Special Issue “Synthesis, Properties and Applications of Polymers”
Conflicts of Interest
References
- Millican, J.M.; Agarwal, S. Plastic pollution: A material problem? Macromolecules 2021, 54, 4455–4469. [Google Scholar] [CrossRef]
- Thompson, R.C.; Courtene-Jones, W.; Boucher, J.; Pahl, S.; Raubenheimer, K.; Koelmans, A.A. Twenty years of microplastic pollution research—What have we learned? Science 2024, 386, eadl2746. [Google Scholar] [CrossRef] [PubMed]
- Jagur-Grodzinski, J. Polymers for tissue engineering, medical devices, and regenerative medicine. Concise general review of recent studies. Polym. Adv. Technol. 2006, 17, 395–418. [Google Scholar] [CrossRef]
- Maitz, M.F. Applications of synthetic polymers in clinical medicine. Biosurface Biotribology 2015, 1, 161–176. [Google Scholar] [CrossRef]
- Chen, X.; Wu, T.; Bu, Y.; Yan, H.; Lin, Q. Fabrication and biomedical application of alginate composite hydrogels in bone tissue engineering: A review. Int. J. Mol. Sci. 2024, 25, 7810. [Google Scholar] [CrossRef]
- El-Ghoul, Y.; Alminderej, F.M.; Alsubaie, F.M.; Alrasheed, R.; Almousa, N.H. Recent advances in functional polymer materials for energy, water, and biomedical applications: A review. Polymers 2021, 13, 4327. [Google Scholar] [CrossRef]
- Farjaminejad, S.; Farjaminejad, R.; Hasani, M.; Garcia-Godoy, F.; Abdouss, M.; Marya, A.; Harsoputranto, A.; Jamilian, A. Advances and challenges in polymer-based scaffolds for bone tissue engineering: A path towards personalized regenerative medicine. Polymers 2024, 16, 3303. [Google Scholar] [CrossRef]
- Englert, C.; Brendel, J.C.; Majdanski, T.C.; Yildirim, T.; Schubert, S.; Gottschaldt, M.; Windhab, N.; Schubert, U.S. Pharmapolymers in the 21st century: Synthetic polymers in drug delivery applications. Prog. Polym. Sci. 2018, 87, 107–164. [Google Scholar] [CrossRef]
- Sánchez-Cid, P.; Jiménez-Rosado, M.; Romero, A.; Pérez-Puyana, V. Novel trends in hydrogel development for biomedical applications: A review. Polymers 2022, 14, 3023. [Google Scholar] [CrossRef]
- Wu, X.; Chen, X.; Zhang, Q.M.; Tan, D.Q. Advanced dielectric polymers for energy storage. Energy Storage Mater. 2022, 44, 29–47. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, L.; Han, Z.; Li, Q.; He, J.; Wang, Q. Self-healing polymers for electronics and energy devices. Chem. Rev. 2022, 123, 558–612. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama-Sato, K.; Oyaizu, K. Redox: Organic robust radicals and their polymers for energy conversion/storage devices. Chem. Rev. 2023, 123, 11336–11391. [Google Scholar] [CrossRef]
