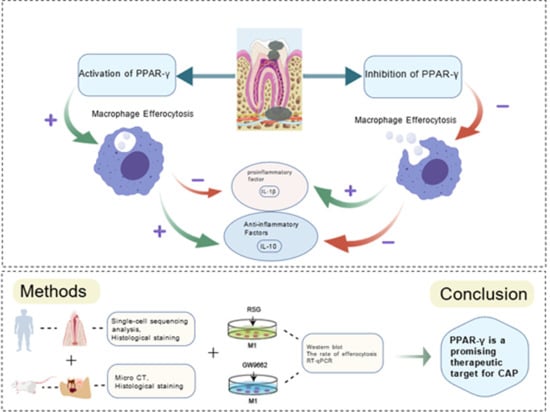
PPAR-γ Inhibits Chronic Apical Periodontitis by Facilitating Macrophage Efferocytosis
Abstract
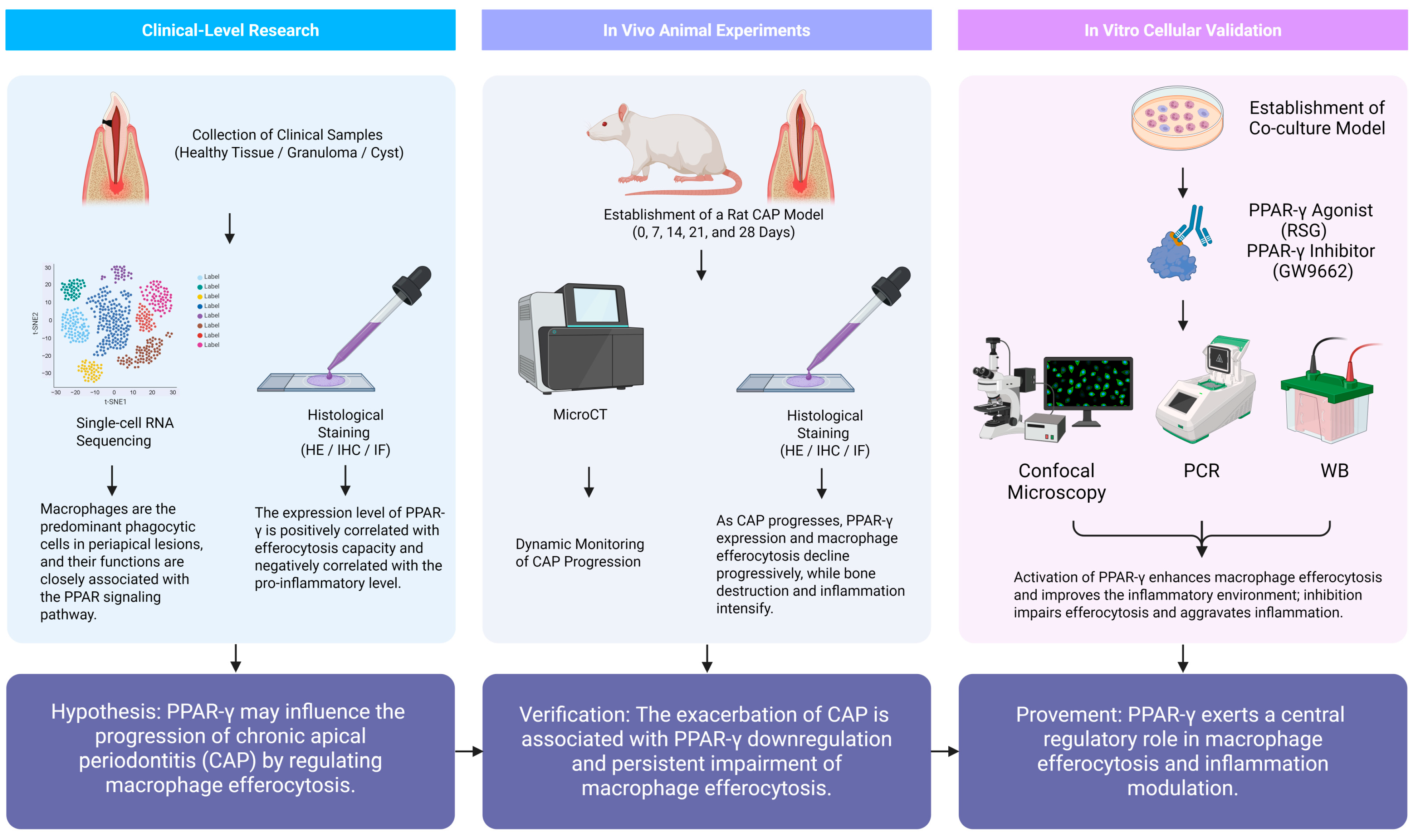
1. Introduction
2. Results
2.1. Single-Cell Sequencing
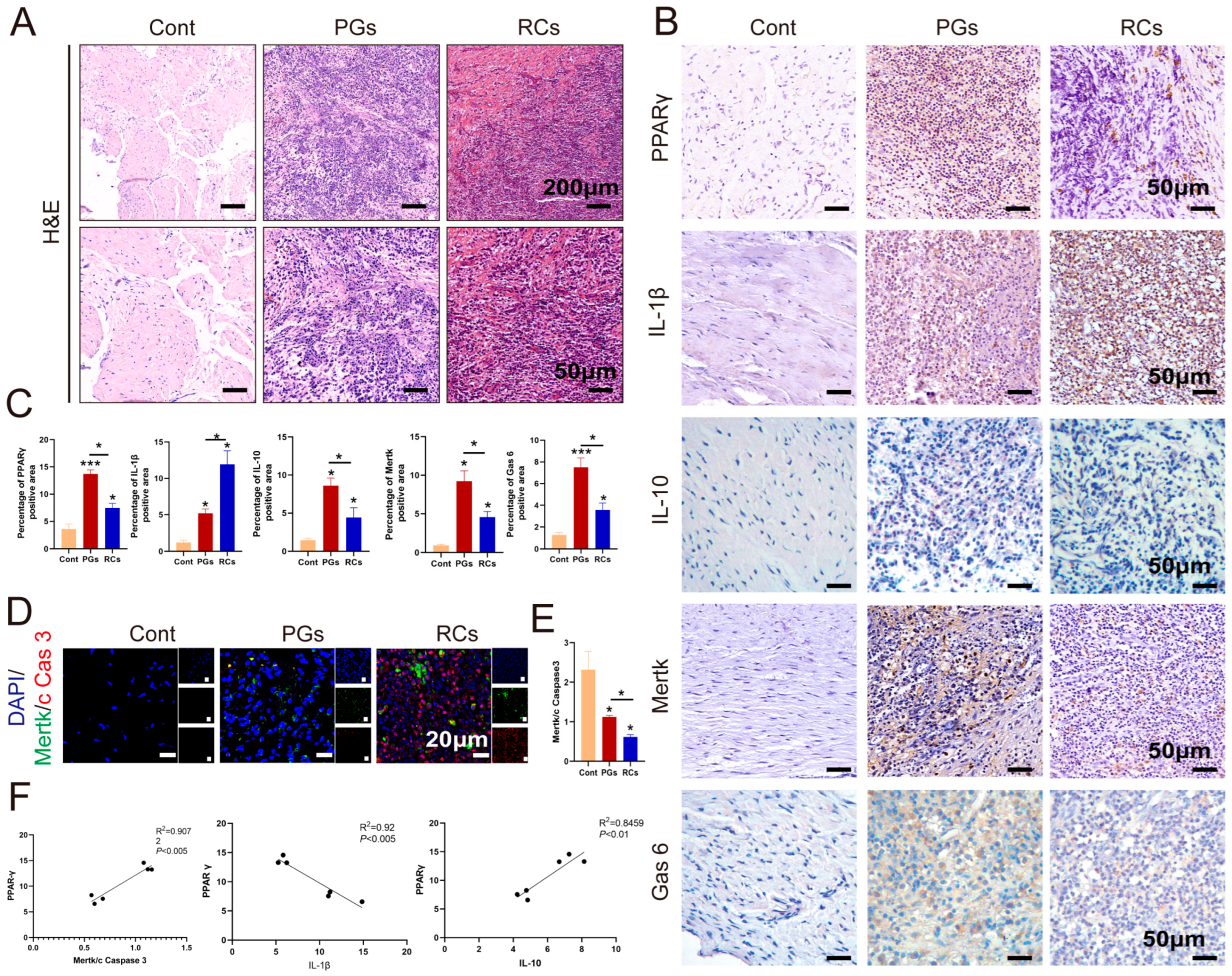
2.2. The Expression of PPAR-γ Varies Depending on Chronic Apical Periodontitis Development in Clinical Samples
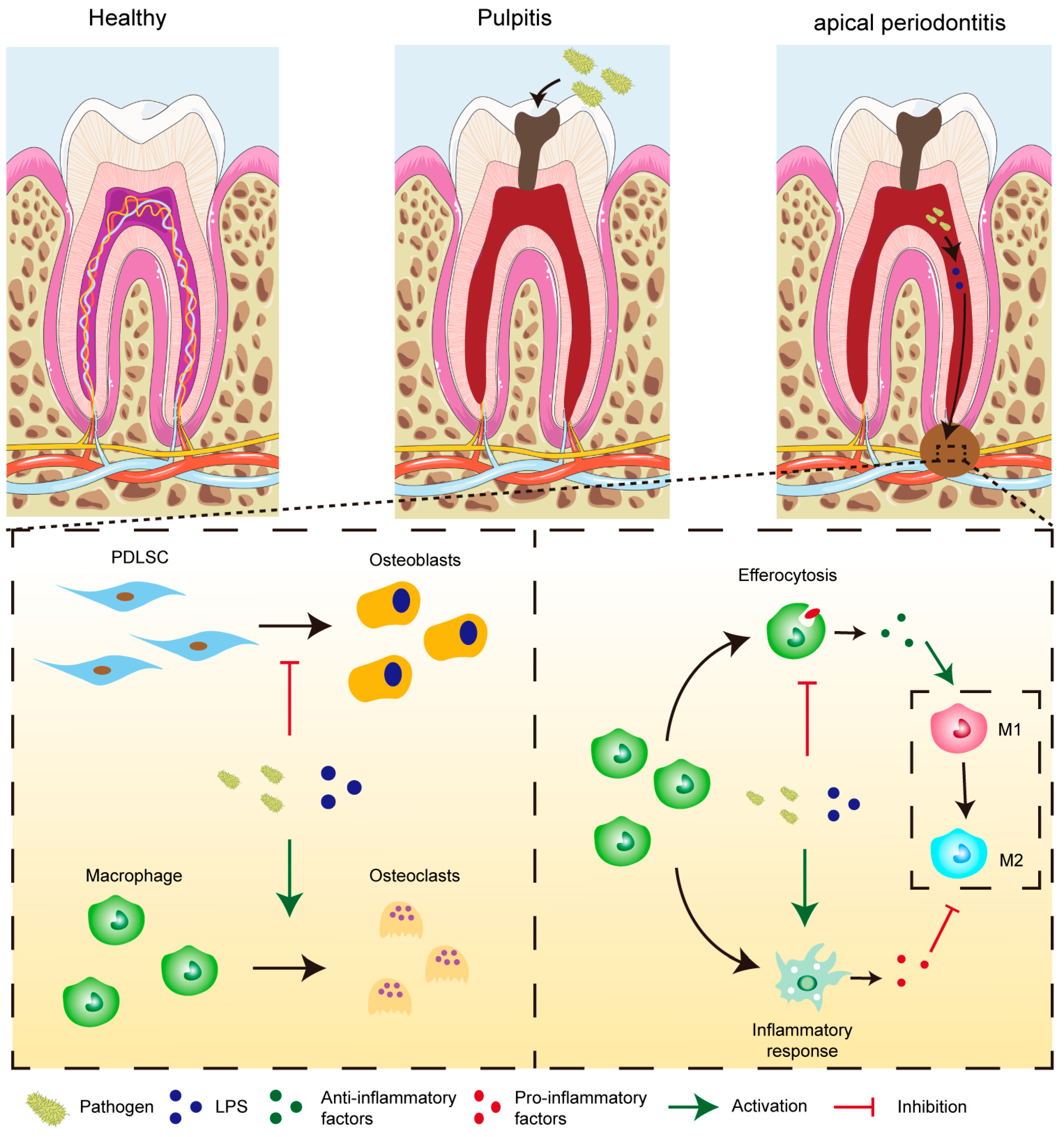
2.3. The Aggravation of CAP in the Animal Model Was Associated with the Down-Regulation of PPAR-γ and a Sustained Dysfunction in Macrophage Efferocytosis
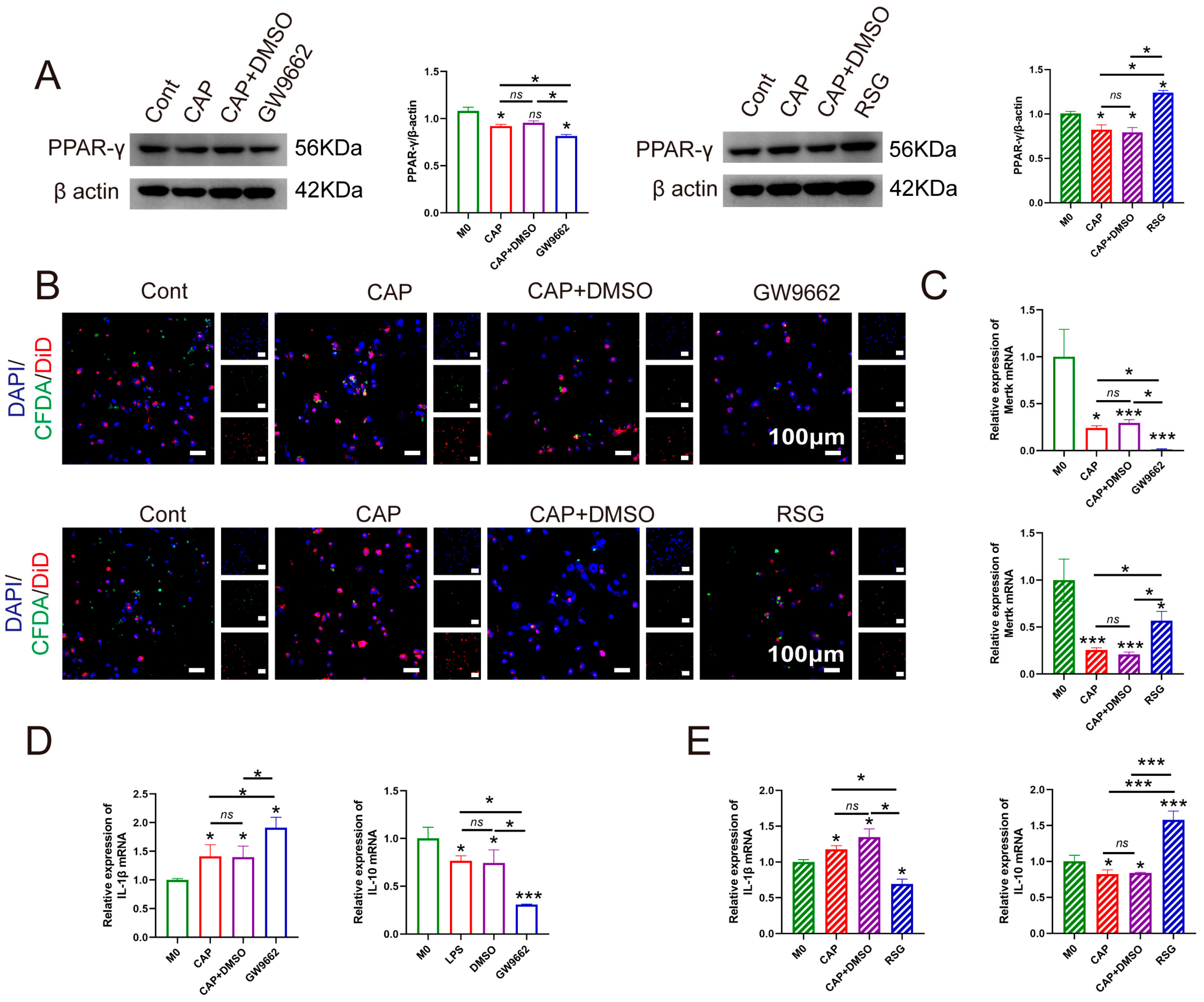
2.4. Interfering with the Expression of PPAR-γ Effectively Modulates Macrophage Efferocytosis in an In Vitro Model Mimicking the Environment of CAP
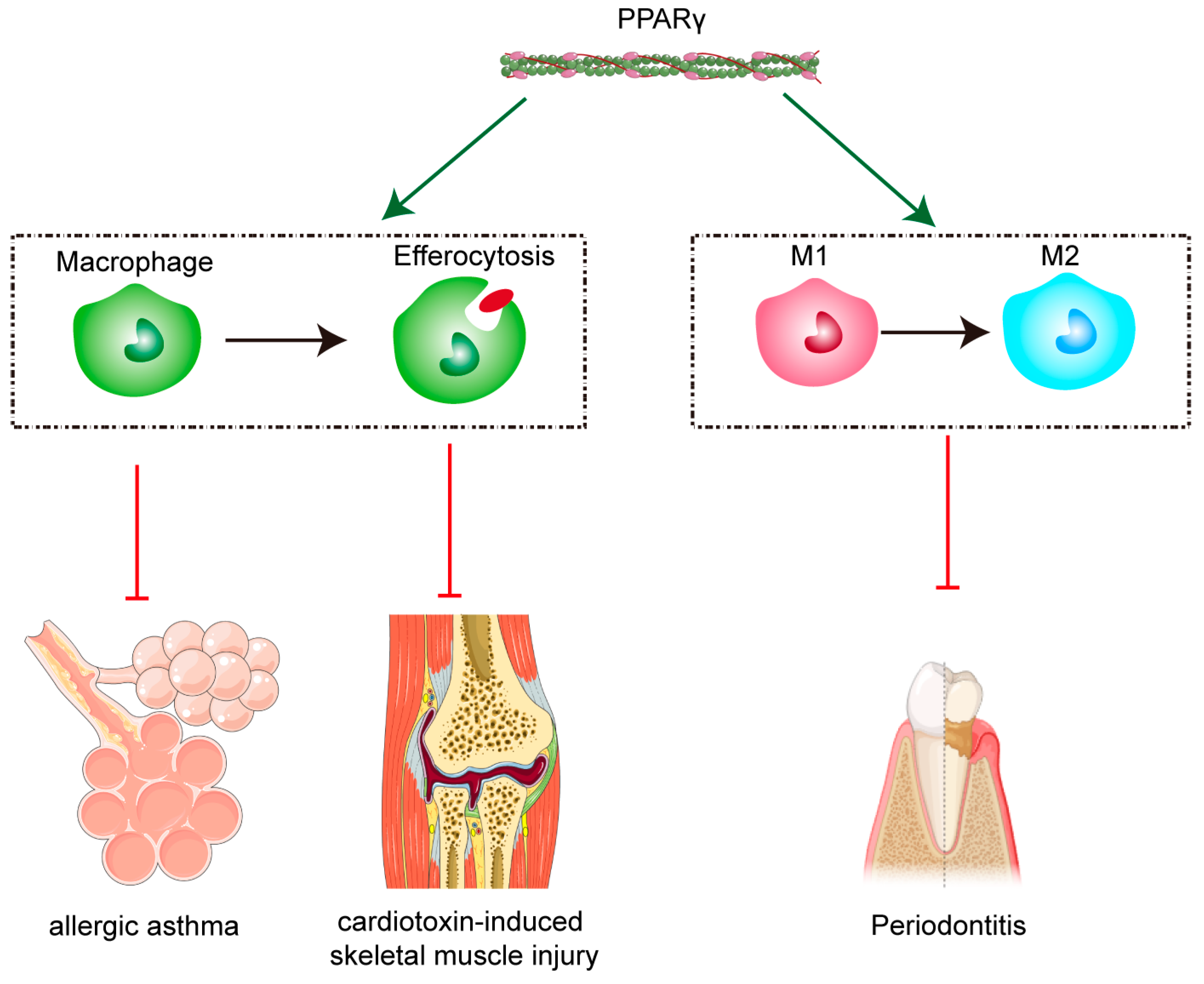
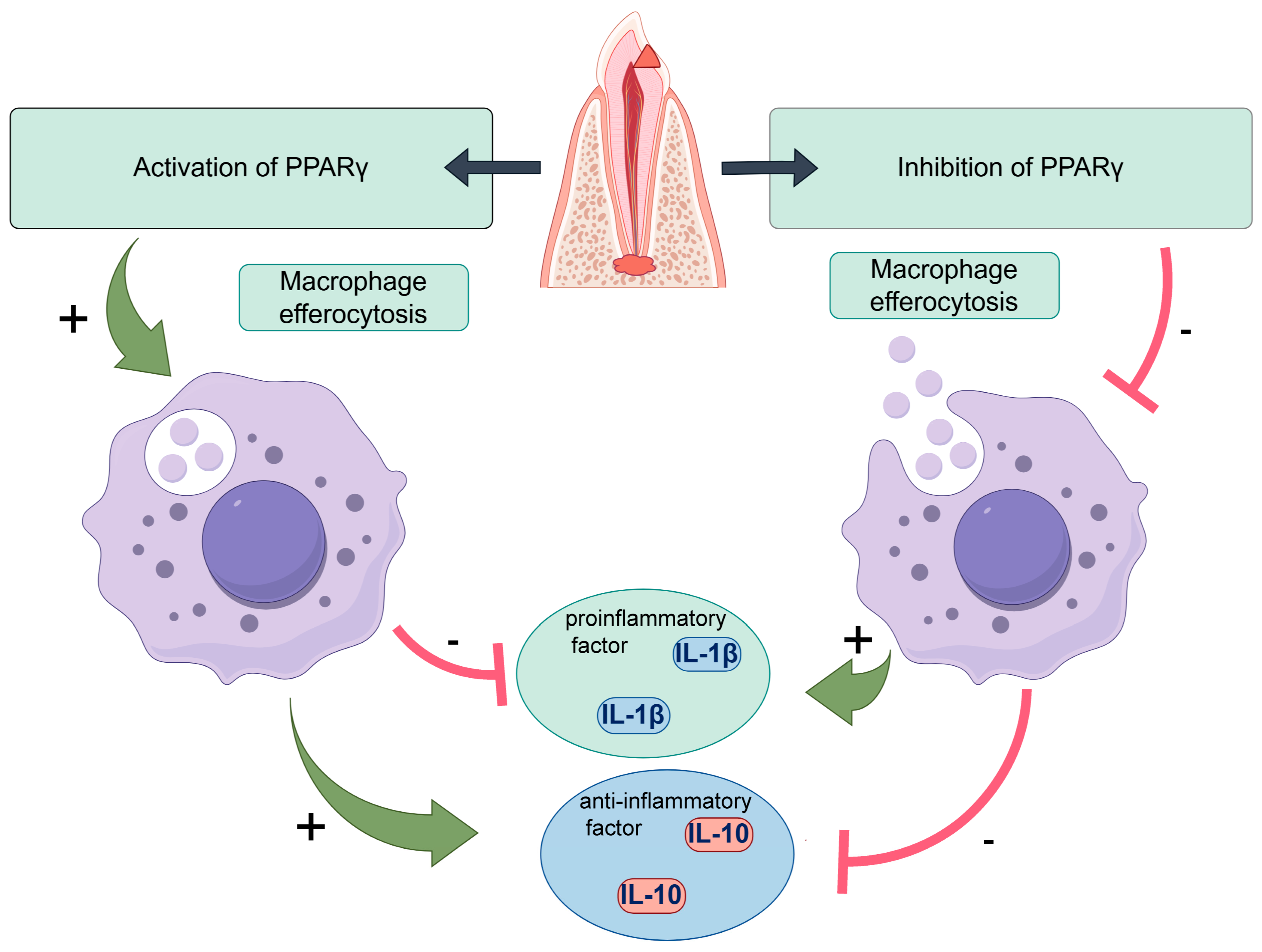
3. Discussion
4. Materials and Methods
4.1. Ethical Statements
4.2. Clinical Sample Collection and Single-Cell Sequencing
4.3. The Establishment of a CAP Animal Model
4.4. The In Vitro Model Establishment
4.5. Micro-Computed Tomography (Micro-CT) Analysis
4.6. Hematoxylin and Eosin (HE) Staining
4.7. Immunohistochemical (IHC) or Double Immunofluorescence (IF) Staining
4.8. The Ratio of Efferocytosis
4.9. Quantitative Real-Time PCR (qRT-PCR)
4.10. Western Blot (WB) Analysis
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CAP | Chronic apical periodontitis |
| RSG | Rosiglitazone |
References
- Yang, M.M.; Shen, Z.S.; Zhang, X.F.; Song, Z.; Zhang, Y.; Lin, Z.M.; Chen, L.L. Ferroptosis of macrophages facilitates bone loss in apical periodontitis via NRF2/FSP1/ROS pathway. Free Radic. Biol. Med. 2023, 208, 334–347. [Google Scholar] [CrossRef]
