IL-31/33 Axis in Atopic Dermatitis
Abstract
1. Introduction
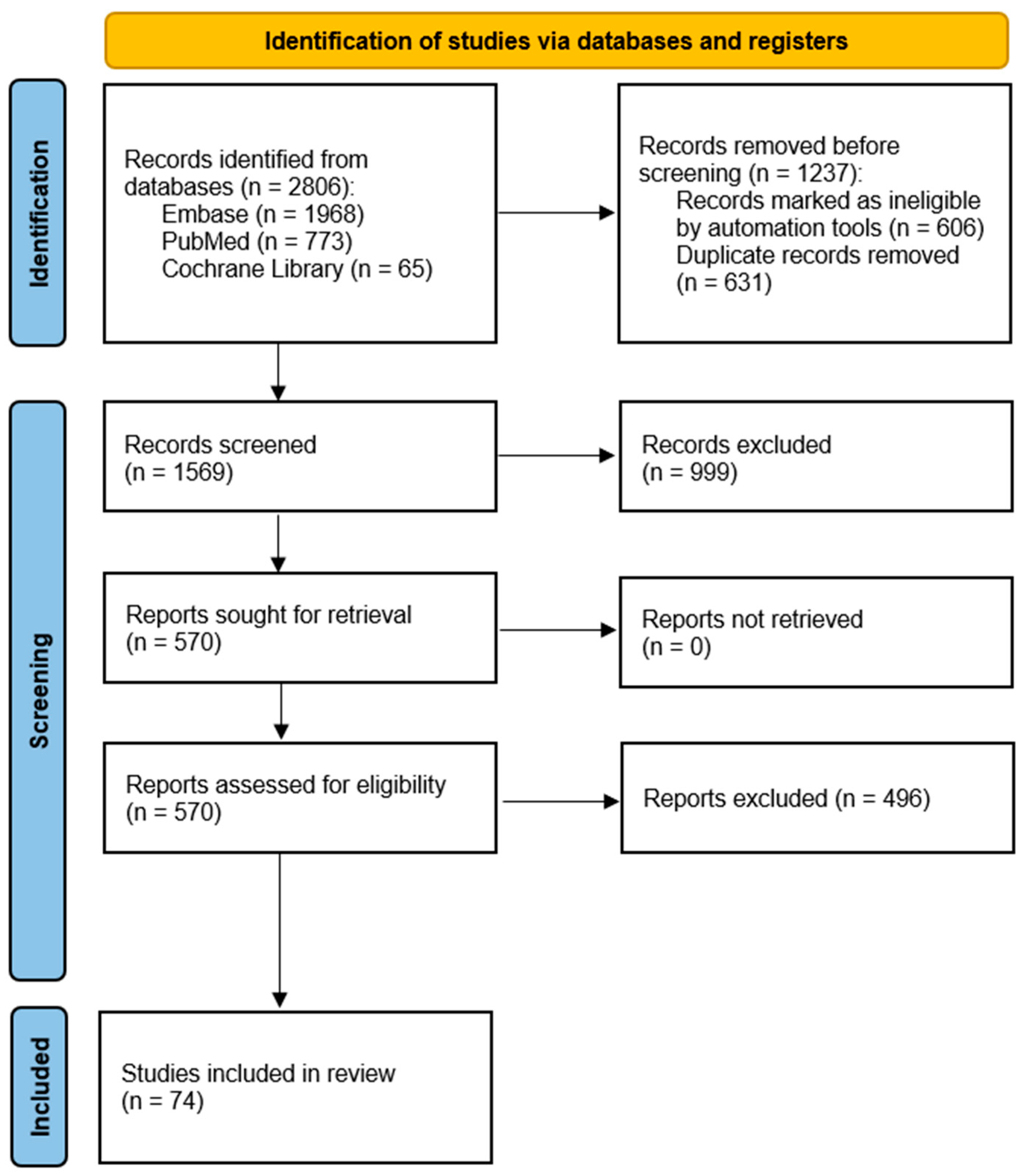
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Literature Selection
3. IL-31 in Atopic Dermatitis
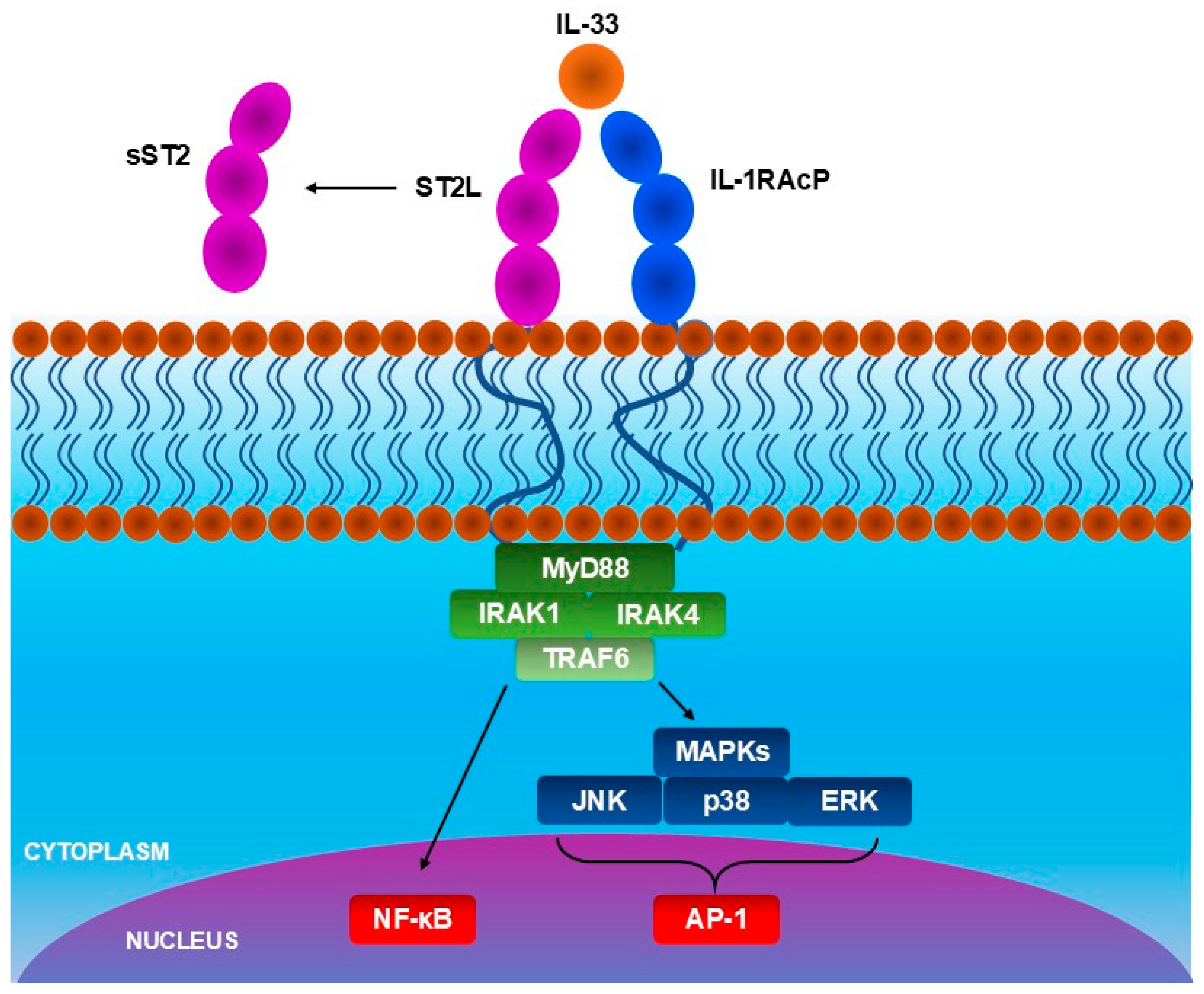
4. IL-33 in Atopic Dermatitis
- IL-33 activates basophils and ILC2s through ST2 receptor, and IL-4 released by basophils further increases the activity of ILC2s. ILC2s produce more IL-5 and IL-13, causing the accumulation of eosinophiles in skin lesions.
