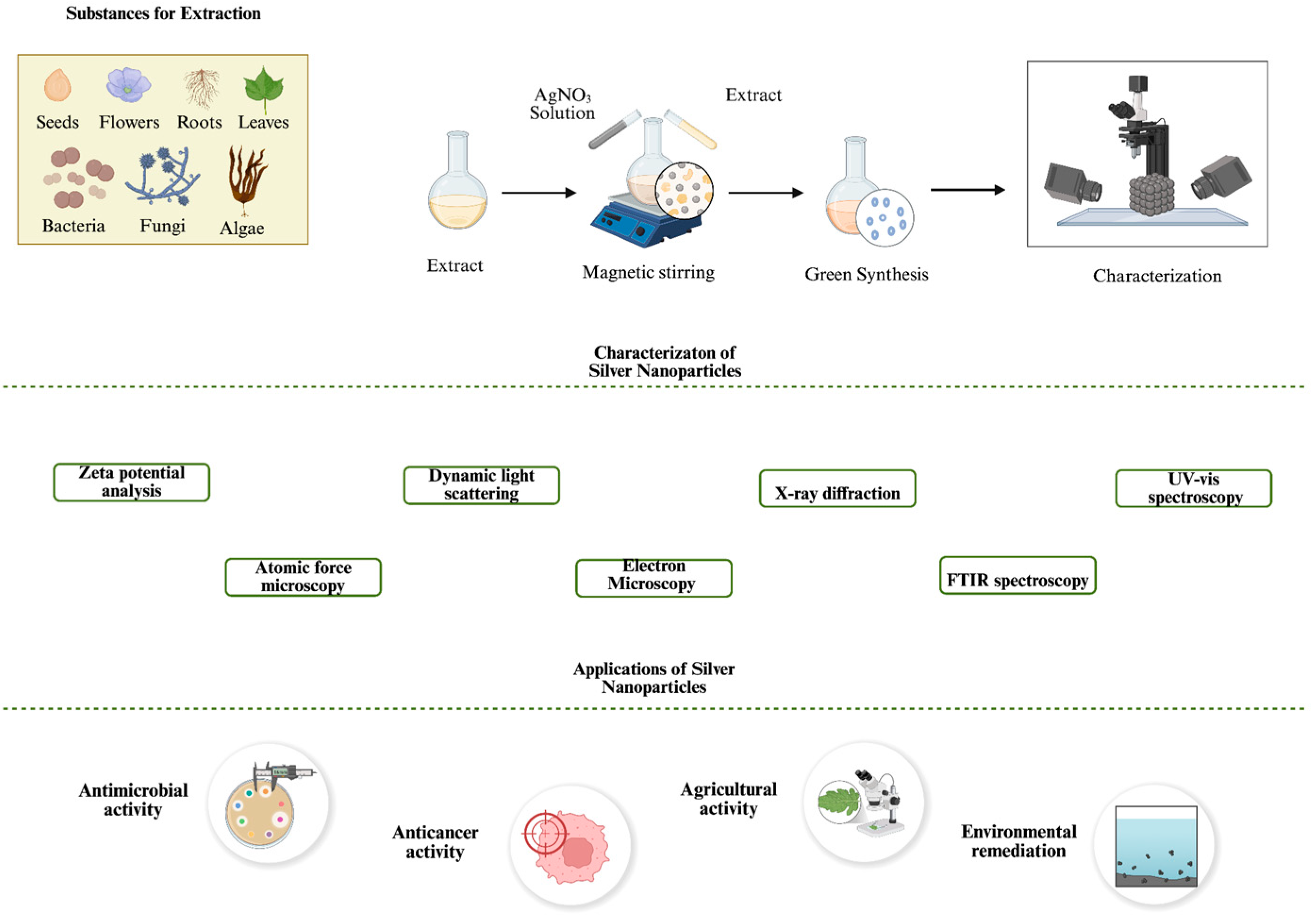
Microbial-Based Green Synthesis of Silver Nanoparticles: A Comparative Review of Bacteria- and Fungi-Mediated Approaches
Abstract
1. Introduction
2. Green Synthesis of AgNPs
3. Bacteria-Based Green Synthesis of AgNPs
3.1. Mechanism of Bacterial Reduction of Silver Ions
3.2. Effect of Synthesis Parameters on Physicochemical Properties and Biological Activities of AgNPs
3.3. Advantages and Limitations of Bacteria-Mediated AgNP Synthesis
4. Fungi-Based Green Synthesis of AgNPs
4.1. Mechanism of Fungal Reduction of Silver Ions
4.2. Effect of Synthesis Parameters on Physicochemical Properties and Biological Activities of AgNPs
4.3. Advantages and Limitations of Fungi-Mediated Synthesis
5. Activity and Application of Microbial-Based Synthesized AgNPs
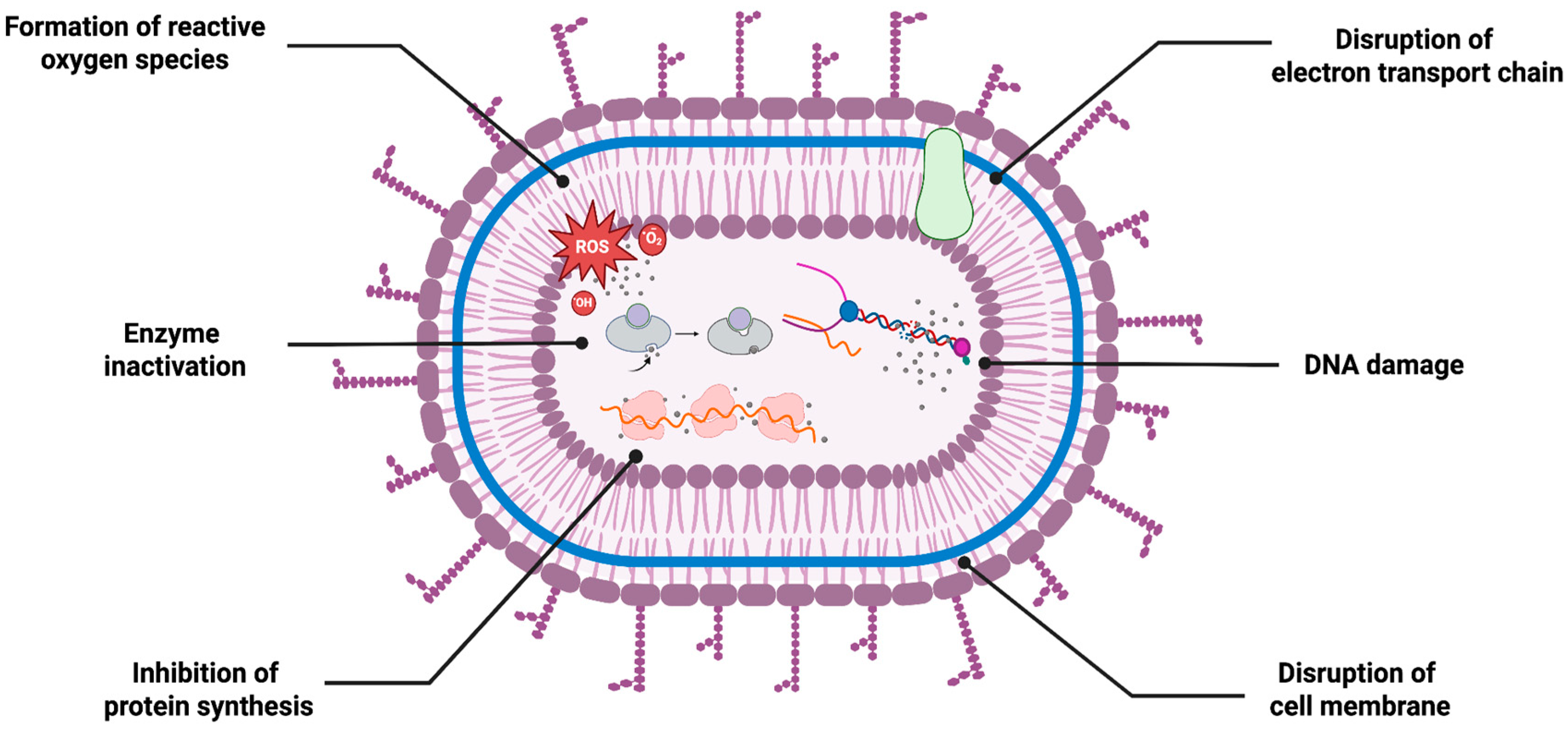
5.1. Antimicrobial Activity
5.1.1. Antimicrobial Activity of Bacteria-Based AgNPs
5.1.2. Antimicrobial Activity of Fungi-Based AgNPs
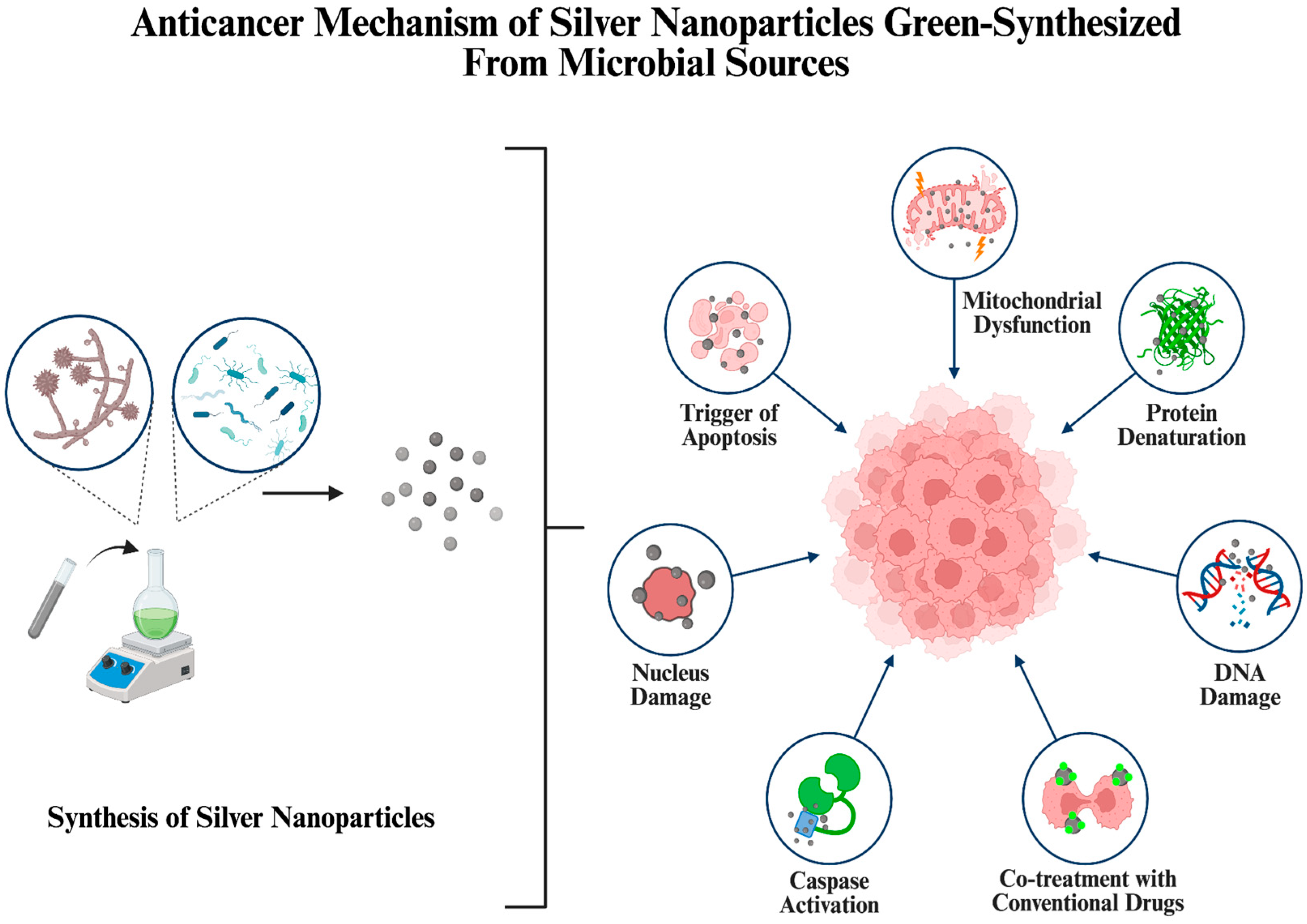
5.2. Anticancer Activity
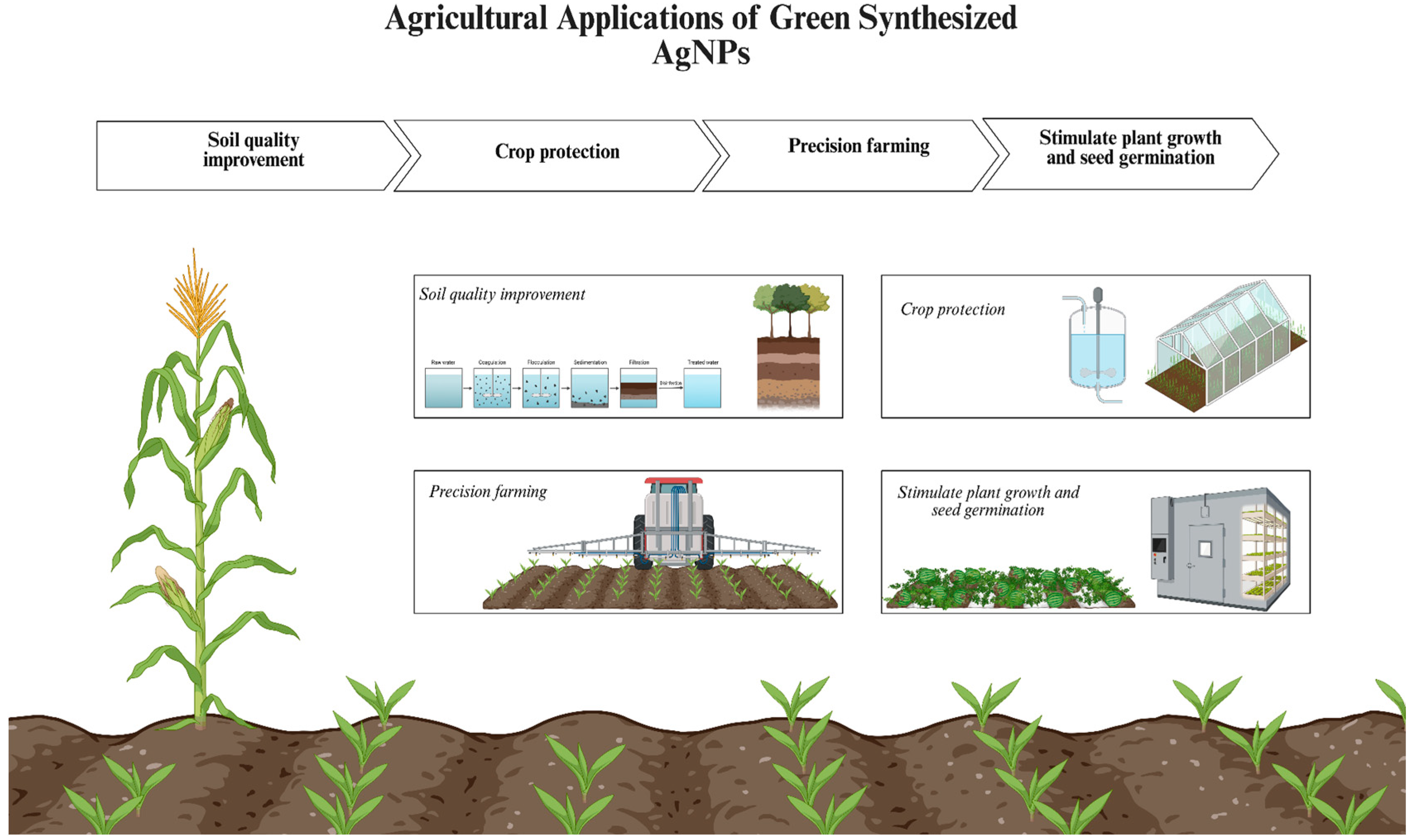
5.3. Agricultural Applications
5.4. Environmental Applications
6. Toxicity & Safety Concerns
7. Conclusions & Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huq, M.A.; Ashrafudoulla, M.; Rahman, M.M.; Balusamy, S.R.; Akter, S. Green Synthesis and Potential Antibacterial Applications of Bioactive Silver Nanoparticles: A Review. Polymers 2022, 14, 742. [Google Scholar] [CrossRef]
- Fahim, M.; Shahzaib, A.; Nishat, N.; Jahan, A.; Bhat, T.A.; Inam, A. Green Synthesis of Silver Nanoparticles: A Comprehensive Review of Methods, Influencing Factors, and Applications. JCIS Open 2024, 16, 100125. [Google Scholar] [CrossRef]
- Singh, R.; Shedbalkar, U.U.; Wadhwani, S.A.; Chopade, B.A. Bacteriagenic Silver Nanoparticles: Synthesis, Mechanism, and Applications. Appl. Microbiol. Biotechnol. 2015, 99, 4579–4593. [Google Scholar] [CrossRef] [PubMed]
- Akhil, T.; Bhavana, V.; Ann Maria, C.G.; Nidhin, M. Role of Biosynthesized Silver Nanoparticles in Environmental Remediation: A Review. Nanotechnol. Environ. Eng. 2023, 8, 829–843. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, L.; Riaz Rajoka, M.S.; Yan, L.; Jiang, C.; Shao, D.; Zhu, J.; Shi, J.; Huang, Q.; Yang, H.; et al. Fungal Silver Nanoparticles: Synthesis, Application and Challenges. Crit. Rev. Biotechnol. 2018, 38, 817–835. [Google Scholar] [CrossRef]
- Khan, A.U.; Malik, N.; Khan, M.; Cho, M.H.; Khan, M.M. Fungi-Assisted Silver Nanoparticle Synthesis and Their Applications. Bioprocess Biosyst. Eng. 2018, 41, 1–20. [Google Scholar] [CrossRef]
- Feroze, N.; Arshad, B.; Younas, M.; Afridi, M.I.; Saqib, S.; Ayaz, A. Fungal Mediated Synthesis of Silver Nanoparticles and Evaluation of Antibacterial Activity. Microsc. Res. Tech. 2020, 83, 72–80. [Google Scholar] [CrossRef]
- Almalki, M.A.; Khalifa, A.Y.Z. Silver Nanoparticles Synthesis from Bacillus sp. KFU36 and Its Anticancer Effect in Breast Cancer MCF-7 Cells via Induction of Apoptotic Mechanism. J. Photochem. Photobiol. B Biol. 2020, 204, 111786. [Google Scholar] [CrossRef]
- Alqaraleh, M.; Khleifat, K.M.; Abu Hajleh, M.N.; Farah, H.S.; Ahmed, K.A.-A. Fungal-Mediated Silver Nanoparticle and Biochar Synergy against Colorectal Cancer Cells and Pathogenic Bacteria. Antibiotics 2023, 12, 597. [Google Scholar] [CrossRef]
- Shaaban, M.T.; Mohamed, B.S.; Zayed, M.; El-Sabbagh, S.M. Antibacterial, Antibiofilm, and Anticancer Activity of Silver-Nanoparticles Synthesized from the Cell-Filtrate of Streptomyces enissocaesilis. BMC Biotechnol. 2024, 24, 8. [Google Scholar] [CrossRef]
- El-Sheikh, S.M.A.; Edrees, N.; EL-Sayed, H.; Khamis, T.; Arisha, A.H.; Metwally, M.M.M.; Eleiwa, N.Z.; Galal, A.A.A. Could Cisplatin Loading on Biosynthesized Silver Nanoparticles Improve Its Therapeutic Efficacy on Human Prostate Cancer Cell Line and Reduce Its In Vivo Nephrotoxic Effects? Biol. Trace Elem. Res. 2022, 200, 582–590. [Google Scholar] [CrossRef]
- Bahrulolum, H.; Nooraei, S.; Javanshir, N.; Tarrahimofrad, H.; Mirbagheri, V.S.; Easton, A.J.; Ahmadian, G. Green Synthesis of Metal Nanoparticles Using Microorganisms and Their Application in the Agrifood Sector. J. Nanobiotechnol. 2021, 19, 86. [Google Scholar] [CrossRef]
- Moustafa, M.T. Removal of Pathogenic Bacteria from Wastewater Using Silver Nanoparticles Synthesized by Two Fungal Species. Water Sci. 2017, 31, 164–176. [Google Scholar] [CrossRef]
- Rostami, H.; Khosravi, F.; Mohseni, M.; Rostami, A.A. Biosynthesis of Ag Nanoparticles Using Isolated Bacteria from Contaminated Sites and Its Application as an Efficient Catalyst for Hydrazine Electrooxidation. Int. J. Biol. Macromol. 2018, 107, 343–348. [Google Scholar] [CrossRef]
- Huston, M.; DeBella, M.; DiBella, M.; Gupta, A. Green Synthesis of Nanomaterials. Nanomaterials 2021, 11, 2130. [Google Scholar] [CrossRef]
- Aarthye, P.; Sureshkumar, M. Green Synthesis of Nanomaterials: An Overview. Mater. Today Proc. 2021, 47, 907–913. [Google Scholar] [CrossRef]
- Jain, N.; Jain, P.; Rajput, D.; Patil, U.K. Green Synthesized Plant-Based Silver Nanoparticles: Therapeutic Prospective for Anticancer and Antiviral Activity. Micro Nano Syst. Lett. 2021, 9, 5. [Google Scholar] [CrossRef]
- Gupta, D.; Boora, A.; Thakur, A.; Gupta, T.K. Green and Sustainable Synthesis of Nanomaterials: Recent Advancements and Limitations. Environ. Res. 2023, 231, 116316. [Google Scholar] [CrossRef]
- Patel, R.R.; Singh, S.K.; Singh, M. Green Synthesis of Silver Nanoparticles: Methods, Biological Applications, Delivery and Toxicity. Mater. Adv. 2023, 4, 1831–1849. [Google Scholar] [CrossRef]
- Banjara, R.A.; Kumar, A.; Aneshwari, R.K.; Satnami, M.L.; Sinha, S.K. A Comparative Analysis of Chemical vs Green Synthesis of Nanoparticles and Their Various Applications. Environ. Nanotechnol. Monit. Manag. 2024, 22, 100988. [Google Scholar] [CrossRef]
- Alsaiari, N.S.; Alzahrani, F.M.; Amari, A.; Osman, H.; Harharah, H.N.; Elboughdiri, N.; Tahoon, M.A. Plant and Microbial Approaches as Green Methods for the Synthesis of Nanomaterials: Synthesis, Applications, and Future Perspectives. Molecules 2023, 28, 463. [Google Scholar] [CrossRef]
- Bhardwaj, B.; Singh, P.; Kumar, A.; Kumar, S.; Budhwar, V. Eco-Friendly Greener Synthesis of Nanoparticles. Adv. Pharm. Bull. 2020, 10, 566–576. [Google Scholar] [CrossRef]
- Rauwel, P.; Küünal, S.; Ferdov, S.; Rauwel, E. A Review on the Green Synthesis of Silver Nanoparticles and Their Morphologies Studied via TEM. Adv. Mater. Sci. Eng. 2015, 2015, 1–9. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Mofijur, M.; Rafa, N.; Chowdhury, A.T.; Chowdhury, S.; Nahrin, M.; Islam, A.B.M.S.; Ong, H.C. Green Approaches in Synthesising Nanomaterials for Environmental Nanobioremediation: Technological Advancements, Applications, Benefits and Challenges. Environ. Res. 2022, 204, 111967. [Google Scholar] [CrossRef]
- Salem, S.S.; Fouda, A. Green Synthesis of Metallic Nanoparticles and Their Prospective Biotechnological Applications: An Overview. Biol. Trace Elem. Res. 2021, 199, 344–370. [Google Scholar] [CrossRef]
- Choudhary, S.; Sangela, V.; Saxena, P.; Saharan, V.; Pugazhendhi, A. Harish Recent Progress in Algae-Mediated Silver Nanoparticle Synthesis. Int. Nano Lett. 2023, 13, 193–207. [Google Scholar] [CrossRef]
- Khanna, P.; Kaur, A.; Goyal, D. Algae-Based Metallic Nanoparticles: Synthesis, Characterization and Applications. J. Microbiol. Methods 2019, 163, 105656. [Google Scholar] [CrossRef]
- Jafarzadeh, S.; Nooshkam, M.; Zargar, M.; Garavand, F.; Ghosh, S.; Hadidi, M.; Forough, M. Green Synthesis of Nanomaterials for Smart Biopolymer Packaging: Challenges and Outlooks. J. Nanostruct. Chem. 2024, 14, 113–136. [Google Scholar] [CrossRef]
