Investigation of the Relevance of CYP3A4 Inhibition on the Pharmacokinetics of the Novel P2X3 Antagonist Filapixant: Results of In Vitro Explorations and a Fixed-Sequence Clinical Trial with Itraconazole in Healthy Volunteers
Abstract
1. Introduction
2. Results
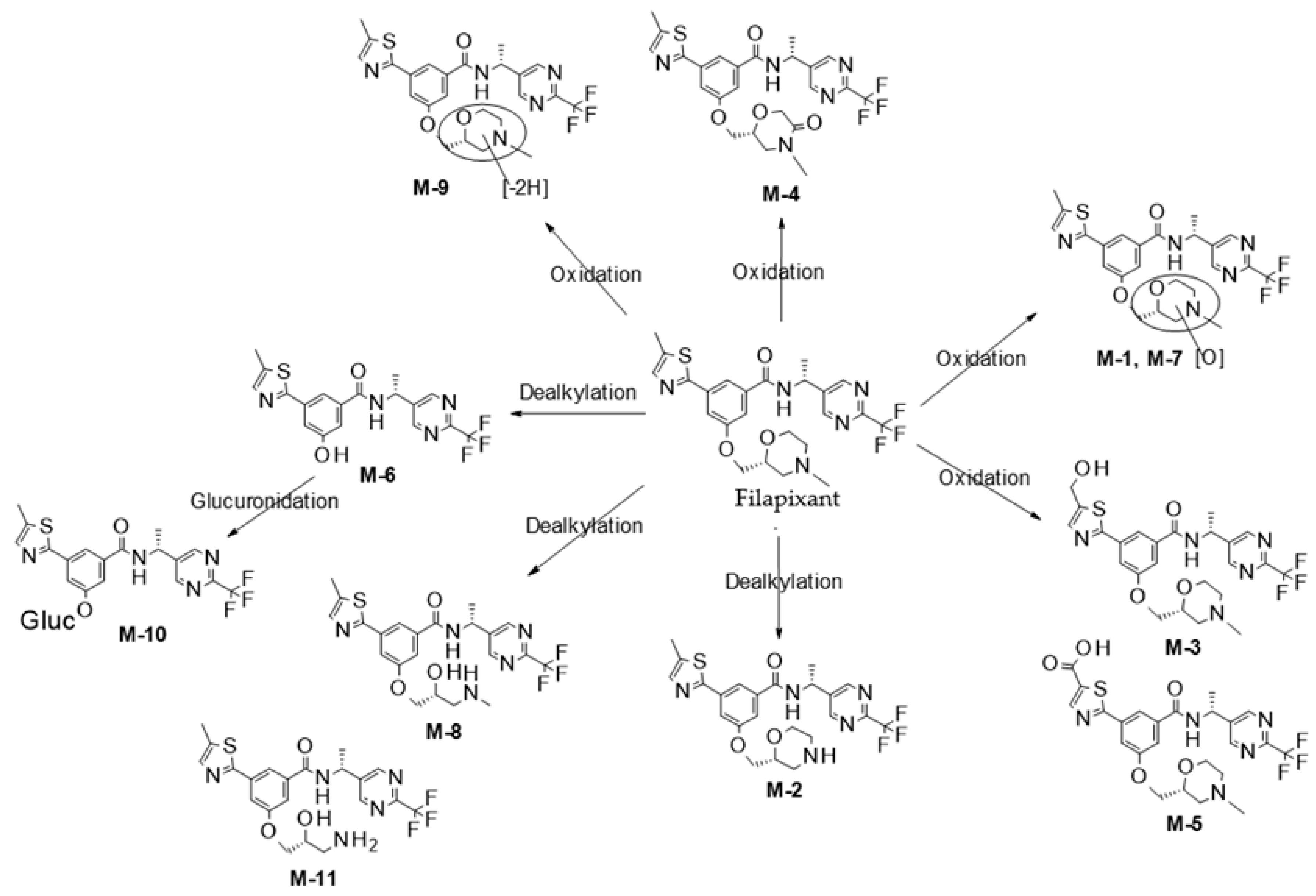
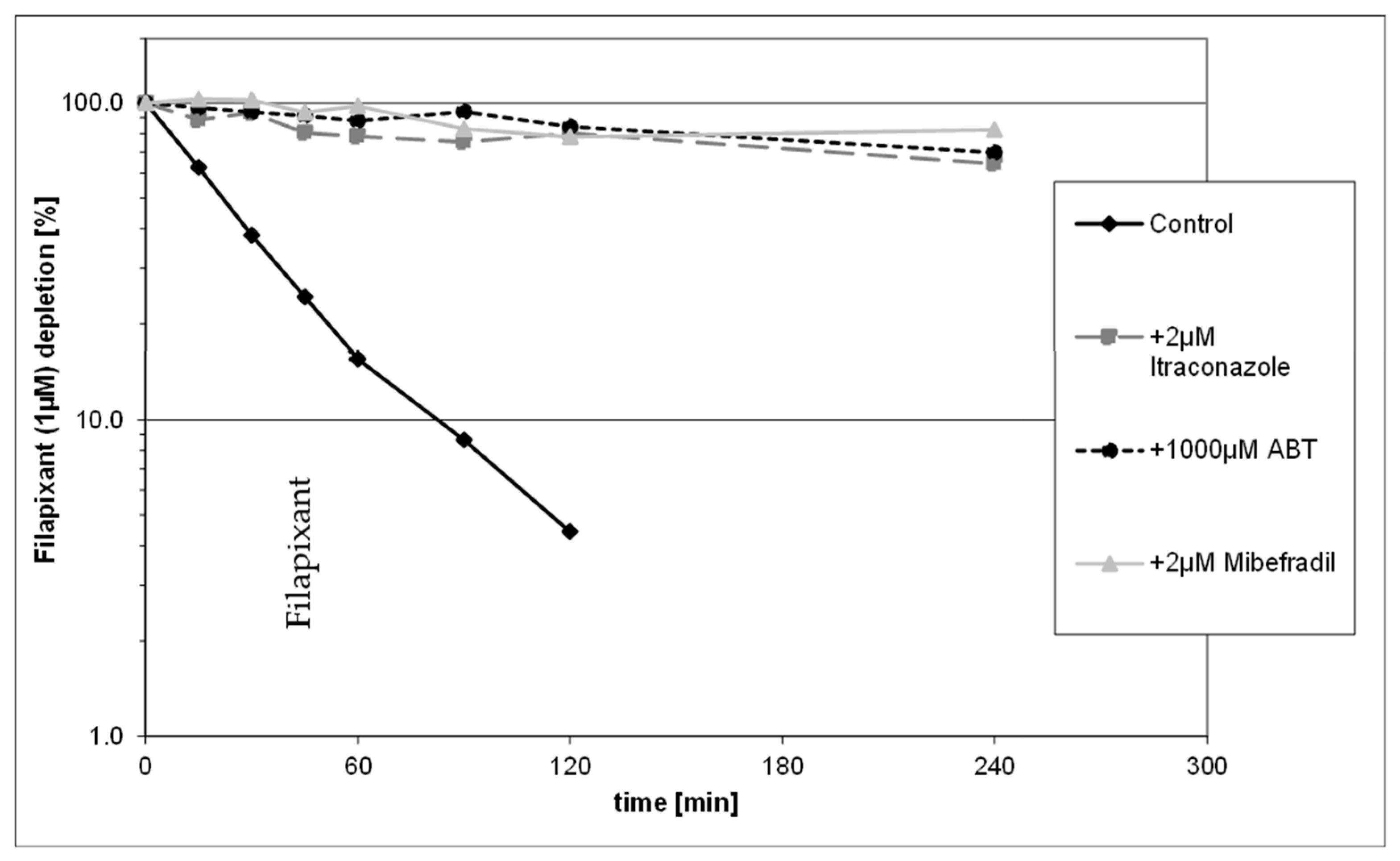
2.1. In Vitro Metabolite Profiling and Enzymes Involved in Metabolism of Filapixant