- Wu, W.; Tang, R.; Li, Q.; Li, Z. Functional hyperbranched polymers with advanced optical, electrical and magnetic properties. Chem. Soc. Rev. 2015, 44, 3997–4022. [Google Scholar] [CrossRef]
- Ando, S.; Tomita, I.; Takagi, K. Polymers for Optical Communications; CRC Press: Boca Raton, FL, USA, 2025. [Google Scholar]
- Mizuno, Y.; Theodosiou, A.; Kalli, K.; Liehr, S.; Lee, H.; Nakamura, K. Distributed polymer optical fiber sensors: A review and outlook. Photonics Res. 2021, 9, 1719–1733. [Google Scholar] [CrossRef]
- Aslam Khan, M.U.; Abd Razak, S.I.; Al Arjan, W.S.; Nazir, S.; Sahaya Anand, T.J.; Mehboob, H.; Amin, R. Recent advances in biopolymeric composite materials for tissue engineering and regenerative medicines: A review. Molecules 2021, 26, 619. [Google Scholar] [CrossRef]
- Santhamoorthy, M.; Kim, S.C. A Review of the Development of Biopolymer Hydrogel-Based Scaffold Materials for Drug Delivery and Tissue Engineering Applications. Gels 2025, 11, 178. [Google Scholar] [CrossRef]
- Wu, D.T.; Munguia-Lopez, J.G.; Cho, Y.W.; Ma, X.; Song, V.; Zhu, Z.; Tran, S.D. Polymeric scaffolds for dental, oral, and craniofacial regenerative medicine. Molecules 2021, 26, 7043. [Google Scholar] [CrossRef]
- Phutane, P.; Telange, D.; Agrawal, S.; Gunde, M.; Kotkar, K.; Pethe, A. Biofunctionalization and applications of polymeric nanofibers in tissue engineering and regenerative medicine. Polymers 2023, 15, 1202. [Google Scholar] [CrossRef]
- Opriș, O.; Mormile, C.; Lung, I.; Stegarescu, A.; Soran, M.L.; Soran, A. An overview of biopolymers for drug delivery applications. Appl. Sci. 2024, 14, 1383. [Google Scholar] [CrossRef]
- Dubashynskaya, N.V.; Petrova, V.A.; Skorik, Y.A. Biopolymer drug delivery systems for oromucosal application: Recent trends in pharmaceutical R&D. Int. J. Mol. Sci. 2024, 25, 5359. [Google Scholar] [CrossRef] [PubMed]
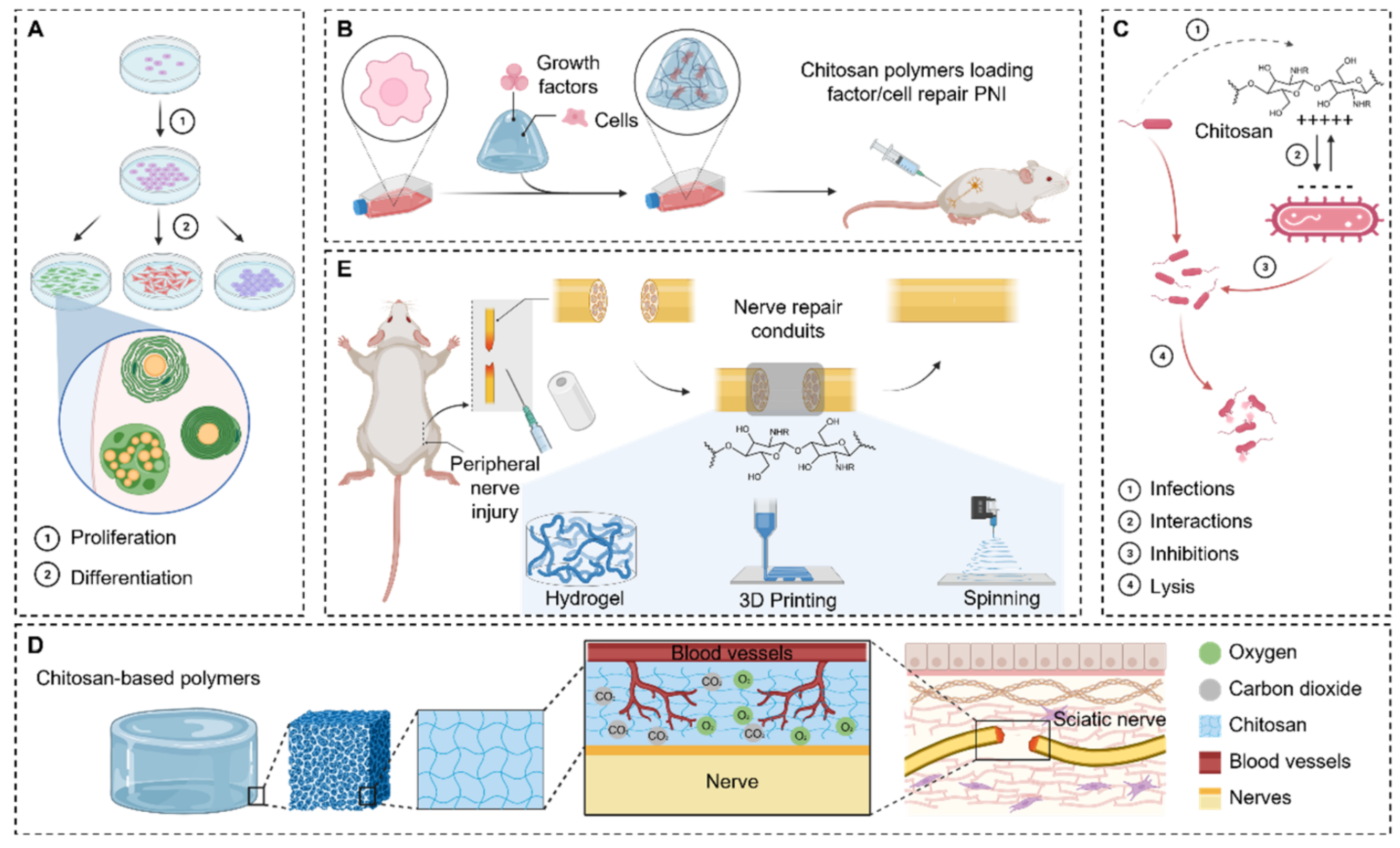
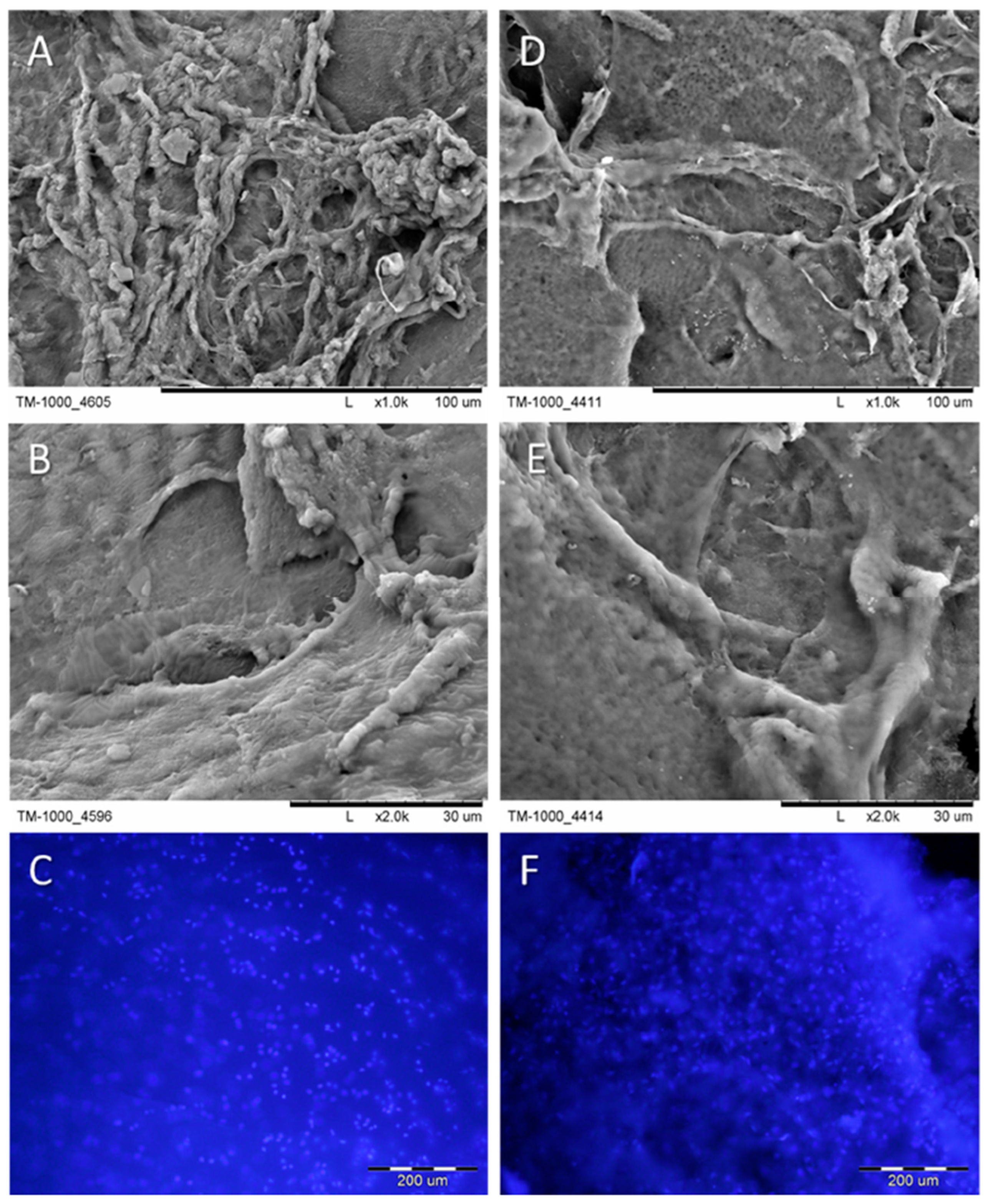
- Zhang, M.; An, H.; Zhang, F.; Jiang, H.; Wan, T.; Wen, Y.; Han, N.; Zhang, P. Prospects of using chitosan-based biopolymers in the treatment of peripheral nerve injuries. Int. J. Mol. Sci. 2023, 24, 12956. [Google Scholar] [CrossRef]