- Baraketi, S.; Khwaldia, K. Nanoparticles from Agri-Food by-Products: Green Technology Synthesis and Application in Food Packaging. Curr. Opin. Green Sustain. Chem. 2024, 49, 100953. [Google Scholar] [CrossRef]
- Thomas, S.; Gonsalves, R.A.; Jose, J.; Zyoud, S.H.; Prasad, A.R.; Garvasis, J. Plant-Based Synthesis, Characterization Approaches, Applications and Toxicity of Silver Nanoparticles: A Comprehensive Review. J. Biotechnol. 2024, 394, 135–149. [Google Scholar] [CrossRef]
- Larrañaga-Tapia, M.; Betancourt-Tovar, B.; Videa, M.; Antunes-Ricardo, M.; Cholula-Díaz, J.L. Green Synthesis Trends and Potential Applications of Bimetallic Nanoparticles towards the Sustainable Development Goals 2030. Nanoscale Adv. 2024, 6, 51–71. [Google Scholar] [CrossRef]
- Gebreslassie, Y.T.; Gebremeskel, F.G. Green and Cost-Effective Biofabrication of Copper Oxide Nanoparticles: Exploring Antimicrobial and Anticancer Applications. Biotechnol. Rep. 2024, 41, e00828. [Google Scholar] [CrossRef]
- Mahesh, B. A Comprehensive Review on Current Trends in Greener and Sustainable Synthesis of Ferrite Nanoparticles and Their Promising Applications. Results Eng. 2024, 21, 101702. [Google Scholar] [CrossRef]
- Eddy, D.R.; Rahmawati, D.; Permana, M.D.; Takei, T.; Noviyanti, A.R.; Rahayu, I. A Review of Recent Developments in Green Synthesis of TiO2 Nanoparticles Using Plant Extract: Synthesis, Characterization and Photocatalytic Activity. Inorg. Chem. Commun. 2024, 165, 112531. [Google Scholar] [CrossRef]
- Ahmad, S.; Ahmad, S.; Ali, S.; Esa, M.; Khan, A.; Yan, H. Recent Advancements and Unexplored Biomedical Applications of Green Synthesized Ag and Au Nanoparticles: A Review. Int. J. Nanomed. 2024, 19, 3187–3215. [Google Scholar] [CrossRef]
- Gaur, J.; Kumar, S.; Pal, M.; Kaur, H.; Batoo, K.M.; Momoh, J.O. Supreet Current Trends: Zinc Oxide Nanoparticles Preparation via Chemical and Green Method for the Photocatalytic Degradation of Various Organic Dyes. Hybrid Adv. 2024, 5, 100128. [Google Scholar] [CrossRef]
- Kummara, S.; Patil, M.B.; Uriah, T. Synthesis, Characterization, Biocompatible and Anticancer Activity of Green and Chemically Synthesized Silver Nanoparticles—A Comparative Study. Biomed. Pharmacother. 2016, 84, 10–21. [Google Scholar] [CrossRef]
- Vanlalveni, C.; Ralte, V.; Zohmingliana, H.; Das, S.; Anal, J.M.H.; Lallianrawna, S.; Rokhum, S.L. A Review of Microbes Mediated Biosynthesis of Silver Nanoparticles and Their Enhanced Antimicrobial Activities. Heliyon 2024, 10, e32333. [Google Scholar] [CrossRef]
- Tarannum, N.; Divya, D.; Gautam, Y.K. Facile Green Synthesis and Applications of Silver Nanoparticles: A State-of-the-Art Review. RSC Adv. 2019, 9, 34926–34948. [Google Scholar] [CrossRef]
- Shah, A.H.; Rather, M.A. Intracellular and Extracellular Microbial Enzymes and Their Role in Nanoparticle Synthesis. In Microbial Nanotechnology: Green Synthesis and Applications; Ansari, M.A., Rehman, S., Eds.; Springer: Singapore, 2021; pp. 41–59. ISBN 978-981-16-1923-6. [Google Scholar]
- De Souza, T.A.J.; Rosa Souza, L.R.; Franchi, L.P. Silver Nanoparticles: An Integrated View of Green Synthesis Methods, Transformation in the Environment, and Toxicity. Ecotoxicol. Environ. Saf. 2019, 171, 691–700. [Google Scholar] [CrossRef]
- Ren Loi, H.; Abbasiliasi, S.; Bothi Raja, P.; Shamzi Mohamed, M.; Tan, W.-N.; Suan Ng, H.; Chi-Wei Lan, J.; Shun Tan, J. Biosynthesis of Silver Nanoparticles Using Nitrate Reductase Produced by Lactobacillus plantarum CAM 4: Characterization and in Vitro Evaluation of Its Antimicrobial Efficiency. J. Mol. Liq. 2023, 376, 121476. [Google Scholar] [CrossRef]
- Saba, M.; Farooq, S.; Alessa, A.H.; Bektas, K.I.; Belduz, A.O.; Khan, A.Z.; Shah, A.A.; Badshah, M.; Khan, S. Green Synthesis of Silver Nanoparticles Using Keratinase from Pseudomonas Aeruginosa-C1M, Characterization and Applications as Novel Multifunctional Biocatalyst. BMC Biotechnol. 2025, 25, 27. [Google Scholar] [CrossRef]
- AbdelGawwad, M.R.; Mahmutović, E.; Al Farraj, D.A.; Elshikh, M.S. In Silico Prediction of Silver Nitrate Nanoparticles and Nitrate Reductase A (NAR A) Interaction in the Treatment of Infectious Disease Causing Clinical Strains of E. Coli. J. Infect. Public Health 2020, 13, 1580–1585. [Google Scholar] [CrossRef]
- Mukherjee, K.; Gupta, R.; Kumar, G.; Kumari, S.; Biswas, S.; Padmanabhan, P. Synthesis of Silver Nanoparticles by Bacillus clausii and Computational Profiling of Nitrate Reductase Enzyme Involved in Production. J. Genet. Eng. Biotechnol. 2018, 16, 527–536. [Google Scholar] [CrossRef]
- Annamalai, J.; Ummalyma, S.B.; Pandey, A.; Bhaskar, T. Recent Trends in Microbial Nanoparticle Synthesis and Potential Application in Environmental Technology: A Comprehensive Review. Environ. Sci. Pollut. Res. 2021, 28, 49362–49382. [Google Scholar] [CrossRef]
- Plokhovska, S.; García-Villaraco, A.; Lucas, J.A.; Gutierrez-Mañero, F.J.; Ramos-Solano, B. Silver Nanoparticles Coated with Metabolites of Pseudomonas sp. N5.12 Inhibit Bacterial Pathogens and Fungal Phytopathogens. Sci. Rep. 2025, 15, 1522. [Google Scholar] [CrossRef]
- Alam, A.; Tanveer, F.; Khalil, A.T.; Zohra, T.; Khamlich, S.; Alam, M.M.; Salman, M.; Ali, M.; Ikram, A.; Shinwari, Z.K.; et al. Silver Nanoparticles Biosynthesized from Secondary Metabolite Producing Marine Actinobacteria and Evaluation of Their Biomedical Potential. Antonie Leeuwenhoek 2021, 114, 1497–1516. [Google Scholar] [CrossRef]
- Dey, G.; Patil, M.P.; Banerjee, A.; Sharma, R.K.; Banerjee, P.; Maity, J.P.; Singha, S.; Taharia, M.; Shaw, A.K.; Huang, H.-B.; et al. The Role of Bacterial Exopolysaccharides (EPS) in the Synthesis of Antimicrobial Silver Nanomaterials: A State-of-the-Art Review. J. Microbiol. Methods 2023, 212, 106809. [Google Scholar] [CrossRef]
- Sambalova, O.; Thorwarth, K.; Heeb, N.V.; Bleiner, D.; Zhang, Y.; Borgschulte, A.; Kroll, A. Carboxylate Functional Groups Mediate Interaction with Silver Nanoparticles in Biofilm Matrix. ACS Omega 2018, 3, 724–733. [Google Scholar] [CrossRef]
- Kwon, Y.M.; Cho, E.-S.; Kim, K.W.; Chung, D.; Bae, S.S.; Yu, W.-J.; Kim, J.Y.H.; Choi, G. Synthesis of Silver Nanoparticles Using Aggregatimonas Sangjinii F202Z8T and Their Biological Characterization. Microorganisms 2023, 11, 2975. [Google Scholar] [CrossRef]
- Adebayo-Tayo, B.; Salaam, A.; Ajibade, A. Green Synthesis of Silver Nanoparticle Using Oscillatoria sp. Extract, Its Antibacterial, Antibiofilm Potential and Cytotoxicity Activity. Heliyon 2019, 5, e02502. [Google Scholar] [CrossRef]
- Cekuolyte, K.; Gudiukaite, R.; Klimkevicius, V.; Mazrimaite, V.; Maneikis, A.; Lastauskiene, E. Biosynthesis of Silver Nanoparticles Produced Using Geobacillus spp. Bacteria. Nanomaterials 2023, 13, 702. [Google Scholar] [CrossRef]
- Solís-Sandí, I.; Cordero-Fuentes, S.; Pereira-Reyes, R.; Vega-Baudrit, J.R.; Batista-Menezes, D.; De Oca-Vásquez, G.M. Optimization of the Biosynthesis of Silver Nanoparticles Using Bacterial Extracts and Their Antimicrobial Potential. Biotechnol. Rep. 2023, 40, e00816. [Google Scholar] [CrossRef]
- Sharma, S.; Sharma, N.; Kaushal, N. Comparative Account of Biogenic Synthesis of Silver Nanoparticles Using Probiotics and Their Antimicrobial Activity Against Challenging Pathogens. BioNanoScience 2022, 12, 833–840. [Google Scholar] [CrossRef]
- Abdelmoneim, H.M.; Taha, T.H.; Elnouby, M.S.; AbuShady, H.M. Extracellular Biosynthesis, OVAT/Statistical Optimization, and Characterization of Silver Nanoparticles (AgNPs) Using Leclercia Adecarboxylata THHM and Its Antimicrobial Activity. Microb. Cell Fact. 2022, 21, 277. [Google Scholar] [CrossRef]