2.2. Clinical Study
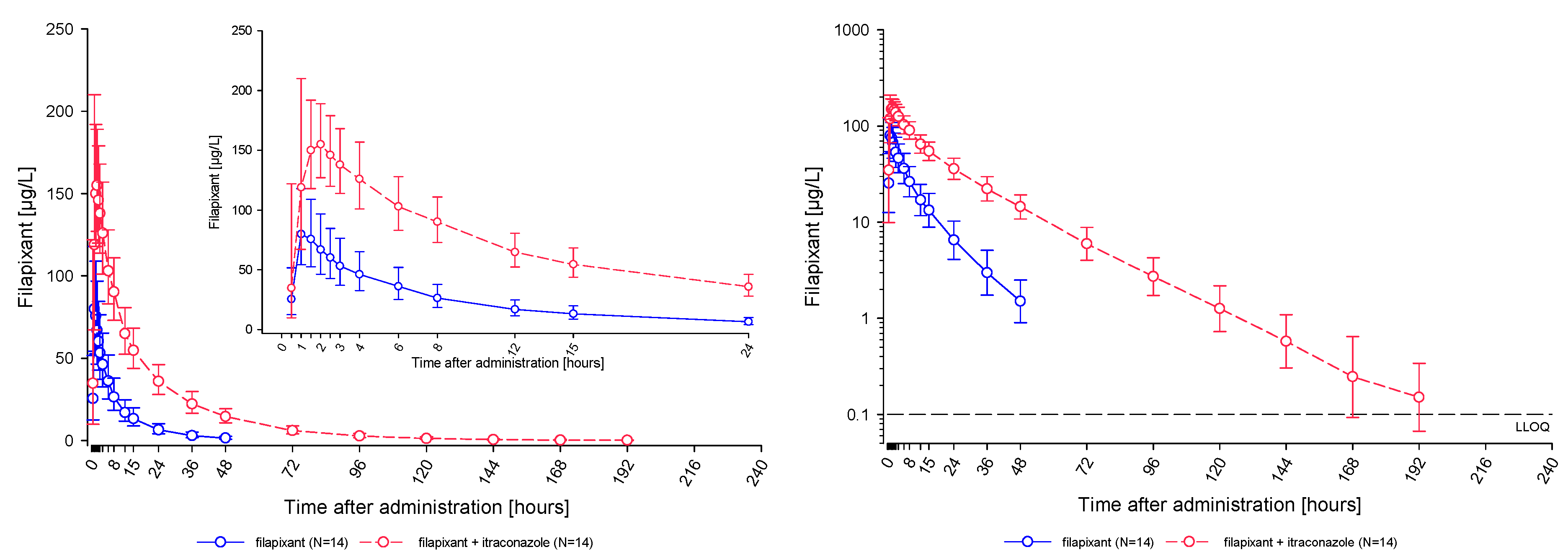
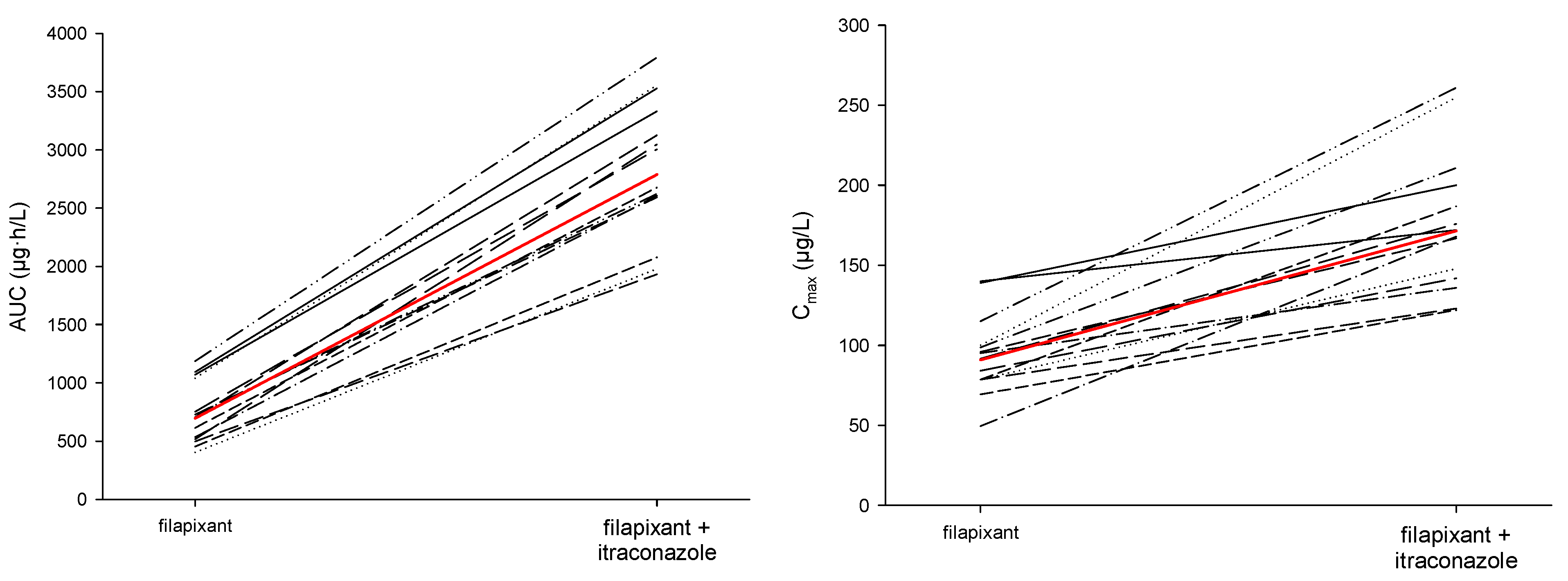
2.2.1. Pharmacokinetic Results
2.2.2. Safety Results
3. Discussion
4. Materials and Methods
4.1. In Vitro Studies Investigating Metabolism and Metabolizing Enzymes of Filapixant
4.1.1. Biotransformation of Filapixant in Human Liver Microsomes and Hepatocytes
