1. Introduction
Type II diabetes mellitus (T2DM) has emerged as one of the most pressing global health challenges worldwide [
1]. T2DM, characterized by insulin resistance and relative insulin deficiency, accounts for approximately 90–95% of all diabetes cases [
1]. The condition is closely associated with lifestyle factors such as poor diet, physical inactivity, and obesity, and the prevalence continues to rise, particularly with rapid economic development and urbanization [
2]. A key contributor to the development and progression of T2DM is chronic hyperglycemia caused by various nutritional factors, such as the excessive intake of carbohydrates, especially refined sugar and simple saccharides [
3]. Unlike complex carbohydrates or fiber-rich foods, simple sugars are rapidly absorbed, causing sharp spikes in blood glucose levels and placing chronic stress on insulin production and function. Over time, this contributes to insulin resistance [
4]. To prevent this disease, dietary intake strategies that modulate carbohydrate digestion and glucose absorption have garnered significant attention for their potential to preclude or delay the onset of the disease. In particular, inhibiting the enzymatic activity of α-amylase and α-glucosidase enzymes responsible for breaking down complex carbohydrates into absorbable sugars can slow glucose release into the bloodstream [
5]. Additionally, moderate suppression of the sodium-glucose cotransporter1 (SGLT1), which facilitates intestinal glucose uptake, may further aid in controlling postprandial blood glucose spikes [
6,
7]. Natural food components such as polyphenols, flavonoids, and certain dietary fibers have demonstrated such effects, positioning functional foods as a promising tool in the dietary management and prevention of T2DM [
8,
9].
Boesenbergia rotunda (Linn.) Mansf, commonly known as fingerroot, is a medicinal plant traditionally and widely used in Southeast Asia and highly valued for its potent and diverse therapeutic properties. Among its bioactive constituents, the flavonoids pinostrobin and pinocembrin, which are among the major bioactive compounds, have recently attracted considerable scientific attention for their potential antidiabetic effects [
10]. Research indicates that pinocembrin not only exhibits significant α-glucosidase inhibitory activity against maltase and sucrase but also inhibits pancreatic amylase enzyme activity, suggesting its role in modulating postprandial hyperglycemia by delaying carbohydrate digestion [
11]. Additionally, pinocembrin has demonstrated the ability to inhibit the formation of advanced glycation end-products (AGEs), which are implicated in the progression of diabetic complications [
11]. Pinostrobin, on the other hand, has shown anti-inflammatory and antioxidant properties, which may contribute to its protective effects against diabetes-induced oxidative stress and inflammation [
10]. Additionally, pinostrobin and pinocembrin have attracted significant interest due to their roles in modulating glucose metabolism. Recent studies suggest that these compounds may influence glucose homeostasis not only by inhibiting carbohydrate-digesting enzymes like α-glucosidase and α-amylase but also by regulating the activity or expression of glucose transporters such as GLUT4 and SGLT1 [
11]. Notably, pinocembrin has been shown to exert a markedly selective and potentially inhibitory effect on SGLT1-mediated glucose uptake [
12,
13] while also possibly enhancing GLUT4 translocation in a context-dependent and pathway-specific manner and promoting increased cellular glucose uptake, which is a key mechanism in improving insulin sensitivity. Meanwhile, antioxidant and anti-inflammatory activities of pinostrobin may indirectly support glucose transporter function by reducing oxidative stress, which impairs insulin signaling pathways.
Although many studies have demonstrated the antidiabetic properties of pinostrobin and pinocembrin, identifying them as effective anti-glycation agents, as well as α-amylase and α-glucosidase inhibitors, their precise molecular mechanisms remain insufficiently explored. These findings suggest their potential role in modulating postprandial glucose levels. However, research on B. rotunda extracts and their bioactive flavonoids is still in the early stage, particularly concerning their molecular interactions and functional mechanisms. To date, no studies have specifically examined the direct effects of pinostrobin or pinocembrin on membrane-bound α-glucosidase activity, which mimics the enzyme’s physiological function in the human small intestine, providing a relevant in vitro model for assessing intestinal glucose digestion and absorption. by GLUT- and SGLT1-mediated glucose uptake in Caco-2 cell models. So, the present study demonstrates the hypoglycemic activity of pinostrobin and pinocembrin through in vitro assays targeting Caco-2 cells’ intestinal membrane-bound enzymes and glucose transporters. Additionally, computational modeling was employed to predict the molecular interactions of these compounds with human target enzymes and transporters. These findings collectively support the comprehensive antidiabetic potential of B. rotunda-derived compounds, pinostrobin and pinocembrin.
3. Discussion
The isolated compounds were identified based on their