- Atalah, J.; Espina, G.; Blamey, L.; Muñoz-Ibacache, S.A.; Blamey, J.M. Advantages of Using Extremophilic Bacteria for the Biosynthesis of Metallic Nanoparticles and Its Potential for Rare Earth Element Recovery. Front. Microbiol. 2022, 13, 855077. [Google Scholar] [CrossRef]
- Garg, D.; Sarkar, A.; Chand, P.; Bansal, P.; Gola, D.; Sharma, S.; Khantwal, S.; Surabhi; Mehrotra, R.; Chauhan, N.; et al. Synthesis of Silver Nanoparticles Utilizing Various Biological Systems: Mechanisms and Applications—A Review. Prog. Biomater. 2020, 9, 81–95. [Google Scholar] [CrossRef]
- Ahmad, S.; Munir, S.; Zeb, N.; Ullah, A.; Khan, B.; Ali, J.; Bilal, M.; Omer, M.; Alamzeb, M.; Salman, S.M.; et al. Green Nanotechnology: A Review on Green Synthesis of Silver Nanoparticles—An Ecofriendly Approach. Int. J. Nanomed. 2019, 14, 5087–5107. [Google Scholar] [CrossRef]
- Khan, F.; Shahid, A.; Zhu, H.; Wang, N.; Javed, M.R.; Ahmad, N.; Xu, J.; Alam, A.; Mehmood, M.A. Prospects of Algae-Based Green Synthesis of Nanoparticles for Environmental Applications. Chemosphere 2022, 293, 133571. [Google Scholar] [CrossRef]
- Dorcheh, S.K.; Vahabi, K. Biosynthesis of Nanoparticles by Fungi: Large-Scale Production. In Fungal Metabolites; Springer: Cham, Germany, 2016; pp. 1–20. ISBN 978-3-319-19456-1. [Google Scholar]
- Jain, A.S.; Pawar, P.S.; Sarkar, A.; Junnuthula, V.; Dyawanapelly, S. Bionanofactories for Green Synthesis of Silver Nanoparticles: Toward Antimicrobial Applications. Int. J. Mol. Sci. 2021, 22, 11993. [Google Scholar] [CrossRef]
- Guilger-Casagrande, M.; Lima, R.D. Synthesis of Silver Nanoparticles Mediated by Fungi: A Review. Front. Bioeng. Biotechnol. 2019, 7, 287. [Google Scholar] [CrossRef]
- Rai, M.; Bonde, S.; Golinska, P.; Trzcińska-Wencel, J.; Gade, A.; Abd-Elsalam, K.A.; Shende, S.; Gaikwad, S.; Ingle, A.P. Fusarium as a Novel Fungus for the Synthesis of Nanoparticles: Mechanism and Applications. J. Fungi 2021, 7, 139. [Google Scholar] [CrossRef]
- Barabadi, H.; Tajani, B.; Moradi, M.; Damavandi Kamali, K.; Meena, R.; Honary, S.; Mahjoub, M.A.; Saravanan, M. Penicillium Family as Emerging Nanofactory for Biosynthesis of Green Nanomaterials: A Journey into the World of Microorganisms. J. Clust. Sci. 2019, 30, 843–856. [Google Scholar] [CrossRef]
- Zomorodian, K.; Pourshahid, S.; Sadatsharifi, A.; Mehryar, P.; Pakshir, K.; Rahimi, M.J.; Arabi Monfared, A. Biosynthesis and Characterization of Silver Nanoparticles by Aspergillus Species. BioMed Res. Int. 2016, 2016, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Beltrán Pineda, M.E.; Lizarazo Forero, L.M.; Sierra, Y.C.A. Mycosynthesis of Silver Nanoparticles: A Review. BioMetals 2023, 36, 745–776. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.A.; Osman, M.E.; Mohamed, E.T. Eco-Friendly Biosynthesis of Silver Nanoparticles Using Marine-Derived Fusarium Exquisite: Optimization, Characterization, and Evaluation of Antimicrobial, Antioxidant, and Cytotoxic Activities. World J. Microbiol. Biotechnol. 2025, 41, 1–8. [Google Scholar] [CrossRef]
- Lotfy, W.A.; Alkersh, B.M.; Sabry, S.A.; Ghozlan, H.A. Biosynthesis of Silver Nanoparticles by Aspergillus terreus: Characterization, Optimization, and Biological Activities. Front. Bioeng. Biotechnol. 2021, 9, 633468. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, K.; Kumar, V. Green Synthesis of Silver Nanoparticles Using Penicillium Camemberti and Its Biological Applications. BioNanoScience 2024, 14, 5179–5193. [Google Scholar] [CrossRef]
- Saxena, J.; Sharma, P.K.; Sharma, M.M.; Singh, A. Process Optimization for Green Synthesis of Silver Nanoparticles by Sclerotinia Sclerotiorum MTCC 8785 and Evaluation of Its Antibacterial Properties. SpringerPlus 2016, 5, 861. [Google Scholar] [CrossRef]
- Osorio-Echavarría, J.; Osorio-Echavarría, J.; Ossa-Orozco, C.P.; Gómez-Vanegas, N.A. Synthesis of Silver Nanoparticles Using White-Rot Fungus Anamorphous Bjerkandera sp. R1: Influence of Silver Nitrate Concentration and Fungus Growth Time. Sci. Rep. 2021, 11, 3842. [Google Scholar] [CrossRef]
- Nayak, R.R.; Pradhan, N.; Behera, D.; Pradhan, K.M.; Mishra, S.; Sukla, L.B.; Mishra, B.K. Green Synthesis of Silver Nanoparticle by Penicillium Purpurogenum NPMF: The Process and Optimization. J. Nanopart. Res. 2011, 13, 3129–3137. [Google Scholar] [CrossRef]
- Soleimani, P.; Mehrvar, A.; Michaud, J.P.; Vaez, N. Optimization of Silver Nanoparticle Biosynthesis by Entomopathogenic Fungi and Assays of Their Antimicrobial and Antifungal Properties. J. Invertebr. Pathol. 2022, 190, 107749. [Google Scholar] [CrossRef]
- Zhu, Y.; Hu, X.; Qiao, M.; Zhao, L.; Dong, C. Penicillium Polonicum-Mediated Green Synthesis of Silver Nanoparticles: Unveiling Antimicrobial and Seed Germination Advancements. Heliyon 2024, 10, e28971. [Google Scholar] [CrossRef]
- Smirnov, O.; Dzhagan, V.; Yeshchenko, O.; Kovalenko, M.; Kapush, O.; Vuichyk, M.; Dzhagan, V.; Mazur, N.; Kalynovskyi, V.; Skoryk, M.; et al. Effect of pH of Ganoderma Lucidum Aqueous Extract on Green Synthesis of Silver Nanoparticles. Adv. Nat. Sci. Nanosci. Nanotechnol. 2023, 14, 035009. [Google Scholar] [CrossRef]
- Barabadi, H.; Honary, S.; Ebrahimi, P.; Alizadeh, A.; Naghibi, F.; Saravanan, M. Optimization of Myco-Synthesized Silver Nanoparticles by Response Surface Methodology Employing Box-Behnken Design. Inorg. Nano Met. Chem. 2019, 49, 33–43. [Google Scholar] [CrossRef]
- Hoseini-Nilaki, S.F.; Ashengroph, M.; Zorab, M.M. Eco-Friendly Synthesis of Silver Nanoparticles Using the Fungus Alternaria sp. OP242500: Optimization through Box-Behnken Design. Results Chem. 2025, 15, 102265. [Google Scholar] [CrossRef]
- Sarkar, J.; Naskar, A.; Nath, A.; Gangopadhyay, B.; Tarafdar, E.; Das, D.; Chakraborty, S.; Chattopadhyay, D.; Acharya, K. Innovative Utilization of Harvested Mushroom Substrate for Green Synthesis of Silver Nanoparticles: A Multi–Response Optimization Approach. Environ. Res. 2024, 248, 118297. [Google Scholar] [CrossRef]
- Rose, G.K.; Thakur, B.; Soni, R.; Soni, S.K. Biosynthesis of Silver Nanoparticles Using Nitrate Reductase from Aspergillus terreus N4 and Their Potential Use as a Non-Alcoholic Disinfectant. J. Biotechnol. 2023, 373, 49–62. [Google Scholar] [CrossRef]
- Othman, A.M.; Elsayed, M.A.; Elshafei, A.M.; Hassan, M.M. Application of Response Surface Methodology to Optimize the Extracellular Fungal Mediated Nanosilver Green Synthesis. J. Genet. Eng. Biotechnol. 2017, 15, 497–504. [Google Scholar] [CrossRef]
- Ashraf, N.; Ahmad, F.; Jing Jie, C.; Tuo Di, Z.; Feng-Zhu, Z.; Yin, D.-C. Optimization of Enterobacter Cloacae Mediated Synthesis of Extracellular Silver Nanoparticles by Response Surface Methodology and Their Characterization. Part. Sci. Technol. 2020, 38, 931–943. [Google Scholar] [CrossRef]
- Ibrahim, S.; Ahmad, Z.; Manzoor, M.Z.; Mujahid, M.; Faheem, Z.; Adnan, A. Optimization for Biogenic Microbial Synthesis of Silver Nanoparticles through Response Surface Methodology, Characterization, Their Antimicrobial, Antioxidant, and Catalytic Potential. Sci. Rep. 2021, 11, 770. [Google Scholar] [CrossRef]
- Mechouche, M.S.; Merouane, F.; Addad, A.; Karmazin, L.; Boukherroub, R.; Lakhdari, N. Enhanced Biosynthesis of Coated Silver Nanoparticles Using Isolated Bacteria from Heavy Metal Soils and Their Photothermal-Based Antibacterial Activity: Integrating Response Surface Methodology (RSM) Hybrid Artificial Neural Network (ANN)-Genetic Algorithm (GA) Strategies. World J. Microbiol. Biotechnol. 2024, 40, 252. [Google Scholar] [CrossRef]
- Ahmad, F.; Ashraf, N.; Zhou, R.-B.; Chen, J.J.; Liu, Y.-L.; Zeng, X.; Zhao, F.-Z.; Yin, D.-C. Optimization for Silver Remediation from Aqueous Solution by Novel Bacterial Isolates Using Response Surface Methodology: Recovery and Characterization of Biogenic AgNPs. J. Hazard. Mater. 2019, 380, 120906. [Google Scholar] [CrossRef]