- Wang, Y.T.; Li, W.L.; Mu, W.L.; Seyam, A.; Guan, Y.H.; Tang, Y.F.; Wang, M.F.; Xin, Y.; Guo, X.M.; Hou, T.Z.; et al. Identification of JNK-JUN-NCOA axis as a therapeutic target for macrophage ferroptosis in chronic apical periodontitis. Int. J. Med. Sci. 2025, 22, 53–70. [Google Scholar] [CrossRef]
- Tibúrcio-Machado, C.S.; Michelon, C.; Zanatta, F.B.; Gomes, M.S.; Marin, J.A.; Bier, C.A. The global prevalence of apical periodontitis: A systematic review and meta-analysis. Int. Endod. J. 2021, 54, 712–735. [Google Scholar] [CrossRef]
- Dou, J.G.; Chen, X.; Zhang, J.L.; Yang, L.; Lin, J.; Zhu, W.L.; Huang, D.M.; Tan, X.L. P. Gingivalis induce macrophage polarization by regulating hepcidin expression in chronic apical periodontitis. Int. Immunopharmacol. 2024, 142, 113139. [Google Scholar] [CrossRef]
- Guan, X.Y.; Zhao, R.; Wang, Y.T.; Li, W.L.; Pan, L.F.; Yang, Y.; Mu, W.L.; Hou, T.Z. Ginsenoside Rb1 ameliorates apical periodontitis via suppressing macrophage pyroptosis. Oral Dis. 2025, 31, 541–554. [Google Scholar] [CrossRef]
- Burns, L.E.; Kim, J.; Wu, Y.X.; Alzwaideh, R.; McGowan, R.; Sigurdsson, A. Outcomes of primary root canal therapy: An updated systematic review of longitudinal clinical studies published between 2003 and 2020. Int. Endod. J. 2022, 55, 714–731. [Google Scholar] [CrossRef] [PubMed]
