J. 2013, 17, 22–28. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dludla, P.V.; Nkambule, B.B.; Nyambuya, T.M.; Ziqubu, K.; Mabhida, S.E.; Mxinwa, V.; Mokgalaboni, K.; Ndevahoma, F.; Hanser, S.; Mazibuko-Mbeje, S.E.; et al. Vitamin C intake potentially lowers total cholesterol to improve endothelial function in diabetic patients at increased risk of cardiovascular disease: A systematic review of randomized controlled trials. Front. Nutr. 2022, 9, 1011002. [Google Scholar] [CrossRef]
- Ashor, A.W.; Siervo, M.; Lara, J.; Oggioni, C.; Afshar, S.; Mathers, J.C. Effect of vitamin C and vitamin E supplementation on endothelial function: A systematic review and meta-analysis of randomised controlled trials. Br. J. Nutr. 2015, 113, 1182–1194. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Li, J.; Yuan, Z. Effect of antioxidant vitamin supplementation on cardiovascular outcomes: A meta-analysis of randomized controlled trials. PLoS ONE 2013, 8, e56803. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lonn, E.; Bosch, J.; Yusuf, S.; Sheridan, P.; Pogue, J.; Arnold, J.M.; Ross, C.; Arnold, A.; Sleight, P.; Probstfield, J.; et al. Effects of long-term vitamin E supplementation on cardiovascular events and CancerA randomized controlled trial. J. Am. Med. Assoc. 2005, 293, 1338–1347. [Google Scholar]
- Lee, I.M.; Cook, N.R.; Gaziano, J.M.; Gordon, D.; Ridker, P.M.; Manson, J.E.; Hennekens, C.H.; Buring, J.E. Vitamin E in the primary prevention of cardiovascular disease and cancer: The Women’s Health Study: A randomized controlled trial. JAMA 2005, 294, 56–65. [Google Scholar] [CrossRef]
- Miller III, E.R.; Pastor-Barriuso, R.; Dalal, D.; Riemersma, R.A.; Appel, L.J.; Guallar, E. Meta-analysis: High-dosage vitamin E supplementation may increase all-cause mortality. Ann. Intern. Med. 2005, 142, 37–46. [Google Scholar] [CrossRef]
- Simon, J.A. Combined vitamin E and vitamin C supplement use and risk of cardiovascular disease mortality. Arch. Intern. Med. 2002, 162, 2630. [Google Scholar] [CrossRef]
- Koltermann, A. Influence of Ginkgo Biloba Extract EGb 761 on Signaling Pathways in Endothelial Cells. Ph.D. Dissertation, Ludwig-Maximilians-University, Munich, Germany, 2008. [Google Scholar] [CrossRef]
- Ma, L.; Liu, X.; Zhao, Y.; Chen, B.; Li, X.; Qi, R. Ginkgolide B reduces LOX-1 expression by inhibiting Akt phosphorylation and increasing Sirt1 expression in oxidized LDL-stimulated human umbilical vein endothelial cells. PLoS ONE 2013, 8, e74769. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, J.S.; Huang, P.H.; Wang, C.H.; Lin, F.Y.; Tsai, H.Y.; Wu, T.C.; Lin, S.J.; Chen, J.W. Nrf-2 mediated heme oxygenase-1 expression, an antioxidant-independent mechanism, contributes to anti-atherogenesis and vascular protective effects of Ginkgo biloba extract. Atherosclerosis 2011, 214, 301–309. [Google Scholar] [CrossRef]
- Wu, T.-C.; Chen, J.-S.; Wang, C.-H.; Huang, P.-H.; Lin, F.-Y.; Lin, L.-Y.; Lin, S.-J.; Chen, J.-W. Activation of heme oxygenase-1 by Ginkgo biloba extract differentially modulates endothelial and smooth muscle-like progenitor cells for vascular repair. Sci. Rep. 2019, 9, 17316. [Google Scholar] [CrossRef] [PubMed]
- Gardner, C.D.; Taylor-Piliae, R.E.; Kiazand, A.; Nicholus, J.; Rigby, A.J.; Farquhar, J.W. Effect of Ginkgo biloba (EGb 761) on treadmill walking time among adults with peripheral artery disease: A randomized clinical trial. J. Cardiopulm. Rehabil. Prev. 2008, 28, 258–265. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Wu, Y.; Li, S.; Cui, W.; Zu, X.; Wang, F.; Du, J. Ginkgo biloba extract improves coronary blood flow in patients with coronary artery disease: Role of endothelium-dependent vasodilation. Planta Med. 2007, 73, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Kuller, L.H.; Ives, D.G.; Fitzpatrick, A.L.; Carlson, M.C.; Mercado, C.; Lopez, O.L.; Burke, G.L.; Furberg, C.D.; DeKosky, S.T. Does Ginkgo biloba reduce the risk of cardiovascular events? Circ. Cardiovasc. Qual. Outcomes 2010, 3, 41–47. [Google Scholar] [CrossRef]
- Snitz, B.E.; O’Meara, E.S.; Carlson, M.C.; Arnold, A.M.; Ives, D.G.; Rapp, S.R.; Saxton, J.; Lopez, O.L.; Dunn, L.O.; Sink, K.M.; et al. Ginkgo biloba for preventing cognitive decline in older adults: A randomized trial. JAMA 2009, 302, 2663–2670. [Google Scholar] [CrossRef]
- Morató, X.; Marquié, M.; Tartari, J.P.; Lafuente, A.; Abdelnour, C.; Alegret, M.; Jofresa, S.; Buendía, M.; Pancho, A.; Aguilera, N.; et al. A randomized, open-label clinical trial in mild cognitive impairment with EGb 761 examining blood markers of inflammation and oxidative stress. Sci. Rep. 2023, 13, 5406. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dong, H.; Liu, M.; Rong, L.; Yang, S.; Wang, J. Efficacy and Hemorheology of Ginkgo biloba Extract (EGb 761) in the Treatment of Sudden Sensorineural Hearing Loss: A Retrospective Study. Noise Health 2024, 26, 383–389. [Google Scholar] [CrossRef]
