Genetic Dissection of Plant Height Variation Between the Parental Lines of the Elite Japonica Hybrid Rice ‘Shenyou 26’
Abstract
1. Introduction
2. Results
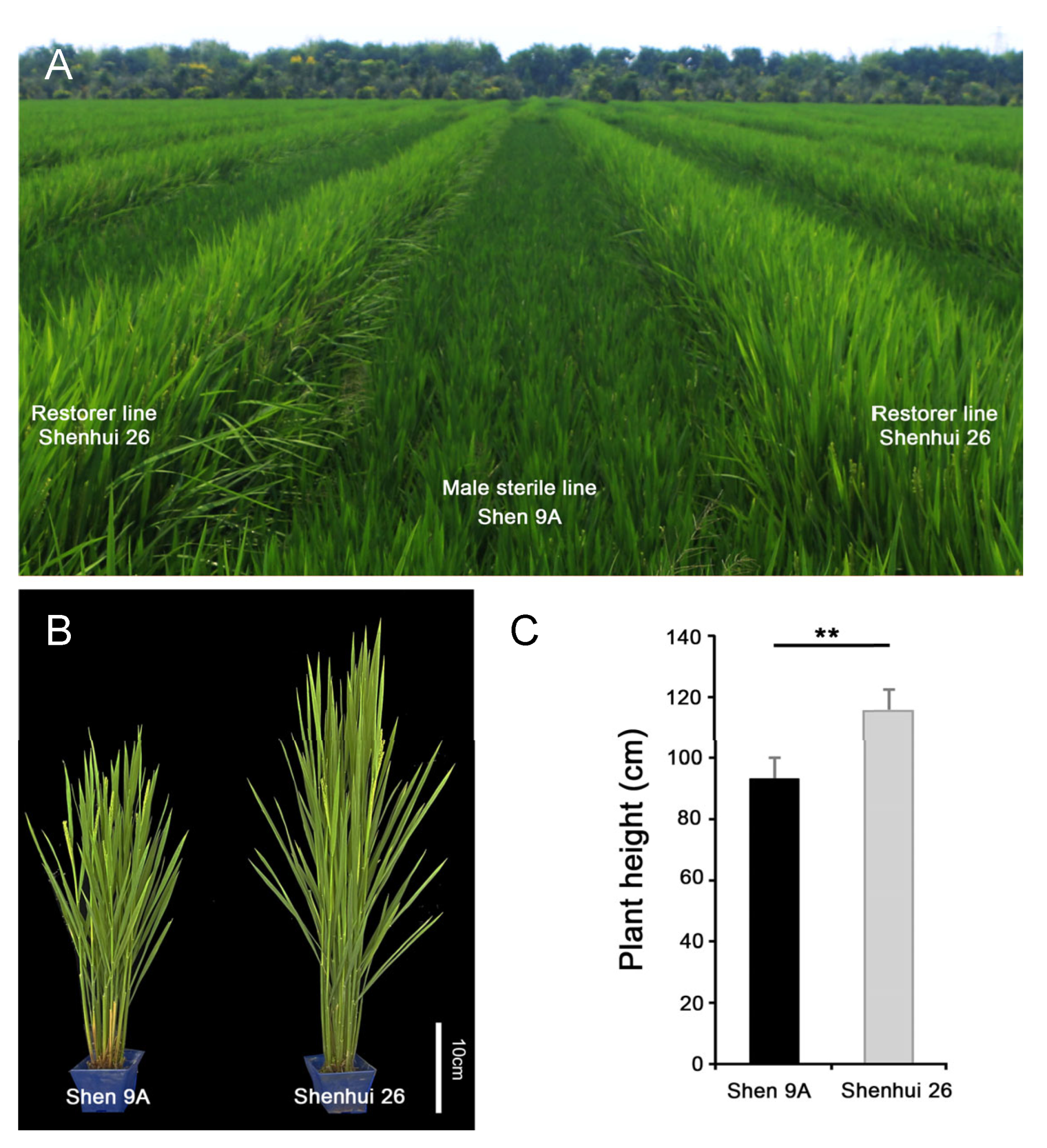
2.1. Height Variation Between the Male-Sterile (Shen 9A) and Restorer (Shenhui 26) Lines in the ‘Shenyou 26’ Hybrid System
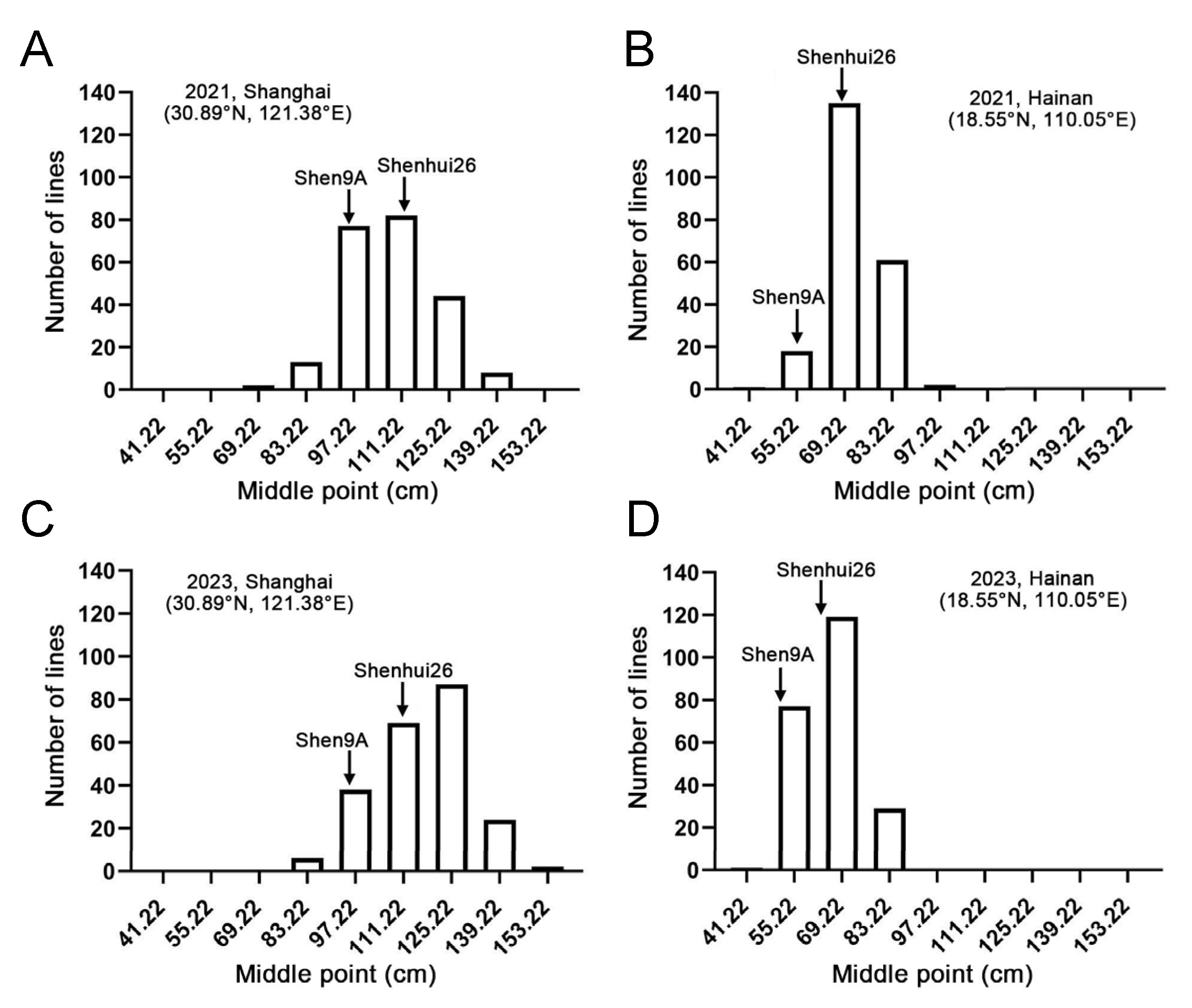
2.2. Genetic Basis of Plant Height Differentiation Between ‘Shen 9A’ and ‘Shenhui 26’
2.3. QTL Identified for the Plant Height in the DH Population
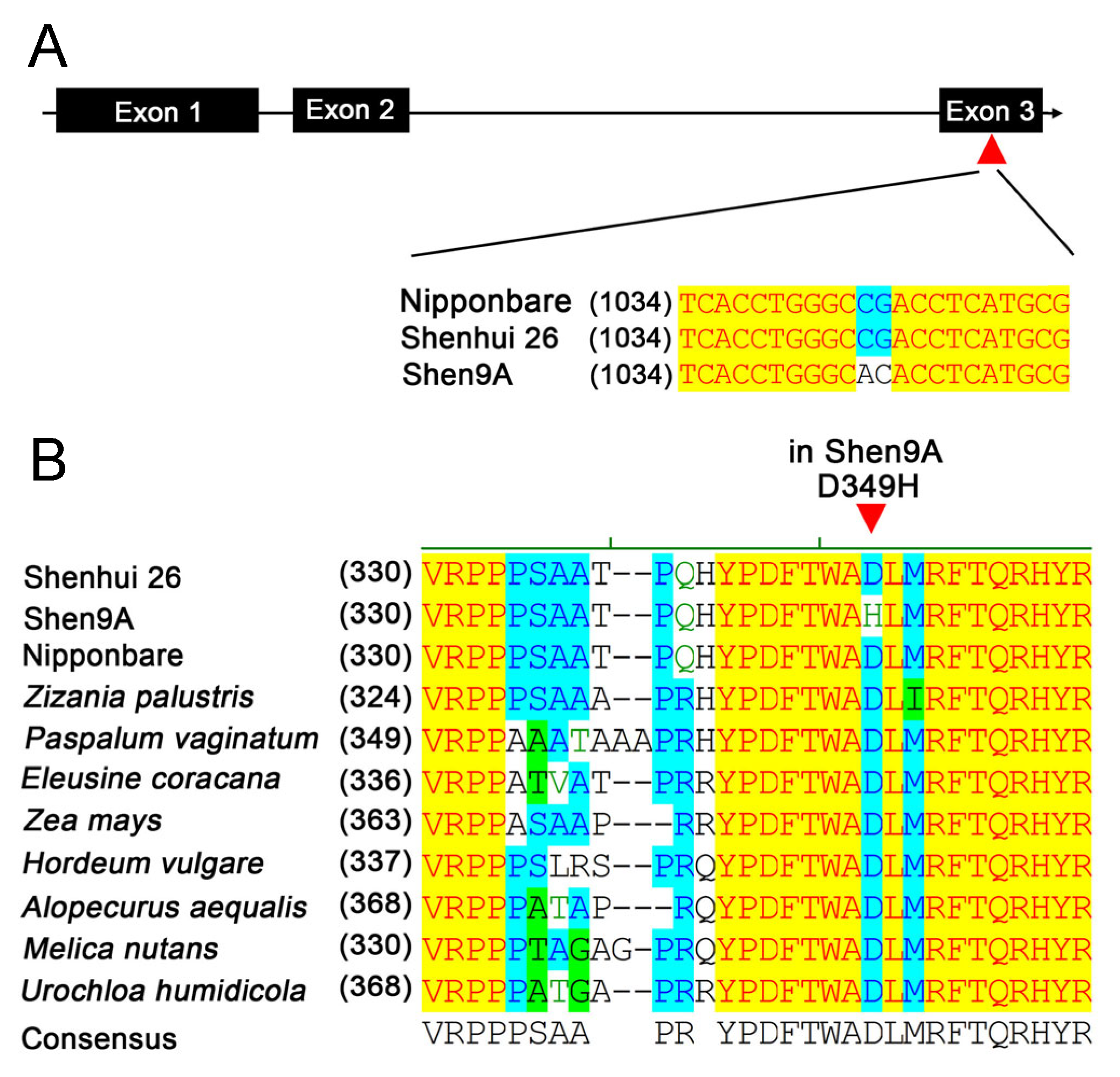
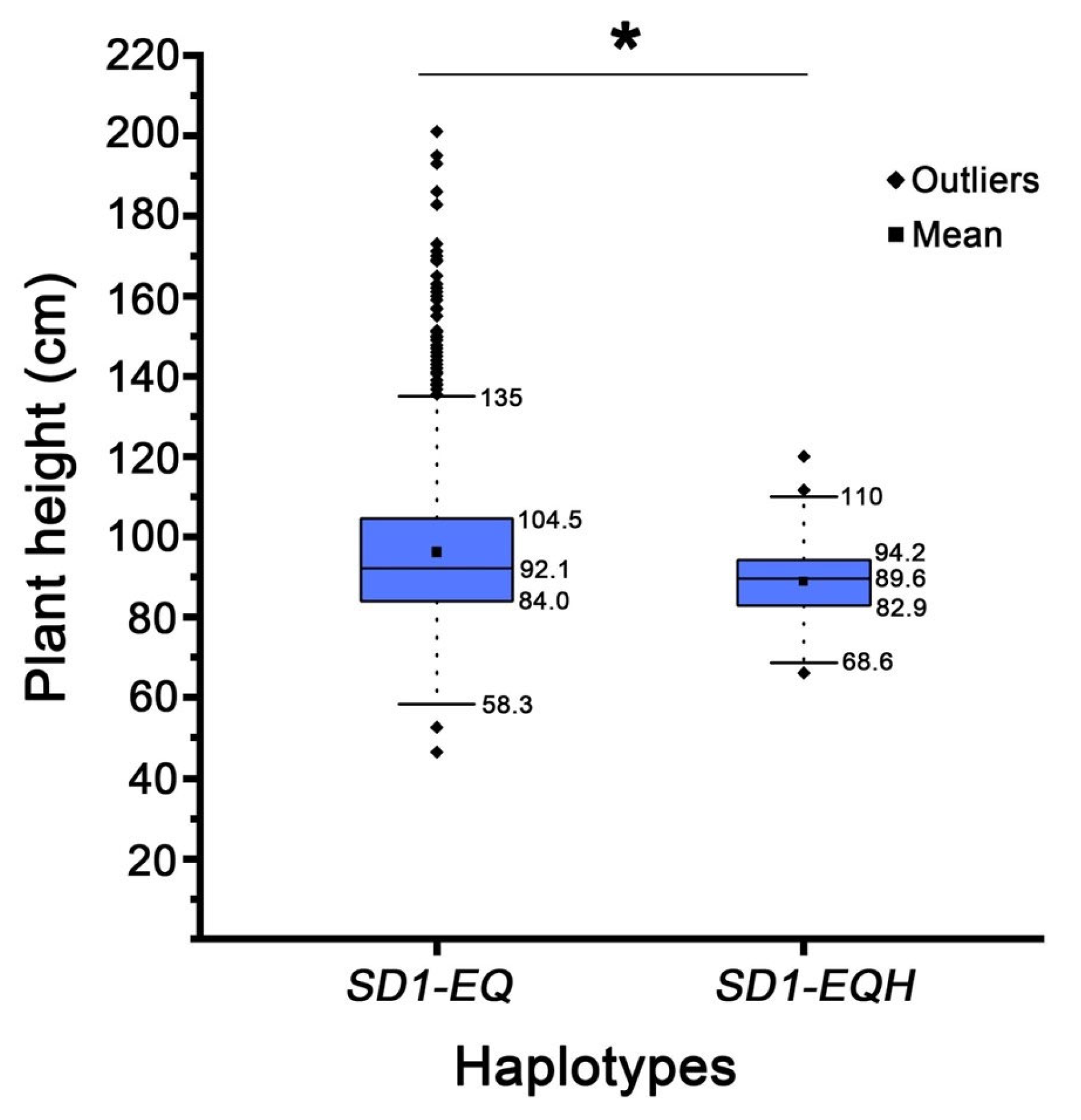
2.4. The SD1 Gene Is a Probable Candidate for the qPH1.1
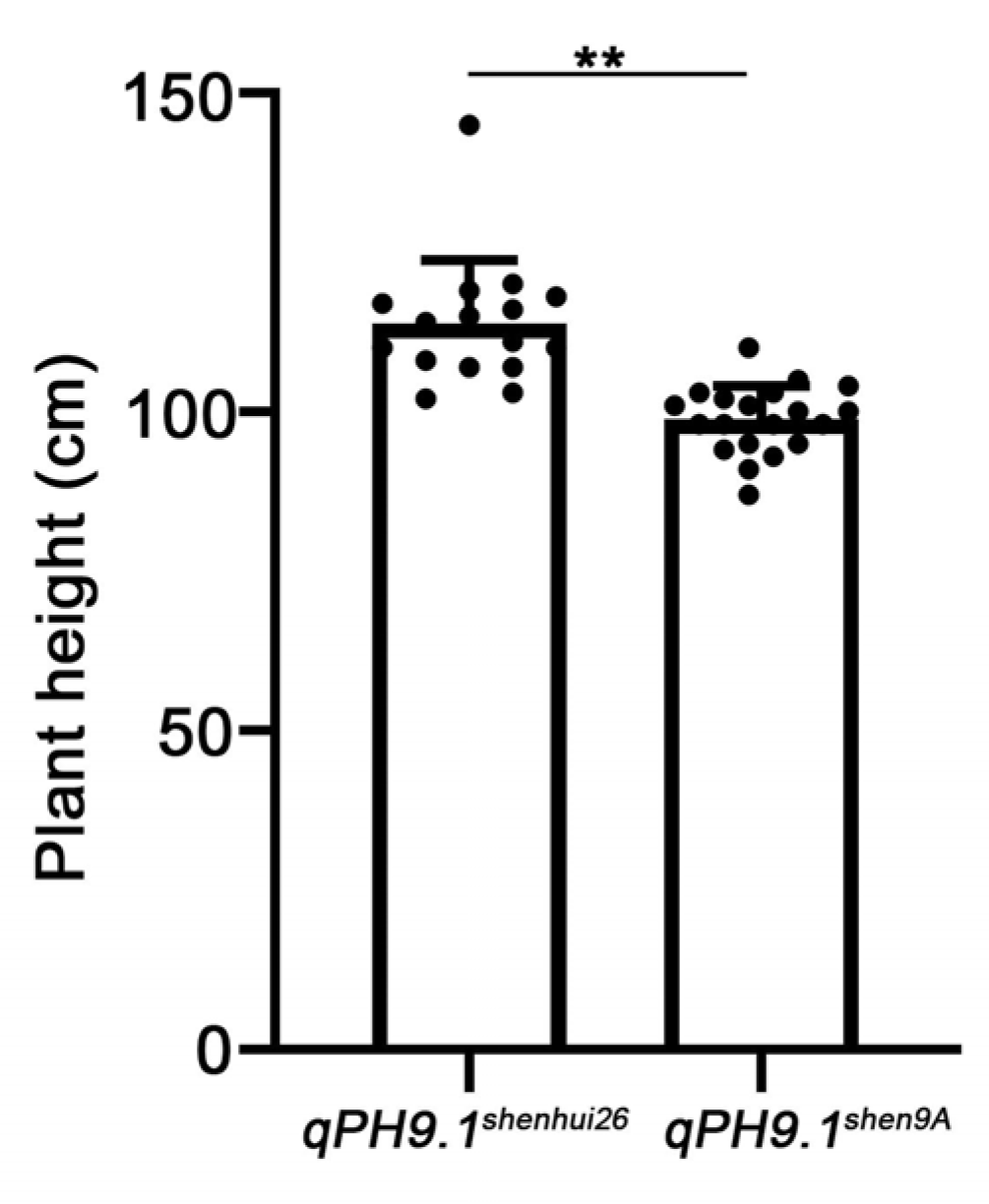
2.5. Genetic Validation for the qPH9.1 Locus
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Field Trials
4.2. ANOVA Analysis
4.3. QTL Mapping
4.4. Validation for the qPH9.1 Locus
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, Y.; Xie, Z.; Chen, D.; Chen, H.; Zeng, Y.; Dai, S. Prediction of Rice Plant Height Using Linear Regression Model by Pyramiding Plant Height-Related Alleles. Int. J. Mol. Sci. 2025, 26, 6249. [Google Scholar] [CrossRef] [PubMed]
