Developing Endogenous Autophagy Reporters in Caenorhabditis elegans to Monitor Basal and Starvation-Induced Autophagy
Abstract
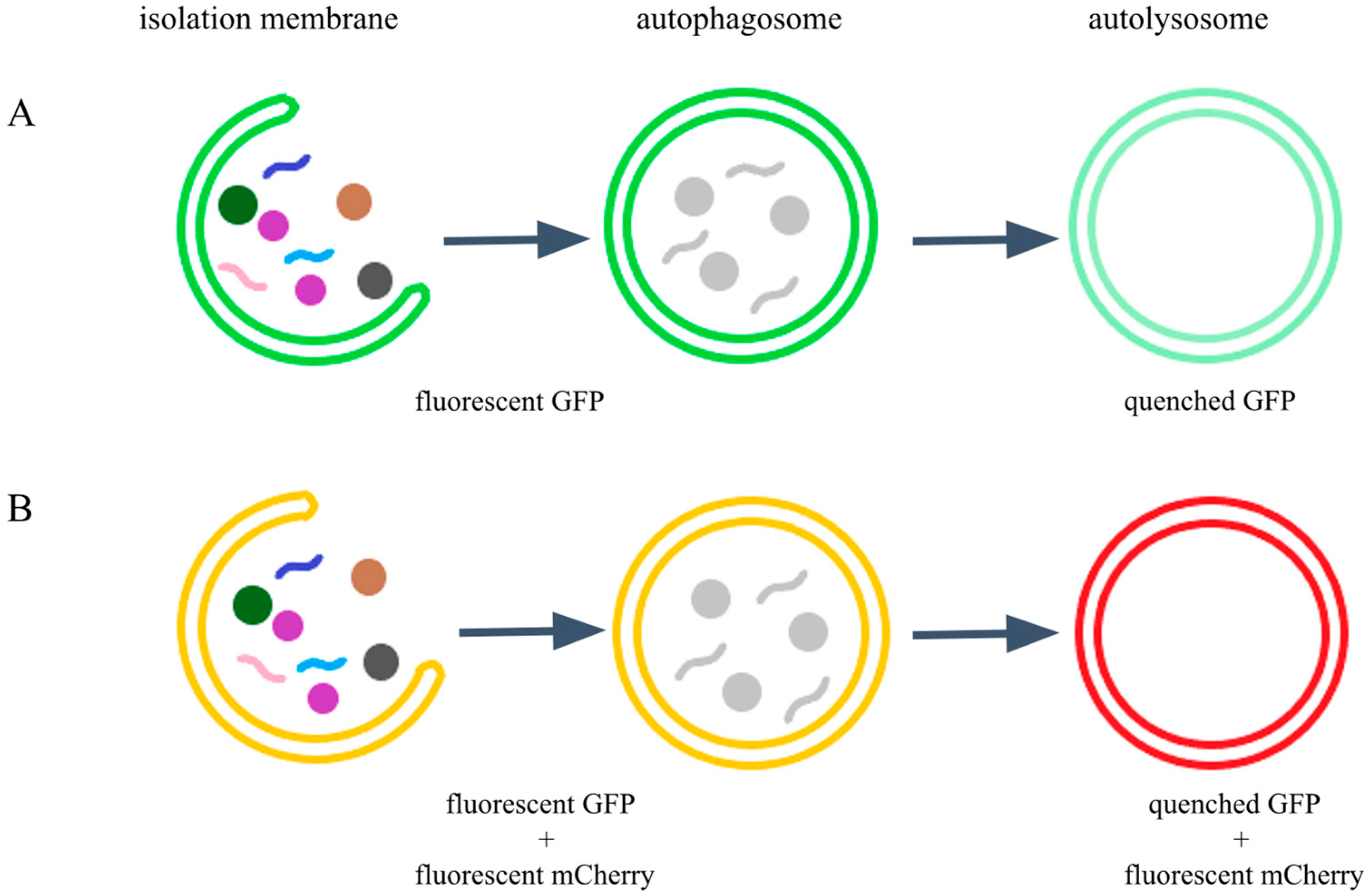
1. Introduction
2. Results
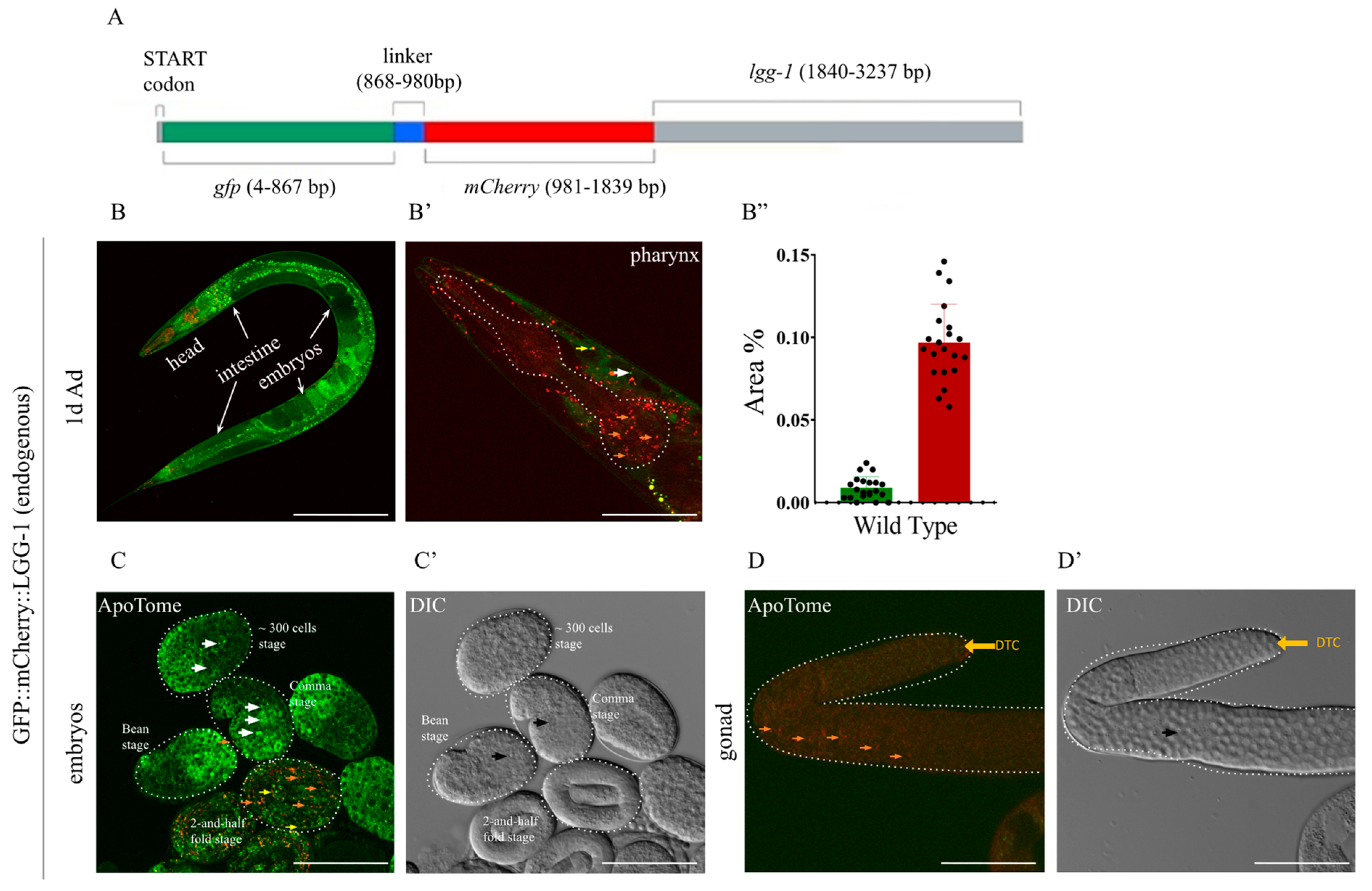
2.1. The Endogenous GFP::mCherry::LGG-1/ATG-8 Reporter Is Expressed Ubiquitously in C. elegans Tissues from Early Embryonic Stages Onward
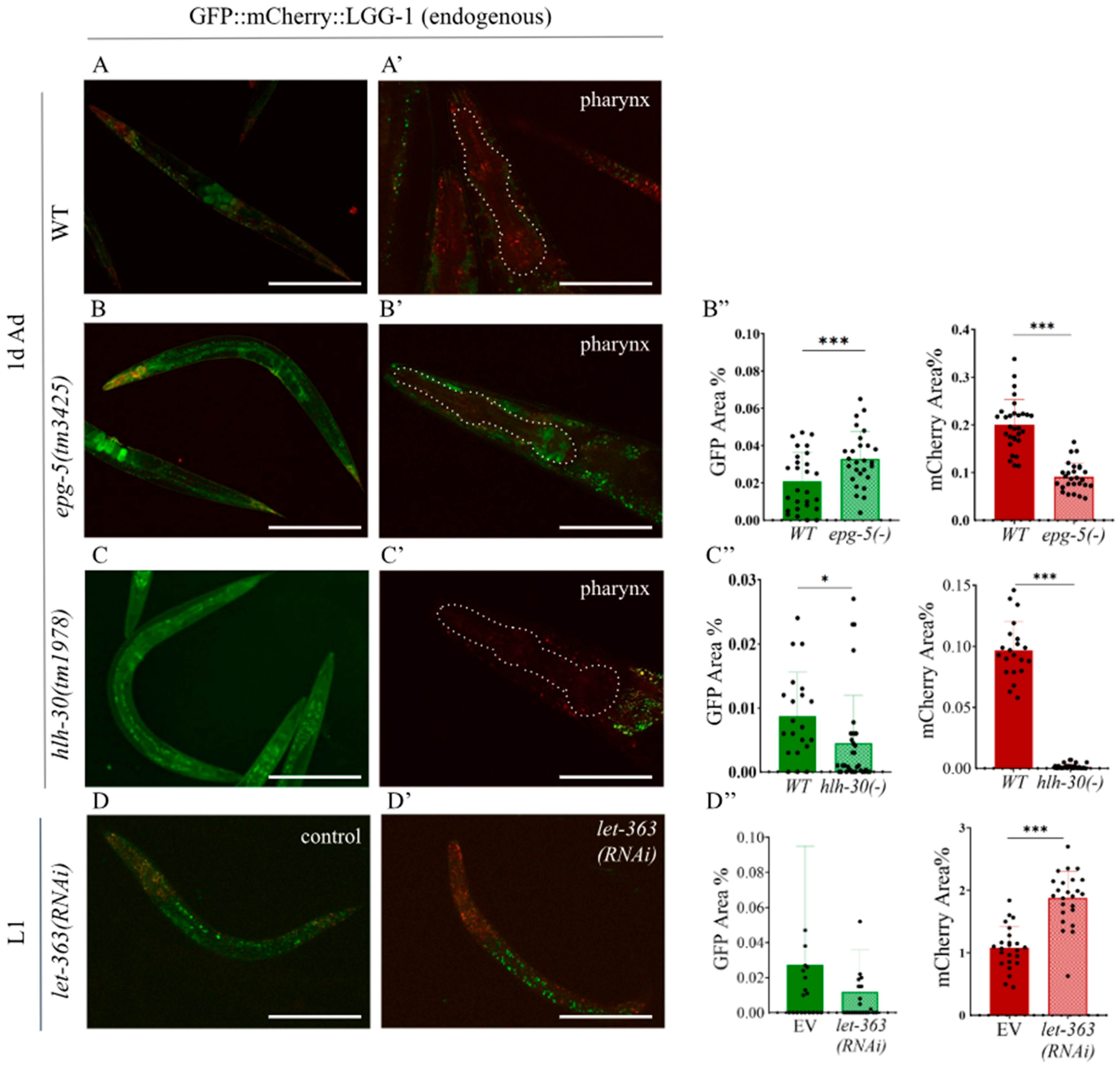
2.2. The Endogenous GFP::mCherry::LGG-1 Reporter Responds to Autophagy-Modulating Stimuli
2.3. Silencing of Let-363/TOR Increases the Number of Autolysosomes in the Endogenous GFP::mCherry::LGG-1 Reporter Strain
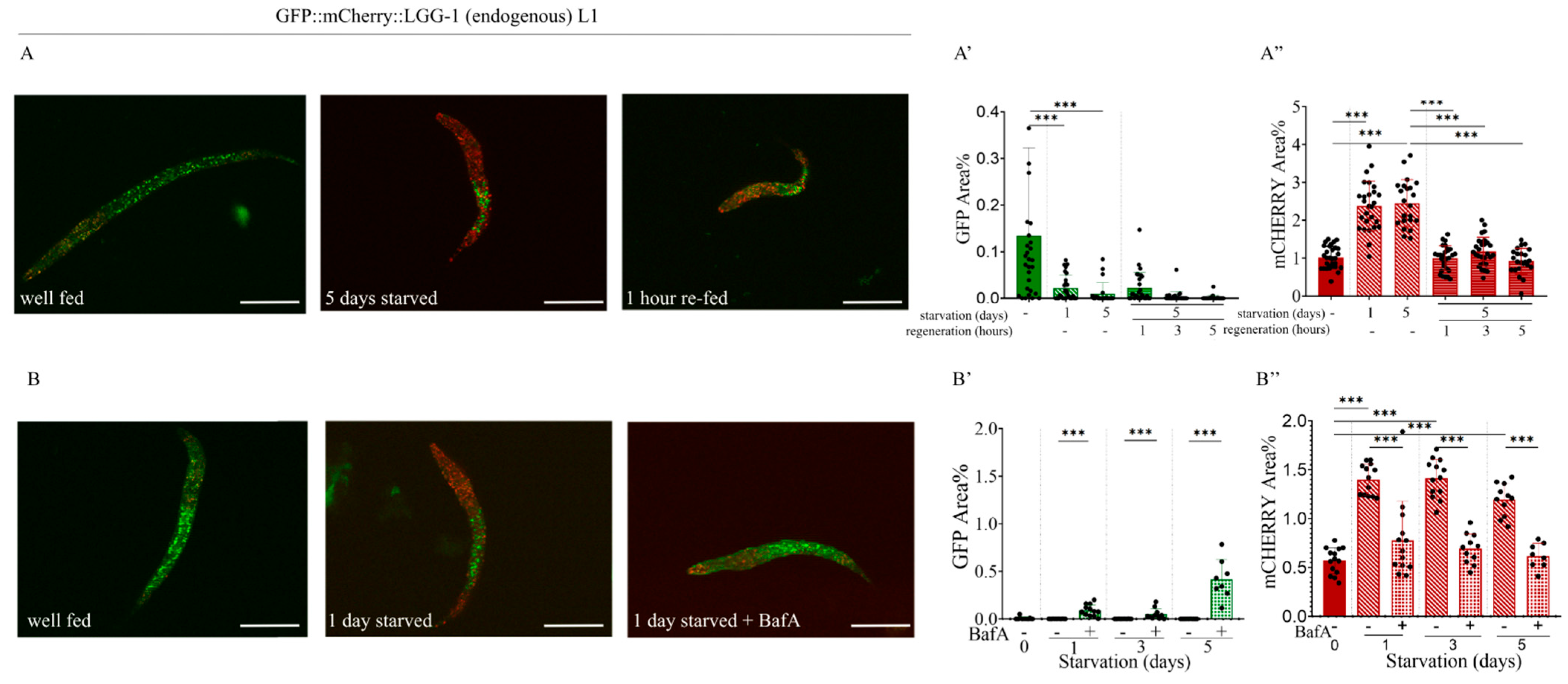
2.4. Starvation Increases the Number of Autolysosomes in L1 Larvae and Adult Animals
2.5. Effect of the Autophagy Inhibitor Bafilomycin A1 on the Expression of the Endogenous GFP::mCherry::LGG-1 Reporter
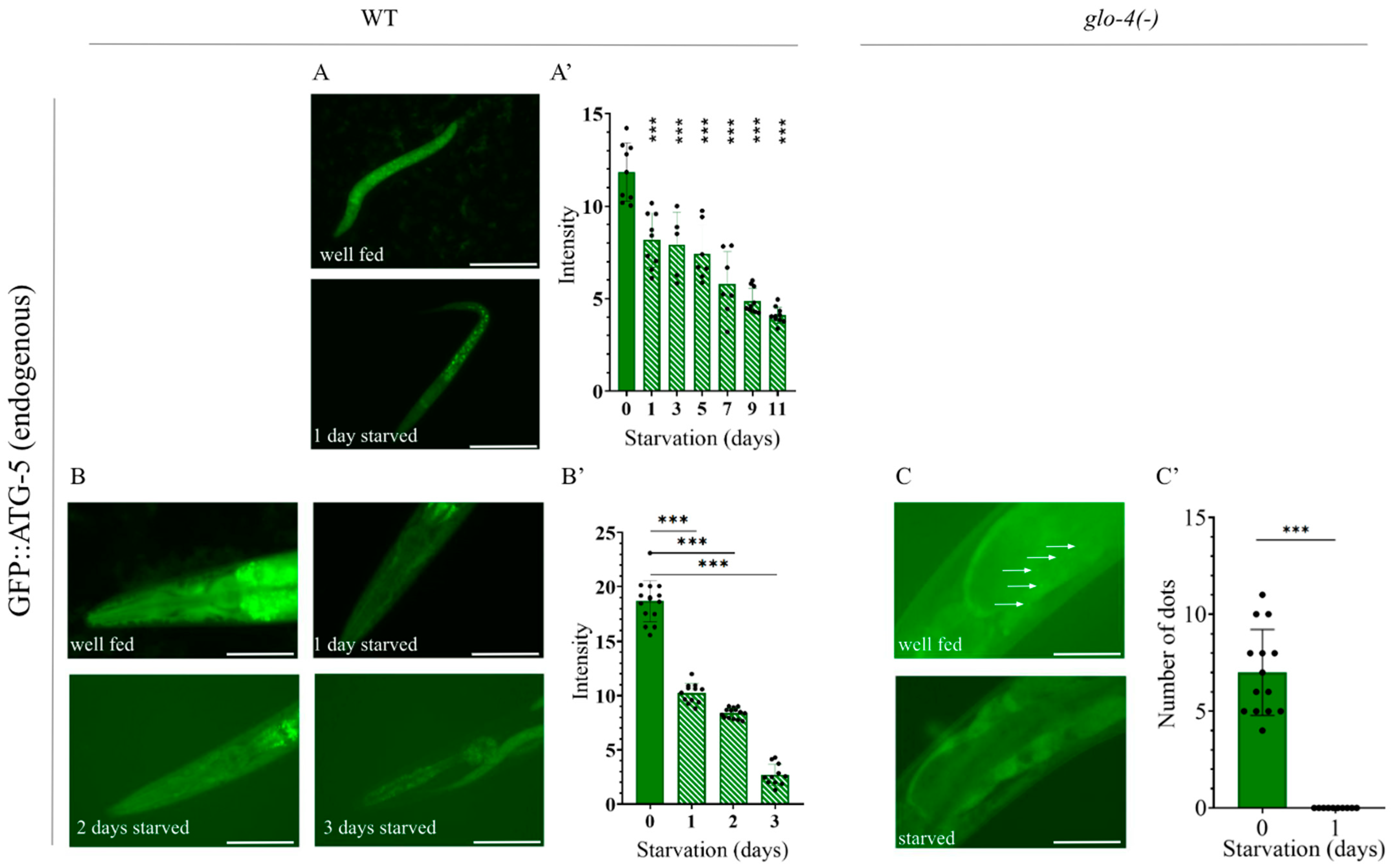
2.6. Analysis of Autophagy Using the Endogenous GFP::ATG-5 Reporter
3. Discussion
4. Materials and Methods
4.1. Strains and Maintenance
4.2. Western Blot Analysis
4.3. Starvation Assay
4.4. RNA Interference
4.5. Bafilomycin A1 Treatment
4.6. Fluorescent Microscopy
4.7. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tóth, M.L.; Sigmond, T.; Borsos, E.; Barna, J.; Erdélyi, P.; Takács-Vellai, K.; Orosz, L.; Kovács, A.L.; Csikós, G.; Sass, M.; et al. Longevity pathways converge on autophagy genes to regulate life span in Caenorhabditis elegans. Autophagy 2008, 4, 330–338. [Google Scholar] [CrossRef]
- Mizushima, N.; Levine, B.; Cuervo, A.M.; Klionsky, D.J. Autophagy fights disease through cellular self-digestion. Nature 2008, 451, 1069–1075. [Google Scholar] [CrossRef]
- Klionsky, D.J. The molecular machinery of autophagy: Unanswered questions. J. Cell Sci. 2005, 118, 7–18. [Google Scholar] [CrossRef]
