Parameters of Micro- and Macrocirculation in Young Uncomplicated Type 1 Diabetic Patients—The Role of Metabolic Memory
Abstract
1. Introduction
2. Results
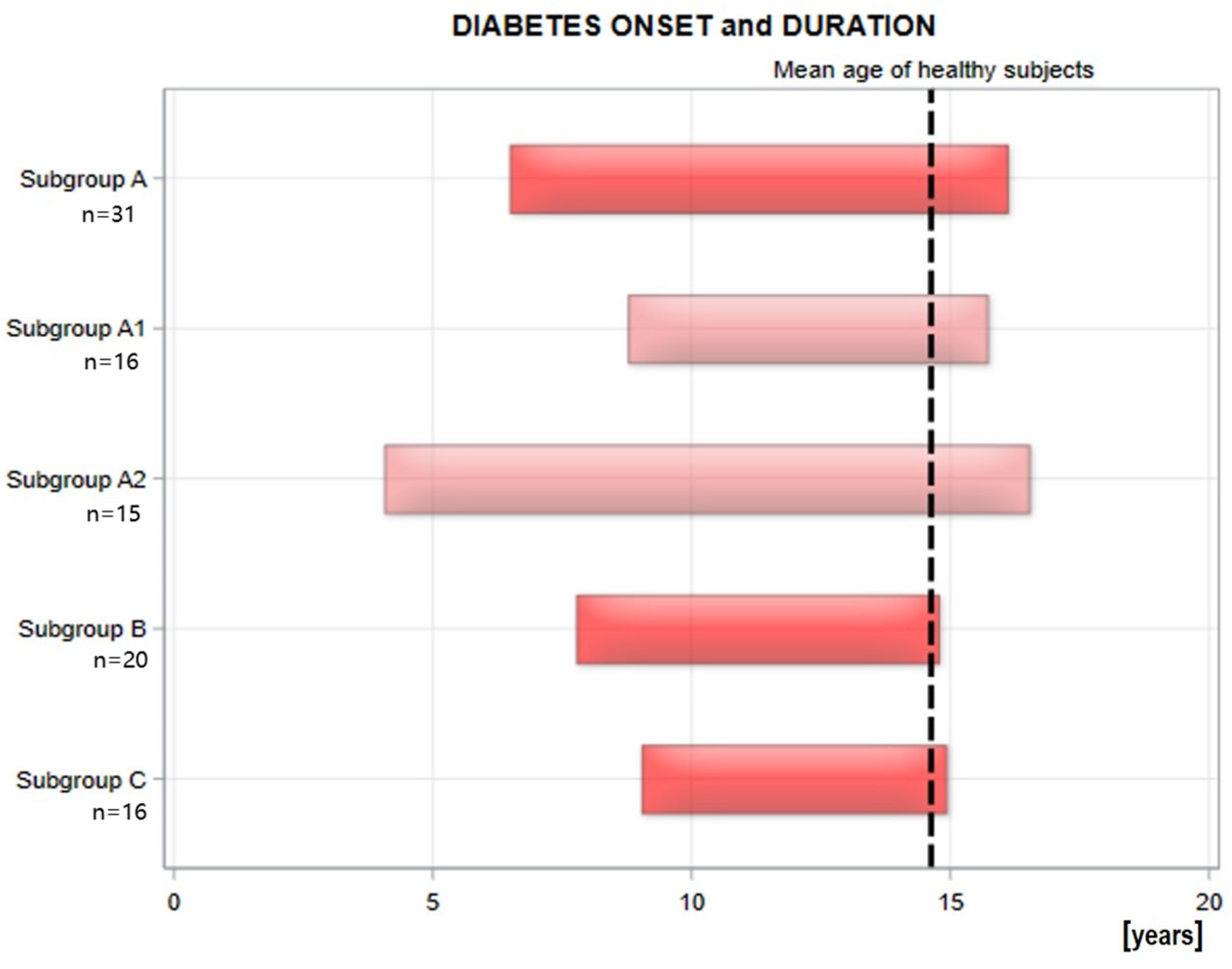
2.1. The Whole Study Group and Subgroups (A1, B, C) [20]
2.2. Comparison of A1 and A2 Subgroups
2.2.1. Characteristics of Groups A1 and A2
2.2.2. Laboratory Examination
2.2.3. Biomarkers Examination
2.2.4. Pulsatility Indices and Pulse Pressure
2.2.5. Microcirculation
3. Discussion
4. Materials and Methods
4.1. Study Design and Population
4.2. Laboratory Analysis
4.3. Pulsatility and Ankle–Brachial Indices, and Pulse Pressure
4.4. Capillaroscopy
4.5. Transcutaneous Oxygen Pressure
4.6. Optical Coherence Tomography
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HbA1c | glycated hemoglobin |
| VEGF | vascular endothelial growth factor |
| BMI | body mass index |
| T1D | diabetes mellitus |
| TSH | thyroid-stimulating hormone |
| fT4 | free thyroxine |
| TNF-α | tumor necrosis factor |
| TGF-β | transforming growth factor beta |
| ratio TNF–α/IL-35 | the ratio of TNF–α and IL-35 |
| IL- | interleukin |
| ICAM-1 | Intercellular Adhesion Molecule–1 |
| sVCAM–1 | Soluble Vascular Cell Adhesion Molecule–1 |
| sP–selectin | Soluble Platelet Selectin |
| AGEs | Advanced Glycation End Products |
| sRAGE | Receptors for Advanced Glycation End Products |
| CRP | C-reactive protein |
| LDL-cholesterol | low-density cholesterol |
| HDL-cholesterol | high-density cholesterol |
| IMT | intima-media thickness |
| CCA_PI | pulsatility index for carotid arteries |
| brachial_PI | pulsatility index for brachial arteries |
| thigh_PI | pulsatility index for femoral arteries |
| above_knee_PI | pulsatility index for muscular arteries above the knee |
| below_knee_PI | pulsatility index for muscular arteries below the knee |
| ankle_PI | pulsatility index for muscular arteries at the ankle level |
| ABI | Ankle–brachial index |
| SBP | Systolic blood pressure |
| DBP | Diastolic blood pressure |
| PP | pulse pressure |
| PORH | post-reactive hyperemia |
| CoverageBASE | ratio of the capillary area to the total area of the determined rows in the baseline condition |
| CoveragePORH | ratio of the capillary area to the total area of the determined rows after PORH |
| ∆CoveragePB | CoveragePORH–CoverageBASE |
| Capillary reactivity | ∆CoveragePB/CoverageBASE |
| DistanceBASE | mean distance between successive capillaries in baseline condition |
| DistancePORH | average distance between successive capillaries after PORH |
| ∆DistancePB | DistancePORH–DistanceBASE |
| TcPO2_base | mean value of TcPO2 within 60 s before T_base |
| TcPO2_zero | mean value of TcPO2 within 60 s before T_zero |
| TTR | time to reach the baseline value after occlusion |
References
- Ogle, G.D.; Wang, F.; Haynes, A.; Gregory, G.A.; King, T.W.; Deng, K.; Dabelea, D.; James, S.; Jenkins, A.J.; Li, X.; et al. Global Type 1 Diabetes Prevalence, Incidence, and Mortality Estimates 2025: Results from the International Diabetes Federation Atlas, 11th Edition, and the T1D Index Version 3.0. Diabetes Res. Clin. Pract. 2025, 225, 112277. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Dong, X.; Zhang, Y.; Jiang, Y.; Lu, D.; Wu, Q.; Liang, Z.; Yang, G.; Chen, B. Transcutaneous Oxygen Pressure (TcPO2): A Novel Diagnostic Tool for Peripheral Neuropathy in Type 2 Diabetes Patients. Diabetes Res. Clin. Pract. 2014, 105, 336–343. [Google Scholar] [CrossRef]