- Raedel, M.; Hartmann, A.; Bohm, S.; Walter, M.H. Three-year outcomes of root canal treatment: Mining an insurance database. J. Dent. 2015, 43, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Villalobos, V.; Garrido, M.; Reyes, A.; Fernández, C.; Diaz, C.; Torres, V.A.; González, P.A.; Cáceres, M. Aging envisage imbalance of the periodontium: A keystone in oral disease and systemic health. Front. Immunol. 2022, 13, 1044334. [Google Scholar] [CrossRef] [PubMed]
- Siebeler, R.; de Winther, M.P.J.; Hoeksema, M.A. The regulatory landscape of macrophage interferon signaling in inflammation. J. Allergy Clin. Immun. 2023, 152, 326–337. [Google Scholar] [CrossRef]
- Lin, J.T.; Xu, A.K.; Jin, J.K.; Zhang, M.; Lou, J.A.; Qian, C.; Zhu, J.; Wang, Y.T.; Yang, Z.M.; Li, X.M.; et al. MerTK-mediated efferocytosis promotes immune tolerance and tumor progression in osteosarcoma through enhancing M2 polarization and PD-L1 expression. Oncoimmunology 2022, 11, 2024941. [Google Scholar] [CrossRef]
- Huang, Y.M.; Wang, B.Z.; Ma, Z.J.; Chen, T.Z.; Zou, H.T.; Chen, Y.T.; Dong, Z.; Chen, J.Y.; Zhang, H.; Ding, Y.J.; et al. Sulforaphane promotes diabetic wound healing by regulating macrophage efferocytosis and polarization. Int. Immunopharmacol. 2025, 150, 114243. [Google Scholar] [CrossRef]
- Miao, L.Y.; Yu, C.Q.; Guan, G.; Luan, X.Y.; Jin, X.S.; Pan, M.Q.; Yang, Y.Z.; Yan, J.Y.; Chen, P.; Di, G.H. Extracellular vesicles containing GAS6 protect the liver from ischemia-reperfusion injury by enhancing macrophage efferocytosis via MerTK-ERK-COX2 signaling. Cell Death Discov. 2024, 10, 401. [Google Scholar] [CrossRef]