- Kirubakaran, D.; Wahid, J.B.A.; Karmegam, N.; Jeevika, R.; Sellapillai, L.; Rajkumar, M.; SenthilKumar, K.J. A Comprehensive Review on the Green Synthesis of Nanoparticles: Advancements in Biomedical and Environmental Applications. Biomedical. Mater. Devices 2025, 4, 388–413. [Google Scholar] [CrossRef]
- Zhao, X.; Xu, X.; Ai, C.; Yan, L.; Jiang, C.; Shi, J. Chapter 22—Advantages of Silver Nanoparticles Synthesized by Microorganisms in Antibacterial Activity. In Green Synthesis of Silver Nanomaterials; Abd-Elsalam, K.A., Ed.; Nanobiotechnology for Plant Protection; Elsevier: Amsterdam, The Netherlands, 2022; pp. 571–586. ISBN 978-0-12-824508-8. [Google Scholar]
- Gudikandula, K.; Vadapally, P.; Singara Charya, M.A. Biogenic Synthesis of Silver Nanoparticles from White Rot Fungi: Their Characterization and Antibacterial Studies. OpenNano 2017, 2, 64–78. [Google Scholar] [CrossRef]
- Ibrahim, N.A.A.; Saeed, H.A.; Saeed, S.M.; Mohamed, O.; Suliman, O.H.; Ibrahim, S.A.E.; Mohamed, S.B. Green Synthesis of Silver Nanoparticles Using Sudanese Candida Parapsilosis: A Sustainable Approach to Combat Antimicrobial Resistance. BMC Microbiol. 2025, 25, 312. [Google Scholar] [CrossRef]
- Ali, E.M.; Rajendran, P.; Abdallah, B.M. Mycosynthesis of Silver Nanoparticles from Endophytic Aspergillus parasiticus and Their Antibacterial Activity against Methicillin-Resistant Staphylococcus aureus in Vitro and in Vivo. Front. Microbiol. 2024, 15, 1483637. [Google Scholar] [CrossRef]
- Oyebamiji, A.K.; Akintelu, S.A.; Afolabi, S.O.; Ebenezer, O.; Akintayo, E.T.; Akintayo, C.O. A Comprehensive Review on Mycosynthesis of Nanoparticles, Characteristics, Applications, and Limitations. Plasmonics 2025, 20, 6919–6937. [Google Scholar] [CrossRef]
- Elbahnasawy, M.A.; Shehabeldine, A.M.; Khattab, A.M.; Amin, B.H.; Hashem, A.H. Green Biosynthesis of Silver Nanoparticles Using Novel Endophytic Rothia Endophytica: Characterization and Anticandidal Activity. J. Drug Deliv. Sci. Technol. 2021, 62, 102401. [Google Scholar] [CrossRef]
- Kumar, V.; Parida, S.N.; Dhar, S.; Bisai, K.; Sarkar, D.J.; Panda, S.P.; Das, B.K. Biogenic Synthesis of Silver Nanoparticle by Cytobacillus firmus Isolated from the River Sediment with Potential Antimicrobial Properties against Edwardsiella tarda. Front. Microbiol. 2024, 15, 1416411. [Google Scholar] [CrossRef]
- Prema, P.; Subha Ranjani, S.; Ramesh Kumar, K.; Veeramanikandan, V.; Mathiyazhagan, N.; Nguyen, V.-H.; Balaji, P. Microbial Synthesis of Silver Nanoparticles Using Lactobacillus plantarum for Antioxidant, Antibacterial Activities. Inorg. Chem. Commun. 2022, 136, 109139. [Google Scholar] [CrossRef]
- Singh, P.; Pandit, S.; Jers, C.; Joshi, A.S.; Garnæs, J.; Mijakovic, I. Silver Nanoparticles Produced from Cedecea sp. Exhibit Antibiofilm Activity and Remarkable Stability. Sci. Rep. 2021, 11, 12619. [Google Scholar] [CrossRef]
- Huq, A.; Akter, S. Biosynthesis, Characterization and Antibacterial Application of Novel Silver Nanoparticles against Drug Resistant Pathogenic Klebsiella Pneumoniae and Salmonella Enteritidis. Molecules 2021, 26, 5996. [Google Scholar] [CrossRef]
- Nainangu, P.; Mothilal, S.N.; Subramanian, K.; Thanigaimalai, M.; Kandasamy, R.; Srinivasan, G.P.; Gopal, S.; Shaik, M.R.; Kari, Z.A.; Guru, A.; et al. Characterization and Antibacterial Evaluation of Eco-Friendly Silver Nanoparticles Synthesized by Halophilic Streptomyces Rochei SSCM102 Isolated from Mangrove Sediment. Mol. Biol. Rep. 2024, 51, 730. [Google Scholar] [CrossRef]
- Defaei, A.; Shahrian, M.; Karimi, J. Eco-Friendly Synthesis of Silver Nanoparticles from Filamentous Cyanobacteria Arthrospira Platensis Phycocyanin and Its Antifungal and Antibacterial Activities. S. Afr. J. Chem. Eng. 2025, 53, 495–499. [Google Scholar] [CrossRef]
- Li, Q.; Feng, T.; Li, H.; Wang, Z.; Wei, X.; Liu, J. Green Synthesis of Silver Nanoparticles Using Endophytic Bacterium Bacillus zanthoxyli GBE11 and Their Antimicrobial Activity. Biomass Conv. Bioref. 2024, 14, 13173–13185. [Google Scholar] [CrossRef]
- Mollania, H.; Oloomi-buygi, M.; Mollania, N. Catalytic and Anti-Cancer Properties of Platinum, Gold, Silver, and Bimetallic Au-Ag Nanoparticles Synthesized by Bacillus sp. Bacteria. J. Biotechnol. 2024, 379, 33–45. [Google Scholar] [CrossRef]
- Wafy, K.R.; El-Aswar, E.I.; Mohamed, W.S.E.; El-Sabbagh, S.M. Water Disinfection Using Durable Ceramic Filter Coated with Silver Nanoparticles Synthesized Using Actinomycetes. Appl. Water Sci. 2023, 13, 140. [Google Scholar] [CrossRef]
- Banerjee, A.; Das, D.; Andler, R.; Bandopadhyay, R. Green Synthesis of Silver Nanoparticles Using Exopolysaccharides Produced by Bacillus anthracis PFAB2 and Its Biocidal Property. J. Polym. Environ. 2021, 29, 2701–2709. [Google Scholar] [CrossRef]
- El Deeb, B.A.; Faheem, G.G.; Bakhit, M.S. Biosynthesis of Silver Nanoparticles by Talaromyces Funiculosus for Therapeutic Applications and Safety Evaluation. Sci. Rep. 2025, 15, 13750. [Google Scholar] [CrossRef]
- Rosyidah, A.; Weeranantanapan, O.; Chudapongse, N.; Limphirat, W.; Nantapong, N. Streptomyces chiangmaiensis SSUT88A Mediated Green Synthesis of Silver Nanoparticles: Characterization and Evaluation of Antibacterial Action against Clinical Drug-Resistant Strains. RSC Adv. 2022, 12, 4336–4345. [Google Scholar] [CrossRef] [PubMed]
- Dawoud, T.M.; Yassin, M.A.; El-Samawaty, A.R.M.; Elgorban, A.M. Silver Nanoparticles Synthesized by Nigrospora Oryzae Showed Antifungal Activity. Saudi J. Biol. Sci. 2021, 28, 1847–1852. [Google Scholar] [CrossRef]
- Mwangi, N.V.; Madivoli, S.E.; Kangogo, M.; Wangui, M.C.; Wanakai, I.S.; Nzilu, M.D.; Waudo, W. An Evaluation of Antimicrobial Potency of Silver Nanoparticles Synthesised from Fusarium sp. Discov. Appl. Sci. 2024, 6, 201. [Google Scholar] [CrossRef]
- Yassin, M.A.; Elgorban, A.M.; El-Samawaty, A.E.-R.M.A.; Almunqedhi, B.M.A. Biosynthesis of Silver Nanoparticles Using Penicillium Verrucosum and Analysis of Their Antifungal Activity. Saudi J. Biol. Sci. 2021, 28, 2123–2127. [Google Scholar] [CrossRef]
- Jana, S.; Das, D.; Bhattacharyya, S.; Raha, S. Mycogenic Synthesis of Silver Nanoparticles Using Endophytic Fungi and Their Characterization, Biological Activities, Including in-Silico Studies with Special Reference to Fusarium Wilt of Tomato. Fungal Biol. 2025, 129, 101610. [Google Scholar] [CrossRef]
- Kalyanasundaram, P.; Ravi, Y.; Velu, R.K. Sustainable Production of Ag-NPs Using Aristolochia Indica Endophytic Fungi and Exploration of Their Anti-Angiogenic and Cytotoxic Potential. J. Inorg. Organomet. Polym. 2025. [Google Scholar] [CrossRef]
- Ali, S.; Fatima, L.; Ahmad, M.U.; Khan, Q.F.; Hayyat, M.U.; Siddiq, Z.; Patel, S.; Shah, T.A.; Jardan, Y.A.B.; Younous, Y.A.; et al. Green Synthesis of Agaricus Avensis-Mediated Silver Nanoparticles for Improved Catalytic Efficiency of Tyrosine Hydroxylase towards Potential Biomedical Implications. Discov. Life 2024, 54, 4. [Google Scholar] [CrossRef]
- Khalil, T.; Sarwar, S.; Javed, S.; Abid, A.; Hanif, M.; Khalil, H.; Abrar, A. Green Synthesis of Silver Nanoparticles Using Wild Mushroom Lepista Sordida and Its Antifungal Activity. Pak. J. Bot. 2025, 57, 931–938. [Google Scholar] [CrossRef]
- Shankar, A.; Kaushik, N.K.; Kumar, V.; Malik, V.; Singh, D.; Kumar, V.; Singh, B. Green Mycosynthesis of Silver Nanoparticles Exhibiting Antimalarial Activity. J. Taibah Univ. Sci. 2024, 18, 2381286. [Google Scholar] [CrossRef]
- Mikhailova, E.O. Green Silver Nanoparticles: An Antibacterial Mechanism. Antibiotics 2025, 14, 5. [Google Scholar] [CrossRef] [PubMed]