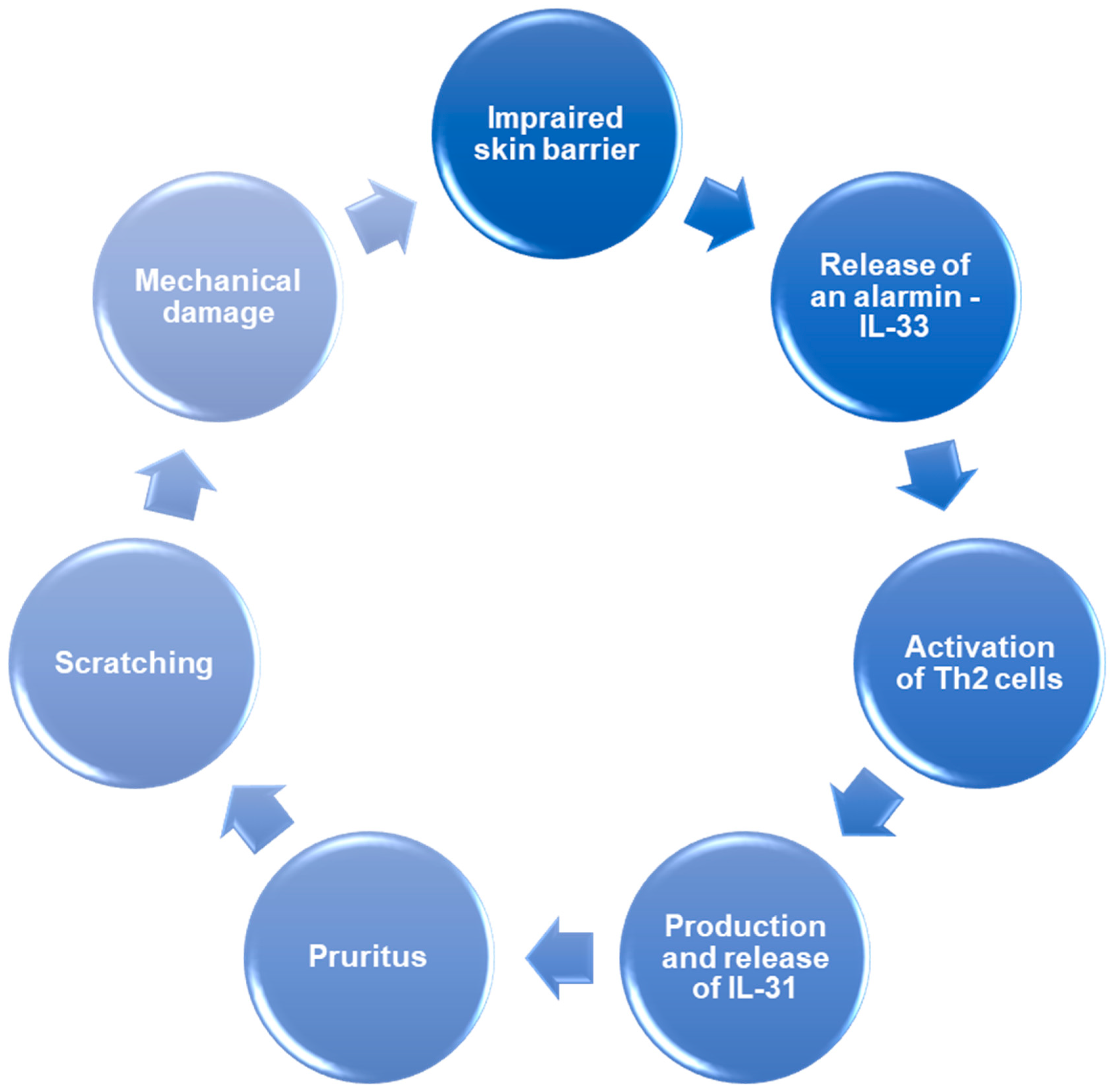
- IL-33 activates Th2 cells, inducing the release of IL-31, which directly activates sensory neurons, causing pruritus. Moreover, IL-33 activates mast cells, which release histamine—another agent responsible for pruritus. In response to the pruritus, scratching occurs, which causes further damage to keratinocytes and the release of IL-33 again, exacerbating the inflammation.
- IL-33 and cytokines which are releases due to IL-33-dependent immune cell activation—IL-4 and IL-13, reduce filaggrin expression impairing the skin barrier. Moreover, IL-33 activates the signal transducer and activator of transcription 3 (STAT3) pathway, reducing expression of claudin-1. Skin with disrupted barrier function is more susceptible to allergens and other harmful factors, leading to damage to keratinocytes and an increase in IL-33 release.
5. The IL-31/IL-33 Axis
6. Therapeutic Targeting of IL-31/IL-33 Axis
6.1. Therapies Targeting IL-31
6.2. Therapies Targeting IL-33
7. Current Challenges and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sroka-Tomaszewska, J.; Trzeciak, M. Molecular Mechanisms of Atopic Dermatitis Pathogenesis. Int. J. Mol. Sci. 2021, 22, 4130. [Google Scholar] [CrossRef]
- Facheris, P.; Jeffery, J.; Del Duca, E.; Guttman-Yassky, E. The Translational Revolution in Atopic Dermatitis: The Paradigm Shift from Pathogenesis to Treatment. Cell Mol. Immunol. 2023, 20, 448–474. [Google Scholar] [CrossRef]
- Afshari, M.; Kolackova, M.; Rosecka, M.; Čelakovská, J.; Krejsek, J. Unraveling the Skin; a Comprehensive Review of Atopic Dermatitis, Current Understanding, and Approaches. Front. Immunol. 2024, 15, 1361005. [Google Scholar] [CrossRef]
- de Lusignan, S.; Alexander, H.; Broderick, C.; Dennis, J.; McGovern, A.; Feeney, C.; Flohr, C. The Epidemiology of Eczema in Children and Adults in England: A Population-Based Study Using Primary Care Data. Clin. Exp. Allergy 2021, 51, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Choi, A.; Noh, Y.; Oh, I.S.; Jeon, J.Y.; Yoo, H.J.; Shin, J.Y.; Son, S.W. Real-World Treatment Patterns for Atopic Dermatitis in South Korea. Sci. Rep. 2022, 12, 13626. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, K.; Kishimoto, M.; Sugai, J.; Komine, M.; Ohtsuki, M. Risk Factors for the Development of Psoriasis. Int. J. Mol. Sci. 2019, 20, 4347. [Google Scholar] [CrossRef] [PubMed]
- Langan, S.M.; Irvine, A.D.; Weidinger, S. Atopic Dermatitis. Lancet 2020, 396, 345–360. [Google Scholar] [CrossRef]
- Schram, M.E.; Tedja, A.M.; Spijker, R.; Bos, J.D.; Williams, H.C.; Spuls, P.I. Is There a Rural/Urban Gradient in the Prevalence of Eczema? A Systematic Review. Br. J. Dermatol. 2010, 162, 964–973. [Google Scholar] [CrossRef]
- Guttman-Yassky, E.; Renert-Yuval, Y.; Brunner, P.M. Atopic Dermatitis. Lancet 2025, 405, 583–596. [Google Scholar] [CrossRef]
- Steinhoff, M.; Ahmad, F.; Pandey, A.; Datsi, A.; AlHammadi, A.; Al-Khawaga, S.; Al-Malki, A.; Meng, J.; Alam, M.; Buddenkotte, J. Neuroimmune Communication Regulating Pruritus in Atopic Dermatitis. J. Allergy Clin. Immunol. 2022, 149, 1875–1898. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Gelfand, J.M.; Margolis, D.J.; Boguniewicz, M.; Fonacier, L.; Grayson, M.H.; Simpson, E.L.; Ong, P.Y.; Chiesa Fuxench, Z.C. Patient Burden and Quality of Life in Atopic Dermatitis in US Adults: A Population-Based Cross-Sectional Study. Ann. Allergy Asthma Immunol. 2018, 121, 340–347. [Google Scholar] [CrossRef]
- Son, S.W.; Lee, J.H.; Ahn, J.; Chang, S.E.; Choi, E.H.; Han, T.Y.; Jang, Y.H.; Kim, H.O.; Kim, M.B.; Kim, Y.C.; et al. Assessment of Disease Severity and Quality of Life in Patients with Atopic Dermatitis from South Korea. Ann. Dermatol. 2022, 34, 419–430, Erratum in Ann. Dermatol. 2023, 35, 86. [Google Scholar] [CrossRef]
- Nicholas, M.N.; Gooderham, M.J. Atopic Dermatitis, Depression, and Suicidality. J. Cutan. Med. Surg. 2017, 21, 237–242. [Google Scholar] [CrossRef]
- Cao, L.; Su, J.; Tian, F.; Zhou, Y.; Liu, S.; Lou, F. Risk of Depression in Patients with Atopic Dermatitis: An Updated Systematic Review and Meta-Analysis of Children, Adolescent and Adult Groups. J. Paediatr. Child. Health 2024, 60, 640–647. [Google Scholar] [CrossRef]
- Silverberg, J.I. Comorbidities and the Impact of Atopic Dermatitis. Ann. Allergy Asthma Immunol. 2019, 123, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Boothe, W.D.; Tarbox, J.A.; Tarbox, M.B. Atopic Dermatitis: Pathophysiology. Adv. Exp. Med. Biol. 2024, 1447, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Bonamonte, D.; Filoni, A.; Vestita, M.; Romita, P.; Foti, C.; Angelini, G. The Role of the Environmental Risk Factors in the Pathogenesis and Clinical Outcome of Atopic Dermatitis. Biomed Res. Int. 2019, 2019, 2450605. [Google Scholar] [CrossRef] [PubMed]
- Torres, T.; Ferreira, E.O.; Gonçalo, M.; Mendes-Bastos, P.; Selores, M.; Filipe, P. Update on Atopic Dermatitis. Acta Med. Port. 2019, 32, 606–613. [Google Scholar] [CrossRef]
- Nakatsuji, T.; Chen, T.H.; Narala, S.; Chun, K.A.; Two, A.M.; Yun, T.; Shafiq, F.; Kotol, P.F.; Bouslimani, A.; Melnik, A.V.; et al. Antimicrobials from Human Skin Commensal Bacteria Protect against Staphylococcus Aureus and Are Deficient in Atopic Dermatitis. Sci. Transl. Med. 2017, 9, eaah4680. [Google Scholar] [CrossRef]
