Differential Expression of Complement Pathway Components in Unexplained Infertility Versus Male Factor Infertility: Insights from an Exploratory Pilot Study
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
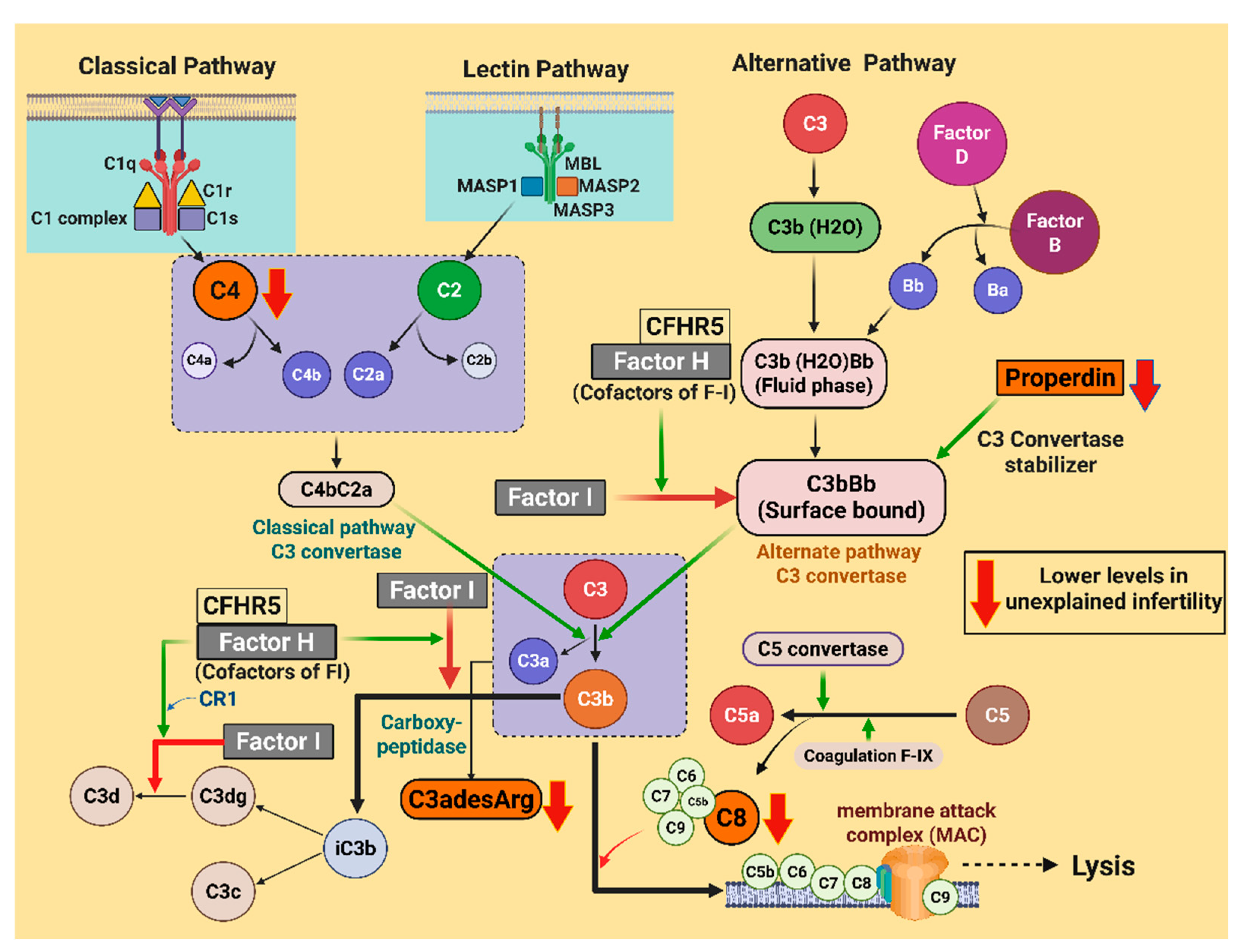
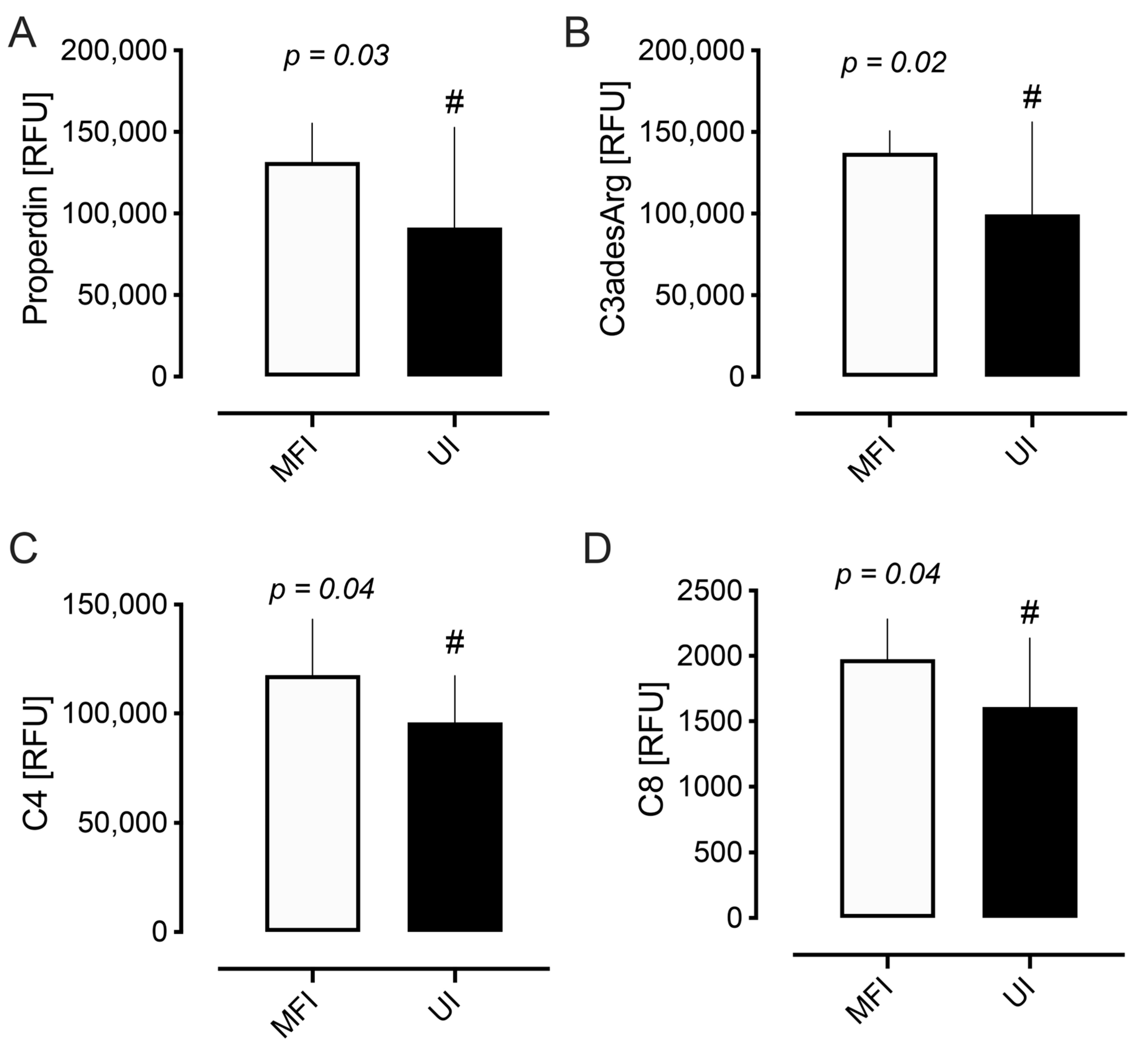
2.2. Complement Proteins
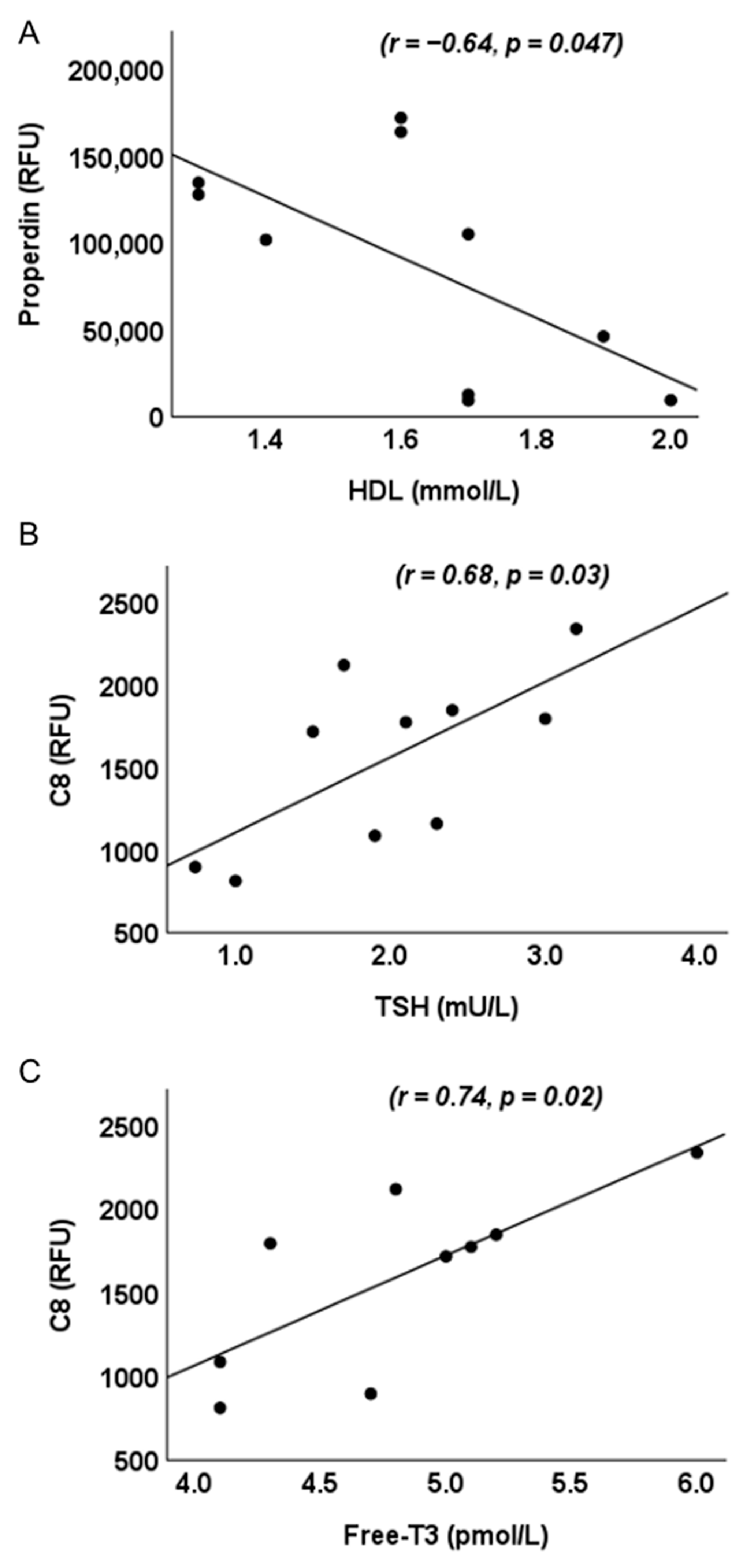
2.3. Correlation Analysis
3. Discussion
4. Materials and Methods
4.1. Study Design and Participants
4.2. IVF Protocol
4.3. Sample Collection
4.4. Biochemical and Hormonal Assays
4.4.1. Metabolic Measures
4.4.2. Reproductive Hormones
4.4.3. Complement Protein Quantification
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IVF | In Vitro Fertilization |
| UI | Unexplained Infertility |
| MFI | Male Factor Infertility |
| SOMA | Slow Off-rate Modified Aptamer |
| C3adesArg | C3a Anaphylatoxin |
| MBL | Mannose-Binding Lectin |
| MAC | Membrane Attack Complex |
| rFSH | Recombinant Follicle-Stimulating Hormone |
| AMH | Anti-Müllerian Hormone |
| ASP | Acylation-Stimulating Protein |
| PCOS | Polycystic Ovary Syndrome |
| BMI | Body Mass Index |
| HOMA-IR | Homeostatic Model Assessment of Insulin Resistance |
| HbA1c | Glycosylated Haemoglobin |
| CRP | C-Reactive Protein |
| WCC | White Cell Count |
| LH | Luteinizing Hormone |
| FBG | Fasting Blood Glucose |
| HDL-c | High-Density Lipoprotein Cholesterol |
| LDL-c | Low-Density Lipoprotein Cholesterol |
| SHBG | Sex Hormone-Binding Globulin |
| FAI | Free Androgen Index |
| TSH | Thyroid Stimulating Hormone |
| Free-T3 | Free-Triiodothyronine |
| Free-T4 | Free-Thyroxine |
| iC3B | Inactivated C3b |
| C3a | C3 Anaphylatoxin |
| C3adesArg | C3a Anaphylatoxin Des Arginine |
| CFHR5 | Complement Factor H-Related Protein 5 |
| C5a | C5 Anaphylatoxin |
| C5b, 6 Complex | Complement C5b-C6 Complex |
| MASP3 | Mannan-Binding Lectin Serine Protease 1 |
| DAF | Complement Decay-Accelerating Factor |
References
- Awonuga, A.O.; Camp, O.G.; Biernat, M.M.; Abu-Soud, H.M. Overview of infertility. Syst. Biol. Reprod. Med. 2025, 71, 116–142. [Google Scholar] [CrossRef] [PubMed]
- Rowe, P.J.; Comhaire, F.H.; Hargreave, T.B. WHO Manual for the Standardized Investigation and Diagnosis of the Infertile Male; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Agarwal, A.; Mulgund, A.; Hamada, A.; Chyatte, M.R. A unique view on male infertility around the globe. Reprod. Biol. Endocrinol. 2015, 13, 37. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.H.; Tobler, K.J. Female Infertility. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- The Guideline Group on Unexplained Infertility; Romualdi, D.; Ata, B.; Bhattacharya, S.; Bosch, E.; Costello, M.; Gersak, K.; Homburg, R.; Mincheva, M.; Norman, R.J.; et al. Evidence-based guideline: Unexplained infertilitydagger. Hum. Reprod. 2023, 38, 1881–1890. [Google Scholar] [CrossRef] [PubMed]