- Lu, L.; Zhu, M.; Wu, Q.; Sun, Z.; Chen, X.; Miao, C. Sirt7/HIC1 Complex Participates in Hyperglycaemia-Mediated EndMT via Modulation of SDC1 Expression in Diabetic Kidney Disease and Metabolic Memory. J. Cell Mol. Med. 2024, 28, e18336. [Google Scholar] [CrossRef]
- Yang, T.; Qi, F.; Guo, F.; Shao, M.; Song, Y.; Ren, G.; Linlin, Z.; Qin, G.; Zhao, Y. An Update on Chronic Complications of Diabetes Mellitus: From Molecular Mechanisms to Therapeutic Strategies with a Focus on Metabolic Memory. Mol. Med. 2024, 30, 71. [Google Scholar] [CrossRef]
- Engerman, R.L.; Kern, T.S. Progression of Incipient Diabetic Retinopathy during Good Glycemic Control. Diabetes 1987, 36, 808–812. [Google Scholar] [CrossRef]
- Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group; Lachin, J.M.; White, N.H.; Hainsworth, D.P.; Sun, W.; Cleary, P.A.; Nathan, D.M. Effect of Intensive Diabetes Therapy on the Progression of Diabetic Retinopathy in Patients with Type 1 Diabetes: 18 Years of Follow-up in the DCCT/EDIC. Diabetes 2015, 64, 631–642. [Google Scholar] [CrossRef]
- Lachin, J.M.; Nathan, D.M. Understanding Metabolic Memory: The Prolonged Influence of Glycemia During the Diabetes Control and Complications Trial (DCCT) on Future Risks of Complications During the Study of the Epidemiology of Diabetes Interventions and Complications (EDIC). Diabetes Care 2021, 44, 2216–2224. [Google Scholar] [CrossRef]
- Giordano, C.; Amato, M.C.; Ciresi, A.; Citarrella, R.; Mantione, L.; Accidenti, M.; Pantò, F.; Guarnotta, V.; Allotta, M.L.; Criscimanna, A.; et al. Predictors of Microvascular Complications in Type 1 Diabetic Patients at Onset: The Role of Metabolic Memory. Eur. J. Intern. Med. 2011, 22, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Cé, G.V.; Rohde, L.E.; da Silva, A.M.V.; Coutinho, M.K.P.; de Castro, A.C.; Bertoluci, M.C. Endothelial Dysfunction Is Related to Poor Glycemic Control in Adolescents with Type 1 Diabetes under 5 Years of Disease: Evidence of Metabolic Memory. J. Clin. Endocrinol. Metab. 2011, 96, 1493–1499. [Google Scholar] [CrossRef] [PubMed]
- Wautier, M.-P.; Chappey, O.; Corda, S.; Stern, D.M.; Schmidt, A.M.; Wautier, J.-L. Activation of NADPH Oxidase by AGE Links Oxidant Stress to Altered Gene Expression via RAGE. Am. J. Physiol.-Endocrinol. Metab. 2001, 280, E685–E694. [Google Scholar] [CrossRef]
- Loomans, C.J.M.; De Koning, E.J.P.; Staal, F.J.T.; Rookmaaker, M.B.; Verseyden, C.; De Boer, H.C.; Verhaar, M.C.; Braam, B.; Rabelink, T.J.; Van Zonneveld, A.J. Endothelial Progenitor Cell Dysfunction: A Novel Concept in the Pathogenesis of Vascular Complications of Type 1 Diabetes. Diabetes 2004, 52, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Aronson, D. Cross-Linking of Glycated Collagen in the Pathogenesis of Arterial and Myocardial Stiffening of Aging and Diabetes. J. Hypertens. 2003, 21, 3–12. [Google Scholar] [CrossRef] [PubMed]
- McCarter, R.J.; Hempe, J.M.; Gomez, R.; Chalew, S.A. Biological Variation in HbA1c Predicts Risk of Retinopathy and Nephropathy in Type 1 Diabetes. Diabetes Care 2004, 27, 1259–1264. [Google Scholar] [CrossRef] [PubMed]