- Al Kindi, A.; Williams, H.; Matsuda, K.; Alkahtani, A.M.; Saville, C.; Bennett, H.; Alshammari, Y.; Tan, S.Y.; O’Neill, C.; Tanaka, A.; et al. Staphylococcus Aureus Second Immunoglobulin-Binding Protein Drives Atopic Dermatitis via IL-33. J. Allergy Clin. Immunol. 2021, 147, 1354–1368.e3. [Google Scholar] [CrossRef]
- Chiricozzi, A.; Maurelli, M.; Peris, K.; Girolomoni, G. Targeting IL-4 for the Treatment of Atopic Dermatitis. Immunotargets Ther. 2020, 9, 151. [Google Scholar] [CrossRef]
- Bonzano, L.; Borgia, F.; Casella, R.; Miniello, A.; Nettis, E.; Gangemi, S. Microbiota and IL-33/31 Axis Linkage: Implications and Therapeutic Perspectives in Atopic Dermatitis and Psoriasis. Biomolecules 2023, 13, 1100. [Google Scholar] [CrossRef]
- Nedoszytko, B.; Reszka, E.; Gutowska-Owsiak, D.; Trzeciak, M.; Lange, M.; Jarczak, J.; Niedoszytko, M.; Jablonska, E.; Romantowski, J.; Strapagiel, D.; et al. Genetic and Epigenetic Aspects of Atopic Dermatitis. Int. J. Mol. Sci. 2020, 21, 6484. [Google Scholar] [CrossRef]
- Trier, A.M.; Mack, M.R.; Fredman, A.; Tamari, M.; Ver Heul, A.M.; Zhao, Y.; Guo, C.J.; Avraham, O.; Ford, Z.K.; Oetjen, L.K.; et al. IL-33 Signaling in Sensory Neurons Promotes Dry Skin Itch. J. Allergy Clin. Immunol. 2022, 149, 1473–1480.e6. [Google Scholar] [CrossRef] [PubMed]
- Huang, I.H.; Chung, W.H.; Wu, P.C.; Chen, C.B. JAK–STAT Signaling Pathway in the Pathogenesis of Atopic Dermatitis: An Updated Review. Front. Immunol. 2022, 13, 1068260. [Google Scholar] [CrossRef] [PubMed]
- Biazus Soares, G.; Hashimoto, T.; Yosipovitch, G. Atopic Dermatitis Itch: Scratching for an Explanation. J. Investig. Dermatol. 2024, 144, 978–988. [Google Scholar] [CrossRef]
- Staughton, R.C.D.; Bridgett, C.K.; Norén, P.; Goulding, J.M.R.; Affleck, A.; Walsh, S. Habitual Scratching Amplifies and Perpetuates Atopic Dermatitis. Br. J. Dermatol. 2020, 183, 403. [Google Scholar] [CrossRef] [PubMed]
- Frazier, W.; Bhardwaj, N. Atopic Dermatitis: Diagnosis and Treatment. Am. Fam. Physician 2020, 101, 590–598. [Google Scholar]
- Tanei, R. Atopic Dermatitis in Older Adults: A Review of Treatment Options. Drugs Aging 2020, 37, 149. [Google Scholar] [CrossRef]
- Werfel, T.; Heratizadeh, A.; Aberer, W.; Augustin, M.; Biedermann, T.; Bauer, A.; Fölster-Holst, R.; Kahle, J.; Kinberger, M.; Nemat, K.; et al. S3 Guideline Atopic Dermatitis: Part 2—Systemic Treatment. J. Dtsch. Dermatol. Ges. 2024, 22, 307–320. [Google Scholar] [CrossRef]
- Elahi, N.; Astaneh, M.E.; Ai, J.; Rizwan, M. Atopic Dermatitis Treatment: A Comprehensive Review of Conventional and Novel Bioengineered Approaches. Int. J. Biol. Macromol. 2024, 282, 137083. [Google Scholar] [CrossRef]
- Datsi, A.; Steinhoff, M.; Ahmad, F.; Alam, M.; Buddenkotte, J. Interleukin-31: The “Itchy” Cytokine in Inflammation and Therapy. Allergy 2021, 76, 2982–2997. [Google Scholar] [CrossRef]
- Bağci, I.S.; Ruzicka, T. IL-31: A New Key Player in Dermatology and Beyond. J. Allergy Clin. Immunol. 2018, 141, 858–866. [Google Scholar] [CrossRef]
- Furue, M.; Yamamura, K.; Kido-Nakahara, M.; Nakahara, T.; Fukui, Y. Emerging Role of Interleukin-31 and Interleukin-31 Receptor in Pruritus in Atopic Dermatitis. Allergy 2018, 73, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Bang, C.H.; Song, J.Y.; Song, Y.M.; Lee, J.H.; Park, Y.M.; Lee, J.Y. Production of IL-31 in CD45RO+CLA+H4R+ T Cells in Atopic Dermatitis. J. Clin. Med. 2021, 10, 1976. [Google Scholar] [CrossRef]
- Raap, U.; Gehring, M.; Kleiner, S.; Rüdrich, U.; Eiz-Vesper, B.; Haas, H.; Kapp, A.; Gibbs, B.F. Human Basophils Are a Source of—And Are Differentially Activated by—IL-31. Clin. Exp. Allergy 2017, 47, 499–508. [Google Scholar] [CrossRef]
- Xu, J.; Zanvit, P.; Hu, L.; Tseng, P.-Y.; Liu, N.; Wang, F.; Liu, O.; Zhang, D.; Jin, W.; Guo, N.; et al. The Cytokine TGF-β Induces Interleukin-31 Expression from Dermal Dendritic Cells to Activate Sensory Neurons and Stimulate Wound Itching. Immunity 2020, 53, 371–383.e5. [Google Scholar] [CrossRef]
- Kunsleben, N.; Rüdrich, U.; Gehring, M.; Novak, N.; Kapp, A.; Raap, U. IL-31 Induces Chemotaxis, Calcium Mobilization, Release of Reactive Oxygen Species, and CCL26 in Eosinophils, Which Are Capable to Release IL-31. J. Investig. Dermatol. 2015, 135, 1908–1911. [Google Scholar] [CrossRef]
- Niyonsaba, F.; Ushio, H.; Hara, M.; Yokoi, H.; Tominaga, M.; Takamori, K.; Kajiwara, N.; Saito, H.; Nagaoka, I.; Ogawa, H.; et al. Antimicrobial Peptides Human β-Defensins and Cathelicidin LL-37 Induce the Secretion of a Pruritogenic Cytokine IL-31 by Human Mast Cells. J. Immunol. 2010, 184, 3526–3534. [Google Scholar] [CrossRef] [PubMed]
- Hofbauer, G.; Nobbe, S.; Dziunycz, P.; Mühleisen, B.; Bilsborough, J.; Dillon, S.; French, L. IL-31 Expression by Inflammatory Cells Is Preferentially Elevated in Atopic Dermatitis. Acta Derm. Venereol. 2012, 92, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.D.; Oussedik, E.; D’Amber, V.; Feldman, S.R. Interleukin-31 Pathway and Its Role in Atopic Dermatitis: A Systematic Review. J. Dermatol. Treat. 2017, 28, 591–599. [Google Scholar] [CrossRef]
- Yamamura, Y.; Nakashima, C.; Otsuka, A. Interplay of Cytokines in the Pathophysiology of Atopic Dermatitis: Insights from Murin Models and Human. Front. Med. 2024, 11, 1342176. [Google Scholar] [CrossRef]