1H-NMR spectra, which were consistent with previously reported data for pinocembrin (C
15H
12O
4) and pinostrobin (C
16H
14O
4). Pinostrobin was further confirmed by its molecular mass of 270.284 Da, in agreement with literature values [
15]. Structural elucidation of the two compounds, pinostrobin and pinocembrin, was unequivocally supported by MS and NMR analyses. Both compounds have previously been reported from the rhizomes of
B. rotunda, and are recognized as major bioactive markers for the standardization of
B. rotunda extracts [
16]. The HPLC method used for quantitative analysis was validated according to the International Council for Harmonisation (ICH) guideline Q2(R1) [
17], and all validation parameters met the acceptable criteria, confirming the suitability of the assay for analytical purposes. To enrich the bioactive content, solvent fractionation was employed, with the ethyl acetate fraction yielding a high concentration of chalcone derivatives. This fraction contained 21.4 ± 0.05%
w/
w of pinostrobin and 9.3 ± 0.02%
w/
w of pinocembrin. These values align with previous reports, which also demonstrated that pinostrobin tends to occur at approximately 1–2 times the concentration of pinocembrin in
B. rotunda extracts [
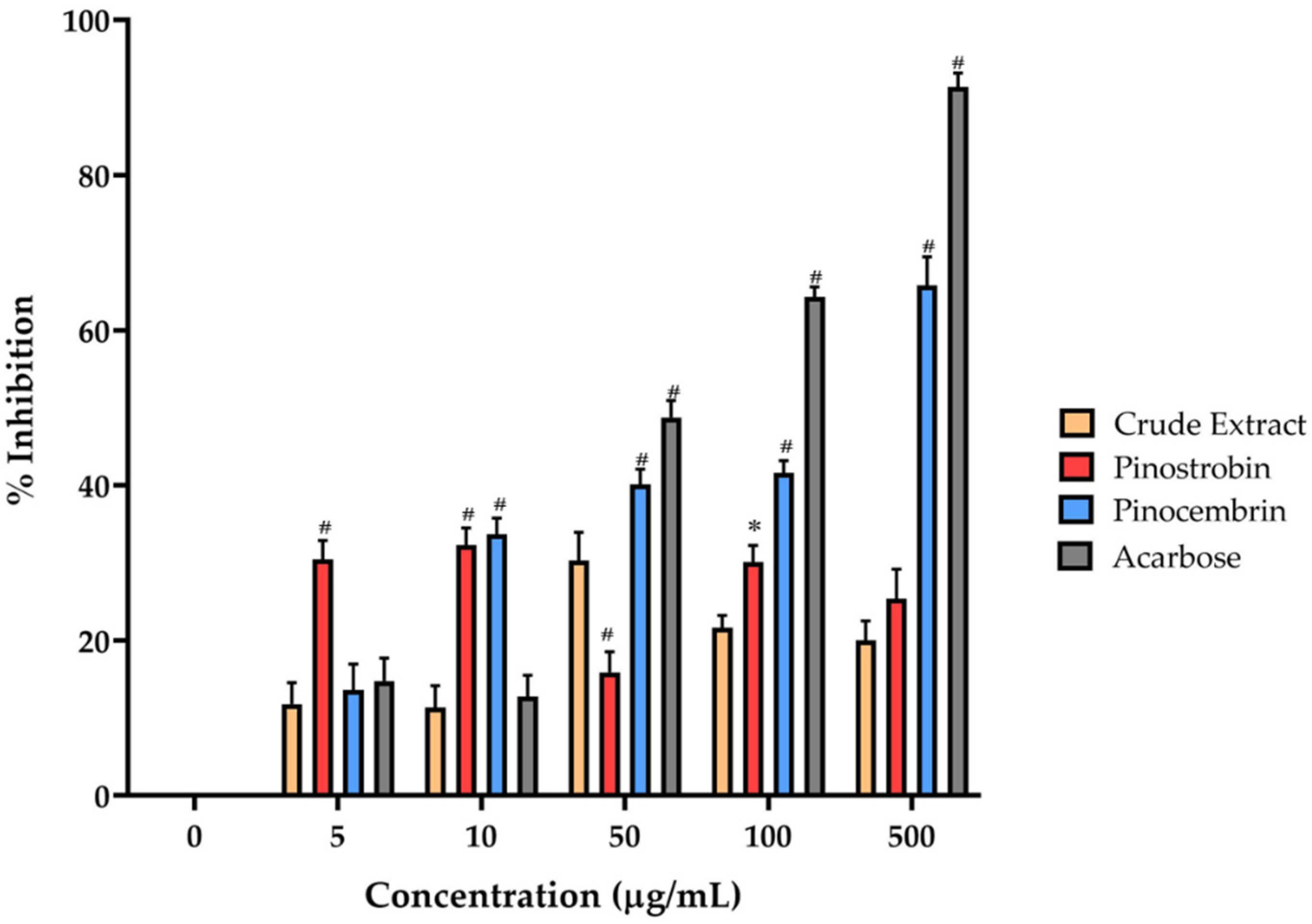
16]. The α-amylase inhibitory activities of pinostrobin, pinocembrin, and the crude extract of
B. rotunda may suggest potential antidiabetic properties, as these compounds can modulate carbohydrate digestion. Among the tested substances, pinocembrin demonstrated the most highly significant pancreatic α-amylase inhibition in all tested compounds. Previous reports on the α-amylase inhibitory activity of pinocembrin have shown that racemic pinocembrin exhibits concentration-dependent inhibition, with both S- and R-enantiomers contributing additively to the overall activity [
18]. This suggests that pinocembrin can effectively inhibit α-amylase activity, supporting its potential role in managing postprandial hyperglycemia. In contrast, pinostrobin and the crude extract of
B. rotunda showed mild α-amylase inhibitory activity in the current study. However, other research has highlighted the antidiabetic potential of
B. rotunda extracts, demonstrating that a polyphenol-rich fraction of
B. rotunda exhibited antidiabetic properties in high fructose/streptozotocin-induced diabetic rats [
19], suggesting that major bioactive compounds in the extract may contribute to its hypoglycemic effects. The observed differences in α-amylase inhibitory activities among these compounds may be attributed to their distinct chemical structures and affinities for the enzyme’s active site. Pinocembrin, a flavanone, may interact more effectively with α-amylase compared to pinostrobin, leading to higher inhibitory activity. These findings indicate that pinocembrin possesses notable α-amylase inhibitory activity, which may contribute to its antidiabetic potential. While pinostrobin and the crude extract of
B. rotunda showed limited activity in this assay, their overall antidiabetic effects may involve additional mechanisms or other bioactive constituents, warranting further investigation to fully elucidate their comprehensive antidiabetic properties.
The in vitro α-glucosidase inhibitory activities suggest their potential as antidiabetic agents by modulating disaccharidase digestion. Pinocembrin showed the most potent α-glucosidase inhibition among the tested compounds. This finding aligns with previous studies where pinocembrin exhibited moderate α-glucosidase inhibitory activity, with IC
50 values of 0.35 ± 0.021 mM against maltase and 0.39 ± 0.020 mM against sucrase activity [
11]. The inhibition mechanism was identified through which pinocembrin interacts with both the active site and allosteric sites of the enzyme, thereby affecting both the binding affinity and the maximum reaction rate. However, pinostrobin exhibited moderate α-glucosidase inhibitory activity, though less potent than pinocembrin. Its structural similarity to pinocembrin may suggest potential for enzyme interaction, albeit with lower efficacy. The crude extract of
B.
rotunda showed the weakest α-glucosidase inhibitory activity among the tested samples. However, it is noteworthy that certain fractions of
B.
rotunda extracts not only contain an amount of specific bioactive constituents within the extract that contribute to its inhibitory effects, but they also suggest possible synergistic effects. Pinostrobin and pinocembrin inhibit maltase activity and may be influenced by specific hydroxylation patterns in their flavonoids [
20]. Some studies have shown that hydroxylation at certain positions enhances inhibitory effects [
21]. The structural features of pinostrobin and pinocembrin may not favor strong interactions with the enzyme’s active site, resulting in lower inhibitory activity.
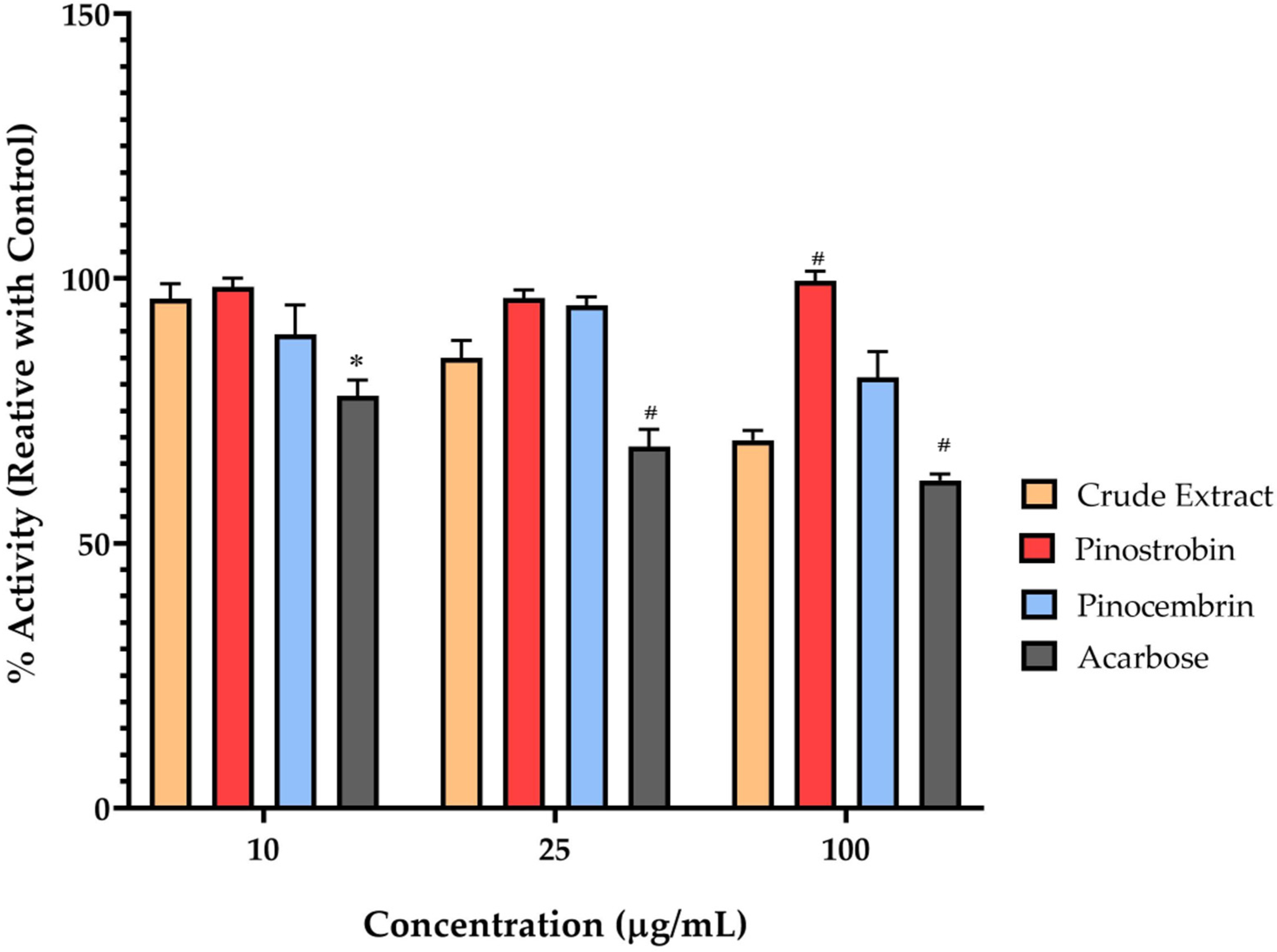
Furthermore, the use of Caco-2 cells as a model for human intestinal absorption provides a relevant system for assessing the potential of compounds to inhibit α-glucosidase activity in the human intestine that closely mimics human intestinal physiology. However, it is important to consider that in vitro results may not always directly translate to in vivo efficacy due to factors such as bioavailability and metabolism. In this study, the crude extract of
B.