- Xia, F.; Tao, X.; Wang, H.; Shui, J.; Min, C.; Xia, Y.; Li, J.; Tang, M.; Liu, Z.; Hu, Y.; et al. Biosynthesis of Silver Nanoparticles Using the Biofilm Supernatant of Pseudomonas Aeruginosa PA75 and Evaluation of Their Antibacterial, Antibiofilm, and Antitumor Activities. Int. J. Nanomed. 2023, 18, 2485–2502. [Google Scholar] [CrossRef] [PubMed]
- Algburi, A.; Mubarak, R.M.; Mubarak, T.H.; Fathi-karkan, S.; Rahdar, A.; Romanholo Ferreira, L.F. Lactobacillus acidophilus and Mixed Probiotic-Mediated Synthesis of Silver Nanoparticles: Antibacterial Efficacy Against Multidrug-Resistant Otopathogens. BioNanoScience 2025, 15, 419. [Google Scholar] [CrossRef]
- Khairnar, S.V.; Das, A.; Oupický, D.; Sadykov, M.; Romanova, S. Strategies to Overcome Antibiotic Resistance: Silver Nanoparticles and Vancomycin in Pathogen Eradication. RSC Pharm. 2025, 2, 455–479. [Google Scholar] [CrossRef]
- Abeer Mohammed, A.B.; Abd Elhamid, M.M.; Khalil, M.K.M.; Ali, A.S.; Abbas, R.N. The Potential Activity of Biosynthesized Silver Nanoparticles of Pseudomonas Aeruginosa as an Antibacterial Agent against Multidrug-Resistant Isolates from Intensive Care Unit and Anticancer Agent. Environ. Sci. Eur. 2022, 34, 109. [Google Scholar] [CrossRef]
- Haji, S.H.; Ali, F.A.; Aka, S.T.H. Synergistic Antibacterial Activity of Silver Nanoparticles Biosynthesized by Carbapenem-Resistant Gram-Negative Bacilli. Sci. Rep. 2022, 12, 15254. [Google Scholar] [CrossRef] [PubMed]
- Ahamad, I.; Aziz, N.; Zaki, A.; Fatma, T. Synthesis and Characterization of Silver Nanoparticles Using Anabaena Variabilis as a Potential Antimicrobial Agent. J. Appl. Phycol. 2021, 33, 829–841. [Google Scholar] [CrossRef]
- Ajaz, S.; Ahmed, T.; Shahid, M.; Noman, M.; Shah, A.A.; Mehmood, M.A.; Abbas, A.; Cheema, A.I.; Iqbal, M.Z.; Li, B. Bioinspired Green Synthesis of Silver Nanoparticles by Using a Native Bacillus sp. Strain AW1-2: Characterization and Antifungal Activity against Colletotrichum Falcatum Went. Enzym. Microb. Technol. 2021, 144, 109745. [Google Scholar] [CrossRef]
- Pallath, N.; Francis, B.; Devanesan, S.; Asemi, N.; AlSalhi, M.S.; Natarajan, S. Synthesis of Silver Nanoparticles from Marine Bacteria and Evaluation of Antimicrobial, Antifungal and Cytotoxic Effects. J. King Saud Univ. Sci. 2024, 36, 103073. [Google Scholar] [CrossRef]
- Chatterjee, N.; Pal, S.; Dhar, P. Green Silver Nanoparticles from Bacteria- Antioxidant, Cytotoxic and Antifungal Activities. Next Nanotechnol. 2024, 6, 100089. [Google Scholar] [CrossRef]
- Kthiri, A.; Hamimed, S.; Othmani, A.; Landoulsi, A.; O’Sullivan, S.; Sheehan, D. Novel Static Magnetic Field Effects on Green Chemistry Biosynthesis of Silver Nanoparticles in Saccharomyces Cerevisiae. Sci. Rep. 2021, 11, 20078. [Google Scholar] [CrossRef]
- Ilahi, N.; Haleem, A.; Iqbal, S.; Fatima, N.; Sajjad, W.; Sideeq, A.; Ahmed, S. Biosynthesis of Silver Nanoparticles Using Endophytic Fusarium Oxysporum Strain NFW16 and Their in Vitro Antibacterial Potential. Microsc. Res. Tech. 2022, 85, 1568–1579. [Google Scholar] [CrossRef]
- Abdel-Kareem, M.M.; Ali, M.M.A.; Hesham, A.E.-L.; Abdel-Raheam, H.E.F.; Obiedallah, M. Mycosynthesis of Silver Nanoparticles by Aspergillus templicola OR480102: A Multifaceted Approach for Antibacterial, Anticancer, and Scratch Assay Applications. BMC Biotechnol. 2025, 25, 46. [Google Scholar] [CrossRef] [PubMed]
- Nongthombam, K.S.; Kannaiah, S.; Raju, P.; Govindan, L.; Mutum, S.S.; Pandey, R.R. Biogenic Production of Silver Nanoparticles Using Neocosmospora Solani Endophytic Fungal Extract: Their In Vitro Antibacterial and Anticancer Properties. J. Inorg. Organomet. Polym. 2025, 35, 1603–1614. [Google Scholar] [CrossRef]
- Dadayya, M.; Thippeswamy, M.G.; Shivaiah, N.; Siddaraju, T.R.; Jayaramaiah, P.; Veeranna, S.H.; Basaiah, T.; Mathad, S.N.; Hemagiri Gowda, R.; Naik, S.; et al. Pharmacological Properties of Biomimetic Synthesized Silver Nanoparticles from Endophytic Fungus Coniothyrium Chaingmaiense: KUMBMDBT-25. Sci. Rep. 2025, 15, 606. [Google Scholar] [CrossRef]
- Ahmad, N.; Malik, M.A.; Wani, A.H.; Bhat, M.Y. Biogenic Silver Nanoparticles from Fungal Sources: Synthesis, Characterization, and Antifungal Potential. Microb. Pathog. 2024, 193, 106742. [Google Scholar] [CrossRef]
- Ishfaq, S.; Liang, X.; Ding, Y.; Zhang, J.; Zhang, F.; Anjum, A.; Guo, W. Integrated Transcriptomic and Metabolomic Analysis Reveals the Antifungal Effects of Myco-Silver Nanoparticles against Toxigenic Fusarium Verticillioides. J. Environ. Manag. 2025, 390, 126360. [Google Scholar] [CrossRef]
- Oluranti, O.O.; Ogundeji, B.A.; Orisajo, S.B. Comparative Impacts of Myco-Synthesized Nanoparticles against Strains of Aspergillus spp. Causing Biodeterioration of Stored Cocoa Beans in Nigeria. Discov. Nano 2024, 19, 207. [Google Scholar] [CrossRef]
- Tian, S.; Saravanan, K.; Mothana, R.A.; Ramachandran, G.; Rajivgandhi, G.; Manoharan, N. Anti-Cancer Activity of Biosynthesized Silver Nanoparticles Using Avicennia Marina against A549 Lung Cancer Cells through ROS/Mitochondrial Damages. Saudi J. Biol. Sci. 2020, 27, 3018–3024. [Google Scholar] [CrossRef]
- Pallavi, S.S.; Rudayni, H.A.; Bepari, A.; Niazi, S.K.; Nayaka, S. Green Synthesis of Silver Nanoparticles Using Streptomyces Hirsutus Strain SNPGA-8 and Their Characterization, Antimicrobial Activity, and Anticancer Activity against Human Lung Carcinoma Cell Line A549. Saudi J. Biol. Sci. 2022, 29, 228–238. [Google Scholar] [CrossRef]
- Souza, T.A.J.; Franchi, L.P.; Rosa, L.R.; Da Veiga, M.A.M.S.; Takahashi, C.S. Cytotoxicity and Genotoxicity of Silver Nanoparticles of Different Sizes in CHO-K1 and CHO-XRS5 Cell Lines. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2016, 795, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Fattah, W.I.; Ali, W.G. On the Anti-Cancer Activities of Silver Nanoparticles. J. Appl. Biotechnol. Bioeng. 2018, 5, 43–46. [Google Scholar] [CrossRef]
- Wypij, M.; Jędrzejewski, T.; Trzcińska-Wencel, J.; Ostrowski, M.; Rai, M.; Golińska, P. Green Synthesized Silver Nanoparticles: Antibacterial and Anticancer Activities, Biocompatibility, and Analyses of Surface-Attached Proteins. Front. Microbiol. 2021, 12, 632505. [Google Scholar] [CrossRef]
- Rudrappa, M.; Kumar, R.S.; Nagaraja, S.K.; Hiremath, H.; Gunagambhire, P.V.; Almansour, A.I.; Perumal, K.; Nayaka, S. Myco-Nanofabrication of Silver Nanoparticles by Penicillium Brasilianum NP5 and Their Antimicrobial, Photoprotective and Anticancer Effect on MDA-MB-231 Breast Cancer Cell Line. Antibiotics 2023, 12, 567. [Google Scholar] [CrossRef]
- El-Ansary, A.E.; Omran, A.A.A.; Mohamed, H.I.; El-Mahdy, O.M. Green Synthesized Silver Nanoparticles Mediated by Fusarium Nygamai Isolate AJTYC1: Characterizations, Antioxidant, Antimicrobial, Anticancer, and Photocatalytic Activities and Cytogenetic Effects. Environ. Sci. Pollut. Res. 2023, 30, 100477–100499. [Google Scholar] [CrossRef]
- Aboul-Nasr, M.B.; Yasien, A.A.; Mohamed, S.S.; Aboul-Nasr, Y.B.; Obiedallah, M. Exploring the Anticancer Potential of Green Silver Nanoparticles–Paclitaxel Nanocarrier on MCF-7 Breast Cancer Cells: An in Vitro Approach. Sci. Rep. 2025, 15, 20198. [Google Scholar] [CrossRef]
- Lan Chi, N.T.; Veeraragavan, G.R.; Brindhadevi, K.; Chinnathambi, A.; Salmen, S.H.; Alharbi, S.A.; Krishnan, R.; Pugazhendhi, A. Fungi Fabrication, Characterization, and Anticancer Activity of Silver Nanoparticles Using Metals Resistant Aspergillus niger. Environ. Res. 2022, 208, 112721. [Google Scholar] [CrossRef] [PubMed]
- Akther, T.; Vabeiryureilai, M.; Nachimuthu, S.K.; Davoodbasha, M.; Srinivasan, H. Fungal-Mediated Synthesis of Pharmaceutically Active Silver Nanoparticles and Anticancer Property against A549 Cells through Apoptosis. Environ. Sci. Pollut. Res. 2019, 26, 13649–13657. [Google Scholar] [CrossRef]
