Dietary Exposure to 2,2′,4,4′-Tetrabromodiphenyl Ether (BDE-47) Causes Inflammation in the Liver of Common Carp (Cyprinus carpio) and Affects Lipid Metabolism by Interfering with Steroid Hormone Biosynthesis Pathways
Abstract
1. Introduction
2. Results and Discussion
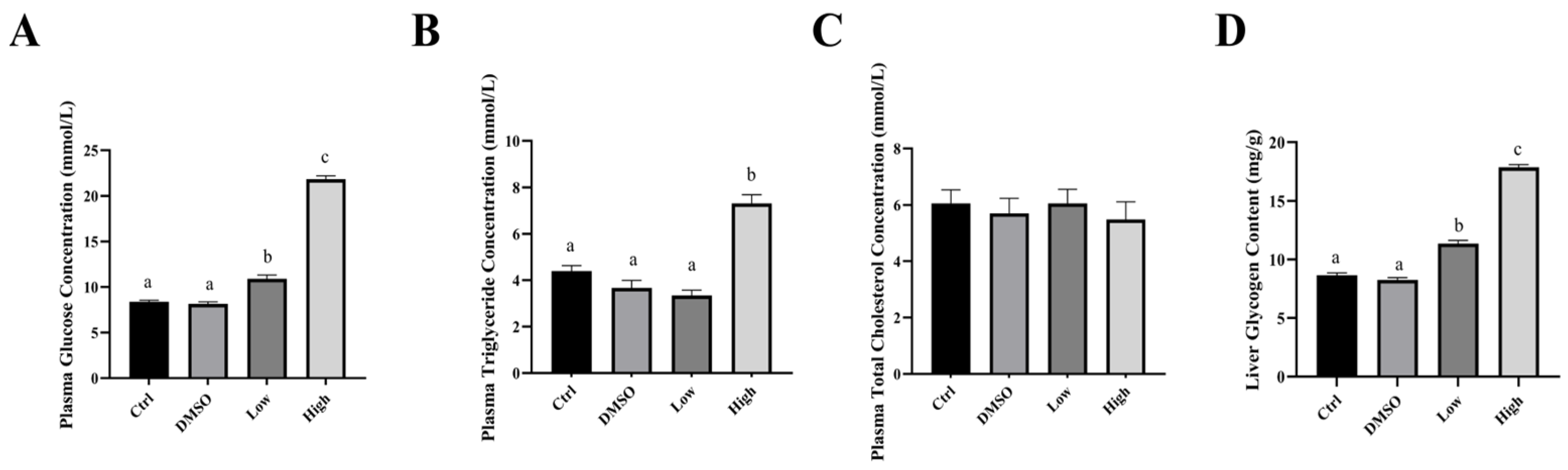
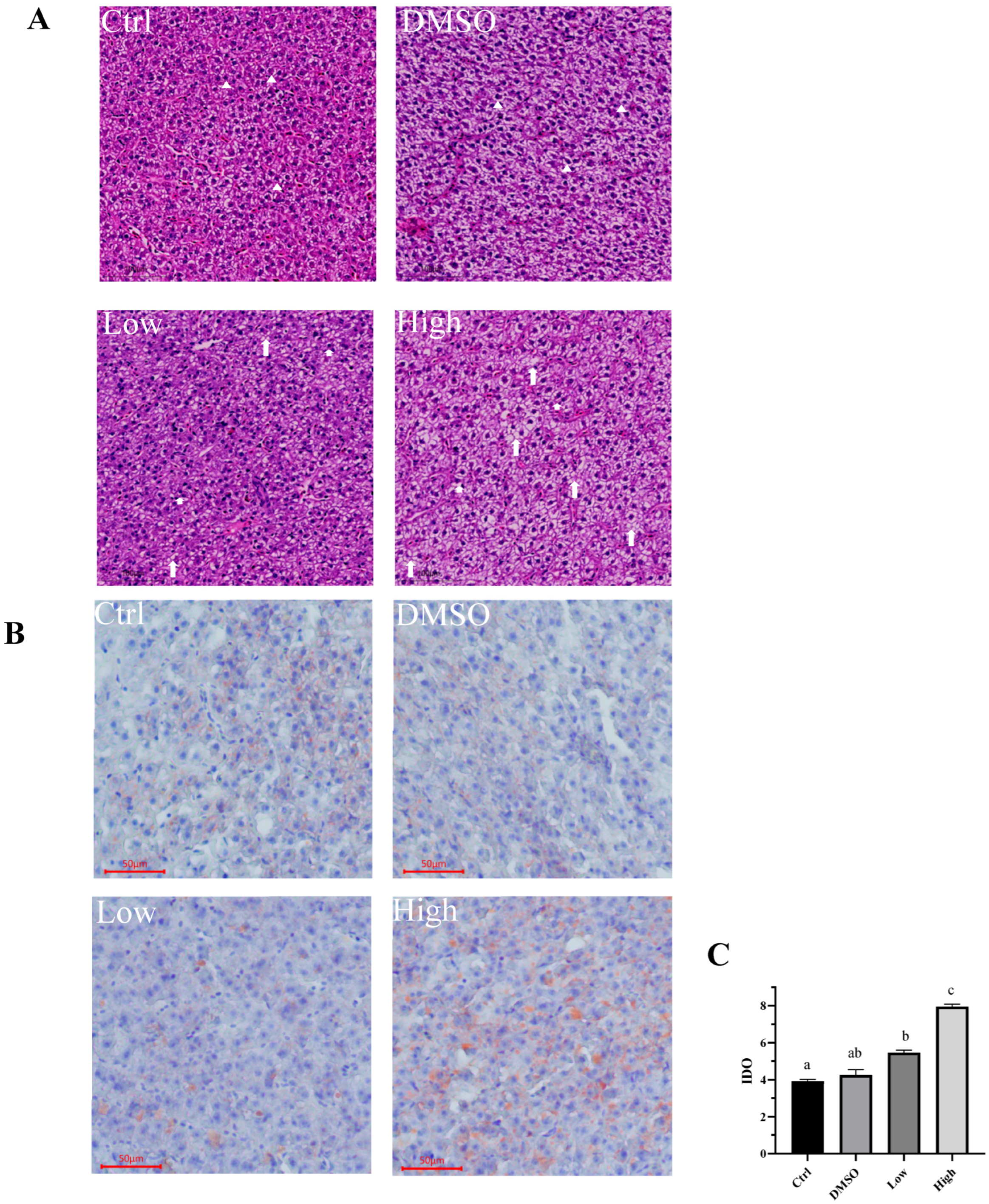
2.1. BDE-47 Exposure Results in the Accumulation of Lipids in the Carp Liver
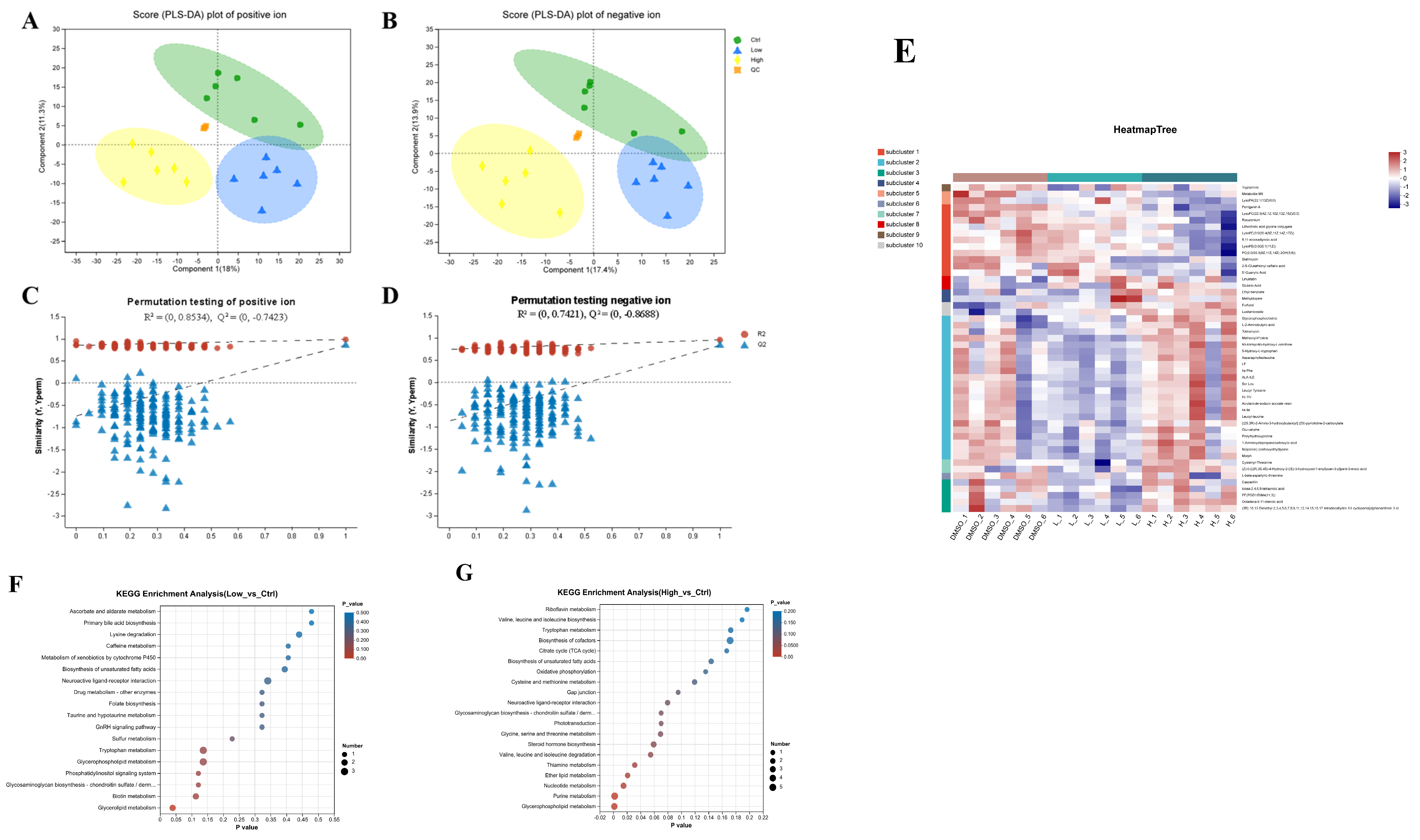
2.2. BDE-47 Affects the Metabolites Involved in Glucose and Lipid Metabolism in the Carp Liver
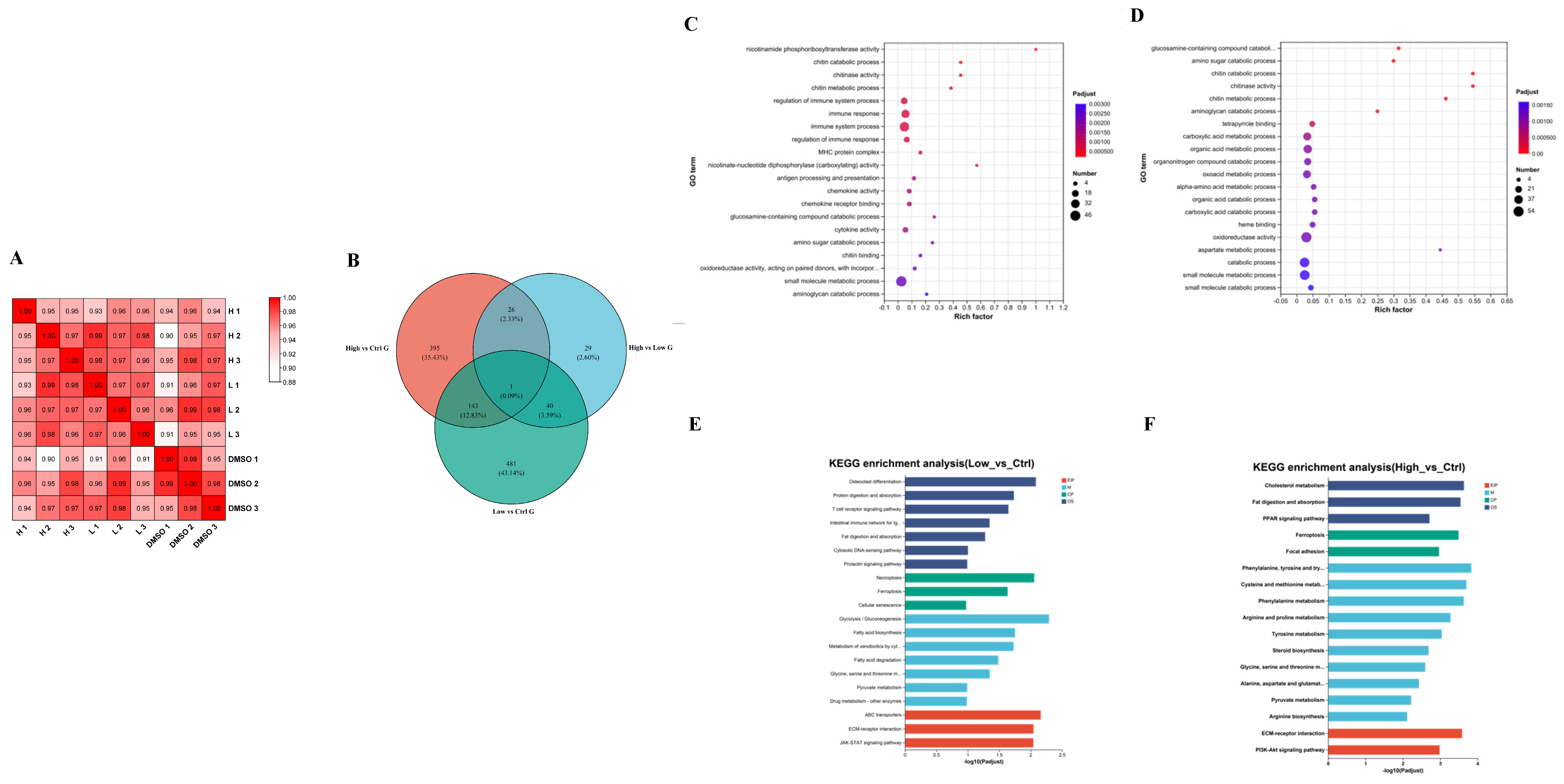
2.3. Transcriptome Data Reveal That BDE-47 Affects Lipid Accumulation Through Glucose- and Lipid Metabolic-Related Pathways
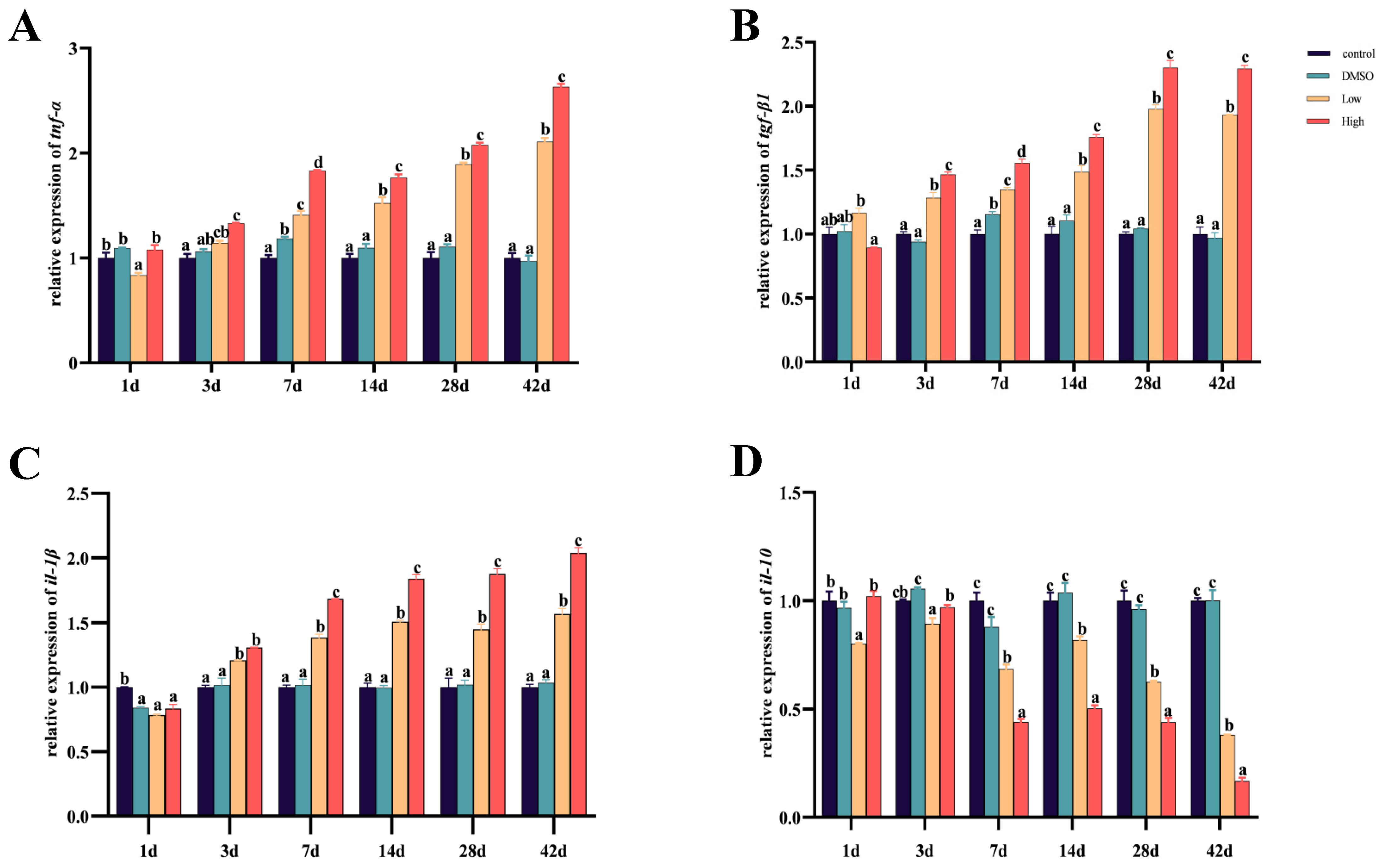
2.4. BDE-47 Induces an Inflammatory Response in the Liver of Carp
2.5. Transcriptome and Metabolomic Joint Analyses Reveal That the BDE-47 Pathway Induces Lipid Deposition in the Carp Liver
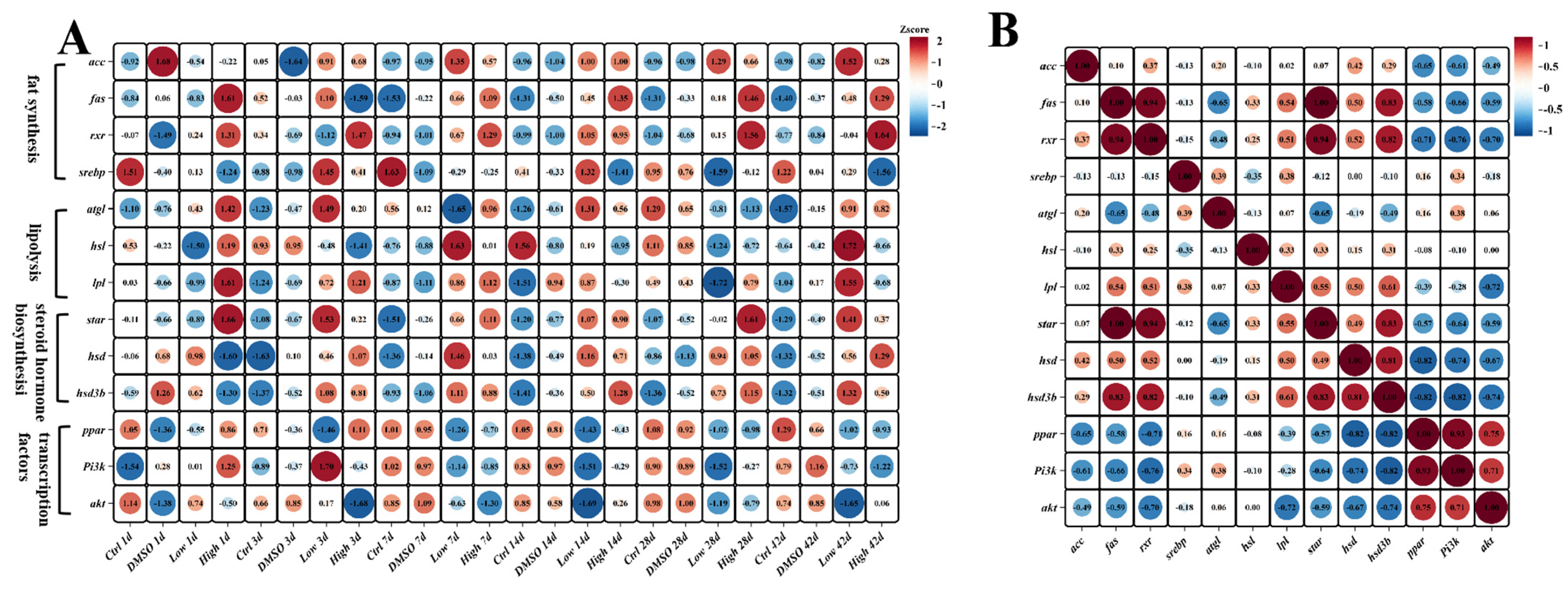
2.6. BDE-47 Induces Lipid Deposition Through the Steroid Hormone Biosynthetic Pathway in the Carp Liver
3. Materials and Methods
3.1. Chemical and Experimental Fish
3.2. Experimental Design
3.3. Biochemical Analysis
3.4. Histopathological Analysis
3.5. Validation of the Correlation Between Oil Red O and Biochemical Data
3.6. Metabolomic Analysis
3.7. Transcriptomic Analysis
3.8. Integration Analysis of the Transcriptome and Metabolome
3.9. qPCR
3.10. Statistical Analysis
3.11. Distribution of Experiment Personnel
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adamek, Z.; Moessmer, M.; Hauber, M. Current Principles and Issues Affecting Organic Carp (Cyprinus carpio) Pond Farming. Aquaculture 2019, 512, 734261. [Google Scholar] [CrossRef]
- Li, B.; Shi, J.; Zhang, J.; Tao, H.; Ge, H.; Zhang, M.; Xu, Z.; Xiao, R. Occurrence and Ecological Risk Assessment of 2,2′,4,4′-Tetrabromodiphenyl Ether and Decabromodiphenyl Ether in Surface Waters across China. Chemosphere 2023, 312, 137215. [Google Scholar] [CrossRef] [PubMed]
- Andrade, N.A.; McConnell, L.L.; Torrents, A.; Ramirez, M. Persistence of Polybrominated Diphenyl Ethers in Agricultural Soils after Biosolids Applications. J. Agric. Food Chem. 2010, 58, 3077–3084. [Google Scholar] [CrossRef]
- Wang, T.; Yu, J.C.; Wang, P.; Zhang, Q.H. Levels and Distribution of Polybrominated Diphenyl Ethers in the Aquatic and Terrestrial Environment around a Wastewater Treatment Plant. Environ. Sci. Pollut. Res. 2016, 23, 16440–16447. [Google Scholar] [CrossRef]
- Zhou, Y.H.; Chen, Q.F.; Du, X.Y.; Yin, G.; Qiu, Y.L.; Ye, L.; Zhu, Z.L.; Zhao, J.F. Occurrence and Trophic Magnification of Polybrominated Diphenyl Ethers (Pbdes) and Their Methoxylated Derivatives in Freshwater Fish from Dianshan Lake, Shanghai, China. Environ. Pollut. 2016, 219, 932–938. [Google Scholar] [CrossRef]
- Chen, G.Z.; Deng, X.Y.; Wang, J.Z. Pollution Level, Spatial Distribution, and Congener Fractionation Characteristics of Low-Brominated Polybrominated Diphenyl Ethers (Pbdes) in Sediments around Chaohu Lake, China. Environ. Monit. Assess. 2022, 194, 15. [Google Scholar] [CrossRef]