4.1.2. CYP Phenotyping Studies in Human Hepatocytes, Liver Microsomes, and Recombinant Human CYP Enzymes
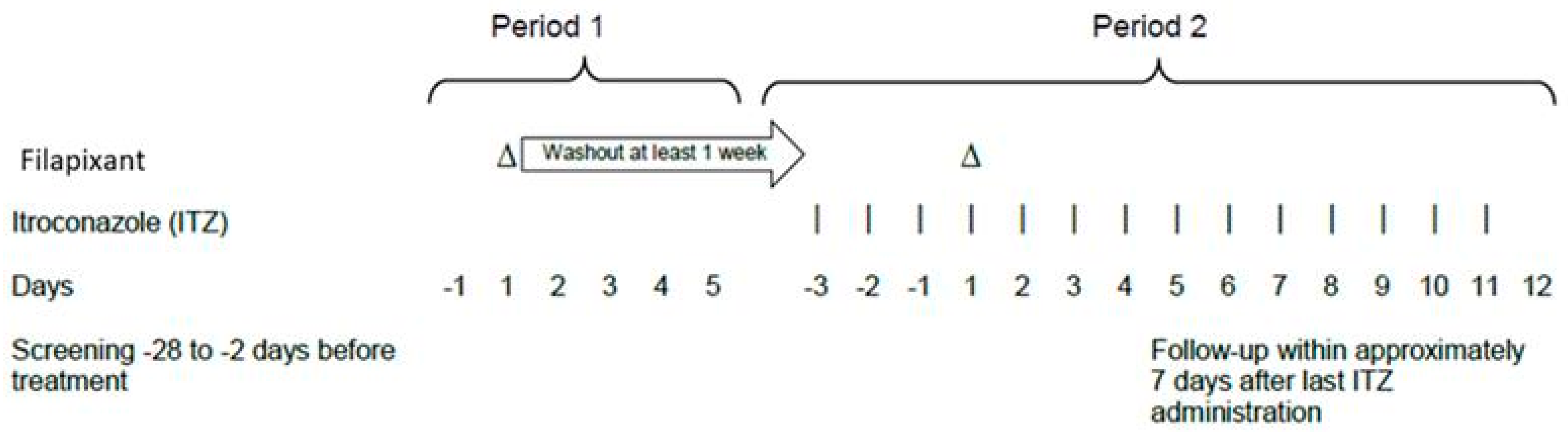
4.2. Clinical Study
5. Conclusions
- Metabolism by CYP3A4 was found to be the main elimination pathway for filapixant in vitro. Drug depletion and formation of metabolites were inhibited in the presence of strong CYP3A4 inhibitors.