- Ballester, P.; Cerdá, B.; Arcusa, R.; Marhuenda, J.; Yamedjeu, K.; Zafrilla, P. Effect of Ginger on Inflammatory Diseases. Molecules 2022, 27, 7223. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, S.; Song, X.; Gao, H.; Zhang, Y.; Zhou, X.; Wang, F. 6-Gingerol Inhibits Ferroptosis in Endothelial Cells in Atherosclerosis by Activating the NRF2/HO-1 Pathway. Appl. Biochem. Biotechnol. 2025, 197, 3890–3906. [Google Scholar] [CrossRef] [PubMed]
- Morvaridzadeh, M.; Fazelian, S.; Agah, S.; Khazdouz, M.; Rahimlou, M.; Agh, F.; Potter, E.; Heshmati, S.; Heshmati, J. Effect of ginger (Zingiber officinale) on inflammatory markers: A systematic review and meta-analysis of randomized controlled trials. Cytokine 2020, 135, 155224. [Google Scholar] [CrossRef] [PubMed]
- Preciado-Ortiz, M.E.; Gembe-Olivarez, G.; Martínez-López, E.; Rivera-Valdés, J.J. Immunometabolic Effects of Ginger (Zingiber officinale Roscoe) Supplementation in Obesity: A Comprehensive Review. Molecules 2025, 30, 2933. [Google Scholar] [CrossRef]
- Askari, G.; Aghajani, M.; Salehi, M.; Najafgholizadeh, A.; Keshavarzpour, Z.; Fadel, A.; Venkatakrishnan, K.; Salehi-Sahlabadi, A.; Hadi, A.; Pourmasoumi, M. The effects of ginger supplementation on biomarkers of inflammation and oxidative stress in adults: A systematic review and meta-analysis of randomized controlled trials. J. Herb. Med. 2020, 22, 100364. [Google Scholar] [CrossRef]
- Garza, M.C.; Pérez-Calahorra, S.; Rodrigo-Carbó, C.; Sánchez-Calavera, M.A.; Jarauta, E.; Mateo-Gallego, R.; Gracia-Rubio, I.; Lamiquiz-Moneo, I. Effect of aromatic herbs and spices present in the Mediterranean diet on the glycemic profile in type 2 diabetes subjects: A systematic review and meta-analysis. Nutrients 2024, 16, 756. [Google Scholar] [CrossRef]
- Lee, C.H.; Kim, J.H. A review on the medicinal potentials of ginseng and ginsenosides on cardiovascular diseases. J. Ginseng Res. 2014, 38, 161–166. [Google Scholar] [CrossRef]
- Song, X.; Gao, W.; Shi, Y.; Li, J.; Zheng, Z. Panax Ginseng and its derivatives: Promoting angiogenesis in ischemic dis-eases–A mechanistic overview. J. Funct. Foods 2023, 109, 105762. [Google Scholar] [CrossRef]
- Rodthongdee, K.; Watanapa, W.B.; Ruamyod, K.; Semprasert, N.; Nambundit, P.; Kooptiwut, S.; Boontaveekul, L. Ginsenoside Re increases human coronary artery endothelial SKCa current and nitric oxide release via glucocorticoid recep-tor-PI3K-Akt/PKB pathway. J. Ginseng Res. 2025, 49, 523–531. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, C.; Xue, J.; Jin, D.; Tian, L.; Zhao, D.; Li, X.; Qi, W. Ginseng in vascular dysfunction: A review of therapeutic potentials and molecular mechanisms. Phytother. Res. 2022, 36, 857–872. [Google Scholar] [CrossRef]
- Jovanovski, E.; Peeva, V.; Sievenpiper, J.L.; Jenkins, A.L.; Desouza, L.; Rahelic, D.; Sung, M.K.; Vuksan, V. Modulation of en-dothelial function by Korean red ginseng (Panax ginseng CA Meyer) and its components in healthy individuals: A randomized controlled trial. Cardiovasc. Ther. 2014, 32, 163–169. [Google Scholar] [CrossRef]
- Chung, I.M.; Lim, J.W.; Chung, H.Y.; Seo, J.Y.; Shin, G.J.; Park, S.H.; Kim, H. 7. Korean Panax Red Ginseng Improves Endothelial Dysfunction and Arterial Stiffness in Patients with Coronary Artery Disease Probably by Decreasing Rho-Associated Kinase Activity of Peripheral Blood Mononuclear Cells. Artery Res. 2009, 3, 93. [Google Scholar] [CrossRef]
- Cha, T.W.; Kim, M.; Kim, M.; Chae, J.S.; Lee, J.H. Blood pressure-lowering effect of Korean red ginseng associated with decreased circulating Lp-PLA2 activity and lysophosphatidylcholines and increased dihydrobiopterin level in prehy-pertensive subjects. Hypertens. Res. 2016, 39, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, A.; Khalili, N.; Najafi, N.; Hajizadeh-Sharafabad, F. Ginseng supplementation and vascular function: A sys-tematic review and meta-analysis of clinical trials. BMC Complement. Med. Ther. 2025, 25, 259. [Google Scholar] [CrossRef] [PubMed]
- Jafari, A.; Kordkatuli, K.; Mardani, H.; Mehdipoor, F.; Jami, P.B.; Abbastabar, M.; Vakili, M.; Besharat, S.; Alaghi, A. Ginseng supplementation and cardiovascular disease risk factors: A protocol for GRADE-assessed systematic review and dose-response meta-analysis. BMJ Open 2024, 14, e080926. [Google Scholar] [CrossRef]