- Matsukami, H.; Suzuki, G.; Someya, M.; Uchida, N.; Tue, N.M.; Tuyen, L.H.; Viet, P.H.; Takahashi, S.; Tanabe, S.; Takigami, H. Concentrations of Polybrominated Diphenyl Ethers and Alternative Flame Retardants in Surface Soils and River Sediments from an Electronic Waste-Processing Area in Northern Vietnam, 2012–2014. Chemosphere 2017, 167, 291–299. [Google Scholar] [CrossRef]
- Khalil, A.; Parker, M.; Mpanga, R.; Cevik, S.E.; Thorburn, C.; Suvorov, A. Developmental Exposure to 2,2′,4,4′–Tetrabromodiphenyl Ether Induces Long-Lasting Changes in Liver Metabolism in Male Mice. J. Endocr. Soc. 2017, 1, 323–344. [Google Scholar] [CrossRef]
- Liu, X.H.; Wang, J.; Lu, C.Q.; Zhu, C.Y.; Qian, B.; Li, Z.W.; Liu, C.; Shao, J.; Yan, J.S. The Role of Lysosomes in Bde 47-Mediated Activation of Mitochondrial Apoptotic Pathway in Hepg2 Cells. Chemosphere 2015, 124, 10–21. [Google Scholar] [CrossRef]
- Pazin, M.; Pereira, L.C.; Dorta, D.J. Toxicity of Brominated Flame Retardants, Bde-47 and Bde-99 Stems from Impaired Mitochondrial Bioenergetics. Toxicol. Mech. Methods 2015, 25, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Sen, P.; Qadri, S.; Luukkonen, P.K.; Ragnarsdottir, O.; McGlinchey, A.; Jäntti, S.; Juuti, A.; Arola, J.; Schlezinger, J.J.; Webster, T.F.; et al. Exposure to Environmental Contaminants Is Associated with Altered Hepatic Lipid Metabolism in Non-Alcoholic Fatty Liver Disease. J. Hepatol. 2022, 76, 283–293. [Google Scholar] [CrossRef]
- Yang, C.X.; Zhu, L.; Kang, Q.Z.; Lee, H.K.; Li, D.P.; Chung, A.C.K.; Cai, Z.W. Chronic Exposure to Tetrabromodiphenyl Ether (Bde-47) Aggravates Hepatic Steatosis and Liver Fibrosis in Diet-Induced Obese Mice. J. Hazard. Mater. 2019, 378, 120766. [Google Scholar] [CrossRef] [PubMed]
- Casella, M.; Lori, G.; Coppola, L.; La Rocca, C.; Tait, S. Bde-47,-99,-209 and Their Ternary Mixture Disrupt Glucose and Lipid Metabolism of Hepg2 Cells at Dietary Relevant Concentrations: Mechanistic Insight through Integrated Transcriptomics and Proteomics Analysis. Int. J. Mol. Sci. 2022, 23, 14465. [Google Scholar] [CrossRef] [PubMed]
- Tung, E.W.Y.; Boudreau, A.; Wade, M.G.; Atlas, E. Induction of Adipocyte Differentiation by Polybrominated Diphenyl Ethers (Pbdes) in 3t3-L1 Cells. PLoS ONE 2014, 9, e94583. [Google Scholar] [CrossRef]
- Suvorov, A.; Battista, M.C.; Takser, L. Perinatal Exposure to Low-Dose 2,2′,4,4′-Tetrabromodiphenyl Ether Affects Growth in Rat Offspring: What Is the Role of Igf-1? Toxicology 2009, 260, 126–131. [Google Scholar] [CrossRef]
- Kamstra, J.H.; Hruba, E.; Blumberg, B.; Janesick, A.; Mandrup, S.; Hamers, T.; Legler, J. Transcriptional and Epigenetic Mechanisms Underlying Enhanced in Vitro Adipocyte Differentiation by the Brominated Flame Retardant Bde-47. Environ. Sci. Technol. 2014, 48, 4110–4119. [Google Scholar] [CrossRef]
- Albrektsen, S.; Kortet, R.; Skov, P.V.; Ytteborg, E.; Gitlesen, S.; Kleinegris, D.; Mydland, L.T.; Hansen, J.O.; Lock, E.J.; Morkore, T.; et al. Future Feed Resources in Sustainable Salmonid Production: A Review. Rev. Aquac. 2022, 14, 1790–1812. [Google Scholar] [CrossRef]