- Ortega, M.A.; Fraile-Martinez, O.; de Leon-Oliva, D.; Boaru, D.L.; Lopez-Gonzalez, L.; García-Montero, C.; Alvarez-Mon, M.A.; Guijarro, L.G.; Torres-Carranza, D.; Saez, M.A.; et al. Autophagy in Its (Proper) Context: Molecular Basis, Biological Relevance, Pharmacological Modulation, and Lifestyle Medicine. Int. J. Biol. Sci. 2024, 20, 2532–2554. [Google Scholar] [CrossRef] [PubMed]
- Anding, A.L.; Baehrecke, E.H. Cleaning House: Selective Autophagy of Organelles. Dev. Cell 2017, 41, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Mariño, G.; Levine, B. Autophagy and the Integrated Stress Response. Mol. Cell 2010, 40, 280–293. [Google Scholar] [CrossRef]
- Jo, E.K.; Yuk, J.M.; Shin, D.M.; Sasakawa, C. Roles of autophagy in elimination of intracellular bacterial pathogens. Front. Immunol. 2013, 4, 97. [Google Scholar] [CrossRef]
- Pang, Y.; Wu, L.; Tang, C.; Wang, H.; Wei, Y. Autophagy-Inflammation Interplay During Infection: Balancing Pathogen Clearance and Host Inflammation. Front. Pharmacol. 2022, 13, 832750. [Google Scholar] [CrossRef]
- Glick, D.; Barth, S.; Macleod, K.F. Autophagy: Cellular and molecular mechanisms. J. Pathol. 2010, 221, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Aman, Y.; Schmauck-Medina, T.; Hansen, M.; Morimoto, R.I.; Simon, A.K.; Bjedov, I.; Palikaras, K.; Simonsen, A.; Johansen, T.; Tavernarakis, N.; et al. Autophagy in healthy aging and disease. Nat. Aging 2021, 1, 634–650. [Google Scholar] [CrossRef]
- Guo, F.; Liu, X.; Cai, H.; Le, W. Autophagy in neurodegenerative diseases: Pathogenesis and therapy. Brain Pathol. 2017, 28, 3–13. [Google Scholar] [CrossRef]
- Nah, J.; Yuan, J.; Jung, Y.K. Autophagy in neurodegenerative diseases: From mechanism to therapeutic approach. Mol. Cells 2015, 38, 381–389. [Google Scholar] [CrossRef]