- Jakutowicz, T.; Wasyłeczko, M.; Płończak, M.; Wojciechowski, C.; Chwojnowski, A.; Czubak, J. Comparative Study of Autogenic and Allogenic Chondrocyte Transplants on Polyethersulfone Scaffolds for Cartilage Regeneration. Int. J. Mol. Sci. 2024, 25, 9075. [Google Scholar] [CrossRef]
- Lu, D.; Chai, A.; Hu, X.; Zhong, P.; Kang, N.; Kuang, X.; Yang, Z. Conformational Transition of Semiflexible Ring Polyelectrolyte in Tetravalent Salt Solutions: A Simple Numerical Modeling without the Effect of Twisting. Int. J. Mol. Sci. 2024, 25, 8268. [Google Scholar] [CrossRef]
- Lu, X.; Wang, Y.; Xu, X.; Yan, B.; Wu, T.; Lu, L. Polymer-based solid-state electrolytes for high-energy-density lithium-ion batteries–review. Adv. Energy Mater. 2023, 13, 2301746. [Google Scholar] [CrossRef]
- Janek, J.; Zeier, W.G. Challenges in speeding up solid-state battery development. Nat. Energy 2023, 8, 230–240. [Google Scholar] [CrossRef]
- Zheng, F.; Kotobuki, M.; Song, S.; Lai, M.O.; Lu, L. Review on solid electrolytes for all-solid-state lithium-ion batteries. J. Power Sources 2018, 389, 198–213. [Google Scholar] [CrossRef]
- Shaplov, A.S.; Ponkratov, D.O.; Vygodskii, Y.S. Poly (ionic liquid) s: Synthesis, properties, and application. Polym. Sci. Ser. B 2016, 58, 73–142. [Google Scholar] [CrossRef]
- Shaplov, A.S.; Marcilla, R.; Mecerreyes, D. Recent advances in innovative polymer electrolytes based on poly (ionic liquid) s. Electrochim. Acta 2015, 175, 18–34. [Google Scholar] [CrossRef]
- Machín, A.; Morant, C.; Márquez, F. Advancements and challenges in solid-state battery technology: An in-depth review of solid electrolytes and anode innovations. Batteries 2024, 10, 29. [Google Scholar] [CrossRef]
- Kiriy, N.; Özenler, S.; Voigt, P.; Kobsch, O.; Meier-Haack, J.; Arnhold, K.; Janke, A.; Muza, U.L.; Geisler, M.; Lederer, A.; et al. Optimizing the ion conductivity and mechanical stability of polymer electrolyte membranes designed for use in lithium ion batteries: Combining imidazolium-containing poly (ionic liquids) and poly (propylene carbonate). Int. J. Mol. Sci. 2024, 25, 1595. [Google Scholar] [CrossRef]
- Andjela, L.; Abdurahmanovich, V.M.; Vladimirovna, S.N.; Mikhailovna, G.I.; Yurievich, D.D.; Alekseevna, M.Y. A review on Vat Photopolymerization 3D-printing processes for dental application. Dent. Mater. 2022, 38, e284–e296. [Google Scholar] [CrossRef] [PubMed]