- Aragao, C.; Gonçalves, A.T.; Costas, B.; Azeredo, R.; Xavier, M.J.; Engrola, S. Alternative Proteins for Fish Diets: Implications Beyond Growth. Animals 2022, 12, 1211. [Google Scholar] [CrossRef]
- Liu, H.; Tang, S.; Zheng, X.; Zhu, Y.; Ma, Z.; Liu, C.; Hecker, M.; Saunders, D.M.V.; Giesy, J.P.; Zhang, X.; et al. Bioaccumulation, Biotransformation, and Toxicity of Bde-47, 6-Oh-Bde-47, and 6-Meo-Bde-47 in Early Life-Stages of Zebrafish (Danio rerio). Environ. Sci. Technol. 2015, 49, 1823–1833. [Google Scholar] [CrossRef]
- Linares, V.; Belles, M.; Domingo, J.L. Human Exposure to Pbde and Critical Evaluation of Health Hazards. Arch. Toxicol. 2015, 89, 335–356. [Google Scholar] [CrossRef] [PubMed]
- Yao, B.; Luo, Z.R.; Zhi, D.; Hou, D.M.; Luo, L.; Du, S.Z.; Zhou, Y.Y. Current Progress in Degradation and Removal Methods of Polybrominated Diphenyl Ethers from Water and Soil: A Review. J. Hazard. Mater. 2021, 403, 123674. [Google Scholar] [CrossRef]
- Van der Schyff, V.; Kalina, J.; Govarts, E.; Gilles, L.; Schoeters, G.; Castaño, A.; Esteban-López, M.; Kohoutek, J.; Kukučka, P.; Covaci, A.; et al. Exposure to Flame Retardants in European Children—Results from the Hbm4eu Aligned Studies. Int. J. Hyg. Environ. Health 2023, 247, 114070. [Google Scholar] [CrossRef]
- Riaz, R.; Malik, R.N.; de Wit, C.A. Soil-Air Partitioning of Semivolatile Organic Compounds in the Lesser Himalaya Region: Influence of Soil Organic Matter, Atmospheric Transport Processes and Secondary Emissions. Environ. Pollut. 2021, 291, 118006. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Liu, B.; Yu, Y.; Li, H.; Li, Q.; Cui, Y.; Ma, Y. Polybrominated Diphenyl Ethers (Pbdes) in Household Dust: A Systematic Review on Spatio-Temporal Distribution, Sources, and Health Risk Assessment. Chemosphere 2023, 314, 137641. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.X.; Song, N.N.; Jiang, T.; Wu, J.; Zhang, L.; Ge, W.; Chai, C. Polybrominated Diphenyl Ethers in Surface Sediments from Fishing Ports Along the Coast of Bohai Sea, China. Mar. Pollut. Bull. 2021, 164, 112037. [Google Scholar] [CrossRef] [PubMed]
- Macklin, R.; Brazier, B.; Harrison, S.; Chapman, D.; Vilizzi, L. A Review of the Status and Range Expansion of Common Carp (Cyprinus carpio L.) in Ireland. Aquat. Invasions 2016, 11, 75–82. [Google Scholar] [CrossRef]
- Yick, J.L.; Wisniewski, C.; Diggle, J.; Patil, J.G. Eradication of the Invasive Common Carp, Cyprinus carpio from a Large Lake: Lessons and Insights from the Tasmanian Experience. Fishes 2021, 6, 6. [Google Scholar] [CrossRef]
- Rypel, A.L. Do Invasive Freshwater Fish Species Grow Better When They Are Invasive? Oikos 2013, 123, 279–289. [Google Scholar] [CrossRef]