- Huang, J.; Yi, Q.; Chen, Y.; Li, Y.; Xu, G.; Zhang, J.; Niu, T.; You, Y.; Zou, W.; Qian, S.; et al. Curcumin suppresses oxidative stress via regulation of ROS/NF-κB signaling pathway to protect retinal vascular endothelial cell in diabetic reti-nopathy. Mol. Cell. Toxicol. 2021, 17, 367–376. [Google Scholar] [CrossRef]
- Shahcheraghi, S.H.; Salemi, F.; Peirovi, N.; Ayatollahi, J.; Alam, W.; Khan, H.; Saso, L. Nrf2 regulation by curcumin: Molecular aspects for therapeutic prospects. Molecules 2021, 27, 167. [Google Scholar] [CrossRef]
- Tang, W.W.; Huang, F.F.; Haedi, A.R.; Shi, Q.Y. The effect of curcumin supplementation on endothelial function and blood pressure in patients with metabolic disorders: A meta-analysis of meta-analyses. Prostaglandins Other Lipid Mediat. 2024, 175, 106900. [Google Scholar] [CrossRef]
- Hegde, M.; Girisa, S.; BharathwajChetty, B.; Vishwa, R.; Kunnumakkara, A.B. Curcumin formulations for better bioavailability: What we learned from clinical trials thus far? ACS Omega 2023, 8, 10713–10746. [Google Scholar] [CrossRef]
- Yakubu, J.; Pandey, A.V. Innovative delivery systems for curcumin: Exploring nanosized and conventional formulations. Pharmaceutics 2024, 16, 637. [Google Scholar] [CrossRef]
- Dehzad, M.J.; Ghalandari, H.; Askarpour, M. Curcumin/turmeric supplementation could improve blood pressure and endothelial function: A grade-assessed systematic review and dose–response meta-analysis of randomized controlled trials. Clin. Nutr. ESPEN 2024, 59, 194–207. [Google Scholar] [CrossRef]
- Karimi, A.; Jazani, A.M.; Darzi, M.; Azgomi, R.N.; Vajdi, M. Effects of curcumin on blood pressure: A systematic review and dose-response meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2023, 33, 2089–2101. [Google Scholar] [CrossRef]
- Gimblet, C.J.; Kruse, N.T.; Geasland, K.; Michelson, J.; Sun, M.; Ten Eyck, P.; Linkenmeyer, C.; Mandukhail, S.R.; Rossman, M.J.; Sambharia, M.; et al. Curcumin supplementation and vascular and cognitive function in chronic kidney disease: A randomized controlled trial. Antioxidants 2024, 13, 983. [Google Scholar] [CrossRef]
- Lazo, O.L.; White, P.F.; Lee, C.; Eng, H.C.; Matin, J.M.; Lin, C.; Del Cid, F.; Yumul, R. Use of herbal medication in the perioperative period: Potential adverse drug interactions. J. Clin. Anesth. 2024, 95, 111473. [Google Scholar] [CrossRef]
- Chow, S.L.; Bozkurt, B.; Baker, W.L.; Bleske, B.E.; Breathett, K.; Fonarow, G.C.; Greenberg, B.; Khazanie, P.; Leclerc, J.; Morris, A.A.; et al. Complementary and alternative medicines in the management of heart failure: A scientific statement from the American Heart Association. Circulation 2023, 147, e4–e30. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kang, S.; Go, G.W. Exploring the multifaceted role of ginkgolides and bilobalide from Ginkgo biloba in mitigating metabolic disorders. Food Sci. Biotechnol. 2024, 33, 2903–2917. [Google Scholar] [CrossRef] [PubMed]
- Vogensen, S.B.; Strømgaard, K.; Shindou, H.; Jaracz, S.; Suehiro, M.; Ishii, S.; Shimizu, T.; Nakanishi, K. Preparation of 7-substituted ginkgolide derivatives: Potent platelet activating factor (PAF) receptor antagonists. J. Med. Chem. 2003, 46, 601–608. [Google Scholar] [CrossRef] [PubMed]
- National Center for Complementary and Integrative Health (NCCIH). Ginkgo: Usefulness and Safety. Updated February 2025. Available online: https://www.nccih.nih.gov/health/ginkgo (accessed on 8 October 2025).
- Hatfield, J.; Saad, S.; Housewright, C. Dietary supplements and bleeding. Bayl. Univ. Med. Cent. Proc. 2022, 35, 802–807. [Google Scholar] [CrossRef]
- Stoddard, G.J.; Archer, M.; Shane-McWhorter, L.; Bray, B.E.; Redd, D.F.; Proulx, J.; Zeng-Treitler, Q. Ginkgo and warfarin interaction in a large veterans administration population. AMIA Annu. Symp. Proc. 2015, 2015, 1174. [Google Scholar]
- Mai, N.T.; Hieu, N.V.; Ngan, T.T.; Van Anh, T.; Van Linh, P.; Thu Phuong, N.T. Impact of Ginkgo biloba drug interactions on bleeding risk and coagulation profiles: A comprehensive analysis. PLoS ONE 2025, 20, e0321804. [Google Scholar] [CrossRef]
- Bent, S.; Goldberg, H.; Padula, A.; Avins, A.L. Spontaneous bleeding associated with Ginkgo biloba: A case report and systematic review of the literature. J. Gen. Intern. Med. 2005, 20, 657–661. [Google Scholar] [CrossRef]
- Marx, W.; McKavanagh, D.; McCarthy, A.L.; Bird, R.; Ried, K.; Chan, A.; Isenring, L. The effect of ginger (Zingiber officinale) on platelet aggregation: A systematic literature review. PLoS ONE 2015, 10, e0141119, Correction in PLoS ONE 2015, 10, e0143675. [Google Scholar]
- Modi, M.; Modi, K. Ginger Root. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK565886/ (accessed on 29 August 2025).