- Caussin, E.; Moussally, C.; Le Goff, S.; Fasham, T.; Troizier-Cheyne, M.; Tapie, L.; Dursun, E.; Attal, J.-P.; François, P. Vat photopolymerization 3D printing in dentistry: A comprehensive review of actual popular technologies. Materials 2024, 17, 950. [Google Scholar] [CrossRef] [PubMed]
- Generalova, A.N.; Demina, P.A.; Akasov, R.A.; Khaydukov, E.V. Photopolymerization in 3D printing of tissue-engineered constructs for regenerative medicine. Russ. Chem. Rev. 2023, 92, RCR5068. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Zhang, X.; Zhang, Y.; Hou, D. Recent progress of the vat photopolymerization technique in tissue engineering: A brief review of mechanisms, methods, materials, and applications. Polymers 2023, 15, 3940. [Google Scholar] [CrossRef]
- Hu, Y.; Luo, Z.; Bao, Y. Trends in photopolymerization 3D printing for advanced drug delivery applications. Biomacromolecules 2024, 26, 85–117. [Google Scholar] [CrossRef]
- Ribas-Massonis, A.; Cicujano, M.; Duran, J.; Besalú, E.; Poater, A. Free-radical photopolymerization for curing products for refinish coatings market. Polymers 2022, 14, 2856. [Google Scholar] [CrossRef]
- Pagac, M.; Hajnys, J.; Ma, Q.P.; Jancar, L.; Jansa, J.; Stefek, P.; Mesicek, J. A review of vat photopolymerization technology: Materials, applications, challenges, and future trends of 3D printing. Polymers 2021, 13, 598. [Google Scholar] [CrossRef]
- Layani, M.; Wang, X.; Magdassi, S. Novel materials for 3D printing by photopolymerization. Adv. Mater. 2018, 30, 1706344. [Google Scholar] [CrossRef]
- Kholkhoev, B.C.; Bardakova, K.N.; Epifanov, E.O.; Matveev, Z.A.; Shalygina, T.A.; Atutov, E.B.; Voronina, S.Y.; Timashev, P.S.; Burdukovskii, V.F. A photosensitive composition based on an aromatic polyamide for LCD 4D printing of shape memory mechanically robust materials. Chem. Eng. J. 2023, 454, 140423. [Google Scholar] [CrossRef]
- He, X.; Zang, L.; Xin, Y.; Zou, Y. An overview of photopolymerization and its diverse applications. Appl. Res. 2023, 2, e202300030. [Google Scholar] [CrossRef]
- Sapozhnikov, D.A.; Volkova, T.V.; Sakharova, A.A.; Gasanov, R.G.; Voytekunas, V.Y.; Abadie, M.J.M.; Sanchez, J.-Y.; Vygodskii, Y.S. Photopolymerization of (meth) acrylates in the presence of polyheteroarylenes. Polym. Sci. Ser. B 2009, 51, 1–12. [Google Scholar] [CrossRef]