- Hedden, P. The genes of the Green Revolution. Trends Genet. 2003, 19, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Richards, D.E.; Hartley, N.M.; Murphy, G.P.; Devos, K.M.; Flintham, J.E.; Beales, J.; Fish, L.J.; Worland, A.J.; Pelica, F.; et al. ‘Green revolution’ genes encode mutant gibberellin response modulators. Nature 1999, 400, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Huang, Y.; Tan, Y.; Tan, L.; Yin, J.; Zou, G. Potentially Useful Dwarfing or Semi-dwarfing Genes in Rice Breeding in Addition to the sd1 Gene. Rice 2022, 15, 66. [Google Scholar] [CrossRef]
- Asano, K.; Yamasaki, M.; Takuno, S.; Miura, K.; Katagiri, S.; Ito, T.; Doi, K.; Wu, J.; Ebana, K.; Matsumoto, T.; et al. Artificial selection for a green revolution gene during japonica rice domestication. Proc. Natl. Acad. Sci. USA 2011, 108, 11034–11039. [Google Scholar] [CrossRef]
- Zhang, L.; Bian, Z.; Ma, B.; Li, X.; Zou, Y.; Xie, D.; Liu, J.; Ren, Y.; Zhang, C.; Wang, J.; et al. Exploration and selection of elite Sd1 alleles for rice design breeding. Mol. Breed. 2020, 40, 79. [Google Scholar] [CrossRef]
- Yu, Y.; Hu, X.; Zhu, Y.; Mao, D. Re-evaluation of the rice ‘Green Revolution’ gene: The weak allele SD1-EQ from japonica rice may be beneficial for super indica rice breeding in the post-Green Revolution era. Mol. Breed. 2020, 40, 84. [Google Scholar] [CrossRef]