- Merle, N.S.; Church, S.E.; Fremeaux-Bacchi, V.; Roumenina, L.T. Complement System Part I—Molecular Mechanisms of Activation and Regulation. Front. Immunol. 2015, 6, 262. [Google Scholar] [CrossRef]
- Hatzirodos, N.; Hummitzsch, K.; Irving-Rodgers, H.F.; Rodgers, R.J. Transcriptome comparisons identify new cell markers for theca interna and granulosa cells from small and large antral ovarian follicles. PLoS ONE 2015, 10, e0119800. [Google Scholar] [CrossRef]
- Fan, X.; Bialecka, M.; Moustakas, I.; Lam, E.; Torrens-Juaneda, V.; Borggreven, N.V.; Trouw, L.; Louwe, L.A.; Pilgram, G.S.K.; Mei, H.; et al. Single-cell reconstruction of follicular remodeling in the human adult ovary. Nat. Commun. 2019, 10, 3164. [Google Scholar] [CrossRef]
- Jarkovska, K.; Martinkova, J.; Liskova, L.; Halada, P.; Moos, J.; Rezabek, K.; Gadher, S.J.; Kovarova, H. Proteome Mining of Human Follicular Fluid Reveals a Crucial Role of Complement Cascade and Key Biological Pathways in Women Undergoing in Vitro Fertilization. J. Proteome Res. 2010, 9, 1289–1301. [Google Scholar] [CrossRef]
- Teirila, L.; Heikkinen-Eloranta, J.; Kotimaa, J.; Meri, S.; Lokki, A.I. Regulation of the complement system and immunological tolerance in pregnancy. Semin. Immunol. 2019, 45, 101337. [Google Scholar] [CrossRef]
- Cunnion, K.M.; Hair, P.S.; Buescher, E.S. Cleavage of complement C3b to iC3b on the surface of Staphylococcus aureus is mediated by serum complement factor I. Infect. Immun. 2004, 72, 2858–2863. [Google Scholar] [CrossRef]
- Palomino, W.A.; Argandoña, F.; Azúa, R.; Kohen, P.; Devoto, L. Complement C3 and decay-accelerating factor expression levels are modulated by human chorionic gonadotropin in endometrial compartments during the implantation window. Reprod. Sci. 2013, 20, 1103–1110. [Google Scholar] [CrossRef]
- Madhukaran, S.P.; Alhamlan, F.S.; Kale, K.; Vatish, M.; Madan, T.; Kishore, U. Role of collectins and complement protein C1q in pregnancy and parturition. Immunobiology 2016, 221, 1273–1288. [Google Scholar] [CrossRef]
- Agostinis, C.; Bulla, R.; Tripodo, C.; Gismondi, A.; Stabile, H.; Bossi, F.; Guarnotta, C.; Garlanda, C.; De Seta, F.; Spessotto, P.; et al. An Alternative Role of C1q in Cell Migration and Tissue Remodeling: Contribution to Trophoblast Invasion and Placental Development. J. Immunol. 2010, 185, 4420–4429. [Google Scholar] [CrossRef]
- Burwick, R.M.; Feinberg, B.B. Complement activation and regulation in preeclampsia and hemolysis, elevated liver enzymes, and low platelet count syndrome. Am. J. Obstet. Gynecol. 2022, 226, S1059–S1070. [Google Scholar] [CrossRef] [PubMed]
- Langer, H.F.; Chung, K.J.; Orlova, V.V.; Choi, E.Y.; Kaul, S.; Kruhlak, M.J.; Alatsatianos, M.; DeAngelis, R.A.; Roche, P.A.; Magotti, P.; et al. Complement-mediated inhibition of neovascularization reveals a point of convergence between innate immunity and angiogenesis. Blood 2010, 116, 4395–4403. [Google Scholar] [CrossRef]
- Kwak-Kim, J.; Bao, S.; Lee, S.K.; Kim, J.W.; Gilman-Sachs, A. Immunological modes of pregnancy loss: Inflammation, immune effectors, and stress. Am. J. Reprod. Immunol. 2014, 72, 129–140. [Google Scholar] [CrossRef]