rotunda exhibits some inhibitory activity against membrane-bound α-glucosidase, and its efficacy is limited compared to established inhibitors, e.g., acarbose. The individual constituents, pinostrobin and pinocembrin, showed minimal activity, suggesting that the crude extract’s effect may result from a combination of compounds or other minor constituents. Many studies initially screen compounds for α-glucosidase inhibitory activity using the enzyme derived from
Saccharomyces cerevisiae (yeast) due to its availability and cost-effectiveness. However, the yeast α-glucosidase differs structurally and functionally from the human intestinal membrane-bound α-glucosidase, leading to discrepancies in inhibitory efficacy observed in vitro versus in vivo. For instance, a study by Wongon and Limpeanchob demonstrated that oxyresveratrol exhibited potent inhibition of yeast α-glucosidase but showed significantly reduced efficacy against the membrane-bound α-glucosidase in differentiated Caco-2 cells [
22]. Similarly, research on 9-O-berberrubine carboxylate derivatives revealed strong inhibitory activity against yeast α-glucosidase, with IC
50 values ranging from 1.61 to 32.84 μM [
23]. However, the authors emphasized the necessity of further studies involving human intestinal enzymes and cell-based experiments to validate these findings.
In the context of B. rotunda, the crude extract demonstrated a concentration-dependent inhibition of maltase activity in Caco-2 cells; the residual maltase activity was higher than pinocembrin, which exhibited minimal inhibitory effects on maltase activity at all tested concentrations. These findings suggest that while the crude extract contains components capable of inhibiting human membrane-bound α-glucosidase, the isolated flavonoids pinostrobin and pinocembrin may not be the primary active constituents responsible for this activity. This underscores the importance of evaluating compounds in human-relevant models, as results from yeast-based assays may not accurately reflect efficacy in human systems.
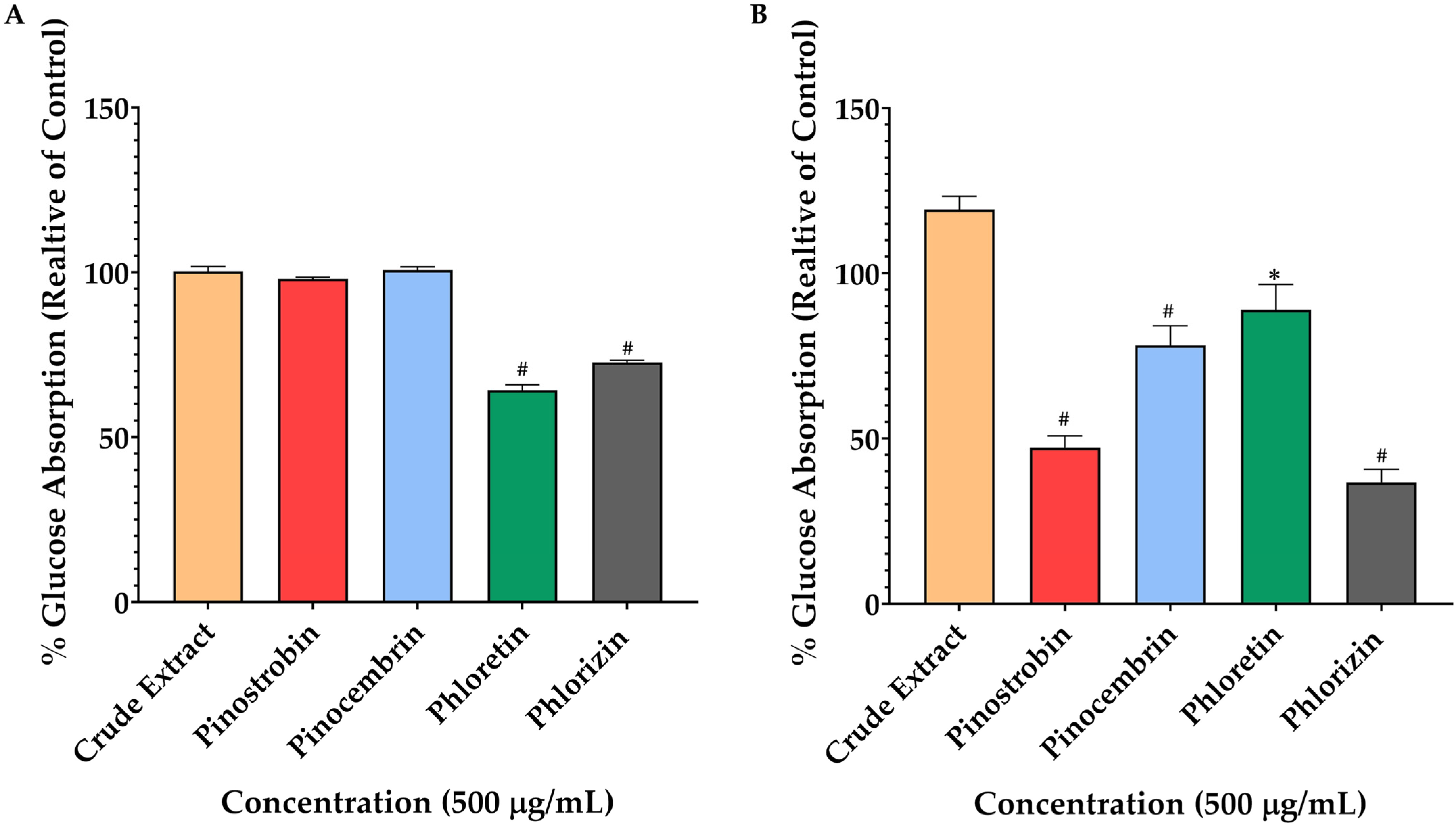
The observed inhibitory effects of
B.
rotunda extract and its constituents, pinostrobin and pinocembrin, on glucose uptake in Caco-2 cells suggest a potential in vitro model for studying intestinal glucose absorption, as it expresses key glucose transporters such as SGLT and GLUT. In the present study, the crude extract of
B.
rotunda significantly reduced glucose uptake in the differentiated Caco-2 model. Pinostrobin exhibited the most pronounced inhibitory effect, reducing 2-NBDG absorption. Pinocembrin also demonstrated notable inhibition, as well as the positive control, phlorizin, which showed strong inhibitory activity, reducing glucose uptake, while phloretin had a milder effect. These findings align with previous research indicating that certain flavonoids can modulate glucose transport in intestinal cells [
24]. The study by Manzano and Williamson demonstrated that polyphenols and phenolic acids from strawberry and apple decreased glucose uptake and transport in human intestinal Caco-2 cells [
25]. Similarly, 6-shogaol, a compound found in ginger, was shown to reduce glucose uptake in Caco-2 cells by downregulating the expression of SGLT1 and GLUT2 transporters [
26]. The differential effects observed among the compounds tested may be attributed to their varying affinities for glucose transporters or their influence on transporter expression by their mechanisms of action and efficacy in vivo models. Using high-glucose environments, known inhibitors such as phlorizin (targeting SGLT) and phloretin (targeting GLUT) significantly reduced 2-NBDG transport, indicating effective inhibition of glucose transporter activity. However, the crude extract, pinostrobin, and pinocembrin did not exhibit significant effects under these conditions, suggesting limited interaction with glucose transporters when glucose is abundant. Conversely, under glucose-free conditions, pinostrobin and pinocembrin significantly reduced 2-NBDG transport, with pinostrobin at 500 μg/mL achieving a 49% reduction in absorption, comparable to phlorizin’s 45% reduction. This indicates that these compounds may modulate glucose transport mechanisms, particularly under low-glucose conditions. These findings align with previous research indicating that certain flavonoids can modulate glucose transport in intestinal cells. For instance, studies have shown that polyphenol-rich extracts can inhibit glucose uptake in Caco-2 cells by modulating the expression of glucose transporters such as GLUT2 and SGLT1 [
27].