- Asano, K.; Takashi, T.; Miura, K.; Qian, Q.; Kitano, H.; Matsuoka, M.; Ashikari, M. Genetic and Molecular Analysis of Utility of sd1 Alleles in Rice Breeding. Breed. Sci. 2007, 57, 53–58. [Google Scholar] [CrossRef]
- Peng, Y.; Hu, Y.; Qian, Q.; Ren, D. Progress and Prospect of Breeding Utilization of Green Revolution Gene SD1 in Rice. Agriculture 2021, 11, 611. [Google Scholar] [CrossRef]
- Spielmeyer, W.; Ellis, M.H.; Chandler, P.M. Semidwarf (sd-1), “green revolution” rice, contains a defective gibberellin 20-oxidase gene. Proc. Natl. Acad. Sci. USA 2002, 99, 9043–9048. [Google Scholar] [CrossRef]
- Wang, F.; Yoshida, H.; Matsuoka, M. Making the ‘Green Revolution’ Truly Green: Improving Crop Nitrogen Use Efficiency. Plant Cell Physiol. 2021, 62, 942–947. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Wang, W.; Chen, J.; Liu, Y.; Chu, C. Genetic improvement toward nitrogen-use efficiency in rice: Lessons and perspectives. Mol. Plant 2023, 16, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Ferrero-Serrano, Á.; Cantos, C.; Assmann, S. The Role of Dwarfing Traits in Historical and Modern Agriculture with a Focus on Rice. Cold Spring Harb. Perspect. Biol. 2019, 11, a034645. [Google Scholar] [CrossRef] [PubMed]
- Murai, M.; Hirose, S.; Sato, S.; Takebe, M. Effects of Dwarfing Genes from Dee-geo-woo-gen and Other Varieties on Cool Temperature Tolerance at Booting Stage in Rice. Jpn. J. Breed. 1991, 41, 241–254. [Google Scholar] [CrossRef]