- Simeunovic, A.; Brunborg, C.; Heier, M.; Seljeflot, I.; Dahl-Jørgensen, K.; Margeirsdottir, H.D. Sustained Low-Grade Inflammation in Young Participants with Childhood Onset Type 1 Diabetes: The Norwegian Atherosclerosis and Childhood Diabetes (ACD) Study. Atherosclerosis 2023, 379, 117151. [Google Scholar] [CrossRef]
- dos Santos Haber, J.F.; Barbalho, S.M.; Sgarbi, J.A.; de Argollo Haber, R.S.; de Labio, R.W.; Laurindo, L.F.; Chagas, E.F.B.; Payão, S.L.M. The Relationship between Type 1 Diabetes Mellitus, TNF-α, and IL-10 Gene Expression. Biomedicines 2023, 11, 1120. [Google Scholar] [CrossRef]
- Miao, F.; Smith, D.D.; Zhang, L.; Min, A.; Feng, W.; Natarajan, R. Lymphocytes from Patients with Type 1 Diabetes Display a Distinct Profile of Chromatin Histone H3 Lysine 9 Dimethylation: An Epigenetic Study in Diabetes. Diabetes 2008, 57, 3189–3198. [Google Scholar] [CrossRef]
- Alnek, K.; Kisand, K.; Heilman, K.; Peet, A.; Varik, K.; Uibo, R. Increased Blood Levels of Growth Factors, Proinflammatory Cytokines, and Th17 Cytokines in Patients with Newly Diagnosed Type 1 Diabetes. PLoS ONE 2015, 10, e0142976. [Google Scholar] [CrossRef] [PubMed]
- Miao, F.; Chen, Z.; Zhang, L.; Liu, Z.; Wu, X.; Yuan, Y.-C.; Natarajan, R. Profiles of Epigenetic Histone Post-Translational Modifications at Type 1 Diabetes Susceptible Genes. J. Biol. Chem. 2012, 287, 16335–16345. [Google Scholar] [CrossRef]
- Deng, J.-Y.; Wu, X.-Q.; He, W.-J.; Liao, X.; Tang, M.; Nie, X.-Q. Targeting DNA Methylation and Demethylation in Diabetic Foot Ulcers. J. Adv. Res. 2023, 54, 119–131. [Google Scholar] [CrossRef]
- Neubauer-Geryk, J.; Wielicka, M.; Myśliwiec, M.; Zorena, K.; Bieniaszewski, L. The Impact of Metabolic Memory on Immune Profile in Young Patients with Uncomplicated Type 1 Diabetes. Int. J. Mol. Sci. 2024, 25, 3190. [Google Scholar] [CrossRef]
- Neubauer-Geryk, J.; Myśliwiec, M.; Zorena, K.; Bieniaszewski, L. Soluble P-Selectin as an Indicator of Cutaneous Microangiopathy in Uncomplicated Young Patients with Type 1 Diabetes. Life 2024, 14, 1587. [Google Scholar] [CrossRef]
- Roy, B. Pathophysiological Mechanisms of Diabetes-Induced Macrovascular and Microvascular Complications: The Role of Oxidative Stress. Med. Sci. 2025, 13, 87. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.-R.; Wang, M.-Y.; Zhang, C.-L.; Wang, Y. Endothelial Dysfunction in Vascular Complications of Diabetes: A Comprehensive Review of Mechanisms and Implications. Front. Endocrinol. 2024, 15, 1359255. [Google Scholar] [CrossRef]
- Kostolanská, J.; Jakuš, V.; Barák, Ľ. Monitoring of Early and Advanced Glycation in Relation to the Occurrence of Microvascular Complications in Children and Adolescents with Type 1 Diabetes Mellitus. Physiol. Res. 2009, 58, 553–561. [Google Scholar] [CrossRef]
- Monnier, V.M.; Sell, D.R.; Gao, X.; Genuth, S.M.; Lachin, J.M.; Bebu, I. Plasma Advanced Glycation End Products and the Subsequent Risk of Microvascular Complications in Type 1 Diabetes in the DCCT/EDIC. BMJ Open Diabetes Res. Care 2022, 10, e002667. [Google Scholar] [CrossRef]
- Akın Kağızmanlı, G.; Aydın, T.; Yüksek Acinikli, K.; İşgüder, R.; Kızıldağ Karabacak, Z.; Demir, K.; Böber, E.; Ünsal, Ş.E.; Abacı, A. Nailfold Capillaroscopy: A Non-Invasive Tool for Early Detection of Microvascular Alterations in Children with Type 1 Diabetes Mellitus. J. Clin. Res. Pediatr. Endocrinol. 2025. Epub ahead of print. [Google Scholar] [CrossRef]