- Guan, X.Y.; Wang, Y.T.; Li, W.L.; Mu, W.L.; Tang, Y.F.; Wang, M.F.; Seyam, A.; Yang, Y.; Pan, L.F.; Hou, T.Z. The Role of Macrophage Efferocytosis in the Pathogenesis of Apical Periodontitis. Int. J. Mol. Sci. 2024, 25, 3854. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xue, X.Y.; Zhao, X.T.; Luo, L.; Liu, J.; Dai, S.; Zhang, F.; Wu, R.; Liu, Y.F.; Peng, C.; et al. Forsythiaside A alleviates acute lung injury by inhibiting inflammation and epithelial barrier damages in lung and colon through PPAR-γ/RXR-α complex. J. Adv. Res. 2024, 60, 183–200. [Google Scholar] [CrossRef]
- Hou, J.L.; Wang, X.Y.; Zhang, J.; Shen, Z.J.; Li, X.; Yang, Y.X. Chuanxiong Renshen Decoction Inhibits Alzheimer’s Disease Neuroinflammation by Regulating PPAR-γ/NF-κB Pathway. Drug Des. Dev. Ther. 2024, 18, 3209–3232. [Google Scholar] [CrossRef]
- Kotzbeck, P.; Taschler, U.; Haudum, C.; Foessl, I.; Schoiswohl, G.; Boulgaropoulos, B.; Bounab, K.; Einsiedler, J.; Pajed, L.; Tilp, A.; et al. Rosiglitazone Reverses Inflammation in Epididymal White Adipose Tissue in Hormone-Sensitive Lipase-Knockout Mice. J. Lipid Res. 2023, 64, 100305. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.X.; Meng, X.Q.; Wang, Y.L.; Liu, Y.; Shi, X.R.; Shao, S.; Duan, S.Z.; Lu, H.X. Deficiency Promotes Osteoclastogenesis and Exacerbates Periodontitis. J. Dent. Res. 2023, 102, 72–81. [Google Scholar] [CrossRef]
- Karatas, O.; Yuce, H.B.; Taskan, M.M.; Gevrek, F.; Alkan, C.; Kara, G.I.; Temiz, C. Cinnamic acid decreases periodontal inflammation and alveolar bone loss in experimental periodontitis. J. Periodontal Res. 2020, 55, 676–685. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Luo, W.X.; Zhao, C.Z.; Yu, M.Q.; Li, H.Y.; Zhou, F.; Wang, D.Y.; Bai, F.W.; Chen, T.; Xiong, Y.; et al. FoxO1-modulated macrophage polarization regulates osteogenesis via PPAR-γ signaling. BBA-Mol. Basis Dis. 2024, 1870, 167333. [Google Scholar] [CrossRef]