- Wong, E.; Cuervo, A.M. Autophagy gone awry in neurodegenerative diseases. Nat. Neurosci. 2010, 13, 805–811. [Google Scholar] [CrossRef]
- Shen, Y.; Li, M.; Wang, K.; Qi, G.; Liu, H.; Wang, W.; Ji, Y.; Chang, M.; Deng, C.; Xu, F.; et al. Diabetic Muscular Atrophy: Molecular Mechanisms and Promising Therapies. Front. Endocrinol. 2022, 13, 917113. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Z.; Ma, Q.; Sun, C. The role of autophagy in fibrosis: Mechanisms, progression and therapeutic potential. Int. J. Mol. Med. 2025, 55, 1–16. [Google Scholar] [CrossRef]
- Palikaras, K.; Lionaki, E.; Tavernarakis, N. Coordination of mitophagy and mitochondrial biogenesis during ageing in C. elegans. Nature 2015, 521, 525–528. [Google Scholar] [CrossRef] [PubMed]
- Korolchuk, V.I.; Mansilla, A.; Menzies, F.M.; Rubinsztein, D.C. Autophagy Inhibition Compromises Degradation of Ubiquitin-Proteasome Pathway Substrates. Mol. Cell 2009, 33, 517–527. [Google Scholar] [CrossRef]
- Chen, Y.; Klionsky, D.J. The regulation of autophagy—Unanswered questions. J. Cell Sci. 2011, 124, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Hurley, J.H.; Young, L.N. Mechanisms of autophagy initiation. Annu. Rev. Biochem. 2017, 86, 225–244. [Google Scholar] [CrossRef]
- Corsi, A.K.; Wightman, B.; Chalfie, M. A transparent window into biology: A primer on Caenorhabditis elegans. Genetics 2015, 200, 387–407, Erratum in: Genetics 2015, 201, 339. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, T.K.; Sewell, A.K.; Wu, Z.; Han, M. TOR signaling in caenorhabditis elegans development, metabolism, and aging. Genetics 2019, 213, 329–360. [Google Scholar] [CrossRef]