- Cisło, M.; Wąsikowa, R.; Iwanicka, Z.; Wojakiewicz, R.; Sajewicz, E. Results of Capillaroscopy of Nail Plate in Children with Diabetes. Pediatr. Pol. 1984, 59, 623–630. [Google Scholar] [PubMed]
- Kuryliszyn-Moskal, A.; Dubicki, A.; Zarzycki, W.; Zonnenberg, A.; Górska, M. Microvascular Abnormalities in Capillaroscopy Correlate with Higher Serum IL-18 and SE-Selectin Levels in Patients with Type 1 Diabetes Complicated by Microangiopathy. Folia Histochem. Cytobiol. 2011, 49, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Abdelmaksoud, A.A.; Daifallah, S.M.; Salah, N.Y.; Saber, A.S. Nail Fold Microangiopathy in Adolescents with Type 1 Diabetes: Relation to Diabetic Vascular Complications. Microcirculation 2022, 29, e12771. [Google Scholar] [CrossRef]
- Kuryliszyn-Moskal, A.; Zarzycki, W.; Dubicki, A.; Zonnenberg, A.; Górska, M. A Study on Microvascular Abnormalities in Capillaroscopy in Patients with Type 1 Diabetes Mellitus. Diabetol. Doświadczalna I Klin. 2006, 6, 98–103. [Google Scholar]
- Kuryliszyn-Moskal, A.; Ciołkiewicz, M.; Dubicki, A. Morphological Alterations in Nailfold Capillaroscopy and the Clinical Picture of Vascular Involvement in Autoimmune Diseases: Systemic Lupus Erythematosus and Type 1 Diabetes. Ann. Acad. Med. Stetin. 2010, 56 (Suppl. S1), 73–79. [Google Scholar]
- Kuryliszyn-Moskal, A.; Zarzycki, W.; Dubicki, A.; Moskal, D.; Kosztyła-Hojna, B.; Hryniewicz, A. Clinical Usefulness of Videocapillaroscopy and Selected Endothelial Cell Activation Markers in People with Type 1 Diabetes Mellitus Complicated by Microangiopathy. Adv. Med. Sci. 2017, 62, 368–373. [Google Scholar] [CrossRef]
- Kaminska-Winciorek, G.; Deja, G.; Polańska, J.; Jarosz-Chobot, P. Diabetic Microangiopathy in Capillaroscopic Examination of Juveniles with Diabetes Type 1. Postepy Hig. Med. Dosw. 2012, 30, 51–59. [Google Scholar]
- Tibiriçá, E.; Rodrigues, E.; Cobas, R.A.; Gomes, M.B. Endothelial Function in Patients with Type 1 Diabetes Evaluated by Skin Capillary Recruitment. Microvasc. Res. 2007, 73, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Tooke, J.E.; Lins, P.E.; Ostergren, J.; Fagrell, B. Skin Microvascular Autoregulatory Responses in Type I Diabetes: The Influence of Duration and Control. Int. J. Microcirc. Clin. Exp. 1985, 4, 249–256. [Google Scholar] [PubMed]
- Trapp, R.G.; Soler, N.G.; Spencer-Green, G. Nailfold Capillaroscopy in Type I Diabetics with Vasculopathy and Limited Joint Mobility. J. Rheumatol. 1986, 13, 917–920. [Google Scholar] [PubMed]
- Neubauer-Geryk, J.; Kozera, G.M.; Wolnik, B.; Szczyrba, S.; Nyka, W.M.; Bieniaszewski, L. Decreased Reactivity of Skin Microcirculation in Response to L-Arginine in Later-Onset Type 1 Diabetes. Diabetes Care 2013, 36, 950–956. [Google Scholar] [CrossRef][Green Version]
- Gołębiewska, J.; Olechowski, A.; Wysocka-Mincewicz, M.; Odrobina, D.; Baszyńska-Wilk, M.; Groszek, A.; Szalecki, M.; Hautz, W. Optical Coherence Tomography Angiography Vessel Density in Children with Type 1 Diabetes. PLoS ONE 2017, 12, e0186479. [Google Scholar] [CrossRef]
- Dan, A.O.; Mocanu, C.L.; Bălășoiu, A.T.; Tănasie, C.A.; Puiu, I.; Târtea, A.E.; Sfredel, V. Correlations between Retinal Microvascular Parameters and Clinical Parameters in Young Patients with Type 1 Diabetes Mellitus: An Optical Coherence Tomography Angiography Study. Diagnostics 2024, 14, 317. [Google Scholar] [CrossRef]