- Huang, Q.; Weng, D.L.; Yao, S.F.; Shen, H.L.; Gao, S.; Zhang, Y.Y.; Huang, W.J.; Wang, Y.; Wang, H.; Xu, W.C. Progranulin deficiency suppresses allergic asthma and enhances efferocytosis via PPAR-γ/MFG-E8 regulation in macrophages. Immun. Inflamm. Dis. 2023, 11, e779. [Google Scholar] [CrossRef]
- Garabuczi, É.; Tarban, N.; Fige, É.; Patsalos, A.; Halász, L.; Szendi-Szatmári, T.; Sarang, Z.; Király, R.; Szondy, Z. Nur77 and PPAR-γ regulate transcription and polarization in distinct subsets of M2-like reparative macrophages during regenerative inflammation. Front. Immunol. 2023, 14, 1139204. [Google Scholar] [CrossRef]
- Guan, X.Y.; Wei, Z.C.; Wang, Y.T.; Li, W.L.; Mu, W.L.; Seyam, A.; Shi, C.; Hou, T.Z. Blocking Gremlin1 inhibits M1 macrophage polarization through Notch1/Hes1 signaling pathway in apical periodontitis. Immunopharm. Immunot. 2024, 46, 703–714. [Google Scholar] [CrossRef]
- Chen, X.; Dou, J.G.; Fu, Z.H.; Qiu, Y.; Zou, L.; Huang, D.M.; Tan, X.L. Macrophage M1 polarization mediated via the IL-6/STAT3 pathway contributes to apical periodontitis induced by. J. Appl. Oral Sci. 2022, 30, e20220316. [Google Scholar] [CrossRef]
- Jia, T.T.; Yuan, F.; Tao, J.Q.; Wang, G.; Zhang, X.H.; Zhang, B.; Li, H.B. CRISPR/Cas13d targeting GZMA in PARs pathway regulates the function of osteoclasts in chronic apical periodontitis. Cell. Mol. Biol. Lett. 2023, 28, 70. [Google Scholar] [CrossRef]