- Kuebutornye, F.K.A.; Roy, K.; Folorunso, E.A.; Mraz, J. Plant-Based Feed Additives in Cyprinus carpio Aquaculture. Rev. Aquac. 2023, 16, 309–336. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, L.; Huang, Y.; Chen, S.; Liu, Y.; Tang, N.; Li, Z.; Zhang, X.; Li, L.; Chen, D. Dietary Exposure to 2,2 ′,4,4 ′-Tetrabromodiphenyl Ether (Bde-47) Induces Oxidative Damage Promoting Cell Apoptosis Primarily Via Mitochondrial Pathway in the Hepatopancreas of Carp, Cyprinus carpio. Ecotoxicol. Environ. Saf. 2024, 274, 116192. [Google Scholar] [CrossRef]
- Wang, C.-Q.; Su, Z.; Dai, C.-G.; Song, J.-L.; Qian, B. Multi-Omics Analysis Reveals Bde47 Induces Depression-Like Behaviors in Mice by Interfering with the 2-Arachidonoyl Glycerol-Associated Microbiota-Gut-Brain Axis. Ecotoxicol. Environ. Saf. 2023, 259, 115041. [Google Scholar] [CrossRef]
- Emereole, C.; Jansen, R.; Okonkwo, O.J. Polybrominated Diphenyl Ethers in the Grey-Headed Gull (Larus cirrocephalus) and African Sacred Ibis (Threskiornis aethiopicus). Environ. Sci.-Adv. 2023, 2, 1651–1661. [Google Scholar] [CrossRef]
- Cao, M.; Xu, T.; Zhang, H.; Wei, S.; Wang, H.; Song, Y.; Guo, X.; Chen, D.; Yin, D. Bde-47 Causes Depression-Like Effects in Zebrafish Larvae Via a Non-Image-Forming Visual Mechanism. Environ. Sci. Technol. 2023, 57, 9592X–9602X. [Google Scholar] [CrossRef]
- Yang, C.; Wei, J.; Cao, G.; Cai, Z. Lipid Metabolism Dysfunction and Toxicity of Bde-47 Exposure in White Adipose Tissue Revealed by the Integration of Lipidomics and Metabolomics. Sci. Total Environ. 2022, 806, 150350. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, M.; Fan, Y.; Wang, J.; Jiang, S.; Abudureman, H. Bidirectional Regulation of Bde-47 on 3t3-L1 Cell Differentiation Based on a Restricted Cubic Spline Model. Toxicol. Ind. Health 2022, 38, 481–492. [Google Scholar] [CrossRef]
- Pietron, W.J.; Malagocki, P.; Warenik-Bany, M. Feed as a Source of Polybrominated Diphenyl Ethers (Pbdes). Environ. Res. 2023, 231, 116257. [Google Scholar] [CrossRef]
- Hossain, M.M.; Shahjahan, M.; Rahman, M.H.; Khan, S.; Saha, N. Development of a Sustainable Polyculture Technique Using Asian Watergrass as Fish Feed in the Southern Coastal Region of Bangladesh. Aquac. Stud. 2024, 24, AQUAST1309. [Google Scholar] [CrossRef]
- Shen, N.; Song, Z.; Xia, C.; Mu, H.; Chen, X.; Cheng, H.; Xu, J.; Sun, Y.; Wei, C.; Zhang, L. Comparative Evaluation of Soybean Meal Vs. Extruded Soybean Meal as a Replacer for Fishmeal in Diets of Olive Flounder (Paralichthys olivaceus): Effects on Growth Performance and Muscle Quality. Aquaculture 2024, 578, 740136. [Google Scholar] [CrossRef]
- Wei, J.T.; Li, X.N.; Xiang, L.; Song, Y.Y.; Liu, Y.C.; Jiang, Y.Y.; Cai, Z.W. Metabolomics and Lipidomics Study Unveils the Impact of Polybrominated Diphenyl Ether-47 on Breast Cancer Mice. J. Hazard. Mater. 2020, 390, 121451. [Google Scholar] [CrossRef] [PubMed]
- Soulen, B.K.; Venables, B.J.; Johnston, D.W.; Roberts, A.P. Accumulation of Pbdes in Stranded Harp (Pagophilus groenlandicus) and Hooded Seals (Cystophora cristata) from the Northeastern United States. Mar. Environ. Res. 2018, 138, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Erratico, C.; Currier, H.; Szeitz, A.; Bandiera, S.; Covaci, A.; Elliott, J. Levels of Pbdes in Plasma of Juvenile Starlings (Sturnus vulgaris) from British Columbia, Canada and Assessment of Pbde Metabolism by Avian Liver Microsomes. Sci. Total Environ. 2015, 518, 31–37. [Google Scholar] [CrossRef]