- Qian, J.; Haq, Z.; Yang, D.; Jin, J.Q.; Stewart, J.M. Duration of Diabetes as a Risk Factor for Retinal Microvasculature Alterations Detected with Optical Coherence Tomography Angiography in Patients without Clinical Retinopathy. Diagnostics 2022, 12, 3020. [Google Scholar] [CrossRef]
- Assmann, T.S.; Brondani, L.A.; Bouças, A.P.; Rheinheimer, J.; de Souza, B.M.; Canani, L.H.; Bauer, A.C.; Crispim, D. Nitric Oxide Levels in Patients with Diabetes Mellitus: A Systematic Review and Meta-Analysis. Nitric Oxide 2016, 61, 1–9. [Google Scholar] [CrossRef]
- Rawshani, A.; Sattar, N.; Franzén, S.; Rawshani, A.; Hattersley, A.T.; Svensson, A.-M.; Eliasson, B.; Gudbjörnsdottir, S. Excess Mortality and Cardiovascular Disease in Young Adults with Type 1 Diabetes in Relation to Age at Onset: A Nationwide, Register-Based Cohort Study. Lancet 2018, 392, 477–486. [Google Scholar] [CrossRef]
- Bell, K.J.; Lain, S.J. The Changing Epidemiology of Type 1 Diabetes: A Global Perspective. Diabetes Obes. Metab. 2025, 27, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Giannopoulou, E.Z.; Doundoulakis, I.; Antza, C.; Christoforidis, A.; Haidich, A.B.; Kotsis, V.; Stabouli, S. Subclinical Arterial Damage in Children and Adolescents with Type 1 Diabetes: A Systematic Review and Meta-Analysis. Pediatr. Diabetes 2019, 20, pedi.12874. [Google Scholar] [CrossRef] [PubMed]
- Dalla Pozza, R.; Bechtold, S.; Bonfig, W.; Putzker, S.; Kozlik-Feldmann, R.; Netz, H.; Schwarz, H.-P. Age of Onset of Type 1 Diabetes in Children and Carotid Intima Medial Thickness. J. Clin. Endocrinol. Metab. 2007, 92, 2053–2057. [Google Scholar] [CrossRef] [PubMed]
- Benitez-Aguirre, P.Z.; Januszewski, A.S.; Cho, Y.H.; Craig, M.E.; Jenkins, A.J.; Donaghue, K.C. Early Changes of Arterial Elasticity in Type 1 Diabetes with Microvascular Complications—A Cross-Sectional Study from Childhood to Adulthood. J. Diabetes Complicat. 2017, 31, 1674–1680. [Google Scholar] [CrossRef]
- ElSayed, N.A.; McCoy, R.G.; Aleppo, G.; Balapattabi, K.; Beverly, E.A.; Briggs Early, K.; Bruemmer, D.; Ebekozien, O.; Echouffo-Tcheugui, J.B.; Ekhlaspour, L.; et al. 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes—2025. Diabetes Care 2025, 48, S27–S49. [Google Scholar] [CrossRef]
- Dyck, P.J. Detection, Characterization, and Staging of Polyneuropathy: Assessed in Diabetics. Muscle Nerve 1988, 11, 21–32. [Google Scholar] [CrossRef]
- Neubauer-Geryk, J.; Myśliwiec, M.; Zorena, K.; Bieniaszewski, L. The Relationship Between Cholesterol Level, Cytokine Profile, and Arterial Stiffness in Young Patients with Uncomplicated Type 1 Diabetes. Int. J. Mol. Sci. 2025, 26, 5513. [Google Scholar] [CrossRef]
- Lurbe, E.; Regueiro-Ons, C.; Mancia, G.; Düzova, A.; Erdine, S.; Herceg-Cavrak, V.; Kulaga, Z.; Litwin, M.; Pall, D.; Petricevic, N.; et al. Blood Pressure Values in Healthy Normal Weight Children and Adolescents in Eight European Countries: Auscultatory and Oscillometric Measurements. Eur. J. Intern. Med. 2025, 139, 106382. [Google Scholar] [CrossRef]
- Kernick, D.P.; Tooke, J.E.; Shore, A.C. The Biological Zero Signal in Laser Doppler Fluximetry—Origins and Practical Implications. Pflugers Arch. 1999, 437, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Triton Plus Manual. Available online: https://www.manualslib.com/products/Topcon-Dri-Oct-1-Triton-Plus-10613501.html (accessed on 27 October 2024).