- Rubin, D.; Patel, V.; Dietrich, E. Effects of oral ginger supplementation on the INR. Case Rep. Med. 2019, 2019, 8784029. [Google Scholar] [CrossRef]
- Chua, Y.T.; Ang, X.L.; Zhong, X.M.; Khoo, K.S. Interaction between warfarin and Chinese herbal medicines. Singap. Med. J. 2015, 56, 11. [Google Scholar] [CrossRef]
- Mar, P.L.; Gopinathannair, R.; Gengler, B.E.; Chung, M.K.; Perez, A.; Dukes, J.; Ezekowitz, M.D.; Lakkireddy, D.; Lip, G.Y.; Miletello, M.; et al. Drug interactions affecting oral anticoagulant use. Circ. Arrhythmia Electrophysiol. 2022, 15, e007956. [Google Scholar] [CrossRef]
- Drugs.com. Drug Interaction Report: Clopidogrel and Ginger (Professional Version). Available online: https://www.drugs.com/interactions-check.php?drug_list=705-0,1173-0&professional=1 (accessed on 8 October 2025).
- Hussain, Y.; Abdullah Khan, F.; Alsharif, K.F.; Alzahrani, K.J.; Saso, L.; Khan, H. Regulatory effects of curcumin on platelets: An update and future directions. Biomedicines 2022, 10, 3180. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.H.; Nawaz, Z.; Pertani, S.A.; Roomi, A.; Mahmood, H.; Saeed, S.A.; Gilani, A.H. Inhibitory effect of curcumin, a food spice from turmeric, on platelet-activating factor-and arachidonic acid-mediated platelet aggregation through inhibition of thromboxane formation and Ca2+ signaling. Biochem. Pharmacol. 1999, 58, 1167–1172. [Google Scholar] [CrossRef] [PubMed]
- Talasaz, A.H.; McGonagle, B.; HajiQasemi, M.; Ghelichkhan, Z.A.; Sadeghipour, P.; Rashdi, S.; Cuker, A.; Lech, T.; Goldhaber, S.Z.; Jennings, D.L.; et al. Pharmacokinetic and pharmacodynamic interactions between food or herbal products and oral anticoagulants: Evidence review, practical recommendations, and knowledge gaps. In Seminars in Thrombosis and Hemostasis; Thieme Medical Publishers, Inc.: New York, NY, USA, 2024. [Google Scholar]
- Medsafe. Turmeric. Early Warning System. 2018. Available online: https://medsafe.govt.nz/safety/ews/2018/Turmeric.asp (accessed on 8 October 2025).
- Flory, S.; Männle, R.; Frank, J. The inhibitory activity of curcumin on P-glycoprotein and its uptake by and efflux from LS180 cells is not affected by its galenic formulation. Antioxidants 2021, 10, 1826. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.W.; Huang, C.Y.; Yang, S.Y.; Peng, Y.H.; Yu, C.P.; Chao, P.D.; Hou, Y.C. Oral intake of curcumin markedly activated CYP 3A4: In vivo and ex-vivo studies. Sci. Rep. 2014, 4, 6587. [Google Scholar] [CrossRef]
- Nowinski, K.; Chaireti, R. Discrepancies in Recommendations on Pharmacokinetic Drug Interactions for Anticancer Medications and Direct Oral Anticoagulants (DOAC): A Comparative Analysis of Different Clinical Decision Support Systems and Sources. Pharmaceuticals 2025, 18, 1044. [Google Scholar] [CrossRef]
- Cummings, K.C., III; Keshock, M.; Ganesh, R.; Sigmund, A.; Kashiwagi, D.; Devarajan, J.; Grant, P.J.; Urman, R.D.; Mauck, K.F. Preoperative management of surgical patients using dietary supplements: Society for Perioperative Assessment and Quality Improvement (SPAQI) consensus statement. Mayo Clin. Proc. 2021, 96, 1342–1355. [Google Scholar] [CrossRef]
- Yang, Y.M.; Chen, J.Z.; Wang, X.X.; Wang, S.J.; Hu, H.; Wang, H.Q. Resveratrol attenuates thromboxane A2 receptor ago-nist-induced platelet activation by reducing phospholipase C activity. Eur. J. Pharmacol. 2008, 583, 148–155. [Google Scholar] [CrossRef]
- Gresele, P.; Pignatelli, P.; Guglielmini, G.; Carnevale, R.; Mezzasoma, A.M.; Ghiselli, A.; Momi, S.; Violi, F. Resveratrol, at Concentrations Attainable with Moderate Wine Consumption, Stimulates Human Platelet Nitric Oxide Production3. J. Nutr. 2008, 138, 1602–1608. [Google Scholar] [CrossRef] [PubMed]
- Chiba, T.; Kimura, Y.; Suzuki, S.; Tatefuji, T.; Umegaki, K. Trans-resveratrol enhances the anticoagulant activity of warfarin in a mouse model. J. Atheroscler. Thromb. 2016, 23, 1099–1110. [Google Scholar] [CrossRef] [PubMed]
- Shaito, A.; Posadino, A.M.; Younes, N.; Hasan, H.; Halabi, S.; Alhababi, D.; Al-Mohannadi, A.; Abdel-Rahman, W.M.; Eid, A.H.; Nasrallah, G.K.; et al. Potential adverse effects of resveratrol: A literature review. Int. J. Mol. Sci. 2020, 21, 2084. [Google Scholar] [CrossRef] [PubMed]
- Chow, H.S.; Garland, L.L.; Hsu, C.H.; Vining, D.R.; Chew, W.M.; Miller, J.A.; Perloff, M.; Crowell, J.A.; Alberts, D.S. Resveratrol modulates drug-and carcinogen-metabolizing enzymes in a healthy volunteer study. Cancer Prev. Res. 2010, 3, 1168–1175. [Google Scholar] [CrossRef]