- Gao, Q.; Zhou, Z.Y.; He, Y.N.; Dong, M.H.; Wang, Z.N.; Chen, H.M. Bde-47 Induces Immunotoxicity in Raw264.7 Macrophages through the Reactive Oxygen Species-Mediated Mitochondrial Apoptotic Pathway. Molecules 2023, 28, 2036. [Google Scholar] [CrossRef]
- Wang, B.Y.; Guo, Y.; Zhou, B.; Zhang, H.; Cui, X.Y.; Sun, Y.; Wang, Y. A Possible Speculation on the Involvement of Ros and Lysosomes Mediated Mitochondrial Pathway in Apoptosis of Rotifer Brachionus plicatilis with Bde-47 Exposure. Sci. Total Environ. 2021, 787, 147315. [Google Scholar] [CrossRef]
- Yang, J.; Chan, K.M. Evaluation of the Toxic Effects of Brominated Compounds (Bde-47, 99, 209, Tbbpa) and Bisphenol a (Bpa) Using a Zebrafish Liver Cell Line, ZFL. Aquat. Toxicol. 2015, 159, 138–147. [Google Scholar] [CrossRef]
- Lu, G.H.; Qi, P.D.; Chen, W. Integrated Biomarker Responses of Carassius auratus Exposed to Bde-47, Bde-99 and Their Mixtures. Int. J. Environ. Res. 2013, 7, 907–916. [Google Scholar]
- Ji, C.; Wu, H.; Wei, L.; Zhao, J.; Lu, H.; Yu, J. Proteomic and Metabolomic Analysis of Earthworm Eisenia fetida Exposed to Different Concentrations of 2,2′,4,4′-Tetrabromodiphenyl Ether. J. Proteom. 2013, 91, 405–416. [Google Scholar] [CrossRef]
- McIntyre, R.L.; Kenerson, H.L.; Subramanian, S.; Wang, S.A.; Kazami, M.; Stapleton, H.M.; Yeung, R.S. Polybrominated Diphenyl Ether Congener, Bde-47, Impairs Insulin Sensitivity in Mice with Liver-Specific Pten Deficiency. BMC Obes. 2015, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, G.; Li, Y.; Zhang, Y. Biotransformation Kinetics and Pathways of 2,2′,4,4′-Tetrabromodiphenyl Ether (Bde-47) and Its Hydroxylated and Methoxylated Derivatives (6-Oh-Bde-47 and 6-Meo-Bde-47) in Earthworms (Eisenia fetida). Sci. Total Environ. 2023, 855, 158934. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Wei, S.; Hu, M.; Wei, H.; Wang, X.; Shang, Y.; Li, L.; Shi, H.; Wang, Y. Microplastics Aggravate the Adverse Effects of Bde-47 on Physiological and Defense Performance in Mussels. J. Hazard. Mater. 2020, 398, 122909. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Zhao, J.; Wu, H. Gender-Specific Metabolic Responses in Gonad of Mussel Mytilus galloprovincialis to 2,2′,4,4′-Tetrabromodiphenyl Ether. Environ. Toxicol. Pharmacol. 2014, 37, 1116–1122. [Google Scholar] [CrossRef]
- Yang, C.; Wong, C.M.; Wei, J.; Chung, A.C.K.; Cai, Z. The Brominated Flame Retardant Bde 47 Upregulates Purine Metabolism and Mitochondrial Respiration to Promote Adipocyte Differentiation. Sci. Total Environ. 2018, 644, 1312–1322. [Google Scholar] [CrossRef]
- Saquib, Q.; Siddiqui, M.A.; Ahmed, J.; Al-Salim, A.; Ansari, S.M.; Faisal, M.; Al-Khedhairy, A.A.; Musarrat, J.; AlWathnani, H.A.; Alatar, A.A.; et al. Hazards of Low Dose Flame-Retardants (Bde-47 and Bde-32): Influence on Transcriptome Regulation and Cell Death in Human Liver Cells. J. Hazard. Mater. 2016, 308, 37–49. [Google Scholar] [CrossRef]