- Filapixant was found to be a moderately sensitive substrate of CYP3A4/P-gp. Single oral dosing of 80 mg filapixant concomitantly with multiple daily oral doses of 200 mg itraconazole, a strong CYP3A4/P-gp inhibitor, led to a 1.89-fold higher Cmax and 4.01-fold higher AUC of filapixant compared to the exposure without itraconazole co-administration. Additionally, the geometric mean terminal half-life (t1/2) of filapixant after a single-dose administration was prolonged by co-administration with itraconazole. The changes in both Cmax and half-life, together with the overall change in AUC, indicate that both clearance and bioavailability were affected by co-administration of itraconazole.
- Single dose oral administration of 80 mg filapixant, with and without itraconazole, was well tolerated by all participants.
- For subsequent studies, the co-administration of filapixant at therapeutic doses with strong inhibitors of CYP3A4/P-gp should be avoided, or an appropriate dose adjustment needs to be implemented.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABT | 1-Aminobenzotriazol |
| CI | Confidence interval |
| CLint | Intrinsic clearance |
| CV | Coefficient of variation |
| CYP | Cytochrome P450 |
| CYP3A4 | Cytochrome P450 3A4 |
| GCP | Good Clinical Practice |
| GLP | Good Laboratory Practice |
| HPLC | High-performance liquid chromatography |
| ICH | International Council for Harmonization |
| LLOQ | Lower limit of quantification |
| MS/MS | Tandem mass spectrometry |
| P-gp | P-glycoproteinP |
| PK | Pharmacokinetics |
| QC | Quality control |
| SD | Standard deviation |
| TEAE | Treatment-emergent adverse event |
| ULOQ | Upper limit of quantification |
| Definitions of Pharmacokinetic Terms | |
| AUC | Area under the concentration–time curve from zero to infinity after single dose |
| AUC0 tlast | AUC from time zero to last data point above LLOQ |
| Cmax | Maximum observed drug concentration after single dose administration |
| t1/2 | Half-life associated with the terminal slope |
| tmax | Time to reach Cmax after single dosing |
Appendix A. In- and Exclusion Criteria
Appendix A.1. The Inclusion Criteria
- The informed consent must be signed before any study specific tests or procedures are performed.
- Ability to understand and follow study-related instructions.
- Participant must be 18 to 55 years of age (inclusive), at the time of signing the informed consent.
- Male.
- Participant is healthy as determined by the investigator (including assessment of medical history, physical examination, blood pressure, pulse rate, 12-lead electrocardiogram, body temperature, and clinical laboratory).
- Confirmation of the participant’s health insurance coverage at screening.
- Body mass index (BMI) above or equal 18 and below or equal 30.0 kg/m2 at screening.
- Body weight of at least 50 kg at screening.
- Agreement by the participant that he and his female partner of childbearing potential will use an accepted method of contraception for the duration of the study. Note: The parallel application of two of the following methods of contraception is considered adequately reliable:
Appendix A.2. Exclusion Criteria
- History or evidence of any clinically significant cardiovascular, gastrointestinal, endocrine, hematologic, hepatic, immunologic, metabolic, urologic, pulmonary, neurologic, dermatologic, psychiatric, renal, and/or other major disease or malignancy, as judged by the investigator.
- Clinically relevant history of allergic conditions (including drug allergies, asthma, eczema, or anaphylactic reactions, but excluding untreated, asymptomatic, seasonal allergies at the time of dosing).
- Clinically relevant abnormalities in the medical examination (including medical history, physical examination, vital signs, laboratory tests, and electrocardiogram) as judged by the investigator at screening or upon admission to the clinical unit.