- Wołoszyn-Durkiewicz, A.; Iwaszkiewicz-Grześ, D.; Świętoń, D.; Kujawa, M.J.; Jankowska, A.; Durawa, A.; Glasner, P.; Trzonkowski, P.; Glasner, L.; Szurowska, E.; et al. The Complex Network of Cytokines and Chemokines in Pediatric Patients with Long-Standing Type 1 Diabetes. Int. J. Mol. Sci. 2024, 25, 1565. [Google Scholar] [CrossRef] [PubMed]
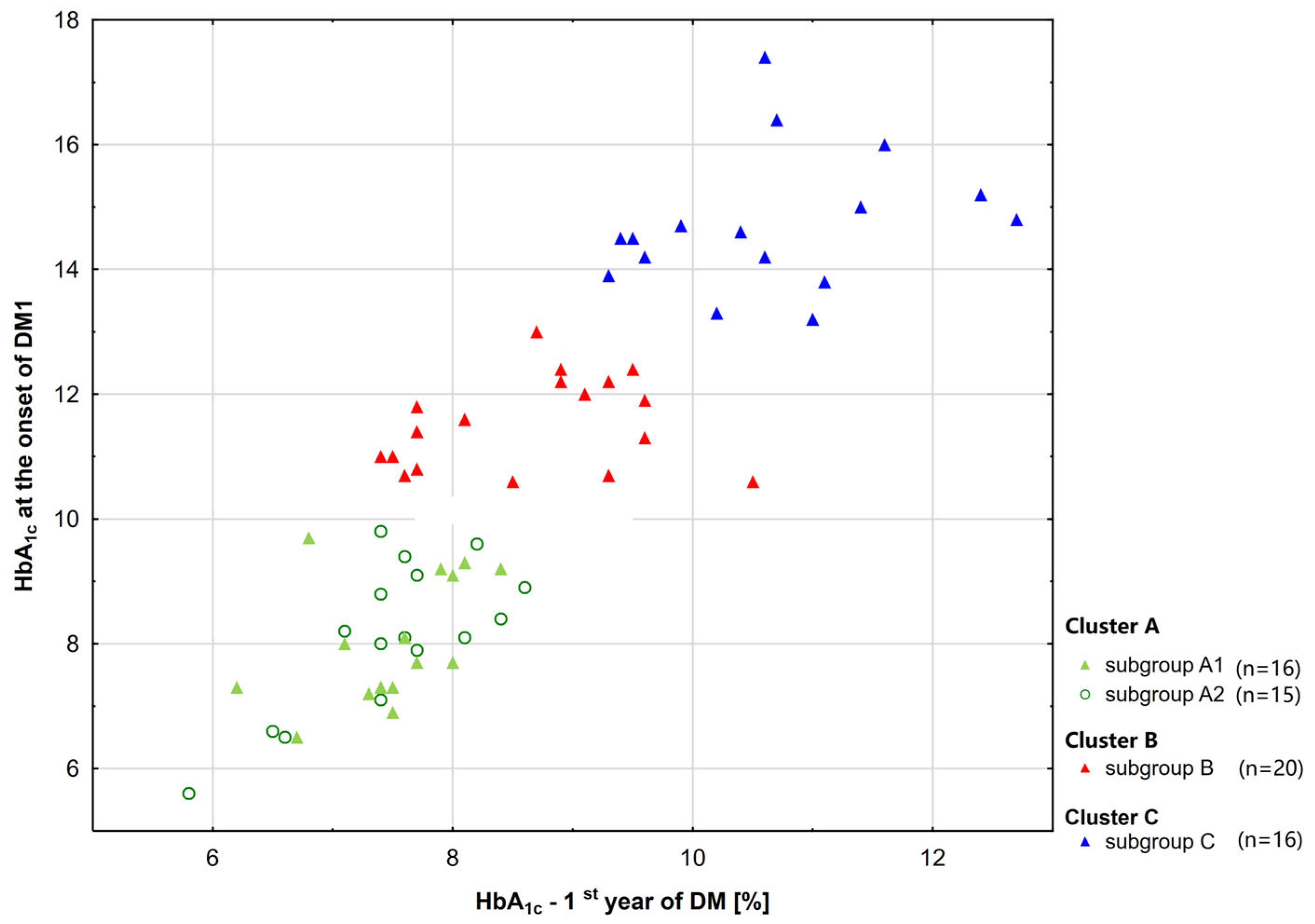

| Characteristics | Diabetic Patients | p | |
|---|---|---|---|
| Group A1 n = 16 | Group A2 n = 15 | ||
| Males, n (%) | 4 (27) | 9 (56) | 0.17 |
| BMI [kg/m2] | 20.4 (15.3–26.0) | 22.6 (16.1–25.6) | 0.30 |
| Age [years] | 17.1 (8.4–18.0)/15.7 ± 2.9 | 17.1 (13–17.9)/16.5 ± 1.6 | 0.65 |
| Onset of diabetes [age] | 9.1 (3.1–13.5)/9 ± 3.2 | 3.8 (1.2–7.6)/4.2 ± 2 | *** |
| Diabetes duration [years] | 7 (1.2–9.4)/6.7 ± 2.4 | 12.2 (9.9–15.9)/12.3 ± 1.8 | *** |
| Insulin dose units/24 h | 40 (21–70) | 54 (30–100) | * |
| Insulin dose units/kg | 0.7 (0.4–1) | 0.8 (0.6–1.4) | 0.10 |
| Treatment with pump [%] | 80 (0–90) | 76 (0–100) | 0.45 |
| HbA1c at onset of T1D [%] | 7.7 (6.5–9.7) | 8.15 (5.6–9.8) | 0.63 |
| Mean HbA1c 1st year of T1D [%] | 7.5 (6.2–8.4) | 7.5 (5.8–8.6) | 0.98 |
| Mean HbA1c 2nd year of T1D [%] | 7.8 (5.5–10.1) | 7.0 (6.2–8.6) | 0.30 |
| HbA1c current [%] | 6.8 (5.9–11.6) | 8.6 (6.2–9.8) | 0.07 |
| Episodes of mild hypoglycemia [N/last month] | 10 (0–16) | 10 (0–20) | 0.83 |
| Episodes of severe hypoglycemia [N/last year] | 0 (0–1) | 0 (0–1) | 0.74 |
| Autoimmune thyroiditis, n [%] | 5 (31) | 4 (27) | 0.90 |
| Celiac disease, n [%] | 4 (25) | 4 (27) | 0.76 |
| Characteristics | Diabetic Patients | p | |
|---|---|---|---|
| Group A1 n = 16 | Group A2 n = 15 | ||
| CRP [mg/L] | 0.6 (0.2–4.8) | 0.3 (0.1–1.3) | 0.09 |
| Serum creatinine [mg/dL] | 0.71 (0.45–0.86) | 0.77 (0.5–0.95) | 0.57 |
| Albuminuria [mg/dL] | 14 (3–33) | 7.1 (2.5–88) | 0.28 |
| Total cholesterol [mg/dL] | 164 (129–248) | 182 (127–247) | 0.25 |
| Cholesterol LDL [mg/dL] | 95 (61–138) | 106 (61–170) | 0.30 |
| Cholesterol HDL [mg/dL] | 52 (33–63) | 53 (39–75) | 0.77 |