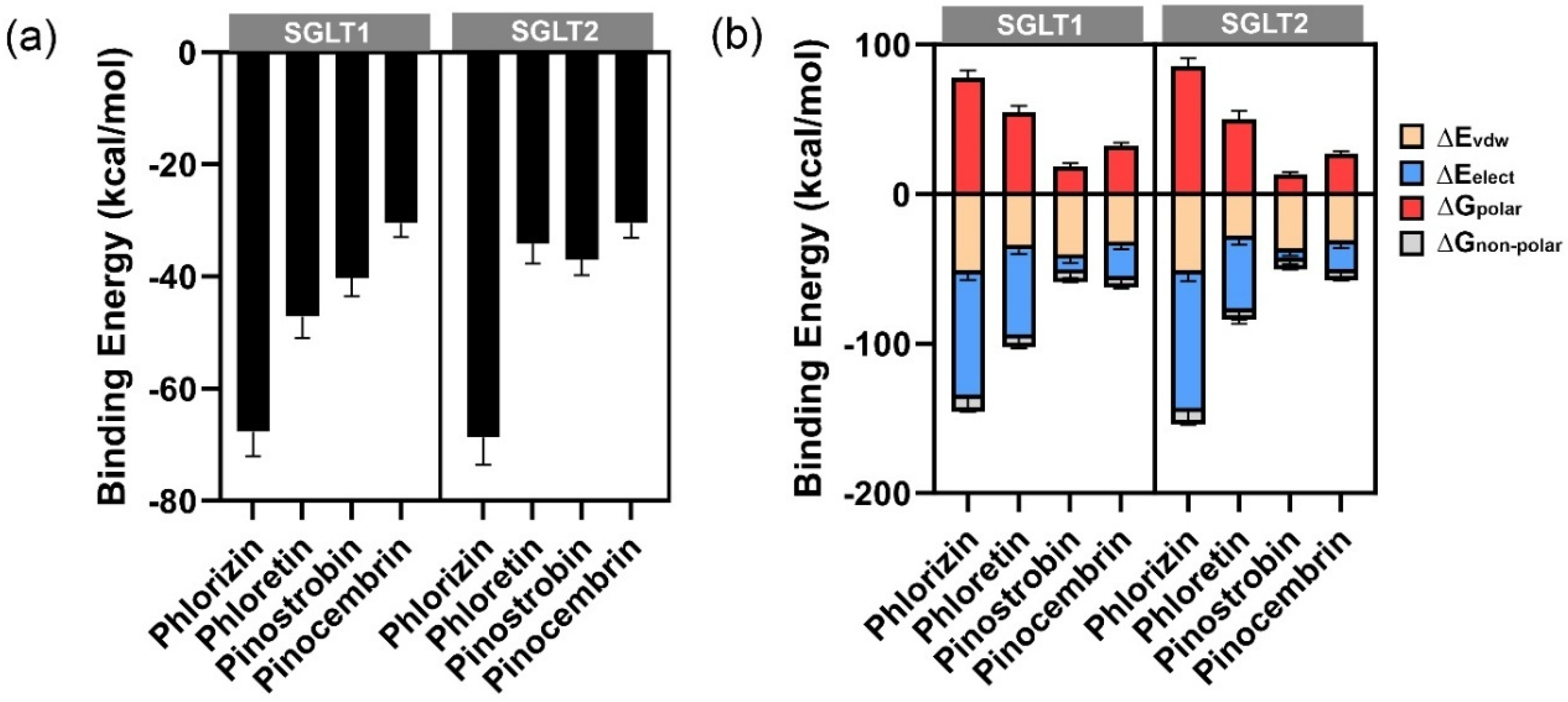
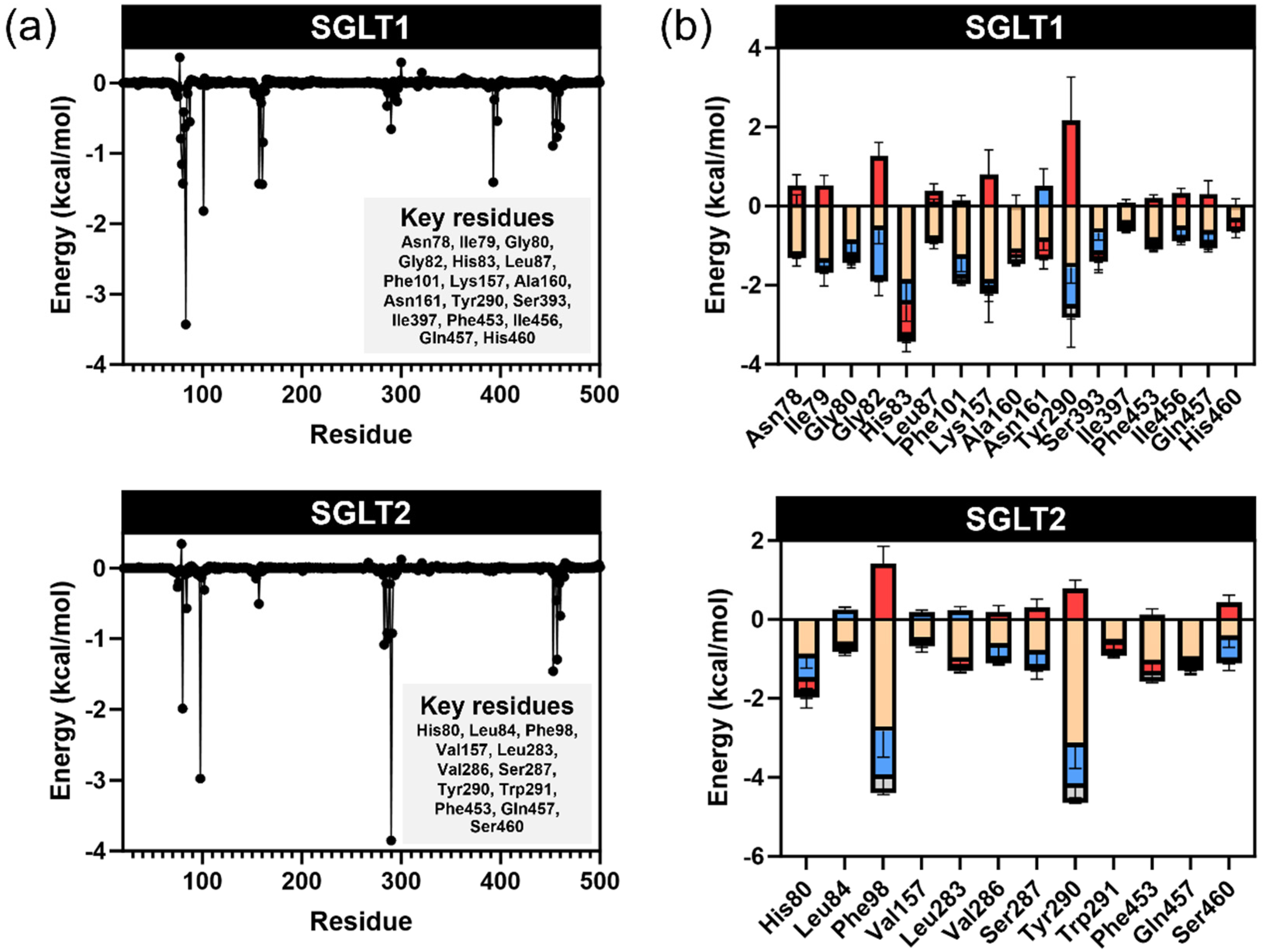
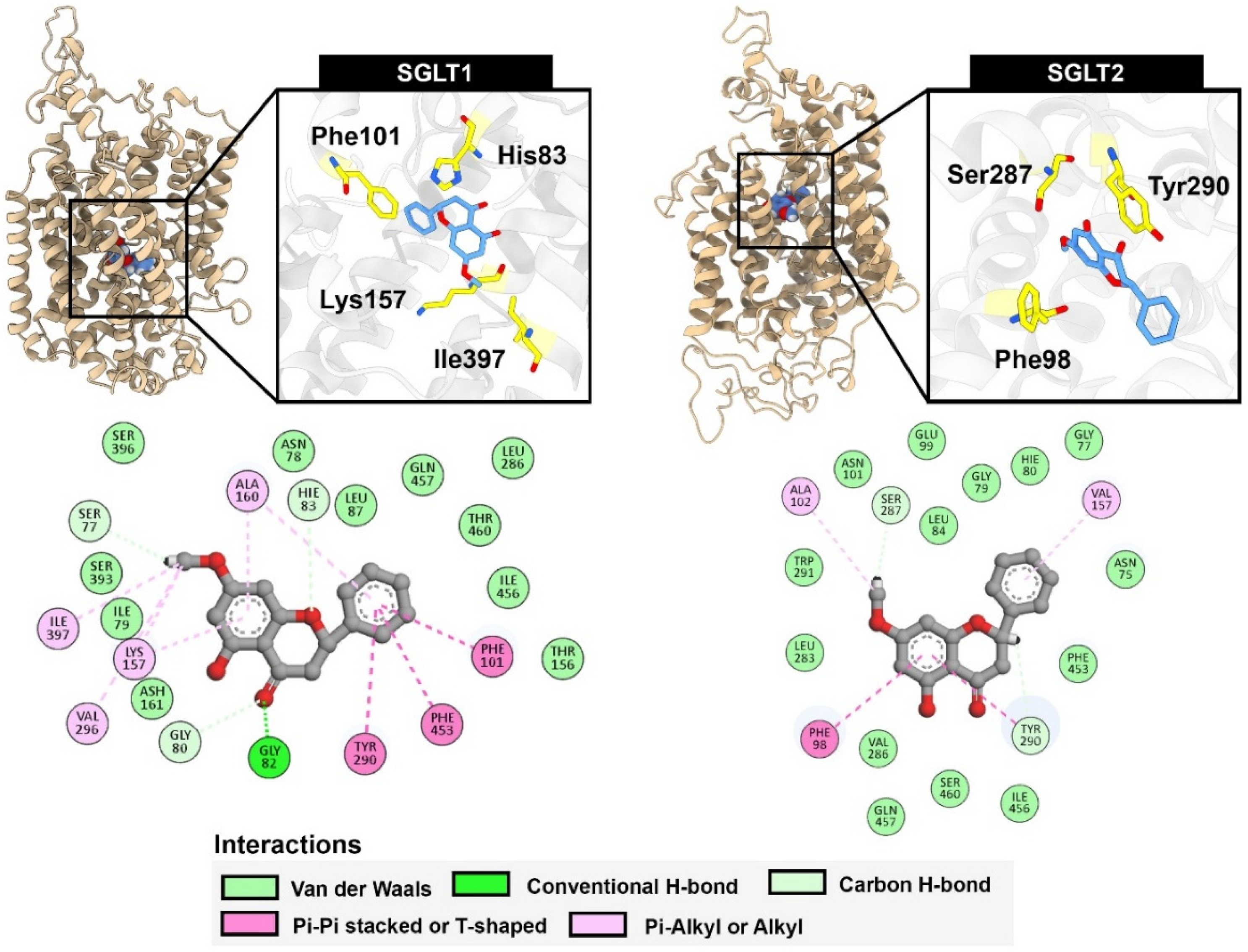
Understanding the specific interactions between these compounds and glucose transporters could provide insights into their potential as natural modulators of glucose absorption. The binding of compounds within the glucose transporter channels of SGLTs leads to the blockage of glucose from passage through the cells. We employed an in silico study to highlight the key interactions between our potential compound, pinostrobin, and the glucose transporters SGLT1 and SGLT2. The relative binding energies calculated using GNINA and MM-GBSA methods indicated the strength of binding. Lower binding energies suggested greater binding affinity, which correlates with inhibitory activity against enzymes. Compared with the positive control, phlorizin, pinostrobin showed lower binding ability in both docking scores and MM-GBSA calculations. However, pinostrobin exhibited a stronger binding ability than pinocembrin, highlighting the role of its methoxy group in interacting with amino acid residues within the binding site of SGLTs. Per-residue energy decomposition analysis revealed that the methoxy groups of pinostrobin formed various π–alkyl and alkyl interactions with key amino acids such as Lys157, Val296, and Ile397 (in the case of SGLT1), and Ala102 (in the case of SGLT2). These particular interactions were not observed with pinocembrin, suggesting that the methoxy group may represent a key pharmacophore for the development of SGLT inhibitors. In high-glucose environments, glucose transport across intestinal epithelial cells, such as Caco-2, is predominantly facilitated by facilitative glucose transporters GLUT and GLU11. GLUT2, a low-affinity, high-capacity transporter, can translocate from the basolateral to the