- Guthrie, A.R.; Chow, H.H.; Martinez, J.A. Effects of resveratrol on drug-and carcinogen-metabolizing enzymes, implica-tions for cancer prevention. Pharmacol. Res. Perspect. 2017, 5, e00294. [Google Scholar] [CrossRef]
- Aires, V.; Colin, D.J.; Doreau, A.; Di Pietro, A.; Heydel, J.M.; Artur, Y.; Latruffe, N.; Delmas, D. P-glycoprotein 1 affects chemoactivities of resveratrol against human colorectal cancer cells. Nutrients 2019, 11, 2098. [Google Scholar] [CrossRef]
- Javaid, M.; Kadhim, K.; Bawamia, B.; Cartlidge, T.; Farag, M.; Alkhalil, M. Bleeding Risk in Patients Receiving Omega-3 Polyunsaturated Fatty Acids: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. J. Am. Heart Assoc. 2024, 13, e032390. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khan, S.U.; Lone, A.N.; Khan, M.S.; Virani, S.S.; Blumenthal, R.S.; Nasir, K.; Miller, M.; Michos, E.D.; Ballantyne, C.M.; Boden, W.E.; et al. Effect of omega-3 fatty acids on cardiovascular outcomes: A systematic review and meta-analysis. eClinicalMedicine 2021, 38, 100997. [Google Scholar] [CrossRef]
- Chang, J.P.-C.; Tseng, P.-T.; Ze, B.-S. Safety of Supplementation of Omega-3 Polyunsaturated Fatty Acids: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2023, 14, 1326–1336. [Google Scholar] [CrossRef]
- Begtrup, K.M.; Krag, A.E.; Hvas, A.M. No impact of fish oil supplements on bleeding risk: A systematic review. Dan. Med. J. 2017, 64, A5366. [Google Scholar] [PubMed]
- Ouagueni, A.; Al-Zoubi, R.M.; Zarour, A.; Al-Ansari, A.; Bawadi, H. Effects of Omega-3 Polyunsaturated Fatty Acids, Docosahexaenoic Acid and Eicosapentaenoic Acid, on Post-Surgical Complications in Surgical Trauma Patients: Mechanisms, Nutrition, and Challenges. Mar. Drugs 2024, 22, 207. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, J.; Sterpu, I.; Lindqvist, P.G. Does Omega-3 supplementation increase profuse postpartum hemorrhage? A hospital-based register study. Acta Obstet. Gynecol. Scand. 2024, 103, e14987. [Google Scholar] [CrossRef] [PubMed]
- Gross, B.W.; Gillio, M.; Rinehart, C.D.; Lynch, C.A.; Rogers, F.B. Omega-3 Fatty Acid Supplementation and Warfarin: A Lethal Combination in Traumatic Brain Injury. J. Trauma Nurs. 2017, 24, 15–18. [Google Scholar] [CrossRef]
- Ried, K.; Toben, C.; Fakler, P. Effect of Garlic on Serum Lipids: An Updated Meta-Analysis. Nutr. Rev. 2013, 71, 282–299. [Google Scholar] [CrossRef]
- Macan, H.; Uykimpang, R.; Alconcel, M.; Takasu, J.; Razon, R.; Amagase, H.; Niihara, Y. Aged garlic extract may be safe for patients on warfarin therapy. J. Nutr. 2006, 136, 793S–795S. [Google Scholar] [CrossRef]
- Tan, C.S.S.; Lee, S.W.H. Warfarin and Food, Herbal or Dietary Supplement Interactions: A Systematic Review. Br. J. Clin. Pharmacol. 2020, 86, 1165–1182. [Google Scholar] [CrossRef]
- Li, X.; Long, J.; Gao, Q.; Pan, M.; Wang, J.; Yang, F.; Zhang, Y. Nattokinase Supplementation and Cardiovascular Risk Factors: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Rev. Cardiovasc. Med. 2023, 24, 234. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhou, Y.; Fu, Z.; Dou, P.; Yu, C.; Dong, X.; Hong, H.; Luo, Y.; Tan, Y. Decoding nattokinase efficacy: From digestion and absorption to lipid pathway modulation in high-fat diet-induced atherosclerosis. Food Biosci. 2025, 66, 106203. [Google Scholar] [CrossRef]
- Chen, H.; McGowan, E.M.; Ren, N.; Lal, S.; Nassif, N.; Shad-Kaneez, F.; Qu, X.; Lin, Y. Nattokinase: A Promising Alternative in Prevention and Treatment of Cardiovascular Diseases. Biomark. Insights 2018, 13, 1177271918785130. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, H.; Zhang, Q.; Suo, H.; Xu, F.; Huang, W.; Wang, D.O. Nattokinase as a Functional Food Ingredient: Therapeutic Ap-plications and Mechanisms in Age-Related Diseases. Food Sci. Hum. Wellness 2024, 13, 2401–2409. [Google Scholar] [CrossRef]
- Zubiaur, P.; Rodríguez-Antona, C.; Boone, E.C.; Daly, A.K.; Tsermpini, E.E.; Khasawneh, L.Q.; Sangkuhl, K.; Duconge, J.; Botton, M.R.; Savieo, J.; et al. PharmVar GeneFocus: CYP4F2. Clin. Pharmacol. Ther. 2024, 116, 963–975. [Google Scholar] [CrossRef] [PubMed]
- Medsafe. Can Vitamin E Cause Bleeding? 2022. Available online: https://www.medsafe.govt.nz/profs/PUArticles/June2022/Can-Vitamin-E-cause-bleeding.html (accessed on 8 October 2025).