- Xing, X.; Kang, J.; Qiu, J.; Zhong, X.; Shi, X.; Zhou, B.; Wei, Y. Waterborne Exposure to Low Concentrations of Bde-47 Impedes Early Vascular Development in Zebrafish Embryos/Larvae. Aquat. Toxicol. 2018, 203, 19–27. [Google Scholar] [CrossRef] [PubMed]
- De Guia, R.M.; Rose, A.J.; Herzig, S. Glucocorticoid Hormones and Energy Homeostasis. Horm. Mol. Biol. Clin. Investig. 2014, 19, 117–128. [Google Scholar] [CrossRef]
- Vengerovskii, A.I.; Baturina, N.O.; Saratikov, A.S. The Effect of Glucocorticoid Preparations on Liver Metabolism. Eksperimental’naia I Klin. Farmakol. 1999, 62, 75–80. [Google Scholar]
- Kiani, P.; Khodadadi, E.S.; Nikdasti, A.; Yarahmadi, S.; Gheibi, M.; Yousefi, Z.; Ehtiati, S.; Yahyazadeh, S.; Shafiee, S.M.; Taghizadeh, M.; et al. Autophagy and the Peroxisome Proliferator-Activated Receptor Signaling Pathway: A Molecular Ballet in Lipid Metabolism and Homeostasis. Mol. Cell. Biochem. 2025, 480, 3477–3499. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, Z.-H.; Zabed, H.M.; Yun, J.; Zhang, G.; Qi, X. An Insight into the Roles of Dietary Tryptophan and Its Metabolites in Intestinal Inflammation and Inflammatory Bowel Disease. Mol. Nutr. Food Res. 2021, 65, 2000461. [Google Scholar] [CrossRef]
- Li, Z.; Liang, Z.; Zhang, J.; Liu, S. Role of Glycerophospholipid Metabolism in Epilepsy. Curr. Neuropharmacol. 2025, 23, 1–17. [Google Scholar] [CrossRef]
- Yuan, X.; Ferrari, D.; Mills, T.; Wang, Y.; Czopik, A.; Doursout, M.-F.; Evans, S.E.; Idzko, M.; Eltzschig, H.K. Editorial: Purinergic Signaling and Inflammation. Front. Immunol. 2021, 12, 699069. [Google Scholar] [CrossRef]
- Clark, D.A.; Munshi, U.K. Feeding Associated Neonatal Necrotizing Enterocolitis (Primary Nec) Is an Inflammatory Bowel Disease. Pathophysiol. Off. J. Int. Soc. Pathophysiol. 2014, 21, 29–34. [Google Scholar] [CrossRef]
- Lin, J.; Lin, K.; Huang, L.; Jiang, Y.; Ding, X.; Luo, W.; Samorodov, A.V.; Pavlov, V.N.; Liang, G.; Qian, J.; et al. Heme Induces Inflammatory Injury by Directly Binding to the Complex of Myeloid Differentiation Protein 2 and Toll-Like Receptor 4. Toxicol. Lett. 2022, 370, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, S.; Thadathil, N.; Selvarani, R.; Nicklas, E.H.; Wang, D.; Miller, B.F.; Richardson, A.; Deepa, S.S. Necroptosis Contributes to Chronic Inflammation and Fibrosis in Aging Liver. Aging Cell 2021, 20, e13512. [Google Scholar] [CrossRef] [PubMed]
- Parveen, S.; Fatma, M.; Mir, S.S.; Dermime, S.; Uddin, S. Jak-Stat Signaling in Autoimmunity and Cancer. ImmunoTargets Ther. 2025, 14, 523–554. [Google Scholar] [CrossRef]
- Masenga, S.K.; Desta, S.; Hatcher, M.; Kirabo, A.; Lee, D.L. How Ppar-Alpha Mediated Inflammation May Affect the Pathophysiology of Chronic Kidney Disease. Curr. Res. Physiol. 2025, 8, 100133. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Colby, J.K.; Zuo, X.; Jaoude, J.; Wei, D.; Shureiqi, I. The Role of Ppar-Δ in Metabolism, Inflammation, and Cancer: Many Characters of a Critical Transcription Factor. Int. J. Mol. Sci. 2018, 19, 3339. [Google Scholar] [CrossRef]