- Lafitte, H.R.; Yongsheng, G.; Yan, S.; Li, Z.K. Whole plant responses, key processes, and adaptation to drought stress: The case of rice. J. Exp. Bot. 2007, 58, 169–175. [Google Scholar]
- Asano, K.; Hirano, K.; Ueguchi-Tanaka, M.; Angeles-Shim, R.B.; Komura, T.; Satoh, H.; Kitano, H.; Matsuoka, M.; Ashikari, M. Isolation and characterization of dominant dwarf mutants, Slr1-d, in rice. Mol. Genet. Genom. 2009, 281, 223–231. [Google Scholar] [CrossRef]
- Liu, C.; Zheng, S.; Gui, J.; Fu, C.; Yu, H.; Song, D.; Shen, J.; Qin, P.; Liu, X.; Han, B.; et al. Shortened Basal Internodes Encodes a Gibberellin 2-Oxidase and Contributes to Lodging Resistance in Rice. Mol. Plant 2018, 11, 288–299. [Google Scholar] [CrossRef]
- Ruan, B.; Jiang, Y.; Ma, Y.; Zhou, M.; Chen, F.; Zhang, Y.; Yu, Y.; Wu, L. Characterization of the ddt1 Mutant in Rice and Its Impact on Plant Height Reduction and Water Use Efficiency. Int. J. Mol. Sci. 2024, 25, 7629. [Google Scholar] [CrossRef]
- Fu, Y.; Zhao, H.; Huang, J.; Zhu, H.; Luan, X.; Bu, S.; Liu, Z.; Wang, X.; Peng, Z.; Meng, L.; et al. Dynamic analysis of QTLs on plant height with single segment substitution lines in rice. Sci. Rep. 2022, 12, 5465. [Google Scholar] [CrossRef]