- Pastori, D.; Carnevale, R.; Cangemi, R.; Saliola, M.; Nocella, C.; Bartimoccia, S.; Vicario, T.; Farcomeni, A.; Violi, F.; Pignatelli, P. Vitamin E serum levels and bleeding risk in patients receiving oral anticoagulant therapy: A retrospective cohort study. J. Am. Heart Assoc. 2013, 2, e000364. [Google Scholar] [CrossRef] [PubMed]
- Abrol, R.; Kaushik, R.; Goel, D.; Sama, S.; Kaushik, R.M.; Kala, M. Vitamin E-induced coagulopathy in a young patient: A case report. J. Med. Case Rep. 2023, 17, 107. [Google Scholar] [CrossRef] [PubMed]
- Özkan, M.; Güneş, H. Do the natural and herbal remedies used for fighting against COVID-19 pose a risk for surgical patients? J. Herb. Med. 2024, 46, 100902. [Google Scholar] [CrossRef]
- van Heeswijk, R.P.; Cooper, C.L.; Foster, B.C.; Chauhan, B.M.; Shirazi, F.; Seguin, I.; Phillips, E.J.; Mills, E. Effect of high-dose vitamin C on hepatic cytochrome P450 3A4 activity. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2005, 25, 1725–1728. [Google Scholar] [CrossRef]
- Sharma, Y.; Sumanadasa, S.; Shahi, R.; Woodman, R.; Mangoni, A.A.; Bihari, S.; Thompson, C. Efficacy and safety of vitamin C supplementation in the treatment of community-acquired pneumonia: A systematic review and meta-analysis with trial sequential analysis. Sci. Rep. 2024, 14, 11846. [Google Scholar] [CrossRef]
- Yuan, C.S.; Wei, G.; Dey, L.; Karrison, T.; Nahlik, L.; Maleckar, S.; Kasza, K.; Ang-Lee, M.; Moss, J. Brief communication: American ginseng reduces warfarin’s effect in healthy patients: A randomized, controlled trial. Ann. Intern. Med. 2004, 141, 23–27. [Google Scholar] [CrossRef]
- Malati, C.Y.; Robertson, S.M.; Hunt, J.D.; Chairez, C.; Alfaro, R.M.; Kovacs, J.A.; Penzak, S.R. Influence of Panax ginseng on cytochrome P450 (CYP) 3A and P-glycoprotein (P-gp) activity in healthy participants. J. Clin. Pharmacol. 2012, 52, 932–939. [Google Scholar] [CrossRef] [PubMed]
- Park, J.D. Metabolism and drug interactions of Korean ginseng based on the pharmacokinetic properties of ginseno-sides: Current status and future perspectives. J. Ginseng Res. 2024, 48, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Ma, J.; Li, T.; Xiao, Y.; Zheng, N.; Liu, J.; Gao, Y.; Shao, J.; Jia, L. Global deregulation of ginseng products may be a safety hazard to warfarin takers: Solid evidence of ginseng-warfarin interaction. Sci. Rep. 2017, 7, 5813. [Google Scholar] [CrossRef] [PubMed]
- Sood, B.; Patel, P.; Keenaghan, M. Coenzyme Q10. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK531491/ (accessed on 5 September 2025).