- Ramanjaneya, M.; Diboun, I.; Rizwana, N.; Dajani, Y.; Ahmed, L.; Butler, A.E.; Almarzooqi, T.A.; Shahata, M.; Al Bader, M.K.; Elgassim, E.; et al. Elevated Adipsin and Reduced C5a Levels in the Maternal Serum and Follicular Fluid During Implantation Are Associated With Successful Pregnancy in Obese Women. Front. Endocrinol. 2022, 13, 918320. [Google Scholar] [CrossRef]
- Rahal, D.; Andrade, F.; Nisihara, R. Insights into the role of complement system in the pathophysiology of endometriosis. Immunol. Lett. 2021, 231, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Regal, J.F.; Gilbert, J.S.; Burwick, R.M. The complement system and adverse pregnancy outcomes. Mol. Immunol. 2015, 67, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Hasty, L.A.; Lambris, J.D.; Lessey, B.A.; Pruksananonda, K.; Lyttle, C.R. Hormonal regulation of complement components and receptors throughout the menstrual cycle. Am. J. Obstet. Gynecol. 1994, 170, 168–175. [Google Scholar] [CrossRef]
- Koshak, E.A.; Aljohani, H.; Atwah, A.; Aljedani, R.; Aljaied, Y.; Gaddoury, M.A. Preconception Immunoglobulins and Complements as Potential Biomarkers in Unexplained Female Infertility in Saudi Arabia. Cureus 2023, 15, e48322. [Google Scholar] [CrossRef]
- Ricklin, D.; Reis, E.S.; Mastellos, D.C.; Gros, P.; Lambris, J.D. Complement component C3—The “Swiss Army Knife” of innate immunity and host defense. Immunol. Rev. 2016, 274, 33–58. [Google Scholar] [CrossRef]
- Farries, T.C.; Lachmann, P.J.; Harrison, R.A. Analysis of the interactions between properdin, the third component of complement (C3), and its physiological activation products. Biochem. J. 1988, 252, 47–54. [Google Scholar] [CrossRef]
- Fearon, D.T.; Austen, K.F. Properdin: Binding to C3b and stabilization of the C3b-dependent C3 convertase. J. Exp. Med. 1975, 142, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, C.; Balduit, A.; Mangogna, A.; Zito, G.; Romano, F.; Ricci, G.; Kishore, U.; Bulla, R. Immunological Basis of the Endometriosis: The Complement System as a Potential Therapeutic Target. Front. Immunol. 2020, 11, 599117. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, C.; Zorzet, S.; Balduit, A.; Zito, G.; Mangogna, A.; Macor, P.; Romano, F.; Toffoli, M.; Belmonte, B.; Morello, G.; et al. The Inflammatory Feed-Forward Loop Triggered by the Complement Component C3 as a Potential Target in Endometriosis. Front. Immunol. 2021, 12, 693118. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, N.; Wolf, H.N.; Enzbrenner, A.; Schikora, J.; Reichenthaler, M.; Enzmann, V.; Pauly, D. Properdin Modulates Complement Component Production in Stressed Human Primary Retinal Pigment Epithelium Cells. Antioxidants 2020, 9, 793. [Google Scholar] [CrossRef]
- Blatt, A.Z.; Pathan, S.; Ferreira, V.P. Properdin: A tightly regulated critical inflammatory modulator. Immunol. Rev. 2016, 274, 172–190. [Google Scholar] [CrossRef]