- Wu, Z.; Tang, D.; Liu, K.; Miao, C.; Zhuo, X.; Li, Y.; Tan, X.; Sun, M.; Luo, Q.; Cheng, Z. Characterization of a new semi-dominant dwarf allele of SLR1 and its potential application in hybrid rice breeding. J. Exp. Bot. 2018, 69, 4703–4713. [Google Scholar] [CrossRef]
- Sun, B.; Niu, F.; Zhou, J.; Chu, H.; Luo, Z.; Cheng, C.; Cao, L. Characteristics and Key Cultivation Techniques of New Japonica Three-line Hybrid Rice ‘Shenyou26’. Mol. Plant Breed. 2020, 18, 3449–3454. [Google Scholar]
- Sun, B.; Ding, X.; Ye, J.; Dai, Y.; Cheng, C.; Zhou, J.; Niu, F.; Tu, R.; Hu, Q.; Xie, K.; et al. Unveiling the Genetic Basis Underlying Rice Anther Culturability via Segregation Distortion Analysis in Doubled Haploid Population. Genes 2023, 14, 2086. [Google Scholar] [CrossRef]
- Peng, H.; Wang, K.; Chen, Z.; Cao, Y.; Gao, Q.; Li, Y.; Li, X.; Lu, H.; Du, H.; Lu, M.; et al. MBKbase for rice: An integrated omics knowledgebase for molecular breeding in rice. Nucleic Acids Res. 2020, 48, D1085–D1092. [Google Scholar] [CrossRef]
- Yuan, L.-p. Development of Hybrid Rice to Ensure Food Security. Rice Sci. 2014, 21, 1–2. [Google Scholar] [CrossRef]
- Sha, H.; Liu, H.; Zhao, G.; Han, Z.; Chang, H.; Wang, J.; Zheng, H.; Zhang, J.; Yu, Y.; Liu, Y.; et al. Elite sd1 alleles in japonica rice and their breeding applications in northeast China. Crop J. 2022, 10, 224–233. [Google Scholar] [CrossRef]