- Almeida-Junior, L.A.; de Carvalho, M.S.; Almeida, L.K.Y.; Silva-Sousa, A.C.; Sousa-Neto, M.D.; Silva, R.A.B.; Silva, L.A.B.; Paula-Silva, F.W.G. TNF-α-TNFR1 Signaling Mediates Inflammation and Bone Resorption in Apical Periodontitis. J. Endod. 2023, 49, 1319–1328.e1312. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.T.; Tang, Y.F.; Wang, M.F.; Li, W.L.; Mu, W.L.; Seyam, A.; Dai, Y.T.; Hou, T.Z.; Guan, X.Y. ARA290 Attenuates Apical Periodontitis via SIRT1/NF kB/IL-1b Pathway Modulation-. Int. Dent. J. 2025, 75, 100863. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.Y.; Liu, S.; Wu, S.X.; Xia, M.; Liu, W.Q.; Wang, L.N.; Dong, M.; Niu, W.D. Milk-Derived Small Extracellular Vesicles Promote Osteogenic Differentiation and Inhibit Inflammation via microRNA-21. Int. J. Mol. Sci. 2023, 24, 13873. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wang, X.X.; Ma, L.; Wang, H.Y.; Peng, Y.; Liu, H.Y.; Xiao, J.H.; Cao, Z.G. M2 macrophages with inflammation tropism facilitate cementoblast mineralization. J. Periodontol. 2023, 94, 290–300. [Google Scholar] [CrossRef]
- Wang, B.Z.; Huang, Y.M.; Ding, Y.J.; Chen, J.Y.; Chen, Y.T.; Zhang, H.; Tan, Q. Naringenin Accelerates Diabetic Wound Healing via Regulating Macrophage M2 Polarization and Efferocytosis. Food Sci. Nutr. 2025, 13, e70688. [Google Scholar] [CrossRef]
- Tang, Y.D.; Hu, H.B.; Xie, Q.L.; Shen, J. GAS6/AXL signaling promotes M2 microglia efferocytosis to alleviate neuroinflammation in sepsis-associated encephalopathy. Cell Death Discov. 2025, 11, 268. [Google Scholar] [CrossRef]