- Arffman, R.K.; Saraswat, M.; Joenväärä, S.; Khatun, M.; Agarwal, R.; Tohmola, T.; Sundström-Poromaa, I.; Renkonen, R.; Piltonen, T.T. Thromboinflammatory changes in plasma proteome of pregnant women with PCOS detected by quantitative label-free proteomics. Sci. Rep. 2019, 9, 17578. [Google Scholar] [CrossRef]
- Ishak, G.M.; Feugang, J.M.; Pechanova, O.; Pechan, T.; Peterson, D.G.; Willard, S.T.; Ryan, P.L.; Gastal, E.L. Follicular-fluid proteomics during equine follicle development. Mol. Reprod. Dev. 2022, 89, 298–311. [Google Scholar] [CrossRef]
- Paes, V.M.; Liao, S.F.; Figueiredo, J.R.; Willard, S.T.; Ryan, P.L.; Feugang, J.M. Proteome changes of porcine follicular fluid during follicle development. J. Anim. Sci. Biotechnol. 2019, 10, 94. [Google Scholar] [CrossRef]
- Topdagi, S.K.; Topdagi, Y.E.; Ozdemir, I.; Borekci, B. The Role of Total Oxidant and Antioxidant Levels in Follicular Fluid in Unexplained İnfertility. Niger. J. Clin. Pract. 2024, 27, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-González, D.; García-González, M.; Gómez-Bernal, F.; Quevedo-Abeledo, J.C.; González-Rivero, A.F.; Jiménez-Sosa, A.; González-López, E.; Heras-Recuero, E.; Ocejo-Vinyals, J.G.; González-Gay, M.; et al. Relationship between the complement system and serum lipid profile in patients with rheumatoid arthritis. Front. Immunol. 2024, 15, 1420292. [Google Scholar] [CrossRef]
- Zhu, X.; Hong, X.; Wu, J.; Zhao, F.; Wang, W.; Huang, L.; Li, J.; Wang, B. The Association between Circulating Lipids and Female Infertility Risk: A Univariable and Multivariable Mendelian Randomization Analysis. Nutrients 2023, 15, 3130. [Google Scholar] [CrossRef] [PubMed]
- Rosales, C.; Yelamanchili, D.; Gillard, B.K.; Liu, J.; Gotto, A.M., Jr.; Pownall, H.J. Serum opacity factor rescues fertility among female Scarb1(−/−) mice by reducing HDL-free cholesterol bioavailability. J. Lipid Res. 2023, 64, 100327. [Google Scholar] [CrossRef]
- Janeway, C.A., Jr.; Travers, P.; Walport, M.; Shlomchik, M.J. Immunobiology: The Immune System in Health and Disease, 5th ed.; Garland Publishing: New York, NY, USA, 2001. [Google Scholar]
- Cui, W.; Lapointe, M.; Gauvreau, D.; Kalant, D.; Cianflone, K. Recombinant C3adesArg/acylation stimulating protein (ASP) is highly bioactive: A critical evaluation of C5L2 binding and 3T3-L1 adipocyte activation. Mol. Immunol. 2009, 46, 3207–3217. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, C.; Zorzet, S.; Balduit, A.; Zito, G.; Mangogna, A.; Macor, P.; Romano, F.; Toffoli, M.; Belmonte, B.; Martorana, A.; et al. Complement Component 3 expressed by the endometrial ectopic tissue is involved in the endometriotic lesion formation through mast cell activation. bioRxiv 2020. [Google Scholar] [CrossRef]
- Lynch, A.M.; Gibbs, R.S.; Murphy, J.R.; Giclas, P.C.; Salmon, J.E.; Holers, V.M. Early elevations of the complement activation fragment C3a and adverse pregnancy outcomes. Obstet. Gynecol. 2011, 117, 75–83. [Google Scholar] [CrossRef]