- Participant has/had febrile illness or symptomatic, viral, bacterial (including upper respiratory infection), or fungal (non-cutaneous) infection within 1 week prior to admission to the clinical unit.
- History or current condition for which it can be assumed that the absorption, distribution, excretion and effect of the study interventions will be influenced., e.g., history of malabsorption, esophageal reflux, peptic ulcer disease, erosive esophagitis frequent, or surgical interventions including cholecystectomy, impaired renal function.
- Any malignant tumor and history thereof.
- Acute or chronic progressive liver diseases, e.g., disturbances of the bilirubin excretion (Dubin-Johnson and Rotor syndromes); disturbances of the bile secretion and the flow (cholestasis); presence or history of liver tumors (benign or malignant); between the subsidence of a viral hepatitis (normalization of liver parameters) and the screening for this study there must be an interval of at least six months.
- Clinically relevant kidney diseases (e.g., glomerulonephritis), or renal injury associated with multisystem diseases/disorders (e.g., systemic lupus erythematosus, diabetic nephropathy); severe renal insufficiency or acute renal failure; a history of a single episode of uncomplicated nephrolithiasis does not prevent participation.
- Known or suspected allergy or hypersensitivity, to filapixant, to itraconazole, or any of their excipients.
- Contraindications to itraconazole (symptoms or history of ventricular dysfunction, heart failure, liver disease).
- History of alcohol or drug abuse within two years prior to screening.
- Any use of systemic or topically active medication or herbal remedies, prescription or non-prescription, within one week prior to the first study drug administration or during the trial until follow-up (occasional use of ibuprofen is permissible). Particularly, this includes drugs that might affect the PK of filapixant, e.g., laxatives, loperamide, metoclopramide, antacids, H2-receptor antagonists, CYP3A4 inhibitors, or inducers.
- Regular use of alimentary supplements, e.g., carnitine products, anabolics, high-dose vitamins.
- Not able or willing to abstain from consumption of the following:
- Caffeine containing beverages or food (e.g., tea, coffee, cola, chocolate, mate) from 24 h before each filapixant administration and on the PK profile days (Day 1 of Period 1 and 2)
- Quinine containing beverages or food (bitter lemon, tonic water) from 1 week before the first study intervention administration until the last PK blood sample
- Food and beverages containing furanocoumarin derivatives (e.g., grapefruit, pomelos, limes, Seville oranges) from 1 week before the first study intervention administration until the last PK blood sample
- Poppy seeds containing food from 1 week before the first study intervention administration until last PK blood sample
- History of drinking more than 14 units of alcohol per week (1 unit = 10 g pure alcohol), i.e., on average approximately 20 g/day, or 400 mL of beer [5%], or 60 mL of spirits [35%], or 170 mL of wine [12%]) within three months prior to admission to the clinical unit.
- Smoking more than 10 cigarettes per day, and not willing or able to abstain from smoking from 10 h before until 8 h after filapixant administration.
- Significant blood loss, e.g., by donation of more than 100 mL of whole blood or plasma within four weeks or 500 mL whole blood within three months before the first study intervention administration until follow-up.
- Plasmapheresis within four weeks prior to screening and until the follow-up visit.
- PQ > 220 ms, QT interval corrected using Bazett’s formula (QTcB) > 450 ms, QRS >120 ms.
- Pulse rate <50 or >90 bpm (a lower heart rate between 50 bpm and 45 bpm is acceptable in case of normal thyroid function and absence of symptoms of bradycardia), systolic blood pressure <100 or >140 mmHg, and blood pressure <60 or >90 mmHg.
- Clinically relevant findings in the physical examination.
- Poor venous access.
- Clinically relevant abnormalities in the laboratory parameters at screening and upon admission to the clinical unit. In particular:
- -
- ALT, aspartate aminotransferase (AST), or Bilirubin outside normal ranges;
- -
- Thyroid stimulating hormone (TSH) > the ULN;
- -
- Positive results for hepatitis B virus surface antigen (HBsAg), hepatitis C virus antibodies (anti-HCV), and human immune deficiency virus antigen-/antibody (anti-HIV 1 + 2).
- Positive urine drug screening.
- Positive alcohol breath test.
- Previous assignment to study intervention during this study.
- Previous (within two months prior to administration) or concomitant participation in another clinical study.
- Participant is in custody by order of an authority or a court of law.
- Close affiliation with the investigational site, e.g., a close relative of the investigator or a dependent person (e.g., employee or student of the investigation site).
- Participant is an employee of Bayer AG.
- Criteria which in the opinion of the investigator preclude participation for scientific reasons, for reasons of compliance, or for reasons of the participant’s safety.