- Meng, L.; Li, H.; Zhang, L.; Wang, J. QTL IciMapping: Integrated software for genetic linkage map construction and quantitative trait locus mapping in biparental populations. Crop J. 2015, 3, 269–283. [Google Scholar] [CrossRef]
- Yin, C.; Li, H.; Li, S.; Xu, L.; Zhao, Z.; Wang, J. Genetic dissection on rice grain shape by the two-dimensional image analysis in one japonica × indica population consisting of recombinant inbred lines. Theor. Appl. Genet. 2015, 128, 1969–1986. [Google Scholar] [CrossRef]
| Environment 1 | Parents (cm) | DH Population | ||||
|---|---|---|---|---|---|---|
| Shen9A | Shenhui26 | Mean (cm) | Range (cm) | Skewness | Kurtosis | |
| 21SH | 96.59 | 113.93 | 109.38 | 74.5–143.0 | 0.07 | −0.17 |
| 21HN | 55.47 | 67.90 | 72.40 | 46.5–98.5 | 0.16 | 0.32 |
| 23SH | 93.16 | 110.36 | 117.93 | 84.5–150.5 | −0.15 | −0.46 |
| 23HN | 50.37 | 62.27 | 66.74 | 44.0–90.0 | 0.23 | −0.37 |
| Variance Components | Heritability (H2) | |||
|---|---|---|---|---|
| Environment (σ2E) | Genotype (σ2G) | G by E (σ2G×E) | Random Error (σ2ε) | |
| 662.36 | 86.01 | 48.21 | 1.51 | 0.88 |
| Environment | QTL | Chr. | Position 1 | LOD | Flank Marker 2 | PVE (%) | Add 3 | CI (cM) 4 |
|---|---|---|---|---|---|---|---|---|