- Butler, A.E.; Moin, A.S.M.; Begam, H.H.; Waris, S.; Azeez, J.M.; Sathyapalan, T.; Atkin, S.L.; Brennan, E. Association of Complement Proteins with C Reactive Protein in Non-Obese Women with and Without Polycystic Ovary Syndrome. Int. J. Mol. Sci. 2025, 26, 3008. [Google Scholar] [CrossRef]
- Saleh, J.; Al-Khanbashi, M.; Al-Maarof, M.; Al-Lawati, M.; Rizvi, S.G.; Cianflone, K. Acylation-stimulating protein increases and correlates with increased progesterone levels during the luteal phase of the menstrual cycle. Eur. J. Endocrinol. 2009, 160, 301–307. [Google Scholar] [CrossRef]
- Devi, K.; Malleshappa, K.; Jeyalakshmi, L. Association of acylation stimulating protein with endogenous sex hormones & lipid profile during menstrual cycle. Indian J. Physiol. Pharmacol. 2012, 56, 147–153. [Google Scholar]
- Coulthard, L.G.; Woodruff, T.M. Is the complement activation product C3a a proinflammatory molecule? Re-evaluating the evidence and the myth. J. Immunol. 2015, 194, 3542–3548. [Google Scholar] [CrossRef] [PubMed]
- Merle, N.S.; Noe, R.; Halbwachs-Mecarelli, L.; Fremeaux-Bacchi, V.; Roumenina, L.T. Complement System Part II: Role in Immunity. Front. Immunol. 2015, 6, 257. [Google Scholar] [CrossRef]
- Bartosik, D.; Damjanov, I.; Viscarello, R.R.; Riley, J.A. Immunoproteins in the endometrium: Clinical correlates of the presence of complement fractions C3 and C4. Am. J. Obstet. Gynecol. 1987, 156, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Esteve-Valverde, E.; Alijotas-Reig, J.; Belizna, C.; Marques-Soares, J.; Anunciacion-Llunell, A.; Feijóo-Massó, C.; Sáez-Comet, L.; Mekinian, A.; Ferrer-Oliveras, R.; Lefkou, E.; et al. Low complement levels are related to poor obstetric outcomes in women with obstetric antiphospholipid syndrome. The EUROAPS Registry Study Group. Placenta 2023, 138, 20, Errutum in Placenta 2023, 136, 29–34. [Google Scholar] [CrossRef]
- Crisafulli, F.; Andreoli, L.; Zucchi, D.; Reggia, R.; Gerardi, M.C.; Lini, D.; Tani, C.; Zatti, S.; Franceschini, F.; Mosca, M.; et al. Variations of C3 and C4 Before and During Pregnancy in Systemic Lupus Erythematosus: Association With Disease Flares and Obstetric Outcomes. J. Rheumatol. 2023, 50, 1296–1301. [Google Scholar] [CrossRef]
- Micheloud, D.; Sarmiento, E.; Teijeiro, R.; Jensen, J.; Rodríguez Molina, J.J.; Fernández-Cruz, E.; Carbone, J. Hypocomplementemia in the absence of autoantibodies in women with recurrent pregnancy loss. Allergol. Immunopathol. 2007, 35, 90–94. [Google Scholar] [CrossRef][Green Version]
- Taru, M.; Katoh, T.; Koshimizu, K.; Kuribayashi, S.; Miura, R.; Hamano, S.; Shirasuna, K. Inflammatory uterine microenvironment in long-term infertility repeat breeder cows compared with normal fertile cows. Vet. Anim. Sci. 2024, 25, 100369. [Google Scholar] [CrossRef]
- Meek, S.C.; Hodge, D.D.; Musich, J.R. Autoimmunity in infertile patients with endometriosis. Am. J. Obstet. Gynecol. 1988, 158, 1365–1373. [Google Scholar] [CrossRef]