- Bishoyi, A.K.; Mandhata, C.P.; Sahoo, C.R.; Samal, P.; Dubey, D.; Jali, B.R.; Alamri, A.M.; Khan, M.S.; Padhy, R.N. Biogenic Synthesis and Characterization of Silver Nanoparticles With Cyanobacterium Oscillatoria salina Using Against MDR Pathogenic Bacteria and Their Antiproliferative and Toxicity Study. Cell Biochem. Funct. 2025, 43, e70043. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Zahoor, M.; Khan, R.S.; Ikram, M.; Islam, N.U. The Impact of Silver Nanoparticles on the Growth of Plants: The Agriculture Applications. Heliyon 2023, 9, e16928. [Google Scholar] [CrossRef] [PubMed]
- Kale, S.K.; Parishwad, G.V.; Husainy, A.S.N.; Patil, A.S. Emerging Agriculture Applications of Silver Nanoparticles. ES Food Agrofor. 2021, 3, 17–22. [Google Scholar] [CrossRef]
- Ibrahim, E.; Fouad, H.; Zhang, M.; Zhang, Y.; Qiu, W.; Yan, C.; Li, B.; Mo, J.; Chen, J. Biosynthesis of Silver Nanoparticles Using Endophytic Bacteria and Their Role in Inhibition of Rice Pathogenic Bacteria and Plant Growth Promotion. RSC Adv. 2019, 9, 29293–29299. [Google Scholar] [CrossRef]
- Khoa, N.Đ.; Xạ, T.V.; Hào, L.T. Disease-Reducing Effects of Aqueous Leaf Extract of Kalanchoe Pinnata on Rice Bacterial Leaf Blight Caused by Xanthomonas Oryzae Pv. Oryzae Involve Induced Resistance. Physiol. Mol. Plant Pathol. 2017, 100, 57–66. [Google Scholar] [CrossRef]
- Marpu, S.; Kolailat, S.S.; Korir, D.; Kamras, B.L.; Chaturvedi, R.; Joseph, A.; Smith, C.M.; Palma, M.C.; Shah, J.; Omary, M.A. Photochemical Formation of Chitosan-Stabilized near-Infrared-Absorbing Silver Nanoworms: A “Green” Synthetic Strategy and Activity on Gram-Negative Pathogenic Bacteria. J. Colloid Interface Sci. 2017, 507, 437–452. [Google Scholar] [CrossRef] [PubMed]
- Masum, M.I.; Siddiqa, M.; Ali, K.A.; Zhang, Y.; Abdallah, Y.; Ibrahim, E.; Qiu, W.; Yan, C.; Li, B. Biogenic Synthesis of Silver Nanoparticles Using Phyllanthus Emblica Fruit Extract and Its Inhibitory Action Against the Pathogen Acidovorax Oryzae Strain RS-2 of Rice Bacterial Brown Stripe. Front. Microbiol. 2019, 10, 820. [Google Scholar] [CrossRef]
- Rivas-Cáceres, R.R.; Luis Stephano-Hornedo, J.; Lugo, J.; Vaca, R.; Del Aguila, P.; Yañez-Ocampo, G.; Mora-Herrera, M.E.; Camacho Díaz, L.M.; Cipriano-Salazar, M.; Alaba, P.A. Bactericidal Effect of Silver Nanoparticles against Propagation of Clavibacter Michiganensis Infection in Lycopersicon Esculentum Mill. Microb. Pathog. 2018, 115, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, M.; Prabhuram, S.R.R.; Kumar, G.V.; Chamseddine, A.; Velusamy, P.; Bajaber, M.A.; Gopinath, S.C.B. Pseudomonas Fluorescens–Based Biogenic Silver Nanoparticles: A Green Solution for Brown Leaf Spot Disease in Rice. BioNanoScience 2025, 15, 1–11. [Google Scholar] [CrossRef]
- Hulkoti, N.I.; Taranath, T.C. Biosynthesis of Nanoparticles Using Microbes—A Review. Colloids Surf. B Biointerfaces 2014, 121, 474–483. [Google Scholar] [CrossRef]
- Saravanan, M.; Barik, S.K.; MubarakAli, D.; Prakash, P.; Pugazhendhi, A. Synthesis of Silver Nanoparticles from Bacillus brevis (NCIM 2533) and Their Antibacterial Activity against Pathogenic Bacteria. Microb. Pathog. 2018, 116, 221–226. [Google Scholar] [CrossRef]
- Ahmed, T.; Shahid, M.; Noman, M.; Niazi, M.B.K.; Mahmood, F.; Manzoor, I.; Zhang, Y.; Li, B.; Yang, Y.; Yan, C.; et al. Silver Nanoparticles Synthesized by Using Bacillus cereus SZT1 Ameliorated the Damage of Bacterial Leaf Blight Pathogen in Rice. Pathogens 2020, 9, 160. [Google Scholar] [CrossRef]
- Sun, D.; Chen, R.; Lei, L.; Zhang, F. Green Synthesis of Silver Nanoparticles from the Endophytic Fungus Panax Notoginseng and Their Antioxidant and Antimicrobial Activities and Effects on Cherry Tomato Preservation. Int. J. Food Microbiol. 2025, 431, 111083. [Google Scholar] [CrossRef]
- Osman, A.I.; Zhang, Y.; Farghali, M.; Rashwan, A.K.; Eltaweil, A.S.; Abd El-Monaem, E.M.; Mohamed, I.M.A.; Badr, M.M.; Ihara, I.; Rooney, D.W.; et al. Synthesis of Green Nanoparticles for Energy, Biomedical, Environmental, Agricultural, and Food Applications: A Review. Environ. Chem. Lett. 2024, 22, 841–887. [Google Scholar] [CrossRef]
- Sharma, R.K.; Yadav, S.; Dutta, S.; Kale, H.B.; Warkad, I.R.; Zbořil, R.; Varma, R.S.; Gawande, M.B. Silver Nanomaterials: Synthesis and (Electro/Photo) Catalytic Applications. Chem. Soc. Rev. 2021, 50, 11293–11380. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Savanur, M.R.S.; Singh, S.K.; Singh, J.; Bhattacharya, S.; Asnani, K.; Kumar, B.; Kumar, M.; Kumar, S. Recent Advances in Silver Nanoparticles-Catalyzed Reactions. ChemistrySelect 2025, 10, e202403655. [Google Scholar] [CrossRef]
- Mechouche, M.S.; Merouane, F.; Messaad, C.E.H.; Golzadeh, N.; Vasseghian, Y.; Berkani, M. Biosynthesis, Characterization, and Evaluation of Antibacterial and Photocatalytic Methylene Blue Dye Degradation Activities of Silver Nanoparticles from Streptomyces Tuirus Strain. Environ. Res. 2022, 204, 112360. [Google Scholar] [CrossRef]
- Saied, E.; Hashem, A.H.; Ali, O.M.; Selim, S.; Almuhayawi, M.S.; Elbahnasawy, M.A. Photocatalytic and Antimicrobial Activities of Biosynthesized Silver Nanoparticles Using Cytobacillus firmus. Life 2022, 12, 1331. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, H.K.; Anwer, S.S.; Zrary, T.J. Dye Removal Efficiency of Three Fungal Isolates Using Green-Synthesized Silver Nanoparticles. A Comparative Study. Bioremediat. J. 2025, 1–22. [Google Scholar] [CrossRef]
- Saied, E.; Abdel-Maksoud, M.A.; Alfuraydi, A.A.; Kiani, B.H.; Bassyouni, M.; Al-Qabandi, O.A.; Bougafa, F.H.E.; Badawy, M.S.E.M.; Hashem, A.H. Endophytic Aspergillus hiratsukae Mediated Biosynthesis of Silver Nanoparticles and Their Antimicrobial and Photocatalytic Activities. Front. Microbiol. 2024, 15, 1345423. [Google Scholar] [CrossRef] [PubMed]
- Sutar, A.A.; More, S.V. Textile Dye Degradation and Antibacterial Potential of Myco-Synthesized Silver Nano Particles (AgNPs). Int. J. Innov. Sci. Res. Technol. 2022, 7, 395–404. [Google Scholar] [CrossRef]
- Abd Elghaffar, R.Y.; Emam, A.M.; Taher, E.S.; Baz, M.M.; Nayel, H.; Abdeen, A.; El-Nablaway, M.; Alwutayd, K.M.; Mihaela, O.; Ioan, B.-D.; et al. The Potential Biological Activities of Aspergillus luchuensis-Aided Green Synthesis of Silver Nanoparticles. Front. Microbiol. 2024, 15, 1381302. [Google Scholar] [CrossRef]
- Basheer, M.A.; Abutaleb, K.; Abed, N.N.; Mekawey, A.A.I. Mycosynthesis of Silver Nanoparticles Using Marine Fungi and Their Antimicrobial Activity against Pathogenic Microorganisms. J. Genet. Eng. Biotechnol. 2023, 21, 127. [Google Scholar] [CrossRef]
- Namasivayam, S.K.R.; Kumar, S.N.; Kamil, T.M.; Ravi, T. Biopolymer-Mediated Coating Influence on Wastewater Treatment Efficacy of Silver Nanoparticles Synthesized from Fungal Consortium. Natl. Acad. Sci. Lett. 2020, 43, 557–561. [Google Scholar] [CrossRef]
- Jaswal, T.; Gupta, J. A Review on the Toxicity of Silver Nanoparticles on Human Health. Mater. Today Proc. 2023, 81, 859–863. [Google Scholar] [CrossRef]
- Moon, J.; Kwak, J.I.; An, Y.-J. The Effects of Silver Nanomaterial Shape and Size on Toxicity to Caenorhabditis Elegans in Soil Media. Chemosphere 2019, 215, 50–56. [Google Scholar] [CrossRef]
- Das, B.; Tripathy, S.; Adhikary, J.; Chattopadhyay, S.; Mandal, D.; Dash, S.K.; Das, S.; Dey, A.; Dey, S.K.; Das, D.; et al. Surface Modification Minimizes the Toxicity of Silver Nanoparticles: An in Vitro and in Vivo Study. J. Biol. Inorg. Chem. 2017, 22, 893–918. [Google Scholar] [CrossRef]