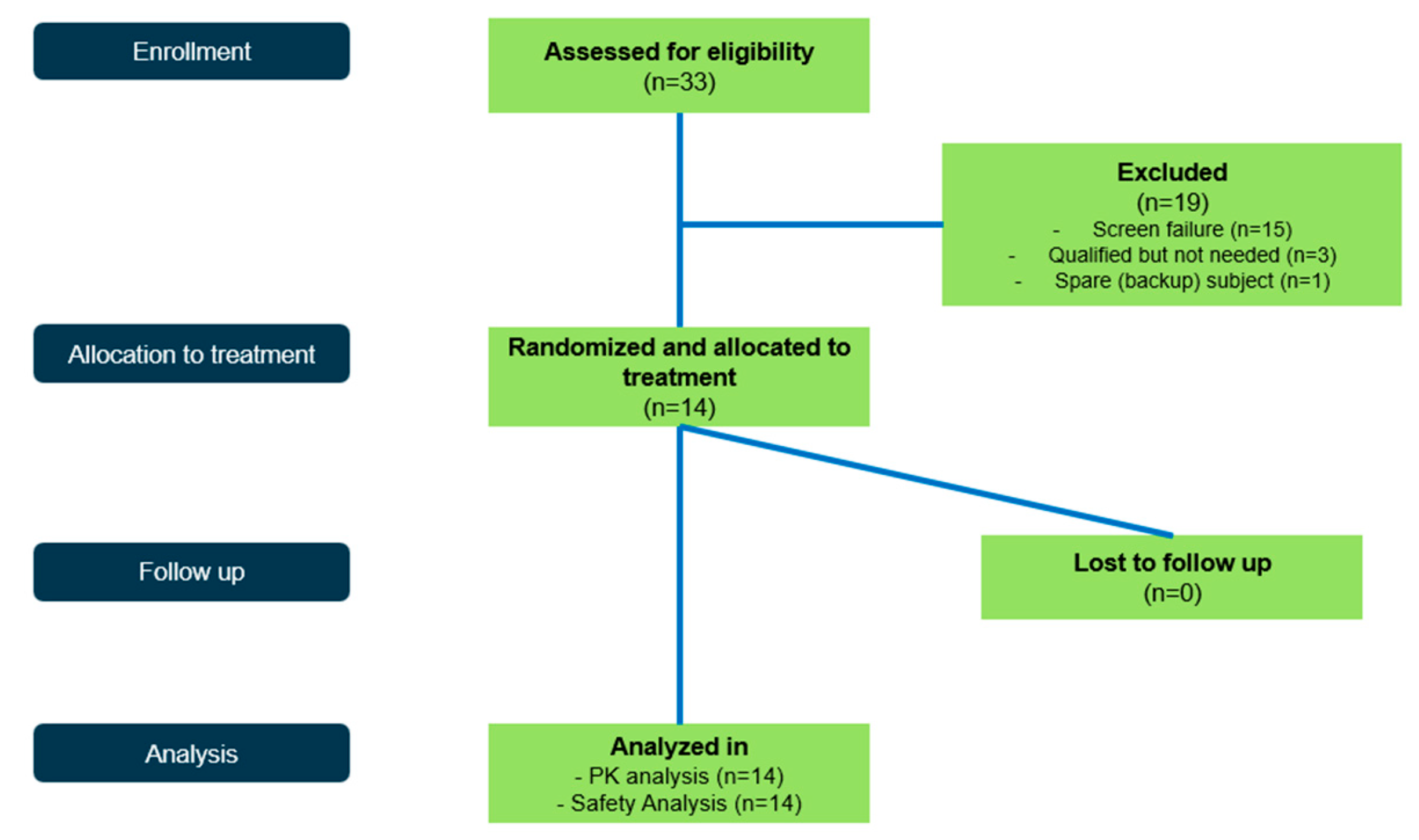
Appendix B. Participant Disposition
Appendix C. Human Liver Microsome and Hepatocyte Data
| Filapixant/Metabolite | Liver Microsomes a,b | Hepatocytes c |
|---|---|---|
| Filapixant | 45 | 68–87 |
| M-1 | 1.8 | 2.6–3.6 |
| M-2 | 15 | 1.7–7.6 |
| M-3 | 0.9 | 0.4–0.8 |
| M-4 | 7.6 | 1.0–7.5 |
| M-5 | n.d. | 0–0.2 |
| M-6 | 4.4 | n.d. |
| M-7 | 9.1 | 2.3–3.2 |
| M-8 | 2.4 | 0–1.7 |
| M-9 | 6.7 | 0–1.3 |
| M-10 | 0.2–0.9 | |
| M-11 | 0–0.3 |
References
- Morice, A.; Smith, J.A.; McGarvey, L.; Birring, S.S.; Parker, S.M.; Turner, A.; Hummel, T.; Gashaw, I.; Fels, L.; Klein, S.; et al. Eliapixant (BAY 1817080), a P2X3 receptor antagonist, in refractory chronic cough: A randomised, placebo-controlled, crossover phase 2a study. Eur. Respir. J. 2021, 58, 2004240. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, C.; Francke, K.; Birring, S.S.; Van Den Berg, J.W.K.; Marsden, P.A.; McGarvey, L.; Turner, A.M.; Wielders, P.; Gashaw, I.; Klein, S.; et al. The P2X3 Receptor Antagonist Filapixant in Patients with Refractory Chronic Cough: A Randomized Controlled Trial. Respir. Res. 2023, 24, 109. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, C.; Singh, D.; Francke, K.; Klein, S.; Hetzel, T.; Zolk, O.; Gashaw, I.; Scheerans, C.; Morice, A. Pharmacodynamics, Pharmacokinetics and CYP3A4 Interaction Potential of the Selective P2X3 Receptor Antagonist Filapixant: A Randomized Multiple Ascending-dose Study in Healthy Young Men. Br. J. Clin. Pharmacol. 2024, 90, 2004–2018. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Guideline on the Investigation of Drug Interactions. 2012. Available online: www.ema.europa.eu/contact (accessed on 1 June 2025).