apical membrane in response to elevated luminal glucose, enhancing glucose absorption. GLUT1, a high-affinity transporter, contributes to basal glucose uptake and remains active across varying glucose conditions. In this study, the significant reduction in 2-NBDG transport observed with phloretin treatment supports the role of GLUT2 and GLUT1 in mediating glucose transport under high-glucose conditions, as phloretin is a known inhibitor of these transporters. In contrast, pinostrobin, pinocembrin, and the crude extract of
B.
rotunda did not significantly inhibit glucose transport in the presence of high glucose, suggesting that their effects may not involve direct inhibition of GLUT2/GLUT1 or that their activity may be more prominent under glucose-depleted conditions. This aligns with previous findings that glucose transporter activity and localization are dynamically regulated by glucose availability [
28,
29]. In low-glucose environments, sodium-dependent glucose transporters (SGLT1 and SGLT2) play a critical role in active glucose uptake across the intestinal epithelium. Unlike facilitative GLUT transporters, SGLTs utilize the sodium gradient to actively transport glucose against its concentration gradient, ensuring efficient absorption when luminal glucose levels are low. SGLT1 is primarily expressed in the small intestine and is responsible for most dietary glucose uptake, while SGLT2 is mainly found in the kidneys but may have minor intestinal roles [
30]. In this study, the notable reduction in 2-NBDG transport by pinostrobin, pinocembrin, and known inhibitors phlorizin and phloretin under glucose-deprived conditions suggests that these compounds may inhibit SGLT-mediated glucose uptake. Phlorizin, a well-characterized SGLT inhibitor, confirms the involvement of these transporters. This supports the hypothesis that
B.