- Ramzan, U.; Majeed, W.; Hussain, A.A.; Qurashi, F.; Qamar, S.U.R.; Naeem, M.; Uddin, J.; Khan, A.; Al-Harrasi, A.; Razak, S.I.A.; et al. New Insights for Exploring the Risks of Bioaccumulation, Molecular Mechanisms, and Cellular Toxicities of AgNPs in Aquatic Ecosystem. Water 2022, 14, 2192. [Google Scholar] [CrossRef]
- Reijnders, L. Is ‘Green’ Gold and Silver Nanoparticle Synthesis Environmentally Friendly? Nanomaterials 2025, 15, 1095. [Google Scholar] [CrossRef]
- Castro, V.L.; Jonsson, C.M.; Silva, M.S.G.M.; Castanha, R.; Vallim, J.H.; Da Silva, L.A.G.; De Oliveira, R.M.D.; Correa, D.S.; Ferreira, M.D. Estimates of AgNP Toxicity Thresholds in Support of Environmental Safety Policies. J. Nanopart. Res. 2022, 24, 9. [Google Scholar] [CrossRef]
- Khan, S.K.; Dutta, J.; Rather, M.A.; Ahmad, I.; Nazir, J.; Khan, I.A.; Wani, G.B. Silver Nanoparticle Toxicity in Rainbow Trout: Insights into Physiological and Molecular Responses. Toxicol. Mech. Methods 2025, 35, 382–397. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.-I.; Pak, S.-W.; Lee, S.-J.; Park, S.-H.; Shin, I.-S.; Moon, C.; Yu, W.-J.; Kim, S.-H.; Kim, J.-C. In Vitro Study of Silver Nanoparticles-Induced Embryotoxicity Using a Rat Whole Embryo Culture Model. Toxicol. Res. 2025, 41, 189–197. [Google Scholar] [CrossRef]
- Tareq, M.; Khadrawy, Y.A.; Rageh, M.M.; Mohammed, H.S. Dose-Dependent Biological Toxicity of Green Synthesized Silver Nanoparticles in Rat’s Brain. Sci. Rep. 2022, 12, 22642. [Google Scholar] [CrossRef]
- Balamurugan, V.; Ragavendran, C.; Arulbalachandran, D. Eco-Friendly Green Synthesis of AgNPs from Elaeocarpus Serratus Fruit Extract: Potential to Antibacterial, Antioxidant, Cytotoxic Effects of Colon Cancerous Cells (HT-29) and Its Toxicity Assessments of Marine Microcrustacean Artemia Nauplii. Mol. Biol. Rep. 2024, 51, 418. [Google Scholar] [CrossRef]
- Okuthe, G.E.; Siguba, B. Silver Nanoparticle-Induced Nephrotoxicity in Zebrafish (Danio Rerio). Int. J. Mol. Sci. 2025, 26, 4216. [Google Scholar] [CrossRef]
- Tończyk, A.; Niedziałkowska, K.; Lisowska, K. Ecotoxic Effect of Mycogenic Silver Nanoparticles in Water and Soil Environment. Sci. Rep. 2025, 15, 10815. [Google Scholar] [CrossRef]
- Sani Aliero, A.; Hasmoni, S.H.; Haruna, A.; Isah, M.; Malek, N.A.N.N.; Ahmad Zawawi, N. Bibliometric Exploration of Green Synthesized Silver Nanoparticles for Antibacterial Activity. Emerg. Contam. 2025, 11, 100411. [Google Scholar] [CrossRef]
- Hano, C.; Abbasi, B.H. Plant-Based Green Synthesis of Nanoparticles: Production, Characterization and Applications. Biomolecules 2022, 12, 31. [Google Scholar] [CrossRef]
- Nie, P.; Zhao, Y.; Xu, H. Synthesis, Applications, Toxicity and Toxicity Mechanisms of Silver Nanoparticles: A Review. Ecotoxicol. Environ. Saf. 2023, 253, 114636. [Google Scholar] [CrossRef] [PubMed]
- Vadakkan, K.; Rumjit, N.P.; Ngangbam, A.K.; Vijayanand, S.; Nedumpillil, N.K. Novel Advancements in the Sustainable Green Synthesis Approach of Silver Nanoparticles (AgNPs) for Antibacterial Therapeutic Applications. Coord. Chem. Rev. 2024, 499, 215528. [Google Scholar] [CrossRef]
- Sati, A.; Ranade, T.N.; Mali, S.N.; Ahmad Yasin, H.K.; Pratap, A. Silver Nanoparticles (AgNPs): Comprehensive Insights into Bio/Synthesis, Key Influencing Factors, Multifaceted Applications, and Toxicity—A 2024 Update. ACS Omega 2025, 10, 7549–7582. [Google Scholar] [CrossRef]
- Islam, A.K.M.S.; Bhuiyan, R.; Nihad, S.A.I.; Akter, R.; Khan, M.A.I.; Akter, S.; Islam, R.; Khokon, A.R.; Latif, M.A. Green Synthesis and Characterization of Silver Nanoparticles and Its Efficacy against Rhizoctonia Solani, a Fungus Causing Sheath Blight Disease in Rice. PLoS ONE 2024, 19, e0304817. [Google Scholar] [CrossRef] [PubMed]
- Aboelghait, K.M.; Abdallah, W.E.; Abdelfattah, I.; El-Shamy, A.M. Green Synthesis of Silver Nanoparticles by Waste of Murcott Mandarin Peel as a Sustainable Approach for Efficient Heavy Metal Removal from Metal Industrial Wastewater. Sep. Purif. Technol. 2024, 347, 127609. [Google Scholar] [CrossRef]
- Elazab, N.T.; Baka, Z.A.M.; Saleh, H.H.; El-Zahed, M.M. Green Synthesis of Silver Nanoparticles Using Cakile Maritima Seed Extract: Molecular, Antifungal and Physiological Studies. Physiol. Mol. Plant Pathol. 2024, 129, 102183. [Google Scholar] [CrossRef]
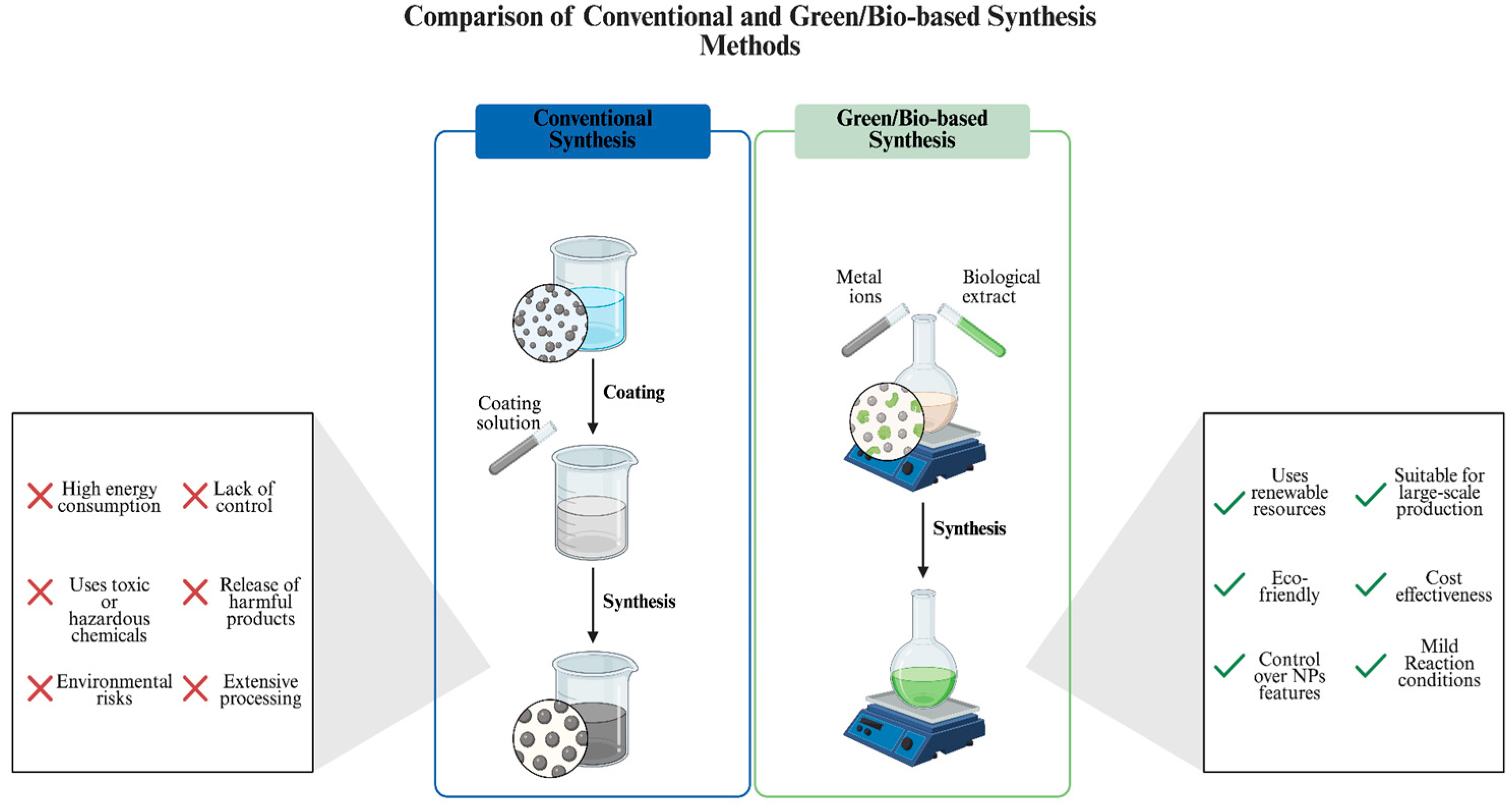

| Synthesis Method | Advantages | Limitations | References |
|---|---|---|---|
| Chemical | Rapid synthesis Significant control over physicochemical property of NPs | High energy consumption Use of toxic chemicals High heat generation Limited applicability for clinical use | [12,22] |
| Plant-based | Cost effective High availability of source Eco-friendly and sustainable Effective phytochemical act as reducing and stabilizing agents | Variability and limited availability in certain plant species Limited control over the physicochemical properties | [18,23,24] |
| Bacteria-based | Convenience in purification Metabolically versatile strains Tunable physicochemical properties with diverse choice of strains and synthesis parameter manipulation | Possibility of contamination Longer synthesis time, especially compared to plant-based approach | [18,25] |
| Fungi-based | High metal tolerance Capable of generating large amounts of extracellular enzymes Controllable during downstream processing | Possibility of contamination Longer synthesis time, especially compared to plant-based approach | [18,23] |
| Algae-based | Rich functional biomolecules Producing NPs with diverse physicochemical properties Fast growth and easily scalable Low-cost cultivation Potential for bioremediation | Limited knowledge of synthesis mechanisms High maintenance | [24,26,27] |
| Key Process Parameter | Bacterial-Based Synthesis | Fungi-Based Synthesis |
|---|---|---|
| Size/shape | pH, temperature, incubation period, metal precursor concentration, and bacterial strain substantially affect nanoparticle size and form. Controlling these factors allows the creation of spherical, triangular, and rod-like forms, but strain variability can cause less uniform distributions without process control. | Fungal systems facilitate more consistent size and shape distributions through the production of extracellular proteins and polysaccharides that function as natural reducing and capping agents. Under mild circumstances, these biomolecules facilitate the production of stable, precisely defined NPs. |
| Ease of scaling | Scaling up is easy with fast growth, cheap media, and proven bioreactor technology. Removing endotoxins and recovering intracellular products can complicate downstream processing. | Extracellular synthesis simplifies downstream recovery and may reduce costs at larger scales, despite the necessity of longer cultivation times and enriched media. |
| Stability | Moderate stability is attained via the cell wall and extracellular polymeric compounds; supplementary stabilizers may be necessary. | Enhanced colloidal stability is attributed to secreted proteins that function as natural stabilizers, albeit with potential partial obstruction of catalytic sites. |
| Localization of process | Predominantly intracellular or periplasmic, with some strains capable of extracellular synthesis. | Primarily extracellular, facilitating easier nanoparticle recovery and purification. |
| Reproducibility | Even though strain variability may have an effect on the results, it is possible to obtain high repeatability. This can be accomplished under specific conditions and through statistical optimization (for example, RSM). | The secretion patterns change depending on the growth phase and the composition of the medium, which might result in batch-to-batch variability if the process is not well regulated. |