- Hsu, W.H.; Lee, B.H.; Pan, T.M. Monascin Attenuates Oxidative Stress-Mediated Lung Inflammation Via Peroxisome Proliferator-Activated Receptor-Gamma (Ppar-Γ) and Nuclear Factor-Erythroid 2 Related Factor 2 (Nrf-2) Modulation. J. Agric. Food Chem. 2014, 62, 5337–5344. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, Y.; Lv, G.; Wang, H. Ferroptosis as a Therapeutic Target for Inflammation-Related Intestinal Diseases. Front. Pharmacol. 2023, 14, 1095366. [Google Scholar] [CrossRef]
- Li, C.; Yuan, M.; Du, J.; Chen, Z.; Chen, S.; Ji, X.; Tang, N.; Chen, D.; Li, Z.; Zhang, X. New Insights on the Protection of Endangered Aquatic Species: Embryotoxicity Effects of 2,2′,4,4′-Tetrabromodiphenyl Ether (Bde-47) Via Integrin-Mediated Oxidative Stress and Inflammatory Pathways in Siberian Sturgeon, Acipenser baerii. Fish Shellfish Immunol. 2025, 161, 110307. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Zhang, Y.Q.; Fan, S.H.; Zhuang, J.; Zheng, Y.L.; Lu, J.; Wu, D.M.; Shan, Q.; Hu, B. Troxerutin Protects against 2,2′,4,4′-Tetrabromodiphenyl Ether (Bde-47)-Induced Liver Inflammation by Attenuating Oxidative Stress-Mediated Nad+-Depletion. J. Hazard. Mater. 2015, 283, 98–109. [Google Scholar] [CrossRef]
- Wu, J.; Hao, Z.; Wang, Y.; Yan, D.; Meng, J.; Ma, H. Melatonin Alleviates Bde-209-Induced Cognitive Impairment and Hippocampal Neuroinflammation by Modulating Microglia Polarization Via Sirt1-Mediated Hmgb1/Tlr4/Nf-Κb Pathway. Food Chem. Toxicol. 2023, 172, 113561. [Google Scholar] [CrossRef] [PubMed]
- Karpeta, A.; Rak-Mardyla, A.; Jerzak, J.; Gregoraszczuk, E.L. Congener-Specific Action of Pbdes on Steroid Secretion, Cyp17, 17β-Hsd and Cyp19 Activity and Protein Expression in Porcine Ovarian Follicles. Toxicol. Lett. 2011, 206, 258–263. [Google Scholar] [CrossRef]
- Saquib, Q.; Siddiqui, M.A.; Ahmad, J.; Ansari, S.M.; Al-Wathnani, H.A.; Rensing, C. 6-Ohbde-47 Induces Transcriptomic Alterations of Cyp1a1, Xrcc2, Hspa1a, Egr1 Genes and Trigger Apoptosis in Hepg2 Cells. Toxicology 2018, 400, 40–47. [Google Scholar] [CrossRef]
- Dungar, B.M.; Schupbach, C.D.; Jacobson, J.R.; Kopf, P.G. Adrenal Corticosteroid Perturbation by the Endocrine Disruptor Bde-47 in a Human Adrenocortical Cell Line and Male Rats. Endocrinology 2021, 162, bqab160. [Google Scholar] [CrossRef]
- Sharthiya, H.; Schupbach, C.; Kopf, P.; Dineley, K.; Malaiyandi, L. The Polybrominated Flame Retardant Bde-47 Increases Steroidogenesis and Alters Mitochondrial Activity and Morphology in the Hac15 Human Adrenocortical Cell Line. Faseb J. 2018, 32, 831.1. [Google Scholar] [CrossRef]
- Maria Beltran, E.; Gonzalez-Doncel, M.; Enrique Garcia-Maurino, J.; Garcia Hortiguela, P.; Victoria Pablos, M. Effects of Life Cycle Exposure to Dietary 2,2′, 4,4′-Tetrabromodiphenyl Ether (Bde-47) on Medaka Fish (Oryzias latipes). Aquat. Toxicol. 2022, 245, 106133. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.D. Study on Cytotoxicity Effects of Bde-47 and Its Induction Mechanism to Rxr Receptors in Human L02 Liver Cells. Master’s Thesis, Sun Yat-sen University, Guangzhou, China, 2011. [Google Scholar]