rotunda compounds exert their effects predominantly by targeting SGLT function in low-glucose states, consistent with reports that active glucose transport via SGLTs is critical for maintaining glucose homeostasis when extracellular glucose is scarce [
31,
32]. Although our in vitro findings demonstrate that pinostrobin and pinocembrin possess promising antidiabetic activity through inhibition of glucose uptake, the clinical relevance of these results requires further investigation. Preclinical studies have demonstrated favorable safety profiles for both pinocembrin and pinostrobin. Pinocembrin exhibited no acute or sub-chronic toxicity in male Wistar rats at oral doses up to 500 mg/kg, with no mortality, significant changes in body or organ weights, or alterations in blood biochemistry [
33]. Genotoxicity assessments, including liver micronucleus formation and mitotic index analysis, indicated no mutagenic effects. Additionally, pinocembrin showed organ-specific protective properties, such as hepatoprotection against carbon tetrachloride-induced liver fibrosis and neuroprotection against β-amyloid-induced neuronal damage [
34]. Similarly, pinostrobin demonstrated low acute toxicity in male Wistar rats, with doses up to 500 mg/kg causing no significant adverse effects. Sub-chronic studies using fingerroot extract containing pinostrobin confirmed its safety at doses up to 100 mg/kg/day [
35]. Genotoxicity evaluations revealed no mutagenic activity, and pinostrobin also exhibited renal protective effects against gentamicin-induced toxicity, likely mediated by its anti-inflammatory and antioxidant mechanisms [
36]. Overall, both compounds appear to be safe in preclinical models. However, comprehensive clinical studies are necessary to confirm their safety, pharmacokinetics, and therapeutic potential in humans.
4. Materials and Methods
4.1. Plant Material and Plant Extraction
B. rotunda was collected in January 2024 from Srithep, Phetchabun, Thailand, and the specimen was identified by a botanist and kept in both Queen Sirikit Botanical Gardens (QSBG), Chiang Mai, Thailand, and School of Pharmaceutical Sciences, University of Phayao, Thailand, under the same code of PHARCOSUP45. Fresh rhizomes were collected. Rhizomes were collected, dried in a hot-air oven at 55 °C for 2 days, and then ground. The size of the ground powder was selected in the range of 100–250 µm and kept at reduced pressure until use. The dried plant (300 g) was macerated with 99% ethanol (2 L) and assisted by an ultrasonic bath (KQ3200DE, Kunshan Ultrasonic Instruments, Shanghai, China) at 40 kHz for 30 min. It was repeated by the new solvent three times. After that, the extract was filtered through a cellulose membrane (Whatman filter paper no. 1), (Merck, Göttingen, Germany) and the solvent was then evaporated under reduced pressure using a rotary evaporator (EYELA, N-1001, Tokyo, Japan) to obtain the crude extract (12% w/w of dried plant).
4.2. Isolation of Pinostrobin and Pinocembrin
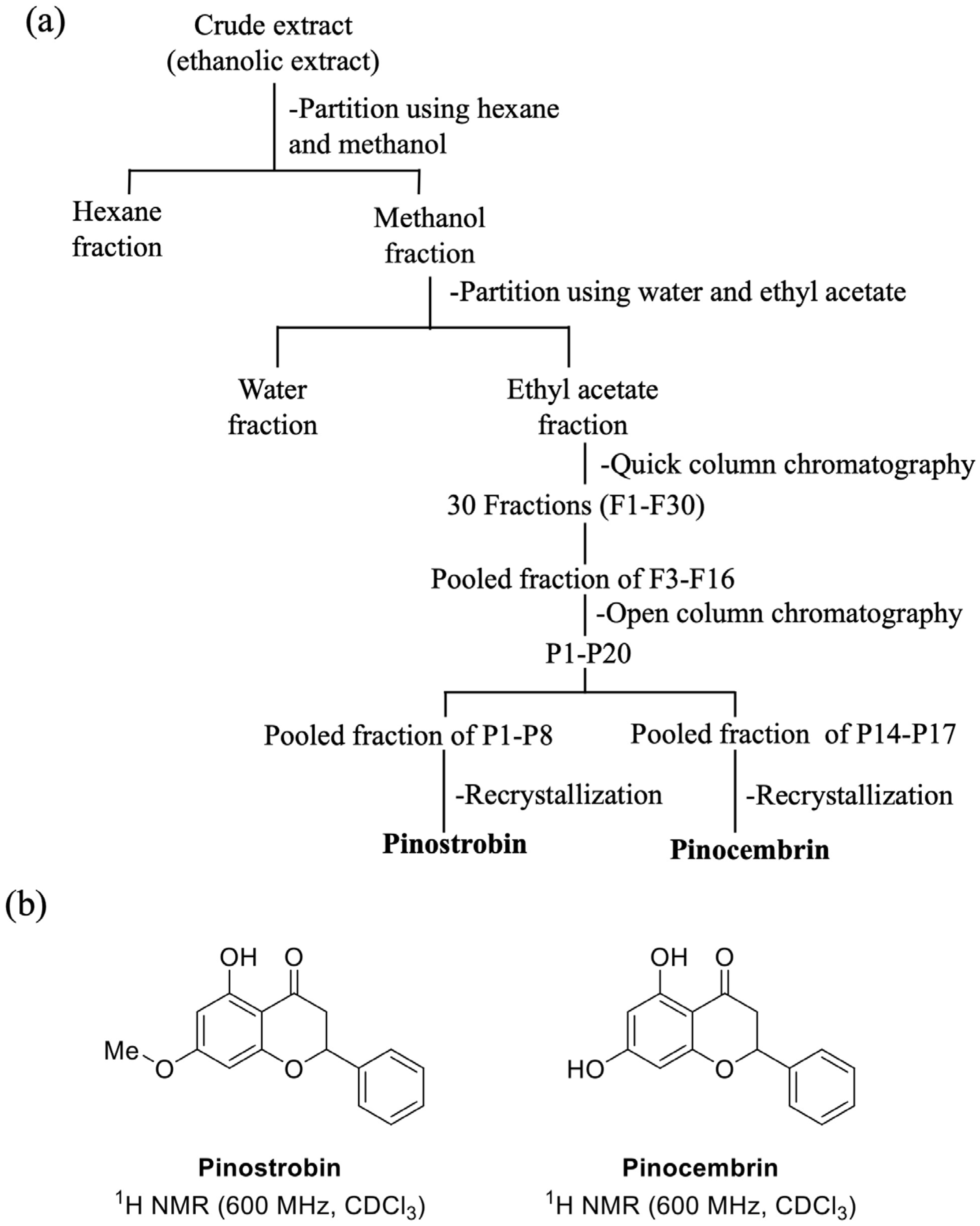
The crude extract (25 g) was partitioned by hexane (1 L) and methanol (1 L) to obtain the hexane (7.5 g) and methanol fractions. The methanol fraction was then partitioned using water (500 mL) and ethyl acetate (EtOAc) (1 L) to obtain the EtOAc (17 g) and water fractions (4.2 g). The EtOAc fraction was then subjected to fractionation using a quick column chromatography (column: 10 × 13 cm) with gradient elution of hexane–EtOAc (100:0 to 0:100) followed by hexane to EtOAc (100:0 to 0:100) and then EtOAc to MeOH (100:0 to 50:50). Thirty fractions (250 mL each) were collected and named as F1-F30 (
Figure 1a). The fractions 13 to 16 (P15) were pooled, evaporated, and purified by silica gel column chromatography (EtOAc–hexane, 1:9), yielding 20 fractions (P15-1 to P15-20). P15-1 to P15-8 were pooled and evaporated from the solvent and then recrystallized using DCM and MeOH (3:7) at 4 °C to provide white crystals of pinostrobin (98 mg). While P15-14 to P15-17 fractions were pooled and then recrystallized by DCM and MeOH (3:7) at 4 °C to provide yellowish powder of pinocembrin (41 mg), The isolated compounds were identified by NMR (Nuclear Magnetic Resonance Spectroscopy AVANCE NEO Bruker, (CryoProbe Prodigy) 600 MHz (Liquid)–600 MHz Prodigy NMR) (Bruker, MA, USA) and mass spectroscopy (Agilent-6540 UHD accurate-mass quadrupole-time-of-flight (QTOF) mass spectrometer (Agilent Technologies, CA, USA).