| Cost | The use of simple media and rapid production cycles results in low operating costs; however, the addition of extra purification stages (such as the removal of endotoxins and the disruption of cells) can result in an increase in the overall cost. | Higher expenses for the medium and cultivation, but reduced costs for downstream processing as a result of extracellular production; typically cost-effective when produced in large quantities. |
| Synthesized Bacterial Strain | Application Type | NP Property | Results | References |
|---|---|---|---|---|
| Streptomyces enissocaesilis BS1 | Antimicrobial Biomedical | Average particle size of 32.2 nm Spherical morphology SPR peak at 434 nm | Antibacterial activity against multiple strains Antibiofilm activity Anticancer activity against MCF-7 and Caco-2 cancer cell lines | [10] |
| Rothia endophytica | Antimicrobial | Particle size between 47 and 72 nm Cubical morphology Zeta potential of −5.06 ± 0.52 mV SPR peak at 410 nm | Antifungal activity against Candida albicans ATCC 10231 | [92] |
| Cytobacillus firmus | Antimicrobial | Average particle size of 20 nm Spherical morphology SPR peaks at a range of 400–470 nm | Antibacterial activity against Edwardsiella tarda Dose-dependent antibiofilm activity against Edwardsiella tarda | [93] |
| Lactobacillus plantarum | Biomedical Antimicrobial | Particle size between 40 and 50 nm Spherical morphology Zeta potential of −78.8 mV SPR peak at 436 nm | Antioxidant activity Antibacterial activity against multiple strains | [94] |
| Cedecea lapagei | Antimicrobial | Particle size between 10 and 40 nm Spherical, triangular and hexagonal Zeta potential of −15.3 mV SPR peaks at a range of 400–500 nm | Antibacterial activity against multiple strains Antibiofilm activity against E. coli and P. aeruginosa | [95] |
| Massilia sp. MAHUQ-52 | Antimicrobial | Particle size between 15 and 55 nm Spherical morphology Zeta potential of −18.4 mV SPR peak at 435 nm | Antibacterial activity against drug resistant Klebsiella pneumoniae and Salmonella enteritidis | [96] |
| Streptomyces rochei SSCM102 | Antimicrobial | Particle size between 11 and 21 nm Cubic morphology SPR peak at 380 nm | Antibacterial activity against multiple strains of human pathogens | [97] |
| Arthrospira platensis | Antimicrobial | Average particle size of 50 nm Spherical morphology SPR peak at 450 nm | Antifungal activity against Aspergillus fumigatus and F. oxysporum | [98] |
| Bacillus zanthoxyli GBE11 | Antimicrobial | Particle size between 3.68 and 31.60 nm Spherical morphology Zeta potential of −23.53 ± 1.46 mV SPR peak at 439 nm | Antibacterial activity against multiple strains | [99] |
| Native bacterium GFCr-4 | Environmental Biomedical | Average particle size of 25 nm Spherical morphology SPR peak at 420 nm | Catalytic activity on the production of 2-aminothiophene derivatives Anticancer activity against MCF-7 cancer cells | [100] |
| Streptomycetes parvulus strain K2 | Environmental | Particle size between 5 and 45 nm Nearly spherical morphology SPR peak at 420 nm | Water treatment capability on drinking water infected with multiple bacterial strains | [101] |
| Bacillus anthracis PFAB2 | Antimicrobial | Average particle size of 84 nm Nearly spherical morphology Zeta potential of −15.5 mV SPR peaks at a range of 350–400 nm | Antibacterial activity against multiple strains Antifungal activity against multiple strains | [102] |
| Synthesized Fungal Strain | Application Type | NP Property | Results | References |
|---|---|---|---|---|
| Talaromyces funiculosus | Antimicrobial Biomedical | Average particle size of 34.32 nm Spherical morphology Zeta potential of −18.41 mV SPR peak at 422.5 nm | Antibacterial activity against multiple strains Antifungal activity against multiple strains Anticancer activity against Hep-G2 and HEK-293 cancer cell lines Antioxidant activity by increasing GSH and reducing MDA levels Anti-inflammatory activity by increasing IL-10 production and reducing TNF-α levels | [103] |
| Streptomyces chiangmaiensis SSUT88A | Antimicrobial | Average particle size of 13.57 nm for Intracellular cell-free supernatant (IS-AgNPs) and 30.47 nm for Extracellular cell-free supernatant (ES-AgNPs) Spherical morphology for both IS- and ES-AgNPs Zeta potential of −32.0 mV for IS-AgNPs and −27.9 mV for ES-AgNPs SPR peaks at 418 nm for IS-AgNPs and 422 nm for ES-AgNPs | Antibacterial activity against multiple drug-resistant strains by IS-AgNPs | [104] |
| Nigrospora oryzae | Antimicrobial | Particle size between 3 and 13 nm Spherical morphology SPR peak at 420 nm | Antifungal activity against Fusarium spp. | [105] |
| Fusarium sp. | Antimicrobial | Average particle size of 38.5 nm Spherical morphology SPR peak at 418 nm | Antibacterial activity against multiple strains | [106] |
| Penicillium verrucosum | Antimicrobial | Particle size between 10 and 12 nm Spherical morphology SPR peak at 420 nm | Antifungal activity against Fusarium chlamydosporum and Aspergillus flavus | [107] |
| F. oxysporum Fusarium proliferatum | Antimicrobial Biomedical Agricultural | Particle sizes between 14 to 27 nm for F. oxysporum derived AgNPs (FoAgNPs) and 18 to 40 nm for F. proliferatum derived AgNPs (FpAgNPs) Spherical to globose morphology for FpAgNPs and rectangular to spherical morphology for FoAgNPs SPR peaks at 450 nm for FpAgNPs and 435 nm for FoAgNPs | Antibacterial activity against multiple strains of human pathogens Antifungal activity against multiple strains Antioxidant activity Larvicidal activity Phyto-stimulatory activity on Vigna radiata seeds | [108] |
| Aristolochia indica | Antimicrobial Biomedical | Particle size between 15 and 40 nm Spherical morphology Zeta potential of −70.0 mV SPR peak at 426 nm | Antibacterial activity Antioxidant activity Anticancer activity against MCF-7 cancer cells | [109] |
| Lepista sordida | Antimicrobial | Particle size between 65 to 75 nm Hexagonal morphology SPR peak at 345 nm | Antifungal activity against Aspergillus flavus and Alternaria alternata | |
| Agaricus avensis | Environmental | Average particle size of 88.49 ± 3.83 nm Zeta potential of −9.16 mV Spherical and irregular morphology SPR peak at 260 nm | Catalytic activity on the conversion of L-tyrosine to L-dopa | [110,111] |
| Thermomyces lanuginosus BJMDU1 | Antimicrobial Environmental | Average particle size of 80 nm Spherical and oval morphology SPR peak at 430 nm | Antibacterial activity against multiple strains Anti-malarial activity Catalytic activity on the conversion of p-nitrophenol to p-aminophenol | [112] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akdaşçi, E.; Eker, F.; Duman, H.; Bechelany, M.; Karav, S. Microbial-Based Green Synthesis of Silver Nanoparticles: A Comparative Review of Bacteria- and Fungi-Mediated Approaches. Int. J. Mol. Sci. 2025, 26, 10163. https://doi.org/10.3390/ijms262010163
Akdaşçi E, Eker F, Duman H, Bechelany M, Karav S. Microbial-Based Green Synthesis of Silver Nanoparticles: A Comparative Review of Bacteria- and Fungi-Mediated Approaches. International Journal of Molecular Sciences. 2025; 26(20):10163. https://doi.org/10.3390/ijms262010163
Chicago/Turabian StyleAkdaşçi, Emir, Furkan Eker, Hatice Duman, Mikhael Bechelany, and Sercan Karav. 2025. "Microbial-Based Green Synthesis of Silver Nanoparticles: A Comparative Review of Bacteria- and Fungi-Mediated Approaches" International Journal of Molecular Sciences 26, no. 20: 10163. https://doi.org/10.3390/ijms262010163
APA StyleAkdaşçi, E., Eker, F., Duman, H., Bechelany, M., & Karav, S. (2025). Microbial-Based Green Synthesis of Silver Nanoparticles: A Comparative Review of Bacteria- and Fungi-Mediated Approaches. International Journal of Molecular Sciences, 26(20), 10163. https://doi.org/10.3390/ijms262010163