- Nakamura, S.; Yoshimori, T. Autophagy and Longevity. Mol. Cells 2018, 41, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Prasher, D.C. Using GFP to see the light. Trends Genet. 1995, 8, 320–323. [Google Scholar] [CrossRef]
- Piatkevich, K.D.; Verkhusha, V.V. Guide to red fluorescent proteins and biosensors for flow cytometry. Methods Cell Biol. 2011, 102, 431–461. [Google Scholar] [CrossRef]
- Palmisano, N.J.; Meléndez, A. Detection of autophagy in Caenorhabditis elegans using GFP::LGG-1 as an autophagy marker. Cold Spring Harb. Protoc. 2016, 2016, 68–75. [Google Scholar] [CrossRef]
- Chen, Y.; Scarcelli, V.; Legouis, R. Approaches for studying autophagy in caenorhabditis elegans. Cells 2017, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.T.; Kumsta, C.; Hellman, A.B.; Adams, L.M.; Hansen, M. Spatiotemporal regulation of autophagy during Caenorhabditis elegans aging. eLife 2017, 6, 1–23. [Google Scholar] [CrossRef]
- Mizushima, N.; Yoshimori, T. Autophagic flux measurement: Cargo degradation versus autophagosome formation. Trends Cell Biol. 2025, 35, 87–98. [Google Scholar] [CrossRef]
- Kimura, S.; Noda, T.; Yoshimori, T. Dissection of the autophagosome maturation process by a novel reporter protein, tandem fluorescent-tagged LC3. Autophagy 2007, 3, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Meléndez, A.; Tallóczy, Z.; Seaman, M.; Eskelinen, E.-L.; Hall, D.H.; Levine, B. Autophagy genes are essential for dauer development and life-span extension in C. elegans. Science 2003, 301, 1387–1391. [Google Scholar] [CrossRef]
- Noma, K.; Jin, Y. Rapid integration of multi-copy transgenes using optogenetic mutagenesis in Caenorhabditis elegans. G3 Genes Genomes Genet. 2018, 8, 2091–2097. [Google Scholar] [CrossRef]
- Chichili, V.P.R.; Kumar, V.; Sivaraman, J. Linkers in the structural biology of protein-protein interactions. Protein Sci. 2013, 22, 153–167. [Google Scholar] [CrossRef]