- He, X.L.; Li, Z.R.; Li, S.J.; Zhang, X.J.; Liu, D.H.; Han, X.W.; He, H.; Chen, J.H.; Dong, X.M.; Long, W.J.; et al. Huoxue Tongluo tablet enhances atherosclerosis efferocytosis by promoting the differentiation of Trem2+macrophages via PPAR-γ signaling pathway. Phytomedicine 2025, 140, 156579. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, J.M.; Jin, J.; Hu, Q.Q.; Zhao, T.; Wang, J.; Gao, J.B.; Man, J. S100A9 deletion in microglia/macrophages ameliorates brain injury through the STAT6/PPAR-γ pathway in ischemic stroke. CNS Neurosci. Ther. 2024, 30, e14881. [Google Scholar] [CrossRef]
- de Oliveira, K.M.H.; Garlet, G.P.; De Rossi, A.; Barreiros, D.; Queiroz, A.M.; da Silva, L.A.B.; Nelson, P.; da Silva, R.A.B. Effects of Rosiglitazone on the Outcome of Experimental Periapical Lesions in Mice. J. Endod. 2017, 43, 2061–2069. [Google Scholar] [CrossRef]
- Justo, M.P.; Cardoso, C.D.M.; Cantiga-Silva, C.; de Oliveira, P.H.C.; Sivieri-Araújo, G.; Azuma, M.M.; Ervolino, E.; Cintra, L.T.A. Curcumin reduces inflammation in rat apical periodontitis. Int. Endod. J. 2022, 55, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.Q.; Zhang, J.; Liu, G.J.; Zhu, L.X.; Peng, B. Expression of PINK1 and Parkin in human apical periodontitis. Int. Endod. J. 2022, 55, 870–881. [Google Scholar] [CrossRef] [PubMed]



| Therapeutic Strategy | Model/Study | Key Findings | Reference |
|---|---|---|---|
| Rosiglitazone (PPAR-γ agonist, synthetic drug) | Mouse model of CAP | Reduced inflammatory cytokine expression, decreased tissue damage | de Oliveira. et al. J. Endod. 2017 [33] |