| Triglycerides [mg/dL] | 66 (34–294) | 82 (45–159) | 0.32 |
| TSH [mIU/L] | 1.6 (0.6–3.9) | 2 (1–4.3) | 0.57 |
| fT4 [pmol/L] | 12.8 (11.3–15) | 12.9 (10.4–15.0) | 0.83 |
| Characteristics | Diabetic Patients | p | |
|---|---|---|---|
| Group A1 n = 16 | Group A2 n = 15 | ||
| anti-inflammatory cytokines [20] | |||
| IL-35 [ng/mL] | 11.9 (3.9–22.7) 11.95 # | 4.7 (0.8–11.4) 3.81 # | *** |
| IL-4 [pg/mL] | 14.2 (2.2–29)/ 8.4 # | 4.2 (0.7–17.8) 6.5 # | ** |
| IL-10 [pg/mL] | 1.8 (0.5–4.7) | 1.6 (0–3.8) | 0.47 |
| pro-inflammatory cytokines [20] | |||
| TNF–α [pg/mL] | 1.6 (0–3.5) | 1.9 (0–4.9) | 0.63 |
| IL-12 [pg/mL] | 4.4 (0–16.3) 5.3 # | 3.9 (0–9.2) 7.6 # | * |
| IL-18 [pg/mL] | 78.2 (34.6–146) | 77.2 (45–124.6) | 0.87 |
| ratio TNF–α/IL-35 | 0.09 (0–0.43) 0.15 # | 0.4 (0–2.97) 0.6 # | *** |
| Vascular and inflammatory biomarkers | |||
| Serum angiogenin [ng/mL] | 298.3 (124–799.6) | 257.6 (103–985) | 0.50 |
| VEGF [pg/mL] | 132 (55.6–6848) | 180.6 (75.5–363.8) | 0.23 |
| sVCAM–1 [ng/mL] | 301 (77–337.9) | 326.5 (98.5–654) | 0.71 |
| ICAM–1 [ng/mL] | 263.6 (115.6–389.4) | 228.9 (177.5–471.9) | 0.68 |
| sP-Selectin [ng/mL] | 298.3 (98.4–521.6) | 260.9 (146–731.6) | 0.68 |
| AGEs [pg/mL] | 13,450 (5,48–24,560) 9174 # | 22,033 (7650–32,520) 11,595 # | ** |
| sRAGE [pg/mL] | 1287 (788–2483) | 1304 (1106–1982) | 0.50 |
| Characteristics | Diabetic Patients | p | |
|---|---|---|---|
| Group A1 n = 16 | Group A2 n = 15 | ||
| CCA_PI | 2 (1.4–2.7) | 1.8 (1.4–2.9) | 0.49 |
| brachial_PI | 2.5 (1.8–3) | 2.4 (1.8–3.7) | 0.83 |
| thigh_PI | 2.3 (1.9–2.9) | 2.3 (2.0–2.9) | 0.77 |
| above_knee_PI | 2.3 (2.0–3.2) | 2.4 (1.8–3.2) | 0.42 |
| below_knee_PI | 2.5 (2.1–3.6) | 2.7 (2.0–3.6) | 0.19 |
| ankle_PI | 2.6 (2–3.4) | 2.7 (2.2–3.8) | 0.42 |
| ABI | 1.1 (0.9–1.4) | 1.1 (0.9–1.3) | 0.68 |
| SBP [mmHg] | 107 (89–132) | 113 (84–124) | 0.19 |
| DBP [mmHg] | 58 (49–71) | 61 (50–76) | 0.28 |
| PP [mmHg] | 47 (32–61) | 51 (34–70) | 0.49 |
| Characteristics | Diabetic Patients | p | |
|---|---|---|---|
| Group A1 n = 16 | Group A2 n = 15 | ||
| Capillaroscopy | |||