- Kamata, Y.; Tominaga, M.; Takamori, K. Mechanisms of Itch in Atopic Dermatitis. Juntendo Med. J. 2025, 71, JMJ24-0036-R. [Google Scholar] [CrossRef]
- Oyama, S.; Kitamura, H.; Kuramochi, T.; Higuchi, Y.; Matsushita, H.; Suzuki, T.; Goto, M.; Adachi, H.; Kasutani, K.; Sakamoto, A.; et al. Cynomolgus Monkey Model of Interleukin-31-induced Scratching Depicts Blockade of Human Interleukin-31 Receptor A by a Humanized Monoclonal Antibody. Exp. Dermatol. 2018, 27, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Dillon, S.R.; Sprecher, C.; Hammond, A.; Bilsborough, J.; Rosenfeld-Franklin, M.; Presnell, S.R.; Haugen, H.S.; Maurer, M.; Harder, B.; Johnston, J.; et al. Interleukin 31, a Cytokine Produced by Activated T Cells, Induces Dermatitis in Mice. Nat. Immunol. 2004, 5, 752–760, Erratum in Nat. Immunol. 2005, 114, 6. [Google Scholar] [CrossRef]
- Orfali, R.L.; Aoki, V. Blockage of the IL-31 Pathway as a Potential Target Therapy for Atopic Dermatitis. Pharmaceutics 2023, 15, 577. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, C.; Otsuka, A.; Kabashima, K. Interleukin-31 and Interleukin-31 Receptor: New Therapeutic Targets for Atopic Dermatitis. Exp. Dermatol. 2018, 27, 327–331. [Google Scholar] [CrossRef]
- Cornelissen, C.; Marquardt, Y.; Czaja, K.; Wenzel, J.; Frank, J.; Lüscher-Firzlaff, J.; Lüscher, B.; Baron, J.M. IL-31 Regulates Differentiation and Filaggrin Expression in Human Organotypic Skin Models. J. Allergy Clin. Immunol. 2012, 129, 426–433.e8. [Google Scholar] [CrossRef] [PubMed]
- Hänel, K.H.; Pfaff, C.M.; Cornelissen, C.; Amann, P.M.; Marquardt, Y.; Czaja, K.; Kim, A.; Lüscher, B.; Baron, J.M. Control of the Physical and Antimicrobial Skin Barrier by an IL-31–IL-1 Signaling Network. J. Immunol. 2016, 196, 3233–3244. [Google Scholar] [CrossRef]
- Dai, X.; Okazaki, H.; Hanakawa, Y.; Murakami, M.; Tohyama, M.; Shirakata, Y.; Sayama, K. Eccrine Sweat Contains IL-1α, IL-1β and IL-31 and Activates Epidermal Keratinocytes as a Danger Signal. PLoS ONE 2013, 8, e67666. [Google Scholar] [CrossRef]
- Sonkoly, E.; Muller, A.; Lauerma, A.I.; Pivarcsi, A.; Soto, H.; Kemeny, L.; Alenius, H.; Dieu-Nosjean, M.-C.; Meller, S.; Rieker, J.; et al. IL-31: A New Link between T Cells and Pruritus in Atopic Skin Inflammation. J. Allergy Clin. Immunol. 2006, 117, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Combarros, D.; Brahmi, R.; Musaefendic, E.; Heit, A.; Kondratjeva, J.; Moog, F.; Pressanti, C.; Lecru, L.A.; Arbouille, S.; Laffort, C.; et al. Reconstructed Epidermis Produced with Atopic Dog Keratinocytes Only Exhibit Skin Barrier Defects after the Addition of Proinflammatory and Allergic Cytokines. JID Innov. 2025, 5, 100330. [Google Scholar] [CrossRef]
- Feld, M.; Garcia, R.; Buddenkotte, J.; Katayama, S.; Lewis, K.; Muirhead, G.; Hevezi, P.; Plesser, K.; Schrumpf, H.; Krjutskov, K.; et al. The Pruritus- and TH2-Associated Cytokine IL-31 Promotes Growth of Sensory Nerves. J. Allergy Clin. Immunol. 2016, 138, 500–508.e24. [Google Scholar] [CrossRef]
- Cevikbas, F.; Wang, X.; Akiyama, T.; Kempkes, C.; Savinko, T.; Antal, A.; Kukova, G.; Buhl, T.; Ikoma, A.; Buddenkotte, J.; et al. A Sensory Neuron-Expressed IL-31 Receptor Mediates T Helper Cell-Dependent Itch: Involvement of TRPV1 and TRPA1. J. Allergy Clin. Immunol. 2014, 133, 448–460.E7. [Google Scholar] [CrossRef]
- Ozceker, D.; Bulut, M.; Ozbay, A.C.; Dilek, F.; Koser, M.; Tamay, Z.; Guler, N. Assessment of IL-31 Levels and Disease Severity in Children with Atopic Dermatitis. Allergol. Immunopathol. 2018, 46, 322–325. [Google Scholar] [CrossRef]
- Siniewicz-Luzeńczyk, K.; Stańczyk-Przyłuska, A.; Zeman, K. Correlation between Serum Interleukin 31 Level and the Severity of Disease in Children with Atopic Dermatitis. Adv. Dermatol. Allergol. 2013, 5, 282–285. [Google Scholar] [CrossRef] [PubMed]
- Ezzat, M.; Hasan, Z.; Shaheen, K. Serum Measurement of Interleukin-31 (IL-31) in Paediatric Atopic Dermatitis: Elevated Levels Correlate with Severity Scoring. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Byeon, J.H.; Yoon, W.; Ahn, S.H.; Lee, H.S.; Kim, S.; Yoo, Y. Correlation of Serum Interleukin-31 with Pruritus and Blood Eosinophil Markers in Children with Atopic Dermatitis. Allergy Asthma Proc. 2020, 41, 59–65. [Google Scholar] [CrossRef]
- Raap, U.; Wichmann, K.; Bruder, M.; Ständer, S.; Wedi, B.; Kapp, A.; Werfel, T. Correlation of IL-31 Serum Levels with Severity of Atopic Dermatitis. J. Allergy Clin. Immunol. 2008, 122, 421–423. [Google Scholar] [CrossRef]
- Bodoor, K.; Al-Qarqaz, F.; Heis, L.A.; Alfaqih, M.A.; Oweis, A.O.; Almomani, R.; Obeidat, M.A. IL-33/13 Axis and IL-4/31 Axis Play Distinct Roles in Inflammatory Process and Itch in Psoriasis and Atopic Dermatitis. Clin. Cosmet. Investig. Dermatol. 2020, 13, 419–424. [Google Scholar] [CrossRef]
- Kim, S.; Kim, H.-J.; Yang, H.S.; Kim, E.; Huh, I.-S.; Yang, J.-M. IL-31 Serum Protein and Tissue MRNA Levels in Patients with Atopic Dermatitis. Ann. Dermatol. 2011, 23, 468. [Google Scholar] [CrossRef]
- Cayrol, C.; Girard, J.P. Interleukin-33 (IL-33): A Nuclear Cytokine from the IL-1 Family. Immunol. Rev. 2018, 281, 154–168. [Google Scholar] [CrossRef]
- Liew, F.Y.; Girard, J.P.; Turnquist, H.R. Interleukin-33 in Health and Disease. Nat. Rev. Immunol. 2016, 16, 676–689. [Google Scholar] [CrossRef] [PubMed]