- Food and Drug Administration. Clinical Drug Interaction Studies-Cytochrome P450 Enzyme-and Transporter-Mediated Drug Interactions Guidance for Industry. 2020. Available online: https://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/default.htm (accessed on 1 June 2025).
- Hardin, T.C.; Graybill, J.R.; Fetchick, R.; Woestenborghs, R.; Rinaldi, M.G.; Kuhn, J.G. Pharmacokinetics of Itraconazole Following Oral Administration to Normal Volunteers. Antimicrob. Agents Chemother. 1988, 32, 1310–1313. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.; Gashaw, I.; Baumann, S.; Chang, X.; Hummel, T.; Thuß, U.; Friedrich, C. First-in-human Study of Eliapixant (BAY 1817080), a Highly Selective P2X3 Receptor Antagonist: Tolerability, Safety and Pharmacokinetics. Br. J. Clin. Pharmacol. 2022, 88, 4552–4564. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, C.; Francke, K.; Gashaw, I.; Scheerans, C.; Klein, S.; Fels, L.; Smith, J.A.; Hummel, T.; Morice, A. Safety, Pharmacodynamics, and Pharmacokinetics of P2X3 Receptor Antagonist Eliapixant (BAY 1817080) in Healthy Subjects: Double-Blind Randomized Study. Clin. Pharmacokinet. 2022, 61, 1143–1156. [Google Scholar] [CrossRef] [PubMed]
- The International Council for Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. Harmonised Tripartite Guideline, Guideline for Good Clinical Practice E6 (R2), 2016.
- Principles on Good Laboratory Practice, OECD (ENV/MC/CHEM(98)17).
- G.I.W.G. European Medicines Agency. Reflection Paper for Laboratories that Perform the Analysis or Evaluation of Clinical Trial Samples, 2012.
- Food and Drug Administration. DA CDER. Guidance for Industry: Bioanalytical Method Validation, 2018. [Google Scholar]
- Committee for Medicinal Products for Human Use. European Medicines Agency. Guideline on Bioanalytical Method Validation, 2011.
| Filapixant | M-1 a | M-2 | M-3 | M-4 | M-5 a | M-6 | M-7 | M-8 | M-9 | M-11 a | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Enzyme | Peak Area [% of Radioactivity] | ||||||||||
| Reference | 97.1 | - | - | - | - | - | 1.3 | - | - | - | |
| CYP1A1 | 49.2 | - | 12.8 | 2.9 | 6.5 | - | 0.5 | 17.1 | 0.7 | 1.2 | |
| CYP1A2 | 96.9 | 0.5 | - | - | - | - | 1.2 | - | - | - | |
| CYP1B1 | 97.0 | 0.9 | - | - | - | - | - | 1.1 | - | - | - |
| CYP2A6 | 97.4 | - | - | - | - | - | - | 1.0 | - | - | - |
| CYP2B6 | 97.2 | - | - | - | - | - | 0.4 | 1.0 | - | - | - |
| CYP2C8 | 97.0 | - | 0.3 | - | - | - | - | 1.0 | - | - | - |
| CYP2C9 | 96.3 | - | 0.7 | - | - | 0.2 | - | 1.0 | 0.2 | - | - |
| CYP2C18 | 96.1 | - | 0.5 | - | - | - | - | 1.0 | - | - | - |
| CYP2C19 | 92.0 | - | 2.0 | - | - | - | - | 2.3 | 0.2 | 0.9 | - |
| CYP2D6 | 86.0 | 0.8 | 6.6 | 5.4 | - | - | - | 0.8 | - | - | |
| CYP2E1 | 97.8 | - | - | - | - | - | 0.8 | - | - | ||
| CYP2J2 | 72.7 | 1.1 | 19.4 | 0.9 | - | - | - | 1.1 | 0.7 | 1.8 | - |
| CYP3A4 | 37.9 | 1.5 | 15.5 | 0.5 | 6.1 | 0.4 | 6.0 | 7.0 | 3.4 | 9.2 | 1.9 |
| CYP3A5 | 84.8 | 1.0 | 1.7 | - | 1.5 | - | 0.8 | 5.3 | - | 1.9 | - |
| CYP3A7 | 96.6 | 1.1 | 0.5 | - | - | - | - | 1.2 | - | - | - |
| CYP4A11 | 97.8 | - | - | - | - | - | - | 0.6 | - | - | - |
| CYP4F2 | 97.0 | 0.9 | - | - | - | 0.5 | - | 1.0 | - | - | - |
| CYP4F3A | 97.4 | 0.9 | - | - | - | - | - | 1.0 | - | - | - |
| CYP4F3B | 97.6 | - | - | - | - | - | - | 1.0 | - | - | - |
| CYP4F12 | 97.5 | - | - | - | - | - | - | 1.2 | - | - | - |
| Aromatase | 97.7 | - | 0.2 | - | - | - | - | 0.7 | - | - | - |
| Reductase | 97.8 | - | - | - | - | - | - | 0.8 | - | - | - |
| insect cell control | 97.9 | 0.8 | - | - | - | - | - | 0.7 | - | - | 0.2 |