| 2021 Shanghai | qPH1.1 qPH5.1 qPH9.1 | 1 5 9 | 160 112 95 | 5.6490 6.9651 5.0192 | Chr1-35346977~Chr1-37122278 Chr5-25700930~Chr5-26419104 Chr9-17208953~Chr9-17730888 | 8.4004 10.1566 7.4181 | 3.7806 −4.1561 3.577 | 154.5~161.5 110.5~113.5 92.5~100.5 |
| 2021 Hainan | qPH1.1 qPH2.1 qPH5.1 qPH9.1 qPH12.1 | 1 2 5 9 12 | 163 59 115 94 69 | 10.7983 3.1405 6.3262 19.0114 5.9827 | Chr1-37122278~Chr1-38859748 Chr2-8900156~Chr2-8940541 Chr5-26419104~Chr5-27331103 Chr9-17208953~Chr9-17730888 Chr12-24973861~Chr12-25345406 | 12.0605 3.1628 6.7287 22.7247 6.2037 | 3.0255 −1.5828 −2.2572 4.1779 −2.1937 | 158.5~165.5 56.5~59.5 111.5~115.5 92.5~96.5 67.5~72.5 |
| 2023 Shanghai | qPH1.1 qPH5.1 qPH9.2 | 1 5 9 | 163 111 100 | 14.1927 8.4175 9.6674 | Chr1-37122278~Chr1-38859748 Chr5-25616977~Chr5-25700930 Chr9-17730888~Chr9-18211888 | 18.6952 10.0683 11.7154 | 5.5662 −4.0886 4.4217 | 158.5~164.5 109.5~111.5 97.5~100.5 |
| 2023 Hainan | qPH1.1 qPH6.1 qPH9.1 | 1 6 9 | 163 137 93 | 11.4845 3.0276 5.6217 | Chr1-37122278~Chr1-38859748 Chr6-29076596~Chr6-30825274 Chr9-17208953~Chr9-17730888 | 18.6777 4.4325 8.3606 | 3.7294 −1.8182 2.5112 | 158.5~165.5 135.5~142.5 91.5~96.