- Saluja, R.; Khan, M.; Church, M.K.; Maurer, M. The Role of IL-33 and Mast Cells in Allergy and Inflammation. Clin. Transl. Allergy 2015, 5, 33. [Google Scholar] [CrossRef] [PubMed]
- Murdaca, G.; Greco, M.; Tonacci, A.; Negrini, S.; Borro, M.; Puppo, F.; Gangemi, S. Il-33/Il-31 Axis in Immune-Mediated and Allergic Diseases. Int. J. Mol. Sci. 2019, 20, 5856. [Google Scholar] [CrossRef]
- Travers, J.; Rochman, M.; Miracle, C.E.; Habel, J.E.; Brusilovsky, M.; Caldwell, J.M.; Rymer, J.K.; Rothenberg, M.E. Chromatin Regulates IL-33 Release and Extracellular Cytokine Activity. Nat. Commun. 2018, 9, 3244. [Google Scholar] [CrossRef] [PubMed]
- Carriere, V.; Roussel, L.; Ortega, N.; Lacorre, D.A.; Americh, L.; Aguilar, L.; Bouche, G.; Girard, J.P. IL-33, the IL-1-like Cytokine Ligand for ST2 Receptor, Is a Chromatin-Associated Nuclear Factor in Vivo. Proc. Natl. Acad. Sci. USA 2007, 104, 282–287. [Google Scholar] [CrossRef]
- Lüthi, A.U.; Cullen, S.P.; McNeela, E.A.; Duriez, P.J.; Afonina, I.S.; Sheridan, C.; Brumatti, G.; Taylor, R.C.; Kersse, K.; Vandenabeele, P.; et al. Suppression of Interleukin-33 Bioactivity through Proteolysis by Apoptotic Caspases. Immunity 2009, 31, 84–98. [Google Scholar] [CrossRef]
- Wong, C.K.; Leung, K.M.L.; Qiu, H.N.; Chow, J.Y.S.; Choi, A.O.K.; Lam, C.W.K. Activation of Eosinophils Interacting with Dermal Fibroblasts by Pruritogenic Cytokine IL-31 and Alarmin IL-33: Implications in Atopic Dermatitis. PLoS ONE 2012, 7, e29815. [Google Scholar] [CrossRef]
- Wierzbicka, J.M.; Piotrowska, A.; Purzycka-Bohdan, D.; Olszewska, A.; Nowak, J.I.; Szczerkowska-Dobosz, A.; Nedoszytko, B.; Nowicki, R.J.; Żmijewski, M.A. The Effects of Vitamin d on the Expression of Il-33 and Its Receptor St2 in Skin Cells; Potential Implication for Psoriasis. Int. J. Mol. Sci. 2021, 22, 12907. [Google Scholar] [CrossRef]
- Savinko, T.; Matikainen, S.; Saarialho-Kere, U.; Lehto, M.; Wang, G.; Lehtimäki, S.; Karisola, P.; Reunala, T.; Wolff, H.; Lauerma, A.; et al. IL-33 and ST2 in Atopic Dermatitis: Expression Profiles and Modulation by Triggering Factors. J. Investig. Dermatol. 2012, 132, 1392–1400. [Google Scholar] [CrossRef]
- Gao, T.C.; Wang, C.H.; Wang, Y.Q.; Mi, W.L. IL-33/ST2 Signaling in the Pathogenesis of Chronic Pain and Itch. Neuroscience 2023, 529, 16–22. [Google Scholar] [CrossRef]
- Tamagawa-Mineoka, R.; Okuzawa, Y.; Masuda, K.; Katoh, N. Increased Serum Levels of Interleukin 33 in Patients with Atopic Dermatitis. J. Am. Acad. Dermatol. 2014, 70, 882–888. [Google Scholar] [CrossRef]
- Liu, B.; Tai, Y.; Achanta, S.; Kaelberer, M.M.; Caceres, A.I.; Shao, X.; Fang, J.; Jordt, S.E. IL-33/ST2 Signaling Excites Sensory Neurons and Mediates Itch Response in a Mouse Model of Poison Ivy Contact Allergy. Proc. Natl. Acad. Sci. USA 2016, 113, E7572–E7579. [Google Scholar] [CrossRef]
- Maurer, M.; Cheung, D.S.; Theess, W.; Yang, X.; Dolton, M.; Guttman, A.; Choy, D.F.; Dash, A.; Grimbaldeston, M.A.; Soong, W. Phase 2 Randomized Clinical Trial of Astegolimab in Patients with Moderate to Severe Atopic Dermatitis. J. Allergy Clin. Immunol. 2022, 150, 1517–1524. [Google Scholar] [CrossRef]
- Schuler, C.F.; Gudjonsson, J.E. IL-33 Antagonism Does Not Improve Chronic Atopic Dermatitis: What Can We Learn? J. Allergy Clin. Immunol. 2022, 150, 1410–1411. [Google Scholar] [CrossRef]
- Imai, Y. Interleukin-33 in Atopic Dermatitis. J. Dermatol. Sci. 2019, 96, 2–7. [Google Scholar] [CrossRef]
- Borek, F.; Nagashima, S.; Villalobos, W.R.; Gmyterco, V.C.; Sell, T.; de Farias, M.R.; Bechara, G.H. Immunoexpression of IL-33 in the Different Clinical Aspects of Canine Atopic Dermatitis. Vet. Immunol. Immunopathol. 2024, 273, 110786. [Google Scholar] [CrossRef]
- Seltmann, J.; Werfel, T.; Wittmann, M. Evidence for a Regulatory Loop between IFN-γ and IL-33 in Skin Inflammation. Exp. Dermatol. 2013, 22, 102–107. [Google Scholar] [CrossRef]
- Xuan, Z.; Chen, X.; Zhou, W.; Shen, Y.; Sun, Z.; Zhang, H.; Yao, Z. Exploring Causal Correlations between Circulating Cytokines and Atopic Dermatitis: A Bidirectional Two-Sample Mendelian Randomization Study. Front. Immunol. 2024, 15, 1367958. [Google Scholar] [CrossRef]
- Zaryczańska, A.; Gleń, J.; Zabłotna, M.; Nowicki, R.J.; Trzeciak, M. Serum Levels and Single Nucleotide Polymorphisms of the Interleukin-33 Gene in Atopic Dermatitis. Postep. Dermatol. Alergol. 2022, 39, 959–964. [Google Scholar] [CrossRef]
- Gunji, Y.; Matsumura, T.; Karasawa, T.; Komada, T.; Baatarjav, C.; Komori, S.; Aizawa, H.; Mizushina, Y.; Tsuda, H.; Miyake, K.; et al. IL-33-Primed NLRP3 Inflammasome in Basophils Drives IL-1β Production and Initiates Atopic Dermatitis Inflammation. Cell Death Discov. 2025, 11, 346. [Google Scholar] [CrossRef]
- Chen, Z.A.; Yang, Y.T.; Wang, X.X.; Xia, L.L.; Wang, W.B.; Wu, X.L.; Gao, Z. Keloids and Inflammation: The Crucial Role of IL-33 in Epidermal Changes. Front. Immunol. 2025, 16, 1514618. [Google Scholar] [CrossRef]
- Guo, M.M.-H.; Chen, K.-D.; Kuo, H.-C. Semaphorin 7a Regulates the Expression of IL-4 and IL-33 in a Cell Model of Atopic Dermatitis and Is Associated with Disease Severity. Exp. Dermatol. 2025, 34, e70087. [Google Scholar] [CrossRef]