- Xie, C.B.; Jane-Wit, D.; Pober, J.S. Complement Membrane Attack Complex: New Roles, Mechanisms of Action, and Therapeutic Targets. Am. J. Pathol. 2020, 190, 1138–1150. [Google Scholar] [CrossRef]
- Nemerow, G.R.; Yamamoto, K.-I.; Lint, T.F. Restriction of Complement-Mediated Membrane Damage by the Eighth Component of Complement: A Dual Role for C8 in the Complement Attack Sequence1. J. Immunol. 1979, 123, 1245–1252. [Google Scholar] [CrossRef] [PubMed]
- Klein, C.; Bruce, P.; Hammermueller, J.; Hayes, T.; Lillie, B.; Betteridge, K. Transcriptional profiling of equine endometrium before, during and after capsule disintegration during normal pregnancy and after oxytocin-induced luteostasis in non-pregnant mares. PLoS ONE 2021, 16, e0257161. [Google Scholar] [CrossRef]
- McMurray, J.C.; Schornack, B.J.; Weskamp, A.L.; Park, K.J.; Pollock, J.D.; Day, W.G.; Brockshus, A.T.; Beakes, D.E.; Schwartz, D.J.; Mikita, C.P.; et al. Immunodeficiency: Complement disorders. Allergy Asthma Proc. 2024, 45, 305–309. [Google Scholar] [CrossRef]
- Ducolomb, Y.; González-Márquez, H.; Fierro, R.; Jiménez, I.; Casas, E.; Flores, D.; Bonilla, E.; Salazar, Z.; Betancourt, M. Effect of porcine follicular fluid proteins and peptides on oocyte maturation and their subsequent effect on in vitro fertilization. Theriogenology 2013, 79, 896–904. [Google Scholar] [CrossRef]
- Orouji Jokar, T.; Fourman, L.T.; Lee, H.; Mentzinger, K.; Fazeli, P.K. Higher TSH Levels Within the Normal Range Are Associated With Unexplained Infertility. J. Clin. Endocrinol. Metab. 2018, 103, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Jalili, M.; Azizi, R.; Aflatoonian, A.; Ghadiri-Anari, A. The association of higher thyroid stimulating hormone levels in the normal range with unexplained infertility: A cross-sectional study. Int. J. Reprod. Biomed. 2024, 22, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Sowiński, J.; Sawicka-Gutaj, N.; Gutaj, P.; Ruchała, M. The role of free triiodothyronine in pathogenesis of infertility in levothyroxine-treated women with thyroid autoimmunity—A preliminary observational study. Gynecol. Endocrinol. 2015, 31, 116–118. [Google Scholar] [CrossRef]
- Pietzner, M.; Engelmann, B.; Kacprowski, T.; Golchert, J.; Dirk, A.L.; Hammer, E.; Iwen, K.A.; Nauck, M.; Wallaschofski, H.; Führer, D.; et al. Plasma proteome and metabolome characterization of an experimental human thyrotoxicosis model. BMC Med. 2017, 15, 6. [Google Scholar] [CrossRef]
- Landi, C.; Cantara, S.; Shaba, E.; Vantaggiato, L.; Marzocchi, C.; Maino, F.; Bombardieri, A.; Carleo, A.; Di Giuseppe, F.; Angelucci, S.; et al. Alteration of Serum Proteome in Levo-Thyroxine-Euthyroid Thyroidectomized Patients. J. Clin. Med. 2022, 11, 1676. [Google Scholar] [CrossRef]
- Aghajanova, L.; Stavreus-Evers, A.; Lindeberg, M.; Landgren, B.M.; Sparre, L.S.; Hovatta, O. Thyroid-stimulating hormone receptor and thyroid hormone receptors are involved in human endometrial physiology. Fertil. Steril. 2011, 95, 230–237.e2. [Google Scholar] [CrossRef] [PubMed]