- Engelsen, J.; Nielsen, J.D.; Hansen, K.F. Effect of Coenzyme Q10 and Ginkgo biloba on warfarin dosage in patients on long-term warfarin treatment. A randomized, double-blind, placebo-controlled cross-over trial. Ugeskr. Laeger 2003, 165, 1868–1871. [Google Scholar] [PubMed]
- Volak, L.P.; Ghirmai, S.; Cashman, J.R.; Court, M.H. Curcuminoids inhibit multiple human cytochromes P450, UDP-glucuronosyltransferase, and sulfotransferase enzymes, whereas piperine is a relatively selective CYP3A4 inhibitor. Drug Metab. Dispos. 2008, 36, 1594–1605. [Google Scholar] [CrossRef]
- Sanchez-Fuentes, A.; Rivera-Caravaca, J.M.; Lopez-Galvez, R.; Marin, F.; Roldan, V. Non-vitamin K antagonist oral anticoagulants and drug-food interactions: Implications for clinical practice and potential role of probiotics and prebiotics. Front. Cardiovasc. Med. 2022, 8, 787235. [Google Scholar] [CrossRef]
- Shi, L.; Wang, W.; Jing, C.; Hu, J.; Liao, X. Berberine and health outcomes: An overview of systematic reviews. BMC Complement. Med. Ther. 2025, 25, 147. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Paul, M.; Hemshekhar, M.; Kemparaju, K.; Girish, K.S. Berberine mitigates high glucose-potentiated platelet aggregation and apoptosis by modulating aldose reductase and NADPH oxidase activity. Free Radic. Biol. Med. 2019, 130, 196–205. [Google Scholar] [CrossRef]
- Rui, R.; Yang, H.; Liu, Y.; Zhou, Y.; Xu, X.; Li, C.; Liu, S. Effects of Berberine on Atherosclerosis. Front. Pharmacol. 2021, 12, 764175. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, C.; Wu, Y.-B.; Wang, A.-P.; Jiang, J.-D.; Kong, W.-J. Evaluation of Anticoagulant and Antithrombotic Activities of Berberine: A Focus on the Ameliorative Effect on Blood Hypercoagulation. Int. J. Pharmacol. 2018, 14, 1087–1098. [Google Scholar] [CrossRef]
- Ye, Y.; Liu, X.; Wu, N.; Han, Y.; Wang, J.; Yu, Y.; Chen, Q. Efficacy and Safety of Berberine Alone for Several Metabolic Disorders: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Front. Pharmacol. 2021, 12, 653887. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, Z.; Wang, Y.; Xu, Q.; Ma, J.; Li, X.; Yan, J.; Tian, Y.; Wen, Y.; Chen, T. Berberine and health outcomes: An umbrella review. Phytother Res. 2023, 37, 2051–2066. [Google Scholar] [CrossRef] [PubMed]
- The Therapeutic Effects of Statins and Berberine on the Hyperlipemia. Available online: https://clinicaltrials.gov/study/NCT01697735 (accessed on 20 September 2025).
- Zhuang, W.; Liu, S.; Zhao, X.; Sun, N.; He, T.; Wang, Y.; Jia, B.; Lin, X.; Chu, Y.; Xi, S. Interaction Between Chinese Medicine and Warfarin: Clinical and Research Update. Front. Pharmacol. 2021, 12, 751107. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, T.L.; Yeh, C.C.; Lin, C.S.; Shih, C.C.; Liao, C.C. Effects of red yeast rice prescription (LipoCol Forte) on adverse outcomes of surgery. QJM 2019, 112, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Mazzanti, G.; Moro, P.A.; Raschi, E.; Da Cas, R.; Menniti-Ippolito, F. Adverse reactions to dietary supplements containing red yeast rice: Assessment of cases from the Italian surveillance system. Br. J. Clin. Pharmacol. 2017, 83, 894–908. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Twarużek, M.; Ałtyn, I.; Kosicki, R. Dietary Supplements Based on Red Yeast Rice—A Source of Citrinin? Toxins 2021, 13, 497. [Google Scholar] [CrossRef]
- Rowan, C.G.; Brunelli, S.M.; Munson, J.; Flory, J.; Reese, P.P.; Hennessy, S.; Lewis, J.; Mines, D.; Barrett, J.S.; Bilker, W.; et al. Clinical importance of the drug interaction between statins and CYP3A4 inhibitors: A retrospective cohort study in the Health Improvement Network. Pharmacoepidemiol. Drug Saf. 2012, 21, 494–506. [Google Scholar] [CrossRef]
- Fogacci, F.; Banach, M.; Mikhailidis, D.P.; Bruckert, E.; Toth, P.P.; Watts, G.F.; Reiner, Ž.; Mancini, J.; Rizzo, M.; Mitchenko, O.; et al. Safety of red yeast rice supplementation: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2019, 143, 1–16. [Google Scholar] [CrossRef]
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA); Turck, D.; Bohn, T.; Cámara, M.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Jos, Á.; Mangelsdorf, I.; McNulty, B.; et al. Scientific Opinion on additional scientific data related to the safety of monacolins from red yeast rice submitted pursuant to Article 8 of Regulation (EC) No 1925/2006. EFSA J. 2025, 23, 9276. [Google Scholar] [CrossRef]
- Renaud, D.; Höller, A.; Michel, M. Potential Drug–Nutrient Interactions of 45 Vitamins, Minerals, Trace Elements, and Associated Dietary Compounds with Acetylsalicylic Acid and Warfarin—A Review of the Literature. Nutrients 2024, 16, 950. [Google Scholar] [CrossRef]
- Fang, F.; Yang, H.; Wang, X.; Zhao, T.; Zhao, P.; Liu, X. Extracellular Vesicles in Atherosclerosis: From Pathogenesis to Theranostic Applications. Small 2025, 21, e2504761. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, W.; Wu, Z.; Chen, Y. Diversity of extracellular vesicle sources in atherosclerosis: Role and therapeutic application. Angiogenesis 2025, 28, 34. [Google Scholar] [CrossRef] [PubMed]