- Di Salvo, E.; Ventura-Spagnolo, E.; Casciaro, M.; Navarra, M.; Gangemi, S. IL-33/IL-31 Axis: A Potential Inflammatory Pathway. Mediat. Inflamm. 2018, 2018, 858032. [Google Scholar] [CrossRef]
- De Martinis, M.; Sirufo, M.M.; Suppa, M.; Ginaldi, L. IL-33/IL-31 Axis in Osteoporosis. Int. J. Mol. Sci. 2020, 21, 1239. [Google Scholar] [CrossRef]
- Murdaca, G.; Paladin, F.; Orsi, A.; Gangemi, S. Interleukin-31: A Pro-Inflammatory Oriented Cytokine. Front. Biosci. (Landmark Ed.) 2025, 30, 37462. [Google Scholar] [CrossRef]
- Lee, C.Y.; Sung, C.H.; Wu, M.C.; Chang, Y.C.; Chang, J.C.; Fang, Y.P.; Wang, N.M.; Chou, T.Y.; Chan, Y.J. Clinical Characteristics and Differential Cytokine Expression in Hospitalized Taiwanese Children with Respiratory Syncytial Virus and Rhinovirus Bronchiolitis. J. Microbiol. Immunol. Infect. 2023, 56, 282–291. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.; Qu, J. Serum IL-31 Is Related to the Severity and 3-Month Prognosis of Patients with Intracerebral Hemorrhage. Medicine 2024, 103, E35760. [Google Scholar] [CrossRef]
- Miao, Y.; Zhang, Z.X.; Feng, X.; Sun, W.M. IL-33 as a Novel Serum Prognostic Marker of Intracerebral Hemorrhage. Oxid. Med. Cell Longev. 2021, 2021, 5597790. [Google Scholar] [CrossRef]
- Takaoka, A.; Arai, I.; Sugimoto, M.; Honma, Y.; Futaki, N.; Nakamura, A.; Nakaike, S. Involvement of IL-31 on Scratching Behavior in NC/Nga Mice with Atopic-like Dermatitis. Exp. Dermatol. 2006, 15, 161–167. [Google Scholar] [CrossRef]
- Che, D.N.; Shin, J.Y.; Kang, H.J.; Cho, B.O.; Kim, Y.S.; Jang, S. Il Luteolin Suppresses IL-31 Production in IL-33-Stimulated Mast Cells through MAPK and NF-ΚB Signaling Pathways. Int. Immunopharmacol. 2020, 83, 106403. [Google Scholar] [CrossRef]
- Gangemi, S.; Franchina, T.; Minciullo, P.L.; Profita, M.; Zanghì, M.; David, A.; Kennez, I.; Adamo, V. IL-33/IL-31 Axis: A New Pathological Mechanisms for EGFR Tyrosine Kinase Inhibitors-Associated Skin Toxicity. J. Cell Biochem. 2013, 114, 2673–2676. [Google Scholar] [CrossRef]
- Rizzo, J.M.; Oyelakin, A.; Min, S.; Smalley, K.; Bard, J.; Luo, W.; Nyquist, J.; Guttman-Yassky, E.; Yoshida, T.; De Benedetto, A.; et al. ΔNp63 Regulates IL-33 and IL-31 Signaling in Atopic Dermatitis. Cell Death Differ. 2016, 23, 1073–1085. [Google Scholar] [CrossRef]
- Yi, L.; Cheng, D.; Zhang, K.; Huo, X.; Mo, Y.; Shi, H.; Di, H.; Zou, Y.; Zhang, H.; Zhao, J.; et al. Intelectin Contributes to Allergen-Induced IL-25, IL-33, and TSLP Expression and Type 2 Response in Asthma and Atopic Dermatitis. Mucosal Immunol. 2017, 10, 1491–1503. [Google Scholar] [CrossRef]
- Kasraie, S.; Niebuhr, M.; Werfel, T. Interleukin (IL)-31 Induces Pro-inflammatory Cytokines in Human Monocytes and Macrophages Following Stimulation with Staphylococcal Exotoxins. Allergy 2010, 65, 712–721. [Google Scholar] [CrossRef]
- Prajapati, S.; Flemming, J.P.; Khan, D.; Han, H.; How, B.; Rozenberg, S.S.; Feldman, S.R. The Role of Nemolizumab in the Treatment of Atopic Dermatitis for the Adult Population. Immunotherapy 2024, 16, 925–935. [Google Scholar] [CrossRef]
- Kaneda, N. Pharmacological Profiles and Clinical Findings of Nemolizumab as Treatment for Pruritus Associated with Atopic Dermatitis. Folia Pharmacol. Jpn. 2023, 158, 22150. [Google Scholar] [CrossRef]
- Kabashima, K.; Furue, M.; Hanifin, J.M.; Pulka, G.; Wollenberg, A.; Galus, R.; Etoh, T.; Mihara, R.; Nakano, M.; Ruzicka, T. Nemolizumab in Patients with Moderate-to-Severe Atopic Dermatitis: Randomized, Phase II, Long-Term Extension Study. J. Allergy Clin. Immunol. 2018, 142, 1121–1130.e7. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Pinter, A.; Alavi, A.; Lynde, C.; Bouaziz, J.-D.; Wollenberg, A.; Murrell, D.F.; Alpizar, S.; Laquer, V.; Chaouche, K.; et al. Nemolizumab Is Associated with a Rapid Improvement in Atopic Dermatitis Signs and Symptoms: Subpopulation (EASI ≥ 16) Analysis of Randomized Phase 2B Study. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1562–1568. [Google Scholar] [CrossRef]
- Ruzicka, T.; Hanifin, J.M.; Furue, M.; Pulka, G.; Mlynarczyk, I.; Wollenberg, A.; Galus, R.; Etoh, T.; Mihara, R.; Yoshida, H.; et al. Anti–Interleukin-31 Receptor A Antibody for Atopic Dermatitis. N. Engl. J. Med. 2017, 376, 826–835. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Pinter, A.; Pulka, G.; Poulin, Y.; Bouaziz, J.-D.; Wollenberg, A.; Murrell, D.F.; Alexis, A.; Lindsey, L.; Ahmad, F.; et al. Phase 2B Randomized Study of Nemolizumab in Adults with Moderate-to-Severe Atopic Dermatitis and Severe Pruritus. J. Allergy Clin. Immunol. 2020, 145, 173–182. [Google Scholar] [CrossRef]
- Sidbury, R.; Alpizar, S.; Laquer, V.; Dhawan, S.; Abramovits, W.; Loprete, L.; Krishnaswamy, J.K.; Ahmad, F.; Jabbar-Lopez, Z.; Piketty, C. Pharmacokinetics, Safety, Efficacy, and Biomarker Profiles During Nemolizumab Treatment of Atopic Dermatitis in Adolescents. Dermatol. Ther. 2022, 12, 631–642. [Google Scholar] [CrossRef]
- Kabashima, K.; Matsumura, T.; Komazaki, H.; Kawashima, M. Trial of Nemolizumab and Topical Agents for Atopic Dermatitis with Pruritus. N. Engl. J. Med. 2020, 383, 141–150. [Google Scholar] [CrossRef]