- Vygodskii, Y.S.; Sapozhnikov, D.A.; Bayminov, B.A.; Semjonov, S.L.; Kosolapov, A.F.; Plastinin, E.A. In situ synthesis of copolymers based on polyvinylpyrrolidone and condensation polymers and their use as optical fiber coatings. Prog. Org. Coat. 2016, 99, 210–215. [Google Scholar] [CrossRef]
- Vygodskii, Y.S.; Volkova, T.V.; Sakharova, A.A.; Sapozhnikov, D.A.; Nikiforova, G.G.; Matieva, A.M. Three-dimensional free-radical copolymerization of methyl methacrylate with diunsaturated monomers in the presence of an aromatic polyimide. Polym. Sci. Ser. A 2006, 48, 683–688. [Google Scholar] [CrossRef]
- Kholkhoev, B.C.; Nikishina, A.N.; Bardakova, K.N.; Matveev, Z.A.; Sapozhnikov, D.A.; Efremov, Y.M.; Timashev, P.S.; Burdukovskii, V.F. 4D-printing of high-temperature shape-memory polymers based on polyimide, N, N-dimethylacrylamide and photoactive cross-linkers. Polymer 2024, 299, 126978. [Google Scholar] [CrossRef]
- Sapozhnikov, D.A.; Melnik, O.A.; Chuchalov, A.V.; Kovylin, R.S.; Chesnokov, S.A.; Khanin, D.A.; Nikiforova, G.G.; Kosolapov, A.F.; Semjonov, S.L.; Vygodskii, Y.S. Soluble Fluorinated Cardo Copolyimide as an Effective Additive to Photopolymerizable Compositions Based on Di (meth) acrylates: Application for Highly Thermostable Primary Protective Coating of Silica Optical Fiber. Int. J. Mol. Sci. 2024, 25, 5494. [Google Scholar] [CrossRef] [PubMed]
- Vygodskii, Y.S.; Matieva, A.; Volkova, T.; Sakharova, A.; Sapozhnikov, D. (Co)polymerization of styrene in the presence of polyheteroarylenes. Polym. Sci. Ser. A 2004, 46, 352–360. [Google Scholar]
- Vygodskii, Y.S.; Volkova, T.; Sakharova, A.; Sapozhnikov, D.; Nikiforova, G.; Buzin, M. Three-dimensional copolymerization of methyl methacrylate and allyl methacrylate in the presence of aromatic polyimide. Polym. Sci. Ser. A 2004, 46, 681–687. [Google Scholar]
- Vygodskii, Y.S.; Volkova, T.Y.V.; Batalova, T.Y.L.; Zabegaeva, O.N.; Belavtseva, E.M.; Sakharova, A.A.; Gasanov, R.G.; Sapozhnikov, D.A.; Voytekunas, V.Y. Copolymers obtained by ε-caprolactam and methyl methacrylate polymerization in the presence of polyimides. High Perform. Polym. 2009, 21, 579–595. [Google Scholar] [CrossRef]