- Zhu, Y.; Tan, Y.Q.; Wang, C.C.; Leung, L.K. The Flame Retardant 2,2’,4,4’-Tetrabromodiphenyl Ether Enhances the Expression of Corticotropin-Releasing Hormone in the Placental Cell Model Jeg-3. Chemosphere 2017, 174, 499–505. [Google Scholar] [CrossRef]
- Nakamura, M.T.; Yudell, B.E.; Loor, J.J. Regulation of Energy Metabolism by Long-Chain Fatty Acids. Prog. Lipid Res. 2014, 53, 124–144. [Google Scholar] [CrossRef]
- Krumm, E.A.; Patel, V.J.; Tillery, T.S.; Yasrebi, A.; Shen, J.; Guo, G.L.; Marco, S.M.; Buckley, B.T.; Roepke, T.A. Organophosphate Flame-Retardants Alter Adult Mouse Homeostasis and Gene Expression in a Sex-Dependent Manner Potentially through Interactions with Erα. Toxicol. Sci. 2018, 162, 212–224. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Wu, R.; Wang, X.; Huang, W.; Zheng, S.; Zhang, Q.; Peng, J.; Tan, W.; Wu, K. Effects of 2,2′,4,4′-Tetrabromodiphenyl Ether (Bde-47) on Reproductive and Endocrine Function in Female Zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2022, 248, 114326. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, Y.B.; Hao, J.; Zhu, J.E.Y.; Tang, N.; Qi, J.W.; Wang, S.Y.; Wang, H.; Peng, S.; Liu, J.; et al. Intraperitoneal Injection Urocortin-3 Reduces the Food Intake of Siberian Sturgeon (Acipenser baerii). Peptides 2016, 85, 80–88. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Han, N.; Huang, Y.; Sun, H.; Liu, Y.; Chen, D.; Li, Z.; Zhang, X. Dietary Exposure to 2,2′,4,4′-Tetrabromodiphenyl Ether (BDE-47) Causes Inflammation in the Liver of Common Carp (Cyprinus carpio) and Affects Lipid Metabolism by Interfering with Steroid Hormone Biosynthesis Pathways. Int. J. Mol. Sci. 2025, 26, 10152. https://doi.org/10.3390/ijms262010152
Chen S, Han N, Huang Y, Sun H, Liu Y, Chen D, Li Z, Zhang X. Dietary Exposure to 2,2′,4,4′-Tetrabromodiphenyl Ether (BDE-47) Causes Inflammation in the Liver of Common Carp (Cyprinus carpio) and Affects Lipid Metabolism by Interfering with Steroid Hormone Biosynthesis Pathways. International Journal of Molecular Sciences. 2025; 26(20):10152. https://doi.org/10.3390/ijms262010152
Chicago/Turabian StyleChen, Shuhuang, Nian Han, Yujie Huang, Huimin Sun, Youlian Liu, Defang Chen, Zhiqiong Li, and Xin Zhang. 2025. "Dietary Exposure to 2,2′,4,4′-Tetrabromodiphenyl Ether (BDE-47) Causes Inflammation in the Liver of Common Carp (Cyprinus carpio) and Affects Lipid Metabolism by Interfering with Steroid Hormone Biosynthesis Pathways" International Journal of Molecular Sciences 26, no. 20: 10152. https://doi.org/10.3390/ijms262010152
APA StyleChen, S., Han, N., Huang, Y., Sun, H., Liu, Y., Chen, D., Li, Z., & Zhang, X. (2025). Dietary Exposure to 2,2′,4,4′-Tetrabromodiphenyl Ether (BDE-47) Causes Inflammation in the Liver of Common Carp (Cyprinus carpio) and Affects Lipid Metabolism by Interfering with Steroid Hormone Biosynthesis Pathways. International Journal of Molecular Sciences, 26(20), 10152. https://doi.org/10.3390/ijms262010152