- Kabashima, K.; Matsumura, T.; Komazaki, H.; Kawashima, M. Nemolizumab plus Topical Agents in Patients with Atopic Dermatitis (AD) and Moderate-to-severe Pruritus Provide Improvement in Pruritus and Signs of AD for up to 68 Weeks: Results from Two Phase III, Long-term Studies*. Br. J. Dermatol. 2022, 186, 642–651. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Wollenberg, A.; Reich, A.; Thaçi, D.; Legat, F.J.; Papp, K.A.; Stein Gold, L.; Bouaziz, J.-D.; Pink, A.E.; Carrascosa, J.M.; et al. Nemolizumab with Concomitant Topical Therapy in Adolescents and Adults with Moderate-to-Severe Atopic Dermatitis (ARCADIA 1 and ARCADIA 2): Results from Two Replicate, Double-Blind, Randomised Controlled Phase 3 Trials. Lancet 2024, 404, 445–460. [Google Scholar] [CrossRef]
- Igarashi, A.; Katsunuma, T.; Matsumura, T.; Komazaki, H.; Takahashi, H.; Miura, K.; Horino, S.; Yoshihara, S.; Maeda, S.; Akashi, M.; et al. Efficacy and Safety of Nemolizumab in Paediatric Patients Aged 6–12 Years with Atopic Dermatitis with Moderate-to-Severe Pruritus: Results from a Phase III, Randomized, Double-Blind, Placebo-Controlled, Multicentre Study. Br. J. Dermatol. 2023, 190, 20–28. [Google Scholar] [CrossRef]
- Mima, Y.; Yamamoto, M.; Iozumi, K. Cutaneous Adverse Events Following Nemolizumab Administration: A Review. J. Clin. Med. 2025, 14, 3026. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Filipenko, D.; Dias Barbosa, C.; Rodriguez, D.; Chambenoit, O.; Jack, K.; Piketty, C.; Subramanian, R.; Puelles, J. Patients’ Experiences of Atopic Dermatitis and Nemolizumab Treatment: An In-Trial Interview Study Embedded in a Phase 3 Clinical Trial (ARCADIA). Patient—Patient-Centered Outcomes Res. 2025, 18, 511–521. [Google Scholar] [CrossRef]
- Horimukai, K.; Kinoshita, M.; Takahata, N. Severe Atopic Dermatitis with Increasing Daytime Sleepiness Following Nemolizumab Administration: A Case Report. Cureus 2025, 17, e78214. [Google Scholar] [CrossRef]
- Katsuta, M.; Osawa, A.; Ishiuji, Y.; Fujii, S.; Kanbe, M.; Asahina, A.; Nobeyama, Y. A Case of Concurrent Exacerbation of Manifestations and Asthma after the Initiation of Nemolizumab Treatment for Atopic Dermatitis. JAAD Case Rep. 2025, 61, 89–91. [Google Scholar] [CrossRef]
- Yokozeki, H.; Murota, H.; Matsumura, T. Long-Term (68 Weeks) Administration of Nemolizumab and Topical Corticosteroids for Prurigo Nodularis in Patients Aged ≥ 13 Years: Efficacy and Safety Data from a Phase II/III Study. Br. J. Dermatol. 2025, 193, 56–65. [Google Scholar] [CrossRef]
- Igarashi, A.; Katsunuma, T.; Nagano, Y.; Komazaki, H. Long-Term (68 Weeks) Administration of Nemolizumab in Paediatric Patients Aged 6–12 Years with Atopic Dermatitis with Moderate-to-Severe Pruritus: Efficacy and Safety Data from a Phase III Study. Br. J. Dermatol. 2025, 192, 837–844. [Google Scholar] [CrossRef]
- Masuo, Y.; Yonekura, S.; Nakajima, S.; Sawada, Y.; Kabashima, K. Clinical Factors Predicting Nemolizumab Response in Atopic Dermatitis. J. Allergy Clin. Immunol. Glob. 2025, 4, 100457. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence (NICE). Nemolizumab for Treating Moderate to Severe Atopic Dermatitis in People 12 Years and Over; NICE: London, UK, 2025; ISBN 9781473170827. [Google Scholar]
- Kuznik, A.; Bégo-Le-Bagousse, G.; Eckert, L.; Gadkari, A.; Simpson, E.; Graham, C.N.; Miles, L.; Mastey, V.; Mahajan, P.; Sullivan, S.D. Economic Evaluation of Dupilumab for the Treatment of Moderate-to-Severe Atopic Dermatitis in Adults. Dermatol. Ther. 2017, 7, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-L.; Gutowska-Owsiak, D.; Hardman, C.S.; Westmoreland, M.; MacKenzie, T.; Cifuentes, L.; Waithe, D.; Lloyd-Lavery, A.; Marquette, A.; Londei, M.; et al. Proof-of-Concept Clinical Trial of Etokimab Shows a Key Role for IL-33 in Atopic Dermatitis Pathogenesis. Sci. Transl. Med. 2019, 11, eaax2945. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Mustapa, M.N.; Reid, F.; Lei, A.; Smith, R.; Moate, R.; Kelly, A.; Chen, R.; Gavala, M.; Jimenez, E.; et al. Efficacy and Safety of Tozorakimab in Moderate-to-severe Atopic Dermatitis: A Phase 2a Randomized Controlled Trial (FRONTIER-2). J. Eur. Acad. Dermatol. Venereol. 2025, 39, 1126–1133. [Google Scholar] [CrossRef]
- Saraiva, G.; LI, J.; Brooks, D.; Gorseth, E.; Moate, R.; Patel, R.; Kell, C.; Pandya, H.C.; Hofherr, A.; Jenkins, M.; et al. Safety Profile of Tozorakimab (an Anti-IL-33 Monoclonal Antibody): Data from the FRONTIER Phase 2 Program of 1076 Patients. Am. J. Respir. Crit. Care Med. 2025, 211, A1373. [Google Scholar] [CrossRef]
- Waters, M.; McKinnell, J.A.; Kalil, A.C.; Martin, G.S.; Buchman, T.G.; Theess, W.; Yang, X.; Lekkerkerker, A.N.; Staton, T.; Rosenberger, C.M.; et al. Astegolimab or Efmarodocokin Alfa in Patients with Severe COVID-19 Pneumonia: A Randomized, Phase 2 Trial*. Crit. Care Med. 2023, 51, 103–116. [Google Scholar] [CrossRef]
- Alska, E.; Łaszczych, D.; Napiórkowska-Baran, K.; Szymczak, B.; Rajewska, A.; Rubisz, A.E.; Romaniuk, P.; Wrzesień, K.; Mućka, N.; Bartuzi, Z. Advances in Biologic Therapies for Allergic Diseases: Current Trends, Emerging Agents, and Future Perspectives. J. Clin. Med. 2025, 14, 1079. [Google Scholar] [CrossRef] [PubMed]
- Nnane, I.; Frederick, B.; Yao, Z.; Raible, D.; Shu, C.; Badorrek, P.; van den Boer, M.; Branigan, P.; Duffy, K.; Baribaud, F.; et al. The First-in-human Study of CNTO 7160, an Anti-interleukin-33 Receptor Monoclonal Antibody, in Healthy Subjects and Patients with Asthma or Atopic Dermatitis. Br. J. Clin. Pharmacol. 2020, 86, 2507–2518. [Google Scholar] [CrossRef] [PubMed]