4.3. HPLC Method Validation for Quantification of Pinostrobin and Pinocembrin
Pinostrobin and pinocembrin were simultaneously analyzed using an HPLC-UV assay. The HPLC assay was carried out using the HPLC Shimadzu Prominence UFLC system, hyphenated with a UV-Vis detector (SPD-20A 230 V) (Shimadzu, Tokyo, Japan), and an LC-20AD pump (Shimadzu, Tokyo, Japan)was used. The sample injection volume was 20 μL, and a C-18(2) column (Phenomenex) (Santa Clara, CA, USA), 250 mm in length and 4.6 mm in diameter, was used. A flow rate of 1.50 mL/min under room temperature conditions was employed. The gradient elution was applied at different mobile phases. The mobile phase consisted of 1.0% (v/v) formic acid in water (A) and acetonitrile (B). The gradient that was eluted was as follows: 0–5 min (40% A), 5–7 min (10% A), 7–12 min (5% A) for a total analysis time of 12 min, followed by a post-run of 40% A for another 3 min. The mobile phase was filtered through a 0.45 μm filter and degassed using an ultrasonic bath before use. A UV-Vis detector was set at a wavelength of 300 nm. To ensure the reliability of the analysis assay, the HPLC method was validated based on linearity and range, lowest detection limit concentration (LOD), the lowest quantification limit concentration (LOQ), accuracy (%recovery), and precision (%RSD) following the q2(R1) ICH guideline (2005). This validated HPLC analysis was applied to standardize the bioactive compound content, pinostrobin, and pinocembrin in % w/w in the extract before bioactivity evaluation.
4.4. In Vitro α-Amylase Inhibition Assay
The α-amylase assay was performed following the method described by Wongon and Limpeanchob, with slight modifications [
22]. The reaction product was quantified colorimetrically using reducing sugar oxidation. Briefly, 50 μL of various concentrations of
B. rotunda extract (PE), pinostrobin, pinocembrin, or acarbose (Sigma-Aldrich, St. MO, USA) was incubated with 100 μL of starch solution (12.5 mg/mL in PBS) at 37 °C for 5 min. Subsequently, 100 μL of α-amylase solution (Sigma-Aldrich, St. MO, USA) (1.5 U/mL) was added and incubated for an additional 10 min. Then, 50 μL of the reaction mixture was transferred to react with 100 μL of 1% 3,5-dinitrosalicylic acid (DNS) reagent (Sigma-Aldrich, St. MO, USA). The mixture was heated at 95 °C for 5 min, allowed to cool, and the absorbance was measured at 540 nm.
4.5. In Vitro α-Glucosidase Inhibition Assay
The α-glucosidase inhibitory activity was evaluated based on the method of Wongon and Limpeanchob, with minor modifications [
22]. The assay measured the release of p-nitrophenol from p-nitrophenyl-α-D-glucopyranoside (PNPG) (Sigma-Aldrich, St. MO, USA). In brief, 20 μL of α-glucosidase solution (Sigma-Aldrich, St. MO, USA) (125 mU/mL in phosphate-buffered saline, pH 6.8) was mixed with 80 μL of various concentrations of
B. rotunda extract (PE), pinostrobin, or pinocembrin in a 96-well microplate. The mixtures were incubated at 37 °C for 10 min, followed by the addition of 100 μL of 0.2 mM PNPG. After a further incubation at 37 °C for 30 min, the absorbance was measured at 405 nm using a microplate reader (Thermo Fisher Scientific, MA, USA). Acarbose, prepared in distilled water at various concentrations, was used as the positive control.
4.6. Cell Culture
Caco-2 cells were obtained from the American Type Culture Collection (ATCC) and cultured in DMEM/F12 medium (Cytiva, UT, USA) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin–streptomycin (Thermo Fisher Scientific, MA, USA). Cells were maintained at 37 °C in a humidified atmosphere containing 95% air and 5% CO
2. For experiments, Caco-2 cells were seeded in 96-well plates for cell viability assays and in 24-well plates for α-glucosidase inhibition studies. Upon reaching confluence, the cells were allowed to differentiate, during which they expressed brush border enzymes such as lactase, sucrase-isomaltase, and alkaline phosphatase within 2–3 weeks, as described by Van Beers [
37]. Throughout the differentiation period, the culture medium was replaced with fresh medium every two days. Fully differentiated cells (18–21 days post-seeding) were used for most experimental procedures.
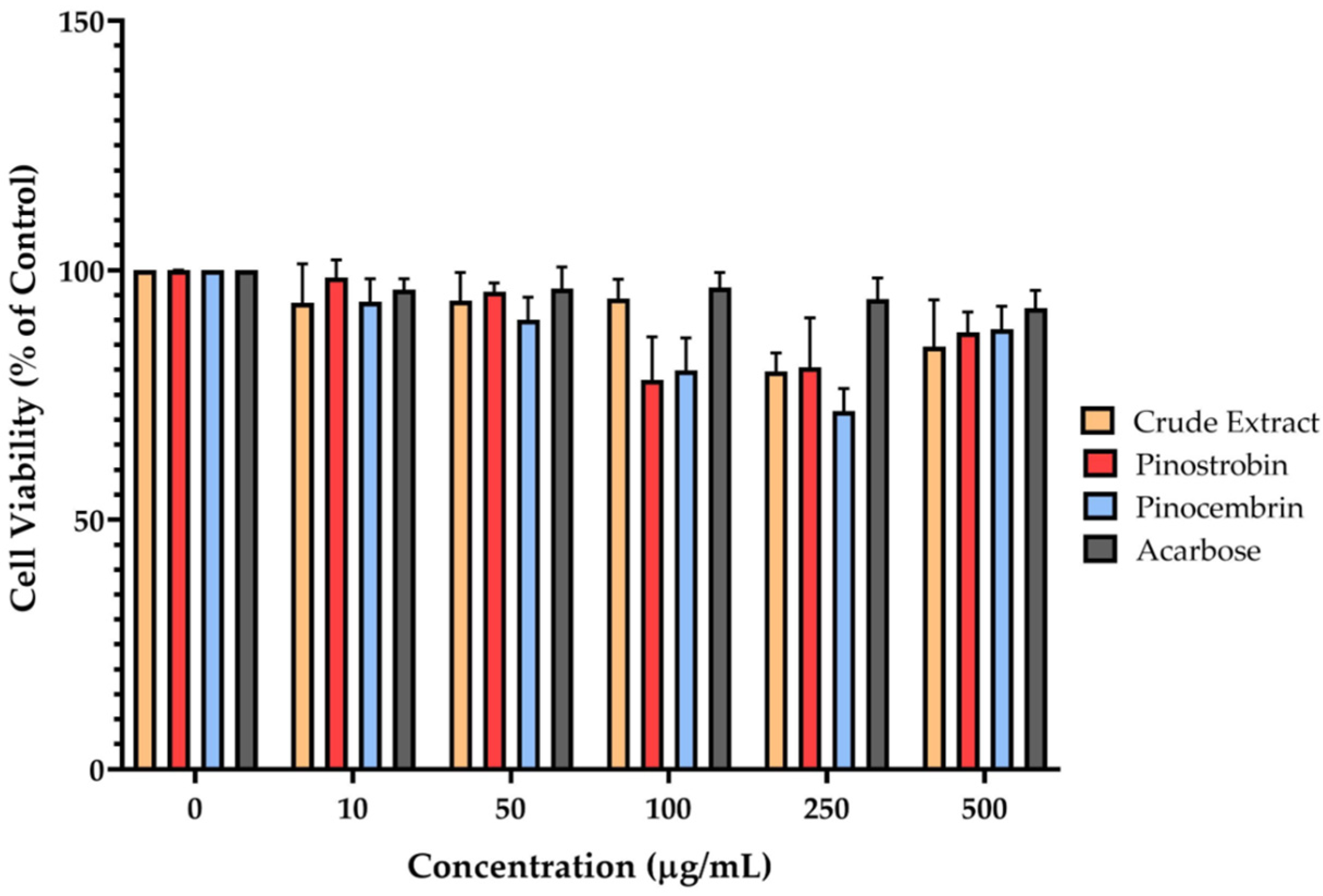
4.7. Determination of Cell Viability
Cell viability was evaluated using the MTT assay, which is based on the conversion of MTT to an insoluble purple formazan product by mitochondrial dehydrogenases in viable cells. No such conversion occurs in non-viable (dead) cells. Caco-2 cells were treated with various concentrations of B. rotunda rhizome extract (PE), pinostrobin, or pinocembrin or acarbose for 24 h. Two hours before the end of the treatment period, 10 μL of MTT solution (Tokyo Chemical Industry, TK, JP) (5 mg/mL) was added to each well. Following incubation, the culture medium was removed, and the resulting formazan crystals were solubilized using a 1:1 (v/v) mixture of dimethyl sulfoxide (RCI Labscan, BKK, TH) (DMSO) and ethanol. Absorbance was measured at 595 nm using a microplate reader.