- Malik, H.; Zamouri, S.; Akkawi, S.; Mehra, S.; Mouaki, R.; Sathyapalan, T.; Nandakumar, M.; Butler, A.E.; Atkin, S.L. Endothelial Protein Changes Indicative of Endometriosis in Unexplained Infertility, an Exploratory Study. Int. J. Mol. Sci. 2025, 26, 6485. [Google Scholar] [CrossRef]
- Alpha Scientists in Reproductive Medicine. The Alpha consensus meeting on cryopreservation key performance indicators and benchmarks: Proceedings of an expert meeting. Reprod. Biomed. Online 2012, 25, 146–167. [Google Scholar] [CrossRef] [PubMed]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the Concentration of Low-Density Lipoprotein Cholesterol in Plasma, Without Use of the Preparative Ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Gold, L.; Ayers, D.; Bertino, J.; Bock, C.; Bock, A.; Brody, E.N.; Carter, J.; Dalby, A.B.; Eaton, B.E.; Fitzwater, T.; et al. Aptamer-based multiplexed proteomic technology for biomarker discovery. PLoS ONE 2010, 5, e15004. [Google Scholar] [CrossRef]
- Suhre, K.; Arnold, M.; Bhagwat, A.M.; Cotton, R.J.; Engelke, R.; Raffler, J.; Sarwath, H.; Thareja, G.; Wahl, A.; DeLisle, R.K.; et al. Connecting genetic risk to disease end points through the human blood plasma proteome. Nat. Commun. 2017, 8, 14357. [Google Scholar] [CrossRef]
- Kraemer, S.; Vaught, J.D.; Bock, C.; Gold, L.; Katilius, E.; Keeney, T.R.; Kim, N.; Saccomano, N.A.; Wilcox, S.K.; Zichi, D.; et al. From SOMAmer-based biomarker discovery to diagnostic and clinical applications: A SOMAmer-based, streamlined multiplex proteomic assay. PLoS ONE 2011, 6, e26332. [Google Scholar] [CrossRef]
- Kahal, H.; Halama, A.; Aburima, A.; Bhagwat, A.M.; Butler, A.E.; Graumann, J.; Suhre, K.; Sathyapalan, T.; Atkin, S.L. Effect of induced hypoglycemia on inflammation and oxidative stress in type 2 diabetes and control subjects. Sci. Rep. 2020, 10, 4750. [Google Scholar] [CrossRef] [PubMed]
| MFI | UI | p Value | |
|---|---|---|---|
| Age (years) | 32.6 (4.0) | 33.8 (5.3) | 0.51 |
| BMI (kg/m2) | 25.7 (2.6) | 25.3 (4.9) | 0.84 |
| HOMA-IR | 1.5 (0.7) | 1.9 (1.4) | 0.45 |
| Cholesterol (mmol/L) | 4.8 (0.8) | 4.6 (0.7) | 0.44 |
| Triglycerides (mmol/L) | 0.9 (0.5) | 1.0 (0.4) | 0.67 |
| HDL-c (mmol/L) | 1.6 (0.4) | 1.6 (0.2) | 0.97 |
| LDL-c (mmol/L) | 2.8 (0.7) | 1.0 (0.4) | 0.24 |
| CRP (mg/L) | 1.9 (1.3) | 2.4 (2.0) | 0.51 |
| WCC × 109/L | 5.9 (1.7) | 7.2 (2.2) | 0.12 |
| AMH (ng/mL) | 22.4 (15.3) | 24.5 (12.5) | 0.72 |
| FAI | 1.4 (0.7) | 0.8 (0.9) | 0.11 |
| TSH (mU/L) | 2.3 (1.2) | 2.0 (0.8) | 0.53 |
| Free-T3 (pmol/L) | 4.7 (0.7) | 4.8 (0.6) | 0.66 |
| Free-T4 (pmol/L) | 11.2 (1.6) | 11.4 (0.9) | 0.66 |
| Positive pregnancy test | 0.3 (0.5) | 0.3 (0.5) | 0.95 |
| Number of eggs retrieved | 9.0 (7.5) | 8.4 (3.2) | 0.78 |
| Number of embryos created | 3.7 (3.0) | 5.2 (2.4) | 0.18 |
| G3D3 | 3.4 (2.2) | 2.7 (2.6) | 0.49 |