- Jiang, M.; Liu, K.; Luo, J.; Dong, Z. Autophagy Is a Renoprotective Mechanism During in Vitro Hypoxia and In Vivo Ischemia-Reperfusion Injury. Am. J. Pathol. 2010, 176, 1181–1192. [Google Scholar] [CrossRef]
- Tai, H.; Wang, Z.; Gong, H.; Han, X.; Zhou, J.; Wang, X.; Wei, X.; Ding, Y.; Huang, N.; Qin, J.; et al. Autophagy impairment with lysosomal and mitochondrial dysfunction is an important characteristic of oxidative stress-induced senescence. Autophagy 2017, 13, 99–113. [Google Scholar] [CrossRef]
- Tian, Y.; Li, Z.; Hu, W.; Ren, H.; Tian, E.; Zhao, Y.; Lu, Q.; Huang, X.; Yang, P.; Li, X.; et al. C. elegans Screen Identifies Autophagy Genes Specific to Multicellular Organisms. Cell 2010, 141, 1042–1055. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Meléndez, A. Conserved components of the macroautophagy machinery in Caenorhabditis elegans. Genetics 2025, 229, iyaf007. [Google Scholar] [CrossRef] [PubMed]
- Lapierre, L.R.; Daniel De Magalhaes Filho, C.; McQuary, P.R.; Chu, C.-C.; Visvikis, O.; Chang, J.T.; Gelino, S.; Ong, B.; Davis, A.E.; Irazoqui, J.E.; et al. The TFEB orthologue HLH-30 regulates autophagy and modulates longevity in Caenorhabditis elegans. Nat. Commun. 2013, 4, 1–17. [Google Scholar] [CrossRef]
- Mugume, Y.; Kazibwe, Z.; Bassham, D.C. Target of rapamycin in control of autophagy: Puppet master and signal integrator. Int. J. Mol. Sci. 2020, 21, 8259. [Google Scholar] [CrossRef]
- Long, X.; Spycher, C.; Han, Z.S.; Rose, A.M.; Müller, F.; Avruch, J. TOR Deficiency in C. elegans Causes Developmental Arrest and Intestinal Atrophy by Inhibition of mRNA Translation. Curr. Biol. 2002, 12, 1448–1461. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Yoshimori, T.; Levine, B. Methods in Mammalian Autophagy Research. Cell 2010, 140, 313–326. [Google Scholar] [CrossRef]