- Danto, S.I.; Tsamandouras, N.; Reddy, P.; Gilbert, S.; Mancuso, J.; Page, K.; Peeva, E.; Vincent, M.S.; Beebe, J.S. Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of PF-06817024 in Healthy Participants, Participants with Chronic Rhinosinusitis with Nasal Polyps, and Participants with Atopic Dermatitis: A Phase 1, Randomized, Double-Blind, Placebo-Controlled Study. J. Clin. Pharmacol. 2024, 64, 529–543. [Google Scholar] [CrossRef]
- Kosloski, M.P.; Guttman-Yassky, E.; Cork, M.J.; Worm, M.; Nahm, D.; Zhu, X.; Ruddy, M.K.; Harel, S.; Kamal, M.A.; Goulaouic, H.; et al. Pharmacokinetics and Pharmacodynamics of Itepekimab in Adults with Moderate-to-severe Atopic Dermatitis: Results from Two Terminated Phase II Trials. Clin. Transl. Sci. 2024, 17, e13874, Erratum in Clin. Transl. Sci. 2024, 17, e70026. [Google Scholar] [CrossRef]
- Olbrich, H.; Sadik, C.D.; Ludwig, R.J.; Thaçi, D.; Boch, K. Dupilumab in Inflammatory Skin Diseases: A Systematic Review. Biomolecules 2023, 13, 634. [Google Scholar] [CrossRef] [PubMed]
- England, E.; Rees, D.G.; Scott, I.C.; Carmen, S.; Chan, D.T.Y.; Chaillan Huntington, C.E.; Houslay, K.F.; Erngren, T.; Penney, M.; Majithiya, J.B.; et al. Tozorakimab (MEDI3506): An Anti-IL-33 Antibody That Inhibits IL-33 Signalling via ST2 and RAGE/EGFR to Reduce Inflammation and Epithelial Dysfunction. Sci. Rep. 2023, 13, 9825. [Google Scholar] [CrossRef] [PubMed]


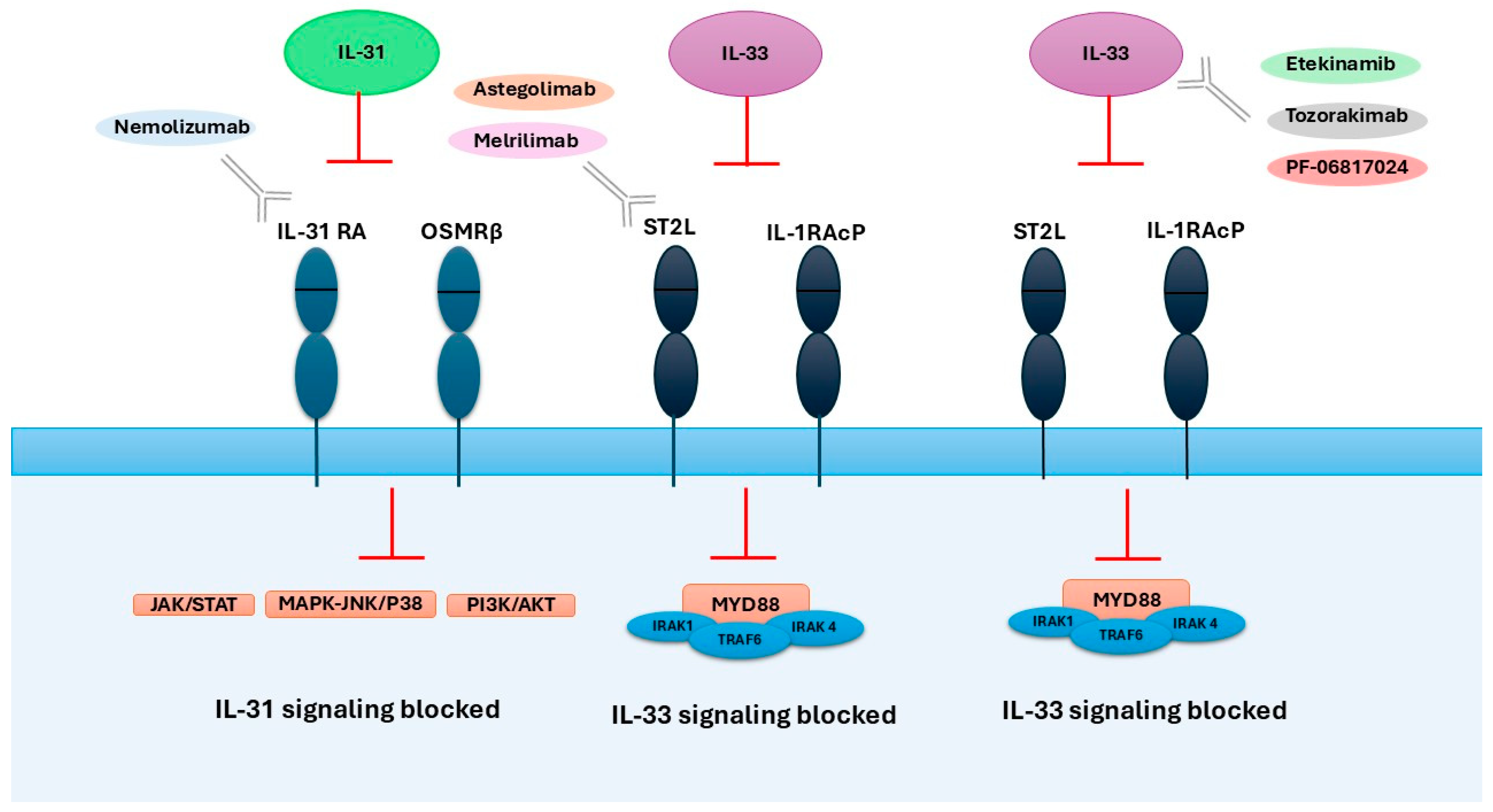
| Drug | Mechanism of Action | Dose | Response to Treatment | Adverse Effects | Ref. |
|---|---|---|---|---|---|
| Nemolizumab | Humanized monoclonal antibody against IL-31RA | 60 mg every 4 weeks (30 mg in children) | Reduction in puritis and AD symptoms; significant improvements in quality of life. | Mild; mainly skin-related adverse events; deterioration in the quality of sleep, emotional sphere, and daily activities. | [104,105,106,107,108,109] |
| Etekinamib | Humanized IgG1 anti-IL-33 monoclonal antibody | Single IV 300 mg dose | Reduction in AD symptoms and skin inflammation; sustained clinical improvement up to day 57; significant improvement in QoL and pruritus. | Mostly mild and transient; common: headache, upper respiratory infections; no serious drug-related safety concerns | [117] |
| Tozorakimab (MEDI3506) | Human monoclonal antibody that neutralizes IL-33 | 60 mg, 300 mg, or 600 mg subcutaneously every 4 weeks (×4 doses) | No statistically significant difference in primary endpoint (EASI score at Week 16); numerical increase in EASI-75 and IGA 0/1 responses in the 600 mg group. | Mostly mild/moderate; 2 discontinuations (600 mg); 1 serious case of thrombosis (300 mg) linked to treatment. | [118,126] |
| Astegolimab | Human IgG2 monoclonal antibody that blocks IL-33 receptor ST2 | 490 mg subcutaneously every 4 weeks for 16 weeks | No significant difference vs. placebo in EASI score improvement at Week 16; similar secondary outcomes and biomarkers. | Mostly mild/moderate AEs; common: worsening AD, infections; few related AEs; 1 serious unrelated AE; no withdrawals due to AEs. | [75] |
| Melrilimab (CNTO 7160) | Monoclonal antibody targeting the IL-33R | 3 mg/kg or 10 mg/kg IV every 2 weeks (3 doses total) | Strong, dose-dependent inhibition of p38 phosphorylation in basophils and sIL-33R neutralization; no clinical improvement (SCORAD, EASI). | Well tolerated; one serious adverse event (cellulitis) in the 3 mg/kg group; moderate immunogenicity; no deaths reported | [122] |
| PF-06817024 | Humanized monoclonal antibody that binds and neutralizes IL-33 | Loading dose: 600 mg IV, then 300 mg IV every 4 weeks (total 4 doses) | Dose-dependent increase in total IL-33 indicating target engagement; exploratory efficacy data (e.g., clinical symptom improvement) to be published separately. | Mostly mild to moderate; included AD exacerbation (15%), eczema (10%), nausea (10%), and infections (20%). One treatment-related serious AE was reported (cervical carcinoma) | [123] |
| Itepekimab | Human monoclonal IgG4P antibody that binds IL-33 | SC: 300 mg every 2 weeks, 300 mg every 4 weeks, 100 mg every 4 weeks, 30 mg every 8 weeks | No clinically significant improvement in EASI scores compared to placebo; no added benefit in combination with dupilumab. | Well tolerated; most common: nasopharyngitis, worsening of AD. | [124] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łacwik, J.; Kraik, K.; Laska, J.; Tota, M.; Sędek, Ł.; Gomułka, K. IL-31/33 Axis in Atopic Dermatitis. Int. J. Mol. Sci. 2025, 26, 10162. https://doi.org/10.3390/ijms262010162
Łacwik J, Kraik K, Laska J, Tota M, Sędek Ł, Gomułka K. IL-31/33 Axis in Atopic Dermatitis. International Journal of Molecular Sciences. 2025; 26(20):10162. https://doi.org/10.3390/ijms262010162
Chicago/Turabian StyleŁacwik, Julia, Krzysztof Kraik, Julia Laska, Maciej Tota, Łukasz Sędek, and Krzysztof Gomułka. 2025. "IL-31/33 Axis in Atopic Dermatitis" International Journal of Molecular Sciences 26, no. 20: 10162. https://doi.org/10.3390/ijms262010162
APA StyleŁacwik, J., Kraik, K., Laska, J., Tota, M., Sędek, Ł., & Gomułka, K. (2025). IL-31/33 Axis in Atopic Dermatitis. International Journal of Molecular Sciences, 26(20), 10162. https://doi.org/10.3390/ijms262010162