| PK Metric (unit) | Treatment | n | Geom. Mean | Geom. CV (%) | Min | Median | Max |
|---|---|---|---|---|---|---|---|
| AUC (µg·h/L) | filapixant | 14 | 695 | 36.4 | 402 | 715 | 1190 |
| filapixant + itraconazole | 14 | 2790 | 22.1 | 1930 | 2840 | 3790 | |
| AUC0-tlast (µg·h/L) | filapixant | 14 | 687 | 37.4 | 388 | 713 | 1180 |
| filapixant + itraconazole | 14 | 2780 | 22.2 | 1930 | 2840 | 3790 | |
| Cmax (µg/L) | filapixant | 14 | 90.8 | 27.6 | 49.6 | 93.4 | 140 |
| filapixant + itraconazole | 14 | 172 | 24.3 | 122 | 170 | 261 | |
| t1/2 (h) | filapixant | 14 | 12.1 | 14.5 | 9.52 | 12.2 | 14.5 |
| filapixant + itraconazole | 14 | 22.8 | 20.2 | 16.8 | 22.9 | 35.2 | |
| tmax (h) | filapixant | 14 | 1.00 | 1.00 | 2.00 | ||
| filapixant + itraconazole | 14 | 1.00 | 1.25 | 4.02 |
| Ratio | PK Metric | N | Geom. CV (%) | Least Square Mean Ratio | 90% CI |
|---|---|---|---|---|---|
| Filapixant + itraconazole/ Filapixant only | AUC | 14 | 13.61 | 4.01 | 3.66; 4.39 |
| Cmax | 14 | 19.07 | 1.89 | 1.67; 2.15 |
| Filapixant | |
| Calibration standards mean inter-assay accuracy of back-calculated concentrations | 99.50% to 100.50% |
| Calibration standards precision | ≤4.13% |
| Accuracy at the lowest calibration standard (LLOQ) | 99.90% |
| Precision at the lowest calibration standard (LLOQ) | 3.67% |
| Concentration range of Quality control (QC) samples (μg/L) | 0.300 to 150 |
| QC accuracy | 94.33% to 98.40% |
| QC precision | 2.21% to 4.35% |
| Itraconazole | |
| Calibration standards mean inter-assay accuracy of back-calculated concentrations | 98.00% to 102.00% |
| Calibration standards precision | ≤5.82% |
| Accuracy at the lowest calibration standard (LLOQ) | 99.90% |
| Precision at the lowest calibration standard (LLOQ) | 2.59% |
| Concentration range of QC samples (μg/L) | 3.00 to 750 |
| QC accuracy | 90.40% to 96.33% |
| QC precision | 1.87% to 5.16% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Francke, K.; Rottmann, A.; Klein, S.; Höchel, J.; Friedrich, C. Investigation of the Relevance of CYP3A4 Inhibition on the Pharmacokinetics of the Novel P2X3 Antagonist Filapixant: Results of In Vitro Explorations and a Fixed-Sequence Clinical Trial with Itraconazole in Healthy Volunteers. Int. J. Mol. Sci. 2025, 26, 10177. https://doi.org/10.3390/ijms262010177
Francke K, Rottmann A, Klein S, Höchel J, Friedrich C. Investigation of the Relevance of CYP3A4 Inhibition on the Pharmacokinetics of the Novel P2X3 Antagonist Filapixant: Results of In Vitro Explorations and a Fixed-Sequence Clinical Trial with Itraconazole in Healthy Volunteers. International Journal of Molecular Sciences. 2025; 26(20):10177. https://doi.org/10.3390/ijms262010177
Chicago/Turabian StyleFrancke, Klaus, Antje Rottmann, Stefan Klein, Joachim Höchel, and Christian Friedrich. 2025. "Investigation of the Relevance of CYP3A4 Inhibition on the Pharmacokinetics of the Novel P2X3 Antagonist Filapixant: Results of In Vitro Explorations and a Fixed-Sequence Clinical Trial with Itraconazole in Healthy Volunteers" International Journal of Molecular Sciences 26, no. 20: 10177. https://doi.org/10.3390/ijms262010177
APA StyleFrancke, K., Rottmann, A., Klein, S., Höchel, J., & Friedrich, C. (2025). Investigation of the Relevance of CYP3A4 Inhibition on the Pharmacokinetics of the Novel P2X3 Antagonist Filapixant: Results of In Vitro Explorations and a Fixed-Sequence Clinical Trial with Itraconazole in Healthy Volunteers. International Journal of Molecular Sciences, 26(20), 10177. https://doi.org/10.3390/ijms262010177