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, B.; Ding, X.; Xie, K.; Zhang, X.; Cheng, C.; Dai, Y.; Zhang, A.; Zhou, J.; Niu, F.; Tu, R.; et al. Genetic Dissection of Plant Height Variation Between the Parental Lines of the Elite Japonica Hybrid Rice ‘Shenyou 26’. Int. J. Mol. Sci. 2025, 26, 10155. https://doi.org/10.3390/ijms262010155
Sun B, Ding X, Xie K, Zhang X, Cheng C, Dai Y, Zhang A, Zhou J, Niu F, Tu R, et al. Genetic Dissection of Plant Height Variation Between the Parental Lines of the Elite Japonica Hybrid Rice ‘Shenyou 26’. International Journal of Molecular Sciences. 2025; 26(20):10155. https://doi.org/10.3390/ijms262010155
Chicago/Turabian StyleSun, Bin, Xiaorui Ding, Kaizhen Xie, Xueqing Zhang, Can Cheng, Yuting Dai, Anpeng Zhang, Jihua Zhou, Fuan Niu, Rongjian Tu, and et al. 2025. "Genetic Dissection of Plant Height Variation Between the Parental Lines of the Elite Japonica Hybrid Rice ‘Shenyou 26’" International Journal of Molecular Sciences 26, no. 20: 10155. https://doi.org/10.3390/ijms262010155
APA StyleSun, B., Ding, X., Xie, K., Zhang, X., Cheng, C., Dai, Y., Zhang, A., Zhou, J., Niu, F., Tu, R., Qiu, Y., Feng, Z., Hu, B., Shao, C., Li, H., Shen, T., Cao, L., & Chu, H. (2025). Genetic Dissection of Plant Height Variation Between the Parental Lines of the Elite Japonica Hybrid Rice ‘Shenyou 26’. International Journal of Molecular Sciences, 26(20), 10155. https://doi.org/10.3390/ijms262010155