| Curcumin (plant-derived polyphenol) | Mouse model of CAP | Activated PPAR-γ signaling suppressed NF-κB, mitigating periapical bone resorption | Justo MP. et al. Int. Endod. J. 2022 [34] |
| Gene | Forward Primer (from 5′ to 3′) | Reverse Primer (from 3′ to 5′) |
|---|---|---|
| Gapdh | AGAAGGCTGGGGCTCATTTG | CTTCTGACACCTACCGGGGA |
| IL-1β | CAGAAGTACCTGAGCTCGCC | GAAGCCCTTGCTGTAGTGGT |
| IL-10 | GCCTTGTCTGAGATGATCCAGTT | ACAGTAGCTAAAGAAGGGACACT |
| Mertk | CCGCCCCACCTTTTCAGTAT | TTACCCCCGTCACTCCTTAC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wang, M.; Jia, X.; Tang, Y.; Wang, J.; Zhang, W.; Hou, T.; Guan, X. PPAR-γ Inhibits Chronic Apical Periodontitis by Facilitating Macrophage Efferocytosis. Int. J. Mol. Sci. 2025, 26, 10157. https://doi.org/10.3390/ijms262010157
Wang Y, Wang M, Jia X, Tang Y, Wang J, Zhang W, Hou T, Guan X. PPAR-γ Inhibits Chronic Apical Periodontitis by Facilitating Macrophage Efferocytosis. International Journal of Molecular Sciences. 2025; 26(20):10157. https://doi.org/10.3390/ijms262010157
Chicago/Turabian StyleWang, Yuting, Mingfei Wang, Xiaowen Jia, Yifei Tang, Jiayi Wang, Wenjiao Zhang, Tiezhou Hou, and Xiaoyue Guan. 2025. "PPAR-γ Inhibits Chronic Apical Periodontitis by Facilitating Macrophage Efferocytosis" International Journal of Molecular Sciences 26, no. 20: 10157. https://doi.org/10.3390/ijms262010157
APA StyleWang, Y., Wang, M., Jia, X., Tang, Y., Wang, J., Zhang, W., Hou, T., & Guan, X. (2025). PPAR-γ Inhibits Chronic Apical Periodontitis by Facilitating Macrophage Efferocytosis. International Journal of Molecular Sciences, 26(20), 10157. https://doi.org/10.3390/ijms262010157