| Feature | ASCEND Trial [39] | REDUCE-IT Trial [40,41] | STRENGTH Trial [42] | VITAL Trial [43] |
|---|---|---|---|---|
| Patient Characteristics | Diabetic, no prior CV events | Diabetic with additional CV risk factors or patients with established CVD | High-risk CVD patients, statin-treated | Generally healthy, men ≥ 50 yrs, women ≥ 55 yrs |
| Number of Patients | 15,480 | 8179 | 13,078 | 25,871 |
| Dosage | 1 g/day Omega-3 (EPA 460 mg + DHA 380 mg) and placebo -a subgroup with 100 mg/day Aspirin | 4 g/day Icosapent Ethyl (EPA only) and placebo-mineral oil | 4 g/day Omega-3 (EPA + DHA) and placebo—corn oil | 1 g/day Omega-3 (Omacor, fish oil) with or without Vitamin D3: 2000 IU/day and placebo |
| Follow-up Duration | ~7.4 years | 4.9 years | ~3.5 years | 5.3 years |
| Main Findings | Aspirin reduced CV events Omega-3 showed no CV benefit | Strong evidence supporting this dose of EPA for reducing cardio-vascular events in high-risk patients | Omega-3 showed no CV benefit | Omega-3 showed no reduction in major cardiovascular events, but a modest reduction in myocardial infarction in those with low baseline fish intake |
| Mechanism of action for cardiovascular benefits | Aspirin: antithrombotic Omega-3: the relatively low dose may have been inadequate to produce meaningful cardiovascular benefit | Reduced triglycerides anti-inflammatory improved endothelial function plaque stabilization antioxidant antithrombotic | DHA may counteract EPA’s beneficial effects, so formulation rather than dose explains the lack of cardiovascular benefits | Omega-3: the relatively low dose may have been inadequate to produce meaningful cardiovascular benefit |
| Safety/ Adverse Effects | Increased major bleeding after aspirin intake No major issues with omega-3 | Increased atrial fibrillation risk, a trend toward higher serious bleeding risk | Increased atrial fibrillation risk, gastrointestinal side effects | No major safety concerns |
| Compound | Key Mechanisms/Actions | References |
|---|---|---|
| Omega-3 fatty acids (EPA, DHA) | ↓ Triglycerides, modest ↑ HDL-C | [27] |
| Hepatic de novo lipogenesis and increased postprandial fatty acid oxidation | [39] | |
| ↑ Resolvin = anti-inflammatory effects | [32] | |
| ↓ TxAz synthesis & GPVI modulation, ↓ Ca2+ influx & membrane fluidity | [29,32,36] | |
| Berberine | ↓ Triglycerides and LDL-C, modest ↑ HDL-C | [46] |
| ↓ ROS anti-inflammatory effects | [47] | |
| Improves insulin sensitivity | [45] | |
| Blocks GPIIb/IIIa, modulates PI3Kβ/Ca2+ | [48] | |
| Red yeast rice | Monacolin K acts like a statin = ↓LDL-C | [50] |
| ↓ TXB2 and ↑ antithrombin III | [51] | |
| Garlic | ↓ TC, ↓ TG, ↓ LDL-C slightly ↑ HDL-C | [57] |
| ↓ ROS, ↓ NF-kB, ↑ NO, ↑ H2S ↑ ANP, ↓ SRAA, ↑ VSMC proliferation ⟶ vasodilation and lower blood pressure | [62] | |
| Decrease the absorption of cholesterol, -HMG-CoA Reductase | [53] | |
| Increased secondary bile acids, increase GLP-1 | [61] | |
| Inhibits platelet activation & GP IIb/IIIa binding | [63] | |
| Inhibits fibrinogen binding and platelet shape change | [60] | |
| Nattokinase (NK) | ↓ TG, ↓ LDL-C, ↓ ox LDL, ↑ HDL-C | [188] |
| Down-regulated PXDN and PNLIP | [189] | |
| -HMGcoA reductase, +LPLase | [64] | |
| ↓ ROS, ↓ IL-6 | [65] | |
| ↓ Blood pressure | [71] | |
| ↑ tPA release, ↓ TXA2, ↓ Fibrinogen & clotting factors (VII, VIII) | [67,69,70] | |
| Ginkgo biloba | ↑ NO bioavailability | [114] |
| ↓ ICAM-1, VCAM-1 | [115] | |
| ↓ ROS, ↓cytokines | [116] | |
| ↓ PAF | [150,151] | |
| Ginger | ↓ CRP, ↓ Il-6, ↓ TNFα | [126] |
| ↓ ROS | [124] | |
| ↓ TXA2 GPVI modulation | [157,158] | |
| Ginseng | ↑ NO | [131] |
| ↓ ICAM-1, VCAM-1 | [132] | |
| ↓ GPIIb/IIIa, ↓ TXB2, GPVI modulation | [133] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobre, M.-Z.; Virgolici, B.; Doicin, I.-C.; Vîrgolici, H.; Stanescu-Spinu, I.-I. Navigating the Effects of Anti-Atherosclerotic Supplements and Acknowledging Associated Bleeding Risks. Int. J. Mol. Sci. 2025, 26, 10183. https://doi.org/10.3390/ijms262010183
Dobre M-Z, Virgolici B, Doicin I-C, Vîrgolici H, Stanescu-Spinu I-I. Navigating the Effects of Anti-Atherosclerotic Supplements and Acknowledging Associated Bleeding Risks. International Journal of Molecular Sciences. 2025; 26(20):10183. https://doi.org/10.3390/ijms262010183
Chicago/Turabian StyleDobre, Maria-Zinaida, Bogdana Virgolici, Ioana-Cristina Doicin, Horia Vîrgolici, and Iulia-Ioana Stanescu-Spinu. 2025. "Navigating the Effects of Anti-Atherosclerotic Supplements and Acknowledging Associated Bleeding Risks" International Journal of Molecular Sciences 26, no. 20: 10183. https://doi.org/10.3390/ijms262010183
APA StyleDobre, M.-Z., Virgolici, B., Doicin, I.-C., Vîrgolici, H., & Stanescu-Spinu, I.-I. (2025). Navigating the Effects of Anti-Atherosclerotic Supplements and Acknowledging Associated Bleeding Risks. International Journal of Molecular Sciences, 26(20), 10183. https://doi.org/10.3390/ijms262010183