| CoverageBASE [%] | 18 (15.3–21) 3.0 # | 15 (12.9–25) 3.1 # | * |
| CoveragePORH [%] | 17 (14.8–21) | 16 (10.3–24) | 0.57 |
| ∆CoveragePB [%] | −1 (−3.1–3) | 1 (−8.1–5) | 0.28 |
| Capillary reactivity | −5 (−16.4–18) | 5 (−41–31) | 0.38 |
| DistanceBASE [µm] | 214.4 (194.5–263.8) 25.4 # | 246.5 (179.4–274.9) 31.3 # | * |
| DistancePORH [µm] | 217.6 (195.4–274.5) | 241.2 (190.2–345) | 0.08 |
| ∆DistancePB [µm] | 2.8 (−32.8–51.7) | 9.7 (−51.5–105.8) | 0.86 |
| Transcutaneous oxygen pressure | |||
| TcPO2_base [mmHg] | 58.9 (37.8–77.9) | 51.2 (28–71.5) | 0.18 |
| TcPO2_zero [mmHg] | 3.3 (1.9–9) | 3.4 (1.1–14) | 0.54 |
| TTR [s] | 90.5 (53–240) | 63 (28–240) | 0.08 |
| Optical coherence tomography indices | |||
| CST | 247 (208–269) | 264 (226–285) | 0.12 |
| Volume cube | 10 (10–11) | 11 (10–11) | 0.06 |
| TAC | 279 (268–310) | 292 (273–316) | 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neubauer-Geryk, J.; Myśliwiec, M.; Zorena, K.; Bieniaszewski, L. Parameters of Micro- and Macrocirculation in Young Uncomplicated Type 1 Diabetic Patients—The Role of Metabolic Memory. Int. J. Mol. Sci. 2025, 26, 10156. https://doi.org/10.3390/ijms262010156
Neubauer-Geryk J, Myśliwiec M, Zorena K, Bieniaszewski L. Parameters of Micro- and Macrocirculation in Young Uncomplicated Type 1 Diabetic Patients—The Role of Metabolic Memory. International Journal of Molecular Sciences. 2025; 26(20):10156. https://doi.org/10.3390/ijms262010156
Chicago/Turabian StyleNeubauer-Geryk, Jolanta, Małgorzata Myśliwiec, Katarzyna Zorena, and Leszek Bieniaszewski. 2025. "Parameters of Micro- and Macrocirculation in Young Uncomplicated Type 1 Diabetic Patients—The Role of Metabolic Memory" International Journal of Molecular Sciences 26, no. 20: 10156. https://doi.org/10.3390/ijms262010156
APA StyleNeubauer-Geryk, J., Myśliwiec, M., Zorena, K., & Bieniaszewski, L. (2025). Parameters of Micro- and Macrocirculation in Young Uncomplicated Type 1 Diabetic Patients—The Role of Metabolic Memory. International Journal of Molecular Sciences, 26(20), 10156. https://doi.org/10.3390/ijms262010156