- Baugh, L.R.; Hu, P.J. Starvation responses throughout the caenorhabditis elegans life cycle. Genetics 2020, 216, 837–878. [Google Scholar] [CrossRef] [PubMed]
- Mata-Cabana, A.; Romero-Expósito, F.J.; Olmedo, M. Aging during C. elegans L1 quiescence. Aging 2020, 12, 17756–17758. [Google Scholar] [CrossRef]
- Sigmond, T.; Barna, J.; Tóth, M.L.; Takács-Vellai, K.; Pásti, G.; Kovács, A.L.; Vellai, T. Chapter 30 Autophagy in Caenorhabditis elegans. Methods Enzymol. 2008, 451, 521–541. [Google Scholar] [CrossRef]
- Kang, C.; You, N.J.; Avery, L. Dual roles of autophagy in the survival of Caenorhabditis elegans during starvation. Genes Dev. 2007, 21, 2161–2171. [Google Scholar] [CrossRef]
- Xie, Z.; Xie, Y.; Xu, Y.; Zhou, H.; Xu, W.; Dong, Q. Bafilomycin A1 inhibits autophagy and induces apoptosis in MG63 osteosarcoma cells. Mol. Med. Rep. 2014, 10, 1103–1107. [Google Scholar] [CrossRef]
- Yuan, N.; Song, L.; Zhang, S.; Lin, W.; Cao, Y.; Xu, F.; Fang, Y.; Wang, Z.; Zhang, H.; Li, X.; et al. Bafilomycin A1 targets both autophagy and apoptosis pathways in pediatric B-cell acute lymphoblastic leukemia. Haematologica 2015, 100, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Zhou, X.J.; Zhang, H. Exploring the role of autophagy-related gene 5 (ATG5) yields important insights into autophagy in autoimmune/autoinflammatory diseases. Front. Immunol. 2018, 9, 2334. [Google Scholar] [CrossRef] [PubMed]
- Currie, E.; King, B.; Lawrenson, A.L.; Schroeder, L.K.; Kershner, A.M.; Hermann, G.J. Role of the Caenorhabditis elegans multidrug resistance gene, mrp-4, in gut granule differentiation. Genetics 2007, 177, 1569–1582. [Google Scholar] [CrossRef] [PubMed]