- Vygodskii, Y.S.; Volkova, T.; Sakharova, A.; Sapozhnikov, D.; Matieva, A. Influence of poly (heteroarylenes) with different molecular masses on free-radical polymerization of methyl methacrylate. Polym. Sci. Ser. A 2002, 44, 1249–1254. [Google Scholar]
- Sapozhnikov, D.A.; Baiminov, B.A.; Vygodskii, Y.S. Highly Heat-Resistant Polymeric Coatings of Optical Fibers. Polym. Sci. Ser. C 2020, 62, 165–171. [Google Scholar] [CrossRef]
- Mota, F.A.; Passos, M.L.; Santos, J.L.; Saraiva, M.L.M. Comparative analysis of electrochemical and optical sensors for detection of chronic wounds biomarkers: A review. Biosens. Bioelectron. 2024, 251, 116095. [Google Scholar] [CrossRef]
- Guliy, O.I.; Karavaeva, O.A.; Smirnov, A.V.; Eremin, S.A.; Bunin, V.D. Optical sensors for bacterial detection. Sensors 2023, 23, 9391. [Google Scholar] [CrossRef]
- Elsherif, M.; Salih, A.E.; Muñoz, M.G.; Alam, F.; AlQattan, B.; Antonysamy, D.S.; Zaki, M.F.; Yetisen, A.K.; Park, S.; Wilkinson, T.D.; et al. Optical fiber sensors: Working principle, applications, and limitations. Adv. Photonics Res. 2022, 3, 2100371. [Google Scholar] [CrossRef]
- Panda, P.; Chakroborty, S. Optical sensor technology and its application in detecting environmental effluents: A review. Int. J. Environ. Anal. Chem. 2024, 104, 4057–4072. [Google Scholar] [CrossRef]
- Qu, X.; Hu, Y.; Xu, C.; Li, Y.; Zhang, L.; Huang, Q.; Moshirian-Farahi, S.S.; Zhang, J.; Xu, X.; Liao, M.; et al. Optical sensors of volatile organic compounds for non-invasive diagnosis of diseases. Chem. Eng. J. 2024, 485, 149804. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sapozhnikov, D.A. Special Issue “Synthesis, Properties and Applications of Polymers”. Int. J. Mol. Sci. 2025, 26, 10147. https://doi.org/10.3390/ijms262010147
Sapozhnikov DA. Special Issue “Synthesis, Properties and Applications of Polymers”. International Journal of Molecular Sciences. 2025; 26(20):10147. https://doi.org/10.3390/ijms262010147
Chicago/Turabian StyleSapozhnikov, Dmitriy A. 2025. "Special Issue “Synthesis, Properties and Applications of Polymers”" International Journal of Molecular Sciences 26, no. 20: 10147. https://doi.org/10.3390/ijms262010147
APA StyleSapozhnikov, D. A. (2025). Special Issue “Synthesis, Properties and Applications of Polymers”. International Journal of Molecular Sciences, 26(20), 10147. https://doi.org/10.3390/ijms262010147