4.8. Membrane Bound α-Glucosidase Inhibition Assay
Membrane-bound α-glucosidase activity in Caco-2 cells was assessed with modifications based on the method described by Wongon and Limpeanchob [
22]. Briefly, Caco-2 cells were seeded in 24-well plates at a density of 100,000 cells per well and cultured in DMEM/F12 medium supplemented with 10% fetal bovine serum (FBS) and 1% penicillin–streptomycin for 18–21 days to allow for differentiation. After reaching confluence, the culture medium was removed, and the cells were washed with 1 mL of PBS, followed by overnight incubation in serum- and glucose-free medium. Subsequently, 160 μL of culture medium containing
B. rotunda extract (PE), pinostrobin, pinocembrin, or acarbose (20 μL, at various concentrations) was added and incubated for 15 min. This was followed by the addition of 20 μL of 250 mM maltose (Sigma-Aldric, HE, DEU) as the α-glucosidase substrate, and incubation continued for 4 h. Afterward, 20 μL of the culture medium was collected to determine glucose production using a glucose assay kit (Stanbio Laboratory, TX, USA.). All experiments were performed in triplicate, and α-glucosidase (maltase) inhibitory activity was expressed as percentage inhibition.
4.9. Glucose Uptake Assay
Caco-2 cells were seeded into black 96-well plates (Thermo Fisher Scientific Inc., MA, USA.) at a density of 20,000 cells per well and cultured for 20 days to allow for full differentiation, with media changes every three days. After the 20-day differentiation period, the cells were incubated overnight in glucose-free medium, followed by a 1 h incubation in buffer. Cells were then treated with test compounds, after which 200 μM of the fluorescent glucose analog 2-NBDG (Sigma-Aldrich, MA, USA) was added in a glucose-free medium. Following a 4 h incubation, the medium was removed, and the cells were washed three times with PBS. Intracellular fluorescence of 2-NBDG was measured using a microplate reader at an excitation wavelength of 485 nm and an emission wavelength of 528 nm. Results were expressed as the percentage of relative fluorescence intensity compared to the control group treated with 2-NBDG in the absence of test compounds.
4.10. Caco-2 Cell Monolayer Glucose Transport Assay
Caco-2 cell monolayers were cultured on Transwell
® inserts (Corning Incorporated, NY, USA.) placed in 24-well plates, following the method described by [
38] with minor modifications. To assess apical-to-basolateral glucose permeability, 1 mL of serum-free, phenol red-free medium (pH 7.4, 37 °C) was added to the basolateral chamber, while 0.2 mL of the test solution containing 200 μM 2-NBDG was applied to the apical side. The experiment was conducted under both glucose-free (0 mM) and high-glucose (25 mM) conditions. After a 4 h incubation at 37 °C, samples were collected from the basolateral side. The amount of 2-NBDG transported across the cell monolayer was quantified using a fluorescence spectrophotometer at an excitation wavelength of 495 nm and an emission wavelength of 535 nm. Results were expressed as the relative amount of 2-NBDG that permeated to the basolateral compartment, with the glucose transport level of the control group set at 100%. Phloretin and phlorizin (Sigma-Aldrich, MA, USA) (2 mM) were used as positive controls.
4.11. Molecular Docking
The structures of macromolecules were retrieved from the RCSB Protein Data Bank, including sodium-glucose cotransporter 1 (SGLT1, PDB ID: 7WMV) [
39] and sodium-glucose cotransporter 2 (SGLT2, PDB ID: 7VSI) [
40]. The protein structures were prepared by removing water molecules, ions, and co-crystallized ligands. Missing residues were modeled using homology modeling via the online tool SWISS-MODEL [
41]. The completed protein structures were exported in PDB format. The structures of ligands, including phlorizin, phloretin, pinostrobin, and pinocembrin, were generated using Chem3D software version 16. These structures were fully optimized using DFT calculations at the B3LYP/6-31++G (d,p) level of theory with the Gaussian16 package [
42]. The optimized structures were exported in PDB format. Molecular docking experiments were conducted using GNINA docking with deep learning [
43], running on Google Colab. The grid box was automatically generated based on the coordinates of the co-crystallized inhibitor, with an additional 5 Å padding in all dimensions. The exhaustiveness was set to 32 for all predictions. The results were visualized using BIOVIA Discovery Studio 2022.
4.12. Molecular Dynamics Simulation
Molecular dynamics (MD) simulations were conducted using the AMBER 23 package [
44]. Three distinct binding poses obtained from molecular docking were selected for MD simulation. The protein–ligand complexes were prepared using the tleap module implemented in the AmberTools23 package [
45]. The macromolecules were assigned standard protonation states at pH 7.4 using the online tool PDB2PQR. The protein structures were parameterized using the AMBER ff14SB force field [
46]. Ligand charges were calculated at the semi-empirical AM1-BCC level of theory and parameterized using the Generalized AMBER Force Field 2 (GAFF2). Each protein–ligand complex was solvated in an octahedral box with a 12 Å spacing between the solute surface and the box edge, using TIP3P water molecules. Sodium (Na+) and chloride (Cl
−) ions were added to neutralize the system and mimic a physiological concentration of 0.15 M. Energy minimization was performed using 5000 steps of the steepest descent algorithm followed by the conjugate gradient method. Hydrogen-involving bonds were constrained using the SHAKE algorithm. Temperature was maintained with a Langevin thermostat at a collision frequency of 2.0 ps-1, and pressure was controlled using a Berendsen barostat with a pressure relaxation time of 2.0 ps. Long-range electrostatic interactions were calculated using the particle mesh Ewald (PME) method with a cutoff of 8 Å. The system was gradually heated from 10 K to 310 K over 500 ps under the NVT ensemble. Pressure equilibration was carried out at 1 atm for 500 ps under the NPT ensemble. System equilibration was performed with positional restraints on solute atoms using force constants of 2.0, 1.0, 0.5, and 0.1 kcal/mol·Å2 for 200 ps each, followed by an unrestrained equilibration for 200 ps. Finally, production MD simulations were performed for 100 ns with three independent simulations using pmemd.cuda.SPFP, with a time step of 2 fs. Trajectories were saved every 10 ps.
4.13. MM-GBSA Calculation
The relative binding free energy was calculated using the Molecular Mechanics Generalized Born Surface Area (MM-GBSA) method with the MMPBSA.py script. A total of 1000 snapshots were extracted from the last 10 ns of each trajectory for the calculations. The solvation energy was computed using the modified Generalized Born model (igb = 2). The salt concentration was set to 0.15 M. The relative binding free energy was calculated using the following equation:
4.14. Statistical Analysis
The results are presented as mean ± standard error of the mean (SEM). Statistical comparisons between groups were performed using one-way analysis of variance (ANOVA), followed by Duncan’s multiple-range post hoc test to identify significant differences among means. All analyses were conducted using IBM SPSS Statistics version 22.