- Hermann, G.J.; Schroeder, L.K.; Hieb, C.A.; Kershner, A.M.; Rabbitts, B.M.; Fonarev, P.; Grant, B.D.; Priess, J.R. Genetic analysis of lysosomal trafficking in Caenorhabditis elegans. Mol. Biol. Cell 2005, 16, 3273–3288. [Google Scholar] [CrossRef]
- Barth, J.M.I.; Szabad, J.; Hafen, E.; Köhler, K. Autophagy in Drosophila ovaries is induced by starvation and is required for oogenesis. Cell Death Differ. 2011, 18, 915–924. [Google Scholar] [CrossRef]
- Shi, K.; Tong, C. Analyzing Starvation-Induced Autophagy in the Drosophila melanogaster Larval Fat Body. JoVE 2022, 186, e64282. [Google Scholar] [CrossRef]
- McPhee, C.K.; Baehrecke, E.H. Autophagy in Drosophila melanogaster. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2009, 1793, 1452–1460. [Google Scholar] [CrossRef]
- Mizushima, N.; Yamamoto, A.; Matsui, M.; Yoshimori, T.; Ohsumi, Y. In Vivo Analysis of Autophagy in Response to Nutrient Starvation Using Transgenic Mice Expressing a Fluorescent Autophagosome Marker. Mol. Biol. Cell 2004, 15, 1101–1111. [Google Scholar] [CrossRef]
- Chapin, H.C.; Okada, M.; Merz, A.J.; Miller, D.L. Tissue-specific autophagy responses to aging and stress in C. elegans. Aging 2015, 6, 419–434. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.Y.; Hansen, M.; Kumsta, C. Molecular Mechanisms of Autophagy Decline during Aging. Cells 2024, 13, 1364. [Google Scholar] [CrossRef] [PubMed]
- Redman, M.; King, A.; Watson, C.; King, D. What is CRISPR/Cas9? Arch. Dis. Child. Educ. Pract. Ed. 2016, 101, 213–215. [Google Scholar] [CrossRef]
- Scott, R.C.; Schuldiner, O.; Neufeld, T.P. Role and Regulation of Starvation-Induced Autophagy in the Drosophila Fat Body Internal Reserve of Nutrients. In This Catabolic Process, Cytoplasm is Engulfed Within Double-Membrane Vesicles Known as Autophagosomes, Which Subsequently Fuse. 2004. Available online: https://www.cell.com/developmental-cell/pdf/S1534-5807(04)00245-X.pdf (accessed on 9 July 2004).