| Fertility rate | 0.6 (0.2) | 0.6 (0.4) | 0.89 |
| Top quality embryo (proportion) | 0.3 (0.2) | 0.4 (0.4) | 0.36 |
| Live birth rate | 0.0 (0.0) | 0.0 (0.0) | 1.00 |
| MFI | UI | FDR p Value | |
|---|---|---|---|
| Properdin | 131,606 (23,723) | 91,387 (61,357) | 0.034 # |
| C3b | 43,399 (25,396) | 45,084 (54,190) | 0.92 |
| iC3b | 6073 (1129) | 5580 (1806) | 0.41 |
| C3 | 48,348 (6443) | 40,796 (25,267) | 0.29 |
| C3adesArg | 137,187 (13,467) | 99,554 (56,569) | 0.024 # |
| C3a | 399 (49) | 402 (142) | 0.94 |
| C3d | 8789 (2301) | 6551 (3966) | 0.09 |
| C4 | 117,538 (25,704) | 95,901 (21,341) | 0.04 # |
| C4a | 72,800 (2391) | 73,259 (2544) | 0.65 |
| Factor I | 41,056 (4648) | 38,334 (3178) | 0.11 |
| Factor D | 722 (83) | 639 (208) | 0.19 |
| C2 | 2874 (194) | 2870 (289) | 0.96 |
| CFHR5 | 1613 (756) | 1331 (442) | 0.25 |
| Factor B | 28,997 (4571) | 28,385 (3906) | 0.73 |
| Factor H | 60,035 (5505) | 57,943 (5549) | 0.36 |
| C5a | 11,127 (3728) | 10,942 (5900) | 0.93 |
| C5b, 6 Complex | 491 (31) | 501 (61) | 0.61 |
| C5 | 6990 (461) | 7117 (1130) | 0.71 |
| C1q | 35,166 (6834) | 33,787 (6928) | 0.62 |
| C1r | 2783 (982) | 3225 (983) | 0.28 |
| MBL | 15,048 (8231) | 11,240 (5960) | 0.21 |
| MASP3 | 5791 (782) | 5275 (1348) | 0.24 |
| DAF | 14,938 (2131) | 13,127 (3505) | 0.12 |
| C4b | 261 (122) | 417 (255) | 0.06 |
| C8 | 1974 (307) | 1609 (528) | 0.04 # |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brennan, E.; Radhi, M.K.E.A.; Husain, Z.A.A.H.; Sathyapalan, T.; Moin, A.S.M.; Butler, A.E.; Atkin, S.L. Differential Expression of Complement Pathway Components in Unexplained Infertility Versus Male Factor Infertility: Insights from an Exploratory Pilot Study. Int. J. Mol. Sci. 2025, 26, 10168. https://doi.org/10.3390/ijms262010168
Brennan E, Radhi MKEA, Husain ZAAH, Sathyapalan T, Moin ASM, Butler AE, Atkin SL. Differential Expression of Complement Pathway Components in Unexplained Infertility Versus Male Factor Infertility: Insights from an Exploratory Pilot Study. International Journal of Molecular Sciences. 2025; 26(20):10168. https://doi.org/10.3390/ijms262010168
Chicago/Turabian StyleBrennan, Edwina, Marya K. E. A. Radhi, Zainab A. A. H. Husain, Thozhukat Sathyapalan, Abu Saleh Md Moin, Alexandra E. Butler, and Stephen L. Atkin. 2025. "Differential Expression of Complement Pathway Components in Unexplained Infertility Versus Male Factor Infertility: Insights from an Exploratory Pilot Study" International Journal of Molecular Sciences 26, no. 20: 10168. https://doi.org/10.3390/ijms262010168
APA StyleBrennan, E., Radhi, M. K. E. A., Husain, Z. A. A. H., Sathyapalan, T., Moin, A. S. M., Butler, A. E., & Atkin, S. L. (2025). Differential Expression of Complement Pathway Components in Unexplained Infertility Versus Male Factor Infertility: Insights from an Exploratory Pilot Study. International Journal of Molecular Sciences, 26(20), 10168. https://doi.org/10.3390/ijms262010168