- Kuma, A.; Hatano, M.; Matsui, M.; Yamamoto, A.; Nakaya, H.; Yoshimori, T.; Ohsumi, Y.; Tokuhisa, T.; Mizushima, N. The role of autophagy during the early neonatal starvation period. Nature 2004, 432, 1032–1036. [Google Scholar] [CrossRef]
- Conte, D.; MacNei, L.T.; Walhout, A.J.M.; Mello, C.C. RNA Interference in Caenorhabditis elegans. Curr. Protoc. Mol. Biol. 2015, 109, 26.3.1–26.3.30. [Google Scholar] [CrossRef] [PubMed]
- Sturm, Á.; Saskoï, É.; Tibor, K.; Weinhardt, N.; Vellai, T. Highly efficient RNAi and Cas9-based auto-cloning systems for C. Elegans research. Nucleic Acids Res. 2018, 46, 17. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bördén, K.; Vellai, T.; Sigmond, T. Developing Endogenous Autophagy Reporters in Caenorhabditis elegans to Monitor Basal and Starvation-Induced Autophagy. Int. J. Mol. Sci. 2025, 26, 10178. https://doi.org/10.3390/ijms262010178
Bördén K, Vellai T, Sigmond T. Developing Endogenous Autophagy Reporters in Caenorhabditis elegans to Monitor Basal and Starvation-Induced Autophagy. International Journal of Molecular Sciences. 2025; 26(20):10178. https://doi.org/10.3390/ijms262010178
Chicago/Turabian StyleBördén, Kincső, Tibor Vellai, and Tímea Sigmond. 2025. "Developing Endogenous Autophagy Reporters in Caenorhabditis elegans to Monitor Basal and Starvation-Induced Autophagy" International Journal of Molecular Sciences 26, no. 20: 10178. https://doi.org/10.3390/ijms262010178
APA StyleBördén, K., Vellai, T., & Sigmond, T. (2025). Developing Endogenous Autophagy Reporters in Caenorhabditis elegans to Monitor Basal and Starvation-Induced Autophagy. International Journal of Molecular Sciences, 26(20), 10178. https://doi.org/10.3390/ijms262010178






